Abstract
Chronic diseases of the biliary tree (cholangiopathies) represent one of the major unmet needs in clinical hepatology and a significant knowledge gap in liver pathophysiology. The common theme in cholangiopathies is that the target of the disease is the biliary tree. After damage to the biliary epithelium, inflammatory changes stimulate a reparative response with proliferation of cholangiocytes and restoration of the biliary architecture, owing to the reactivation of a variety of morphogenetic signals. Chronic damage and inflammation will ultimately result in pathological repair with generation of biliary fibrosis and clinical progression of the disease. The hallmark of pathological biliary repair is the appearance of reactive ductular cells, a population of cholangiocyte-like epithelial cells of unclear and likely mixed origin that are able to orchestrate a complex process that involves a number of different cell types, under joint control of inflammatory and morphogenetic signals. Several questions remain open concerning the histogenesis of reactive ductular cells, their role in liver repair, their mechanism of activation, and the signals exchanged with the other cellular elements cooperating in the reparative process. This review contributes to the current debate by highlighting a number of new concepts derived from the study of the pathophysiology of chronic cholangiopathies, such as congenital hepatic fibrosis, biliary atresia, and Alagille syndrome.
Keywords: cholangiopathies, macrophages, myofibroblasts, hepatic progenitor cells, ductular reaction
chronic diseases of the biliary epithelium, or cholangiopathies, remain one of the major unmet needs in both experimental and clinical hepatology, although the topic has drawn growing attention since the early 1990s (2, 58, 79, 129). Cholangiopathies comprise a large group of diseases of diverse etiologies, including genetic, inflammatory, immune-mediated, toxic, infectious, and ischemic causes. Although the single conditions may be rare, the epidemiological impact of cholangiopathies as a group is relevant and is associated with high morbidity and mortality as well as a significant financial burden to families and health care systems. Cholangiopathies, in fact, are the main indication to liver transplantation in the pediatric population (∼80%) and represent a consistent proportion of liver transplants performed in adults (10–20%) (130). Despite significant advances in the knowledge of the biology of cholangiocytes, the ability to translate them into effective therapeutic strategies has been elusive, and thus the treatment of cholangiopathies remains hitherto unsatisfactory.
Cholangiopathies are generally characterized by a chronically evolving clinical course and share similar pathophysiological mechanisms, including proliferation, apoptosis, cholestasis, inflammation, fibrogenesis, and eventually carcinogenesis (80). A persistent biliary damage triggers a pathological reparative reaction, which is associated with an excessive deposition of scar tissue in the areas surrounding the injured ducts (biliary fibrosis), followed by progression to biliary cirrhosis, portal hypertension, end-stage liver disease, and ultimately liver transplantation or death. Here we will review the current understanding of how progressive biliary fibrosis is generated and evolves in chronic cholangiopathies (80, 130). We will first discuss the mechanisms of liver repair and the cell types engaged in this processes. We will then highlight a number of new concepts emerging from the study of progressive congenital conditions, such as congenital hepatic fibrosis (CHF), biliary atresia (BA), and Alagille syndrome (AGS).
The Biliary Reparative Complex
The liver is equipped with a complex reparative machinery involving multiple cell elements and signals. Depending on the nature and intensity of the damage, epithelial cells, mesenchymal cells (mainly hepatic stellate cells and portal fibroblasts), and inflammatory cells (macrophages, lymphocytes, and neutrophils) are activated to a variable extent, and then they expand thanks to the ability to mutually exchange a variety of paracrine and autocrine signals, including growth factors, cytokines, chemokines, and morphogens (36, 37). It is worth noting that liver repair is a highly dynamic process and that the damaged liver assembles these tools in many different ways, depending also on the anatomic district that is injured and on the quality and duration of the inflammatory process.
In discussing biliary repair, it is important to underline that what is being repaired is an epithelial wound and that the repair mechanism is driven by signals arising from necrosis, apoptosis, or, more in general, loss of the homeostatic equilibrium in biliary epithelial cells. These signals are first detected by macrophages and neutrophils and by the many cell types that come into play to repair the epithelial layer (progenitor cells), its scaffold (myofibroblasts), and vasculature (endothelial cells). Therefore, it seems appropriate to discuss first the several cell types involved in the biliary reparative complex.
Macrophages.
Tissue-resident Kupffer cells (KCs) and macrophages recruited from the circulating bone marrow-derived monocyte lineage represent a major component of the defense mechanisms in the liver. KCs account for ≈15% of the liver nonparenchymal cell population and are located within the periportal area of hepatic sinusoids. They are thought to self-renew and to originate from fetal precursor cells (8). Conversely, liver macrophages derived from circulating monocytes are constantly supplied through a recruitment governed mainly by monocyte chemoattractant protein (MCP)-1/C-C motif chemokine ligand (CCL)2 and its cognate receptor C-C chemokine receptor (CCR)2 (22). Macrophages are actively involved in tissue repair based on their ability to release a wide variety of inflammatory mediators. Macrophages develop distinct (“polarized”) functional programs. These programs in reality represent the extreme points of a continuum that goes from the classical (M1) to the alternative (M2) activation (127).
Depending upon the modulatory effects on the local microenvironment, macrophages play an ambivalent role in liver fibrosis (109, 133). Following liver injury, bone marrow-derived circulating monocytes are recruited via the CCL2/CCR2 and CCL1/CCR8 axis to the inflamed area, whereby they differentiate into macrophages displaying a detrimental, proinflammatory phenotype (9, 55). Profibrogenic effects exerted by macrophages rely on the intense cross-talk they establish with liver myofibroblasts (MF), which is mediated by tumor necrosis factor (TNF)α and interleukin (IL)-1β, which, besides perpetuating hepatocellular injury, stimulate MF survival. Secretion of transforming growth factor (TGF)β and platelet-derived growth factor (PDGF) by macrophages induces MF activation, proliferation, and collagen production.
On the other hand, resolution of fibrosis depends upon a phenotypic switch of macrophages exposed to anti-inflammatory signals, such as, for example, fractalkine/CX3CL1, that are able to turn them into a proresolution (or restorative) phenotype. Proresolution macrophages express TNF-related apoptosis-inducing ligand (TRAIL) and matrix metalloproteinase (MMP)-9, which promote MF apoptosis and release fibrolytic proteases (MMP-12 and MMP-13 in particular), which further shift the extracellular matrix (ECM) remodeling from formation to degradation. As shown by transcriptome analysis-based studies, macrophage heterogeneity, as it develops in liver fibrosis, somehow deviates from a strict M1/M2 categorization (117). Nevertheless, in the liver, the balance between M1 and M2 phenotypes is a critical factor that determines the severity of cholestastic injury; a typical M1 cytokine, IL-6, has been shown to be protective from cholestasis, exerting antiapoptotic effects on hepatocytes and proliferative effects on cholangiocytes (142). The role of macrophages in biliary diseases has not been addressed as extensively as in parenchymal liver diseases; however, recent data in a mouse model of congenital hepatic fibrosis point toward a major fibrogenic role of macrophages attracted by the epithelial dysfunction (see below).
Neutrophils.
Neutrophils around reactive ductules are a typical finding in biliary obstructive lesions, sepsis, and other inflammatory conditions. A prominent neutrophilic infiltration in the portal space in close vicinity to proliferating bile ductules is a classic feature of “cholangitis lenta”, a form of ductular cholestasis during sepsis and endotoxinemia (82). IL-8 [chemokine (C-X-C motif) ligand (CXCL) 8] is a major chemoattractant for neutrophils that are stimulated to produce leucotriens, reactive oxygen species, and defensins, including human neutrophil peptides 1–3 (HNP1-3), all factors that possess strong antimicrobial activity (60, 100). Interestingly, IL-8 is expressed by reactive ductules in septic livers, where it is closely associated with the peribiliary neutrophil infiltration. Furthermore, cultured cholangiocytes expressed and released IL-8 in response to LPS and proinflammatory cytokines such as TNFα and IL-1β (60). Notably, patients with cholestatic liver diseases show increased IL-8 serum levels and intrahepatic IL-8 gene expression associated with neutrophil infiltrations in PBC (150) and PSC (153). Proinflammatory macrophages derived from circulating monocytes are also an important source of IL-8 (150).
Innate lymphoid cells.
Innate lymphoid cells (ILC) are a family of innate immune cells producing many Th cell-associated cytokines but not expressing the classical cell surface markers that characterize the other immune T and B cell lineages (5). Since ILC do not express a T cell receptor, they do not respond in an antigen-specific manner and do not engage in adaptive immune response (5). A specific subset of ILC that exhibit a Th2 response (type 2 ILC or ILC2) is induced by IL-33 and has a protective effect against TNFα-mediated liver injury, as reported in adenovirus-mediated acute hepatitis (86). IL-33 is a nuclear cytokine from the IL-1 family expressed by barrier epithelia and lymphoid cells. IL-33 functions as an alarm signal (alarmin) released upon cellular stress and injury (17). However, in specific settings under the effect of IL-33, ILC2 play a profibrogenic role mediated by the production of IL-13, which stimulated cholangiocyte proliferation in experimental models of biliary atresia (see below). Similar strong profibrogenic effects related to IL-13 release from ILC2 are well recognized in lung fibrosis (52).
Hepatic stellate cells and portal fibroblasts.
Hepatic stellate cells (HSCs) and portal fibroblasts (PFs) are the main resident mesenchymal cell types in the normal liver. Whereas HSC are located in the subendothelial space of Disse, PF reside in the portal tract, closely surrounding the finest portal vein ramifications. In the healthy liver, HSC and PF display a quiescent phenotype. The HSC phenotype is characterized by storage of vitamin A, expression of desmin, β2-macroglobulin, and Hand2, thereby differing from that of PF, which are positive for fibulin-2, elastin, thymocite differentiation antigen-1 (Thy-1), mesothelin (Msln), Gremlin 1 and the ecto-AT-Pase nucleoside triphosphate diphosphohydrolase-2 (NTPD2) (30, 68).
Both HSC and PF respond to inflammatory stimuli, such as oxidative stress, and proinflammatory cytokines, particularly TGFβ and PDGF, which are released by inflammatory cells during chronic liver injury, and are able to transdifferentiate into an activated phenotype (MF). The MF phenotype is characterized by strong expression of α-SMA and enhanced proliferative, migratory, and contractility properties, along with the ability to produce interstitial fibril-forming collagens (mainly type I and III collagens) that increase the stiffness of the ECM scaffolding. A fundamental feature, at a transcriptional level, of the phenotypic switch leading to MF activation is the downregulation of the peroxisome proliferator-activated receptor-γ (PPARγ), a nuclear receptor that inhibits the α1(I) collagen promoter activity (148). MF may also play immune modulatory functions (140) and may promote vascular remodeling, an effect stimulated by hypoxia and mediated by VEGF-A secretion (3).
The question of the origin of the MF that are involved in biliary fibrosis has puzzled researchers for almost two decades, and it is still not fully resolved. It has been proposed that PF are the mesenchymal cells activated following cholangiocyte damage and are responsible for biliary fibrosis, whereas HSC cooperate with hepatocytes in liver fibrogenesis (68). In biliary fibrosis, portal MF are generally localized in the fibrotic tissue, developing around interlobular bile ducts responsible for portal tract enlargement, whereas in chronically evolving hepatitis liver, MF accumulate at the interface between nascent fibrotic septa and hepatocellular parenchyma (16). However, this schematic picture has been challenged by recent lineage-tracing studies suggesting that the majority of MF in biliary cirrhosis derive from HSC (92). A limitation of these studies is that they missed the initial phases of fibrogenesis, as they analyzed liver fibrosis at the cirrhotic stage, when some other fibrogenic cell types could have already been replaced. Therefore, an emerging and intriguing view is that portal MFs may be early players of biliary fibrosis and contribute to recruit and activate HSCs, which then become the main fibrogenic cells as liver damage progresses, extending outside the portal tract and involving the liver lobule. Furthermore, additional inflammatory cell types infiltrating the portal area, such as macrophages, might be actively involved in the scarring process by orchestrating the stage-dependent contribution of PF and HSC. In addition to HSC and PF, bone marrow-derived mesenchymal stem cells have been suggested as putative sources of MF in the liver, although their role is less clear (122) and will not be addressed here further. Also, epithelial to mesenchymal transition (EMT) has been claimed to contribute further to MF generation, but cell fate mapping-based studies argue against this hypothesis (13, 19).
Independently from their origin, the behavior of mesenchymal cells in proximity of the bile ducts is profoundly affected by signals originating from the inflamed ducts, which in turn can be modulated by soluble factors released by activated mesenchymal cells. This intensive epithelial-mesenchymal cross-talk is enabled by the high homology of agonists/receptor systems shared by the two cell types. TGFβ2 (97), IL-6 (147), PDGF-BB (71), MCP-1 (75), connective tissue growth factor (CTGF), and osteopontin (112) secreted by reactive cholangiocytes stimulate portal MF activation. Other soluble factors known to stimulate proliferation and migration of portal MF are FGF2 (139) and, more recently, taurocholic acid and IL-25 (61). Furthermore, PFs have been shown to regulate cholangiocyte proliferation via the paracrine action of NTPD2, which inactivates extracellular nucleotides and prevents their interaction with the P2Y receptors mediating proliferative effects in cholangiocytes (64). Upon MCP-1 stimulation, portal MFs downregulate NTPD2 and thus stimulate cholangiocyte proliferation through the activation of P2Y receptors (30). Expression of hyaluronic acid by portal MFs, which is particularly prominent in biliary fibrosis, has been also reported to induce biliary proliferation (54). Other important paracrine mechanisms regulating the cross-talk between reactive cholangiocytes and MFs are the Wingless (Wnt), Hedgehog (Hh) and Notch signaling, and will be discussed below. Finally, it is interesting to note that the most extensively studied model of liver fibrosis (common bile duct ligation in rodents or BDL) is actually an obstructive cholangiopathy. Therefore, the reader is referred to the wealth of information on BDL present in PubMed.
Hepatic progenitor cells and reactive ductular cells.
The liver possesses an intrinsic ability to “regenerate” after acute transitory damages. Liver “regeneration” is sustained mostly by the replicative ability of its mature epithelial cells (hepatocyte and cholangiocytes) and eventually by the activation of hepatic progenitor cells (HPCs). The involvement of HPC depends on the severity and nature of the liver damage and on the residual replicative ability of hepatocytes/cholangiocytes. Even when regeneration is sustained only by HPCs, the final result may still be a “restitutio ad integrum” of the tissue.
HPCs are thought to be located in specialized portions of the biliary tree, corresponding to the terminal ductules and the canals of Hering, the boundary line between hepatocytes and cholangiocytes (121), or to the peribiliary glands (14). Peribiliary glands are mucin-producing glandular elements contained inside the bile duct walls, concentrated particularly in the merging points of the biliary tree, i.e., at the level of the cystic duct, and of the perihilar and periampullar segments. Notably, this progenitor cell population also has the capability to differentiate into the pancreatic lineage, even yielding insulin-producing β-cells (15). Whether HPCs derived from peribiliary glands are actually engaged in liver repair mechanisms is still uncertain.
Regardless of their localization, HPCs show a peculiar morphology, since they appear as small cells, single or in small clumps, with an oval shape and scant cytoplasm (formerly referred to oval cells in rodents), with the ability to differentiate into the biliary or the hepatocyte lineage (120). In humans, differentiation toward hepatocytes occurs via an intermediate cell population with a mixed hepatobiliary phenotype [intermediate hepato-biliary cells (IHBCs)], whereas commitment toward the biliary differentiation leads to the formation of reactive ductular cells (RDCs) (121). RDCs are epithelial cells displaying a biliary phenotype, organized into irregularly shaped structures, richly anastomosed to each other, often without a recognizable lumen, initially localized at the peripheral region of the portal space, from which they may extend depending upon the severity of the biliary injury. Their histogenesis is uncertain, but different lines of evidence suggest that in addition to being the progeny of HPCs, RDCs might also derive from proliferation of preexisting cholangiocytes or from the ductular metaplasia of periportal hepatocytes, whose plasticity is a well-established property during cholestasis (26, 34, 87, 120, 121). Again, it is tempting to speculate that there is not a single mechanism underlying RDC formation but rather that multiple highly adaptable mechanisms may take part, depending on the nature and the intensity of biliary damage.
RDCs progressively accumulate whether the damaging conditions persist, and their presence is actually the hallmark of ongoing pathological repair (121). RDCs possess different biological properties as compared with normal cholangiocytes. RDCs show a certain degree of “plasticity,” i.e., the ability to change and acquire a number of morphological and functional traits typically expressed by mesenchymal cells. De novo expression of S100A4, vimentin, Snail, and MMP-2, associated with downregulation of the epithelial markers E-cadherin and cytokeratin (CK) 19, was observed in RDCs of tissue sections obtained from patients with primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC) (123), and biliary atresia (BA) (28, 53) and in rodent models of biliary fibrosis induced by BDL (107). However, a full phenotypic conversion of RDC into MF was excluded by lineage-tracing experiments (13, 19). Loss of epithelial secretory and barrier functions and expression of EMT markers are necessary to repair the wound, leading to the concept of “activated cholangiocytes” or “partial EMT” to underline the phenotypic plasticity displayed by RDCs (11, 37).
The cell fate decision of HPC between hepatocellular and biliary lineage is finely orchestrated by the type of inflammatory reaction developing around the stem cell niche and the extent of activation directed by fundamental morphogenetic signals such as Wnt/β-catenin, Hedgehog, or Notch (10, 12, 20, 41, 134). It has been proposed that interactions between HPCs and MFs expressing Jagged1 induce Notch-2 activation in HPC, which is responsible for biliary specification (124). Conversely, in hepatocellular regeneration, macrophage engulfment by phagocytosis of debris from apoptotic hepatocytes stimulates them to release Wnt3a, which would promote nuclear translocation of β-catenin in HPCs and, therefore, their commitment toward the hepatocellular differentiation (10, 124).
On the other hand, the relevance of Wnt/β-catenin in HPC activation and proliferation was demonstrated originally in rat and mouse models, with extensive oval cell response caused by strong inhibition of hepatocyte replication. A paracrine mechanism mediated by Wnt-1 expressed by hepatocytes and Frizzled-2 expressed by oval cells was described, and genetic inactivation of β-catenin resulted in a dramatic decrease in oval cell response (4). The signals that stimulate HPCs to leave their niche and expand are currently unknown, and conflicting results have been reported. The number of morphogens, cytokines, and growth factors able to trigger HPC activation and RDC expansion is continuously increasing (Table 1) (43, 51, 70). Among them, TNF-like weak inducer of apoptosis (TWEAK), connective tissue growth factor (CTGF), integrin αvβ6, and lysyl oxidase-like 2 (LOXL2) have emerged recently as putative therapeutic targets. In fact, frontier drugs aiming at these molecules are already in advanced stages of development.
Table 1.
Morphogens, cytokines, and growth factors able to trigger HPC activation and RDC expansion
| Effects on HPC Activation | Effects on RDC Expansion | |
|---|---|---|
| Morphogens | Hedgehog, Notch, Wnt/β-catenin, YAP | Hedgehog, Notch, Wnt/β-catenin, YAP |
| Cytokines | G-CSF, Fn14, IL-6, IL-22, IFNγ, LIF, lymphotoxin-β, OSM, TNFα, TWEAK, SDF-1/CXCL12 | IL-6, LIF, OSM |
| Hormones/peptides/growth factors | CTGF, integrin αvβ6, TGFβ1, EGF, FGF7, HGF, LOXL2, insulin, somatostatin | α-CGRP, acetylcholine, angiopoietin-1, angiotensin II, EGF, estrogen, FSH, GLP-1, GnRH, HGF, progesterone, prolactin, secretin, substance P, taurocholate acid, taurolithocholate acid, VEGF-A, VEGF-C |
HPC, hepatic progenitor cells; RDC, reactive ductular cells; Wnt, wingless; YAP, Yes-associated protein; LIF, leukemia inhibitory factor; OSM, oncostatin M; G-CSF, granulocyte colony-stimulating factor; TWEAK, TNF-like weak inducer of apoptosis; SDF-1/CXCL12, stromal cell-derived factor-1/chemokine (C-X-C motif) ligand; CTGF, connective tissue growth factor; EGF, epidermal growth factor; GnRH, gonadotropin-releasing hormone; HGF, hepatocyte growth factor; FGF7, fibroblast growth factor 7; LOXL2, lysyl oxidase-like 2; α-CGRP, calcitonin gene-related peptide.
TWEAK is a member of the TNF superfamily expressed by inflammatory cells in particular macrophages that selectively stimulates the HPC/RDC proliferation and expansion without any effect on the mature liver epithelial cells that do not express its cognate receptor Fn14 (62). In fact, oval cell expansion induced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) was significantly reduced in Fn14-null mice as well as in wild-type mice with TWEAK blockade. Increased Fn14 expression was also observed in chronic human liver diseases with intense ductular reaction (62). A marker of RDC upmodulated by TWEAK is CK23, which was reported as markedly increased in end-stage PBC (50). Interestingly, elevated CK23 levels were found in serum of cirrhotic patients, thus suggesting CK23 serum fragments as promising noninvasive biomarkers to assess the extent of ductular reaction (50). Notably, oval cell stimulation by recombinant TWEAK was demonstrated in mice undergoing partial hepatectomy (76).
CTGF is a member of the cysteine-rich, secreted, heparin-binding CCN protein family (CCN2), which is able to modulate external signal transduction into cells by interacting with ECM components (like fibronectin), cell surface glycoproteins (like the heterodimeric cellular receptor integrins), and growth factors (including the profibrogenic cytokine TGFβ1). TGFβ1 is secreted in a latent form, which is locally activated by integrin αvβ6. In the liver, αvβ6 is expressed by ductal plate cells during embryonic development and decreases thereafter, disappearing almost entirely shortly after birth. In the adult, αvβ6 is upregulated in response to duct injury and inflammation and is rapidly downregulated as the inflammatory process resolves. Thus its persistence in epithelial cells is typical of “wounds that do not heal” (31). A recent study demonstrates that integrin αvβ6, in association with CTGF, is expressed by HPCs and RDCs after experimental biliary damage, where they regulate in concert oval cell activation and fibrogenesis by interacting with their common partners fibronectin and TGF-β1 (113). TGF-β1 activation dependent on integrin αvβ6 expression is critical for HPC function. In fact, in vitro progenitor capabilities to differentiate into cholangiocyte and hepatocyte lineages are blocked by neutralization of either αvβ6 or TGFβ1 and, conversely, restored after supplementation of bioactive TGFβ1 (110). Moreover, genetic ablation or pharmacological targeting of αvβ6 in mouse models of biliary injury is therapeutically relevant to prevent progression of biliary fibrosis, an effect related to the inactivation of HPC compartment and, consequently, to the attenuation of the ductular reaction, thereby reducing peribiliary collagen deposition (110, 116).
LOXL2 is a member of a family mediating collagen cross-linking and stabilization, which sustain progression of fibrosis. LOXL2 upregulation is observed in scarring conditions of several organs, including the liver, where a recent study showed the efficacy of a selective anti-LOXL2 antibody to inhibit advanced, preestablished biliary fibrosis and to promote reversal of advanced fibrosis in Mdr2 (Abcb4)−/− mice (57). Besides acting on collagen cross-linking, antifibrotic effects of LOXL2 blockade were also dependent on the attenuation of ductular reaction driven by HPC activation, in line with the ability of LOXL2 to direct progenitor cell fate toward biliary differentiation via autocrine and paracrine (HSC-derived) mechanisms (57).
It is important to note that discrimination between RDC and HPC is often neglected, and some recent literature has referred to both elements as “progenitor cells” (21, 37, 118). However, a clear distinction between the two cell types may help to better understand liver repair mechanisms. RDCs are most likely the progeny of HPCs and, differently from HPCs, do not have a regenerative function but rather a reparative one, as indicated by several studies showing a strong correlation between the amount of RDCs and portal fibrosis (21, 35, 118).
Another matter of semantics that may generate misunderstanding is the term “accumulation” of RDCs, which is often mistakenly referred to as “proliferation.” Proliferation of cholangiocytes is actually a common response to many pathological liver conditions. A strong activation is seen in chronic cholangiopathies, where the ability of cholangiocytes to proliferate is critical to maintain and/or replenish the ductal mass (43) and delays the evolution to ductopenia. Therefore, elucidation of the mechanisms of cholangiocyte proliferation has been the subject of extensive investigation in the last 20 years (51). As a general concept, factors increasing intracellular cAMP content (originally identified as secretin and cholinergic innervation) stimulate proliferation of cholangiocytes, acting through the PKA/MEK/ERK1/2 pathway (44). The list of mediators that are able to stimulate cholangiocyte proliferation is expanding continuously. It encompasses several growth factors, including the epidermal growth factor (EGF) (24), hepatocyte growth factor (HGF) (66), insulin-like growth factor I (IGF-I) (96), neurotrophins (48), neurotransmitters belonging both to parasympathetic and adrenergic systems (43), bile salts, including taurocholate and taurolithocholate, cytokines such as IL-6 (96) and histamine (45), and hormones such as secretin, gastrin, and estrogens (2, 13a).
It is worth noting that more than 20 years after Valeer J. Desmet suggested that RDCs are “the pacemaker of biliary fibrosis” (27), several aspects of the pathobiology of RDCs remain elusive. What stimulates the RDC response? What are the signals that sense the damage and activate the RDC response? Is there any role for molecular patterns associated with pathogen infections [pathogen-associated molecular patterns (PAMPs)] or noninfectious damage [damage-associated molecular patterns (DAMPs)] and, relatively, for their recognition by Toll-like receptor (TLR) in triggering HPC/RDC? Although the nature of these signals remains unclear, all evidence points to inflammation as the fuel that drives the reparative response.
Inflammation, “Parainflammation,” and Innate Immunity
In addition to the classical inflammatory triggers (infection and tissue injury), epithelial inflammatory reactions may be stimulated by autonomous cell mechanisms, which are elicited, for example, by genetic defects causing cell or tissue malfunction (stress) rather than an overt cell damage (apoptosis or necrosis). These stress conditions affect the homeostatic balance of physiological systems that are different from those directly controlling host defense or tissue repair and are, therefore, not engaged in classic inflammatory responses directed to neutralize and remove the source of damage. Rather, the attempts at restoring the normal cell/tissue homeostasis sustain a chronic inflammatory response of low magnitude defined by Chovatiya and Medzhitov (18) as well as Medzhitov (98) as “parainflammation.” Parainflammation (akin to autoinflammation) is an adaptive response to a persistent cell dysfunction that shifts the homeostatic set points (18, 74), but if the normal tissue homeostasis is not restored it becomes maladaptive, and the persistent low-grade inflammation stimulates the deposition of scar tissue.
Diseases caused by genetic mutations of critical proteins expressed by the biliary epithelium in different cell compartments, such as the cellular membrane, the primary cilium, or the ER, are paradigmatic of these processes. In cholangiopathy associated with cystic fibrosis (cystic fibrosis liver disease), absence of the cystic fibrosis conductance regulator protein (CFTR) at the apical membrane of cholangiocytes alters the regulation of TLR4-dependent innate immunity response. As a result, the endotoxin tolerance of the biliary epithelium is impaired, and cholangiocytes generate an enhanced inflammatory response when challenged with TLR4 agonists (several PAMPS and DAMPs) Thus cell dysfunction generates a proinflammatory-reactive phenotype in the biliary epithelium with the release of cyto/chemokines and infiltration of the portal spaces with inflammatory cells. Cholangiocytes isolated from CF mice show increased TLR4 phosphorylation, NF-κB activation, and cytoskeletal changes consistent with the cell-autonomous nature of the inflammatory signal in the CF epithelium (40, 42, 125, 146).
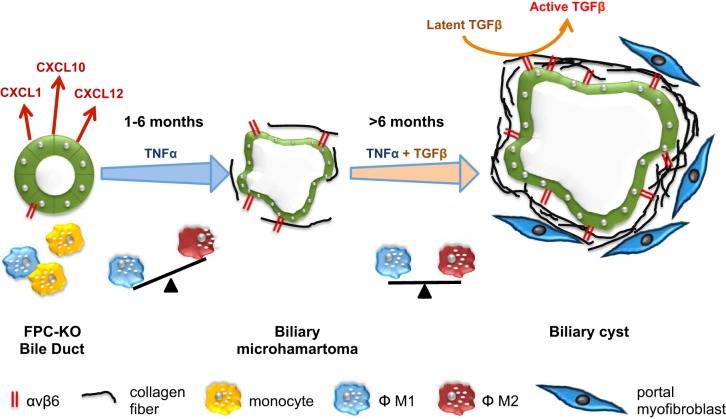
Recent studies in mouse models of congenital hepatic fibrosis (CHF) demonstrate that cholangiocyte dysfunction can be linked to biliary fibrosis through low-grade inflammation derived from impaired control of innate immunity. CHF is a rare disease characterized by altered architecture of the biliary tree and extensive biliary fibrosis, leading to severe portal hypertension and bleeding from esophageal varices (82, 103). CHF is caused by mutations in PKHD1, the gene encoding for fibrocystin (FPC)/polyductin, a ciliary protein that is expressed by ductal epithelial cells, and by cholangiocytes in the liver (137, 149). The function of FPC is still largely unknown, but most evidence indicates that the protein may be involved in a variety of cellular functions, including regulation of proliferation, differentiation, tubulogenesis, planar cell polarity,y and cell-matrix interactions (81). Biliary cysts, which originate from the abnormal remodeling of the ductal plate, progressively enlarge in association with a dense and worsening fibrosis and a smolder portal inflammatory infiltrate. In CHF, overt necroinflammation of the biliary epithelium is absent, indicating that the pathogenetic mechanism is definitely different from that of other cholangiopathies where portal fibrosis is associated with cholangiocyte necrosis or apoptosis. Studies performed using a mouse model of CHF (Pkhd1del4/del4 mice, harboring a deletion of exon 4 of the gene encoding for FPC) showed that in the early phase of the disease, FPC-defective cholangiocytes recruit inflammatory cells, mostly macrophages, by secreting a variety of chemokines, such as CXCL1, CXCL10, and CXCL12 (89). Chemokine secretion was inhibited by blockade of β-catenin, a signaling molecule whose nuclear translocation and activity are increased in Pkhd1del4/del4 mice following cAMP/PKA-mediated phosphorylation of β-catenin at Ser675 (128). In turn, FPC-defective cholangiocytes respond to macrophage-derived cytokines (TNFα and TGFβ) by upregulating integrin αvβ6 (101). In the initial phase of fibrosis, α-SMA+ cells are scarce, and the peribiliary infiltrate is dominated by classically activated, iNOS-expressing M1 macrophages. Switching from a proinflammatory (M1 prevalent) to a reparative behavior (with increased proportion of M2 macrophages), macrophages stimulate a profibrotic response that is further sustained by an increased recruitment of activated portal MFs. In vivo inhibition of macrophage recruitment by clodronate, given before the establishment of portal hypertension, reduced the recruitment of portal MFs, the extent of portal fibrosis, and the development of portal hypertension. These data indicate that CHF is the archetype of a novel model of liver fibrogenesis generated by an adaptive response to the loss of physiological homeostasis in cholangiocytes caused by FPC deficiency (Fig. 1).
Fig. 1.
Biliary repair and fibrosis in congenital hepatic fibrosis. Portal inflammation is driven by the secretion of different chemokines (CXCL1, CXCL10, and CXCL12) by fibrocystin (FPC)-defective cholangiocytes due to an overactivation of β-catenin signaling promoting macrophage recruitment from the circulating monocyte compartment. In turn, macrophage-derived cytokines (TNFα in the first 6 mo and in conjunction with TGFβ afterward) induce upregulation of integrin αvβ6, an activator of latent TGFβ1, in cholangiocytes. In the early phase of fibrosis (1–6 mo), α-smooth muscle actin (α-SMA)+ cells are scarce, and the peribiliary infiltrate is dominated by M1 macrophages. In the 2nd phase (>6 mo), macrophages switch to an M2 phenotype paralleled by an increased recruitment of activated portal myofibroblasts (MFs). In this phase, portal fibrosis extends and associates with portal hypertension. FPC-KO, fibrocystin knockout.
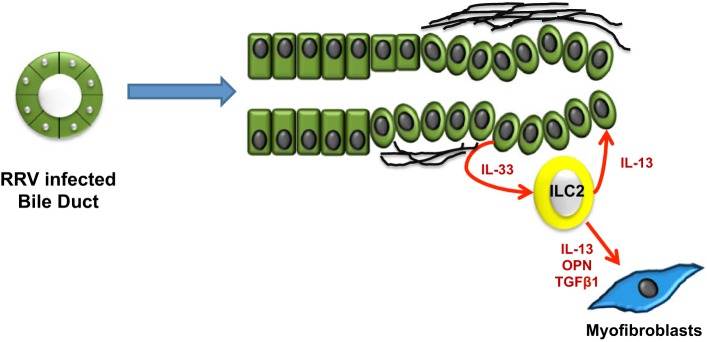
A further example of the role of immune innate-mediated mechanisms in biliary fibrosis is biliary atresia (BA). BA is a developmental cholangiopathy that, if left untreated, rapidly leads to end-stage biliary cirrhosis. Several cases proceed to biliary cirrhosis despite the surgical attempts to reestablish biliary connections and require liver transplantation. BA is characterized by fibro-inflammatory obstruction of the extrahepatic and hilar bile ducts, extensive ductular reaction, and brisk portal fibrosis (23). Evaluation of the cellular elements of liver repair in human samples obtained from BA patients at the time of liver transplant and of the Kasai intervention showed a strong expansion of RDCs and HPCs that closely correlated with the thickness of cirrhotic septa (35). This finding is of interest, since septal thickness was shown to correlate with the severity of cirrhosis and the presence of clinically relevant portal hypertension (102). This represents further circumstantial evidence that expansion of RDCs is functionally associated with evolution to cirrhosis and its major manifestations. Studies in a mouse model of BA [BALB/c mice infected with rhesus rotavirus type A (RRV) in the first 2 postnatal days] further highlight the role of macrophages and dendritic cells as key effectors in triggering biliary injury. By releasing CXCL2, macrophages stimulate neutrophil accumulation, whereas dendritic cells, producing IL-15, activate NK cells. NK cells represent a unique subset of lymphocytes identified originally for their expression of cytoplasmic granules, containing high levels of cytotoxic mediators (Fas L, perforin, granzyme), which provide them with the pronounced ability to kill target cells, a critical function in immune defense and cancer surveillance. In this BA mouse model, NK cell depletion or inactivation immediately after birth prevents development of jaundice after RRV infection (126). Recent studies also demonstrated the pivotal role played by noncytotoxic ILC2. In the RRV infection rodent BA model, accumulation of ILC2 was stimulated by IL-33, which is secreted abundantly in BA (95). Once recruited by IL-33, ILC2 release large amounts of IL-13 in the portal environment. Given its potent mitogenic effects on several epithelial cell types, including cholangiocytes lining the extrahepatic bile ducts, IL-13 in turn promotes and sustains biliary hyperplasia. Furthermore, in the liver, IL-13 stimulates activation of HSC, leading to progressive peribiliary fibrosis and ductal narrowing, in concert with other factors released in the periductal area as a consequence of duct obstruction, such as TGF-β1, osteopontin, and the bile acids (Fig. 2). A feared evolution of this inflammatory process is the malignant progression of injured epithelium. In fact, IL-33 administration in mice with constitutive activation of Akt and Yes-associated protein (YAP) induced cholangiocarcinoma with liver metastases, thus indicating that the IL-33/ILC2/IL-13 paracrine circuit may exert profound oncogenic effects (85). Furthermore, IL-33 by itself is able to stimulate cholangiocyte expression of IL-6 (144), which promotes malignant transformation by increasing the expression of the antiapoptotic protein Mcl-1 via activation of the JAK/STAT pathway (59).
Fig. 2.
Biliary repair and fibrosis in biliary atresia. IL-33 abundantly secreted by cholangiocytes stimulates accumulation of ILC2 (innate lymphoid cells). Once recruited in the peribiliary area, ILC2 release large amounts of IL-13, which exerts potent mitogenic effects on cholangiocytes, thus promoting and sustaining biliary hyperplasia. Furthermore, in concert with other factors released in the periductal area as a consequence of duct obstruction [TGFβ1, osteopontin (OPN), and bile acids], IL-13 stimulates activation of HSC, leading to extensive peribiliary fibrosis and progressive ductal stricturing. RRV, rhesus rotavirus type A.
Morphogenetic Signaling and Biliary Architecture
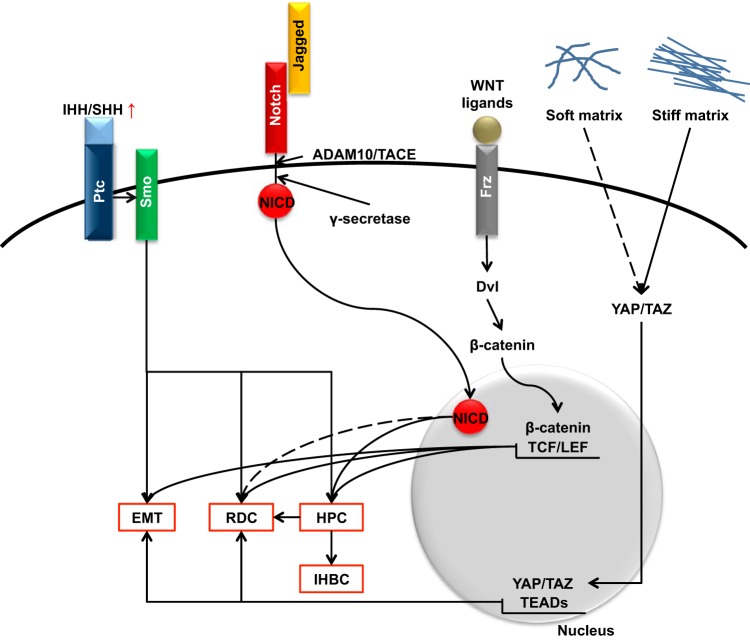
An important requisite of an effective biliary repair is the ability of RDCs, organized in tubeless, highly anastomosed ductular structures at the portal interface, to leave their progenitor cell-like phenotype. Recent studies have unveiled that this mechanism is mediated by the reactivation of morphogenetic pathways involved in biliary development. Among them, Notch, Hedgehog, the Wnt/β-catenin signaling, and, more recently, the YAP/TAZ pathway deserve special consideration (Fig. 3).
Fig. 3.
Morphogenetic signals regulating biliary repair. The reactivation of morphogenetic pathways involved in biliary development [Notch, Hedgehog, Wnt/β-catenin, and Yes-associated protein/transcriptional coactivator with PDZ-binding motif (YAP/TAZ)] regulates activation of hepatic progenitor cells (HPC) and the ability of reactive ductular cells (RDC) to build up functionally active tubular structures. See text for details. EMT, epithelial-to-mesenchymal transition; IHH/SHH, Indian Hedgehog/Sonic Hedgehog; Ptc, patched; Smo, smoothened; NICD, Notch intracellular domain; ADAM/TACE, a disintegrin and metalloproteinase domain-containing protein 10/tumor necrosis factor-α-converting enzyme; Dvl, disheveled; IHBC, intermediate hepato-biliary cells; TCF/LEF, T cell factor/lymphoid enhancer factor; TEADs, TEA domain transcription factors.
Notch.
The Notch pathway is an evolutionarily conserved signaling system that regulates cell fate decisions in stem cells and plays a major role in the development of the biliary tree. Studies in Alagille syndrome (AGS) have clarified that, in addition to its developmental role, Notch signaling is strongly involved in biliary repair and specifically in tubulogenesis and in the biliary phenotypic switch of transdifferentiating hepatocytes. AGS is a rare, genetic multisystem disorder caused by a defective Notch signaling due to JAG1 (50–60%) or, less frequently, NOTCH2 mutations inherited as autosomal dominant disease (1, 84, 97, 104, 138). AGS may affect several organs. Liver disease is characterized by bile duct paucity, with variable degree of cholestasis and eventually intractable pruritus, hypercholesterolemia, and failure to thrive. Liver histology in AGS stands as paradigm of the fundamental role played by Notch signaling in biliary repair. Consistent with a defective biliary branching influencing the postnatal development of the most peripheral bile duct ramifications, ductopenia is more frequently observed at the periphery of the liver (88). Furthermore, ductopenia is associated with a stark imbalance in the epithelial components of the hepatic reparative machinery (35). As compared with biliary atresia, AGS is characterized by a marked accumulation of cells with an IHBC phenotype lacking expression of the Notch-dependent transcription factor HNF1β that contrasts with the nearly complete absence of RDCs. This finding indicates that because of defective Notch signaling, HPCs are forced toward the hepatocyte or, conversely, that hepatocyte-to-cholangiocyte transdifferentiation is blocked at the IHBC level. Notably, this abnormal mechanism of biliary repair has consequences on liver fibrogenesis (Fig. 4). On one side, the reduced number of RDCs is associated with scarce deposition of fibrotic tissue in the portal space, leading to much thinner septa than in BA. On the other side, the increased number of IHBCs is accompanied by a typical pericellular fibrosis developing within the hepatic lobule (“chicken wire” pattern), a fibrotic lesion observed in alcoholic and metabolic liver injury as well. These findings are consistent with the slow clinical progression to cirrhosis observed in AGS patients (33). The ability of Notch signaling to induce biliary specification in liver development was discovered several years ago (46, 47, 71, 73, 151), but its ability to regulate ductal branching during biliary repair has emerged only recently (41, 83, 90, 91, 99).
Fig. 4.
Biliary repair and fibrosis in Alagille syndrome. If Notch signaling is defective, then activation of HPC following biliary damage by Jagged1-expressing PMFs, which instead forms RDC aimed at restoring tubular biliary structures (top), is forced toward the hepatocyte lineage, leading to IHBC accumulation (bottom). This abnormal mechanism of biliary repair has consequences on liver fibrogenesis; whereas the reduced no. of RDC is associated with scarce deposition of fibrotic tissue in the peribiliary area, the increased no. of IHBC is associated with an intralobular, pericellular fibrosis, reproducing the “chicken wire” pattern.
During liver development, Notch signaling is activated in hepatoblasts localized at the parenchymal interface of the nascent portal tract by adjacent mesenchymal cells expressing Jag-1 to stimulate their differentiation toward the biliary phenotype by upregulating the expression of HNF1β and Sox-9. During liver repair, Notch signaling is induced as a default mechanism, HPC conversion to RDC, which is regulated by a direct cell-cell interaction between Notch-expressing HPCs and Jag-1-expressing portal MFs. However, a fine balance between phosphorylation and rapid proteosomal degradation tightly regulates the transcriptional activation of Notch, which physiologically, must be a transient event. Among the regulatory mechanisms antagonizing Notch activation in HPC, Numb is crucial for switching off the defaulting Notch-dependent biliary specification and in turn, upon Wnt/β-catenin activation, stimulates HPC differentiation toward a hepatocyte phenotype (10). Moreover, in biliary repair, Notch signaling plays a wider role, as its effects extend to the generation of branching tubular structures, which requires the coordinated and integrated functions of both Notch-1 and Notch-2 (10, 41). Following biliary injury, if both Notch receptors are defective, then HPC as well as RDC generation are blocked (10, 41). In contrast, when only Notch-2 is defective, biliary specification of HPC in response to biliary injury is preserved, whereas the ability to rebuild biliary tubular structures is severely impaired, thus precluding liver capacity to restore the bile duct mass (41).
If defective Notch function hampers biliary repair, persistent Notch overactivation in the liver may result in liver epithelial cell dysplasia and malignant transformation. During ontogenesis, constitutive activation of Notch-2 intracellular domain (N2ICD) in hepatoblasts is often lethal at birth, leading to an architectural distortion of the liver lobule, which is characterized by ectopic biliary dysgenesis with cystic biliary structures, showing an early malignant-like phenotype (61, 135). In the adult mouse, constitutive expression of N2ICD promotes the expansion of the HPC/RDC compartment (63, 135). Furthermore, specific activation of N2ICD in the adult hepatocytes induces them to transdifferentiate into a biliary phenotype (ductular metaplasia), characterized by the downregulation of HNF4α and albumin (hepatocyte markers), together with the de novo expression of Sox-9 and HNF1β (biliary markers), and to assemble in microcystic structures scattered in the liver parenchyma (63). Both Notch effects on activation of HPC and hepatocyte ductular metaplasia may be oncogenic. In fact, development of dysplastic nodules and then of hepatocellular carcinoma (HCC) were observed in mice bearing Notch-dependent activation of HPC (136). These findings were confirmed in following studies showing that liver-specific N2ICD expression induces biliary hyperplasia and HCC in mice and that N2ICD accelerated DEN-induced HCC formation and growth, with eventual formation of cholangiocarcinoma (CCA); interestingly, in these models HCCs are SOX-9 positive, and have a less differentiated phenotype (29). In a mouse model tracing the hepatocyte cell fate, joint activation of Notch and AKT signaling cooperated to ductular metaplasia of normal hepatocytes, behaving as precursors of rapidly progressing, highly invasive intrahepatic CCA (38). A common observation derived from these studies supports the concept that the oncogenic effects of Notch are strictly dependent upon its interactions with other pathways, including p53, Snail, TNFα, NF-κB, Sox9, and IGF-II (132, 136).
Hedgehog.
Another important morphogenetic mechanism involved in organ development and oncogenesis is the Hedgehog (Hh) pathway. Notably, progenitor and mesenchymal cells respond to Hh stimulation, whereas hepatocytes do not possess this competence. During fetal liver development, Hh, together with its downstream effector Gli1, is expressed by ventral foregut endoderm, the embryonic structure that gives rise to the primordial liver bud; its expression is maintained also in later developmental stages. A reactivation of this signaling system is a fundamental step in the regenerative/reparative response of the liver to injury, and perturbations of Hh signaling have been described in congenital and in chronic cholangiopathies such as Meckel-Gruber syndrome, PBC (67, 106, 107), and BA (108). Human samples of PBC showed an increased immunohistochemical expression of Indian and Sonic Hh, along with Patched (receptor for Hh ligands), and the transactivator Gli2 on portal CK19+/OV6+ RDC compared with controls (67, 106). Activation of the Hh signaling promoted the accumulation of RDC with mesenchymal phenotypic traits characterized by S100A4 and Vimentin expression and increased cell motility (107), accompanied by the expansion of HPC compartment, resulting in the secretion of ECM components and deposition of fibrosis (108). Consistent with the role of PDGF-BB, acquisition of partial EMT features by RDCs was also stimulated by PDGF-BB expressed by both RDCs and portal MFs recruited by cholangiocytes. PDGF-BB stimulated proliferation of MFs in a PI3K/Akt-dependent manner (145); these, in turn, secreted Hh, in particular Sonic Hh ligands, which protected cholangiocytes from apoptosis (106). Overall, these observations indicate that following bile duct injury, activation of Hh signaling in the RDC compartment is an important player in mechanisms aimed to restore the biliary architecture. In addition to stimulate HPC/RDC, Hh signaling modulates several facets of biliary repair in a wide and coordinated fashion. Following liver injury, high amounts of Hh ligands are released within the portal space by different cellular sources, including injured or apoptotic cholangiocytes and hepatocytes, and the various infiltrating inflammatory cells, whereas Hh-responsive cells expand. Hh signaling stimulates activation of MFs (145) as well as the proliferation of liver sinusoidal endothelial cells, their assembly in vascular structures (141), and also their capillarization (143). Moreover, Hh signaling may perpetuate the fibrogenic response associated with the inflammatory response, as shown in human schistosomiasis, where macrophage-derived Hh ligands promots M2 phenotypic conversion of KCs and vascular remodeling (111).
Furthermore, Hh signaling plays a role also in CCA growth and metastasization. In fact, inhibition of Patched 1 receptor by cyclopamine blocked the proliferation of several CCA cell lines in the G1 phase (65). A number of recent papers underline the protumorigenic effects of Hh activation in cholangiocytes. Hh activation via Gli3 inhibited the transcription of death receptor 4, the receptor for TRAIL, increasing the viability and the resistance to apoptosis of CCA cells (77), and downmodulated the antiapoptotic protein Mcl-1 (39). Moreover, the proliferative and invasive potential of CCA cells could be hampered by inhibiting Hh signaling at different levels both in vitro (152) and in vivo (32, 39, 119).
Wingless/β-catenin.
Wingless (Wnt)/β-catenin is a morphogenetic signaling involved in the fetal development of different organs, including the liver, where it controls cell proliferation, cell fate determination, and cell polarity (20, 69, 131). During liver embryogenesis, Wnt ligands are differentially expressed in the portal mesenchyme, where they exert different effects on hepatoblast differentiation. Whereas Wnt3a stimulates the appearance of biliary-like structures around the portal area characterized by the expression of the early biliary marker CK19 (25, 56), Wnt5a induces in vitro a deletion of biliary structures, and its genetic inactivation in mice generates an accumulation of CK19+/Hnf1β+ epithelial biliary cells (72). Mice harboring a specific deletion of β-catenin in the liver showed bile duct hypoplasia (56), whereas conversely, its ectopic overactivation in hepatoblasts induced an abnormal enrichment in CK19+ biliary structures. Beside CHF, perturbation of the Wnt/β-catenin pathway was variably found in liver repair after biliary injury. An increased expression of Wnt3a and Wnt7 is also responsible for the proliferation of RDCs in a mouse model of obstructive cholestasis (BDL model) through the activation of cyclin D1 (124). However, during cholestasis, both autocrine and paracrine effects exerted by Wnt secreted by cholangiocytes contribute to histogenesis of RDCs; whereas Wnt7b and Wnt10a stimulate cholangiocyte proliferation through an autocrine mechanism that is independent from β-catenin, Wnt7a induces paracrine hepatocyte-to-biliary transdifferentiation, which instead is regulated by β-catenin (105). Noteworthy is that mice harboring a genetic inactivation of β-catenin restricted to the hepatocyte (Hep-Ctnnb1−/− mice, showed a markedly decreased HPC expansion following cholestatic damage by DDC intoxication (4), thus confirming that Wnt/β-catenin signaling is essential to build the liver reparative complex.
Hippo/Yes-associated protein pathway.
This is a highly pleiotropic signaling pathway that has a wide range of morphogenic effects in normal as well as in pathological conditions. During fetal development, Hippo modulates a variety of epithelial cell responses involved in organ size determination, epithelial-mesenchymal transition, the balance between cell proliferation and apoptosis, cell adhesion, cell-matrix interactions, and cell polarity. In response to damage and other stimuli, its sustained activation may lead to carcinogenesis and increased tumor invasiveness (78, 114). Yes-associated protein (YAP), the main effector of this signal pathway, and its companion transcriptional coactivator with PDZ-binding motif (TAZ), are both maintained in an inactivated, cytoplasmic state thanks to the activity of LATS1/2 kinases that preserves these two proteins in a phosphorylated form. Dephosphorylation of YAP/TAZ caused by cytoskeletal distress, such as increased cytoskeletal tension or increased matrix stiffening, allows nuclear translocation of both proteins, where they could bind and specifically activate the TEA domain transcription factor (TEAD) 1–4 (6, 115). TEAD activation induces the transcription of several downstream effectors, including CTGF, which in turn, plays a relevant role in development and progression of liver fibrosis (94).
The role of YAP in biliary diseases is gaining increasing attention also because of its possible role in the pathogenesis of CCA. In CCA, upregulation of YAP in the nucleus promoted a more aggressive phenotype in terms of enhanced proliferation, chemoresistance, and metastasization, a mechanism related to the activation of the antiapoptotic pathway PI3K/Akt induced by hyperexpression of IGF-I and miR-29c, whose integrated activity is orchestrated by the oncoprotein Gankirin in conjunction with TEAD. Again, in CCA, by interplaying with the microfibrillar-associated protein 5 (MAP-5), YAP promoted in vitro tubularization and angiogenesis and induced chemoresistance to Nutlin-3 (94). Compared with CCA, YAP involvement in biliary fibrosis is to date less defined. In mice carrying a specific inducible deletion of Yap, Yapflox/flox, a significant reduction in RDC was observed after BDL. This finding was associated with reduced expression levels of Survivin, a member of the inhibitor-of-apoptosis protein family regulating the cell proliferation/apoptosis balance. Increased nuclear expression of YAP was reported in RDCs of human samples obtained from PBC and PSC patients (7), an observation consistent with YAP’s ability to stimulate bile duct secretion of Osteopontin and Sonic Hh, thereby leading to recruitment, activation, and proliferation of portal MFs (93). Altogether, these data indicate YAP as a putative key regulator of biliary repair and carcinogenesis.
Conclusions and Future Perspectives
The growing attention toward cholangiopathies, otherwise viewed as rarely studied rare diseases, has been fostered by the opportunity to probe and model key aspects of liver repair mechanisms and the evidence that a series of “druggable” pathways are perturbed. Central to both physiological and pathological biliary repair is inflammation. In cholangiopathies, chronic inflammation results in the ductular reaction, and this ultimately leads to biliary fibrosis. RDCs have, in fact, the ability to produce a number of soluble mediators directing the recruitment of inflammatory (macrophages, NK cells, ILCs) and mesenchymal cells (PFs, HSCs, MFs), cooperating with the generation of the liver reparative complex. These cell types, in turn, have the ability to reactivate a series of morphogenetic pathways that are essential for a successful wound repair and for the re-stablishment of the proper biliary architecture. In this complex scenario, the study of pathophysiology of CHF, BA, and AGS have unveiled a close relationship of fundamental mechanisms (inflammation, biliary proliferation, and tubular patterning) with specific epithelial dysfunctions, affecting critical morphogenetic pathways (β-catenin, Hh, Notch, YAP/TAZ). The large body of data generated so far has been obtained from studies performed in genetically engineered rodent models faithfully recapitulating the human condition. Besides tracing in vivo the progressive natural course of the disease over the sequential stages, these animal models have offered the opportunity to test the translational relevance of molecular targeting. The recent advances in cell biology technologies have led to the development of patient-derived iPSC and the three-dimensional primary organoids, thus with the fascinating prospect to apply the experimental observations to the human setting. These new tools will inspire future mechanistic studies to improve our understanding of the mechanisms of biliary repair and will hopefully pave the way toward tailored strategies for preserving organ function and prolonging the survival of patients with chronic liver disease, likely including tissue replacement therapies.
GRANTS
This work was supported by Progetto Telethon GGP09189 and Progetto di Ricerca Ateneo (PRAT) 2011 (Grant no. CPD113799/11) to L. Fabris, Projects CARIPLO 2011-0470 and PRIN 2009ARYX4T_005 to M. Strazzabosco, Project Cariplo 2014-1099 to M. Cadamuro, National Institutes of Health Grants DK-079005 and DK-096096 to M. Strazzabosco, RO1-DK-101528 to C. Spirli, and DK-034989 (Silvio O. Conte Digestive Diseases Research Core Centers) to M. Strazzabosco, C. Spirli, and R. Fiorotto.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.F., C.S., M.C., R.F., and M.S. drafted manuscript; L.F., C.S., M.C., and M.S. edited and revised manuscript; L.F., C.S., M.C., R.F., and M.S. approved final version of manuscript; C.S., M.C., and R.F. prepared figures; L.F. and M.S. conceived and designed research.
REFERENCES
- 1.Alagille D, Odièvre M, Gautier M, Dommergues JP. Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr 86: 63–71, 1975. doi: 10.1016/S0022-3476(75)80706-2. [DOI] [PubMed] [Google Scholar]
- 2.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 81: 569–578, 1988. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ankoma-Sey V, Wang Y, Dai Z. Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology 31: 141–148, 2000. doi: 10.1002/hep.510310122. [DOI] [PubMed] [Google Scholar]
- 4.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology 47: 288–295, 2008. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 5.Artis D, Spits H. The biology of innate lymphoid cells. Nature 517: 293–301, 2015. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 6.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158: 157–170, 2014. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, Nayar SK, Cornish T, Alpini G, Bronk S, Pan D, Anders RA. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology 56: 1097–1107, 2012. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogdanos DP, Gao B, Gershwin ME. Liver immunology. Compr Physiol 3: 567–598, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Hérin M, De Baetselier P, Beschin A. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog 6: e1001045, 2010. doi: 10.1371/journal.ppat.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18: 572–579, 2012. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brivio S, Cadamuro M, Fabris L, Strazzabosco M. Epithelial-to-Mesenchymal Transition and Cancer Invasiveness: What Can We Learn from Cholangiocarcinoma? J Clin Med 4: 2028–2041, 2015. doi: 10.3390/jcm4121958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke ZD, Reed KR, Phesse TJ, Sansom OJ, Clarke AR, Tosh D. Liver zonation occurs through a beta-catenin-dependent, c-Myc-independent mechanism. Gastroenterology 136: 2316–2324.e3, 2009. doi: 10.1053/j.gastro.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 13.Cadamuro M, Nardo G, Indraccolo S, Dall’olmo L, Sambado L, Moserle L, Franceschet I, Colledan M, Massani M, Stecca T, Bassi N, Morton S, Spirli C, Fiorotto R, Fabris L, Strazzabosco M. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 58: 1042–1053, 2013. doi: 10.1002/hep.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Caligiuri A, LeSage G, Baiocchi L, Kanno N, Chowdury U, Phinizy JL, Glaser S, Alpini G, Benedetti A, Marucci L, Alvaro D, Papa E. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology 32: 17–25, 2000. doi: 10.1053/jhep.2000.8265. [DOI] [PubMed] [Google Scholar]
- 14.Cardinale V, Wang Y, Carpino G, Reid LM, Gaudio E, Alvaro D. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology 55: 2041–2042, 2012. doi: 10.1002/hep.25587. [DOI] [PubMed] [Google Scholar]
- 15.Carpino G, Puca R, Cardinale V, Renzi A, Scafetta G, Nevi L, Rossi M, Berloco PB, Ginanni Corradini S, Reid LM, Maroder M, Gaudio E, Alvaro D. Peribiliary Glands as a Niche of Extrapancreatic Precursors Yielding Insulin-Producing Cells in Experimental and Human Diabetes. Stem Cells 34: 1332–1342, 2016. doi: 10.1002/stem.2311. [DOI] [PubMed] [Google Scholar]
- 16.Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol 36: 200–209, 2002. doi: 10.1016/S0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 17.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol 31: 31–37, 2014. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell 54: 281–288, 2014. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu AS, Diaz R, Hui JJ, Yanger K, Zong Y, Alpini G, Stanger BZ, Wells RG. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 53: 1685–1695, 2011. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell 149: 1192–1205, 2012. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology 41: 809–818, 2005. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 22.Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology 35: 1093–1103, 2002. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- 23.Davenport M. Biliary atresia. Semin Pediatr Surg 14: 42–48, 2005. doi: 10.1053/j.sempedsurg.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 24.de Groen PC, Vroman B, Laakso K, LaRusso NF. Characterization and growth regulation of a rat intrahepatic bile duct epithelial cell line under hormonally defined, serum-free conditions. In Vitro Cell Dev Biol Anim 34: 704–710, 1998. doi: 10.1007/s11626-998-0066-1. [DOI] [PubMed] [Google Scholar]
- 25.Decaens T, Godard C, de Reyniès A, Rickman DS, Tronche F, Couty JP, Perret C, Colnot S. Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology 47: 247–258, 2008. doi: 10.1002/hep.21952. [DOI] [PubMed] [Google Scholar]
- 26.Demetris AJ, Seaberg EC, Wennerberg A, Ionellie J, Michalopoulos G. Ductular reaction after submassive necrosis in humans. Special emphasis on analysis of ductular hepatocytes. Am J Pathol 149: 439–448, 1996. [PMC free article] [PubMed] [Google Scholar]
- 27.Desmet V, Roskams T, Van Eyken P. Ductular reaction in the liver. Pathol Res Pract 191: 513–524, 1995. doi: 10.1016/S0344-0338(11)80870-8. [DOI] [PubMed] [Google Scholar]
- 28.Díaz R, Kim JW, Hui JJ, Li Z, Swain GP, Fong KS, Csiszar K, Russo PA, Rand EB, Furth EE, Wells RG. Evidence for the epithelial to mesenchymal transition in biliary atresia fibrosis. Hum Pathol 39: 102–115, 2008. doi: 10.1016/j.humpath.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Dill MT, Tornillo L, Fritzius T, Terracciano L, Semela D, Bettler B, Heim MH, Tchorz JS. Constitutive Notch2 signaling induces hepatic tumors in mice. Hepatology 57: 1607–1619, 2013. doi: 10.1002/hep.26165. [DOI] [PubMed] [Google Scholar]
- 30.Dranoff JA, Wells RG. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology 51: 1438–1444, 2010. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659, 1986. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 32.El Khatib M, Kalnytska A, Palagani V, Kossatz U, Manns MP, Malek NP, Wilkens L, Plentz RR. Inhibition of hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis of cholangiocellular carcinoma. Hepatology 57: 1035–1045, 2013. doi: 10.1002/hep.26147. [DOI] [PubMed] [Google Scholar]
- 33.Emerick KM, Rand EB, Goldmuntz E, Krantz ID, Spinner NB, Piccoli DA. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology 29: 822–829, 1999. doi: 10.1002/hep.510290331. [DOI] [PubMed] [Google Scholar]
- 34.Fabris L, Strazzabosco M, Crosby HA, Ballardini G, Hubscher SG, Kelly DA, Neuberger JM, Strain AJ, Joplin R. Characterization and isolation of ductular cells coexpressing neural cell adhesion molecule and Bcl-2 from primary cholangiopathies and ductal plate malformations. Am J Pathol 156: 1599–1612, 2000. doi: 10.1016/S0002-9440(10)65032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fabris L, Cadamuro M, Guido M, Spirli C, Fiorotto R, Colledan M, Torre G, Alberti D, Sonzogni A, Okolicsanyi L, Strazzabosco M. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol 171: 641–653, 2007. doi: 10.2353/ajpath.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fabris L, Strazzabosco M. Epithelial-mesenchymal interactions in biliary diseases. Semin Liver Dis 31: 011–032, 2011. doi: 10.1055/s-0031-1272832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabris L, Brivio S, Cadamuro M, Strazzabosco M. Revisiting Epithelial-to-Mesenchymal Transition in Liver Fibrosis: Clues for a Better Understanding of the “Reactive” Biliary Epithelial Phenotype. Stem Cells Int 2016: 2953727, 2016. doi: 10.1155/2016/2953727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, Willenbring H. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest 122: 2911–2915, 2012. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fingas CD, Mertens JC, Razumilava N, Sydor S, Bronk SF, Christensen JD, Rizvi SH, Canbay A, Treckmann JW, Paul A, Sirica AE, Gores GJ. Polo-like kinase 2 is a mediator of hedgehog survival signaling in cholangiocarcinoma. Hepatology 58: 1362–1374, 2013. doi: 10.1002/hep.26484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiorotto R, Scirpo R, Trauner M, Fabris L, Hoque R, Spirli C, Strazzabosco M. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-κB-mediated inflammatory response in mice. Gastroenterology 141: 1498–1508.e5, 2011. doi: 10.1053/j.gastro.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiorotto R, Raizner A, Morell CM, Torsello B, Scirpo R, Fabris L, Spirli C, Strazzabosco M. Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice. J Hepatol 59: 124–130, 2013. doi: 10.1016/j.jhep.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiorotto R, Villani A, Kourtidis A, Scirpo R, Amenduni M, Geibel PJ, Cadamuro M, Spirli C, Anastasiadis PZ, Strazzabosco M. The cystic fibrosis transmembrane conductance regulator controls biliary epithelial inflammation and permeability by regulating Src tyrosine kinase activity. Hepatology 64: 2118–2134, 2016. doi: 10.1002/hep.28817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franchitto A, Onori P, Renzi A, Carpino G, Mancinelli R, Alvaro D, Gaudio E. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann Transl Med 1: 27, 2013. doi: 10.3978/j.issn.2305-5839.2012.10.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 41: 528–537, 2004. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Francis H, Franchitto A, Ueno Y, Glaser S, DeMorrow S, Venter J, Gaudio E, Alvaro D, Fava G, Marzioni M, Vaculin B, Alpini G. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest 87: 473–487, 2007. doi: 10.1038/labinvest.3700533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, Radtke F, Schmid RM, Siveke JT. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology 48: 607–616, 2008. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 47.Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology 61: 382–392, 2015. doi: 10.1002/hep.27268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gigliozzi A, Alpini G, Baroni GS, Marucci L, Metalli VD, Glaser SS, Francis H, Mancino MG, Ueno Y, Barbaro B, Benedetti A, Attili AF, Alvaro D. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology 127: 1198–1209, 2004. doi: 10.1053/j.gastro.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 50.Guldiken N, Kobazi Ensari G, Lahiri P, Couchy G, Preisinger C, Liedtke C, Zimmermann HW, Ziol M, Boor P, Zucman-Rossi J, Trautwein C, Strnad P. Keratin 23 is a stress-inducible marker of mouse and human ductular reaction in liver disease. J Hepatol 65: 552–559, 2016. doi: 10.1016/j.jhep.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 51.Hall C, Sato K, Wu N, Zhou T, Kyritsi K, Meng F, Glaser S, Alpini G. Regulators of Cholangiocyte Proliferation. Gene Expr 17: 155–171, 2017. doi: 10.3727/105221616X692568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, Fahy RJ, Crotty TB, Hirani N, Flynn RJ, Voehringer D, McKenzie AN, Donnelly SC, Fallon PG. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci USA 111: 367–372, 2014. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harada K, Sato Y, Ikeda H, Isse K, Ozaki S, Enomae M, Ohama K, Katayanagi K, Kurumaya H, Matsui A, Nakanuma Y. Epithelial-mesenchymal transition induced by biliary innate immunity contributes to the sclerosing cholangiopathy of biliary atresia. J Pathol 217: 654–664, 2009. doi: 10.1002/path.2488. [DOI] [PubMed] [Google Scholar]
- 54.He Y, Wu GD, Sadahiro T, Noh SI, Wang H, Talavera D, Wang H, Vierling JM, Klein AS. Interaction of CD44 and hyaluronic acid enhances biliary epithelial proliferation in cholestatic livers. Am J Physiol Gastrointest Liver Physiol 295: G305–G312, 2008. doi: 10.1152/ajpgi.90229.2008. [DOI] [PubMed] [Google Scholar]
- 55.Heymann F, Hammerich L, Storch D, Bartneck M, Huss S, Rüsseler V, Gassler N, Lira SA, Luedde T, Trautwein C, Tacke F. Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology 55: 898–909, 2012. doi: 10.1002/hep.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hussain SZ, Sneddon T, Tan X, Micsenyi A, Michalopoulos GK, Monga SP. Wnt impacts growth and differentiation in ex vivo liver development. Exp Cell Res 292: 157–169, 2004. doi: 10.1016/j.yexcr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 57.Ikenaga N, Peng ZW, Vaid KA, Liu SB, Yoshida S, Sverdlov DY, Mikels-Vigdal A, Smith V, Schuppan D, Popov YV. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017 Jan 10. [Epub ahead of print]. doi: 10.1136/gutjnl-2016-312473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishii M, Vroman B, LaRusso NF. Morphologic demonstration of receptor-mediated endocytosis of epidermal growth factor by isolated bile duct epithelial cells. Gastroenterology 98: 1284–1291, 1990. doi: 10.1016/0016-5085(90)90346-3. [DOI] [PubMed] [Google Scholar]
- 59.Isomoto H, Kobayashi S, Werneburg NW, Bronk SF, Guicciardi ME, Frank DA, Gores GJ. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology 42: 1329–1338, 2005. doi: 10.1002/hep.20966. [DOI] [PubMed] [Google Scholar]
- 60.Isse K, Harada K, Nakanuma Y. IL-8 expression by biliary epithelial cells is associated with neutrophilic infiltration and reactive bile ductules. Liver Int 27: 672–680, 2007. doi: 10.1111/j.1478-3231.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- 61.Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, Liu X, Xu J, Wang P, Paik YH, Meng F, Asagiri M, Murray LA, Hofmann AF, Iida T, Glass CK, Brenner DA, Kisseleva T. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci USA 111: E3297–E3305, 2014. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, Browning B, Michaelson JS, Baestcher M, Wang B, Bissell DM, Burkly LC. TWEAK induces liver progenitor cell proliferation. J Clin Invest 115: 2330–2340, 2005. [Erratum. J Clin Invest 115: 2955, 2005.] doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeliazkova P, Jörs S, Lee M, Zimber-Strobl U, Ferrer J, Schmid RM, Siveke JT, Geisler F. Canonical Notch2 signaling determines biliary cell fates of embryonic hepatoblasts and adult hepatocytes independent of Hes1. Hepatology 57: 2469–2479, 2013. doi: 10.1002/hep.26254. [DOI] [PubMed] [Google Scholar]
- 64.Jhandier MN, Kruglov EA, Lavoie EG, Sévigny J, Dranoff JA. Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem 280: 22986–22992, 2005. doi: 10.1074/jbc.M412371200. [DOI] [PubMed] [Google Scholar]
- 65.Jinawath A, Akiyama Y, Sripa B, Yuasa Y. Dual blockade of the Hedgehog and ERK1/2 pathways coordinately decreases proliferation and survival of cholangiocarcinoma cells. J Cancer Res Clin Oncol 133: 271–278, 2007. doi: 10.1007/s00432-006-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joplin R, Hishida T, Tsubouchi H, Daikuhara Y, Ayres R, Neuberger JM, Strain AJ. Human intrahepatic biliary epithelial cells proliferate in vitro in response to human hepatocyte growth factor. J Clin Invest 90: 1284–1289, 1992. doi: 10.1172/JCI115992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology 45: 1091–1096, 2007. doi: 10.1002/hep.21660. [DOI] [PubMed] [Google Scholar]
- 68.Karin D, Koyama Y, Brenner D, Kisseleva T. The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis. Differentiation 92: 84–92, 2016. doi: 10.1016/j.diff.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 41: 793–799, 2009. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katoonizadeh A, Poustchi H, Malekzadeh R. Hepatic progenitor cells in liver regeneration: current advances and clinical perspectives. Liver Int 34: 1464–1472, 2014. doi: 10.1111/liv.12573. [DOI] [PubMed] [Google Scholar]
- 71.Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest 83: 163–173, 2003. doi: 10.1097/01.LAB.0000054178.01162.E4. [DOI] [PubMed] [Google Scholar]
- 72.Kiyohashi K, Kakinuma S, Kamiya A, Sakamoto N, Nitta S, Yamanaka H, Yoshino K, Fujiki J, Murakawa M, Kusano-Kitazume A, Shimizu H, Okamoto R, Azuma S, Nakagawa M, Asahina Y, Tanimizu N, Kikuchi A, Nakauchi H, Watanabe M. Wnt5a signaling mediates biliary differentiation of fetal hepatic stem/progenitor cells in mice. Hepatology 57: 2502–2513, 2013. doi: 10.1002/hep.26293. [DOI] [PubMed] [Google Scholar]
- 73.Kodama Y, Hijikata M, Kageyama R, Shimotohno K, Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology 127: 1775–1786, 2004. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell 160: 816–827, 2015. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kruglov EA, Nathanson RA, Nguyen T, Dranoff JA. Secretion of MCP-1/CCL2 by bile duct epithelia induces myofibroblastic transdifferentiation of portal fibroblasts. Am J Physiol Gastrointest Liver Physiol 290: G765–G771, 2006. doi: 10.1152/ajpgi.00308.2005. [DOI] [PubMed] [Google Scholar]
- 76.Kuramitsu K, Sverdlov DY, Liu SB, Csizmadia E, Burkly L, Schuppan D, Hanto DW, Otterbein LE, Popov Y. Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol 183: 182–194, 2013. doi: 10.1016/j.ajpath.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurita S, Mott JL, Almada LL, Bronk SF, Werneburg NW, Sun SY, Roberts LR, Fernandez-Zapico ME, Gores GJ. GLI3-dependent repression of DR4 mediates hedgehog antagonism of TRAIL-induced apoptosis. Oncogene 29: 4848–4858, 2010. doi: 10.1038/onc.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA 109: E2441–E2450, 2012. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology 127: 1565–1577, 2004. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 80.Lazaridis KN, LaRusso NF. The Cholangiopathies. Mayo Clin Proc 90: 791–800, 2015. doi: 10.1016/j.mayocp.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee K, Battini L, Gusella GL. Cilium, centrosome and cell cycle regulation in polycystic kidney disease. Biochim Biophys Acta 1812: 1263–1271, 2011. doi: 10.1016/j.bbadis.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lefkowitch JH. Bile ductular cholestasis: an ominous histopathologic sign related to sepsis and “cholangitis lenta”. Hum Pathol 13: 19–24, 1982. doi: 10.1016/S0046-8177(82)80134-2. [DOI] [PubMed] [Google Scholar]
- 83.Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev 14: 582–590, 2004. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 84.Li L, Krantz ID, Deng Y, Genin A, Banta AB, Collins CC, Qi M, Trask BJ, Kuo WL, Cochran J, Costa T, Pierpont ME, Rand EB, Piccoli DA, Hood L, Spinner NB. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 16: 243–251, 1997. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 85.Li J, Razumilava N, Gores GJ, Walters S, Mizuochi T, Mourya R, Bessho K, Wang YH, Glaser SS, Shivakumar P, Bezerra JA. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J Clin Invest 124: 3241–3251, 2014. doi: 10.1172/JCI73742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang Y, Jie Z, Hou L, Aguilar-Valenzuela R, Vu D, Soong L, Sun J. IL-33 induces nuocytes and modulates liver injury in viral hepatitis. J Immunol 190: 5666–5675, 2013. doi: 10.4049/jimmunol.1300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol 13: 389–396, 2002. doi: 10.1016/S1084952102001258. [DOI] [PubMed] [Google Scholar]
- 88.Libbrecht L, Spinner NB, Moore EC, Cassiman D, Van Damme-Lombaerts R, Roskams T. Peripheral bile duct paucity and cholestasis in the liver of a patient with Alagille syndrome: further evidence supporting a lack of postnatal bile duct branching and elongation. Am J Surg Pathol 29: 820–826, 2005. doi: 10.1097/01.pas.0000161325.36348.25. [DOI] [PubMed] [Google Scholar]
- 89.Locatelli L, Cadamuro M, Spirlì C, Fiorotto R, Lecchi S, Morell CM, Popov Y, Scirpo R, De Matteis M, Amenduni M, Pietrobattista A, Torre G, Schuppan D, Fabris L, Strazzabosco M. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology 63: 965–982, 2016. doi: 10.1002/hep.28382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, Matthews RP, Pack M. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development 131: 5753–5766, 2004. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- 91.Lozier J, McCright B, Gridley T. Notch signaling regulates bile duct morphogenesis in mice. PLoS One 3: e1851, 2008. doi: 10.1371/journal.pone.0001851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lua I, Li Y, Zagory JA, Wang KS, French SW, Sévigny J, Asahina K. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J Hepatol 64: 1137–1146, 2016. doi: 10.1016/j.jhep.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Machado MV, Michelotti GA, Pereira TA, Xie G, Premont R, Cortez-Pinto H, Diehl AM. Accumulation of duct cells with activated YAP parallels fibrosis progression in non-alcoholic fatty liver disease. J Hepatol 63: 962–970, 2015. doi: 10.1016/j.jhep.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marti P, Stein C, Blumer T, Abraham Y, Dill MT, Pikiolek M, Orsini V, Jurisic G, Megel P, Makowska Z, Agarinis C, Tornillo L, Bouwmeester T, Ruffner H, Bauer A, Parker CN, Schmelzle T, Terracciano LM, Heim MH, Tchorz JS. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology 62: 1497–1510, 2015. doi: 10.1002/hep.27992. [DOI] [PubMed] [Google Scholar]
- 95.Marvie P, Lisbonne M, L’helgoualc’h A, Rauch M, Turlin B, Preisser L, Bourd-Boittin K, Théret N, Gascan H, Piquet-Pellorce C, Samson M. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med 14: 1726–1739, 2010. doi: 10.1111/j.1582-4934.2009.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsumoto K, Fujii H, Michalopoulos G, Fung JJ, Demetris AJ. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology 20: 376–382, 1994. doi: 10.1002/hep.1840200217. [DOI] [PubMed] [Google Scholar]
- 97.McDaniell R, Warthen DM, Sanchez-Lara PA, Pai A, Krantz ID, Piccoli DA, Spinner NB. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am J Hum Genet 79: 169–173, 2006. doi: 10.1086/505332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Medzhitov R. Origin and physiological roles of inflammation. Nature 454: 428–435, 2008. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 99.Morell CM, Fiorotto R, Fabris L, Strazzabosco M. Notch signalling beyond liver development: emerging concepts in liver repair and oncogenesis. Clin Res Hepatol Gastroenterol 37: 447–454, 2013. doi: 10.1016/j.clinre.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 100.Müller CA, Markovic-Lipkovski J, Klatt T, Gamper J, Schwarz G, Beck H, Deeg M, Kalbacher H, Widmann S, Wessels JT, Becker V, Müller GA, Flad T. Human alpha-defensins HNPs-1, -2, and -3 in renal cell carcinoma: influences on tumor cell proliferation. Am J Pathol 160: 1311–1324, 2002. doi: 10.1016/S0002-9440(10)62558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. A mechanism for regulating pulmonary inflammation and fibrosis: The integrin αvβ6 binds and activates latent TGF β1. Cell 96: 319–328, 1999. doi: 10.1016/S0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 102.Nagula S, Jain D, Groszmann RJ, Garcia-Tsao G. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol 44: 111–117, 2006. doi: 10.1016/j.jhep.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 103.Nakanuma Y, Harada K, Sato Y, Ikeda H. Recent progress in the etiopathogenesis of pediatric biliary disease, particularly Caroli’s disease with congenital hepatic fibrosis and biliary atresia. Histol Histopathol 25: 223–235, 2010. doi: 10.14670/HH-25.223. [DOI] [PubMed] [Google Scholar]
- 104.Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet 16: 235–242, 1997. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 105.Okabe H, Yang J, Sylakowski K, Yovchev M, Miyagawa Y, Nagarajan S, Chikina M, Thompson M, Oertel M, Baba H, Monga SP, Nejak-Bowen KN. Wnt signaling regulates hepatobiliary repair following cholestatic liver injury in mice. Hepatology 64: 1652–1666, 2016. doi: 10.1002/hep.28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, Schuppan D, Diehl AM. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut 57: 1275–1282, 2008. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 107.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, Syn WK, Baroni GS, Benedetti A, Schuppan D, Diehl AM. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest 118: 3331–3342, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Omenetti A, Bass LM, Anders RA, Clemente MG, Francis H, Guy CD, McCall S, Choi SS, Alpini G, Schwarz KB, Diehl AM, Whitington PF. Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology 53: 1246–1258, 2011. doi: 10.1002/hep.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol 14: 181–194, 2014. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 110.Peng ZW, Ikenaga N, Liu SB, Sverdlov DY, Vaid KA, Dixit R, Weinreb PH, Violette S, Sheppard D, Schuppan D, Popov Y. Integrin αvβ6 critically regulates hepatic progenitor cell function and promotes ductular reaction, fibrosis, and tumorigenesis. Hepatology 63: 217–232, 2016. doi: 10.1002/hep.28274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pereira TA, Xie G, Choi SS, Syn WK, Voieta I, Lu J, Chan IS, Swiderska M, Amaral KB, Antunes CM, Secor WE, Witek RP, Lambertucci JR, Pereira FL, Diehl AM. Macrophage-derived Hedgehog ligands promotes fibrogenic and angiogenic responses in human schistosomiasis mansoni. Liver Int 33: 149–161, 2013. doi: 10.1111/liv.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pereira TA, Syn WK, Machado MV, Vidigal PV, Resende V, Voieta I, Xie G, Otoni A, Souza MM, Santos ET, Chan IS, Trindade GV, Choi SS, Witek RP, Pereira FE, Secor WE, Andrade ZA, Lambertucci JR, Diehl AM. Schistosome-induced cholangiocyte proliferation and osteopontin secretion correlate with fibrosis and portal hypertension in human and murine schistosomiasis mansoni. Clin Sci (Lond) 129: 875–883, 2015. doi: 10.1042/CS20150117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pi L, Robinson PM, Jorgensen M, Oh SH, Brown AR, Weinreb PH, Trinh TL, Yianni P, Liu C, Leask A, Violette SM, Scott EW, Schultz GS, Petersen BE. Connective tissue growth factor and integrin αvβ6: a new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology 61: 678–691, 2015. doi: 10.1002/hep.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Piccolo S, Cordenonsi M, Dupont S. Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res 19: 4925–4930, 2013. doi: 10.1158/1078-0432.CCR-12-3172. [DOI] [PubMed] [Google Scholar]
- 115.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 94: 1287–1312, 2014. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 116.Popov Y, Patsenker E, Stickel F, Zaks J, Bhaskar KR, Niedobitek G, Kolb A, Friess H, Schuppan D. Integrin alphavbeta6 is a marker of the progression of biliary and portal liver fibrosis and a novel target for antifibrotic therapies. J Hepatol 48: 453–464, 2008. doi: 10.1016/j.jhep.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 117.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA 109: E3186–E3195, 2012. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, Purdie DM, Demetris AJ, Clouston AD. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology 133: 80–90, 2007. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 119.Riedlinger D, Bahra M, Boas-Knoop S, Lippert S, Bradtmöller M, Guse K, Seehofer D, Bova R, Sauer IM, Neuhaus P, Koch A, Kamphues C. Hedgehog pathway as a potential treatment target in human cholangiocarcinoma. J Hepatobiliary Pancreat Sci 21: 607–615, 2014. doi: 10.1002/jhbp.107. [DOI] [PubMed] [Google Scholar]
- 120.Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis 23: 385–396, 2003. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 121.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 39: 1739–1745, 2004. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 122.Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 130: 1807–1821, 2006. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 123.Rygiel KA, Robertson H, Marshall HL, Pekalski M, Zhao L, Booth TA, Jones DE, Burt AD, Kirby JA. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab Invest 88: 112–123, 2008. doi: 10.1038/labinvest.3700704. [DOI] [PubMed] [Google Scholar]
- 124.Sackett SD, Gao Y, Shin S, Esterson YB, Tsingalia A, Hurtt RS, Brondell K, Kaestner KH, Greenbaum LE. Foxl1 promotes liver repair following cholestatic injury in mice. Lab Invest 89: 1387–1396, 2009. doi: 10.1038/labinvest.2009.103. [DOI] [PubMed] [Google Scholar]
- 125.Scirpo R, Fiorotto R, Villani A, Amenduni M, Spirli C, Strazzabosco M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-γ limits NF-κB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 62: 1551–1562, 2015. doi: 10.1002/hep.28000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shivakumar P, Sabla GE, Whitington P, Chougnet CA, Bezerra JA. Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia. J Clin Invest 119: 2281–2290, 2009. doi: 10.1172/JCI38879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spirli C, Locatelli L, Morell CM, Fiorotto R, Morton SD, Cadamuro M, Fabris L, Strazzabosco M. Protein kinase A-dependent pSer(675) -β-catenin, a novel signaling defect in a mouse model of congenital hepatic fibrosis. Hepatology 58: 1713–1723, 2013. doi: 10.1002/hep.26554. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Strazzabosco M, Mennone A, Boyer JL. Intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest 87: 1503–1512, 1991. doi: 10.1172/JCI115160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol 39, Suppl 2: S90–S102, 2005. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 131.Strazzabosco M, Fabris L. Development of the bile ducts: essentials for the clinical hepatologist. J Hepatol 56: 1159–1170, 2012. doi: 10.1016/j.jhep.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Strazzabosco M, Fabris L. Notch signaling in hepatocellular carcinoma: guilty in association! Gastroenterology 143: 1430–1434, 2012. doi: 10.1053/j.gastro.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol 60: 1090–1096, 2014. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 134.Tanimizu N, Miyajima A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J Cell Sci 117: 3165–3174, 2004. doi: 10.1242/jcs.01169. [DOI] [PubMed] [Google Scholar]
- 135.Tchorz JS, Kinter J, Müller M, Tornillo L, Heim MH, Bettler B. Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology 50: 871–879, 2009. doi: 10.1002/hep.23048. [DOI] [PubMed] [Google Scholar]
- 136.Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, Solé M, Thung S, Stanger BZ, Llovet JM. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology 143: 1660–1669.e7, 2012. doi: 10.1053/j.gastro.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet 12: 2703–2710, 2003. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- 138.Watson GH, Miller V. Arteriohepatic dysplasia: familial pulmonary arterial stenosis with neonatal liver disease. Arch Dis Child 48: 459–466, 1973. doi: 10.1136/adc.48.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett 559: 107–110, 2004. doi: 10.1016/S0014-5793(04)00037-7. [DOI] [PubMed] [Google Scholar]
- 140.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 26: 117–129, 2007. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 141.Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, Cheong Y, Fearing CM, Agboola KM, Chen W, Diehl AM. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology 136: 320–330.e2, 2009. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, Manns MP, Müller W, Trautwein C. Interleukin-6/glycoprotein 130-dependent pathways are protective during liver regeneration. J Biol Chem 278: 11281–11288, 2003. doi: 10.1074/jbc.M208470200. [DOI] [PubMed] [Google Scholar]
- 143.Xie G, Choi SS, Syn WK, Michelotti GA, Swiderska M, Karaca G, Chan IS, Chen Y, Diehl AM. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut 62: 299–309, 2013. doi: 10.1136/gutjnl-2011-301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yamada D, Rizvi S, Razumilava N, Bronk SF, Davila JI, Champion MD, Borad MJ, Bezerra JA, Chen X, Gores GJ. IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism. Hepatology 61: 1627–1642, 2015. doi: 10.1002/hep.27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol 48: 98–106, 2008. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang L, Seki E. Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol 3: 138, 2012. doi: 10.3389/fphys.2012.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yasoshima M, Kono N, Sugawara H, Katayanagi K, Harada K, Nakanuma Y. Increased expression of interleukin-6 and tumor necrosis factor-alpha in pathologic biliary epithelial cells: in situ and culture study. Lab Invest 78: 89–100, 1998. [PubMed] [Google Scholar]
- 148.Yavrom S, Chen L, Xiong S, Wang J, Rippe RA, Tsukamoto H. Peroxisome proliferator-activated receptor gamma suppresses proximal alpha1(I) collagen promoter via inhibition of p300-facilitated NF-I binding to DNA in hepatic stellate cells. J Biol Chem 280: 40650–40659, 2005. doi: 10.1074/jbc.M510094200. [DOI] [PubMed] [Google Scholar]
- 149.Zhang MZ, Mai W, Li C, Cho SY, Hao C, Moeckel G, Zhao R, Kim I, Wang J, Xiong H, Wang H, Sato Y, Wu Y, Nakanuma Y, Lilova M, Pei Y, Harris RC, Li S, Coffey RJ, Sun L, Wu D, Chen XZ, Breyer MD, Zhao ZJ, McKanna JA, Wu G. PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc Natl Acad Sci USA 101: 2311–2316, 2004. doi: 10.1073/pnas.0400073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zimmermann HW, Seidler S, Gassler N, Nattermann J, Luedde T, Trautwein C, Tacke F. Interleukin-8 is activated in patients with chronic liver diseases and associated with hepatic macrophage accumulation in human liver fibrosis. PLoS One 6: e21381, 2011. doi: 10.1371/journal.pone.0021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ. Notch signaling controls liver development by regulating biliary differentiation. Development 136: 1727–1739, 2009. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zuo M, Rashid A, Churi C, Vauthey JN, Chang P, Li Y, Hung MC, Li D, Javle M. Novel therapeutic strategy targeting the Hedgehog signalling and mTOR pathways in biliary tract cancer. Br J Cancer 112: 1042–1051, 2015. doi: 10.1038/bjc.2014.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zweers SJ, Shiryaev A, Komuta M, Vesterhus M, Hov JR, Perugorria MJ, de Waart DR, Chang JC, Tol S, Te Velde AA, de Jonge WJ, Banales JM, Roskams T, Beuers U, Karlsen TH, Jansen PL, Schaap FG. Elevated interleukin-8 in bile of patients with primary sclerosing cholangitis. Liver Int 36: 1370–1377, 2016. doi: 10.1111/liv.13092. [DOI] [PubMed] [Google Scholar]