Abstract
Dopamine decreases Na-K-ATPase (NKA) activity by PKC-dependent phosphorylation and endocytosis of the NKA α1. Dopamine-mediated regulation of NKA is impaired in aging and some forms of hypertension. Using opossum (OK) proximal tubule cells (PTCs), we demonstrated that sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) associates with NKA α1 and dopamine-1 receptor (D1R). This association is required for the dopamine-mediated regulation of NKA. In OK cells, dopamine decreases NHERF-1 association with NKA α1 but increases its association with D1R. However, it is not known whether NHERF-1 plays a role in dopamine-mediated NKA regulation in animal models of hypertension. We hypothesized that defective dopamine-mediated regulation of NKA results from the decrease in NHERF-1 expression in rat renal PTCs isolated from animal models of hypertension [spontaneously hypertensive rats (SHRs) and aged F344 rats]. To test this hypothesis, we isolated and cultured renal PTCs from 22-mo-old F344 rats and their controls, normotensive 4-mo-old F344 rats, and SHRs and their controls, normotensive Wistar-Kyoto (WKY) rats. The results demonstrate that in both hypertensive models (SHR and aged F344), NHERF-1 expression, dopamine-mediated phosphorylation of NKA, and ouabain-inhibitable K+ transport are reduced. Transfection of NHERF-1 into PTCs from aged F344 and SHRs restored dopamine-mediated inhibition of NKA. These results suggest that decreased renal NHERF-1 expression contributes to the impaired dopamine-mediated inhibition of NKA in PTCs from animal models of hypertension.
Keywords: F344, hypertension, Na-K-ATPase, NHERF-1, dopamine
dopamine is a natriuretic catecholamine synthesized by the proximal tubule cells (PTCs) of the kidney where it decreases sodium transport by inhibition of the activities of sodium-hydrogen exchanger type 3 (NHE3) (1, 2, 15, 21, 23, 29), and the Na-K-ATPase (NKA) (20), through activation of protein kinase A (PKA)- and C (PKC)-dependent mechanisms. Activation of PKA and PKC by dopamine acutely inhibits NKA activity by phosphorylating serine 18 (S18) and increasing the endocytosis of the NKA α1-subunit (8–10). The dopamine-mediated inhibition of NKA is impaired in hypertension and aging. However, the mechanisms are not well understood (3, 11, 38).
Our laboratory has demonstrated that dopamine acutely inhibits NKA activity in a pertussis toxin-dependent but ERK-independent pathway in opossum kidney (OK) cells (26). We further demonstrated that treatment of OK cells with dopamine activates PKCβ and PKCζ. However, only PKCζ associates with NKA α1-subunit (42). We and others have demonstrated that sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) associates with dopamine-1 receptor (D1R) to inhibit the activities of NKA (42) and sodium-phosphate cotransporter, NpT2a (47). NHERF-1, a scaffolding protein with two canonical PDZ (postsynaptic protein PSD-95/SAP90, Drosophila septate junction protein Discs-large, and tight junction protein ZO-1) domains and an ezrin-binding domain (43), is highly expressed in both brush-border membranes (BBM) and basolateral membranes (BLM) of the renal proximal tubule (27). NHERF-1 also associates with several other G protein-coupled receptors (GPCR), including the α-adrenergic receptor and parathyroid hormone receptor in renal PTCs to regulate ion transport (14, 31, 41, 42, 47). Our studies in OK cells demonstrated that NKA α1 and D1R associate with NHERF-1 through its PDZ2 domain. In OK PTCs, treatment with dopamine decreases the association of NKA α1-subunit and NHERF-1 but increases the association of NHERF-1 and D1R (42). NHERF-1 also plays an important role in the regulation of the expression, trafficking, and activity of several ion transporters in kidney proximal tubules, including NpT2a (25, 49), NHE3 (35, 50), and electrogenic sodium bicarbonate cotransporter type 1 (NBCe1) (4, 48).
Kobayashi et al. (28) demonstrated a decrease in the NHERF-1 mRNA in spontaneously hypertensive rats (SHR), a well-accepted model of hypertension as compared with their control normotensive Wistar-Kyoto (WKY) rats. However, the role of NHERF-1 in hypertension-, sex-, and age-related regulation of sodium transport, in general, and in the dopamine-mediated regulation of NKA, in particular, is not known (44). The aim of the present study was to determine the role of NHERF-1 in the acute regulation of NKA by dopamine in PTCs from animal models of hypertension, the SHR and aged F344 rats. We found that NHERF-1 protein expression is decreased in renal BBM and BLM from aged F344 rats, a model of hypertension and aging, and in SHR, a model of spontaneous hypertension. Dopamine decreased NKA activity and increased the phosphorylation of the NKA α1-subunit at S18 in PTCs in primary culture from 4-mo-old F344 and WKY rats, well-accepted normotensive rat models that do not develop hypertension, but not in PTCs from aged F344 rats and SHR, well-accepted models of hypertension. Transfection of NHERF-1 in PTCs from 22-mo-old F344 and 4-mo-old SHRs restored the ability of dopamine to inhibit NKA activity. The results confirm that NHERF-1 is required for the acute inhibition of renal PT NKA by dopamine and suggest that changes in NHERF-1 expression or function may play a critical role in renal pathophysiology.
MATERIALS AND METHODS
Chemicals.
Mannitol (M4125), Tris·HCl (T3253), PMSF (78830), protease inhibitor cocktail (535142), phosphatase inhibitor cocktail (P0044), MgCl2 (M8266), NaHCO3 (S5761), sodium dodecyl sulfate (SDS, L3771), dl-dithiothreitol (DTT, D0632), urea (U0631), trypsin (T7575), acetonitrile (574732), bicinchoninic acid (BCA, BCA1), bovine serum albumin (1076192), and dopamine (H8502) were purchased from Sigma-Aldrich (St. Louis, MO). Paraformaldehyde solution 4% (sc-281692, diluted to 3.7% in PBS for use in experiments) was purchased from Santa Cruz Biotechnology (Dallas, TX), and radioactive 86RbCl was purchased from (Perkin Elmer Life and Analytical Sciences (Boston, MA).
Animals.
All animal experiments were performed according to the National Institute of Health (NIH) Guidelines for Animal Research and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Louisville and Howard University. Four-, 10-, and 22-mo-old female F344 rats and 4-mo-old WKY rats and 4-mo-old SHRs were maintained on standard rodent chow and water ad libitum for one week prior to performance of the experiments. The F344 rats were provided by the National Institute of Aging, NIH (Bethesda, MD). The 4-mo-old WKY and SHRs were purchased from Charles River Laboratories (Wilmington, MA). The animals were euthanized and the kidneys were harvested. The renal cortex (2 mm underneath the capsule) from half of the right kidney was used to obtain renal PTCs and the other half of the kidney was fixed in 3.7% paraformaldehyde in PBS for immunohistochemistry. The left kidneys were used to prepare BBM and BLM.
Brush border membrane and basolateral membrane preparation.
Brush-border membrane (BBM) and basolateral membrane (BLM) were obtained simultaneously from the renal homogenate of individual rats following the method of Molitoris and Simon (32), with slight modifications. Briefly, 2 mm sections underneath the capsule of the kidney were homogenized in 4 ml lysis buffer [300 mM mannitol, 5 mM Tris·HCl buffer, containing 0.1 mM PMSF and protease and phosphatase inhibitor cocktails (100 µl each cocktail) at pH 7.4], using a glass Teflon homogenizer (Eberbach, Ann Arbor, MI). The samples were then homogenized further using a polytron homogenizer (Polytron, PT1200E, Kinematica, Bohemia, NY) at 25,000 rpm for 15 s (×3) with a 15-s interval in between. An aliquot of the total homogenate was saved at −80°C. The homogenate was mixed with 9.3 ml water and centrifuged at 48,000 g for 30 min in a fixed angle rotor using a Sorvall centrifuge (RC-28S, Thermo Fisher, Waltham, MA) and SS-34 rotor. The pellet (P1) was resuspended in 1 ml lysis buffer by passing 10 times through an 18g needle, followed by 10 passes through a 26g needle. The sample was transferred to a 4 ml Beckman borosilicate tube (catalog no. 355644, Beckman Coulter, Indianapolis, IN) and 1.4 ml water and 36 μl of ice-cold 1M MgCl2 (15 mM final concentration) were added. The mixture was incubated on ice for 20 min with vigorous shaking at 0, 3, 6, 9, 13, 15, and 18 min. The samples were centrifuged at 2,445 g in a Beckman Optima XE ultracentrifuge, using a 50.3 Ti rotor. The pellets (P2) were saved for BLM preparation. The supernatants were centrifuged for 30 min at 48,000 g for 30 min. Crude BBM pellets (P3) were resuspended in 2 ml of diluted lysis buffer (1 ml lysis buffer and 1 ml water). Twenty microliters ice-cold 1 M MgCl2 (final concentration 10 mM) were added to the samples and incubated for 20 min on ice with vigorous shaking as above. The samples were centrifuged at 48,000 g for 30 min and the final BBM pellets were resuspended in 200 μl lysis buffer and stored at −80°C until further use.
BLMs were prepared by resuspending the pellet P2 in 1 ml lysis buffer, 1.4 ml water, and 36 μl of ice-cold 1 M MgCl2 (15 mM final concentration), using a 26g needle. The samples were incubated on ice for 20 min with vigorous shaking at 0, 3, 6, 9, 12, 15, and 18 min and centrifuged at 2,445 g in a Beckman Optima XE ultracentrifuge, using a 50.3 Ti rotor. The pellets were resuspended in 1 ml lysis buffer and centrifuged at 755 g for 15 min. The supernatant was centrifuged at 48,000 g for 30 min in Beckman Optima XE ultracentrifuge. The pellet was resuspended in 0.31 ml lysis buffer and mixed with 1.53 ml 60% sucrose (final sucrose concentration 50%) and overlaid with 0.49 ml 41% and 1.16 ml 38% sucrose. The samples were centrifuged at 48,000 g in a Beckman Optima XE ultracentrifuge overnight in a swinging bucket (SW 55 Ti) rotor at 4°C. The top layers containing BLMs were collected in a fresh tube and mixed with 1 ml bicarbonate buffer, pH 7.4, with vigorous shaking. The samples were centrifuged at 48,000 g for 30 min and the pellets, containing the BLMs were suspended in 100 μl lysis buffer and stored at −80°C until further use. The isolated BBMs were 7–9-fold enriched in alkaline phosphatase activity and BLMs were 7–9-fold enriched in NKA activity as compared with homogenates (data not shown).
Proteomic sample handling.
BBM and BLM protein samples (50 μg) were diluted into 4% SDS/0.1 M Tris·HCl pH 8.5; 1 M DTT was added before heating at 95°C for 5 min. After cooling to room temperature, 3 volumes of 8 M urea/0.1 M Tris·HCl pH 8.5 were added to each sample before transfer to a Microcon-10 Ultracel YM-10 (10,000 NMWL centrifugal filter, Millipore, Bedford, MA) and digested in solution with 0.1 µg of trypsin (Promega, Madison, WI), according to the filter-assisted sample preparation method of Wisniewski et al. (52). The digested, ultrafiltered samples were trap-cleaned with C18 PROTOTM, 300 Å Ultra MicroSpin columns, lyophilized by vacuum centrifugation, and dissolved into 16 µl of 2% vol/vol acetonitrile for analysis by 1 dimension (1D) liquid chromatographic/mass spectrometric (LC/MS) analysis.
LC/MS data acquisition.
Peptide samples (1.5 µg) were separated with a 3 h 1D gradient using an Proxeon EASY n-LC (Thermo Fisher Scientific, Waltham, MA) UHPLC system and a 10 cm, 100 µm inner diameter fused silica column packed with 3 cm of Luna 5 µ SCX 400Å material (Phenomenex, Torrance, CA), and then 3 cm of Jupiter 5 µm C18 300Å material (Phenomenex). The samples were introduced into an LTQ-Orbitrap ELITE (Thermo-Fisher Scientific), using a Nanospray Flex source with the ion transfer capillary temperature of the mass spectrometer set at 225°C, and the spray voltage set at 1.6 kV. Data were acquired with an approach known as Nth Order Double Play with electron transfer dissociation (ETD) Decision Tree method to exploit peptide fragmentation data acquisition by ETD and collision-induced dissociation (CID) approaches. Scan event one of the method obtained an FTMS MS1 scan (normal mass range; 60,000 resolution, full scan type, positive polarity, profile data type) for the range 300–2000 m/z. Scan event two obtained ITMS MS2 scans (normal mass range, rapid scan rate, centroid data type) on up to 10 peaks with a minimum signal threshold of 10,000 counts from scan event one. A decision tree was used to determine whether CID or ETD activation was used. An ETD scan was triggered if any of the following held: an ion charge state 3 and m/z less than 650; an ion charge state 4 and m/z less than 900; an ion charge state 5 and m/z less than 950; or an ion charge state greater than 5; and a CID scan was triggered in all other cases. The lock mass option was enabled (0% lock mass abundance), using the 371.101236 m/z polysiloxane peak as an internal calibrant.
LCMS data analysis.
Proteome Discoverer v1.4.0.288 (Thermo Fisher) was used to direct the data analysis using Mascot v2.4 (Matrix Science, Boston, MA) and SequestHT (Thermo Fisher) with the 7/01/2014 version of the UniprotKB Rattus norvegicus reference proteome canonical and isoform sequences. To estimate the false discovery rate, a Target Decoy PSM Validator node was included in the Proteome Discoverer workflow. The workflow allows for extraction of MS2 scan data from the Xcalibur RAW file, separate searches of CID and ETD MS2 scans in Mascot and Sequest, and collection of the results into a single file (.msf extension). The resulting .msf files from Proteome Discoverer were loaded into Scaffold Q+S v4.3.4 (Proteome Software, Portland, OR). Scaffold was used to calculate the false discovery rate using the Peptide and Protein Prophet algorithms. Proteins were grouped to satisfy the parsimony principle. The results were annotated with rat gene ontology information from the Gene Ontology Annotations Database (ftp.ebi.ac.uk).
Cell culture media and supplements.
DMEM/F-12 (12634–028), fetal bovine serum (FBS, 10437-028), penicillin-streptomycin (15070-063), and PBS (70011-069) were purchased from GIBCO-Thermo Fisher (Waltham, MA). Plasmocin (ant-mpp) was purchased from Invivogen (San Diego, CA). Collagenase (C5894), transferrin (T8158), hydrocortisone (H0888), epithelial growth factor (EGF, E9644), insulin (I1882), selenium (S5261), and sodium pyruvate (P5280) were purchased from Sigma.
Primary renal proximal tubule cell culture.
Proximal tubule cells (PTCs) from rats were isolated following the protocol described by Breggia and Himmelfarb (5), with minor modifications. The kidneys were removed, decapsulated, and immediately placed in ice-cold PBS. Several 1- to 2-mm thick transverse slices of each kidney were made, followed by removal of the kidney cortex, in a glass Petri dish on ice. The cortex was minced with a razor blade into 1-mm pieces and digested with 1 mg/ml collagenase in PBS for 30 min at 37°C, with rocking. The cortical suspension was pipetted several times with a serologic pipette at the midpoint (15 min) and end of incubation. FBS (1%) was added to the suspension and the tube mixed for 30 s. The suspension was gently pressed through a 100-µm cell strainer (CLS431752-50EA, Sigma, St. Louis, MO), followed by a 70-µm cell strainer (CLS431751-50EA; Sigma) atop a 50-ml conical tube. The filtrate was centrifuged at 120 g for 2 min and the supernatant was discarded. The pellet was resuspended in 1–3 ml PBS (depending on the amount of material) and allowed to sediment for 1–2 min. The supernatant with suspended material was transferred to a new tube, carefully as to not r-suspend the tissue material sediment (containing mostly large pieces of tubules and glomeruli). The transferred suspension (containing smaller fragments of tubules) was visualized by light microscopy, followed by centrifugation at 200 g for 3 min. The pelleted tubule fragments were resuspended in 1:1 DMEM/F-12 media containing 5% heat-inactivated FBS, 50 nM hydrocortisone, 1 ng/ml EGF, 5 μg/ml insulin, 5 μg/ml transferrin, 50 nM selenium, 0.55 mM sodium pyruvate, penicillin 100 IU/ml, streptomycin 100 μg/ml, and 25 μg/ml plasmocin (InvivoGen, San Diego, CA). The cells were cultured in collagen-coated cell culture dishes (Corning, Corning, NY) at 37°C and 95% air-5% CO2 in a standard humidified incubator. Culture media were replaced every 2–3 days. Experiments were performed when the cells reached 70–80% confluence.
86Rb uptake.
NKA activity was measured as ouabain-sensitive 86Rb uptake as described previously (19).
Western blotting and immunoprecipitation.
Western blotting was used to determine the protein expression of NHERF-1 and phosphorylation of NKA α1-subunit exactly as described previously (27), using anti-phospho-(ser18) S18-NKA antibodies (Cell Signaling Technology, Danvers, MA) and NHERF-1 antibodies (45). NKA antibodies (α6F) generated by Dr. D. M. Fambrough were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of National Institute of Child Health and Human Development (NIHCD) and maintained by the University of Iowa, Department of Biological Sciences (Iowa City, IA). Immunoprecipitation of NKA α1-subunit was performed exactly as described previously (42).
NHERF-1 transfection.
Flag-tagged NHERF-1 was transfected into PTCs in primary culture from 22-mo-old F344 rats and 4-mo-old SHR by electroporation as described previously (25).
Protein determination.
Protein concentration was measured by bicinchoninic acid method, using bovine serum albumin as standard.
Statistics.
Data are shown as means ± SE. The n values indicate the number of independent experiments. Each experiment was performed in triplicate. P values were calculated using GraphPad Prism software, and significant differences between or among groups were determined by utilizing Student’s t-test or ANOVA, followed by Bonferroni post hoc test, respectively. A P value < 0.05 was a priori considered statistically significant.
RESULTS
Proteomic identification of proteins in the BBM and BLM from young (4-mo) and old (22-mo) rats.
We have previously demonstrated that dopamine decreases NHERF-1-NKA interaction in OK cells (42). To determine whether aging is associated with differences in expression of proteins associated with dopamine receptor signaling, we performed LC/MS analysis of proteins from the BBM and BLM of kidneys from young (4-mo) and old (22-mo) rats. Proteomic studies identified a total of 1,296 proteins in the BBM and BLM fractions (Supplemental Table S1; Supplemental Material for this article is available at the Journal website). As shown in Table 1, the ion transporters, NHE3, NpT2a, NpT2c, and NHERF2 were predominantly present in the BBM while NKA α1-subunit was predominantly localized to the BLM. NKA β1-subunits and NHERF-1 were identified in both the BBM and BLM fractions. As compared with BBM from young rats, NpT2c expression was significantly lower in the BBM from old rats. There was no significant difference in the expression of NpT2a or NHE3 between 4-mo and 22-mo-old F344 rats. The expression of NKA α1-subunit in the BLM from 22-mo-old F344 rats was not significantly different from 4-mo-old F344 rats. The expression of NHERF-1 in both BBM and BLM fractions from 22-mo-old F344 rats was significantly lower than those from 4-mo-old F344 rats. Interestingly, a post hoc analysis of the shotgun proteomics data for phosphorylation considering variable phosphorylation of serine (S), threonine, and tyrosine, performed as described previously (34), showed increased phosphorylation of NHERF-1 at S277 and S285/287 in BLM from 22-mo-old F344 rats as compared with 4-mo-old rats (Supplemental Tables S2 and S3).
Table 1.
Proteomic identification of BBM and BLM proteins from 22-mo-old and 4-mo-old F344 rats
| BBM (22-mo) |
BLM (22-mo) |
BBM (4-mo) |
BLM (4-mo) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identified Protein | Rat 1 | Rat 2 | Rat 3 | Rat 1 | Rat 2 | Rat3 | Rat 1 | Rat 2 | Rat3 | Rat 1 | Rat 2 | Rat 3 |
| A-kinase anchor protein 2 (Akap2) | 8 | 19 | 0 | 0 | 8 | 0 | 13 | 17 | 31 | 1 | 7 | 3 |
| Aquaporin-1 (Aqp1) | 8 | 7 | 7 | 5 | 9 | 5 | 8 | 8 | 8 | 13 | 8 | 8 |
| Carbonic anhydrase 2 (Ca2) | 14 | 11 | 11 | 1 | 11 | 6 | 14 | 12 | 13 | 11 | 8 | 2 |
| Carbonic anhydrase 4 (Ca4) | 15 | 11 | 11 | 0 | 7 | 2 | 13 | 12 | 10 | 11 | 3 | 8 |
| Caveolin-1 (Cav1) | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 1 |
| Ezrin (Ezr) | 40 | 49 | 22 | 14 | 52 | 25 | 40 | 41 | 51 | 39 | 37 | 34 |
| γ-Glutamyltranspeptidase 1 (Ggt1) | 27 | 33 | 31 | 6 | 7 | 2 | 32 | 32 | 28 | 2 | 5 | 8 |
| Glutamyl aminopeptidase (Enpep) | 50 | 52 | 53 | 9 | 5 | 8 | 48 | 46 | 47 | 4 | 2 | 2 |
| Isoform 5 of sodium/calcium exchanger 1 (Slc8a1) isoform 5 | 4 | 5 | 1 | 0 | 7 | 2 | 8 | 4 | 6 | 11 | 2 | 8 |
| Moesin (Msn) | 53 | 70 | 38 | 19 | 65 | 41 | 53 | 64 | 75 | 53 | 57 | 45 |
| Na+/H+ exchange regulatory cofactor NHERF-1 (Slc9a3r1) | 3 | 4 | 9 | 4 | 8 | 7 | 36 | 42 | 51 | 39 | 37 | 23 |
| Na+/H+exchange regulatory cofactor NHERF-2 (Slc9a3r2) | 8 | 6 | 6 | 0 | 0 | 0 | 3 | 4 | 6 | 0 | 0 | 0 |
| Na+/H+exchange regulatory cofactor NHERF-3 (Pdzk1) | 59 | 72 | 26 | 18 | 68 | 18 | 63 | 71 | 73 | 53 | 51 | 44 |
| Sodium-glucose cotransporter 1 (Slc5a1) | 12 | 10 | 11 | 0 | 12 | 2 | 14 | 21 | 13 | 10 | 9 | 8 |
| Sodium-glucose cotransporter 2 (Slc5a2) | 27 | 28 | 23 | 12 | 14 | 15 | 25 | 27 | 27 | 12 | 13 | 10 |
| Sodium/hydrogen exchanger 3 (NHE3) (Slc9a3) | 5 | 2 | 2 | 0 | 1 | 0 | 3 | 4 | 6 | 0 | 1 | 0 |
| Sodium/potassium-transporting ATPase subunit α-1 (Atp1a1) | 10 | 11 | 9 | 78 | 139 | 110 | 13 | 12 | 10 | 197 | 109 | 160 |
| Sodium/potassium-transporting ATPase subunit β-1(Atp1b1) | 36 | 43 | 36 | 21 | 39 | 37 | 41 | 35 | 36 | 46 | 39 | 50 |
| Sodium/potassium-transporting ATPase subunit β-3 (Atp1b3) | 0 | 1 | 2 | 3 | 4 | 5 | 2 | 2 | 1 | 4 | 2 | 4 |
| Sodium/potassium-transporting ATPase subunit γ (Fxyd2) | 2 | 1 | 1 | 4 | 6 | 4 | 1 | 3 | 1 | 5 | 4 | 5 |
| Sodium-dependent phosphate transport protein 2A, NpT2a (Slc34a1) | 8 | 8 | 5 | 1 | 0 | 2 | 8 | 10 | 9 | 1 | 0 | 1 |
| Sodium-dependent phosphate transport protein 2C, NpoT2c (Slc34a3) | 3 | 1 | 1 | 0 | 0 | 0 | 4 | 4 | 5 | 0 | 1 | 1 |
| Solute carrier family 12 member 3 (Slc12a3) | 11 | 15 | 10 | 2 | 1 | 1 | 6 | 8 | 11 | 4 | 1 | 4 |
| Syntaxin-3 (Stx3) | 5 | 6 | 0 | 0 | 3 | 0 | 5 | 6 | 7 | 2 | 0 | 0 |
Relative abundance (based on peptide spectral count values) for selected membrane proteins (and gene name) identified by high confidence proteomic methods (see materials and methods) in brush-border (BBM, apical) and basolateral (BLM) membrane samples isolated from kidneys from three different 4-mo-old and 22-mo-old F344 rats. The numbers in second row are the identification number of the rats. The numbers in columns represent the relative abundance of the proteins based on peptide spectral counts. The complete list of proteins is shown in Supplemental Table S1.
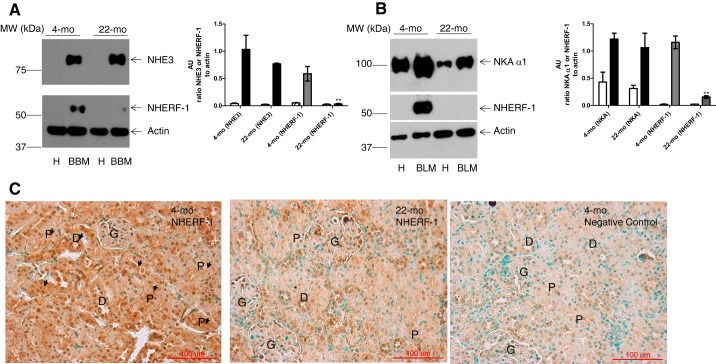
To confirm the proteomics data, the expression of NHERF-1 in the BBM and BLM from kidneys of young (4-mo-old) and old (22-mo-old) F344 rats was determined by Western blotting in isolated BBM and BLM and immunohistochemistry in the kidney. As shown in Fig. 1, NHERF-1 expression was decreased in both BBM (Fig. 1A) and BLM (Fig. 1B) from 22-mo-old rats as compared with 4-mo-old rats. As shown in Fig. 1C, immunohistochemistry corroborated the decreased NHERF-1 expression in the kidneys from 22-mo-old rats as compared with 4-mo-old rats. The expression of NHE3 in the BBM (Fig. 1A) and NKA α1 subunit in the BLM (Fig. 1B) was not different between 4- and 22-mo-old rats.
Fig. 1.
Age-dependent NHERF-1 expression. A: representative blot for NHE3 (top), NHERF-1 (middle), and β-actin (bottom) in homogenates (H) and BBM from 4- and 22-mo-old female F344 rat kidneys (n = 6 in each group). B: representative blot for NKA α1-subunit (top), NHERF-1 (middle), and β-actin (bottom) in homogenates (H) and BLM from 4- and 22-mo-old female F344 rat kidneys. Results are representative from 6 independent observations in each condition. Bar graph in A represents expression of NHE3/actin (white bar, homogenate; black bar, BBM) or NHERF-1/actin (hatched bar, homogenate; gray bar, BBM). Bar graph in B represents expression of NKA/actin (white bar, homogenate; black bar, BLM) or NHERF-1/actin (hatched bar, homogenate; gray bar, BBM) from 6 individual rats (AU, arbitrary units, means ± SE, n = 6 in each group). **P < 0.001 as calculated by one-way ANOVA, followed by Bonferroni post hoc test from 4-mo-old F344 rats. C: representative immunohistochemistry for NHERF-1 in kidneys from 4- and 22-mo-old F344 rats. NHERF-1 immunostaining (brown color) is indicated by arrows or within labeled structures (P, proximal tubule; D, distal tubule; G, glomerulus). Green color is from methyl green nuclear counterstain. The results are representative from six independent observations in each condition. Magnification, ×40.
Effect of age on dopamine-mediated regulation of NKA activity in PTCs in primary culture.
First, to determine the time course of the loss of dopamine-mediated regulation of NKA with aging, NKA activity was measured in primary cultures of PTCs isolated from the kidney cortex from 4-mo, 10-mo, and 22-mo-old female F344 rats as ouabain-sensitive 86Rb uptake. As shown in Fig. 2, treatment with dopamine inhibited NKA-dependent 86Rb uptake in PTCs from 4-mo-old rats but not from 10-mo and 22-mo-old rats.
Fig. 2.
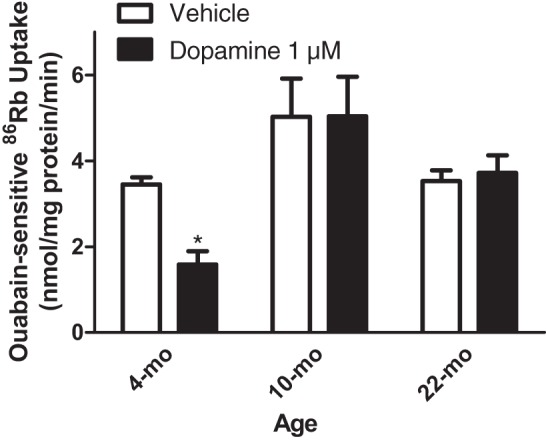
Age differences in dopamine-mediated inhibition of NKA activity. Proximal tubule cells (PTCs) in primary culture from 4-mo, 10-mo, and 22-mo-old F344 rats were treated for 15 min with dopamine (1 μM). Ouabain (4 mM)-sensitive 86Rb uptake was used as a measure of NKA activity. Each bar represents data as nmol 86Rb·mg protein·−1min−1 (means ± SE) in cell cultures from 6 individual rats (n = 6 in each group) performed in triplicate. *P < 0.05 as calculated by one-way ANOVA followed by Bonferroni post hoc test.
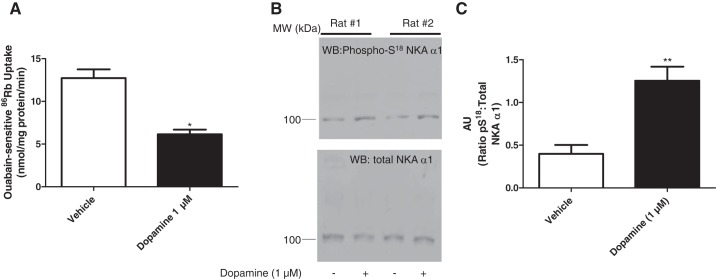
Several studies have demonstrated that the dopamine-mediated inhibition and endocytosis of NKA α1-subunit require phosphorylation of NKA at S18. To confirm that phosphorylation of NKA at S18 is important in the dopamine-mediated regulation of NKA activity and phosphorylation in the F344 rat model, we studied primary cultures of PTCs of 4-mo-old F344 rats and measured NKA activity as ouabain-sensitive 86Rb uptake and NKA phosphorylation by Western blot using anti-phospho-(ser18) S18-NKA antibodies. As shown in Fig. 3, 15-min incubation of the PTCs with dopamine (1 μM) decreased NKA activity and increased NKA α1-subunit S18 phosphorylation without affecting total NKA expression in PTCs from 4-mo-old F344 rats which express NHERF-1.
Fig. 3.
Effect of dopamine on NKA activity. A: PTCs in primary culture from 4-mo-old F344 rats were treated for 15 min with dopamine (1 μM). Ouabain (4 mM)-sensitive 86Rb uptake was used as a measure of NKA activity. Each bar represents data as nmol 86Rb·mg protein·−1min−1 (means ± SE) from 6 individual cultures from 6 different animals performed in triplicate. *P < 0.05 as calculated by Student’s t-test. B: PTCs in primary culture from 4-mo-old F344 rats were treated for 15 min with dopamine (1 μM). Cell membranes were isolated and analyzed by Western blotting using phospho-S18 NKA α1-subunit antibodies (top). Nitrocellulose membranes were stripped and reprobed with NKA antibodies (NKA, α6F, bottom). Representative blots from cells from two different rats are shown. C: bar graph represents data as ratio of band intensity of phospho-S18 (pS18) to total NKA (means ± SE) from 6 cultures from 6 individual rats (n = 6 in each group) as arbitrary units (AU). **P < 0.001 as calculated by Student’s t-test.
Role of NHERF-1 on dopamine-mediated regulation of NKA activity in PTCs of aged rats.
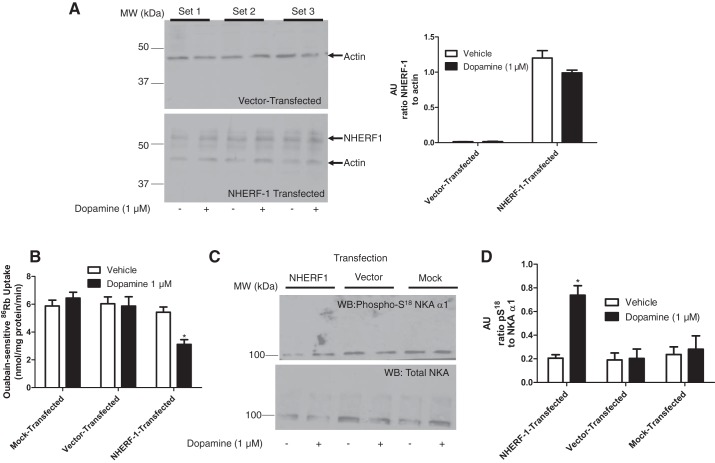
The above proteomic data identified the loss of NHERF-1 in old as compared with young F344 rats. To confirm that loss of NHERF-1 plays a role in the lack of the dopamine-mediated inhibition of NKA activity and increase in NKA α1-subunit S18 phosphorylation in PTCs of aged rats, Flag-tagged NHERF-1 was transiently transfected by electroporation in PTCs from 22-mo-old female F344 rats. Figure 4A shows successful transfection of NHERF-1 in PTCs in primary culture. We have previously demonstrated that the NHERF-1 antibody recognizes different phosphorylation states of NHERF-1 that have different molecular size (25). The band recognized at around >50 kDa by the NHERF-1 antibody was the least phosphorylated form of NHERF-1, as determined by alkaline phosphatase digestion of the membranes (data not shown). This pattern of NHERF-1 is consistent with studies published by our laboratory and by Morales et al., which showed NHERF-1 as a migrating triplet (25, 33). As shown in Fig. 4, B–D, transfection with NHERF-1 restored the ability of dopamine to inhibit NKA activity and increase the S18 phosphorylation of NKA α1-subunit, without affecting total NKA in PTCs from 22-mo-old F344 rats.
Fig. 4.
Effect of NHERF-1 transfection on dopamine-mediated inhibition of NKA. A: PTCs in primary culture from 22-mo-old F344 rats transiently transfected with Flag-tagged NHERF-1 and cell membrane proteins were analyzed by Western blotting. Top: vehicle- or dopamine-treated cells transfected with empty vector from three individual rats. Bottom: vehicle- or dopamine-treated cells transiently transfected with Flag-tagged NHERF-1 from three individual rats. Bar graph represents data as ratio of band intensity of NHERF-1 to actin (means ± SE) of cultures from 6 individual rats (n = 6 in each group) as arbitrary units (AU). B: PTCs (from A) in primary culture from 22-mo-old F344 rats transiently transfected with vector or Flag-tagged NHERF-1 were treated for 15 min with dopamine (1 μM). Ouabain (4 mM)-sensitive 86Rb uptake was measured. Each bar represents data as nmol 86Rb·mg protein·−1min−1 (means ± SE performed in triplicate (n = 3 rats in each group). *P < 0.05 vs. respective vehicle. C: PTCs in primary culture from 22-mo-old F344 rats transiently transfected with vector or Flag-tagged NHERF-1 were treated for 15 min with dopamine (1 μM). Cell membranes were analyzed by Western blotting using phospho-S18 NKA α1-subunit antibodies (top). Nitrocellulose membranes were stripped and reprobed with NKA antibodies (NKA, α6F, bottom). D: bar graph represents data in C as ratio of band intensity of pS18 to total NKA α1 (means ± SE) (n = 3 rats in each group) as arbitrary units (AU). *P < 0.05 vs. respective vehicle-treated group.
Role of NHERF-1 in dopamine-mediated regulation of NKA activity in PTCs of hypertensive rats.
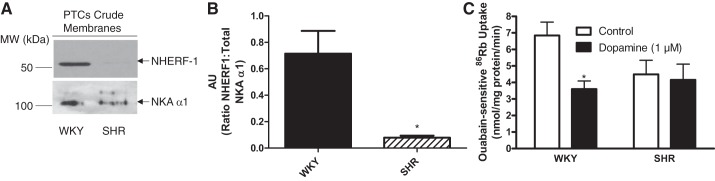
Some models of hypertension including spontaneously hypertensive rats (SHRs) are associated with blunted responses to dopamine (13, 22, 29, 39, 40, 54). Furthermore, as compared with WKY rats, decreased NHERF-1 mRNA was reported in SHR (28). To validate our working hypothesis that NHERF-1 expression is important for the regulation of NKA by dopamine, we compared NHERF-1 expression in PTCs in primary cultures from SHR and its control normotensive WKY rats. As shown in Fig. 5A, NHERF-1 expression was significantly lower in PTCs from SHRs than WKY rats. Figure 5B, represents a way to express the relationship of NHERF-1 and NKA α1 which shows that the NHERF-1-to-NKA α1 ratio was decreased in SHR relative to WKY. Figure 5C shows that the basal NKA-mediated 86Rb uptake was significantly lower in PTCs from SHRs than WKY rats and dopamine decreased NKA-dependent 86Rb uptake in PTCs from WKY but not SHRs.
Fig. 5.
NHERF-1 expression in WKY and SHRs. A: crude membranes from PTCs in primary culture from WKY and SHRs were analyzed by Western blotting using anti-NHERF-1 (top) and NKA α1-subunit (NKA, α6F, bottom). Representative blots from cells from one rat are shown. B: bar graph represents data as ratio of band intensity of NHERF-1 to NKA (means ± SE) from 3 individual rats (n = 3 in each group) as arbitrary units (AU). *P < 0.05 as calculated by one-way ANOVA, followed by Bonferroni post hoc test. C: PTCs in primary culture from WKY and SHRs were treated for 15 min with dopamine (1 μM). Ouabain (4 mM)-sensitive 86Rb uptake was used as a measure of NKA activity. Each bar represents data as nmol 86Rb·mg protein·−1min−1 (means ± SE) from cell cultures from 3 rats (n = 3 in each group) performed in triplicate. *P < 0.05 as calculated by one-way ANOVA, followed by Bonferroni post hoc test.
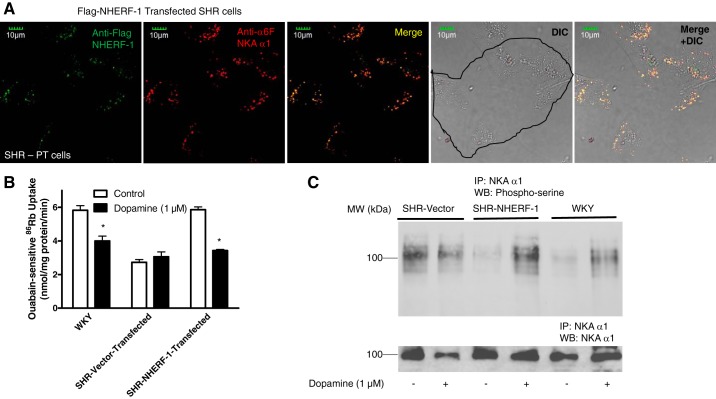
To determine whether NHERF-1 plays a role in dopamine-mediated regulation of NKA in PTCs from SHR, Flag-tagged NHERF-1 was transfected in primary culture of PTCs from SHR (Fig. 6A). As shown in Fig. 6B, transfection of NHERF-1 restored the basal activity of NKA and the dopamine-mediated inhibition of NKA activity in PTCs from SHRs to the same values as those measured in PTCs from WKY rats. As shown in Fig. 6C, basal serine phosphorylation of NKA α1 was higher in PTCs from SHR than WKY rats. Transfection of NHERF-1 decreased basal serine phosphorylation of NKA α1 but restored dopamine-mediated increase in serine phosphorylation of NKA α1 in PTCs from SHR.
Fig. 6.
Effect of NHERF-1 transfection on dopamine-mediated regulation of NKA in SHR. A: PTCs in primary culture from SHRs were transiently transfected with Flag-tagged NHERF-1. Cells were analyzed by confocal microscopy using anti-NHERF-1 (green) and anti-NKA α1-subunit (α6F) antibodies (red). Representative images (red, green, merge, and DIC, differential interference contrast) are shown (n = 3). B: renal PTCs in primary culture from SHRs transiently transfected with vector or Flag-tagged NHERF-1 were treated for 15 min with dopamine (1 μM). Ouabain (4 mM)-sensitive 86Rb uptake was used as a measure of NKA activity. Each bar represents data as nmol 86Rb·mg protein·−1min−1 (means ± SE) from PTCs in culture from 3 individual rats (n = 3 in each group) performed in triplicate. *P < 0.05 as calculated by one-way ANOVA, followed by Bonferroni post hoc test. C: phosphorylation of NKA. Renal PTCs in primary culture from SHRs transiently transfected with empty vector or Flag-tagged NHERF-1 were treated for 15 min with dopamine (1 μM). NKA was immunoprecipitated from crude membranes and subjected to Western blotting using anti-phosphoserine (top, Invitrogen) and NKA α1-subunit (bottom). A representative blot from 2 independent experiments is shown.
DISCUSSION
The present study demonstrates for the first time an association between the reduced expression of NHERF-1 in PTCs and blunted response to dopamine in two well-accepted rat models of hypertension, aged F344 and SHRs. We demonstrated decreased expression of NHERF-1 in BBM and BLM isolated from kidneys of 22-mo-old F344 rats, as compared with 4-mo-old F344 rats and in SHRs as compared with WKY rats. This finding is unique and in conjunction with our recent finding regarding the role of NHERF-1 in the trafficking of the type IIa sodium-phosphate cotransporter, NpT2a (25), suggest that NHERF-1 expression is not static, as was initially proposed (51), but is highly dynamic. Using primary cultures of PTCs from aged F344 and SHRs, we demonstrated that NHERF-1 is essential for the acute inhibition of NKA and phosphorylation of NKA α1-subunit at S18 by dopamine. The results from this study have significant implications for the understanding of the molecular mechanisms by which dopamine regulates sodium transport in PTCs.
Proteomic analysis showed the expression of proteins that are expected in apical and basolateral fractions of renal proximal tubules. NpT2a, NpT2c, and NHE3 were almost exclusively identified in the BBM fractions while NKA α1-subunit was present predominantly in the basolateral fractions corroborating their enrichment in the membrane preparations. Identification of NKA β1-subunits in both the membrane fractions is not surprising as the NKA β1-subunits also associate with the apical H+-K+-ATPase (30).
Using immunoprecipitation and confocal microscopy, we have previously demonstrated that NHERF-1 and NKA associate with one another in OK cells, and that treatment with dopamine decreased this association, while increasing the association between D1R and NHERF-1 (42). In the current study, proteomic analysis suggested that NHERF-1 expression is decreased in BBM and BLM from 22-mo-old relative to 4-mo-old F344 rats. The proteomic data were confirmed by Western blot analysis and immunohistochemistry. We and others have demonstrated that D1R associates with a PDZ-domain containing scaffolding proteins Pals and NHERF-1 (7, 42). The association between D1R and NHERF-1 is essential for the acute inhibition of NKA activity by dopamine (42). Consistent with the decreased expression of NHERF-1 in 22-mo-old F344 rats, our data demonstrated that dopamine reduced NKA activity in primary cultures of PTCs from 4-mo-old rats but not in PTCs from 22-mo-old F344 rats. Transient transfection of NHERF-1 in PTCs from 22-mo-old F344 rats restored the ability of dopamine to acutely inhibit NKA activity. Together, these data suggest that NHERF-1 is critical for acute regulation of NKA by dopamine. However, the mechanisms are not well understood.
Several observations suggest a potential role for differential phosphorylation events in the regulation of renal sodium transport by dopamine. Regulation of dopamine-mediated sodium transport and hypertension are dependent upon intact G protein-coupled receptor kinase 4γ (GRK4γ) (46). Mutations in the GRK4γ are associated with essential hypertension and cause hyperphosphorylation of D1R resulting in diminished trafficking of D1R to the plasma membrane (24). Additionally, D1R has been shown to be hyperphosphorylated in SHR (16, 54). NHERF-1 function is also regulated by phosphorylation at several sites. Weinman and his group (47) showed that NHERF-1 phosphorylation at S77 and T95 are critical for dopamine-mediated inhibition of NpT2a. Cdc2 has been shown to phosphorylate NHERF-1 at S279 (18) during mitosis and G protein-coupled receptor kinase 6A (GRK6A) phosphorylates S289 (17). In vitro studies, including cell cultures, have demonstrated that S289 phosphorylation by GRK6A is important for the oligomerization of NHERF-1 while phosphorylation at S279 prevents its oligomerization with other proteins (17, 18). The post hoc analysis of the shotgun proteomics data for phosphorylation showed increased phosphorylation of NHERF-1 at S277 and S285/287 in BBM and BLM from 22-mo-old animals. These preliminary data suggest that in aging perhaps there is an increased NHERF-1 homodimerization and decreased association with other proteins that could modify dopamine receptor regulation of NKA. It is also possible that there is a defect in NHERF-1 dephosphorylation rather than increased phosphorylation. These possibilities need to be studied further to identify the physiological role of the phosphorylation at these specific sites in overall renal function.
Dopamine is a well-established natriuretic catecholamine that acutely inhibits PTC sodium transport by inhibiting NHE3 and NKA (1, 2, 7, 8, 11, 13, 15, 20–24, 26, 29, 37, 42, 44, 53, 54). Dopamine inhibits sodium transport, in part, by activation of D1R which stimulates PKA- and PKC-dependent pathways (8, 9, 13, 20, 26, 37, 42, 44, 53, 54), as well as G protein-coupled receptor kinases (53). Dopamine is known to phosphorylate NKA at S18 in rat PTCs and OK cells. Dopamine-mediated phosphorylation of NKA at S18 results in the binding of clathrin and AP2 to the NKA α1-subunit. This triggers the endocytosis of NKA α1 and a decrease in its activity in the plasma membrane (6, 9, 12, 37). Nguyen et al. (36) demonstrated decreased NKA activity in microsomes prepared from SHRs, as compared with WKY rats. Consistent with these data, our studies also demonstrate decreased basal NKA activity in the SHR. Our data also demonstrate increased basal NKA phosphorylation in PTCs from SHRs, as compared with WKY rats. Whether or not the decreased basal NKA activity in the SHR is due to increased basal phosphorylation of NKA remains to be determined. Previous studies have demonstrated a decrease in NHERF-1 mRNA in kidneys from SHRs (28). Together, our studies suggest that absence of NHERF-1 may result in increased NKA phosphorylation in SHR as transient transfection with NHERF-1 decreased the basal phosphorylation of NKA and restored the basal activity to levels similar to WKY rats.
Our laboratory has demonstrated a role for NHERF-1 in the acute inhibition of NKA activity by dopamine (42) and PTH (27) in OK cells. We have demonstrated that treatment of OK cells for 15 min with dopamine results in decreased NHERF-1-NKA α1-subunit interaction and increased NHERF-1-D1R and NKA α1-subunit-PKCζ association. The association between NHERF-1-D1R requires an intact PDZ2 domain of NHERF-1. Consistent with our previous data, the data from the current study suggest that NHERF-1 is critical to enable dopamine to inhibit NKA activity in PTCs from aged F344 rats and young SHRs. The inability of dopamine to inhibit NKA activity in PTCs from aged F344 rats and SHRs is restored by increasing NHERF-1 expression. In summary, the findings from this study demonstrating that the absence of NHERF-1 results in loss of the acute inhibition of NKA activity by dopamine in PTCs in a model of aging with hypertension and a model of spontaneous hypertension identify a novel pathophysiologic pathway for these common renal conditions. Further studies are required to determine the role of NHERF-1 in dopamine-regulated sodium homeostasis in vivo.
GRANTS
The work was supported by National Institute on Aging Grant NIH-5R21AG047474 and American Heart Association Grant 16GRNT31030019 (to S. J. Khundmiri), National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK039308 (to P. A. Jose), and Veteran Affairs Merit Review Grant NEPH-016-09S (to E. D. Lederer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.T.B., C.J.K., M.L.M., W.B.K., A.J.L., and S.J.K. performed experiments; M.T.B., M.L.M., E.D.L., and S.J.K. analyzed data; M.T.B., M.L.M., P.A.J., E.J.W., E.D.L., and S.J.K. interpreted results of experiments; M.T.B., C.J.K., M.L.M., W.B.K., P.A.J., E.J.W., A.J.L., E.D.L., and S.J.K. approved final version of manuscript; M.L.M., P.A.J., E.J.W., E.D.L., and S.J.K. edited and revised manuscript; S.J.K. prepared figures; S.J.K. drafted manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
We acknowledge the technical assistance of Daniel Wilkey, Nina Lesousky and Caryl Conklin.
REFERENCES
- 1.Albrecht FE, Xu J, Moe OW, Hopfer U, Simonds WF, Orlowski J, Jose PA. Regulation of NHE3 activity by G protein subunits in renal brush-border membranes. Am J Physiol Regul Integr Comp Physiol 278: R1064–R1073, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Armando I, Villar VAM, Jose PA. Dopamine and renal function and blood pressure regulation. Compr Physiol 1: 1075–1117, 2011. doi: 10.1002/cphy.c100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asghar M, Chillar A, Lokhandwala MF. Renal proximal tubules from old Fischer 344 rats grow into epithelial cells in cultures and exhibit increased oxidative stress and reduced D1 receptor function. Am J Physiol Cell Physiol 295: C1326–C1331, 2008. doi: 10.1152/ajpcell.00367.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardo AA, Kear FT, Santos AV, Ma J, Steplock D, Robey RB, Weinman EJ. Basolateral Na+/HCO3− cotransport activity is regulated by the dissociable Na+/H+ exchanger regulatory factor. J Clin Invest 104: 195–201, 1999. doi: 10.1172/JCI5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breggia AC, Himmelfarb J. Primary mouse renal tubular epithelial cells have variable injury tolerance to ischemic and chemical mediators of oxidative stress. Oxid Med Cell Longev 1: 33–38, 2008. doi: 10.4161/oxim.1.1.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Krmar RT, Dada L, Efendiev R, Leibiger IB, Pedemonte CH, Katz AI, Sznajder JI, Bertorello AM. Phosphorylation of adaptor protein-2 mu2 is essential for Na+,K+-ATPase endocytosis in response to either G protein-coupled receptor or reactive oxygen species. Am J Respir Cell Mol Biol 35: 127–132, 2006. doi: 10.1165/rcmb.2006-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Leibiger I, Katz AI, Bertorello AM. Pals-associated tight junction protein functionally links dopamine and angiotensin II to the regulation of sodium transport in renal epithelial cells. Br J Pharmacol 158: 486–493, 2009. doi: 10.1111/j.1476-5381.2009.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chibalin AV, Katz AI, Berggren PO, Bertorello AM. Receptor-mediated inhibition of renal Na+-K+-ATPase is associated with endocytosis of its α- and β-subunits. Am J Physiol Cell Physiol 273: C1458–C1465, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Chibalin AV, Ogimoto G, Pedemonte CH, Pressley TA, Katz AI, Féraille E, Berggren PO, Bertorello AM. Dopamine-induced endocytosis of Na+,K+ATPase is initiated by phosphorylation of Ser-18 in the rat alpha subunit and is responsible for the decreased activity in epithelial cells. J Biol Chem 274: 1920–1927, 1999. doi: 10.1074/jbc.274.4.1920. [DOI] [PubMed] [Google Scholar]
- 10.Chibalin AV, Pedemonte CH, Katz AI, Féraille E, Berggren PO, Bertorello AM. Phosphorylation of the catalyic alpha-subunit constitutes a triggering signal for Na+,K+ATPase endocytosis. J Biol Chem 273: 8814–8819, 1998. doi: 10.1074/jbc.273.15.8814. [DOI] [PubMed] [Google Scholar]
- 11.Chugh G, Pokkunuri I, Asghar M. Renal dopamine and angiotensin II receptor signaling in age-related hypertension. Am J Physiol Renal Physiol 304: F1–F7, 2013. doi: 10.1152/ajprenal.00441.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cinelli AR, Efendiev R, Pedemonte CH. Trafficking of Na-K-ATPase and dopamine receptor molecules induced by changes in intracellular sodium concentration of renal epithelial cells. Am J Physiol Renal Physiol 295: F1117–F1125, 2008. doi: 10.1152/ajprenal.90317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crambert S, Sjöberg A, Eklöf A-C, Ibarra F, Holtbäck U. Prolactin and dopamine 1-like receptor interaction in renal proximal tubular cells. Am J Physiol Renal Physiol 299: F49–F54, 2010. doi: 10.1152/ajprenal.00582.2009. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham R, Biswas R, Steplock D, Shenolikar S, Weinman E. Role of NHERF and scaffolding proteins in proximal tubule transport. Urol Res 38: 257–262, 2010. doi: 10.1007/s00240-010-0294-1. [DOI] [PubMed] [Google Scholar]
- 15.Escano CS, Armando I, Wang X, Asico LD, Pascua A, Yang Y, Wang Z, Lau YS, Jose PA. Renal dopaminergic defect in C57Bl/6J mice. Am J Physiol Regul Integr Comp Physiol 297: R1660–R1669, 2009. doi: 10.1152/ajpregu.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felder RA, Eisner GM, Jose PA. D1 dopamine receptor signalling defect in spontaneous hypertension. Acta Physiol Scand 168: 245–250, 2000. doi: 10.1046/j.1365-201x.2000.00634.x. [DOI] [PubMed] [Google Scholar]
- 17.Hall RA, Spurney RF, Premont RT, Rahman N, Blitzer JT, Pitcher JA, Lefkowitz RJ. G protein-coupled receptor kinase 6A phosphorylates the Na+/H+ exchanger regulatory factor via a PDZ domain-mediated interaction. J Biol Chem 274: 24328–24334, 1999. doi: 10.1074/jbc.274.34.24328. [DOI] [PubMed] [Google Scholar]
- 18.He J, Lau AG, Yaffe MB, Hall RA. Phosphorylation and cell cycle-dependent regulation of Na+/H+ exchanger regulatory factor-1 by Cdc2 kinase. J Biol Chem 276: 41559–41565, 2001. doi: 10.1074/jbc.M106859200. [DOI] [PubMed] [Google Scholar]
- 19.Holthouser KA, Mandal A, Merchant ML, Schelling JR, Delamere NA, Valdes RR Jr, Tyagi SC, Lederer ED, Khundmiri SJ. Ouabain stimulates Na-K-ATPase through a sodium/hydrogen exchanger-1 (NHE-1)-dependent mechanism in human kidney proximal tubule cells. Am J Physiol Renal Physiol 299: F77–F90, 2010. doi: 10.1152/ajprenal.00581.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horiuchi A, Takeyasu K, Mouradian MM, Jose PA, Felder RA. D1A dopamine receptor stimulation inhibits Na+/K+-ATPase activity through protein kinase A. Mol Pharmacol 43: 281–285, 1993. [PubMed] [Google Scholar]
- 21.Hussain T, Becker M, Beheray S, Lokhandwala MF. Dopamine fails to inhibit Na,H-exchanger in proximal tubules of obese Zucker rats. Clin Exp Hypertens 23: 591–601, 2001. doi: 10.1081/CEH-100107389. [DOI] [PubMed] [Google Scholar]
- 22.Hussain T, Kansra V, Lokhandwala MF. Renal dopamine receptor signaling mechanisms in spontaneously hypertensive and Fischer 344 old rats. Clin Exp Hypertens 21: 25–36, 1999. doi: 10.3109/10641969909068646. [DOI] [PubMed] [Google Scholar]
- 23.Hussain T, Lokhandwala MF. Dopamine-1 receptor G-protein coupling and the involvement of phospholipase A2 in dopamine-1 receptor mediated cellular signaling mechanisms in the proximal tubules of SHR. Clin Exp Hypertens 19: 131–140, 1997. doi: 10.3109/10641969709080810. [DOI] [PubMed] [Google Scholar]
- 24.Jose PA, Soares-da-Silva P, Eisner GM, Felder RA. Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta 1802: 1259–1267, 2010. doi: 10.1016/j.bbadis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ketchem CJ, Khundmiri SJ, Gaweda AE, Murray R, Clark BJ, Weinman EJ, Lederer ED. Role of Na+/H+ exchanger regulatory factor 1 in forward trafficking of the type IIa Na+-Pi cotransporter. Am J Physiol Renal Physiol 309: F109–F119, 2015. doi: 10.1152/ajprenal.00133.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khundmiri SJ, Lederer E. PTH and DA regulate Na-K ATPase through divergent pathways. Am J Physiol Renal Physiol 282: F512–F522, 2002. doi: 10.1152/ajprenal.00111.2000. [DOI] [PubMed] [Google Scholar]
- 27.Khundmiri SJ, Weinman EJ, Steplock D, Cole J, Ahmad A, Baumann PD, Barati M, Rane MJ, Lederer E. Parathyroid hormone regulation of Na+,K+-ATPase requires the PDZ 1 domain of sodium hydrogen exchanger regulatory factor-1 in opossum kidney cells. J Am Soc Nephrol 16: 2598–2607, 2005. doi: 10.1681/ASN.2004121049. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi K, Monkawa T, Hayashi M, Saruta T. Expression of the Na+/H+ exchanger regulatory protein family in genetically hypertensive rats. J Hypertens 22: 1723–1730, 2004. doi: 10.1097/00004872-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Li XX, Xu J, Zheng S, Albrecht FE, Robillard JE, Eisner GM, Jose PA. D1 dopamine receptor regulation of NHE3 during development in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 280: R1650–R1656, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Codina J, Petroske E, Werle MJ, Willingham MC, DuBose TD Jr. The effect of beta-subunit assembly on function and localization of the colonic H+,K+-ATPase alpha-subunit. Kidney Int 66: 1068–1075, 2004. doi: 10.1111/j.1523-1755.2004.00856.x. [DOI] [PubMed] [Google Scholar]
- 31.Mahon MJ, Cole JA, Lederer ED, Segre GV. Na+/H+ exchanger-regulatory factor 1 mediates inhibition of phosphate transport by parathyroid hormone and second messengers by acting at multiple sites in opossum kidney cells. Mol Endocrinol 17: 2355–2364, 2003. doi: 10.1210/me.2003-0043. [DOI] [PubMed] [Google Scholar]
- 32.Molitoris BA, Simon FR. Renal cortical brush-border and basolateral membranes: cholesterol and phospholipid composition and relative turnover. J Membr Biol 83: 207–215, 1985. doi: 10.1007/BF01868695. [DOI] [PubMed] [Google Scholar]
- 33.Morales FC, Takahashi Y, Kreimann EL, Georgescu MM. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci USA 101: 17705–17710, 2004. doi: 10.1073/pnas.0407974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray RD, Merchant ML, Hardin E, Clark B, Khundmiri SJ, Lederer ED. Identification of an RNA-binding protein that is phosphorylated by PTH and potentially mediates PTH-induced destabilization of Npt2a mRNA. Am J Physiol Cell Physiol 310: C205–C215, 2016. doi: 10.1152/ajpcell.00192.2015. [DOI] [PubMed] [Google Scholar]
- 35.Murtazina R, Kovbasnjuk O, Donowitz M, Li X. Na+/H+ exchanger NHE3 activity and trafficking are lipid raft-dependent. J Biol Chem 281: 17845–17855, 2006. doi: 10.1074/jbc.M601740200. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen AT, Hayward-Lester A, Sabatini S, Doris PA. Renal Na+, K+-ATPase in SHR: studies of activity and gene expression. Clin Exp Hypertens 20: 641–656, 1998. doi: 10.3109/10641969809053242. [DOI] [PubMed] [Google Scholar]
- 37.Pedemonte CH, Efendiev R, Bertorello AM. Inhibition of Na,K-ATPase by dopamine in proximal tubule epithelial cells. Semin Nephrol 25: 322–327, 2005. doi: 10.1016/j.semnephrol.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Pelayo JC, Fildes RD, Jose PA. Age-dependent renal effects of intrarenal dopamine infusion. Am J Physiol Regul Integr Comp Physio 247: R212–R216, 1984. [DOI] [PubMed] [Google Scholar]
- 39.Pinho MJ, Serrão MP, Gomes P, Hopfer U, Jose PA, Soares-da-Silva P. Over-expression of renal LAT1 and LAT2 and enhanced L-DOPA uptake in SHR immortalized renal proximal tubular cells. Kidney Int 66: 216–226, 2004. doi: 10.1111/j.1523-1755.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- 40.Pinho MJ, Serrão MP, Soares-da-Silva P. High-salt intake and the renal expression of amino acid transporters in spontaneously hypertensive rats. Am J Physiol Renal Physiol 292: F1452–F1463, 2007. doi: 10.1152/ajprenal.00465.2006. [DOI] [PubMed] [Google Scholar]
- 41.Karim Z, Gérard B, Bakouh N, Alili R, Leroy C, Beck L, Silve C, Planelles G, Urena-Torres P, Grandchamp B, Friedlander G, Prié D. NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 359: 1128–1135, 2008. doi: 10.1056/NEJMoa0802836. [DOI] [PubMed] [Google Scholar]
- 42.Salyer S, Lesousky N, Weinman EJ, Clark BJ, Lederer ED, Khundmiri SJ. Dopamine regulation of Na+-K+-ATPase requires the PDZ-2 domain of sodium hydrogen regulatory factor-1 (NHERF-1) in opossum kidney cells. Am J Physiol Cell Physiol 300: C425–C434, 2011. doi: 10.1152/ajpcell.00357.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shenolikar S, Weinman EJ. NHERF: targeting and trafficking membrane proteins. Am J Physiol Renal Physiol 280: F389–F395, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Vieira-Coelho MA, Hussain T, Kansra V, Serrao MP, Guimaraes JT, Pestana M, Soares-Da-Silva P, Lokhandwala MF. Aging, high salt intake, and renal dopaminergic activity in Fischer 344 rats. Hypertension 34: 666–672, 1999. doi: 10.1161/01.HYP.34.4.666. [DOI] [PubMed] [Google Scholar]
- 45.Wade JB, Liu J, Coleman RA, Cunningham R, Steplock DA, Lee-Kwon W, Pallone TL, Shenolikar S, Weinman EJ. Localization and interaction of NHERF isoforms in the renal proximal tubule of the mouse. Am J Physiol Cell Physiol 285: C1494–C1503, 2003. doi: 10.1152/ajpcell.00092.2003. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z, Armando I, Asico LD, Escano C, Wang X, Lu Q, Felder RA, Schnackenberg CG, Sibley DR, Eisner GM, Jose PA. The elevated blood pressure of human GRK4γ A142V transgenic mice is not associated with increased ROS production. Am J Physiol Heart Circ Physiol 292: H2083–H2092, 2007. doi: 10.1152/ajpheart.00944.2006. [DOI] [PubMed] [Google Scholar]
- 47.Weinman EJ, Biswas R, Steplock D, Douglass TS, Cunningham R, Shenolikar S. Sodium-hydrogen exchanger regulatory factor 1 (NHERF-1) transduces signals that mediate dopamine inhibition of sodium-phosphate co-transport in mouse kidney. J Biol Chem 285: 13454–13460, 2010. doi: 10.1074/jbc.M109.094359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinman EJ, Evangelista CM, Steplock D, Liu MZ, Shenolikar S, Bernardo A. Essential role for NHERF in cAMP-mediated inhibition of the Na+-HCO3− co-transporter in BSC-1 cells. J Biol Chem 276: 42339–42346, 2001. doi: 10.1074/jbc.M106153200. [DOI] [PubMed] [Google Scholar]
- 49.Weinman EJ, Lederer ED. NHERF-1 and the regulation of renal phosphate reabsoption: a tale of three hormones. Am J Physiol Renal Physiol 303: F321–F327, 2012. doi: 10.1152/ajprenal.00093.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinman EJ, Steplock D, Bui G, Yuan N, Shenolikar S. Regulation of renal Na+-H+ exchanger by cAMP-dependent protein kinase. Am J Physiol Renal Physiol 258: F1254–F1258, 1990. [DOI] [PubMed] [Google Scholar]
- 51.Weinman EJ, Steplock D, Cha B, Kovbasnjuk O, Frost NA, Cunningham R, Shenolikar S, Blanpied TA, Donowitz M. PTH transiently increases the percent mobile fraction of Npt2a in OK cells as determined by FRAP. Am J Physiol Renal Physiol 297: F1560–F1565, 2009. doi: 10.1152/ajprenal.90657.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods 6: 359–362, 2009. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 53.Yang J, Villar VA, Jones JE, Jose PA, Zeng C. G protein-coupled receptor kinase 4: role in hypertension. Hypertension 65: 1148–1155, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, Felder RA, Jose PA. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int 70: 1072–1079, 2006. doi: 10.1038/sj.ki.5001708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.