Luminal free fatty acid receptor 2 agonists stimulate enterochromaffin cells and release serotonin, which enhances mucosal defenses in rat duodenum. However, overdriving serotonin release with high luminal concentrations of free fatty acid 2 ligands such as short-chain fatty acids injures the mucosa by decreasing mucosal blood flow. These results are likely implicated in serotonin-related dyspeptic symptom generation because of small intestinal bacterial overgrowth, which is hypothesized to generate excess short-chain fatty acids in the foregut, overdriving serotonin release from enterochromaffin cells.
Keywords: serotonin, bicarbonate secretion, free fatty acid receptor 2, nonsteroidal anti-inflammatory drug-induced enteropathy, short-chain fatty acids, cyclooxygenase
Abstract
Serotonin (5-HT), predominantly synthesized and released by enterochromaffin cells, is implicated in gastrointestinal symptoms such as emesis, abdominal pain, and diarrhea. Because luminal short-chain fatty acids (SCFAs) release 5-HT from enterochromaffin cells, which express the SCFA receptor free fatty acid receptor 2 (FFA2) in rat duodenum, we examined the effects of the selective FFA2 agonist phenylacetamide-1 (PA1) on duodenal 5-HT release with consequent bicarbonate secretion [duodenal bicarbonate secretion (DBS)] and on indomethacin (IND)-induced enteropathy. Intestinal injury was induced by IND (10 mg/kg sc) with or without PA1. We measured DBS in vivo in a duodenal loop perfused with PA1 while measuring 5-HT released in the portal vein. Duodenal blood flow was measured by laser-Doppler flowmetry. IND induced small intestinal ulcers with duodenal sparing. PA1 given with IND (IND + PA1) dose dependently induced duodenal erosions. IND + PA1-induced duodenal lesions were inhibited by the FFA2 antagonist GLPG-0974, ondansetron, or omeprazole but not by RS-23597 or atropine. Luminal perfusion of PA1 augmented DBS accompanied by increased portal blood 5-HT concentrations with approximately eight times more release at 0.1 mM than at 1 µM, with the effects inhibited by coperfusion of GLPG-0974. Luminal PA1 at 1 µM increased, but at 0.1 mM diminished, duodenal blood flow. Cosuperfusion of PA1 (0.1 mM) decreased acid-induced hyperemia, further reduced by IND pretreatment but restored by ondansetron. These results suggest that, although FFA2 activation enhances duodenal mucosal defenses, FFA2 overactivation during ulcerogenic cyclooxygenase inhibition may increase the vulnerability of the duodenal mucosa to gastric acid via excessive 5-HT release and 5-HT3 receptor activation, implicated in foregut-related symptoms such as emesis and epigastralgia.
NEW & NOTEWORTHY Luminal free fatty acid receptor 2 agonists stimulate enterochromaffin cells and release serotonin, which enhances mucosal defenses in rat duodenum. However, overdriving serotonin release with high luminal concentrations of free fatty acid 2 ligands such as short-chain fatty acids injures the mucosa by decreasing mucosal blood flow. These results are likely implicated in serotonin-related dyspeptic symptom generation because of small intestinal bacterial overgrowth, which is hypothesized to generate excess SCFAs in the foregut, overdriving serotonin release from enterochromaffin cells.
irritable bowel syndrome (IBS) is a relatively common disease affecting >15% of the population by some estimates. Although its etiology remains unknown, data support the hypothesis that small intestinal bacterial overgrowth (SIBO) is etiologic, given the effectiveness of nonabsorbable antibiotics and diets low in fermentable oligo-, di-, and monosaccharides and polyols (FODMAPS) in relieving symptoms (11, 27, 49). Other compelling data support the involvement of serotonergic signaling, given the association of treatment with selective serotonin [5-hydroxytryptamine (5-HT)] receptor antagonists with clinical improvement (22, 24). To integrate these findings, we have recently found that enterochromaffin (EC) cells, which store 95% of overall 5-HT content (25), express receptors for the predominant bacterial metabolites, short-chain fatty acids (SCFAs), namely free fatty acid receptor 2 (FFA2) or G protein-coupled receptor 43, in rat duodenum (2). According to our hypothesis, excess SCFAs in the foregut lumen generated from FODMAPS by the overgrowth of fermentative bacteria overactivate FFA2, with resultant supernormal 5-HT generation and consequent symptoms.
Emesis is a common upper gastrointestinal symptom, triggered by central pathways and by vagal afferent nerves (45, 57). 5-HT, a common neurotransmitter and mediator, is involved in the genesis of nausea and emesis (5). The quantity of EC cells and overall mucosal 5-HT content are highest in the duodenum in humans and second highest in rats (28, 56). A variety of stimulants trigger 5-HT release from EC cells, including mechanical stimulation [intraluminal pressure (21), mucosal stroking (41)], diet or food poisoning [bitter tastants (13)], gastric acid (42), bacterial metabolites [SCFAs (23)], infectious diseases [cholera toxin (61), rotavirus (29)], and chemotherapeutic agents [cisplatin (17)]. In health, released 5-HT stimulates enterocytes and enteric neural circuits to increase the rate of ion secretion and the rate and amplitude of smooth muscle contraction with resultant propulsive motility. Nevertheless, excessive release of 5-HT is associated with pathological symptoms such as nausea, vomiting, and diarrhea that occur with chemotherapy, celiac disease, the carcinoid syndrome, and with diarrhea-predominant IBS (8, 15, 17, 64). Conversely, low 5-HT bioavailability is implicated in constipation-predominant IBS (6). Released 5-HT activates 5-HT3 receptors expressed on vagal afferent nerves in the lamina propria mucosa, which transmit neural signals to the vomiting center (area postrema) via the nucleus tractus solitaries, where 5-HT3 receptors are expressed (7). Therefore, nausea, vomiting, and diarrhea due to a variety of causes are commonly treated clinically with anti-emetic 5-HT3 receptor antagonists (57).
Although SCFAs are generally considered to be products of colonic bacterial fermentation, significant SCFA production is present in the upper gastrointestinal lumen, derived from oral flora and from the ingestion of previously fermented foods (12, 32, 35). The density of 5-HT-containing EC cells in the duodenum is second only to that of the proximal colon in rats, perhaps because of the high concentrations of foregut SCFAs that stimulate EC cells to release 5-HT (23, 28). Because duodenal EC cells express the SCFA receptor FFA2 (2), we hypothesized that luminal FFA2 agonists increase 5-HT release from EC cells and that excessive 5-HT release may trigger nausea and vomiting, or unfavorably affect duodenal defense mechanisms.
The duodenal mucosa, regularly exposed to gastric acid, is protected by multilayered defense mechanisms that avert and prevent mucosal injury (39). Cyclooxygenase (COX), the rate-limiting enzyme for prostaglandin (PG) synthesis, is inhibited by nonsteroidal anti-inflammatory drugs (NSAIDs), which in turn induce small intestinal injury in humans, cats, and rodents, with additional production of duodenal lesions in humans and cats (20, 53) but duodenal sparing in rats. These species differences have been related to interspecies variations of the multilayered foregut defense mechanisms consisting of not only the COX-PG pathway but also the “capsaicin pathway,” including activation of capsaicin-sensitive afferent nerves (3).
In contrast to the physiological mucosal defenses of the duodenum, the duodenum may additionally be the origin of dyspeptic symptoms, since infusion of acid or lipid in the duodenum triggers dyspeptic symptoms, including fullness, bloating, nausea, satiety, epigastric burning sensation, and epigastric pain in patients with diagnosed functional dyspepsia (43). Although these symptoms are linked to the release of several gut hormones such as cholecystokinin (CCK), glucagon-like peptide (GLP), and peptide YY (PYY) (10, 63), they are alleviated with 5-HT3 receptor antagonists (9), further implicating 5-HT release, presumably in response to luminal stimuli, in the generation of the symptoms. Therefore, 5-HT release may be correlated with physiological mucosal defense and also with pathological symptom generation.
Here, we examined the effect of a synthetic selective FFA2 agonist on duodenal mucosal defenses, 5-HT release, and indomethacin (IND)-induced enteropathy. Interestingly, an FFA2 agonist increased the rate of duodenal secretion and 5-HT release but induced duodenal mucosal lesions when accompanied by an ulcerogenic dose of IND via 5-HT3 receptor activation. Because rodents cannot vomit because of anatomical and central circuit variants (30), reduced clearance of luminal substances that stimulate excessive 5-HT release from EC cells may injure the rat duodenal mucosa.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats weighing 200–250 g (Harlan, San Diego, CA) were fed a pellet diet and water ad libitum. All studies were performed with approval of the Veterans Affairs Institutional Animal Care and Use Committee. Rats were fasted overnight with free access to water before the experiments. Animals were euthanized by terminal exsanguination under deep isoflurane anesthesia, followed by thoracotomy.
Chemicals.
PA1 [phenylacetamide 1; 4-chloro-α-(1-methylethyl)-N-2-thiazolylbenzene acetamide] (44) and GLPG-0974 (4-[{[(2R)-1-(benzo[b]thien-3-ylcarbonyl)-2-methyl-2-azetidinyl]carbonyl}[(3-chlorophenyl) methyl]amino]-butanoic acid) (50) were synthesized, purified, and verified in the Laboratory of Organic Chemistry, School of Pharmaceutical Sciences, University of Shizuoka, Japan (2); GLPG-0974 was also obtained from Tocris Bioscience (Ellisville, MO). RS-23597 was obtained from Tocris Bioscience. Omeprazole (OPZ), ondansetron (Ond), atropine (ATR), indomethacin (IND), methylcellulose, and other chemicals were purchased from Sigma Chemical (St. Louis, MO). IND was dissolved in ethanol. PA1 and GLPG-0974 were dissolved in DMSO. Omeprazole was suspended in 1% methylcellulose in saline containing 1% NaHCO3 (54). All other chemicals were dissolved in distilled water to make a stock solution.
IND-induced intestinal injury.
IND-induced small intestinal injury was induced as previously described (33). IND (10 mg/kg) was subcutaneously (sc) injected under brief isoflurane anesthesia (4%). After IND treatment, the animals were recovered from the anesthetic and returned to their cages with free access to water and food for 24 h. Portal venous (PV) and arterial blood were collected under isoflurane anesthesia, followed by euthanasia by terminal exsanguination 24 h after IND treatment. The gastrointestinal tract from the stomach to terminal ileum was removed and longitudinally opened along the antimesenteric curvature, since IND-induced intestinal ulcers are principally localized to the mesenteric curvature. After carefully removing the luminal contents and gently rinsing the mucosa in normal saline, the small intestine was cut into ~10-cm segments. The gastric antrum was defined as segment 0, the duodenum as segment 1, the jejunum as segments 2 and 3, and the ileum as segments 4–7. Observed ulcers were typically circular and linearly arranged. The diameter of each ulcer was macroscopically measured with the cumulative ulcer length of all ulcers in each segment calculated. Overall ulcer length was defined by the sum of the cumulative ulcer length in each segment. The sum of ulcer length in the duodenum, the jejunum + ileum, and in all segments was defined as duodenal, nonduodenal, and overall ulcer index (mm), respectively.
After the intestines were photographed, macroscopically normal and ulcerative tissues were fixed with Zamboni’s fixative overnight at 4°C. After cryoprotection in 20% sucrose-containing phosphate-buffered saline, tissues were embedded in OCT compound, and cryostat sections were cut at 8 µm thickness. Sections were counterstained with Alexa-633 phalloidin for F-actin, Alexa-488 wheat germ agglutinin for mucin, and 4′,6-diamidino-2-phenylindole for the nucleus (Invitrogen, Carlsbad, CA).
Drug treatment for the intestinal injury study.
The following drugs were given intragastrically (ig) or intraperitoneally (ip) just before IND treatment; the selective FFA2 agonist PA1 (0.1–1 mg/kg ig) (44), the selective FFA2 antagonist GLPG-0974 (0.1 or 1 mg/kg ig) (50), the 5-HT3 receptor antagonist Ond (3 mg/kg ip) (38), the 5-HT4 receptor antagonist RS-23597 (1 mg/kg ip) (19), OPZ (10 mg/kg ig) (54), and ATR (10 mg/kg ig) (54). All chemicals except OPZ were diluted in saline. All solutions were given at 1 ml/kg. DMSO (0.1% in saline) was used as vehicle control.
Duodenal loop perfusion in vivo.
A duodenal loop and PV cannulation were prepared as previously reported (2, 35). Briefly, under isoflurane anesthesia (2%), the PV was cannulated with a polyethylene (PE)-50 tube attached with a 23-gauge needle and fixed by methylacrylate adhesive at the insertion site. A PE tube was inserted in the incision of the forestomach through the pyloric ring, where it was sutured in place. Another PE tube was inserted in the distal duodenum, creating a 2-cm duodenal loop. The pancreaticobiliary duct was ligated followed by duct cannulation with a PE-10 tube attached with a 30-gauge needle to avoid contaminating the duodenal lumen with pancreatic and biliary secretions. The effluent tube was connected to flow-through pH and CO2 electrodes. The loop was perfused with O2-bubbled normal saline, pH 7, at 1 ml/min by a peristaltic pump. After stabilization for ~30 min, time was set as t = 0 min. The loop was perfused with saline from t = 0 to 10 min, followed by the perfusion of pH 7 Krebs buffer with or without PA1 (1 µM or 0.1 mM) from t = 10 to 35 min. Some animals were coperfused with GLPG-0974 (0.1–10 µM) and PA1 (1 µM). Some animals were pretreated with IND (5 mg/kg sc) 1 h before the experiments to assess the effect of COX inhibition on secretion and 5-HT release. pH and CO2 electrode measurement was recorded every 5 min to calculate total CO2 output as previously described (46). PV blood (200 µl) was collected at t = 10, 15, 20, and 35 min, followed by infusion of equal volume of saline right after each PV blood withdrawal.
5-HT measurement in PV plasma.
After centrifugation at 5,000 g for 5 min, PV plasma was kept at −80°C until use. 5-HT content in PV plasma was measured using a 5-HT ELISA kit (Eagle Bioscience, Nashua, NH) according to the manufacturer’s protocol.
Duodenal blood flow measurement.
Duodenal blood flow was measured by laser Doppler flowmetry as previously described (4). Briefly, under isoflurane anesthesia (2%), the duodenal mucosa was exposed. A superfusion chamber was placed over the mucosa with water-resistant adherent (Silly Putty; Binney & Smith, Easton, PA). The chambered mucosa was topically superfused with pH 7 Krebs solution. After stabilization, time was set to t = 0 min. The mucosa was superfused with pH 7 Krebs for 10 min, followed by superfusion with or without pH 2.2 Krebs solution and/or PA1 (1 µM or 0.1 mM). Some animals were pretreated with IND (5 mg/kg sc), which systemically inhibits COX activity, 1 h before the surgery as previously described (1) to assess the effect of COX inhibition rather than the effect of ulcerogenic IND (10 mg/kg), since the latter reduces intestinal blood flow over time (55). Some animals were intravenously injected with Ond (1 mg/kg) at t = 0 min. Data were collected every 5 min and expressed as percent of basal.
To correlate local 5-HT levels with blood flow responses to 5-HT, we examined the effects of exogenous 5-HT on duodenal blood flow. The abdominal aorta was retrogradely cannulated with a PE-50 tube, whose tip was placed at the entry of celiac and superior mesenteric arteries. After ~30 min stabilization with luminal superfusion of pH 7 Krebs over the duodenal mucosa as described above, in parallel with intra-arterial infusion of saline, 5-HT in saline (0.1 µM–1 mM) was infused intra-arterially at 0.02 ml/min, equivalent to 0.002–20 nmol/min. The doses of 5-HT were increased in a stepwise manner every 5 min.
Statistics.
Values are expressed as means ± SE. The number of animals in each experimental group was n = 4–6. Statistical analysis was performed using GraphPad Prism 6 (La Jolla, CA) using one-way ANOVA or two-way ANOVA followed by Dunnett’s test or Tukey’s multiple comparisons. Differences were considered significant when P values were <0.05.
RESULTS
Effect of PA1 on IND-induced enteropathy.
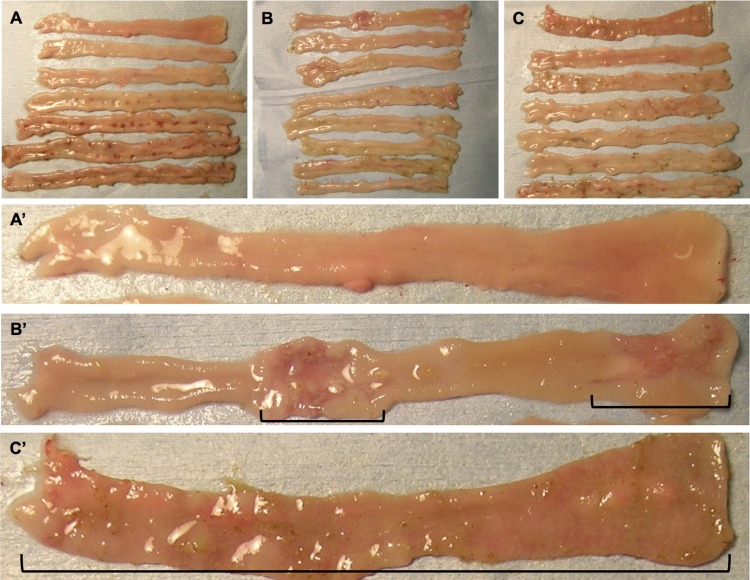
IND (10 mg/kg sc) reproducibly produced small intestinal ulcers, principally in the ileum, whereas duodenal injury was rarely observed (Figs. 1A and 2, A and D). Figure 1A′ depicts grossly normal duodenal mucosa in the IND alone group. In contrast, PA1 + IND treatment dose dependently induced macroscopic duodenal lesions, whereas distal small intestinal (jejunal and ileal) injury was reduced (Figs. 1, B and C, and 2A). Figure 1B′ depicts the proximal and midduodenal mucosal injury in the PA1 (0.3 mg/kg) + IND group. In the most extreme case, PA1 at 1 mg/kg denuded the entire duodenal mucosa (Fig. 1C′).
Fig. 1.
Free fatty acid receptor 2 (FFA2) agonist-induced duodenal mucosal injury and reduction of distal small intestinal injury. Representative macroscopic images of indomethacin (IND)-induced enteropathy with or without FFA2 agonist treatment are shown. The FFA2 agonist phenylacetamide 1 (PA1) was given intragastrically just before IND treatment. IND treatment induced intestinal ulcers in mostly the proximal and midileum (the 4th-6th segments from the top) (A), whereas the duodenum was intact (A′). PA1 treatment at 0.3 (B and B′) and 1 (C and C′) mg/kg induced duodenal lesions (black line delineates the lesions in B′ and C′), whereas the number of ileal ulcers was reduced (B and C). Note that C′ represents maximal mucosal injury with mucosal denudation of the entire duodenum. A′, B′, and C′, magnified images of duodenal segment in A, B, and C, respectively.
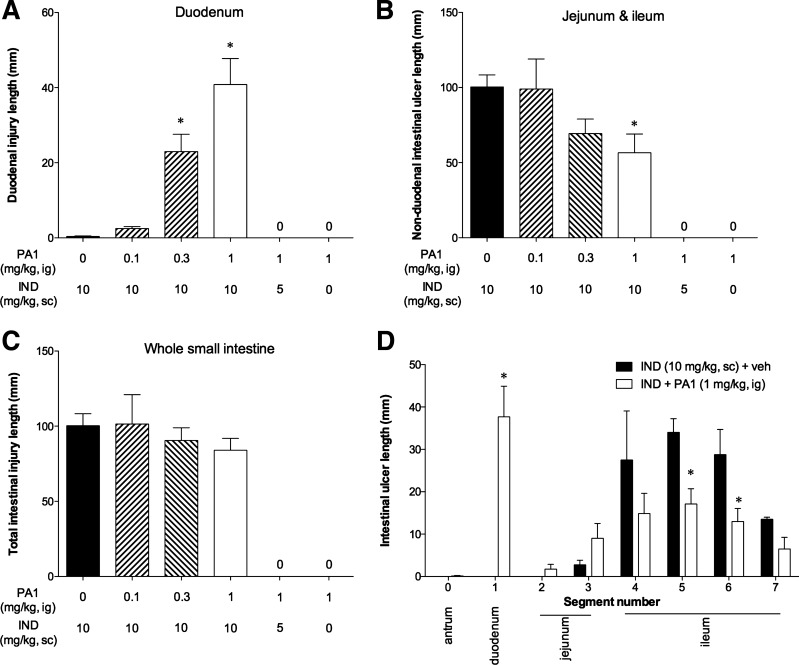
Fig. 2.
Effect of PA1 treatment on IND-induced enteropathy. IND was given at 0, 5, or 10 mg/kg sc. PA1 was given at 0–1 mg/kg ig. A: duodenal ulcer index. B: nonduodenal (jejunum and ileum) ulcer index. C: overall (whole small intestine) ulcer index. D: segmental ulcer indexes from antrum (0) to terminal ileum (7) of IND + vehicle and IND + PA1 (1 mg/kg) groups. Each column is expressed as the mean ± SE (n = 6 experiments). *P < 0.05 vs. IND + vehicle (veh) group. “0” denotes that index was 0.
PA1 (1 mg/kg) alone or PA1 given with a nonulcerogenic though COX inhibitory dose of IND (5 mg/kg sc) did not grossly injure the duodenum or other small intestinal segments (Fig. 2, A–C), suggesting that PA1 is not injurious in and of itself but rather increases the susceptibility to IND-induced injury. Although PA1 induced duodenal lesions (Fig. 2A), it did not affect the overall ulcer index (Fig. 2C) but significantly reduced the ulcer index of the jejunum and ileum (Fig. 2, B and D), suggesting that PA1 has dual effects on IND-induced enteropathy by increasing the injury susceptibility of the duodenal mucosa while protecting the distal small intestinal mucosa from injury.
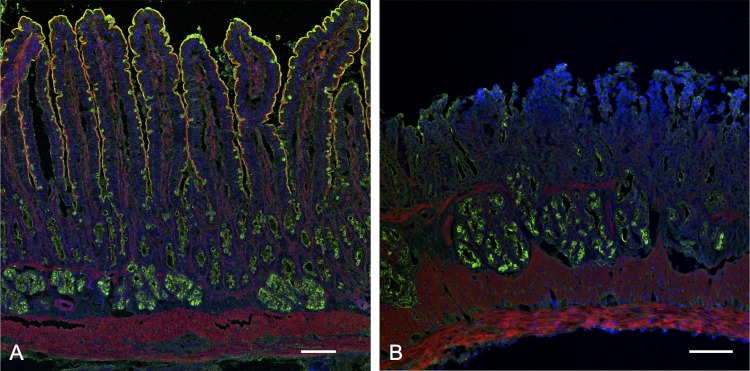
Histochemical analysis confirmed that the macroscopic duodenal lesions were not ulcers but massive superficial erosions, since the muscularis mucosa and submucosal Brunner’s glands remained intact (Fig. 3B), whereas IND treatment alone had no effect on duodenal mucosal histology (Fig. 3A), consistent with the macroscopic observations. These results indicated that PA1 treatment combined with an ulcerogenic IND dose (IND + PA1) induced duodenal erosions but reduced the density and size of jejunal and ileal ulcers.
Fig. 3.
Histological images of the duodenum of rats given an ulcerogenic dose of IND. Duodenal tissues were stained with wheat-germ agglutinin (green), phalloidin (red), and 4′,6-diamidino-2-phenylindole (blue). No obvious damage was observed in the duodenum after IND treatment (A), whereas PA1 (1 mg/kg ig) with IND treatment induced massive villous injury (erosion) in the proximal duodenum (B). Internal bar = 100 µm.
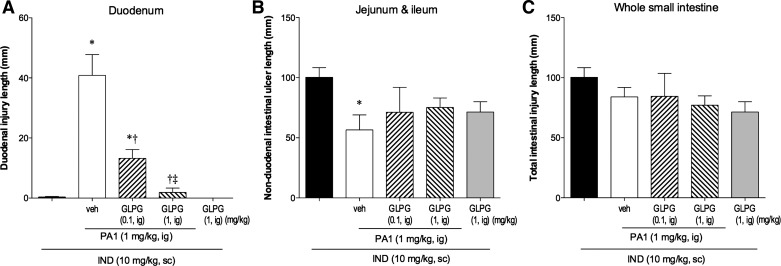
To confirm that the effect of PA1 was mediated via FFA2 activation, the selective FFA2 antagonist GLPG-0974 (0.1–1 mg/kg ig) (50) was simultaneously given with PA1. GLPG-0974 dose dependently reduced IND + PA1-induced duodenal ulcer index (Fig. 4A), whereas the effect of PA1 on the nonduodenal ulcer index was reversed (Fig. 4B), confirming that IND + PA1-induced duodenal injury was mediated by FFA2 activation.
Fig. 4.
Effect of a selective FFA2 antagonist on IND + PA1-induced duodenal lesions. PA1 was given at 1 mg/kg ig. The effect of cotreatment with the FFA2 antagonist GLPG-0974 (0.1 or 1 mg/kg) on IND + PA1-induced intestinal lesions was examined. A: duodenal ulcer index. B: nonduodenal intestinal ulcer index. C: overall ulcer index. Each column is expressed as the mean ± SE (n = 6). P < 0.05 vs. IND alone group (*), IND + PA1 + veh group (†), and IND + PA1 + GLPG-0974 (0.1 mg/kg) group (‡).
Mechanisms of PA1-induced duodenal erosions in IND-induced enteropathy.
To identify the pathway underlying the effect of IND + PA1, we next examined the effect of pharmacological interventions that reduced the effects of endogenous 5-HT and luminal acid, and also reduced motility.
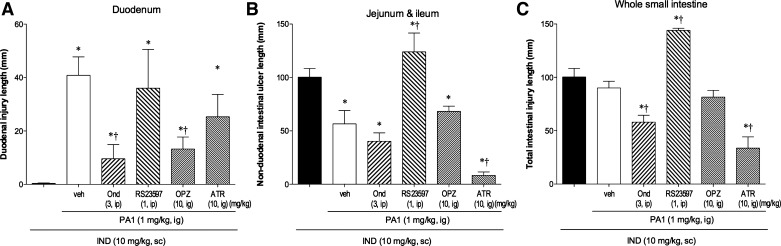
The 5-HT3 receptor antagonist Ond significantly reduced IND + PA1-induced duodenal lesions, whereas the 5-HT4 receptor antagonist RS-23597 had no effect on duodenal lesions (Fig. 5A). In parallel, Ond had no further effect on the PA1-associated reduction of the nonduodenal ulcer index, whereas RS-23597 increased the overall and nonduodenal ulcer indexes (Fig. 5, B and C), consistent with a previous report that 5-HT4 receptor antagonism aggravates IND-induced small intestinal ulcers (38). This result suggests that IND + PA1-induced duodenal lesions are mediated by 5-HT3 rather than 5-HT4 receptor activation, whereas PA1-induced protection of nonduodenal segments from IND-induced ulcers occurs via 5-HT4- rather than 5-HT3-mediated pathways.
Fig. 5.
Effect of drug treatment on IND + PA1-induced duodenal lesions. Effect of ondansetron (Ond, 3 mg/kg ip), RS-23597 (1 mg/kg ig), omeprazole (OPZ, 10 mg/kg ig), or atropine (ATR, 10 mg/kg ig) on IND + PA1-induced intestinal lesions was examined. A: duodenal ulcer index. B: nonduodenal ulcer index. C: overall ulcer index. Each column is expressed as the mean ± SE (n = 6). P < 0.05 vs. IND alone group (*) and IND + PA1 + veh group (†).
The proton pump inhibitor OPZ significantly reduced IND + PA1-induced duodenal lesions (Fig. 5A), whereas no change occurred in the overall ulcer index or with the PA1-associated reduction of the nonduodenal ulcer index (Fig. 5, B and C), suggesting that gastric acid is involved in the induction of duodenal erosions but is not involved in jejunal or ileal injury due to IND + PA1. A single dose of OPZ had no effect on IND-induced enteropathy, consistent with a previous report (54), although 9 days of OPZ treatment aggravated IND-induced enteropathy (65).
The muscarinic receptor antagonist ATR had no effect on IND + PA1-induced duodenal lesions (Fig. 5A), whereas ATR reduced the overall ulcer index and further reduced the nonduodenal ulcer index (Fig. 5, B and C), suggesting that hypermotility is not involved in IND + PA1-induced duodenal erosions but is related to nonduodenal mucosal injury, as previously reported (54, 58).
These results suggest that IND + PA1-induced duodenal erosions are mediated via 5-HT3 activation and gastric acid exposure but not by hypermotility.
Effect of luminal perfusion of PA1 on 5-HT release in PV.
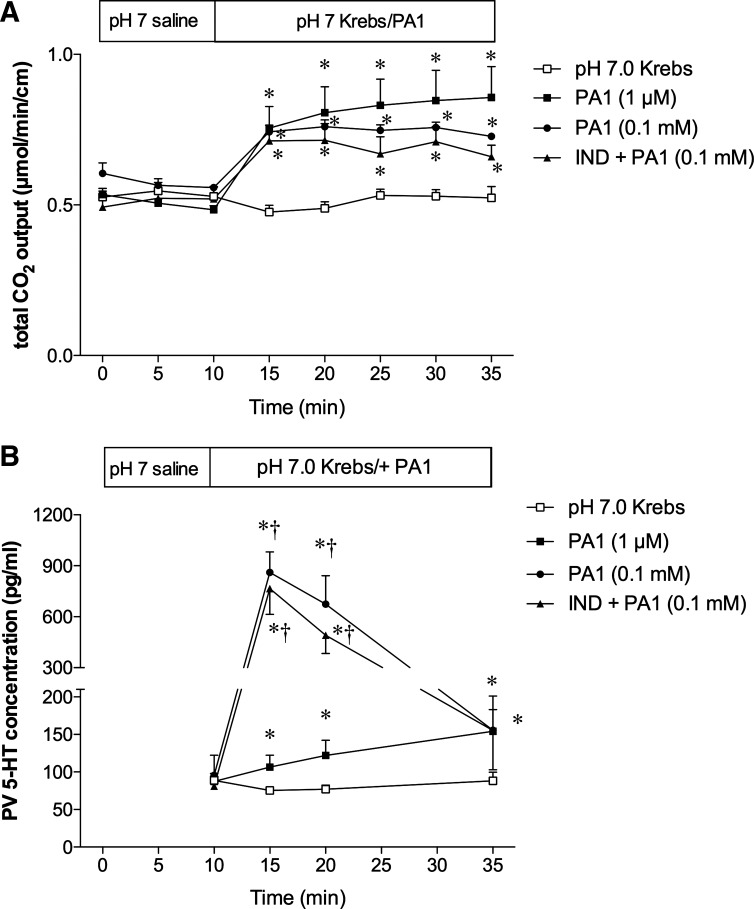
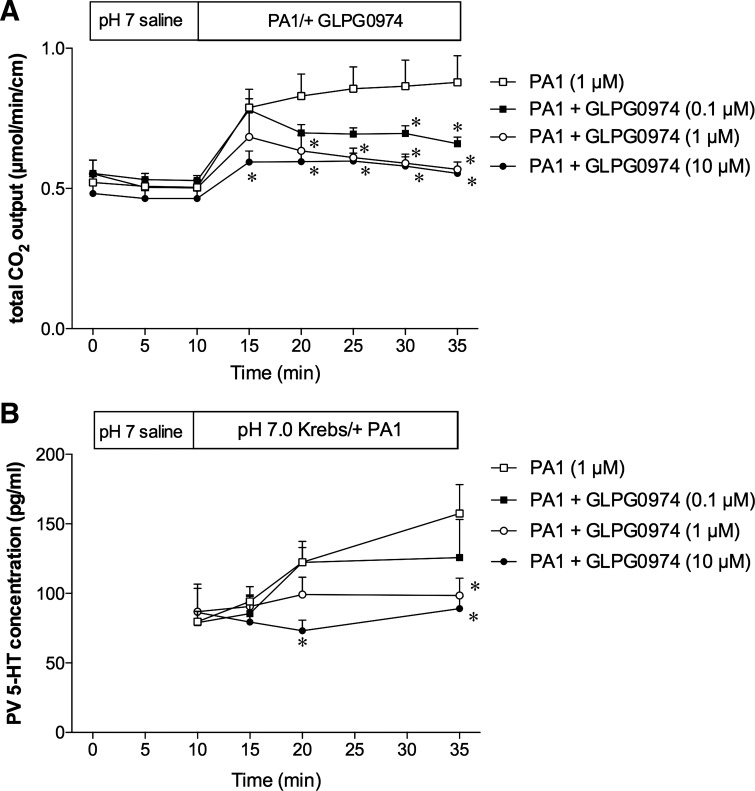
We have reported that luminal perfusion of PA1 (0.1–10 µM) dose dependently increases duodenal bicarbonate secretion (2). Because FFA2 is expressed on 5-HT-containing EC cells (2), and because IND + PA1 induced duodenal erosions via 5-HT3 activation, we examined the effects of luminal PA1 perfusion on secretion and on 5-HT release in the PV. Luminal perfusion of 1 µM or 0.1 mM PA1 increased the rate of secretion to the same extent, measured as total CO2 output (Fig. 6A), consistent with our earlier results (2). PA1 (1 µM)-induced secretion was dose dependently inhibited by coperfusion of the selective FFA2 antagonist GLPG-0974 (0.1–10 µM), confirming that the effect of PA1 was mediated by FFA2 activation (Fig. 7A).
Fig. 6.
Effect of luminal perfusion of PA1 on secretion and serotonin (5-HT) release in portal vein (PV) in rat duodenum. The duodenal loop was perfused with pH 7.0 Krebs buffer with or without PA1 (1 µM or 0.1 mM) in vivo. IND (5 mg/kg sc) was pretreated 1 h before the experiments. A: duodenal secretion was measured with flow-through pH and CO2 electrodes and expressed as total CO2 output. B: during luminal perfusion of PA1 (1 µM or 0.1 mM), PV blood was collected and 5-HT levels were measured. Each data point is expressed as the mean ± SE (n = 5 or 6). P < 0.05 vs. pH 7.0 Krebs group (*) and PA1 (1 µM) group (†).
Fig. 7.
Effect of the FFA2 antagonist on the stimulatory effects of PA1. The duodenal loop was perfused with PA1 (1 µM) with or without GLPG-0974 (0.1–10 µM) in vivo. A: PA1-induced augmented secretion was dose dependently inhibited by coperfusion with GLPG-0974. B: GLPG-0974 dose dependently inhibited PA1-induced augmented 5-HT concentration in PV. Each data point is expressed as the mean ± SE (n = 5 or 6). *P < 0.05 vs. PA1 group.
PV plasma levels of 5-HT were stable during baseline perfusion of pH 7 Krebs buffer (Fig. 6B). PA1 perfusion (1 µM) gradually increased the 5-HT concentrations in PV plasma (Fig. 6B), strongly suggesting that activation of FFA2 expressed on EC cells releases 5-HT. Higher concentrations of PA1 (0.1 mM) markedly increased 5-HT release, approximately eight times more than at 1 µM at peak, then declining to baseline at t = 35 min (Fig. 6B), suggesting that high doses of PA1 may acutely mobilize EC cell 5-HT stores. Furthermore, the COX inhibitory dose of IND (5 mg/kg) had no effect on 0.1 mM PA1-induced secretion and 5-HT release in PV (Fig. 6, A and B), suggesting that the FFA2–5-HT pathway is COX independent. Coperfusion of GLPG-0974 inhibited the PA1-induced -HT release in PV (Fig. 7B), confirming the FFA2 specificity.
Effect of luminal perfusion of PA1 on duodenal blood flow.
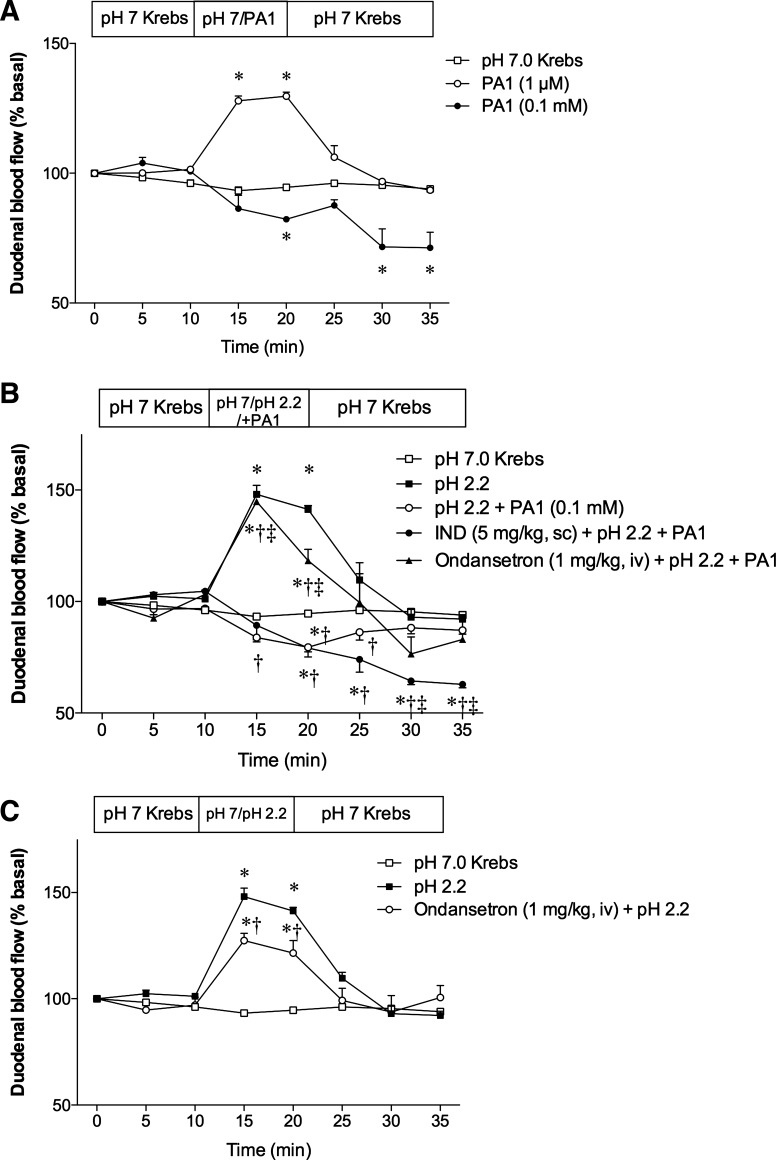
Because one of the factors contributing to the pathogenesis of IND-induced enteropathy is the reduction of mesenteric blood flow (34, 55), we examined the effect of luminal PA1 on duodenal blood flow. Luminal superfusion of 1 µM PA1 increased duodenal blood flow, whereas 0.1 mM PA1 reduced blood flow, compared with the pH 7.0 Krebs control group (Fig. 8A). Luminal acid (pH 2.2) perfusion increased blood flow as previously described (1). The acid-induced hyperemic response was abolished by cosuperfusion of 0.1 mM PA1 (Fig. 8B). Perfusion of acid + PA1 in rats pretreated with a nonulcerogenic dose of IND (5 mg/kg sc) further diminished blood flow. Furthermore, Ond (1 mg/kg iv) injected at t = 0 min mostly reversed the inhibitory effect of PA1 on acid-induced hyperemia without affecting basal blood flow. Interestingly, Ond alone slightly, but significantly, reduced acid-induced hyperemia, suggesting that acid-induced 5-HT release, as reported previously (40), partially contributes to acid-induced hyperemia via 5-HT3 receptor activation. These results suggest that a high luminal concentration of PA1 abolishes acid-induced hyperemia via endogenous 5-HT release, followed by 5-HT3 receptor activation, whereas the physiological response to luminal acid may also involve 5-HT3 receptor activation.
Fig. 8.
Effect of luminal superfusion of PA1 on duodenal blood flow. The duodenal mucosa was topically superfused, and duodenal blood flow was measured using laser Doppler flowmetry. A: luminal perfusion of PA1 at 1 µM increased blood flow, whereas PA1 at 0.1 mM gradually decreased blood flow. Each data point is expressed as the mean ± SE (n = 6). *P < 0.05 vs. pH 7.0 Krebs group. B: mucosa was superfused with pH 2.2 Krebs with or without PA1 (0.1 mM). Some animals were pretreated with IND (5 mg/kg sc) 1 h before experiment or Ond (1 mg/kg iv) at t = 0 min. Each data point is expressed as the mean ± SE (n = 4–6). P < 0.05 vs. pH 7.0 Krebs group (*), pH 2.2 group (†), and pH 2.2 + PA1 group (‡). C: iv Ond injection significantly reduced acid-induced hyperemia. Each data point is expressed as the mean ± SE (n = 5 or 6). P < 0.05 vs. pH 7.0 Krebs group (*) and pH 2.2 group (†).
Effect of intra-arterial infusion of exogenous 5-HT on duodenal blood flow.
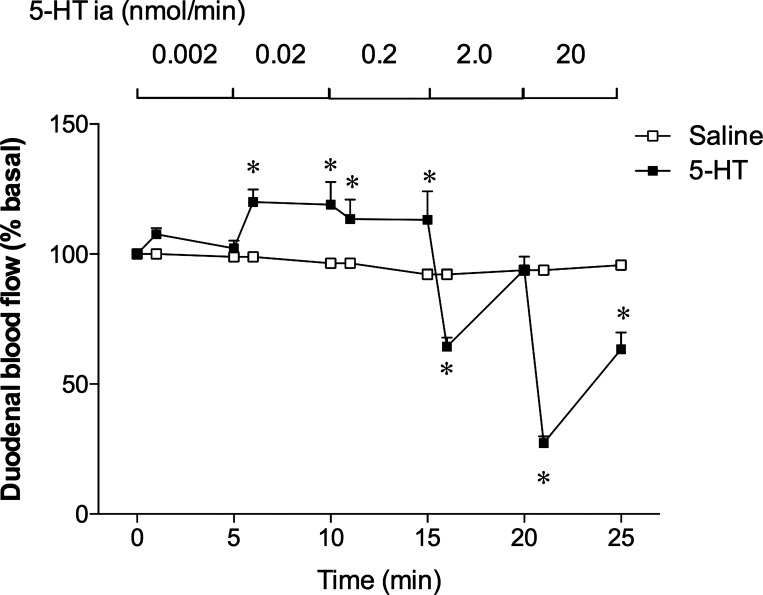
To assess the effects of exogenous 5-HT on duodenal blood flow, we chose intra-arterial infusion of 5-HT, since bolus intravenous injection of 5-HT rapidly decreased duodenal blood flow (data not shown), mostly because of the direct cardiovascular depressant effects followed by peripheral vasoconstriction (67). 5-HT infused intra-arterially at low doses (0.002–0.2 nmol/min corresponding to the original concentrations of 0.1–10 µM) dose dependently increased duodenal blood flow, whereas higher doses of 5-HT (2.0 and 20 nmol/min) rapidly and dose dependently decreased blood flow (Fig. 9). This result suggests that local 5-HT concentrations are correlated with blood flow responses; blood flow is increased at low concentrations but decreased at high concentrations.
Fig. 9.
Effect of ia infusion of 5-HT on duodenal blood flow. The abdominal aorta was retrogradely cannulated. Saline or 5-HT in saline (0.1 µM–1 mM) was infused ia in a stepwise manner from the entry of celiac and superior mesenteric artery at 0.02 ml/min, equivalent to 0.002–20 nmol/min. The duodenal mucosa was topically superfused, and duodenal blood flow was measured. Low doses of 5-HT dose dependently increased blood flow, whereas high doses dose dependently decreased blood flow. Each data point is expressed as the mean ± SE (n = 5). *P < 0.05 vs. the saline group.
DISCUSSION
We demonstrated that an ulcerogenic dose of IND combined with luminal FFA2 activation by PA1 induced duodenal mucosal injury, in contrast to IND treatment alone, which rarely caused duodenal injury. Furthermore, IND + PA1-induced duodenal injury was mediated by 5-HT3 receptor activation and gastric acid but not by hypermotility. We also demonstrated that luminal FFA2 activation increased 5-HT release in the PV and that 5-HT3 receptor activation by endogenous 5-HT release in response to FFA2 activation abolished the hyperemic response to luminal acid. These results suggest that PA1-induced 5-HT release during COX inhibition disrupts duodenal mucosal defenses, increasing the injury susceptibility of the duodenal mucosa.
This is the first study showing that excessive release of endogenous 5-HT is involved in the induction of duodenal erosions due to the impairment of duodenal defense mechanisms, including the hyperemic response to gastric acid, which occurs via 5-HT3 receptor activation, further confirming that acid-induced hyperemia is an important factor of duodenal mucosal protection. Luminal acid exposure stimulates the release of 5-HT in the lumen and in the submucosa and the vasculature (40). Lower concentrations of PA1 (1 or 10 µM) augmented the rate of duodenal secretion via 5-HT4 receptor activation (2) and increased duodenal blood flow, suggesting that physiological levels of 5-HT release in response to luminal acid or low doses of an FFA2 agonist may enhance duodenal mucosal defenses. Interestingly, acid-induced hyperemia was reduced by a 5-HT3 receptor antagonist, suggesting that 5-HT released by luminal acid partially contributes to the hyperemic response via 5-HT3 receptor activation. Furthermore, low doses of exogenous 5-HT infused intra-arterially increased duodenal blood flow, similar to the effect of low concentrations of PA1. Nonetheless, excessive release of 5-HT by higher concentrations of PA1 may be pathogenic because 0.1 mM PA1 not only released an approximately eight times greater concentration of 5-HT in the PV than in response to 1 µM PA1 but also abolished protective acid-induced hyperemia, consistent with a previous report for exogenous 5-HT administration (60). High doses of intra-arterial 5-HT also decreased blood flow, also corresponding to the effect of high concentrations of PA1. These results demonstrate that locally released 5-HT, luminal PA1 concentrations, and duodenal blood flow responses are closely correlated. Therefore, physiological levels of 5-HT release are mucosally protective via 5-HT4 receptor activation and partially via 5-HT3 receptor activation, whereas excessive release of 5-HT may increase duodenal mucosal injury susceptibility to luminal acid via 5-HT3 receptor overactivation. Because COX inhibition by IND reduces secretion in the stomach and duodenum, and then reduces gastric and duodenal luminal pH (59), the injurious effects of gastric acid should be enhanced in the duodenum after IND treatment, although IND alone rarely caused duodenal injury. Moreover, a nonulcerogenic dose of IND at 5 mg/kg with PA1 at 1 mg/kg did not injure the intestine, and PA1-induced 5-HT release was COX independent, consistent with a previous report showing that IND has no effect on toxin-induced 5-HT release in porcine jejunum (62), although 5-HT increases release of PGE2, the effect inhibited by IND in human jejunum (47). These results further suggest that prolonged COX inhibition by an ulcerogenic dose of IND at 10 mg/kg with excessive 5-HT release induced by a high dose of PA1, concomitant with enhanced gastric acid exposure, induces duodenal lesions via disruption of multilayered defense mechanisms in the duodenal mucosa, including hyperemic responses (Fig. 10). Differential actions of 5-HT3 and 5-HT4 receptors are possibly the result of their localization (for instance, 5-HT3 receptor is expressed on afferent nerves vs. 5-HT4 receptor on epithelial cells), receptor affinities to 5-HT, interactions with other 5-HT receptors, and the contribution of the serotonin reuptake transporter that may regulate local 5-HT bioavailability.
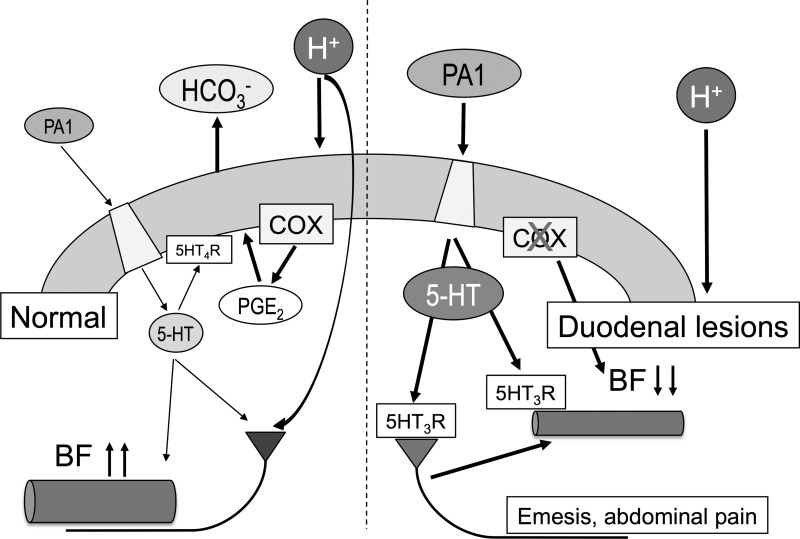
Fig. 10.
Proposed mechanisms underlying luminal FFA2 activation under normal conditions and cyclooxygenase (COX) inhibition in the duodenum. Under normal conditions (left), low doses of the luminal FFA2 agonist PA1 stimulate enterochromaffin (EC) cells and then increase 5-HT release. The physiological concentrations of released 5-HT activate 5-HT4 receptors on epithelial cells and/or cholinergic neurons and then stimulate secretion (2). 5-HT also increases blood flow (BF) via 5-HT3 receptor activation. When gastric acid enters the duodenal lumen, luminal acid increases PGE2 production in parallel with 5-HT release and then further stimulates secretion. Acid also activates acid sensors on afferent nerves and then increases blood flow (1). In contrast, high doses of PA1 release excessive amounts of 5-HT that reduce blood flow via 5-HT3 receptor (over)activation (right). Additional COX inhibition by nonsteroidal anti-inflammatory drugs (NSAIDs) further decreases blood flow. COX inhibition also impairs secretory response and mucus secretion against acid exposure (39). Consequent luminal acid exposure fails to enhance mucosal defense mechanisms, followed by injuring the duodenal mucosa. Furthermore, excessive 5-HT activates 5-HT3 receptors on afferent nerves and then may trigger the abnormal symptoms such as emesis and epigastric pain.
The highest dose of PA1 (1 mg/kg ig) used for the intestinal injury study was given as an intragastric administration of 1 ml/kg of a 3.4 mM solution. We predicted that a <1 mM concentration of PA1 was continuously present in the duodenal lumen by intermittent transfer from the stomach after dilution with gastric juice and content. Therefore, continuous exposure to 0.1 mM PA1, which robustly released 5-HT in the perfusion experiments, may be equivalent to a single intragastric administration of a 1 mg/kg dose of PA1, which injured the duodenal mucosa when combined with 10 mg/kg IND. Because we confirmed that PA1-induced secretion and PV 5-HT release were COX independent in the duodenum, FFA2-mediated 5-HT release and the ulcerogenic effects of IND may occur in parallel. In contrast, in the distal intestine (especially in the ileum), where IND principally induces injury, luminal PA1 concentration likely is present in the micromolar range, which releases lower amounts of 5-HT, which are protective. Because endogenous 5-HT released during the development of IND-induced enteropathy protects the intestinal mucosa via 5-HT4 receptor activation, but aggravates injury via 5-HT3 receptor activation, both shown in this study and in a previous report (38), the levels of locally released 5-HT and the predominant activation of 5-HT4 vs. 5-HT3 receptors are likely vital for the development of IND-induced enteropathy in the distal intestine. The number of EC cells and the 5-HT content, higher in the duodenum than in the ileum (28), also inform the extent of local 5-HT release, which can either protect or injure the mucosa. Furthermore, the disparate effects of atropine on duodenal and distal intestinal injury also implicate the hypermotility induced by IND in the ileum for 5-HT release by increasing luminal pressure, a mechanism unlikely to occur in the duodenum.
Although it is difficult to measure the levels of locally released 5-HT in the mucosa, we could correlate 5-HT infused intra-arterially with blood flow responses. According to the blood flow rate of the rat superior mesenteric artery measured at 13.2 ml/min (18), the predicted peripheral concentrations after intra-arterial 5-HT infusion are 15 nM for an infusion rate of 0.2 nmol/min, which increased blood flow as did PA1 at 1 µM, and 150 nM at 2 nmol/min, which decreased blood flow as did PA1 at 0.1 mM. PV 5-HT levels were 0.57 nM at 100 pg/ml in the 1 µM PA1 group and 4.54 nM at 800 pg/ml in the 0.1 mM PA1 group. A corresponding range of local 5-HT concentrations, predicted to be in the nanomolar range (should be higher in the subepithelial space), may contribute to the local changes measured in this study. Although the 5-HT-induced vasoconstriction is mainly mediated by the 5-HT2A or 5-HT1B receptor, dependent on types of vasculature (67), we observed the involvement of 5-HT3 receptor in PA1-induced reduction of acid-induced hyperemia. Further study will clarify the underlying mechanism and receptor subtypes involved in the regulation of duodenal mucosal blood flow.
This study provides further evidence that luminal FFA2 agonists release 5-HT from EC cells. In the upper gastrointestinal lumen, luminal FFA2 agonists are predominantly SCFAs, derived from fermentation of carbohydrate substrates by oral floral (32) and also from ingestion of previously fermented foodstuffs (2). These SCFAs are also absorbed as nutrients from the duodenal mucosa through the apical sodium-dependent monocarboxylate transporter 1 (SMCT1) and the basolateral monocarboxylate transporter MCT1/MCT4, expressed on the duodenocyte plasma membranes (35). Therefore, under physiological conditions, luminal SCFAs may stimulate FFA2 on EC cells, followed by “physiological” postprandial release of 5-HT, which enhances mucosal defenses and motility. Because SIBO increases the luminal SCFA content in the jejunum severalfold higher than that of healthy subjects (31), pathological 5-HT release via FFA2 overactivation may occur. Furthermore, the microbiota induces the expression of the rate-limiting 5-HT-synthesizing enzyme tryptophan hydroxylase 1 and 5-HT storage in EC cells (48, 51, 68). Because in functional dyspepsia and in IBS SIBO has been detected by culture and by breath testing, and because symptoms respond to nonabsorbable antibiotics, SIBO is implicated in the pathogenesis of these functional diseases (16, 26, 49). Therefore, the increased concentration of luminal SCFAs associated with SIBO may overactivate FFA2 and enhance 5-HT release with the generation of pathological amounts of mucosal 5-HT with resultant emesis and dyspeptic symptoms such as bloating, nausea, fullness, and epigastralgia. Although rodents do not vomit (30) and it is difficult to evaluate dyspeptic symptoms in rodents, our observations that FFA2 activation under COX inhibition induced duodenal erosions suggest that reduced clearance of luminal FFA2 agonists followed by excessive 5-HT release may injure the duodenal mucosa in rats. Interestingly, IND + PA1-induced duodenal injury and PA1 + acid-induced reduction of blood flow were both inhibited by a 5-HT3 receptor antagonist, clinically used in the treatment of nausea and emesis (9). Therefore, IND + PA1-induced duodenal injury holds promise as a rodent model of the foregut effects of 5-HT excess. Further study using animals capable of vomiting such as ferrets will clarify this possibility.
Because SCFAs themselves are nonselective, SCFA can also activate FFA3 expressed on L cells with resultant release of the intestinotrophic gut hormone GLP-2, which increases the rate of duodenal secretion, prevents NSAID-induced enteropathy, and accelerates the healing of small intestinal ulcers (2, 33, 66). Our previous studies combined with the present study suggest that the effects of luminal SCFAs on mucosal defenses are dependent on the balance between FFA2-mediated 5-HT release and FFA3-mediated GLP-2 release. Therefore, luminal SCFA-associated 5-HT-related symptoms such as those associated with SIBO may be treated with a FFA2 antagonist, whereas a FFA3 agonist may be useful to enhance mucosal defenses and repair. The observation that a selective FFA2 antagonist dose-dependently inhibited PA1-induced secretion and PV 5-HT release, and IND + PA1-induced duodenal injury helped confirm that luminal PA1 was FFA2 specific. These findings also suggest that luminal FFA2 antagonists may be therapeutic for IBS patients by inhibiting FFA2 overactivation associated with luminal SCFA, with consequent reduction of 5-HT release.
Although FFA2 is expressed on EC cells in rat duodenum (2), further supported by the present study, FFA2 is colocalized with GLP-1- or PYY-containing L cells, but not with 5-HT-containing EC cells, in rat and human colon (36, 37). This discrepancy may be explained by the different endocrine lineage in the foregut and hindgut mucosa. For instance, 5-HT is often colocalized with CCK in the duodenum (14) but rarely in the colon (52) in mice. Therefore, we predict the expression of FFA2 on human duodenal EC cells. Further study for FFA2-expressing EC cells with 5-HT content in the duodenal biopsy specimens will clarify the correlation between SIBO or functional symptoms and FFA2-mediated 5-HT release.
In conclusion, although physiological FFA2 activation enhances duodenal mucosal defenses by increased secretion via 5-HT4 receptor activation (2) and by increased duodenal blood flow, FFA2 overactivation in the presence of ulcerogenic doses of COX inhibitors may increase the vulnerability of the duodenal mucosa to gastric acid via 5-HT3 receptor activation and the reduction of blood flow. Clinical translation of these novel mechanisms can lead to more effective therapies for 5-HT-releated dyspeptic symptoms.
GRANTS
This work was supported by a Department of Veterans Affairs Merit Review Award and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-54221.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.A. conceived and designed research; Y.A., K.M., and H.S. performed experiments; Y.A., K.M., K.N., H.S., I.K., A. Kuri, and K.-i.I. analyzed data; Y.A., H.S., I.K., A. Kuri, K.-i.I., A. Kuwahara, and J.D.K. interpreted results of experiments; Y.A. prepared figures; Y.A. drafted manuscript; Y.A. and J.D.K. edited and revised manuscript; Y.A., K.M., K.N., H.S., I.K., A. Kuri, K.-i.I., A. Kuwahara, and J.D.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Stacey Jung for assistance with manuscript preparation and Drs. Paul H. Guth and Eli Engel for helpful discussion.
REFERENCES
- 1.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acid-sensing pathways of rat duodenum. Am J Physiol Gastrointest Liver Physiol 277: G268–G274, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Akiba Y, Inoue T, Kaji I, Higashiyama M, Narimatsu K, Iwamoto K, Watanabe M, Guth PH, Engel E, Kuwahara A, Kaunitz JD. Short-chain fatty acid sensing in rat duodenum. J Physiol 593: 585–599, 2015. doi: 10.1113/jphysiol.2014.280792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiba Y, Kaunitz JD. Duodenal luminal chemosensing; acid, ATP, and nutrients. Curr Pharm Des 20: 2760–2765, 2014. doi: 10.2174/13816128113199990565. [DOI] [PubMed] [Google Scholar]
- 4.Akiba Y, Watanabe C, Mizumori M, Kaunitz JD. Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol 297: G781–G791, 2009. doi: 10.1152/ajpgi.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews PL. Physiology of nausea and vomiting. Br J Anaesth 69, Suppl 1: 2S–19S, 1992. doi: 10.1093/bja/69.supplement_1.2S. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 130: 34–43, 2006. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Barnes NM, Costall B, Naylor RJ, Tattersall FD. Identification of 5-HT3 recognition sites in the ferret area postrema. J Pharm Pharmacol 40: 586–588, 1988. doi: 10.1111/j.2042-7158.1988.tb05312.x. [DOI] [PubMed] [Google Scholar]
- 8.Bearcroft CP, Perrett D, Farthing MJ. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut 42: 42–46, 1998. doi: 10.1136/gut.42.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beattie DT, Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol 377: 181–203, 2008. doi: 10.1007/s00210-008-0276-9. [DOI] [PubMed] [Google Scholar]
- 10.Bharucha AE, Camilleri M, Burton DD, Thieke SL, Feuerhak KJ, Basu A, Zinsmeister AR. Increased nutrient sensitivity and plasma concentrations of enteral hormones during duodenal nutrient infusion in functional dyspepsia. Am J Gastroenterol 109: 1910–1920, 2014. doi: 10.1038/ajg.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, Törnblom H, Simrén M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology 149: 1399–1407.e2, 2015. doi: 10.1053/j.gastro.2015.07.054. [DOI] [PubMed] [Google Scholar]
- 12.Botta GA, Radin L, Costa A, Schito G, Blasi G. Gas-liquid chromatography of the gingival fluid as an aid in periodontal diagnosis. J Periodontal Res 20: 450–457, 1985. doi: 10.1111/j.1600-0765.1985.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 13.Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology 132: 1890–1901, 2007. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 14.Cho HJ, Callaghan B, Bron R, Bravo DM, Furness JB. Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res 356: 77–82, 2014. doi: 10.1007/s00441-013-1780-x. [DOI] [PubMed] [Google Scholar]
- 15.Coleman NS, Foley S, Dunlop SP, Wheatcroft J, Blackshaw E, Perkins AC, Singh G, Marsden CA, Holmes GK, Spiller RC. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol 4: 874–881, 2006. doi: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Costa MB, Azeredo IL Jr, Marciano RD, Caldeira LM, Bafutto M. Evaluation of small intestine bacterial overgrowth in patients with functional dyspepsia through H2 breath test. Arq Gastroenterol 49: 279–283, 2012. doi: 10.1590/S0004-28032012000400009. [DOI] [PubMed] [Google Scholar]
- 17.Cubeddu LX. Serotonin mechanisms in chemotherapy-induced emesis in cancer patients. Oncology 53, Suppl 1: 18–25, 1996. doi: 10.1159/000227636. [DOI] [PubMed] [Google Scholar]
- 18.D’Almeida MS, Gaudin C, Lebrec D. Validation of 1- and 2-mm transit-time ultrasound flow probes on mesenteric artery and aorta of rats. Am J Physiol Heart Circ Physiol 268: H1368–H1372, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Eglen RM, Bley K, Bonhaus DW, Clark RD, Hegde SS, Johnson LG, Leung E, Wong EH. RS 23597-190: a potent and selective 5-HT4 receptor antagonist. Br J Pharmacol 110: 119–126, 1993. doi: 10.1111/j.1476-5381.1993.tb13780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliakim R, Ophir M, Rachmilewitz D. Duodenal mucosal injury with nonsteroidal antiinflammatory drugs. J Clin Gastroenterol 9: 395–399, 1987. doi: 10.1097/00004836-198708000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol 108: 105–113, 1997. doi: 10.1007/s004180050151. [DOI] [PubMed] [Google Scholar]
- 22.Fukudo S, Kinoshita Y, Okumura T, Ida M, Akiho H, Nakashima Y, Nishida A, Haruma K. Ramosetron reduces symptoms of irritable bowel syndrome with diarrhea and improves quality of life in women. Gastroenterology 150: 358–66.e8, 2016. doi: 10.1053/j.gastro.2015.10.047. [DOI] [PubMed] [Google Scholar]
- 23.Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, Eubanks S, Harris M, Pappas TN, Takahashi T. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol 284: R1269–R1276, 2003. doi: 10.1152/ajpregu.00442.2002. [DOI] [PubMed] [Google Scholar]
- 24.Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, Henry A, Hall I, Whorwell P, Spiller R. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 63: 1617–1625, 2014. doi: 10.1136/gutjnl-2013-305989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414, 2007. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Ghoshal UC, Srivastava D. Irritable bowel syndrome and small intestinal bacterial overgrowth: meaningful association or unnecessary hype. World J Gastroenterol 20: 2482–2491, 2014. doi: 10.3748/wjg.v20.i10.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghoshal UC, Srivastava D, Misra A, Ghoshal U. A proof-of-concept study showing antibiotics to be more effective in irritable bowel syndrome with than without small-intestinal bacterial overgrowth: a randomized, double-blind, placebo-controlled trial. Eur J Gastroenterol Hepatol 28: 281–289, 2016. doi: 10.1097/MEG.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 28.Glisić R, Koko V, Todorović V, Drndarević N, Cvijić G. Serotonin-producing enterochromaffin (EC) cells of gastrointestinal mucosa in dexamethasone-treated rats. Regul Pept 136: 30–39, 2006. doi: 10.1016/j.regpep.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, Taylor JA, Loitto VM, Magnusson KE, Ahlman H, Lundgren O, Svensson L. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog 7: e1002115, 2011. doi: 10.1371/journal.ppat.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PL. Why can’t rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One 8: e60537, 2013. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Høverstad T, Bjørneklett A, Fausa O, Midtvedt T. Short-chain fatty acids in the small-bowel bacterial overgrowth syndrome. Scand J Gastroenterol 20: 492–499, 1985. doi: 10.3109/00365528509089686. [DOI] [PubMed] [Google Scholar]
- 32.Høverstad T, Bjørneklett A, Midtvedt T, Fausa O, Bøhmer T. Short-chain fatty acids in the proximal gastrointestinal tract of healthy subjects. Scand J Gastroenterol 19: 1053–1058, 1984. [PubMed] [Google Scholar]
- 33.Inoue T, Higashiyama M, Kaji I, Rudenkyy S, Higuchi K, Guth PH, Engel E, Kaunitz JD, Akiba Y. Dipeptidyl peptidase IV inhibition prevents the formation and promotes the healing of indomethacin-induced intestinal ulcers in rats. Dig Dis Sci 59: 1286–1295, 2014. doi: 10.1007/s10620-013-3001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwai T, Ichikawa T, Kida M, Goso Y, Saegusa Y, Okayasu I, Saigenji K, Ishihara K. Vulnerable sites and changes in mucin in the rat small intestine after non-steroidal anti-inflammatory drugs administration. Dig Dis Sci 55: 3369–3376, 2010. doi: 10.1007/s10620-010-1185-6. [DOI] [PubMed] [Google Scholar]
- 35.Kaji I, Iwanaga T, Watanabe M, Guth PH, Engel E, Kaunitz JD, Akiba Y. SCFA transport in rat duodenum. Am J Physiol Gastrointest Liver Physiol 308: G188–G197, 2015. doi: 10.1152/ajpgi.00298.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaji I, Karaki S, Tanaka R, Kuwahara A. Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J Mol Histol 42: 27–38, 2011. doi: 10.1007/s10735-010-9304-4. [DOI] [PubMed] [Google Scholar]
- 37.Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, Kuwahara A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol 39: 135–142, 2008. doi: 10.1007/s10735-007-9145-y. [DOI] [PubMed] [Google Scholar]
- 38.Kato S, Matsuda N, Matsumoto K, Wada M, Onimaru N, Yasuda M, Amagase K, Horie S, Takeuchi K. Dual role of serotonin in the pathogenesis of indomethacin-induced small intestinal ulceration: pro-ulcerogenic action via 5-HT3 receptors and anti-ulcerogenic action via 5-HT4 receptors. Pharmacol Res 66: 226–234, 2012. doi: 10.1016/j.phrs.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Kaunitz JD, Akiba Y. Acid-sensing protective mechanisms of duodenum. J Physiol Pharmacol 54, Suppl 4: 19–26, 2003. [PubMed] [Google Scholar]
- 40.Kellum J, McCabe M, Schneier J, Donowitz M. Neural control of acid-induced serotonin release from rabbit duodenum. Am J Physiol Gastrointest Liver Physiol 245: G824–G831, 1983. [DOI] [PubMed] [Google Scholar]
- 41.Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl− secretion via afferent neurons and 5-HT4 receptors. Am J Physiol Gastrointest Liver Physiol 277: G515–G520, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Kellum JM, Donowitz M, Cerel A, Wu J. Acid and isoproterenol cause serotonin release by acting on opposite surfaces of duodenal mucosa. J Surg Res 36: 172–176, 1984. doi: 10.1016/0022-4804(84)90084-2. [DOI] [PubMed] [Google Scholar]
- 43.Lee KJ, Tack J. Duodenal implications in the pathophysiology of functional dyspepsia. J Neurogastroenterol Motil 16: 251–257, 2010. doi: 10.5056/jnm.2010.16.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee T, Schwandner R, Swaminath G, Weiszmann J, Cardozo M, Greenberg J, Jaeckel P, Ge H, Wang Y, Jiao X, Liu J, Kayser F, Tian H, Li Y. Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol Pharmacol 74: 1599–1609, 2008. doi: 10.1124/mol.108.049536. [DOI] [PubMed] [Google Scholar]
- 45.Minami M, Endo T, Hirafuji M, Hamaue N, Liu Y, Hiroshige T, Nemoto M, Saito H, Yoshioka M. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther 99: 149–165, 2003. doi: 10.1016/S0163-7258(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 46.Mizumori M, Meyerowitz J, Takeuchi T, Lim S, Lee P, Supuran CT, Guth PH, Engel E, Kaunitz JD, Akiba Y. Epithelial carbonic anhydrases facilitate PCO2 and pH regulation in rat duodenal mucosa. J Physiol 573: 827–842, 2006. doi: 10.1113/jphysiol.2006.107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munck LK, Mertz-Nielsen A, Westh H, Bukhave K, Beubler E, Rask-Madsen J. Prostaglandin E2 is a mediator of 5-hydroxytryptamine induced water and electrolyte secretion in the human jejunum. Gut 29: 1337–1341, 1988. doi: 10.1136/gut.29.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nzakizwanayo J, Dedi C, Standen G, Macfarlane WM, Patel BA, Jones BV. Escherichia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Sci Rep 5: 17324, 2015. doi: 10.1038/srep17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 95: 3503–3506, 2000. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 50.Pizzonero M, Dupont S, Babel M, Beaumont S, Bienvenu N, Blanqué R, Cherel L, Christophe T, Crescenzi B, De Lemos E, Delerive P, Deprez P, De Vos S, Djata F, Fletcher S, Kopiejewski S, L’Ebraly C, Lefrançois JM, Lavazais S, Manioc M, Nelles L, Oste L, Polancec D, Quénéhen V, Soulas F, Triballeau N, van der Aar EM, Vandeghinste N, Wakselman E, Brys R, Saniere L. Discovery and optimization of an azetidine chemical series as a free fatty acid receptor 2 (FFA2) antagonist: from hit to clinic. J Med Chem 57: 10044–10057, 2014. doi: 10.1021/jm5012885. [DOI] [PubMed] [Google Scholar]
- 51.Reigstad CS, Salmonson CE, Rainey JF III, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29: 1395–1403, 2015. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roth KA, Kim S, Gordon JI. Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am J Physiol Gastrointest Liver Physiol 263: G174–G180, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Satoh H, Amagase K, Ebara S, Akiba Y, Takeuchi K. Cyclooxygenase (COX)-1 and COX-2 both play an important role in the protection of the duodenal mucosa in cats. J Pharmacol Exp Ther 344: 189–195, 2013. doi: 10.1124/jpet.112.199182. [DOI] [PubMed] [Google Scholar]
- 54.Satoh H, Amagase K, Takeuchi K. Exacerbation of nonsteroidal anti-inflammatory drug-induced small intestinal lesions by antisecretory drugs in rats: the role of intestinal motility. J Pharmacol Exp Ther 343: 270–277, 2012. doi: 10.1124/jpet.112.197475. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu K, Koga H, Iida M, Haruma K. Microcirculatory changes in experimental mesenteric longitudinal ulcers of the small intestine in rats. Dig Dis Sci 52: 3019–3028, 2007. doi: 10.1007/s10620-007-9804-6. [DOI] [PubMed] [Google Scholar]
- 56.Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology 85: 1120–1130, 1983. [PubMed] [Google Scholar]
- 57.Spiller R. Serotonin and GI clinical disorders. Neuropharmacology 55: 1072–1080, 2008. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi K, Miyazawa T, Tanaka A, Kato S, Kunikata T. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion 66: 30–41, 2002. doi: 10.1159/000064419. [DOI] [PubMed] [Google Scholar]
- 59.Takeuchi K, Ohuchi T, Matsumoto J, Okabe S. Regulation of gastroduodenal bicarbonate secretion by capsaicin-sensitive sensory neurons in rats. J Clin Gastroenterol 17, Suppl 1: S33–S39, 1993. doi: 10.1097/00004836-199312001-00009. [DOI] [PubMed] [Google Scholar]
- 60.Tsukamoto Y, Goto H, Hase S, Arisawa T, Ohara A, Suzuki T, Hoshino H. Serotonin-induced decrease of duodenal mucosal blood flow plus acid load produces duodenal mucosal lesion in rats. Digestion 50: 99–103, 1991. doi: 10.1159/000200746. [DOI] [PubMed] [Google Scholar]
- 61.Turvill JL, Connor P, Farthing MJ. The inhibition of cholera toxin-induced 5-HT release by the 5-HT(3) receptor antagonist, granisetron, in the rat. Br J Pharmacol 130: 1031–1036, 2000. doi: 10.1038/sj.bjp.0703414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unmack MA, Hansen MB, Grondahl ML, Olsen JE, Christensen P, Skadhauge E. Effects of indomethacin on Salmonella typhimurium- and cholera toxin-induced fluid accumulation in the porcine small intestine. J Vet Med A Physiol Pathol Clin Med 48: 153–163, 2001. doi: 10.1046/j.1439-0442.2001.00348.x. [DOI] [PubMed] [Google Scholar]
- 63.van Boxel OS, ter Linde JJ, Siersema PD, Smout AJ. Role of chemical stimulation of the duodenum in dyspeptic symptom generation. Am J Gastroenterol 105: 803–811, 2010. doi: 10.1038/ajg.2010.100. [DOI] [PubMed] [Google Scholar]
- 64.von der Ohe MR, Camilleri M, Kvols LK. A 5HT3 antagonist corrects the postprandial colonic hypertonic response in carcinoid diarrhea. Gastroenterology 106: 1184–1189, 1994. doi: 10.1016/0016-5085(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 65.Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM, Verdu E, Ongini E. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 141: 1314–1322, 2011. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 66.Wang JH, Inoue T, Higashiyama M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther 339: 464–473, 2011. doi: 10.1124/jpet.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev 64: 359–388, 2012. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161: 264–276, 2015. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]