Abstract
The depth of our knowledge regarding mast cells has widened exponentially in the last 20 years. Once thought to be only important for allergy-mediated events, mast cells are now recognized to be important regulators of a number of pathological processes. The revelation that mast cells can influence organs, tissues, and cells has increased interest in mast cell research during liver disease. The purpose of this review is to refresh the reader’s knowledge of the development, type, and location of mast cells and to review recent work that demonstrates the role of hepatic mast cells during diseased states. This review focuses primarily on liver diseases and mast cells during autoimmune disease, hepatitis, fatty liver disease, liver cancer, and aging in the liver. Overall, these studies demonstrate the potential role of mast cells in disease progression.
Keywords: cholangiopathies, disease, liver, mast cells
Mast Cell Biology, Development, and Classification
mast cells were first described in 1878 in a doctoral thesis by Dr. Paul Ehrlich, who was awarded the Nobel Prize in 1908 for his discoveries. These immune cells were once thought to only mediate allergic reactions and stimulate wound healing; however, it has been revealed that mast cells may also be players in many autoimmune, inflammatory, infectious, and other disorders (8, 12). Mast cell research during disease progression is critical, because mast cells have been found to potentiate negative and positive effects on tissues and organ function (5, 8, 28).
In both humans and rodents, mast cells originate from CD34+ hematopoietic stem cells stimulated by various stem cell factors (SCFs) and interleukins (ILs); these factors further regulate the development of mast cell subtypes (29, 109). Unlike other immune cells, mast cells do not mature before leaving the bone marrow; instead, the immature progenitors circulate in the lymphatic and vascular systems and complete their development peripherally (80, 109). Mast cells are distributed throughout the body in areas close to nerves, blood vessels, and lymphatic vessels (80, 109). SCF plays a crucial role in the development, migration, growth, survival, and location of mast cells (52, 80). Other factors that determine mast cell growth and survival include various ILs, chemokines, cytokines, transforming growth factor-β (TGF-β), and nerve growth factor (28, 80, 109).
Mast cells can be activated in a receptor-dependent or -independent manner, and the high-affinity IgE receptor (FCεRI) is highly expressed on their surface (28, 45, 53, 80). FCεRI-dependent activation results in a variety of specific signaling cascade mechanisms that lead to intracellular calcium influx, activation of certain transcription factors, mast cell degranulation, and cytokine production (105). Apart from FCεRI, mast cells also express other surface markers, such as complement receptors, Fc′YR, β2-integrin, intracellular adhesion molecule-1, serotonin receptor, and Toll-like receptors, which allow them to respond to diverse stimuli (45). Whether degranulation is through classic FCεRI or novel receptors or is receptor-independent, upon activation, mast cells release newly synthesized (lipid mediators and cytokines) and stored (histamine, heparin, and proteases) bioactive substances that are contained in cytoplasmic lipid bodies and granules into the surrounding tissue (5, 12, 28, 105). The release of these mediators is dependent on numerous factors, such as which protease is expressed by the mast cells and the location at the time of activation (28, 45).
The classification of mast cells is dependent on their phenotypic characteristics and anatomic locations. Reber et al. recently summarized the classification of mast cells in both mice and humans and also described their phenotypic characteristics (80). Similar to mast cells in mice, human mast cells are subcategorized into tryptase-positive and tryptase- and chymase-positive. Tryptase- and chymase-positive mast cells have an affinity for the small intestinal submucosa and muscularis mucosa, whereas tryptase-positive mast cells have a tendency to inhabit the mucosa of the stomach, small intestine, and colon. Within rats, mast cells are distinguished by staining for rat mast cell protease-1, which identifies connective tissue-derived mast cells, or rat mast cell protease-2, which identifies mucosal-derived mast cells (14, 114). The specific mast cell subtypes will determine the anatomic residency, and the positioning of mast cells also secures them as one of the first cells in the line of defense in the immune system.
Liver Biology and Mast Cells
The liver has many roles in the maintenance of systemic function and overall organismal homeostasis. Because of its location and specific anatomy, the liver is a primary player in immune responses against infectious pathogens. These responses occur via interactions between parenchymal cells (hepatocytes), antigen-presenting cells, and effector cells of the innate and adaptive immune systems (13, 26). Mast cells of the liver are mainly associated with the connective tissue that is found near hepatic arteries, veins, and bile ducts of the portal tracts in human and rat livers (52, 53, 57). In normal rodent and human livers, mast cells have been shown to accumulate in small numbers along the portal tracts, indicating that mast cell presence is not solely based on liver injury (52, 53, 57). However, increases in mast cell number have been noted during different hepatic injuries. Specifically, Johnson et al. found that, during human cholangiocarcinoma, the number of chymase-positive mast cells totaled ~70 per portal area and the number of tryptase-positive mast cells totaled ~30 per portal area (52). After injury, the number of hepatic mast cells increases, and they degranulate to release numerous growth mediators such as histamine, heparin, tryptase, TGF-β1, TNFα, ILs, cytokines, and basic fibroblast growth factor (bFGF) (13, 31, 45).
Hepatic inflammation is characterized by the migration of inflammatory cells to the damaged area (26). Resident Kupffer cells release chemical messengers that draw inflammatory cells, such as mast cells, to the surrounding area, where they mediate immunoregulatory events. The similarities between mast cells and Kupffer cells are further illuminated when the integral role of mast cells in inflammation is examined. Surrounding the hepatic sinusoids are the parenchymal cells of the liver, hepatocytes, the major function of which is the production of bile. Because they are in direct contact with the blood supply, hepatocytes are vital in the development and progression of liver pathophysiology (28, 32). As previously stated, mast cells circulate throughout the body via the vascular system and are found in close proximity to blood vessels; since hepatocytes are in contact with the blood supply, it seems plausible that mast cells closely regulate hepatic injury through cross talk with hepatocytes. Hepatocyte-secreted bile is modified by cholangiocytes, which are the cells lining the biliary tract and the target of cholangiopathies, such as primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC) (34, 79). Mast cells have been found in close proximity to bile ducts during various cholangiopathies, indicating that mast cells and cholangiocytes may regulate one another via paracrine signaling. Mast cells can also interact with various liver cell types, including cholangiocytes (biliary ductal cells) and hepatocytes, as shown by Grizzi et al. (39).
Autoimmune Cholangiopathies and Obstructive Bile Duct Injury
Primary biliary cholangitis.
PBC, an autoimmune disease characterized by the injury of small- and medium-sized bile ducts, gradually progresses to liver cirrhosis and, eventually, death (49, 111). As these ducts are damaged, bile builds up in the liver and damages the surrounding tissue. The pathogenesis of PBC is related to autoimmunity, as indicated by cell-mediated responses against self-antigens. Studies have found that PBC predominantly affects women (10:1 female-to-male ratio), and the incidence of PBC is higher in patients who have a relative with PBC or any other autoimmune disorder (64).
One of the first studies regarding mast cells and PBC came from Nakamura et al. They found an increase in the number of mast cells around the portal tract in patients with PBC (140 ± 25 cells/mm2, P < 0.05) compared with patients with chronic hepatitis (72 ± 10 cells/mm2, P < 0.05) (71). Their findings suggest that mast cell activation may regulate the pathogenesis of eosinophilia in PBC progression, because mast cells secrete mediators that are critical for eosinophil differentiation, chemotaxis, and activation, such as IL-3, IL-5, granulocyte-macrophage colony-stimulating factor, and platelet-activating factor (71). Furthermore, PBC patients often present with increased circulating bile acid pools, and it has been demonstrated that specific bile acids can alter mast cell activation in vitro (78, 108).
It has been shown that mast cells are in close contact with nerve fibers and that the liver is innervated by the sympathetic and parasympathetic nervous systems, thus supporting the concept that mast cells may influence or be influenced by nerve fibers. According to Matsunaga et al., mast cells may be stimulated by innervation, and this can increase the release of fibrogenic factors in patients with PBC (68), suggesting that mast cells play an active role in PBC. The authors found a significant increase in the number of chymase- and tryptase-positive mast cells that were in close proximity to S-100-positive nerve fibers. The density of mast cells in contact with nerve fibers was 12.0 ± 10.1 chymase-positive mast cells/mm2 (P < 0.0005) and 10.1 ± 10.7 tryptase-positive mast cells/mm2 (P < 0.000001) in PBC liver compared with 3.4 ± 0.9 chymase-positive mast cells/mm2 and 4.1 ± 0.7 tryptase-positive mast cells/mm2 in normal liver. Furthermore, their study revealed a significant relationship between both chymase- and tryptase-positive mast cell density and stromal fibrosis during PBC. The authors concluded that increased nerve stimulation induces mast cell migration and activation, thus releasing profibrogenic factors into the liver and increasing fibrosis (68).
Similarly, a recent study indicated that mast cells were located in the portal areas and sinusoidal walls in patients with PBC and that these mast cells expressed increased chymase (85). Specifically, the amount of hepatic chymase in PBC liver was 11.67 ± 9.96 ng/mg. Furthermore, Satomura et al. deduced that chymase-positive mast cells colocalized in areas that exhibited extensive hepatic fibrosis. From these findings, it is apparent that chymase-positive mast cells increase fibrosis in patients with PBC. There have been only a few studies of the role of mast cells in both human PBC and rodent models of the disease. However, these few studies suggest that there may be a strong correlation between the presence of mast cells and PBC progression that warrants further examination (67, 70, 77, 84, 107). While these studies demonstrate the increased presence of mast cells, the causal effect of mast cells remains to be fully examined.
Primary sclerosing cholangitis.
PSC is a chronic disease that damages both intra- and extrahepatic bile ducts. The inflammation of the bile ducts that occurs during PSC leads to scarring and narrowing of the affected ducts. Eventually, blockages may cause bile to become trapped within the liver, resulting in fibrosis, cirrhosis, and, potentially, liver failure (44, 61).
In 1995 a 75-yr-old woman was found to have extensive sclerosing cholangitis coupled with a massive infiltration of mast cells. This was the first case to demonstrate that the presence of mast cells may correlate with PSC, but the occurrence of extensive sclerosing cholangitis along with a massive infiltration of mast cells was attributed to systemic mastocytosis (6). Approximately 10 years later, in a separate study, four patients with PSC (class 2 or 3) were found to have increased expression of SCF within bile ducts and enhanced c-Kit-positive mast cell presence near portal tracts (124.8 ± 62.1 mast cells per area of portal tract) (50). Both of these studies further opened the window to investigation of the role of mast cells in PSC development and progression.
Tsuneyama et al. evaluated mast cell infiltration and bFGF expression in patients with PSC (98). They found that mast cells surrounded bile ducts during the early stages of PSC but were located in fibrous septa in late-stage PSC (98). Sclerosing areas in both regions were marked by intense expression of bFGF, a factor that is also secreted by activated mast cells (77).
Similarly, another study demonstrated numerous c-Kit-positive mast cells within periductal and ductal fibrotic areas around intrahepatic large bile ducts and also surrounding the proliferative peribiliary glands (97). Infiltrated mast cells expressed bFGF and/or TNF-α, components that are known to act as promoters of fibrosis. Interestingly, in PSC samples, the aberrant expression of SCF was found on biliary epithelia of dilated and stenotic bile ducts that displayed periductal fibrosis and inflammation, while SCF expression was not found in nonaffected bile ducts in normal livers (97). It may be that the aberrantly expressed SCF on biliary epithelial cells accumulates and attracts/stimulates mast cells via the c-Kit receptor and that these activated mast cells induce progressive periductal and portal fibrosis during PSC.
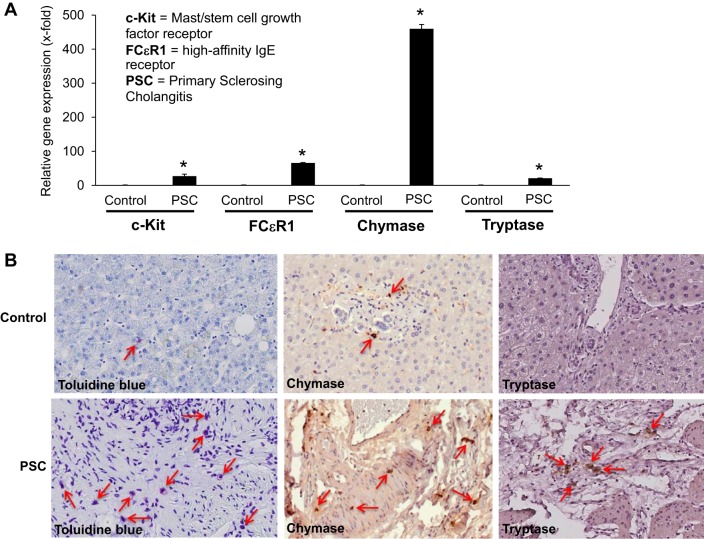
More recently, using both human PSC samples and the multidrug-resistant knockout mouse Mdr2−/−, which histopathologically mimics human PSC, Jones and Hargrove et al. examined the role of mast cells in PSC (53). They found that mast cell number and mast cell markers are increased in both Mdr2−/− mice and PSC patients compared with controls (Fig. 1). Furthermore, when Mdr2−/− mice were treated with cromolyn sodium, a mast cell stabilizer that blocks the release of histamine, mast cell indicators and PSC-associated fibrosis were significantly reduced (53). The authors also examined the effects of mast cells and mast cell-derived histamine on bile flow, the total bile acid pool, and bicarbonate excretion. Interestingly, treatment with cromolyn sodium decreased all these parameters in Mdr2−/− mice, demonstrating for the first time that mast cells and their mediators may influence the function of cholangiocytes and hepatic bile production and flow (53). This is the first study to demonstrate that mast cell-derived histamine may regulate biliary proliferation and hepatic fibrosis in Mdr2−/− mice and human PSC.
Fig. 1.
Mast cells and mast cell markers are upregulated in human PSC. Mast cell presence was assessed in human liver biopsy samples from control (no disease) and PSC patients (late- and advanced-stage PSC) by real-time PCR, toluidine blue staining, and immunohistochemistry for mast cell markers (chymase and tryptase). A: gene expression of c-Kit, FCεR1, chymase, and tryptase increased in samples from advanced- and late-stage PSC compared with normal, nondiseased tissues. Values are means ± SE of ≥6 experiments. *P < 0.05 vs. control. B: immunostaining (toluidine blue) and immunohistochemistry (chymase and tryptase) show infiltration of mast cells surrounding damaged bile ducts in PSC patients compared with normal (control) tissue. Red arrows depict mast cells. Magnification, ×20. [Modified and reprinted with permission from Jones and Hargrove et al. (53).]
The majority of studies of PSC and mast cells demonstrate an upregulation of mast cell number and activation but do not examine the direct, causal effect; however, there is an indication that inhibition of mast cells has a significant causal effect on PSC damage, as shown by Jones and Hargrove et al. (53).
Human and Rodent Bile Duct Obstruction
Biliary obstruction is characterized by blockage of the bile ducts. Upon obstruction, bile begins to build up, causing abdominal pain, itching (pruritus), nausea, and jaundice. If left untreated, bile duct obstruction can lead to chronic liver disease or an increased buildup of bilirubin, which can also be life-threatening.
In a study utilizing surgical biopsy specimens obtained from 50 patients with secondary cholangitis caused by obstruction of the common bile duct, mast cells positive for tryptase, chymase, vasointestinal polypeptide (VIP), and substance P (SP) were detected (42). Numbers of mast cell subtypes were significantly higher in patients who presented with combined exacerbated cholangitis and chronic sclerotic cholangitis than in controls. In patients with combined exacerbated cholangitis, the number of mast cell subtypes was 11.3 tryptase-positive mast cells/mm2, 62.9 tryptase- and chymase-positive mast cells/mm2, 26.7 VIP-positive mast cells/mm2, and 24.7 SP-positive mast cells/mm2; in patients with chronic sclerotic cholangitis, the number of mast cell subtypes was 2.6 tryptase-positive mast cells/mm2, 23.3 chymase- and tryptase-positive mast cells/mm2, 3.7 VIP-positive mast cells/mm2, and 3.9 SP-positive mast cells/mm2. For controls the number of mast cell types was 0.6 tryptase-positive mast cells/mm2, 5.4 chymase- and tryptase-positive mast cells/mm2, 1.0 VIP-positive mast cells/mm2, and 0.7 SP-positive mast cells/mm2. The authors also detected nerve fibers positive for SP and VIP, as well as serotonin-positive endocrine cells, in close proximity to mast cells (42). This study demonstrates the existence of heterogeneous mast cells, nerve structures, and endocrine cells in patients with bile duct obstruction and the potential for mast cells to influence the pathology of obstructive cholangitis.
The rodent model of bile duct ligation (BDL) has been used over the years to study the effects of bile duct obstruction on biliary and hepatic damage (35, 100). This model has also been used to study the role of mast cells in bile duct obstruction. Zhang et al. used the BDL model to investigate the role of the antifibrotic agent N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) in liver damage, fibrosis, and mast cell presence (113). The authors measured mast cell presence by Giemsa staining and visualized numerous mast cells around the portal tract following BDL, whereas mast cell number was reduced in the BDL animals treated with AcSDKP. The authors also found that AcSDKP ameliorated BDL-induced damage and fibrosis (113); however, they failed to demonstrate whether this amelioration was due to the lower mast cell numbers or was a consequence of treatment.
In a more direct study, Lu et al. investigated the effect of the mast cell tryptase inhibitor APC 366 and its influence on BDL-induced hepatic fibrosis (65). Mast cells are known to contribute to fibrosis via the release of tryptase (30) and by increasing fibrogenic factors, such as collagen and laminin (26). The authors found that treatment with APC 366 inhibited hepatic fibrosis, reduced collagen content by twofold (P < 0.01) compared with the BDL group, and lowered serum liver enzymes (65). Inhibition of mast cell tryptase successfully reduced BDL-induced hepatic damage, demonstrating the potential of APC 366 to prevent fibrosis in patients with bile duct obstruction or chronic liver injury.
In a study focusing on isolation of mature hepatic mast cells from rats, Hargrove et al. also examined the migration of mast cells into the liver following BDL (47). Mast cell migration and counts were started 6 h following BDL and continued through 14 days. The authors reported an increase in total mast cell numbers at 3 days following BDL (~500 mast cells) and a peak at 14 days (~700 mast cells) (47). The average number of mast cells per lobe at 14 days was ~200, and mast cells were a varied mix of both chymase- and tryptase-positive (47). In a similar study using BDL-induced liver damage, Kennedy et al. found that inhibition of mast cell-derived histamine decreased biliary proliferation and hepatic fibrosis. They demonstrated mast cell infiltration of the liver following BDL and an increase in mast cell marker (c-Kit, chymase, and tryptase) expression in total liver compared with normal rats subjected to sham surgeries (57). When BDL rats were treated with cromolyn sodium, biliary proliferation, mast cell number, and hepatic fibrosis significantly decreased. The authors found that mast cell number also significantly correlated with bile duct mass, which was increased in BDL rats but decreased in rats treated with cromolyn sodium (Fig. 2). They also found that cromolyn sodium acts solely on mast cells, but not cholangiocytes, demonstrating that these are mast cell-specific events (57) and that the potential exists for cromolyn sodium treatment in patients with complications related to bile duct obstruction.
Fig. 2.
Mast cell numbers increase following bile duct ligation (BDL). A and B: evaluation of hepatic mast cell infiltration and correlation with intrahepatic bile duct mass. Toluidine blue staining shows a significant increase in the number of mast cells in BDL compared with the normal (sham-operated) rat but a reduction in the BDL + cromolyn rat. Values are means ± SE of 6 experiments. *P < 0.05 vs. sham-operated. #P < 0.05 vs. BDL. [Modified and reprinted with permission from Kennedy et al. (57).]
Recently, Hargrove et al. described the effects of BDL on mast cell-deficient (KitW-sh) mice; they found reduced biliary hyperplasia, hepatic fibrosis, and vascular cell activity in mast cell-deficient mice (48). The authors also found a significant upregulation of all these parameters when cultured mast cells were reintroduced into mast cell-deficient mice compared with mice injected with saline (48). These studies are the first to demonstrate that mast cells directly impact the biliary response following injury and highlight the importance of mast cells during biliary obstruction and their key role in mitigating hepatic damage and fibrosis.
A serious complication/side effect of cholestatic liver injury induced by obstruction is chronic pruritus (102). The origin of hepatic pruritus has been surrounded by mystery, and numerous signaling pathways have been investigated to better understand this phenomenon and offer treatment for patients with this debilitating symptom. While it has been shown that the histamine and serotonin pathways play only a minor role (69), mast cells may contribute significantly to pruritus. A study performed to detect the itch response in BDL rats found increased activation of protein-activated receptor 2 (PAR2), which can be activated by mast cells (10), which are known to be upregulated in BDL-induced liver injury (57). Patients with cholestasis and pruritus also presented with increased peripheral neuroinflammation, which is documented by an increase in dermal mast cells (101), again suggesting that mast cells play a role in promoting pruritus.
In contrast to the study from Twycross and colleagues demonstrating that mast cells promote pruritus, O’Keeffe et al. found that cutaneous mast cells did not contribute to cholestatic-induced pruritus (74). The authors analyzed skin biopsies from patients with cholestatic liver disease with pruritus and patients with cholestatic liver disease without pruritus and found no difference in mast cell number between these groups (74). However, the findings of this study are limited, since it analyzed a small sample number (5 or 6 patients per group). Because of the debilitating effects induced by chronic pruritus, further studies are warranted to understand and develop sophisticated tools to combat this condition.
Speculation on the role of mast cells in promoting pruritus is further enhanced when the role of bile acids in chronic itch induced by chronic cholestatic injury is examined. It is well known that bile acids are increased during liver injury, and therapy using ursodeoxycholic acid (UDCA) can somewhat ameliorate pruritus during PBC (33). It has also been demonstrated that UDCA can inhibit the release of histamine from mast cells (unpublished observations), so while traditional antihistamines that block histamine receptors have had only mild success in treating pruritus, UDCA therapy may act on mast cell-derived histamine, thus offering some true relief from chronic itching.
Hepatitis and Alcohol-Induced Liver Injury
In very basic terms, hepatitis is inflammation of the liver that can lead to a broad spectrum of diseases from cirrhosis to liver cancer (7). Hepatitis viruses (A, B, C, D, and E) are the leading activators of hepatitis in the world (with B and C leading to chronic hepatitis), but other infectious modes, including toxins (e.g., alcohol and certain drugs) and autoimmune diseases (7), can also induce hepatitis.
The interplay between mast cells and hepatitis has been highlighted in several studies. Using liver blocks from patients with hepatitis C virus (HCV), Amiot et al. found an increase in histocompatibility antigen, class I, G (HLA-G)-positive cells, and their presence correlated with increased fibrosis (Spearman’s test, r = 0.6102, P < 0.05). Based on costaining for HLA-G and CD-117, the authors deduced that these HLA-G-positive cells were mast cells (1). In vitro, the authors found increased mast cell secretion of HLA-G after stimulation with IL-10 (7.8 ng/ml vs. 4.0 ng/ml in unstimulated controls), further confirming their findings (1). In a separate study using a larger cohort of samples from patients with chronic HCV, Koruk et al. found that as fibrosis increased in the liver, so did the number of mast cells in portal areas (60). Specifically, the number of mast cells per portal area was 0.87 ± 0.86 in chronic HCV. In contrast, no correlation was found between the degree of fibrosis and the number of mast cells in the sinusoids. Furthermore, the increase in the number of portal mast cells correlated with an increase in liver steatosis, suggesting that mast cells can manifest in HCV and contribute to fibrosis and steatosis associated with this chronic disease (60). Li et al. noted that c-Kit and hepatic SCF expression in mast cells was enhanced in rats injected subcutaneously with CCl4 and fed a diet high in cholesterol and alcohol and low in protein and choline to induce chronic hepatitis (63). Expression of c-Kit and SCF was significantly upregulated in livers from the rats with chronic hepatitis compared with the controls. c-Kit expression was 2.783 ± 0.577 in controls and 12.86 ± 3.126 in rats with chronic hepatitis (t = 9.511, P < 0.05), and SCF expression was 3.383 ± 1.583 in controls and 15.58 ± 6.431 in rats with chronic hepatitis (t = 9.625, P < 0.05). Plasma tryptase and hyaluronic acid levels, along with increased fibrosis associated with numerous degranulating and degranulated mast cells located around the liver blood vessels and in fiber intervals, were also increased in the rats with hepatitis (10).
In the United States, two-thirds of the adult population drinks alcohol, but only a minority will develop alcohol-related liver diseases, including alcoholic steatosis, hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) (83). Alcoholic steatosis is the earliest and most common liver disease associated with alcohol abuse (59, 95). This condition is usually asymptomatic and resolves within 6–8 wk of abstinence. Alcoholic liver disease is distinguished from nonalcoholic steatosis and nonalcoholic fatty liver disease (NAFLD), because, unlike NAFLD, it is not associated with metabolic syndrome (83). Serum tryptase levels are often used in the diagnosis of alcohol-induced injury, and baseline serum tryptase concentrations are typically used in the diagnosis and monitoring of mast cell disorders and obesity. Additionally, related metabolic syndromes are found to be associated with increased total tryptase concentrations in adults (37). Beceiro et al. studied the level of mast cell tryptase in individuals who consume large amounts of alcohol and found a low concentration of serum tryptase in these individuals (9). The authors found median serum tryptase levels of 2.23 μg/l in heavy drinkers compared with 3.25 μg/l in healthy controls. While many patients present with abnormally high AST and ALT levels, the levels of tryptase were significantly decreased and not detectable in some patients. To explain these findings, it has been noted that serum tryptase levels are dependent on mast cell burden and mast cell activation (87).
There are a number of reports on the effects of alcohol on mast cell degranulation, including work that demonstrates increased numbers of both total and degranulated mast cells in ethanol-fed rats (70). In vitro, ethanol treatment inhibits mast cell viability by increasing apoptosis, which might offer an explanation for the immunosuppression that is seen with alcohol abuse (73). Furthermore, high doses of ethanol may induce a toxic release of histamine by mast cells that damages surrounding tissues (82). Also, acetaldehyde, which is the metabolite produced in the liver when ethanol is broken down, induces mast cell degranulation and histamine release from isolated rodent mast cells (82). Acetaldehyde-induced mast cell activation in the gut could be partly responsible for excessive endotoxin passage (25) and subsequent systemic immunomodulatory effects. Together, these findings may indicate that low serum tryptase concentrations in heavy drinkers could reflect mast cell exhaustion after chronic alcohol consumption. From a clinical standpoint, these findings may be of importance in light of the common use of serum tryptase levels as a diagnostic tool. The impact of heavy drinking on mast cell degranulation should be considered when tryptase concentrations are analyzed (37).
Recently, the effects of an antioxidant ginger extract, zingerone, were used to evaluate the damage induced by ethanol treatment in rats (67). Mani et al. report a significant increase in mast cell presence and expression of inflammatory markers, such as NF-κB, cyclooxygenase-2, TNF-α, and IL-6, within the liver in rats treated with ethanol. When ethanol-fed rats were treated with zingerone, all these parameters, including mast cell presence, decreased (67). Because of the antioxidant and anti-inflammatory properties of zingerone, it is likely that mast cell presence was reduced via the reduction of inflammatory mediators, and this study contributes to the concept that natural therapies may be an alternative strategy for treatment of patients with ethanol-induced hepatotoxicity.
Finally, alcohol intake may not directly target mast cells but, rather, may act via other cellular signaling pathways and, thereby, influence mast cell function. Ferrier et al. demonstrated that alcohol induces dysregulation of the intestinal barrier, causing the activation of mast cells (25). This activation may be due to increased endotoxin blood levels, which have been noted in 20% of chronic drinkers. To evaluate whether blocking mast cell degranulation could influence alcohol-induced intestinal permeability, Ferrier et al. used the mast cell membrane stabilizer doxantrazole in combination with ethanol treatment. They found that stabilization of mast cells decreased ethanol-induced intestinal barrier permeability (25). Although this study was not directed specifically at the liver, it does highlight the importance of paracellular reactions and permeability of the intestinal barrier via activation of mast cells. Since alcohol consumption induces profound changes in immune function (9), it is conceivable that mast cells could be involved in these changes; further studies are needed to examine this potential interaction.
Steatosis and Steatohepatitis
Obesity and metabolic syndrome are increasing at alarming rates worldwide, and this escalation directly impacts the development of obesity-related illness, including diabetes, heart disease, and liver disease. Fat deposition in the liver can develop into a mild disease, such as steatosis, which can develop into a more progressive and severe pathology, such as nonalcoholic steatohepatitis (NASH) (55). These diseases can lead to liver fibrosis, cirrhosis, and HCC (81). The progression from steatosis to steatohepatitis is still largely not understood. One potential theory is the “two-hit” theory (51): the “first hit” is thought to be driven by insulin resistance, which leads to hepatic lipid accumulation; the “second hit,” made decidedly worse because of the first hit, causes hepatocyte injury, which increases inflammation and fibrosis (51). Many factors, such as proinflammatory cytokines and adipokines, mitochondrial dysfunction, oxidative stress, and endoplasmic reticulum stress, have been suggested in the initiation of the second hit, and some of these factors can be released from mast cells (76, 91).
Mast cells have been implicated in the development of steatosis and are associated with different liver etiologies, including HCV. Steatosis is frequently associated with chronic HCV, although it is unclear whether steatosis is associated with the virus or with host factors. Franceschini et al. evaluated mast cell markers and fibrotic reaction in patients diagnosed with HCV with or without steatosis and found that mast cell density was increased in patients with HCV coupled with steatosis; however, the degree of fibrosis between the two groups was not significantly altered (27). This study sheds light on the role that mast cells may play during fatty liver disease (in this case, induced by HCV) (19, 96). Because of the inflammatory environment around adipose tissue, it is not surprising that mast cells contribute to the pathogenesis of steatosis.
Using the apolipoprotein E-deficient (ApoE−/−) and ApoE−/−/mast cell-deficient (KitW-sh/W-sh) mouse models fed a high-fat diet, Smith et al. evaluated the role of mast cells in the progression of hepatic steatosis (90). ApoE−/−/KitW-sh/W-sh mice developed significantly less hepatic steatosis than ApoE−/− mice after 3 mo of high-fat diet feeding. Using Giemsa staining, they found a significant reduction in mast cell numbers in the ApoE−/−/KitW-sh/W-sh mice compared with the ApoE−/− mice fed the high-fat diet. Analysis of the Th1/Th2/Th17 cytokine profile in the sera revealed a significant reduction of IL-6 and IL-10 in ApoE−/−/KitW-sh/W-sh mice compared with ApoE−/− mice (90). The IL-6 and IL-10 levels were 11.0 ± 1.4 and 74.9 ± 14.2 pg/ml, respectively, in ApoE−/−/KitW-sh/W-sh mice compared with 17.3 ± 1.5 and 111.9 ± 14.4 pg/ml, respectively, in ApoE−/− mice. These results demonstrate the direct involvement of mast cells in the progression of hepatic steatosis following high-fat diet feeding.
Mast cells may also be involved in the pathogenesis of NASH, which is the progressive form of NAFLD. The mast cell protease chymase contributes to the formation of angiotensin (ANG) II and matrix metalloproteinase-9, factors that contribute to liver fibrosis. Tashiro et al. fed hamsters a methionine- and choline-deficient (MCD) diet for 8 wk to induce fatty liver disease and then treated the animals with a chymase inhibitor, TY-51469 (92). The authors report that treatment with TY-51469 ameliorated serum liver enzymes that were increased following the MCD diet and reduced liver steatosis. Fibrosis associated with fatty liver was also decreased in animals treated with TY-51469. Toluidine blue-positive mast cells were increased following the MCD diet and were reduced in animals treated with TY-51469 (92). This study pinpoints mast cells in the progression of NASH and also demonstrates the importance of inhibiting mast cell proteases. In a separate, but similar, study, hamsters were treated for 12 and 24 wk with the same chymase inhibitor and fed a MCD diet. Similar parameters were measured, and hepatic steatosis and fibrosis were more prominent in the placebo-treated hamsters fed the MCD diet for 24 wk than in those fed the MCD diet for 12 wk. TY-51469 ameliorated fibrosis and decreased the gene expression of collagen I, collagen III, and α-smooth muscle actin. These findings demonstrate that blocking mast cell mediators may alter the progressive and damaging course of fatty liver disease.
Hepatic Fibrosis: Congenital and Noncongenital
In the generation of liver fibrosis, three common phases follow injury: inflammation, synthesis of collagenous and noncollagenous extracellular matrix (ECM), and tissue remodeling due to dynamic fibrogenesis (26). Mast cells play an important role in the response to liver fibrosis, which develops as a result of chronic inflammation (60). Fibrosis is the accumulation of interstitial or “scar” ECM after liver injury (66, 103). Given their participation in sinusoidal capillarization, mast cells are thought to play an intricate role in the development and progression of liver fibrosis (26). Sinusoidal capillarization occurs upon transition of hepatic sinusoids to continuous capillaries, which contributes to liver fibrosis by deposition of collagen in extravascular spaces.
In terms of cellular involvement, once hepatic injury has occurred, hepatocellular necrosis leads to recruitment of various inflammatory cells and platelets, activation of Kupffer cells, and release of cytokines and growth factors. During the establishment of liver fibrosis, levels of collagen IV, entacin, and laminin may increase and form continuous basement membrane-like structures, a process that is accompanied by a decrease in the number of fenestrated capillaries (26, 107). Specifically, Franceschini et al. demonstrated that mast cells may be associated with hepatic fibrosis through the activation of fibroblast growth and collagen synthesis and may inhibit ECM degradation through enzymes called tissue inhibitors of metalloproteinases. Their study shows that mast cells may be the primary contributor in the transition from sinusoidal to capillary-type endothelial cells, which leads to the development of sinusoidal basement membrane (26).
Another protease that plays a key role in the development and progression of fibrosis is chymase. Chymase is found in mast cell granules and is thought to provoke the development of fibrosis by aiding in the differentiation of connective tissue and through the production of ANG II from ANG I. ANG II is able to promote fibrotic reaction by inducing hepatic stellate cell proliferation and ECM production in autoimmune hepatitis and PBC (85). Furthermore, blocking ANG II signaling through the use of the ANG II receptor antagonist losartan is able to decrease and even reverse fibrosis associated with chronic HCV (84). Since chymase, which is produced in large quantities by mast cells, is able to produce ANG II, which promotes hepatic fibrosis, it may be of interest to block mast cell degranulation to prevent release of chymase, production of ANG II, and subsequent hepatic stellate cell activation and ECM production.
Aside from noncongenital hepatic fibrosis, there are also forms of hepatic fibrosis that are congenital in nature. Congenital hepatic fibrosis (CHF) is a developmental disorder of the biliary tree and portal veins that is present at birth (43, 89). CHF is histopathologically identified by ductal plate malformation, abnormal branching of the biliary tree, and extensive hepatic fibrosis generally found surrounding the portal tracts (43). If we consider that CHF is characterized by progressive portal fibrosis and that mast cells are found in abundance near portal tracts during hepatic injury (4) and are able to increase hepatic fibrosis (24, 53, 57), it seems intuitive that there may be a link between the degree of CHF-associated portal fibrosis and the number of mast cells. Ozaki et al. noted that patients with CHF tended to have an abundance of mast cells (21.2 ± 9.7 absolute number of mast cells, P < 0.001) in the portal fibrous areas compared with those with other chronic liver diseases: 4.1 ± 2.7 with chronic viral hepatitis F1/F2, 8.5 ± 4.1 with chronic viral hepatitis F3/F4, 7.7 ± 7.2 with alcoholic fibrosis/cirrhosis, 3.7 ± 2.6 with extrahepatic bile duct obstruction, and 2.7 ± 2.3 with nonspecific reactive change (P < 0.001) (75). Based on these findings, the authors concluded that the numerous portal mast cells might be a strong regulator of CHF-related portal fibrosis (75). This is the only study that evaluates the possible role of mast cells in CHF progression and looks only at CHF-related fibrosis, and not biliary-related characteristics. Certainly, more studies are necessary to identify significant findings on the role of mast cells in CHF.
Liver Cancer
Hepatocellular carcinoma.
HCC is one of the most common cancers worldwide and is generally associated with a poor prognosis and high level of recurrence following resection (86). One major side effect associated with tumor development is tumor-associated inflammation, which negatively impacts the immune response and therapeutic efficacy (38). Cell types that can influence tumor-associated inflammation include macrophages, dendritic cells, lymphocytes, neutrophils, eosinophils, and mast cells (28). Specifically, mast cells have been found to be integral in the progression of HCC by promoting angiogenesis and tumor growth (40).
The high expression of IL-17 and IL-17-producing cells has been found to be a poor prognostic factor for HCC (46, 99, 110). A study by Zhang et al. indicates that the density of IL-17-producing cells within the HCC tumor environment correlated with high mortality and reduced survival (112). The median overall survival in patients with a higher density of intratumoral IL-17-producing cells was 34 mo compared with 49 mo in those with a lower density of these cells. Furthermore, the mast cell has also been indicated as an IL-17-producing cell (22, 56). Tu et al. evaluated the role of mast cell-produced IL-17 in the progression of HCC (99). They found that mast cells comprised the majority of IL-7-producing cells within HCC and that increased numbers of mast cells were associated with increased angiogenesis. Furthermore, this study demonstrated that IL-17-positive mast cells were primarily located in peritumoral areas and positively correlated with a poor prognosis (99). This corroborates the findings of Ju et al. that further characterized the negative impact of peritumoral mast cells on HCC prognosis via inflammatory processes (54).
While mast cells do express and secrete IL-17 (16), they are also able to release many other preformed mediators, such as histamine (20, 94). One study found that the human HCC cell line HA22T/VGH expressed the H1 and H2 histamine receptors and that cells treated with mast cell-derived histamine showed a significant increase in growth (62). However, this study also showed that mast cell-derived histamine reduced cell proliferation and viability in the human HCC cell line HuH-6, even though these cells expressed H1 and H2 histamine receptors (62). The difference in cell response to mast cell-derived histamine may be due to the different characteristics of differentiation, biological behavior, and genetic defects of each of these cell lines. In a recent study of patients with HCC who exhibited increased density of tryptase-positive mast cells (MCT), Ammendola et al. found a positive correlation between increased density in tryptase-positive mast cells and microvascular density, showing that mast cells may contribute to increased angiogenesis during HCC (2). This finding is further supported by Goffredo et al., who demonstrated increased serum tryptase levels in patients with HCC (36). In light of the fact that mast cells can secrete a wide variety of mediators following degranulation, these studies only scratch the surface of the impact that mast cell-derived factors may have on HCC growth and angiogenesis. Further studies are necessary to identify the characteristics of HCC tumoral mast cells, their direct role in HCC pathology, and the efficacy of manipulating these mast cells as a therapeutic for patients with HCC.
Aside from their individual contribution to HCC progression, mast cells may also interact with other cell types in the tumor microenvironment to influence HCC stage and prognosis. In the same study by Ju et al. that highlighted the proinflammatory role of mast cells in HCC tumors, a positive correlation was found between the presence of peritumoral Foxp3+ T-regulatory (Treg) cells and mast cell density (54). This study further clarified that the presence of mast cells in combination with Treg cells indicated a better prognosis than the presence of tumors that contained mast cells alone. This study indicates cross talk between mast cells and Treg cells that influences the tumor environment in a positive way; because Treg cells have been deemed anti-inflammatory (58), it is possible that Treg cells are working to counteract the proinflammatory nature of mast cells during HCC.
Hepatic tumors are not always locally derived and may form from various metastatic cancers from other tissue types. Gulubova evaluated the numbers of MCT and tryptase- and chymase-positive mast cells (MCTC) in livers containing metastases from colorectal, gastric, or pancreatic cancer (41). The author found significantly more MCTC and MCT in extra- and peritumoral regions of higher-grade metastases than in regions of lower-grade metastases. The mean numbers of MCT and MCTC were 7.9 ± 5 and 26.4 ± 15.1, respectively, in moderate/high-grade metastases and 3.9 ± 1.4 and 11.0 ± 2.6 in low-grade metastases. Furthermore, the number of MCTC was greater than the number of MCT in both extra- and peritumoral regions. Mast cells positive for SP and VIP were not seen (41). This study highlights the potential role of heterogeneous mast cells in the progression of hepatic metastases.
Cholangiocarcinoma.
Cholangiocarcinoma (CCA) is a rare form of cancer that comprises 2% of all primary neoplasms; however, it is the second-most-common hepatic malignancy after HCC (18). This form of cancer originates from the perihilar, intrahepatic, or extrahepatic bile duct epithelium. The risk factors for developing CCA include chronic inflammation from liver fluke infestation, hepatitis B and C infections, PSC, fatty liver disease, cholelithiasis, and inflammatory bowel disease (17, 106). At the time of diagnosis, only 10–15% of patients with CCA are amenable to potentially curable surgery, as a majority present at an advanced stage. Even with resection, CCA has high rates of recurrence; the 5-yr overall survival rate is 30% (17).
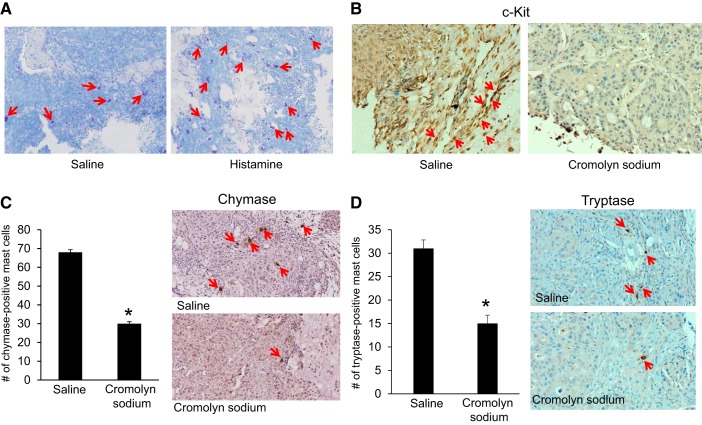
Because mast cells play such a prevalent role in other cholangiocyte-specific diseases (i.e., PBC and PSC) and in HCC, it seems relevant to review whether mast cells play a role in CCA. Johnson et al. evaluated mast cell numbers, SCF/c-Kit signaling, angiogenesis, and epithelial-mesenchymal transition (EMT) in CCA tumors excised from athymic mice and human patients (52). The authors found increased mast cell numbers in both mouse and human tumors compared with normal livers (numbers reported above), and this increase in mast cell numbers was accompanied by increased angiogenesis and EMT markers. In vitro, the link between mast cell-secreted factors and CCA angiogenesis and EMT was shown by treatment of human CCA cells with supernatants from mast cells treated with control, cromolyn sodium (to block mast cell degranulation), or an SCF inhibitor, ISCK03. The authors noted an increase in angiogenesis and EMT markers in CCA cells treated with mast cell supernatants and a decrease in these factors in CCA cells treated with supernatants from mast cells that were pretreated with cromolyn sodium or the SCF inhibitor (52). This strongly implicates mast cells in the progression of CCA via increased angiogenesis and metastatic potential. To evaluate whether these findings could be verified in vivo, athymic mice receiving CCA xenografts were treated with control or cromolyn sodium before evaluation of the same parameters. In cromolyn sodium-treated mice, Johnson et al. reported a decrease in tumor size and mast cell number (Fig. 3), angiogenesis, and EMT compared with control-treated mice (52). To determine the pathway regulating mast cell recruitment to CCA tumors, the authors performed a migration assay in vitro with human CCA cells treated with either control or SCF inhibitor and murine hepatic mast cells and found that inhibition of SCF was able to block mast cell migration. Overall, this study described how mast cells are able to migrate into the CCA tumor microenvironment via SCF/c-Kit signaling and increase tumor progression, angiogenesis, and EMT (52). Furthermore, the authors showed that blocking mast cell migration and degranulation by inhibition of SCF/c-Kit signaling or treatment with cromolyn sodium decreases these parameters.
Fig. 3.
Evaluation of mast cells in human cholangiocarcinoma. Mast cell presence was measured in tumors from mice treated with saline or histamine. Red arrows depict mast cells. A: toluidine blue staining shows an increase in mast cell infiltration in histamine- compared with saline-treated mice. B: immunohistochemistry shows an increase of c-Kit-positive mast cells in tumors from saline-treated mice and a decrease in the number of c-Kit-positive mast cells in tumors from cromolyn sodium-treated mice. C and D: immunohistochemistry and semiquantitative analysis show a decrease in the number of chymase-positive mast cells in tumors from cromolyn sodium- compared with saline-treated mice and similar results for tryptase-positive mast cells. Values are means ± SE of 9 experiments. *P < 0.05 vs. saline (NaCl). Representative images are shown for immunostaining. Original magnification, ×40. [Modified and reprinted with permission from Johnson and Huynh et al. (52).]
Another study examined the number of mast cells in human intrahepatic cholangiocarcinoma (ICC) and identified whether they were MCT or MCTC subsets (93). Terada and Matsunaga noted that, in normal livers, mast cells were located near portal tracts; however, in ICC, mast cells were present within the tumor. They also noted significantly more mast cells in ICC tumors than in normal livers; ~20% of the mast cells were MCT, while the other 80% were MCTC (93). These findings further support the findings of Johnson et al. that mast cells are increased in CCA and may regulate tumor progression (52). Furthermore, this study mimics the finding of Gulubova that HCC tumors were largely infiltrated by MCTC, but not as many MCT (41). These findings help highlight the significant role of mast cells during tumorigenesis and assist in identification of the specific mast cell subsets, which will help future researchers in developing a potential therapeutic target. These studies are highly valuable, as they shed light on a largely understudied field of research; however, more studies are warranted to further prove the pathological features of mast cell infiltration in CCA and to further unveil possible mast cell manipulative therapies.
Senescence and Aging in the Liver
Aging, initially considered an inexorable product of time, is actually related to postmaturation processes that lead to diminished homeostasis and decreased immune responses (39). The aging process is associated with many physiological changes and is generally linked to cellular senescence. Cellular senescence has been deemed the link that connects the visible consequence of aging to its molecular cause, cellular damage (11, 21). Recent studies have shown that senescence can lead to remodeling of the immune system, including changes in cell population, number, migration, and function (21). These senescence-related changes in the immune system may help explain why aging is associated with weakened immune function. Consequently, the incidence of liver disease increases with age, and the ability to overcome a hepatic insult decreases with each decade (39). Although the liver goes through a minor aging process compared with other organs, it still undergoes aging; mast cells have been found to have an important role in this aging process. However, few studies have investigated the role of aging and senescence in hepatic mast cell populations.
Chen et al. evaluated whether age-related senescence was able to affect mast cell development in bone marrow (15). In young (8–12 wk) and old (30–36 wk) normal and senescence-accelerated (SAMP1) mice (which exhibit accelerated senescence at 30 wk of age) subjected to myeloablation (15), they evaluated levels of SCF, a positive regulator of mast cell development, and TGF-β, a negative regulator of mast cell development, as well as the number of mast cell progenitors. Overall, they found an increase in the SCF-to-TGF-β ratio in both young and old mice following myeloablation, but this ratio quickly and drastically decreased in the old myeloablated mice (that exhibit accelerated senescence) but remained high in the young myeloablated mice (that exhibit low senescence). As a consequence of altered SCF-to-TGF-β ratios, recovery of the number of mast cell progenitors was lower in old mice that underwent myeloablation than in young mice, in which recovery was high (15). This study highlights the impact that age-related senescence can have on regulators of mast cell development. This lowered ability to generate mast cells may have an impact on age-related altered immune responses to disease.
To study the impact of aging on mast cell response during injury, one very early study evaluated mast cell numbers in young (2 mo) and old (19 mo) rats following CCl4-induced liver damage (104). Wolfe et al. found considerably increased numbers of hepatic mast cells at 24 h after CCl4 intoxication in the younger rats (6.5 ± 1.0 cells/mm2, P = 0.000076); however, the number of infiltrating mast cells was greatly diminished in the CCl4-treated older rats. No significant changes were noted in hepatic mast cell number between the young and old control rats (104). This suggests that that the older rats have a reduced immune response, which may be due to age-related immune remodeling. When we compare the findings of this study with the findings of Chen et al. (15) described above, it is probable that the decreased mast cell response to injury of these older rats is due to senescence-related impairments in mast cell generation. Further studies are necessary to pinpoint the direct impact of senescence and aging on mast cell numbers, generation, and response.
Liver Rejection
Three principal features of acute liver allograft rejection are portal inflammation, bile duct damage, and venular endothelitis. Chronic rejection is characterized by specific damage and loss of small bile ducts and/or foam cell obilterative arteritis (88). As stated earlier, mast cells in normal hepatic tissue are found in low numbers near portal tracts; after injury, infiltrating hepatic mast cells are found in large numbers near portal tracts and damaged bile ducts. This information may be useful in understanding the pathogenesis of chronic rejection.
Nakano et al. evaluated the role that mast cells may have in immune response to liver transplantation in rats (72). The authors noted increased levels of SCF and c-Kit (integral for mast cell migration), mast cell presence, and histamine release, which may be indicative of mast cell degranulation, in tolerated livers compared with rejected livers (23). Considering that mast cells can interact with other cell types to influence hepatic response to injury, Nakano et al. immunohistochemically evaluated whether mast cells colocalized with other cells following liver transplantation. Based on staining, the authors noted mast cell colocalization with Treg cells, γδ T cells, and hepatic progenitors largely in tolerated livers compared with rejected livers (72). In vitro, the authors used coculture techniques to evaluate whether cross talk between these cell types was occurring and noticed that mast cells were able to increase the γδ T cell population by mitogen stimulation and increase hepatocyte proliferation. These changes in γδ T cell and hepatocyte numbers were accompanied by mast cell degranulation, showing that mast cell-secreted factors, such as histamine, can induce proliferation in various cell types (72). Overall, this study highlights the beneficial role that mast cells may have during immunotolerance of transplanted livers.
In contrast, in their study of portal mast cell presence in normal, acute rejected (AR), and chronic rejected (CR) pediatric livers, Arikan et al. found an increase in the density of portal tract mast cells in AR liver (5.7 ± 4.4 mast cells) compared with control (0.4 ± 0.54 mast cells) and a further increase in CR (34.2 ± 26.2 mast cells) compared with AR liver. Based on their findings, the authors deduced that mast cell-rich portal areas may indicate the potential for CR during allograft liver transplantation (3). In terms of c-Kit expression, El-Refaie and Burt demonstrated significantly elevated levels of MCT and c-Kit-positive mast cells in the livers of both AR and CR patients (23). Their results mimicked those of Arikan et al. and showed that mast cells may play a detrimental role in both acute and chronic allograft rejection through modulation of inflammatory processes. However, neither of these studies evaluated whether mast cells were colocalizing with other cell types, which could potentially influence the hepatic immune response. The role of mast cells during liver tolerance/rejection is controversial and mostly unknown. Further research is necessary to uncover the role that mast cells may have in cell proliferation and immune response following transplantation.
Conclusion
In summary, studies on mast cells and liver disease progression have clearly pinpointed these immune cells as important regulators of pathogenic processes. Mast cells contribute to hepatic fibrosis, cholangiopathies, liver cancer, senescence-impaired aging, alcoholic liver injury, fatty liver disease, and allograft rejection. Since mast cells are recruited in high numbers during injury but reside in normal tissues in low numbers, targeting the migration or activation of mast cells may offer alternative strategies for patients with liver diseases. To date, no specific studies have demonstrated the role of mast cell subtypes in liver diseases; however, the implication that mast cells are present during liver disease is evident. Agents that block mast cell histamine release, such as cromolyn sodium and histamine receptor blockers, are frequently employed in the treatment of other illnesses; these tried-and-true compounds could be easily implemented with minimal negative consequences in therapy for patients with liver disease. It is evident from this litany of potential treatment options that further studies are warranted to explore the full magnitude of the role of mast cells in liver disease.
GRANTS
Portions of this work were supported by Department of Veterans Affairs Biomedical Laboratory Research and Development Service Merit Award 1I01BX003031 and National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK-108959 to H. Francis. This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, Texas.
DISCLAIMERS
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.J., L.K., and H.F. drafted manuscript; V.J., L.K., L.H., J.D., J.T., K.S., and H.F. edited and revised manuscript; V.J., L.K., L.H., J.D., J.T., K.S., and H.F. approved final version of manuscript; H.F. prepared figures.
REFERENCES
- 1.Amiot L, Vu N, Rauch M, L’Helgoualc’h A, Chalmel F, Gascan H, Turlin B, Guyader D, Samson M. Expression of HLA-G by mast cells is associated with hepatitis C virus-induced liver fibrosis. J Hepatol 60: 245–252, 2014. doi: 10.1016/j.jhep.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Ammendola M, Sacco R, Sammarco G, Piardi T, Zuccalà V, Patruno R, Zullo A, Zizzo N, Nardo B, Marech I, Crovace A, Gadaleta CD, Pessaux P, Ranieri G. Mast cells positive to tryptase, endothelial cells positive to protease-activated receptor-2, and microvascular density correlate among themselves in hepatocellular carcinoma patients who have undergone surgery. Onco Targets Ther 9: 4465–4471, 2016. doi: 10.2147/OTT.S105368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arikan C, Nart D, Kilic M, Yuksekkaya HA, Aydogdu S. Association of mast cells and liver allograft rejection. Pediatr Transplant 12: 347–352, 2008. doi: 10.1111/j.1399-3046.2007.00819.x. [DOI] [PubMed] [Google Scholar]
- 4.Armbrust T, Batusic D, Ringe B, Ramadori G. Mast cells distribution in human liver disease and experimental rat liver fibrosis. Indications for mast cell participation in development of liver fibrosis. J Hepatol 26: 1042–1054, 1997. doi: 10.1016/S0168-8278(97)80113-4. [DOI] [PubMed] [Google Scholar]
- 5.Bachelet I, Levi-Schaffer F, Mekori YA. Mast cells: not only in allergy. Immunol Allergy Clin North Am 26: 407–425, 2006. doi: 10.1016/j.iac.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Baron TH, Koehler RE, Rodgers WH, Fallon MB, Ferguson SM. Mast cell cholangiopathy: another cause of sclerosing cholangitis. Gastroenterology 109: 1677–1681, 1995. doi: 10.1016/0016-5085(95)90658-4. [DOI] [PubMed] [Google Scholar]
- 7.Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol 3: 108–113, 2011. doi: 10.4254/wjh.v3.i5.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol 39: 11–25, 2009. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beceiro C, Campos J, Valcarcel MA, Fenger RV, Lojo S, Linneberg A, Vidal C, Gonzalez-Quintela A. Serum concentrations of mast cell tryptase are reduced in heavy drinkers. Alcohol Clin Exp Res 39: 672–678, 2015. doi: 10.1111/acer.12682. [DOI] [PubMed] [Google Scholar]
- 10.Belghiti M, Estévez-Herrera J, Giménez-Garzó C, González-Usano A, Montoliu C, Ferrer-Montiel A, Felipo V, Planells-Cases R. Potentiation of the transient receptor potential vanilloid 1 channel contributes to pruritogenesis in a rat model of liver disease. J Biol Chem 288: 9675–9685, 2013. doi: 10.1074/jbc.M113.455162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia-Dey N, Kanherkar RR, Stair SE, Makarev EO, Csoka AB. Cellular senescence as the causal nexus of aging. Front Genet 7: 13, 2016. doi: 10.3389/fgene.2016.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyce JA. The biology of the mast cell. Allergy Asthma Proc 25: 27–30, 2004. [PubMed] [Google Scholar]
- 13.Bulfone-Paus S, Bahri R. Mast cells as regulators of T cell responses. Front Immunol 6: 394, 2015. doi: 10.3389/fimmu.2015.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan A, Cooley MA, Collins AM. Mast cells in the rat liver are phenotypically heterogeneous and exhibit features of immaturity. Immunol Cell Biol 79: 35–40, 2001. doi: 10.1046/j.1440-1711.2001.00974.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci USA 102: 11408–11413, 2005. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Churchill MJ, Nagar KK, Tailor YH, Chu T, Rush BS, Jiang Z, Wang EB, Renz BW, Wang H, Fung MC, Worthley DL, Mukherjee S, Wang TC. IL-17 producing mast cells promote the expansion of myeloid-derived suppressor cells in a mouse allergy model of colorectal cancer. Oncotarget 6: 32966–32979, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong DQ, Zhu AX. The landscape of targeted therapies for cholangiocarcinoma: current status and emerging targets. Oncotarget 7: 46750–46767, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cidon EU. Resectable cholangiocarcinoma: reviewing the role of adjuvant strategies. Clin Med Insights Oncol 10: 43–48, 2016. doi: 10.4137/CMO.S32821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc 60: 349–356, 2001. doi: 10.1079/PNS2001110. [DOI] [PubMed] [Google Scholar]
- 20.da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem 62: 698–738, 2014. doi: 10.1369/0022155414545334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewan SK, Zheng SB, Xia SJ, Bill K. Senescent remodeling of the immune system and its contribution to the predisposition of the elderly to infections. Chin Med J (Engl) 125: 3325–3331, 2012. [PubMed] [Google Scholar]
- 22.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol 8: 337–348, 2008. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 23.El-Refaie AM, Burt AD. Mast cells and c-Kit expression in liver allograft rejection. Histopathology 47: 375–381, 2005. doi: 10.1111/j.1365-2559.2005.02239.x. [DOI] [PubMed] [Google Scholar]
- 24.Farrell DJ, Hines JE, Walls AF, Kelly PJ, Bennett MK, Burt AD. Intrahepatic mast cells in chronic liver diseases. Hepatology 22: 1175–1181, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Ferrier L, Bérard F, Debrauwer L, Chabo C, Langella P, Buéno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol 168: 1148–1154, 2006. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franceschini B, Ceva-Grimaldi G, Russo C, Dioguardi N, Grizzi F. The complex functions of mast cells in chronic human liver diseases. Dig Dis Sci 51: 2248–2256, 2006. doi: 10.1007/s10620-006-9082-8. [DOI] [PubMed] [Google Scholar]
- 27.Franceschini B, Russo C, Dioguardi N, Grizzi F. Increased liver mast cell recruitment in patients with chronic C virus-related hepatitis and histologically documented steatosis. J Viral Hepat 14: 549–555, 2007. doi: 10.1111/j.1365-2893.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- 28.Francis H, Meininger CJ. A review of mast cells and liver disease: what have we learned? Dig Liver Dis 42: 529–536, 2010. doi: 10.1016/j.dld.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Frieri M, Kumar K, Boutin A. Role of mast cells in trauma and neuroinflammation in allergy immunology. Ann Allergy Asthma Immunol 115: 172–177, 2015. doi: 10.1016/j.anai.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Frungieri MB, Albrecht M, Raemsch R, Mayerhofer A. The action of the mast cell product tryptase on cyclooxygenase-2 (COX2) and subsequent fibroblast proliferation involves activation of the extracellular signal-regulated kinase isoforms 1 and 2 (erk1/2). Cell Signal 17: 525–533, 2005. doi: 10.1016/j.cellsig.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 31.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol 8: 478–486, 2008. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudio E, Franchitto A, Pannarale L, Carpino G, Alpini G, Francis H, Glaser S, Alvaro D, Onori P. Cholangiocytes and blood supply. World J Gastroenterol 12: 3546–3552, 2006. doi: 10.3748/wjg.v12.i22.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology 62: 635–643, 2015. doi: 10.1002/hep.27744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med 11: e7, 2009. doi: 10.1017/S1462399409000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glaser SS, Onori P, Wise C, Yang F, Marzioni M, Alvaro D, Franchitto A, Mancinelli R, Alpini G, Munshi MK, Gaudio E. Recent advances in the regulation of cholangiocyte proliferation and function during extrahepatic cholestasis. Dig Liver Dis 42: 245–252, 2010. doi: 10.1016/j.dld.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goffredo V, Gadaleta CD, Laterza A, Vacca A, Ranieri G. Tryptase serum levels in patients suffering from hepatocellular carcinoma undergoing intra-arterial chemoembolization: possible predictive role of response to treatment. Mol Clin Oncol 1: 385–389, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez-Quintela A, Vizcaino L, Gude F, Rey J, Meijide L, Fernandez-Merino C, Linneberg A, Vidal C. Factors influencing serum total tryptase concentrations in a general adult population. Clin Chem Lab Med 48: 701–706, 2010. doi: 10.1515/CCLM.2010.124. [DOI] [PubMed] [Google Scholar]
- 38.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 140: 883–899, 2010. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grizzi F, Di Caro G, Laghi L, Hermonat P, Mazzola P, Nguyen DD, Radhi S, Figueroa JA, Cobos E, Annoni G, Chiriva-Internati M. Mast cells and the liver aging process. Immun Ageing 10: 9, 2013. doi: 10.1186/1742-4933-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grizzi F, Franceschini B, Chiriva-Internati M, Liu Y, Hermonat PL, Dioguardi N. Mast cells and human hepatocellular carcinoma. World J Gastroenterol 9: 1469–1473, 2003. doi: 10.3748/wjg.v9.i7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulubova MV. Structural examination of tryptase- and chymase-positive mast cells in livers, containing metastases from gastrointestinal cancers. Clin Exp Metastasis 20: 611–620, 2003. doi: 10.1023/A:1027310827655. [DOI] [PubMed] [Google Scholar]
- 42.Gulubova MV, Vlaykova TI. Mast cells in human bile duct obstruction. J Mol Histol 35: 791–801, 2004. doi: 10.1007/s10735-004-0946-y. [DOI] [PubMed] [Google Scholar]
- 43.Gunay-Aygun M, Gahl WA, Heller T. Congenital hepatic fibrosis overview. In: GeneReviews, edited by Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K. Seattle, WA: University of Washington, 1993. [PubMed] [Google Scholar]
- 44.Halilbasic E, Fuchs C, Hofer H, Paumgartner G, Trauner M. Therapy of primary sclerosing cholangitis—today and tomorrow. Dig Dis 33 Suppl 2: 149–163, 2015. doi: 10.1159/000440827. [DOI] [PubMed] [Google Scholar]
- 45.Halova I, Draberova L, Draber P. Mast cell chemotaxis—chemoattractants and signaling pathways. Front Immunol 3: 119, 2012. doi: 10.3389/fimmu.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammad LN, Abdelraouf SM, Hassanein FS, Mohamed WA, Schaalan MF. Circulating IL-6, IL-17 and vitamin D in hepatocellular carcinoma: potential biomarkers for a more favorable prognosis? J Immunotoxicol 10: 380–386, 2013. doi: 10.3109/1547691X.2012.758198. [DOI] [PubMed] [Google Scholar]
- 47.Hargrove L, Graf-Eaton A, Kennedy L, Demieville J, Owens J, Hodges K, Ladd B, Francis H. Isolation and characterization of hepatic mast cells from cholestatic rats. Lab Invest 96: 1198–1210, 2016. doi: 10.1038/labinvest.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hargrove L, Kennedy L, Demieville J, Jones H, Meng F, DeMorrow S, Karstens W, Madeka T, Greene J Jr, Francis H. Bile duct ligation-induced biliary hyperplasia, hepatic injury and fibrosis are reduced in mast cell deficient Kitw-sh mice. Hepatology 65: 1991–2004, 2017. doi: 10.1002/hep.29079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Invernizzi P, Selmi C, Gershwin ME. Update on primary biliary cirrhosis. Dig Liver Dis 42: 401–408, 2010. doi: 10.1016/j.dld.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishii M, Iwai M, Harada Y, Morikawa T, Okanoue T, Kishikawa T, Tsuchihashi Y, Hanai K, Arizono N. A role of mast cells for hepatic fibrosis in primary sclerosing cholangitis. Hepatol Res 31: 127–131, 2005. doi: 10.1016/j.hepres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 51.James O, Day C. Non-alcoholic steatohepatitis: another disease of affluence. Lancet 353: 1634–1636, 1999. doi: 10.1016/S0140-6736(99)00163-4. [DOI] [PubMed] [Google Scholar]
- 52.Johnson C, Huynh V, Hargrove L, Kennedy L, Graf-Eaton A, Owens J, Trzeciakowski JP, Hodges K, DeMorrow S, Han Y, Wong L, Alpini G, Francis H. Inhibition of mast cell-derived histamine decreases human cholangiocarcinoma growth and differentiation via c-Kit/stem cell factor-dependent signaling. Am J Pathol 186: 123–133, 2016. doi: 10.1016/j.ajpath.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones H, Hargrove L, Kennedy L, Meng F, Graf-Eaton A, Owens J, Alpini G, Johnson C, Bernuzzi F, Demieville J, DeMorrow S, Invernizzi P, Francis H. Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2−/− mice. Hepatology 64: 1202–1216, 2016. doi: 10.1002/hep.28704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ju MJ, Qiu SJ, Gao Q, Fan J, Cai MY, Li YW, Tang ZY. Combination of peritumoral mast cells and T-regulatory cells predicts prognosis of hepatocellular carcinoma. Cancer Sci 100: 1267–1274, 2009. doi: 10.1111/j.1349-7006.2009.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juárez-Hernández E, Chávez-Tapia NC, Uribe M, Barbero-Becerra VJ. Role of bioactive fatty acids in nonalcoholic fatty liver disease. Nutr J 15: 72, 2016. doi: 10.1186/s12937-016-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kan J, Mishima S, Kashiwakura J, Sasaki-Sakamoto T, Seki M, Saito S, Ra C, Tokuhashi Y, Okayama Y. Interleukin-17A expression in human synovial mast cells in rheumatoid arthritis and osteoarthritis. Allergol Int 65 Suppl: S11–S16, 2016. doi: 10.1016/j.alit.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy LL, Hargrove LA, Graf AB, Francis TC, Hodges KM, Nguyen QP, Ueno Y, Greene JF, Meng F, Huynh VD, Francis HL. Inhibition of mast cell-derived histamine secretion by cromolyn sodium treatment decreases biliary hyperplasia in cholestatic rodents. Lab Invest 94: 1406–1418, 2014. doi: 10.1038/labinvest.2014.129. [DOI] [PubMed] [Google Scholar]
- 58.Khor B. Regulatory T cells: central concepts from ontogeny to therapy. Transfus Med Rev 31: 36-44, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim MS, Ong M, Qu X. Optimal management for alcoholic liver disease: conventional medications, natural therapy or combination? World J Gastroenterol 22: 8–23, 2016. doi: 10.3748/wjg.v22.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koruk ST, Ozardali I, Dincoglu D, Bitiren M. Increased liver mast cells in patients with chronic hepatitis C. Indian J Pathol Microbiol 54: 736–740, 2011. [DOI] [PubMed] [Google Scholar]
- 61.Kumar A, Wheatley D, Puttanna A. Primary sclerosing cholangitis: therapeutic options and surveillance management. Clin Med Insights Gastroenterol 9: 25–29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lampiasi N, Azzolina A, Montalto G, Cervello M. Histamine and spontaneously released mast cell granules affect the cell growth of human hepatocellular carcinoma cells. Exp Mol Med 39: 284–294, 2007. doi: 10.1038/emm.2007.32. [DOI] [PubMed] [Google Scholar]
- 63.Li H, Zhao LF, Hao YQ, Yin L, Zhao YC, Han DW. [Changes in mast cells and hepatic expression of c-kit and stem cell factor in the rat model of chronic hepatitis]. Zhonghua Gan Zang Bing Za Zhi 21: 869–873, 2013. [DOI] [PubMed] [Google Scholar]
- 64.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ; American Association for Study of Liver Diseases . Primary biliary cirrhosis. Hepatology 50: 291–308, 2009. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 65.Lu J, Chen B, Li S, Sun Q. Tryptase inhibitor APC 366 prevents hepatic fibrosis by inhibiting collagen synthesis induced by tryptase/protease-activated receptor 2 interactions in hepatic stellate cells. Int Immunopharmacol 20: 352–357, 2014. doi: 10.1016/j.intimp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Maher JJ, McGuire RF. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest 86: 1641–1648, 1990. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mani V, Arivalagan S, Siddique AI, Namasivayam N. Antioxidant and anti-inflammatory role of zingerone in ethanol-induced hepatotoxicity. Mol Cell Biochem 421: 169–181, 2016. doi: 10.1007/s11010-016-2798-7. [DOI] [PubMed] [Google Scholar]
- 68.Matsunaga Y, Kawasaki H, Terada T. Stromal mast cells and nerve fibers in various chronic liver diseases: relevance to hepatic fibrosis. Am J Gastroenterol 94: 1923–1932, 1999. doi: 10.1111/j.1572-0241.1999.01232.x. [DOI] [PubMed] [Google Scholar]
- 69.Mela M, Mancuso A, Burroughs AK. Pruritus in cholestatic and other liver diseases. Aliment Pharmacol Ther 17: 857–870, 2003. doi: 10.1046/j.1365-2036.2003.01458.x. [DOI] [PubMed] [Google Scholar]
- 70.Mendes LO, Amorim JP, Teixeira GR, Chuffa LG, Fioruci BA, Pimentel TA, de Mello W Jr, Padovani CR, Pereira S, Martinez M, Pinheiro PF, Oliani SM, Martinez FE. Mast cells and ethanol consumption: interactions in the prostate, epididymis and testis of UChB rats. Am J Reprod Immunol 66: 170–178, 2011. doi: 10.1111/j.1600-0897.2010.00958.x. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura A, Yamazaki K, Suzuki K, Sato S. Increased portal tract infiltration of mast cells and eosinophils in primary biliary cirrhosis. Am J Gastroenterol 92: 2245–2249, 1997. [PubMed] [Google Scholar]
- 72.Nakano T, Lai CY, Goto S, Hsu LW, Kawamoto S, Ono K, Chen KD, Lin CC, Chiu KW, Wang CC, Cheng YF, Chen CL. Immunological and regenerative aspects of hepatic mast cells in liver allograft rejection and tolerance. PLoS One 7: e37202, 2012. doi: 10.1371/journal.pone.0037202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nurmi K, Methuen T, Mäki T, Lindstedt KA, Kovanen PT, Sandler C, Eklund KK. Ethanol induces apoptosis in human mast cells. Life Sci 85: 678–684, 2009. doi: 10.1016/j.lfs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 74.O’Keeffe C, Baird AW, Nolan N, McCormick PA. Cholestatic pruritus—the role of cutaneous mast cells and nerves. Aliment Pharmacol Ther 19: 1293–1300, 2004. doi: 10.1111/j.1365-2036.2004.01997.x. [DOI] [PubMed] [Google Scholar]
- 75.Ozaki S, Sato Y, Yasoshima M, Harada K, Nakanuma Y. Diffuse expression of heparan sulfate proteoglycan and connective tissue growth factor in fibrous septa with many mast cells relate to unresolving hepatic fibrosis of congenital hepatic fibrosis. Liver Int 25: 817–828, 2005. doi: 10.1111/j.1478-3231.2005.01067.x. [DOI] [PubMed] [Google Scholar]
- 76.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol 20: 14205–14218, 2014. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang XN, Ansel JC, Butterfield JH, Planck SR, Rosenbaum JT. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol 147: 564–573, 1995. [PMC free article] [PubMed] [Google Scholar]
- 78.Quist RG, Ton-Nu HT, Lillienau J, Hofmann AF, Barrett KE. Activation of mast cells by bile acids. Gastroenterology 101: 446–456, 1991. doi: 10.1016/0016-5085(91)90024-F. [DOI] [PubMed] [Google Scholar]
- 79.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 43 Suppl 1: S54–S62, 2006. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 80.Reber LL, Sibilano R, Mukai K, Galli SJ. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol 8: 444–463, 2015. doi: 10.1038/mi.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosso N, Chavez-Tapia NC, Tiribelli C, Bellentani S. Translational approaches: from fatty liver to non-alcoholic steatohepatitis. World J Gastroenterol 20: 9038–9049, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruiz CM, Gomes JC. Effects of ethanol, acetaldehyde, and acetic acid on histamine secretion in guinea pig lung mast cells. Alcohol 20: 133–138, 2000. doi: 10.1016/S0741-8329(99)00065-8. [DOI] [PubMed] [Google Scholar]
- 83.Saberi B, Dadabhai AS, Jang YY, Gurakar A, Mezey E. Current management of alcoholic hepatitis and future therapies. J Clin Transl Hepatol 4: 113–122, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salama ZA, Sadek A, Abdelhady AM, Darweesh SK, Morsy SA, Esmat G. Losartan may inhibit the progression of liver fibrosis in chronic HCV patients. Hepatobiliary Surg Nutr 5: 249–255, 2016. doi: 10.21037/hbsn.2016.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Satomura K, Yin M, Shimizu S, Kato Y, Nagano T, Komeichi H, Ohsuga M, Katsuta Y, Aramaki T, Omoto Y. Increased chymase in livers with autoimmune disease: colocalization with fibrosis. J Nippon Med Sch 70: 490–495, 2003. doi: 10.1272/jnms.70.490. [DOI] [PubMed] [Google Scholar]
- 86.Schulze K, Nault JC, Villanueva A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. J Hepatol 65: 1031–1042, 2016. doi: 10.1016/j.jhep.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 87.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am 26: 451–463, 2006. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 88.Shaw BW., Jr Auxiliary liver transplantation for acute liver failure. Liver Transpl Surg 1: 194–200, 1995. doi: 10.1002/lt.500010311. [DOI] [PubMed] [Google Scholar]
- 89.Shorbagi A, Bayraktar Y. Experience of a single center with congenital hepatic fibrosis: a review of the literature. World J Gastroenterol 16: 683–690, 2010. doi: 10.3748/wjg.v16.i6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smith DD, Tan X, Raveendran VV, Tawfik O, Stechschulte DJ, Dileepan KN. Mast cell deficiency attenuates progression of atherosclerosis and hepatic steatosis in apolipoprotein E-null mice. Am J Physiol Heart Circ Physiol 302: H2612–H2621, 2012. doi: 10.1152/ajpheart.00879.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tariq Z, Green CJ, Hodson L. Are oxidative stress mechanisms the common denominator in the progression from hepatic steatosis towards non-alcoholic steatohepatitis (NASH)? Liver Int 34: e180–e190, 2014. doi: 10.1111/liv.12523. [DOI] [PubMed] [Google Scholar]
- 92.Tashiro K, Takai S, Jin D, Yamamoto H, Komeda K, Hayashi M, Tanaka K, Tanigawa N, Miyazaki M. Chymase inhibitor prevents the nonalcoholic steatohepatitis in hamsters fed a methionine- and choline-deficient diet. Hepatol Res 40: 514–523, 2010. doi: 10.1111/j.1872-034X.2010.00627.x. [DOI] [PubMed] [Google Scholar]
- 93.Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol 33: 961–966, 2000. doi: 10.1016/S0168-8278(00)80129-4. [DOI] [PubMed] [Google Scholar]
- 94.Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cells and inflammation. Biochim Biophys Acta 1822: 21–33, 2012. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, Downs N, Gleeson D, MacGilchrist A, Grant A, Hood S, Masson S, McCune A, Mellor J, O’Grady J, Patch D, Ratcliffe I, Roderick P, Stanton L, Vergis N, Wright M, Ryder S, Forrest EH, STOPAH Trial . Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 372: 1619–1628, 2015. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 96.Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis 28: 179–185, 2010. doi: 10.1159/000282083. [DOI] [PubMed] [Google Scholar]
- 97.Tsuneyama K, Kono N, Yamashiro M, Kouda W, Sabit A, Sasaki M, Nakanuma Y. Aberrant expression of stem cell factor on biliary epithelial cells and peribiliary infiltration of c-kit-expressing mast cells in hepatolithiasis and primary sclerosing cholangitis: a possible contribution to bile duct fibrosis. J Pathol 189: 609–614, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 98.Tsuneyama K, Saito K, Ruebner BH, Konishi I, Nakanuma Y, Gershwin ME. Immunological similarities between primary sclerosing cholangitis and chronic sclerosing sialadenitis: report of the overlapping of these two autoimmune diseases. Dig Dis Sci 45: 366–372, 2000. doi: 10.1023/A:1005429130150. [DOI] [PubMed] [Google Scholar]
- 99.Tu JF, Pan HY, Ying XH, Lou J, Ji JS, Zou H. Mast cells comprise the major of interleukin 17-producing cells and predict a poor prognosis in hepatocellular carcinoma. Medicine (Baltimore) 95: e3220, 2016. doi: 10.1097/MD.0000000000003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tuchweber B, Desmoulière A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest 74: 265–278, 1996. [PubMed] [Google Scholar]
- 101.Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: scratching more than the surface. QJM 96: 7–26, 2003. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 102.Weisshaar E, Kucenic MJ, Fleischer AB Jr. Pruritus: a review. Acta Derm Venereol Suppl (Stockh) May: 5–32, 2003. [PubMed] [Google Scholar]
- 103.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology 47: 1394–1400, 2008. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 104.Wolfe AF, Dryden WF, Marshall IG, Pantelouris EM. Pharmacological aspects of neuromuscular transmission in the thymus deficient nude (Nu/Nu) mouse. Exp Neurol 51: 503–509, 1976. doi: 10.1016/0014-4886(76)90273-9. [DOI] [PubMed] [Google Scholar]
- 105.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut 65: 155–168, 2016. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 106.Xie D, Ren Z, Fan J, Gao Q. Genetic profiling of intrahepatic cholangiocarcinoma and its clinical implication in targeted therapy. Am J Cancer Res 6: 577–586, 2016. [PMC free article] [PubMed] [Google Scholar]
- 107.Xu GF, Li PT, Wang XY, Jia X, Tian DL, Jiang LD, Yang JX. Dynamic changes in the expression of matrix metalloproteinases and their inhibitors, TIMPs, during hepatic fibrosis induced by alcohol in rats. World J Gastroenterol 10: 3621–3627, 2004. doi: 10.3748/wjg.v10.i24.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamazaki K, Suzuki K, Nakamura A, Sato S, Lindor KD, Batts KP, Tarara JE, Kephart GM, Kita H, Gleich GJ. Ursodeoxycholic acid inhibits eosinophil degranulation in patients with primary biliary cirrhosis. Hepatology 30: 71–78, 1999. doi: 10.1002/hep.510300121. [DOI] [PubMed] [Google Scholar]
- 109.Yu X, Kasprick A, Petersen F. Revisiting the role of mast cells in autoimmunity. Autoimmun Rev 14: 751–759, 2015. doi: 10.1016/j.autrev.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 110.Zeng Y, Zhang Q, Wang H, Lu M, Kong H, Zhang Y, Shi H. Prognostic significance of interleukin-17 in solid tumors: a meta-analysis. Int J Clin Exp Med 8: 10515–10536, 2015. [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang HC, Hu RF, Zhu T, Tong L, Zhang QQ. Primary biliary cirrhosis degree assessment by acoustic radiation force impulse imaging and hepatic fibrosis indicators. World J Gastroenterol 22: 5276–5284, 2016. doi: 10.3748/wjg.v22.i22.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 50: 980–989, 2009. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 113.Zhang L, Xu LM, Chen YW, Ni QW, Zhou M, Qu CY, Zhang Y. Antifibrotic effect of N-acetyl-seryl-aspartyl-lysyl-proline on bile duct ligation induced liver fibrosis in rats. World J Gastroenterol 18: 5283–5288, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zweifel M, Breu K, Matozan K, Renner E, Welle M, Schaffner T, Clavien PA. Restoration of hepatic mast cells and expression of a different mast cell protease phenotype in regenerating rat liver after 70%-hepatectomy. Immunol Cell Biol 83: 587–595, 2005. doi: 10.1111/j.1440-1711.2005.01368.x. [DOI] [PubMed] [Google Scholar]