Abstract
The intestinal-renal axis for endogenous arginine synthesis is an interorgan process in which citrulline produced in the small intestine is utilized by the kidney for arginine synthesis. The function of this axis in neonates has been questioned because during this period the enzymes needed for arginine synthesis argininosuccinate synthase (ASS1) and lyase (ASL) are present in the gut. However, evidence of high plasma citrulline concentrations in neonates suggests otherwise. We quantified in vivo citrulline production in premature (10 days preterm), neonatal (7 days old), and young pigs (35 days old) using citrulline tracers. Neonatal pigs had higher fluxes (69 µmol·kg−1·h−1, P < 0.001) than premature and young pigs (43 and 45 µmol·kg−1·h−1, respectively). Plasma citrulline concentration was also greater in neonatal pigs than in the other age groups. We also determined the site of synthesis and utilization of citrulline in neonatal and young pigs by measuring organ balances across the gut and the kidney. Citrulline was released from the gut and utilized by the kidney in both neonatal and young pigs. The abundance and localization of the enzymes involved in the synthesis and utilization were determined in intestinal and kidney tissue. Despite the presence of ASS1 and ASL in the neonatal small intestine, the lack of colocalization with the enzymes that produce citrulline results in the release of citrulline by the PDV and its utilization by the kidney to produce arginine. In conclusion, the intestinal-renal axis for arginine synthesis is present in the neonatal pig.
Keywords: arginine, citrulline, interorgan, kidney, small intestine
arginine is an amino acid involved in many physiological processes besides protein synthesis (24). During rapid growth, arginine is considered a conditionally essential amino acid in many species, including pigs, because the high arginine demand for protein, polyamines, creatine, and nitric oxide synthesis exceeds its endogenous production (30). Endogenous arginine synthesis relies on a interorgan process in which citrulline is synthesized by the gut and utilized by two renal enzymes [argininosuccinate synthase (ASS1) and lyase (ASL)] to produce arginine (11, 33) (Fig. 1). Because ASS1 and ASL are present in the small intestine of neonates of different species (3, 9, 15, 18), it is believed that during this period arginine synthesis takes place in the gut, and thus the intestinal-renal axis for “de novo” arginine synthesis is not functional in early life (2, 15, 23). The release of arginine by the portal drained viscera (PDV) (31, 35), together with the in vitro synthesis of arginine by intestinal homogenates (15) and by isolated enterocytes, seems to support this concept (3, 34).
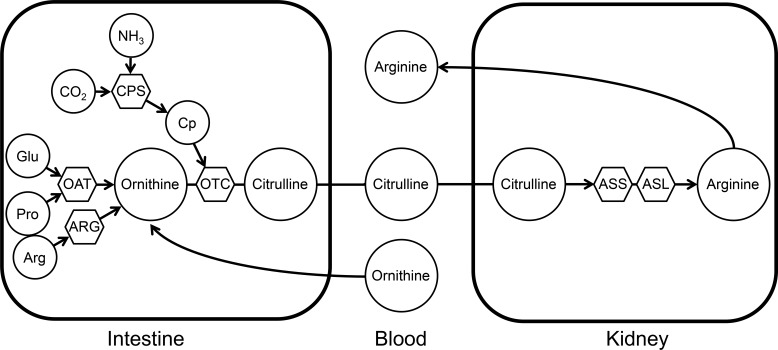
Fig. 1.
Enzymes and precursors involved in the synthesis and utilization of citrulline. The synthesis of citrulline takes place in the small intestine, where ornithine aminotransferase (OAT), carbamoylphosphate synthase I (CPS), ornithine transcarbamylase (OTC), and arginase II (ARG) are present (hexagons). Different precursors may be utilized for citrulline synthesis: glutamate (Glu), proline (Pro), arginine (Arg), and ornithine (circles). Carbamylphosphate (Cp) is produced by the condensation of ammonia (NH3) and carbon dioxide (CO2). In the kidney, by action of argininosuccinate synthase (ASS1) and lyase (ASL), citrulline is converted into arginine, which then enters the circulation.
Circulating citrulline has many sources. There is a small contribution from nitric oxide synthesis and the recycling of posttranslationally modified arginine, but the majority of citrulline originates from the carbamylation of ornithine originating from plasma or produced locally by ornithine aminotransferase (OAT; Fig. 1). The enzymes needed for the synthesis of citrulline from ornithine [carbomyl phosphate synthase (CPS1) and ornithine transcarbamylase (OTC)] are present only in enterocytes (Fig. 1) and hepatocytes. Under normal conditions, due to channeling within the urea cycle, there is no net production or export of citrulline by the liver (6). However, urea cycle disorders can lead to a large increase in plasma citrulline concentration and fluxes due to hepatic export of citrulline (25). Regardless, under normal conditions, most citrulline originates from the small intestine, and for this reason plasma citrulline has been proposed as a marker for gut mass and function, not only in adult subjects (7) but also in neonates (16). The presence of circulating citrulline and the citrulline fluxes measured in fed and fasted neonatal pigs (22) and humans (8) directly conflicts with the concept of a nonfunctional intestinal-renal axis for arginine synthesis in the neonate. To address this apparent paradox, we measured citrulline production in premature, neonatal, and young pigs, thus testing the hypothesis that the intestinal-renal axis for de novo arginine synthesis is present in the neonatal pig. In addition, we determined the site of synthesis and utilization of citrulline in neonatal and young pigs as well as the abundance and localization of the enzymes involved in the synthesis of citrulline and its conversion into arginine.
MATERIALS AND METHODS
General information.
Domestic, conventionally reared cross-bred pigs were purchased from a local commercial swine farm. Premature pigs were obtained at ~90% gestation (day 103) by Cesarean section, as described previously (13). Naturally delivered neonatal pigs obtained from the same farm were transported to the animal facility within 48 h of birth and were studied at 7 days of age. Young pigs were weaned at the farm at 21 days of age, arrived at the animal facility at 4 wk of age, and were studied at 35 days of age. All animal procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Experiment 1: rate of appearance of citrulline and plasma citrulline concentration in neonatal and young pigs.
A jugular and an arterial umbilical catheter were placed on the day of delivery in preterm pigs, and a jugular and a carotid catheter were placed the day after arrival in the neonatal and young pigs. After an overnight fast [except for the premature pigs that received continuous total parenteral nutrition (29)], premature (−10 days preterm; n = 5, 3 females and 2 males), neonatal (7 days old; n = 5 males), and young (35 days old; n = 5 females) pigs were continuously infused with [ureido-15N]citrulline for 4 h (or 6 h for preterm pigs) to determine the rate of appearance of this amino acid (infusion rates were 0.6, 7, and 3.1 µmol·kg−1·h−1, respectively, with a priming dose equivalent to a 1-h infusion). Preterm pigs were infused at a rate of 6 ml·kg−1·h−1 a total parenteral nutrition solution containing arginine (1.43 g/l) and glutamate (7.65 g/l) but free of glutamine, ornithine, and citrulline. Blood samples were collected from the arterial catheter every 30 min for the 4-h duration of the infusion (neonatal and young pigs) or from the jugular vein every 45 min starting at 270 min (premature pigs).
Experiment 2: arteriovenous arginine and citrulline differences across the portal drained viscera and kidney in neonatal and young anesthetized pigs.
Neonatal (7 days; n = 6, 4 females and 2 males) and young (35 days; n = 5 males) pigs were studied while under isoflurane anesthesia. Body temperature was maintained with a water blanket. After the placing of a jugular and a carotid catheter, an arterial blood sample was taken for the determination of isotopic background enrichments (zero time). Immediately after this, a primed, continuous infusion of tracers was started using the jugular catheter. The infusion rates were 7 and 3.1 µmol·kg−1·h−1 [ureido-15N]citrulline, 20 and 10.2 µmol·kg−1·h−1 [13C6]arginine, and 7 and 5 µmol·kg−1·h−1 [5,5-2H2]ornithine for the neonatal and young pigs, respectively. The priming dose was the equivalent to a 1-h infusion. After a midline laparotomy was performed, the renal and portal veins were catheterized. Additional blood samples were collected from the carotid artery and the portal and renal veins at 2.5, 3, 3.5, and 4 h. After the completion of the experiment, pigs were euthanized and tissue samples (proximal jejunum and kidney) collected. Samples were frozen in liquid nitrogen for Western analysis and fixed in formalin for immunohistochemistry.
Sample analysis.
Amino acid enrichments and concentrations were determined as their dansyl derivatives by liquid chromatography-mass spectrometry/ mass spectrometry, as described previously (20).
For Western analysis, proximal jejunum and kidney samples were homogenized and lysed in 300 μl of RIPA buffer (50 mM Tris·HCl, pH 7.4, 1% NP-40, 0.5% Na-deoxycholate, 0.1% SDS, 150 mM NaCl, 2 mM EDTA, and 50 mM NaF) containing protease inhibitor (GenDepot, Katy, TX) and phosphatase inhibitor (Roche Diagnosis, Indianapolis, IN). Samples were centrifuged at 12,000 rpm for 15 min after a 20-min incubation on ice. BCA protein assay was used for quantitation of protein, and 40-μg protein samples were loaded in 4–20% SDS polyacrylamide gel for electrophoresis and then transferred to a PVDF membrane (Bio-Rad, Hercules, CA). After 1 h of blocking in 5% nonfat milk at room temperature, membranes were incubated with primary antibodies overnight at 4°C. Primary antibodies for Western blot analysis were obtained from Abcam (Cambridge, MA; ASS, dilution 1:4,000, ab109753; ASL, dilution 1:1000, ab97370; CPS1, dilution 1:1,000, ab45956; and OAT, dilution 1:1,000, ab137679), Sigma-Aldrich (St. Louis, MO; OTC, dilution 1:400, AV41766), and Santa Cruz Biotechnology (Dallas, TX; β-actin, dilution 1:400, sc-47778). Membranes were then incubated with secondary antibodies for 1 h at room temperature (mouse anti goat, dilution 1:5,000, SC-2354; donkey anti-rabbit, dilution 1:2,000, sc-2305; goat anti-mouse, dilution 1:3,000, sc-2031; Santa Cruz Biotechnology). After incubation with west-Q Pico ECL solution (GenDepot Katy) for 3 min, signals were detected by Quantity One (Bio-Rad), and data were normalized with β-actin and analyzed by ImageJ (26).
For immunohistochemistry, formalin-fixed samples were paraffin-embedded, and 5-µm-thick sections were dewaxed; antigen retrieval was performed with sodium citrate (pH 6.0) for 20 min at 95°C. Sections were incubated in blocking buffer (5% horse serum in PBS with 0.5% Tween 20) for 30 min. Primary antibodies were applied to sections overnight at 4°C (same primary antibodies as those used for Western analysis). After washing with 1× PBST for 3 × 10 min, secondary antibodies were applied to sections at room temperature for 1 h (Alexa Fluor 488 donkey anti goat, A-11055; and Alexa Fluor 555 donkey anti rabbit, A-31572; ThermoFisher Scientific, Waltham, MA). Sections were mounted with vectashield mounting medium with DAPI (H-1200; Vector Laboratories, Burlingame, CA). Signal was detected by deconvolution microscope (DVLive fluorescence microscope; GE Healthcare, Pittsburgh, PA).
Calculations.
Whole body arginine, citrulline, and ornithine fluxes were determined by the isotopic dilution of the respective tracer, as described elsewhere (19). Whole body conversion of citrulline into arginine and ornithine into citrulline was calculated based on the transfer of the label from the infused precursor to the corresponding product (19). Citrulline plasma clearance, the virtual volume of plasma cleared of citrulline per unit of time, was calculated by dividing the citrulline flux by the respective plasma concentration.
Metabolism of arginine (and citrulline) across the PDV and kidney was determined on the basis of arteriovenous concentration and enrichment differences as described below:
| (1) |
| (2) |
| (3) |
| (4) |
where fractional uptake (%) is the fraction of the arterial supply taken up by the organ and organ uptake, release, and net balance are the amount of the amino acid of interest (µmol/l) taken up, released, and balanced across the organ. [A] and [V] are the arterial and venous concentrations, respectively, of arginine (or citrulline), and EA and EV are the arterial and venous isotopic enrichments of [13C6]arginine (or [15N]citrulline) expressed as tracer/tracee ratios.
The utilization of [15N]citrulline and [2H2]ornithine for arginine synthesis was calculated based on the increase of [15N]- or [2H2]arginine across the PDV or kidney as follows:
| (5) |
| (6) |
| (7) |
| (8) |
| (9) |
where [15N]arginine is the amount of labeled arginine (µmol [15N]arginine/l) entering (in), exiting (out), disappearing (uptake), and produced (gain) by the organ. [AArg] and [VArg] are the arterial and venous plasma arginine concentrations, EA_15[N]arg and EV_15[N]arg are the arterial and venous [15N]arginine enrichment, and EA_15[N]cit is the arterial enrichment of the citrulline precursor used for arginine synthesis. Similar calculations can be performed for [2H2]arginine and its precursor [2H2]ornithine.
Data analysis.
Fluxes, plasma concentrations, and clearance data were analyzed statistically utilizing the proc mixed procedure of SAS (version 9.2, SAS Institute, Cary, NC). Post hoc pairwise comparisons between premature, neonatal, and young pigs were conducted using Tukey’s procedure. Plasma concentrations and enrichments were analyzed using a mixed model with age (neonatal or young) and sampling site (arterial, portal, or renal) as fixed effects and pig as the random effect of the model. Preplanned orthogonal contrasts within age group were used to determine differences between the arterial and the two venous (portal and renal) sampling sites. In addition, means were tested to verify that they were different from zero. Western blot data were analyzed utilizing Wilcoxon exact test and the proc NPA 1-way procedure of SAS. Values presented in the text are means ± SE and were tested for significance at the 5% level.
RESULTS
Concentrations, fluxes, and clearances in preterm, neonatal, and young pigs.
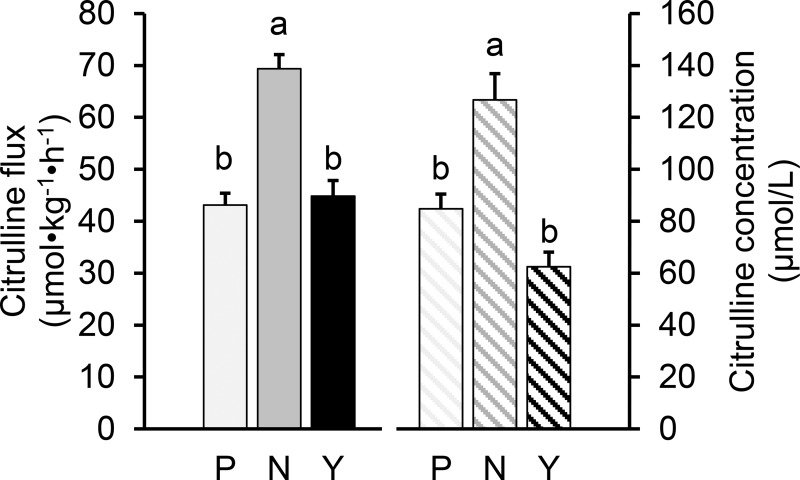
The flux and plasma citrulline concentrations were higher (P < 0.001) in the 7-day-old pigs than in the preterm and young pigs (Fig. 2). No differences were found between the preterm and young animals (flux P = 0.90; concentration P = 0.08). The clearance of plasma citrulline was greater for young pigs (7.3 ± 0.5 dl·kg−1·h−1, P < 0.005) than for perinatal pigs, but no differences were found between the preterm and neonatal pigs (5.1 ± 0.2 vs. 5.3 ± 0.3 dl·kg−1·h−1, P = 0.91). No differences in plasma arginine concentration were found among the different ages (79 ± 5, 62 ± 8, and 57 ± 7 µmol/l for the preterm, neonatal, and young pigs, respectively, P = 0.09), although the premature pigs were receiving total parenteral nutrition when these determinations were made.
Fig. 2.
Citrulline fluxes and concentrations in preterm (P; −10 days preterm), neonatal (N; 7 days old), and young pigs (Y; 35 days old). Bars are means ± SE; n = 5. Means with different letters differ, P < 0.01 (Tukey’s post hoc pairwise comparison).
Concentrations, fluxes, and clearances of citrulline and arginine in anesthetized neonatal and young pigs.
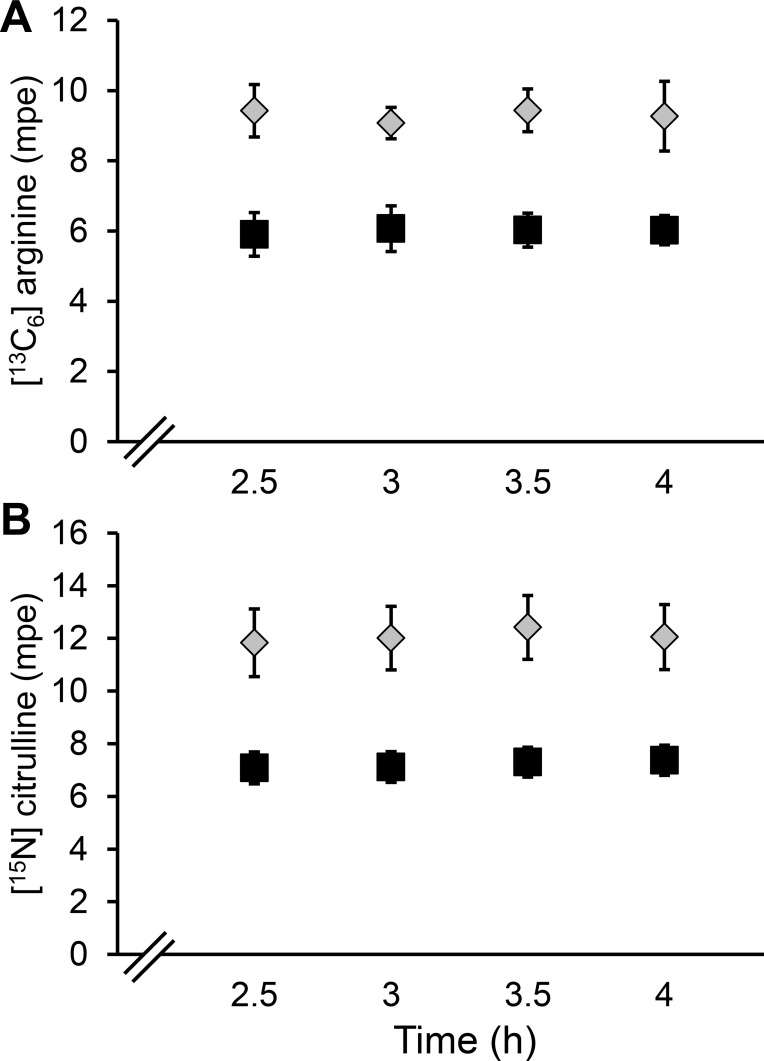
No differences in the fluxes of arginine, citrulline, or ornithine were detected between neonatal and young pigs (P > 0.18; Table 1). The infused arginine and citrulline time course enrichments demonstrated that steady-state conditions were reached within 2.5 h (Fig. 3). The conversion of citrulline into plasma arginine (“de novo” arginine synthesis) was not affected (P = 0.65) by the age of the pigs and accounted for ~40–46% of the citrulline flux (Table 1). The contribution of plasma ornithine to the synthesis of citrulline was similar (~22%, P = 0.79) for both age groups (Table 1). Arterial plasma arginine concentration was not different (P = 0.51) between the two age groups, but citrulline was higher (P < 0.025) in the neonates (Table 1). No differences in the clearance rate of arginine (P = 0.86) or citrulline (P = 0.09) were detected between the two age groups (Table 1).
Table 1.
Arginine, citrulline, and ornithine fluxes, interconversions, plasma concentrations, and clearances in anesthetized neonatal (7-day-old) and young (35-day-old) pigs
| Neonates (n = 6) | Young (n = 5) | P Value | |
|---|---|---|---|
| Fluxes, µmol·kg−1·h−1 | |||
| Arginine | 192.9 ± 13.7 | 165.1 ± 14.4 | <0.20 |
| Citrulline | 54.1 ± 7.6 | 41.1 ± 3.2 | <0.18 |
| Ornithine | 57.2 ± 4.1 | 59.1 ± 4.5 | <0.76 |
| Interconversions | |||
| Citrulline to arginine, µmol·kg−1·h−1 | 20.7 ± 5.2 | 19.1 ± 5.9 | <0.65 |
| Citrulline to arginine as %citrulline flux | 39.6 ± 3.8 | 45.7 ± 3.9 | <0.29 |
| Ornithine to citrulline, µmol·kg−1·h−1 | 11.3 ± 1.6 | 8.9 ± 0.5 | <0.21 |
| Ornithine to citrulline as %citrulline flux | 21.4 ± 2.2 | 22.4 ± 2.9 | <0.79 |
| Arterial concentration, µmol/l | |||
| Arginine | 154.0 ± 23.6 | 130.4 ± 22.9 | <0.51 |
| Citrulline | 196.3 ± 23.8 | 124.6 ± 14.2 | <0.025 |
| Clearances, dl·kg−1·h−1 | |||
| Arginine | 14.0 ± 2.0 | 14.7 ± 3.0 | <0.86 |
| Citrulline | 2.8 ± 0.2 | 3.4 ± 0.2 | <0.091 |
Values are means ± SE.
Fig. 3.
Arginine (A) and citrulline (B) enrichments in neonatal (7-day-old; gray diamonds) and young (35-day-old; ■) pigs. Symbols are means ± SE; n = 6 for neonates and 5 for the young pigs. Because different infusions rates were used for the 2 age groups, no statistical comparison between the 2 age groups was performed.
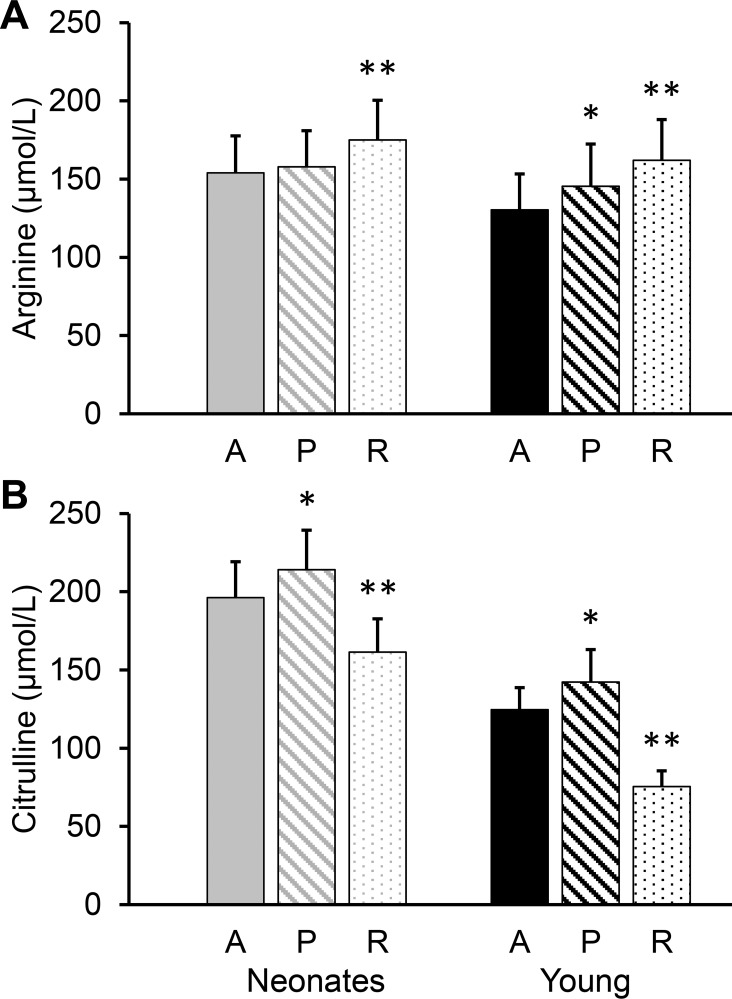
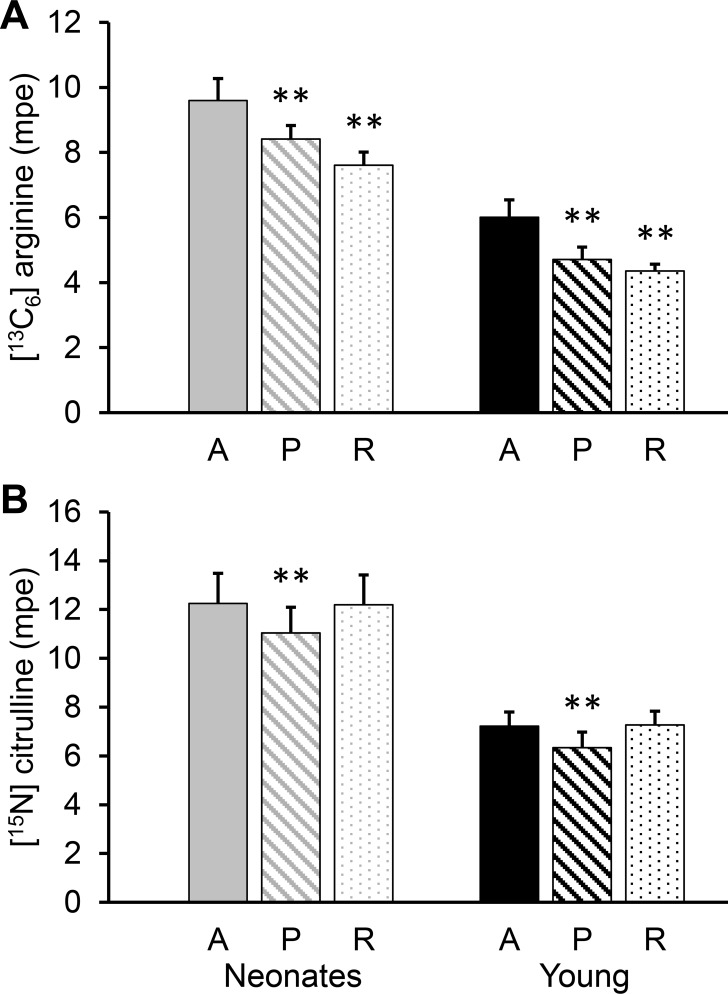
The concentration of plasma arginine differed depending on sampling site (P < 0.001), but there was no age effect (P = 0.64). Arterial plasma arginine concentration was lower than in the portal vein of young pigs (P < 0.027) and renal vein (P < 0.001) of both age groups (Fig. 4A). [13C6]arginine isotopic enrichment followed the opposite pattern, with higher isotopic enrichment in arterial plasma than in plasma sampled from the portal (P < 0.001) or renal vein (P < 0.001; Fig. 5A).
Fig. 4.
Plasma arginine (A) and citrulline (B) concentrations in arterial (A), portal (P), and renal (R) vein plasma of neonatal (7-day-old) and young (35-day-old) pigs. Bars are means ± SE; n = 6 for neonates and 5 for the young pigs. *P < 0.05 and **P < 0.01, significant differences from the corresponding arterial plasma.
Fig. 5.
Arginine (A) and citrulline (B) isotopic enrichments in arterial (A), portal (P), and renal (R) vein plasma of neonatal (7-day-old) and young (35-day-old) pigs. Bars are means ± SE; n = 6 for neonates and 5 for the young pigs. **P < 0.01, significant differences from the corresponding arterial plasma.
There was an effect of age (P < 0.001) and sampling site (P < 0.001) for plasma citrulline. Neonatal pigs had higher citrulline concentrations than young pigs. Whereas portal plasma had higher citrulline concentration than arterial plasma (P < 0.03), renal plasma was lower (P < 0.001; Fig. 4B). The isotopic enrichment of citrulline was lower in portal than in arterial plasma (P < 0.001), but there was no difference between arterial and renal plasma (P > 0.077; Fig. 5B).
Arginine and citrulline arteriovenous differences across the portal drained viscera and the kidney.
No age differences in arginine and citrulline uptake (P = 0.58), fractional uptake (P = 0.21), release (P = 0.16), or net balance (P = 0.31) were detected across the PDV (Table 2). In both age groups, the PDV extracted and released arginine, which resulted in a net release of this amino acid. The uptake of citrulline by the PDV was small and not different from zero. Citrulline was released by the PDV and entered the portal circulation in both neonatal and young pigs (Table 2).
Table 2.
Arginine and citrulline transactions across the PDV and kidney of neonatal (7-day-old) and young (35-day-old) pigs
| Neonates | Young | P Value | |
|---|---|---|---|
| Arginine | |||
| PDV | |||
| Fractional uptake,% | 7.7 ± 1.7 | 12.3 ± 3.2 | <0.21 |
| Uptake, µmol/l | 11.5 ± 3.3 | 14.4 ± 3.6 | <0.58 |
| Release, µmol/l | 15.5 ± 3.7 | 29.6 ± 9.0 | <0.16 |
| Net balance, µmol/l | 4.0 ± 0.7 | 15.2 ± 5.4 | <0.31 |
| Kidney | |||
| Fractional uptake, % | 6.9 ± 1.6 | 5.3 ± 1.9 | <0.56 |
| Uptake, µmol/l | 10.2 ± 3.1 | 6.7 ± 2.9 | <0.44 |
| Release, µmol/l | 31.3 ± 4.8 | 38.4 ± 5.3 | <0.35 |
| Net balance, µmol/l | 21.1 ± 2.9 | 31.7 ± 5.4 | <0.10 |
| Citrulline | |||
| PDV | |||
| Fractional uptake, % | 0.5 ± 0.5a | 1.6 ± 1.2a | <0.40 |
| Uptake, µmol/l | 1.2 ± 0.8a | 1.3 ± 1.3a | <0.94 |
| Release, µmol/l | 18.9 ± 4.7 | 18.8 ± 5.6 | <0.99 |
| Net balance, µmol/l | 17.7 ± 4.8 | 17.5 ± 6.8 | <0.98 |
| Kidney | |||
| Fractional uptake, % | 18.9 ± 2.5 | 39.5 ± 3.5 | <0.001 |
| Uptake, µmol/l | 35.8 ± 4.3 | 48.8 ± 7.1 | <0.14 |
| Release, µmol/l | 0.8 ± 0.3 | −0.5 ± 0.2a | <0.007 |
| Net balance, µmol/l | −35.0 ± 4.2b | −49.3 ± 7.2 | <0.11 |
Values are means ± SE. PDV, portal drained viscera.
Not different from zero.
Negative net balance denotes a net uptake by the organ.
The kidney extracted and released arginine, which resulted in a net renal release of this amino acid without differences between the two age groups (P = 0.10). The fractional renal uptake of citrulline, in contrast, was greater for the young pig than the neonatal animals (P < 0.001); however, this did not translate into a greater absolute µmol/l uptake (P = 0.14; Table 2). A small release of citrulline by the kidney in the neonatal pigs was detected (P < 0.007). These transactions across the kidney resulted in a net uptake of citrulline in both the neonatal and young pigs, but no age differences were found (P = 0.11; Table 2).
Citrulline utilization by the portal drained viscera and the kidney.
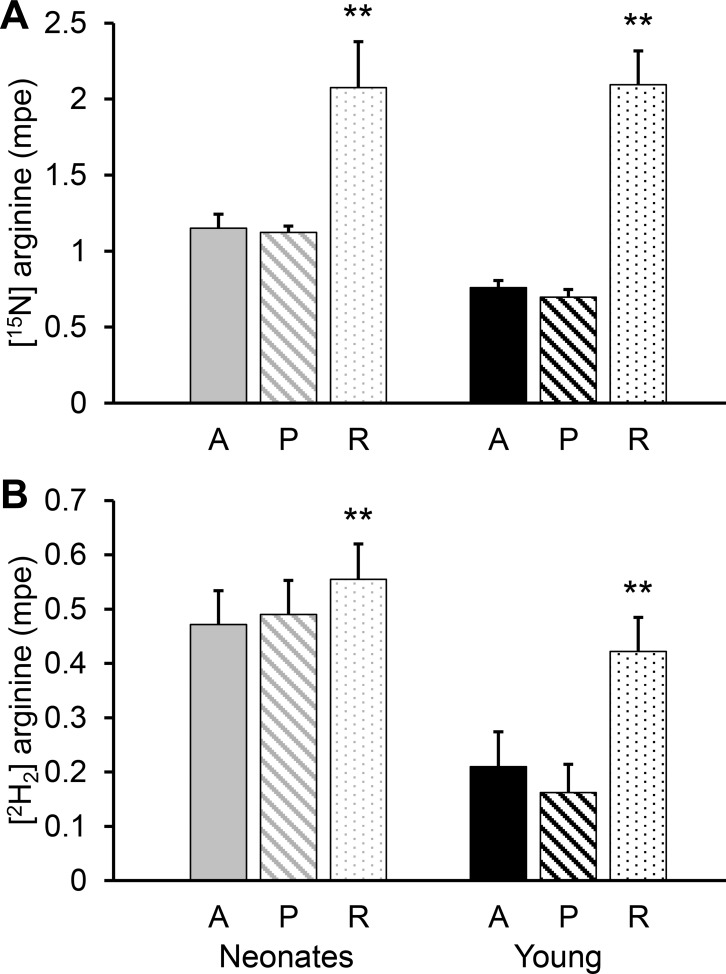
As result of [15N]citrulline utilization for arginine synthesis, [15N]arginine was present in the circulation (Fig. 6A). This [15N]arginine has a metabolic fate similar to unlabeled arginine and was extracted by the PDV and kidney. The [15N]arginine balance across the PDV showed that it was not different from zero for both neonatal and young pigs (P = 0.99), and therefore, there was no synthesis of arginine from citrulline (P = 0.62; Table 3). In contrast, there was a net positive renal [15N]arginine balance in neonatal and young pigs, which indicates citrulline utilization for arginine synthesis by the kidney. There was an age difference (P < 0.032), with young pigs producing more arginine from citrulline than the neonatal animals.
Fig. 6.
[15N]- (A) and [2H2]arginine (B) isotopic enrichments in arterial (A), portal (P), and renal (R) vein plasma of neonatal (7-day-old) and young (35-day-old) pigs. Bars are means ± SE; n = 6 for neonates and 5 for the young pigs. **P < 0.01, significant differences from the corresponding arterial plasma.
Table 3.
[15N]arginine transactions across the PDV and kidney of neonatal (7-day-old) and young (35-day-old) pigs
| Neonates | Young | P Value | |
|---|---|---|---|
| PDV, µmol/l | |||
| [15N]arginine in | 1.74 ± 0.32 | 0.98 ± 0.19 | <0.088 |
| [15N]arginine out | 1.74 ± 0.26 | 1.02 ± 0.23 | <0.071 |
| [15N]arginine uptake | 0.14 ± 0.05 | 0.11 ± 0.03 | <0.62 |
| [15N]arginine through | 1.60 ± 0.28 | 0.87 ± 0.18 | <0.069 |
| [15N]arginine balance | 0.14 ± 0.07a | 0.14 ± 0.07a | <0.99 |
| Arginine balance | 1.62 ± 0.77a | 2.33 ± 1.21a | <0.62 |
| Kidney, µmol/l | |||
| [15N]arginine in | 1.74 ± 0.32 | 0.98 ± 0.19 | <0.088 |
| [15N]arginine out | 3.63 ± 0.74 | 3.11 ± 0.36 | <0.57 |
| [15N]arginine uptake | 0.11 ± 0.02 | 0.05 ± 0.02a | <0.093 |
| [15N]arginine through | 1.64 ± 0.32 | 0.94 ± 0.19 | <0.11 |
| [15N]arginine balance | 1.99 ± 0.48 | 2.17 ± 0.20 | <0.76 |
| Arginine balance | 18.28 ± 4.38 | 32.31 ± 2.94 | <0.032 |
Values are means ± SE. PDV, portal drained viscera.
Not different from zero.
Ornithine utilization for arginine synthesis by the portal drained viscera.
Because ASS1 and ASL are present in neonatal enterocytes, arginine rather than citrulline may be produced by the gut. To quantify this process, we infused a [2H2]ornithine tracer and measured the transactions of [2H2]arginine (Fig. 6B) across the PDV as well as its production. Whereas neonatal pigs had a small positive [2H2]arginine balance across the PDV that was greater (P = 0.036) than the young pigs, the balance for the older animals was not different from zero (P = 0.54). However, when the enrichment of the [2H2]ornithine precursor was used to calculate the total production of arginine, no differences between the two age groups were observed (P = 0.087; Table 4).
Table 4.
[2H2]arginine transactions across the PDV of neonatal (7-day-old) and young (35-day-old) pigs
| Neonates | Young | P Value | |
|---|---|---|---|
| PDV, µmol/l | |||
| [2H2]arginine in | 0.76 ± 0.19 | 0.29 ± 0.10a | <0.070 |
| [2H2]arginine out | 0.81 ± 0.20 | 0.28 ± 0.11a | <0.057 |
| [2H2]arginine uptake | 0.06 ± 0.02 | 0.03 ± 0.01a | <0.26 |
| [2H2]arginine through | 0.70 ± 0.17 | 0.26 ± 0.09a | <0.066 |
| [2H2]arginine balance | 0.10 ± 0.03 | 0.01 ± 0.02a | <0.036 |
| Arginine gain | 1.03 ± 0.24 | 0.20 ± 0.37a | <0.087 |
Values are means ± SE.
Not different from zero.
Abundance of citrulline synthesizing and utilizing enzymes in the gut and kidney of neonatal and young pigs.
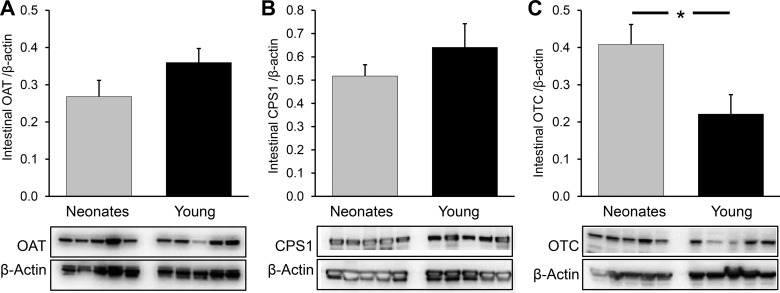
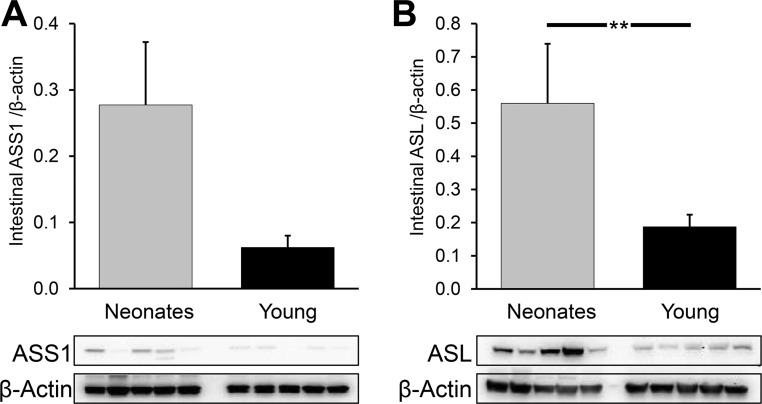
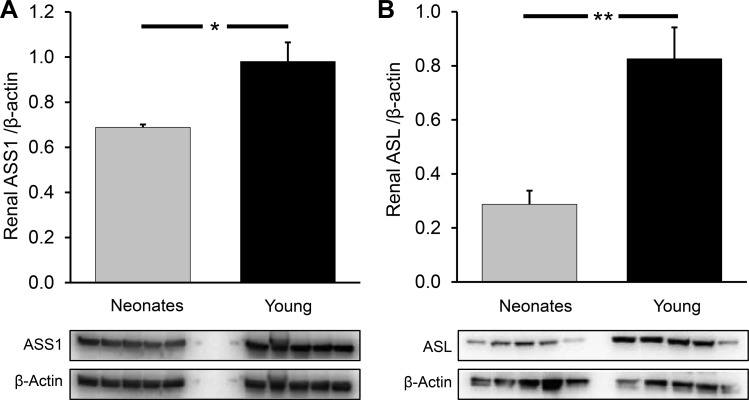
Neonatal pigs had a greater intestinal abundance of OTC (P < 0.04) than young pigs, but no differences were found in the abundance of other intestinal enzymes needed to synthesize citrulline (OAT, P = 0.22; CPS1, P = 0.55; Fig. 7). ASL abundance was greater in the small intestine of neonatal pigs (P < 0.03), but ASS abundance did not differ (P = 0.22) between the two age groups (Fig. 8, A and B).
Fig. 7.
Intestinal abundance of OAT (A), CPS1 (B), and OTC (C) in neonatal (7-day-old) and young (35-day-old) pigs. Bars are means ± SE; n = 5. *P < 0.05, significant differences.
Fig. 8.
Argininosuccinate synthase (ASS1; A) and lyase (ASL; B) abundance in the small intestine of neonatal (7-day-old) and young (35-day-old) pigs. Bars are means ± SE; n = 5. **P < 0.01, significant differences.
As expected, OTC and CPS1 were not detected in pig kidney (not shown). Renal ASS and ASL were more abundant in young pigs than in their neonatal counterparts (P < 0.028 and P < 0.001, respectively; Fig. 9, A and B).
Fig. 9.
Argininosuccinate synthase (ASS1; A) and lyase (ASL; B) abundance in the kidney of neonatal (7-day-old) and young (35-day-old) pigs. Bars are means ± SE; n = 5. *P < 0.05 and **P < 0.01, significant differences.
Localization of citrulline synthesizing and utilizing enzymes in the gut.
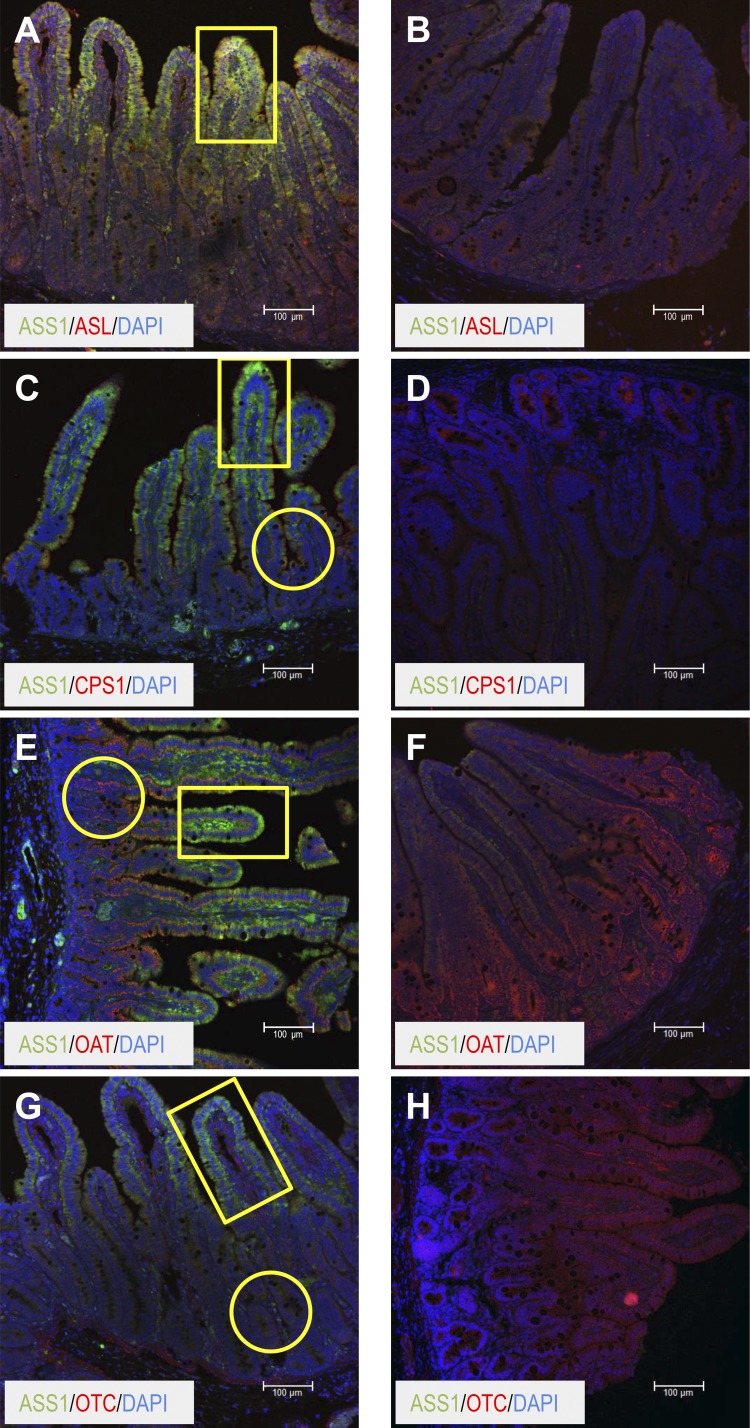
The enzymes involved in the synthesis of citrulline (OAT, CPS1, and OTC) were present in the crypt-basal region of the villus in the neonatal small intestine (Fig. 10, C, E, and G, respectively), whereas the enzymes that utilize citrulline for arginine synthesis were localized toward the apical region of the villus (Fig. 10, A, C, E, and G). Similar distribution was noted for the young pigs (Fig. 10, C, E, and G), but ASS1 and ASL were not distinguishable (Fig. 10B).
Fig. 10.
Colocalization of argininosuccinate lyase (ASL; A and B), carbamoyl phosphate synthase 1 (CPS1; C and D), ornithine aminotransferase (OAT; E and F), and ornithine carbamoyltransferase (OTC; G and H) with argininosuccinate synthase (ASS1) in the small intestine of neonatal (7-day-old; A, C, E, and G) and young (35-day-old; B, D, F, and H) pigs. The different enzymes (in red) are costained with ASS1 in green and DAPI in blue. Colocalization of ASS1 with the different enzymes, if present, appears in yellow. Scale bar, 100 μm. Circles denote the crypt-villus base and rectangles the apical portion of the villus.
DISCUSSION
The endogenous synthesis of arginine in the adult relies on a multiorgan process where citrulline produced in the small intestine is then used for arginine synthesis in the kidney in what has been dubbed the intestinal-renal axis for endogenous arginine synthesis (2, 4). Because citrulline is synthesized mainly by intestinal enterocytes and appears in the peripheral circulation during this interorgan process, the plasma concentration of this amino acid has been proposed as a marker for gut mass and function (7). In neonates, the functionality of this intestinal-renal axis has been questioned due to the presence of ASS1 and ASL in enterocytes and the reduced expression of these enzymes in the kidney during the neonatal period (15, 18, 28, 36). The presence of ASS1 and ASL in the gut, together with the absence of arginase II, has the potential to shift the site of arginine production from the kidney to the small intestine early in life. This neonatal enzymatic pattern is only present for approximately the first 2 wk of life in pigs, rats, and mice (9, 15, 34), but it is likely to persist for longer in humans (18). To capture these changes in the synthesis and utilization of citrulline, we studied premature (−10 days preterm), neonatal (7 days old), and young pigs (35 days old) 2 wk postweaning. Although neither sex was represented uniformly in all age groups and this constitutes a limitation of the study, sex differences in the metabolism of citrulline and arginine are likely to be minimal in prepubertal animals. We measured plasma citrulline and whole body citrulline fluxes in conscious pigs to determine whether the interorgan trafficking of this amino acid was present in the perinatal animals. We also determined the site of citrulline production and utilization in anesthetized neonatal and young pigs.
Premature and neonatal pigs maintain plasma citrulline and citrulline fluxes.
The production of arginine in the neonatal gut and the consequent absence of the intestinal-renal axis would result in little citrulline entering the plasma, and thus plasma concentrations and fluxes should be low or absent. However, this is not the case. Substantial plasma citrulline concentrations have been reported in neonatal pigs (35) and humans, including premature babies (16). Citrulline fluxes in fed and fasted neonatal pigs (22) and humans (8) also show that the rate of citrulline production is comparable (if not larger) than in the adult (5, 21). Here, we have demonstrated that premature and neonatal pigs maintain whole body citrulline fluxes and plasma concentrations similar to those observed in young animals. To the best of our knowledge, this is the first report on citrulline fluxes not only in premature pigs but also in any premature species. Moreover, neonatal pigs may have larger fluxes than young pigs, although we failed to detect significant differences in the anesthetized animals. Furthermore, we determined that “de novo” arginine synthesis, measured by the conversion of citrulline to arginine, was not different between the neonatal and young pigs and that plasma ornithine contributed ~20% of the precursor utilized for citrulline synthesis. Thus, the evidence of de novo arginine synthesis, together with the substantial circulating levels of citrulline, suggests that there is a functional intestinal-renal axis for arginine production in the neonate.
Citrulline is released by the gut of neonatal pigs.
To address directly whether the intestinal-renal axis for endogenous arginine production occurred in neonatal pigs, we measured the release of citrulline by the PDV and its utilization for arginine production in neonatal and young pigs. Due to the invasiveness of the experimental approach and frailty of the preterm pigs, this group was not included in this study. Our results show that there is a substantial net release of plasma citrulline across the PDV and a reduction in the enrichment of the citrulline tracer infused in both neonatal and young pigs. These data demonstrate that citrulline is released by the PDV, diluting the infused [15N]citrulline tracer, which is in agreement with previous reports that showed citrulline release by the jejunum of pigs of different ages (35). Because the release of citrulline from protein turnover and other sources is minimal and the enzymes for its synthesis are present only in enterocytes, it can be inferred that the citrulline appearing in the portal vein was produced by the intestine. Applying portal plasma flow measurements from previous studies (2–2.5 l·kg−1·h−1) (17, 27) to the measured citrulline released by the PDV, we estimated net release rates of citrulline that closely paralleled whole body citrulline fluxes. This strongly suggests that the gut is the primary site for citrulline production and the main source of circulating citrulline in both neonatal and young pigs.
Arginine is released and may be produced by the neonatal PDV.
There was a measurable uptake and release of arginine by the PDV that resulted in a net release of this amino acid, but no age differences were observed. The release of arginine by the PDV, however, does not necessarily imply de novo synthesis, since protein turnover may be responsible, at least partially, for its appearance. Release of arginine by the jejunum and PDV in fasted and fed pigs has been reported previously (31, 35). However, the release of citrulline observed here in neonatal and young pigs does not preclude the production of new arginine by the PDV. This can still be explained by two different mechanisms. The first one is the utilization of circulating citrulline for arginine synthesis during second-pass metabolism due to the presence of ASS1 and ASL in the neonatal gut. In fact, there was a small positive release of [15N]arginine across the PDV in both neonatal and young pigs. These values, however, were not different from zero, and no differences were detected between the two age groups. Note that, whereas citrulline production by the PDV can be attributed to the gut as indicated before, second-pass metabolism of citrulline and its conversion to arginine can take place in other organs drained by the portal vein that also express ASL and ASL (e.g., pancreas, spleen).
The second possible mechanism is the synthesis and release of arginine in addition to citrulline production. To address this possibility, we infused [2H2]ornithine. Here we have shown that plasma ornithine provided ~20% of the precursors used for citrulline synthesis in both neonatal and young pigs. For this reason, an increase in the enrichment of [2H2]arginine across the PDV would indicate that arginine was produced from ornithine and that the complete pathway for arginine synthesis exists in the gut. A small, positive release of arginine (~1 µmol/l) due to the use of the labeled ornithine was detected in the neonatal pig, whereas it was not different from zero in the young animals. These values, however, were not different between the two age groups. Although it is possible that the neonatal gut may produce arginine de novo, the quantification of this process requires nine different analytical measurements and is thus error prone. Thus, if this pathway exists in vivo, its contribution to the whole body arginine economy is small and difficult to quantify.
The neonatal kidney utilizes citrulline for arginine synthesis.
The kidney extracted and released arginine from the circulation in both the neonatal and the young pigs, with a net output of arginine that was not different between the two age groups. In contrast to the PDV, there was a clear increase in the enrichment of [15N] arginine that showed that citrulline was utilized for de novo synthesis of arginine by the kidney. Here we have shown that ASS1 and ASL were present in renal tissue of neonatal pigs, although their abundance were lower than in young animals; this agrees with previous reports on the activity of these enzymes in pigs of different ages (34). The reduced abundance and activity of these two enzymes in the neonate is likely the reason for the lower fractional extraction of citrulline, reduced plasma clearance, and consequent high plasma citrulline concentration reported by us and others (12). Reduced expression and protein abundance of ASS1 and ASL have also been reported in neonatal mice (15) and rats (14). However, there seems to be an excessive capacity to metabolize citrulline into arginine (1, 32), and thus citrulline production is the rate-limiting step in the endogenous synthesis of arginine.
Lack of colocalization of the enzymes that synthesize and utilize citrulline.
The lack of colocalization between the enzymes that produce and utilize citrulline presented here has been described previously in rats (9), pigs (10), and humans (18). However, the functional implications of this lack of colocalization have not been considered in the interpretation of in vitro data. The loss of architecture in intestinal homogenates (15) and isolated enterocyte cultures in vitro (3, 34) brings different epithelial cell types in close proximity, allowing for the exchange of metabolites. For this reason, in vitro systems do not represent in vivo metabolism, and their results should be interpreted with caution. Although the presence of ASS1 and ASL in the neonatal small intestine seems to suggest that the citrulline produced in the gut is utilized for arginine synthesis, the lack of colocalization of these two enzymes with CPS1 and OTC implies that citrulline is exported by the gut, offering an explanation for the presence of circulating citrulline observed in neonates of different species.
In summary, we have shown that there is a substantial citrulline flux and considerable plasma citrulline concentration in neonatal pigs. Despite the presence of ASS1 and ASL in the small intestine, the lack of colocalization with the enzymes that produce citrulline results in the release of citrulline by the PDV and its utilization by the kidney to produce arginine. However, it is possible that a small amount of arginine is produced by the neonatal gut, but more research is needed to quantify this source. In conclusion, the intestinal-renal axis for arginine synthesis is functional in the neonatal pig.
GRANTS
This work was supported by federal funds from the US Department of Agriculture, Agricultural Research Service, under Cooperative Agreement No. 58-3092-5-001 and the National Institutes of Health (R01-GM-108940).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C.M. and D.G.B. conceived and designed research; J.C.M., U.A., J.L.R., I.C.D., and B.S. performed experiments; J.C.M., J.L.R., and Y.Y. analyzed data; J.C.M. and D.G.B. interpreted results of experiments; J.C.M. and Y.Y. prepared figures; J.C.M. drafted manuscript; J.C.M., J.L.R., and D.G.B. edited and revised manuscript; J.C.M., U.A., J.L.R., I.C.D., B.S., and D.G.B. approved final version of manuscript.
REFERENCES
- 1.Agarwal U, Didelija IC, Yuan Y, Wang X, Marini JC. Supplemental citrulline is more efficient than arginine in increasing systemic arginine availability in mice. J Nutr 147: 596–602, 2017. doi: 10.3945/jn.116.240382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertolo RF, Burrin DG. Comparative aspects of tissue glutamine and proline metabolism. J Nutr 138: 2032S–2039S, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Blachier F, M’Rabet-Touil H, Posho L, Darcy-Vrillon B, Duée PH. Intestinal arginine metabolism during development. Evidence for de novo synthesis of L-arginine in newborn pig enterocytes. Eur J Biochem 216: 109–117, 1993. doi: 10.1111/j.1432-1033.1993.tb18122.x. [DOI] [PubMed] [Google Scholar]
- 4.Brosnan ME, Brosnan JT. Renal arginine metabolism. J Nutr 134, 10 Suppl: 2791S–2795S, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, Vogt J, Young VR. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci USA 90: 7749–7753, 1993. doi: 10.1073/pnas.90.16.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung CW, Cohen NS, Raijman L. Channeling of urea cycle intermediates in situ in permeabilized hepatocytes. J Biol Chem 264: 4038–4044, 1989. [PubMed] [Google Scholar]
- 7.Crenn P, Coudray-Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology 119: 1496–1505, 2000. doi: 10.1053/gast.2000.20227. [DOI] [PubMed] [Google Scholar]
- 8.de Betue CTI, Joosten KFM, Deutz NE, Vreugdenhil ACE, van Waardenburg DA. Arginine appearance and nitric oxide synthesis in critically ill infants can be increased with a protein-energy-enriched enteral formula. Am J Clin Nutr 98: 907–916, 2013. doi: 10.3945/ajcn.112.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jonge WJ, Dingemanse MA, de Boer PA, Lamers WH, Moorman AFM. Arginine-metabolizing enzymes in the developing rat small intestine. Pediatr Res 43: 442–451, 1998. doi: 10.1203/00006450-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Dekaney CM, Wu G, Jaeger LA. Gene expression and activity of enzymes in the arginine biosynthetic pathway in porcine fetal small intestine. Pediatr Res 53: 274–280, 2003. doi: 10.1203/00006450-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Dhanakoti SN, Brosnan JT, Herzberg GR, Brosnan ME. Renal arginine synthesis: studies in vitro and in vivo. Am J Physiol Endocrinol Metab 259: E437–E442, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Flynn NE, Knabe DA, Mallick BK, Wu G. Postnatal changes of plasma amino acids in suckling pigs. J Anim Sci 78: 2369–2375, 2000. doi: 10.2527/2000.7892369x. [DOI] [PubMed] [Google Scholar]
- 13.Ghoneim N, Bauchart-Thevret C, Oosterloo B, Stoll B, Kulkarni M, de Pipaon MS, Zamora IJ, Olutoye OO, Berg B, Wittke A, Burrin DG. Delayed initiation but not gradual advancement of enteral formula feeding reduces the incidence of necrotizing enterocolitis (NEC) in preterm pigs. PLoS One 9: e106888, 2014. doi: 10.1371/journal.pone.0106888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goutal I, Fairand A, Husson A. Expression of the genes of arginine-synthesizing enzymes in the rat kidney during development. Biol Neonate 76: 253–260, 1999. doi: 10.1159/000014166. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz R, Kretchmer N. Development of arginine-synthesizing enzymes in mouse intestine. Am J Physiol Gastrointest Liver Physiol 251: G103–G110, 1986. [DOI] [PubMed] [Google Scholar]
- 16.Ioannou HP, Diamanti E, Piretzi K, Drossou-Agakidou V, Augoustides-Savvopoulou P. Plasma citrulline levels in preterm neonates with necrotizing enterocolitis. Early Hum Dev 88: 563–566, 2012. doi: 10.1016/j.earlhumdev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Janeczko MJ, Stoll B, Chang X, Guan X, Burrin DG. Extensive gut metabolism limits the intestinal absorption of excessive supplemental dietary glutamate loads in infant pigs. J Nutr 137: 2384–2390, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Köhler ES, Sankaranarayanan S, van Ginneken CJ, van Dijk P, Vermeulen JLM, Ruijter JM, Lamers WH, Bruder E. The human neonatal small intestine has the potential for arginine synthesis; developmental changes in the expression of arginine-synthesizing and -catabolizing enzymes. BMC Dev Biol 8: 107, 2008. doi: 10.1186/1471-213X-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr 142: 572–580, 2012. doi: 10.3945/jn.111.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marini JC. Quantitative analysis of 15N-labeled positional isomers of glutamine and citrulline via electrospray ionization tandem mass spectrometry of their dansyl derivatives. Rapid Commun Mass Spectrom 25: 1291–1296, 2011. doi: 10.1002/rcm.5007. [DOI] [PubMed] [Google Scholar]
- 21.Marini JC, Agarwal U, Didelija IC, Azamian M, Stoll B, Nagamani SC. Plasma glutamine is a minor precursor for the synthesis of citrulline: A multispecies study. J Nutr 147: 549–555, 2017. doi: 10.3945/jn.116.243592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marini JC, Stoll B, Didelija IC, Burrin DG. De novo synthesis is the main source of ornithine for citrulline production in neonatal pigs. Am J Physiol Endocrinol Metab 303: E1348–E1353, 2012. doi: 10.1152/ajpendo.00399.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris SM., Jr Regulation of enzymes of urea and arginine synthesis. Annu Rev Nutr 12: 81–101, 1992. doi: 10.1146/annurev.nu.12.070192.000501. [DOI] [PubMed] [Google Scholar]
- 24.Morris SM., Jr Arginine: beyond protein. Am J Clin Nutr 83: 508S–512S, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Nagamani SCS, Shchelochkov OA, Mullins MA, Carter S, Lanpher BC, Sun Q, Kleppe S, Erez A, O’Brian Smith E, Marini JC, Lee B; Members of the Urea Cycle Disorders Consortium . A randomized controlled trial to evaluate the effects of high-dose versus low-dose of arginine therapy on hepatic function tests in argininosuccinic aciduria. Mol Genet Metab 107: 315–321, 2012. doi: 10.1016/j.ymgme.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasband WS. ImageJ. Bethesda, MD: National Institutes of Health; https://imagej.nih.gov/ij/, [January 2017]. [Google Scholar]
- 27.Puiman PJ, Stoll B, van Goudoever JB, Burrin DG. Enteral arginine does not increase superior mesenteric arterial blood flow but induces mucosal growth in neonatal pigs. J Nutr 141: 63–70, 2011. doi: 10.3945/jn.110.131888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryall JC, Quantz MA, Shore GC. Rat liver and intestinal mucosa differ in the developmental pattern and hormonal regulation of carbamoyl-phosphate synthetase I and ornithine carbamoyl transferase gene expression. Eur J Biochem 156: 453–458, 1986. doi: 10.1111/j.1432-1033.1986.tb09603.x. [DOI] [PubMed] [Google Scholar]
- 29.Sangild PT, Petersen YM, Schmidt M, Elnif J, Petersen TK, Buddington RK, Greisen G, Michaelsen KF, Burrin DG. Preterm birth affects the intestinal response to parenteral and enteral nutrition in newborn pigs. J Nutr 132: 3786–3794, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Southern LL, Baker DH. Arginine requirement of the young pig. J Anim Sci 57: 402–412, 1983. doi: 10.2527/jas1983.572402x. [DOI] [PubMed] [Google Scholar]
- 31.Stoll B, Henry J, Reeds PJ, Yu H, Jahoor F, Burrin DG. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J Nutr 128: 606–614, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Urschel KL, Shoveller AK, Uwiera RR, Pencharz PB, Ball RO. Citrulline is an effective arginine precursor in enterally fed neonatal piglets. J Nutr 136: 1806–1813, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol Endocrinol Metab 241: E473–E480, 1981. [DOI] [PubMed] [Google Scholar]
- 34.Wu G, Knabe DA. Arginine synthesis in enterocytes of neonatal pigs. Am J Physiol Regul Integr Comp Physiol 269: R621–R629, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Wu G, Borbolla AG, Knabe DA. The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J Nutr 124: 2437–2444, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J 336: 1–17, 1998. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]