Abstract
Contractile activity (e.g., exercise) evokes numerous metabolic adaptations in human skeletal muscle, including enhanced insulin action and substrate oxidation. However, there is intersubject variation in the physiological responses to exercise, which may be linked with factors such as the degree of obesity. Roux-en-Y gastric bypass (RYGB) surgery reduces body mass in severely obese (body mass index ≥ 40 kg/m2) individuals; however, it is uncertain whether RYGB can potentiate responses to contractile activity in this potentially exercise-resistant population. To examine possible interactions between RYGB and contractile activity, muscle biopsies were obtained from severely obese patients before and after RYGB, differentiated into myotubes, and electrically stimulated, after which changes in insulin action and glucose oxidation were determined. Before RYGB, myotubes were unresponsive to electrical stimulation, as indicated by no changes in insulin-stimulated glycogen synthesis and basal glucose oxidation. However, myotubes from the same patients at 1 mo after RYGB increased insulin-stimulated glycogen synthesis and basal glucose oxidation when subjected to contraction. While unresponsive before surgery, contraction improved insulin-stimulated phosphorylation of AS160 (Thr642, Ser704) after RYGB. These data suggest that RYGB surgery may enhance the ability of skeletal muscle from severely obese individuals to respond to contractile activity.
Keywords: exercise, glycogen synthesis, glucose oxidation, insulin signaling, AS160
exercise training induces a wide range of benefits that improves whole body metabolism in individuals with metabolic diseases, such as type 2 diabetes and obesity (16, 19, 22). At the level of skeletal muscle, exercise training enhances insulin action, fuel oxidation, and mitochondrial function, along with other adaptations (19). There are also acute effects from a single training bout, most notably an increased sensitivity to insulin (42). However, large-scale prospective studies have reported substantial intersubject variation in responses to exercise training, with some individuals exhibiting minimal to even negative changes in health-related parameters (9). In addition, Stephens and Sparks (38) examined published and unpublished exercise training studies in subjects with comorbidities (e.g., severe obesity and type 2 diabetes) and concluded that 15–20% of these individuals do not improve glucose homeostasis with the intervention. Such findings suggest that there may be metabolic phenotypes where the acute exercise/exercise training responses are compromised.
The skeletal muscle of severely obese [body mass index (BMI) ≥ 40 kg/m2] individuals’ displays defects in the capacity for substrate oxidation, along with insulin resistance (18, 23). The defects are retained in primary skeletal muscle cell cultures, thus implying a genetic and/or epigenetic origin (1, 5, 8, 11, 17, 23). Such inherent metabolic deficiencies could impair the ability of severely obese individuals to respond to interventions such as exercise. However, Roux-en-Y gastric bypass surgery (RYGB) leads to a rapid improvement in metabolic health, including the reversion of type 2 diabetes in severely obese patients (32, 33). In relation to exercise, it has been hypothesized that implementing contractile activity after RYGB may provide a milieu that synergistically enhances the positive effects of contractile activity; this hypothesis, however, has not been extensively tested (15).
The purpose of the present study was to determine whether RYGB alters the ability of skeletal muscle from severely obese individuals to respond to muscle contraction in terms of enhancing insulin action and glucose oxidation. Primary human skeletal muscle myotubes retain the metabolic phenotype of the donor (5–8, 11, 17, 24), and electrical stimulation of these myotubes has been utilized as an in vitro model of contractile activity (26, 31, 34). We, therefore, subjected primary muscle cells derived from severely obese patients before and after RYGB to electrical stimulation to determine whether the surgery could potentiate responses to muscle contraction.
MATERIALS AND METHODS
Subject recruitment.
Severely obese (BMI > 40 kg/m2) female patients (N = 6) were recruited from the East Carolina University bariatric surgery center. All patients were sedentary and were not prescribed an exercise program. Participants were excluded if they had a diagnosis of diabetes, heart disease, or history of cancer within the past 5 yr. Participants were also excluded if they were using medications that would alter glucose metabolism. Subjects provided written consent before completing any experimental procedures. A muscle biopsy was obtained from the vastus lateralis after an 8- to 12-h fast on a visit solely for blood and muscle sampling. Before the muscle biopsy procedure, a fasting venous blood sample was obtained for analysis of plasma glucose and insulin, and severity of insulin resistance was determined by calculation of the homeostatic model assessment of insulin resistance (HOMA-IR). All procedures were submitted to and approved by the Institutional Review Board of East Carolina University. Subject characteristics are presented in Table 1.
Table 1.
Subject characteristics before and after RYGB surgery
| Weight, kg | BMI, kg/m2 | Glucose, mg/dl | Insulin, mU/l | HOMA-IR | |
|---|---|---|---|---|---|
| Pre | 137.0 ± 8.4 | 49.4 ± 2.5 | 92.6 ± 2.3 | 15.5 ± 1.5 | 3.5 ± 0.3 |
| RYGB | 120.3 ± 9.1* | 43.2 ± 2.8* | 86.8 ± 3.2 | 12.7 ± 3.5 | 2.8 ± 0.9 |
Values are means ± SE. HOMA-IR, homeostatic model assessment of insulin resistance.
P < 0.05 vs. Pre.
Primary human muscle cell cultures and electrical stimulation.
Skeletal muscle biopsies were obtained from the vastus lateralis of fasted patients before and 1 mo following RYGB using the percutaneous needle biopsy technique. Primary skeletal muscle cells were isolated and cultured into myoblasts, as described previously (7, 30). Briefly, myoblasts were subcultured onto six-well type I collagen-coated plates at densities of 60 × 103 or 40 × 103 cells/well for metabolic function and immunoblot analysis, respectively. On reaching 80–90% confluency, differentiation to myotubes was induced by switching from growth to differentiation media (Dulbecco’s modified Eagle’s medium; Thermo Fisher Scientific), supplemented with 2% horse serum (Thermo Fisher Scientific), 0.3% bovine serum albumin (Sigma Aldrich), 0.05% fetuin (Sigma Aldrich), and 100 mg/ml penicillin/streptomycin). On day 7 of differentiation, myotubes were electrically stimulated to contract for 24 h (11.5 V and 1 Hz) using a cell culture stimulator (C-PACE EP, IonOptix, Westwood, MA). A similar stimulation protocol has been used by others to elicit contraction in human myotubes (26, 31); accordingly, we observed visible contraction as well as calcium transients (fluo-8 fluorescence) evoked by the electrical pulses (data not shown) (28). In preliminary experiments, we observed no differences in cell viability (as determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay) under control (no stimulation) and after 24 h of electrical stimulation with the protocol used in the present study in primary skeletal muscle cells from severely obese subjects (data not shown).
In vitro glucose metabolism.
In vitro glucose metabolism was assessed using methods previously described (1, 26). Briefly, following 3-h serum starvation, cells were incubated in a sealed plate with reaction media containing d-[U-14C] glucose (Perkin-Elmer; 1 µCi/ml, 5.0 mM glucose) in the presence or absence of 100 nM insulin for 2 h at 37°C. Following incubation, reaction media was transferred to a modified 48-well microtiter plate with fabricated grooves between two adjoining wells to allow for acid-driven 14CO2 from media to be trapped by 1 M NaOH. Cells were washed with ice-cold PBS and solubilized in 0.05% SDS, after which an aliquot was transferred to a 2-ml tube containing carrier glycogen (2 mg) and heated for 1 h at 100°C. The remaining lysate was used to assess protein concentration (bicinchoninic acid assay; Pierce Biotechnology, Rockford, IL). Glycogen was precipitated by the addition of 100% ethanol and overnight rotation at 4°C. Glycogen pellets were centrifuged (11,100 g for 15 min at 4°C), washed with 70% ethanol, and resuspended in dH2O. Incorporation of radioactive glucose into CO2 or glycogen was determined with liquid scintillation counting.
Tricarboxylic acid cycle flux.
To determine whether electrical stimulation alters flux through the tricarboxylic acid (TCA) cycle, we examined the oxidation of 2-[14C]pyruvate. Following contraction, cells were serum starved for 3 h and then treated with reaction media containing 2-[14C]pyruvate (0.5 µCi/ml, 1 mM sodium pyruvate) for 2 h. In comparison to 1-[14C]pyruvate, which is utilized to assess pyruvate dehydrogenase complex activity, the 14CO2 liberated following treatment with 2-[14C]pyruvate is derived exclusively from the TCA cycle. Following the 2-h incubation, acid-driven CO2 production was examined as described above.
Immunoblot analysis.
Following 3 h of serum starvation, myotubes were treated with 100 nM of insulin for 10 min, after which cells were harvested in ice-cold lysis buffer (50 mM HEPES, 12 mM sodium pyrophosphate, 100 mM sodium fluoride, 100 mM EDTA, 10 mM sodium orthovanate, 1% Triton X-100), supplemented with protease and phosphatase (1, 2) inhibitor cocktails (Sigma-Aldrich, St. Louis, MO). Lysates were sonicated, rotated (~1 h at 4°C), and centrifuged (12,000 rpm for 15 min at 4°C). Protein concentration was determined in supernatants (bicinchoninic acid assay). Supernatants were mixed with Laemmli’s buffer and heated at 95°C for 5 min.
Equal amounts of protein were subjected to SDS-PAGE, after which proteins were transferred to polyvinylidene difluoride membranes. Membranes were incubated with the following primary antibodies: phospho-acetyl CoA carboxylase 2 (ACC2; Ser79; Cell Signaling, Beverly, MA); total ACC2 (Cell Signaling); phospho-Akt (Ser473; Cell Signaling, Danvers, MA); total Akt (Cell Signaling); phospho-Akt-substrate at 160 kDa (AS160; Thr642; Abcam, Cambridge, MA); total AS160 (Millipore, Billerica, MA); citrate synthase (Millipore); GLUT-4 (Millipore); mitofusin 2 (MFN2; Abnova, Walnut, CA); myosin heavy chain I (MHC I) slow isoform (Developmental Studies Hybridoma Bank, Iowa City, IA); and peroxisome proliferator-activated receptor γ coactivator α (PGC-1α) (Abcam). The phospho-specific antibody for the Ser704 residue of AS160 was customized and generated by Capra Science (Sweden) and validated via overexpression in mouse tibialis anterior muscle. Membranes were probed with IRDye secondary antibodies (LI-COR Biosciences, Lincoln, NE), and band intensities quantified using Odyssey software (LI-COR Biosciences). Equal protein loading was verified by use of β-actin (LI-COR Biosciences).
Statistical analysis.
Two- and three-way ANOVA with repeated measures, and Student’s t-test were used to assess whether the combined effects of RYGB and electrical stimulation altered glucose metabolism and intracellular signaling in primary myotubes. Statistical significance was defined as P ≤ 0.05, and data are presented as means ± SE.
RESULTS
Subject characteristics.
Subject characteristics before and after RYGB surgery are presented in Table 1. Body mass decreased at 1 mo after the surgery (~17 kg, P < 0.05); however, subjects were still classified as severely obese, as BMI was >40 kg/m2. No changes in fasting blood glucose or insulin were apparent and resulted in a HOMA-IR index >2.5, implying a state of insulin resistance.
Insulin action.
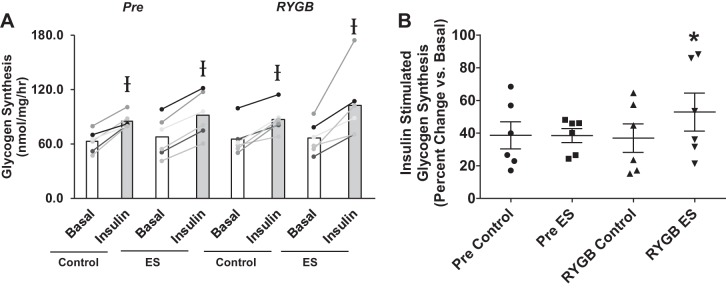
There were no changes in basal (noninsulin) glycogen synthesis rate following electrical stimulation or RYGB (Fig. 1A). Glycogen synthesis rate (an index of insulin action) increased with insulin exposure, regardless of condition (electrical stimulation, RYGB) (Fig. 1A). When data were expressed as relative change (percent increase with insulin vs. basal), the ability of insulin to stimulate glycogen synthesis above the basal condition was unaltered with electrical stimulation in myotubes before surgery (Pre) (Fig. 1B). However, cells derived from the same patients following RYGB surgery demonstrated an increase in insulin-stimulated glycogen synthesis after 24 h of electrical stimulation compared with no stimulation (control condition) (Fig. 1B).
Fig. 1.
Absolute (A) and relative (B) basal and insulin-stimulated glycogen synthesis rates in myotubes derived from RYGB patients before (Pre) and after surgery (RYGB) under control conditions and after 24 h of pulsed electrical stimulation (ES). Values are means ± SE; n = 6 subjects/group. Ɨ P < 0.05 vs. basal, denoting an insulin effect. *P < 0.05 vs. RYGB Control, denoting an electrical stimulation effect after RYGB.
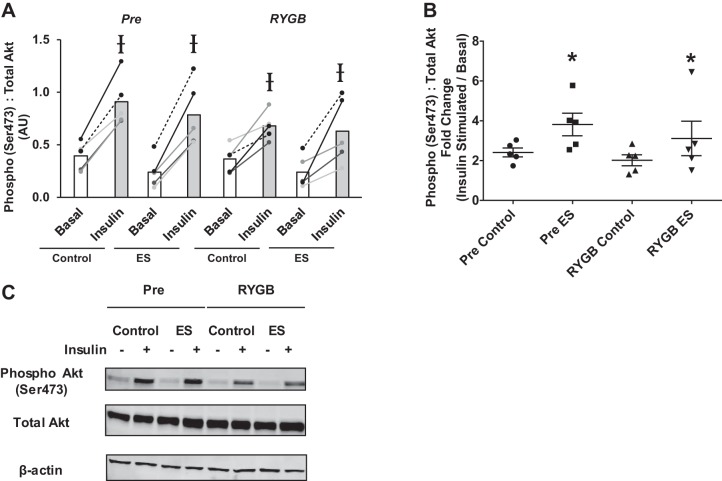
There were no changes in non-insulin (basal)-stimulated Akt Ser473 phosphorylation with either electrical stimulation or RYGB; however, insulin exposure consistently increased phosphorylation (Fig. 2A). When data were expressed as relative change (insulin-stimulated divided by basal), insulin exposure increased Akt phosphorylation at the Ser473 residue by two- to fourfold (Fig. 2B). As presented in Fig. 2B, electrical stimulation enhanced insulin-mediated Akt phosphorylation at the Ser473 residue to the same extent both before and after RYGB.
Fig. 2.
Absolute (A) and relative (B) basal and insulin-stimulated phosphorylation of Akt (Ser473) in myotubes derived from RYGB patients before (Pre) and after surgery (RYGB) under control conditions and after 24 h of pulsed electrical stimulation (ES). Values are means ± SE in arbitrary units (AU; A) and fold change (insulin stimulated/basal; B); n = 5 subjects/group. Ɨ P < 0.05 vs. basal, denoting an insulin effect. *P < 0.05 vs. Control, denoting an electrical stimulation effect. C: representative immunoblot images for phospho-Akt, total Akt, and β-actin.
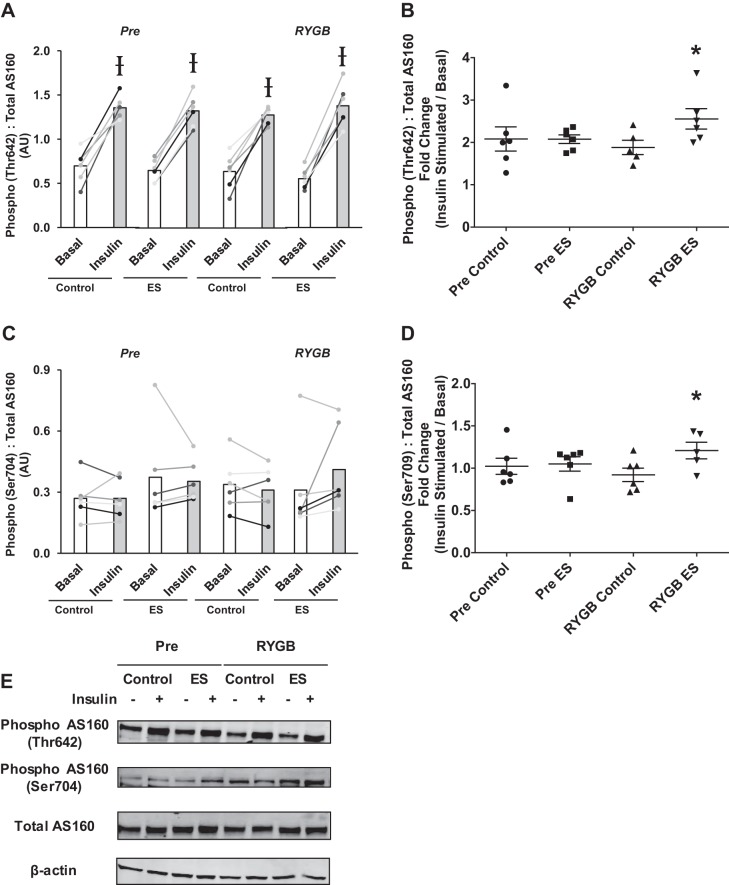
AS160, also known as TBC1D4, has been considered a major target of both insulin and contraction-mediated glucose metabolism in skeletal muscle (12). There were no changes in the basal phosphorylation levels of AS160 at two different residues (Thr642 and Ser704) with RYGB (Fig. 3, A and C). Insulin consistently increased the phosphorylation of Thr642, but not Ser704 (Fig. 3, A and C). Relative (fold-change) insulin-stimulated AS160 phosphorylation was enhanced following electrical stimulation in myotubes derived from patients following RYGB surgery compared with no electrical stimulation (control), but there was no increase with electrical stimulation Pre (Fig. 3, B and D). There were no changes in the protein abundance of GLUT-4 with electrical stimulation or surgery (see Fig. 5).
Fig. 3.
Absolute (A and C) and relative (B and D) insulin-stimulated phosphorylation of AS160 at residues Thr642 (A and B) and Ser704 (C and D) in myotubes derived from RYGB patients before (Pre) and after surgery (RYGB) under control conditions and after 24 h of pulsed electrical stimulation (ES). Values are means ± SE in arbitrary units (A and C) and fold change (insulin stimulated/basal; B and D); n = 6 subjects/group. E: representative immunoblot images for phosphor-AS160 (Thr642 and Ser704), total AS160, and β-actin. Ɨ P < 0.05 vs. basal, denoting an effect of insulin. *P < 0.05 vs. RYGB Control, denoting an effect of electrical stimulation after RYGB.
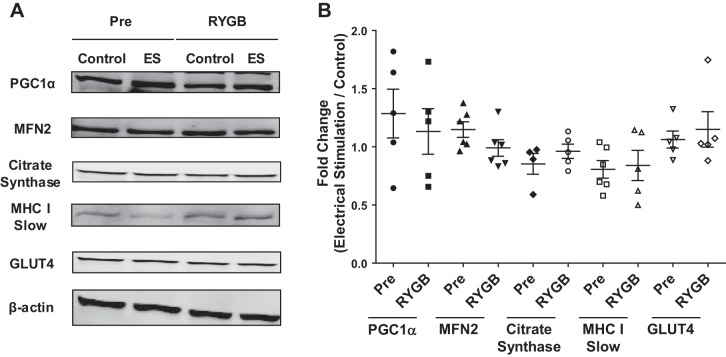
Fig. 5.
Representative immunoblot images for (from top to bottom) PGC-1α, MFN2, citrate synthase, MHC I (slow) isoform, GLUT-4, and β-actin protein content following electrical stimulation (ES) of myotubes derived from RYGB patients before (Pre) and after (RYGB) surgery (A) and expressed as fold change over control with ES (B). Values are means ± SE; n = 6 subjects/group. None of these parameters was altered with either surgery or electrical stimulation.
Substrate oxidation.
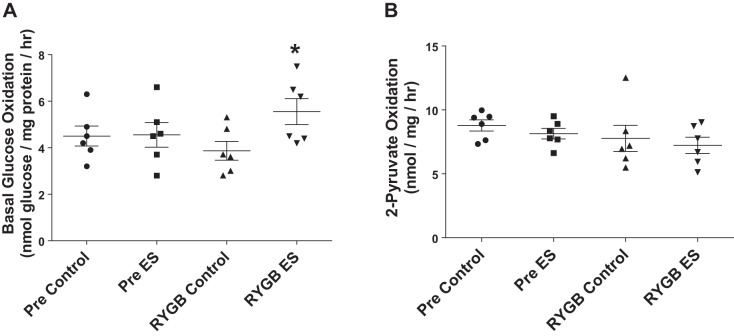
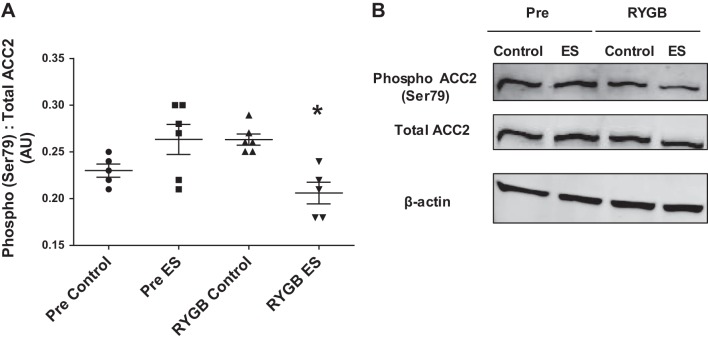
Basal (noninsulin) glucose oxidation was unaltered following electrical stimulation in cells derived from patients Pre (Fig. 4A). In contrast, myotubes derived from patients after RYGB displayed an ~1.5-fold increase in basal glucose oxidation following electrical stimulation (Fig. 4A). In an attempt to determine whether changes in basal glucose oxidation were due to increased TCA cycle flux, we examined the rate of 2-[14C]pyruvate oxidation following electrical stimulation; however, 2-[14C]pyruvate oxidation was not altered following electrical stimulation either before or after RYGB (Fig. 4B). There were no changes in the protein abundance of factors linked with mitochondrial function/content with electrical stimulation or gastric bypass (Fig. 5) (PGC-1α, MFN2, citrate synthase, MHC I). Phosphorylation of ACC2 was significantly reduced following electrical stimulation in myotubes derived from RYGB patients, with no change Pre (Fig. 6).
Fig. 4.
Oxidation of [U-14C]glucose (A) and 2-[14C]pyruvate (B) in myotubes derived from RYGB patients before (Pre) and after (RYGB) surgery under control conditions and after 24 h of pulsed electrical stimulation (ES). Values are means ± SE; n = 6 subjects/group. *P < 0.05 vs. RYGB Control, denoting an effect of electrical stimulation after RYGB.
Fig. 6.
Absolute (A) and representative immunoblot images (B) for ACC2 Ser79 phosphorylation before (Pre) and after RYGB (RYGB) under control conditions and after 24 h of pulsed electrical stimulation (ES). Values are means ± SE; n = 6 subjects/group. *P < 0.05 vs. RYBG Control, denoting an effect of electrical stimulation after RYGB.
DISCUSSION
Individuals who are severely obese may be resistant to some of the positive metabolic effects gained with exercise/exercise training (38). RYGB is an effective surgical tool to aid in weight loss and metabolic improvement, and recent evidence suggests that the combined effects of the surgery and exercise can enhance whole body and skeletal muscle metabolism (6, 14, 15). To specifically focus on skeletal muscle, we utilized the primary cell culture model, in combination with electrical stimulation, to examine alterations in insulin action and fuel oxidation with contraction. Our data reveal that, following RYGB surgery, skeletal muscle cells are more responsive to electrical stimulation in terms of increasing insulin-stimulated glycogen synthesis and basal glucose oxidation (Figs. 1 and 4). These data suggest that RYGB surgery can enhance the ability of the skeletal muscle from severely obese individuals to improve some components of carbohydrate metabolism with contractile activity.
A dampened capacity to respond to exercise signals has been shown in the skeletal muscle of severely obese/insulin-resistant patients under both in vivo and in vitro conditions. After a single bout of exercise, the skeletal muscle of insulin-resistant obese individuals exhibited a minimized response of nuclear-encoded mitochondrial genes and a more transient activation of AMP-activated protein kinase (AMPK) compared with lean individuals (20). Similarly, skeletal muscle AMPK activity and AS160 phosphorylation were attenuated after a single bout of exercise in obese subjects (37). Moreover, treatment of primary myotubes derived from severely obese subjects with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside, a pharmacological agonist of AMPK, did not rescue the insulin-resistant phenotype (8). PGC-1α is an transcription factor involved with contraction-induced adaptations, including improved oxidative metabolism and glucose utilization (27). In support of possible exercise resistance, it was reported that overexpression of PGC-1α did not increase lipid oxidation in myotubes from severely obese individuals to the same extent as in tissue from lean subjects (17). Collectively, these results, along with the present data, suggest that skeletal muscle of severely obese individuals is potentially resistant to some alterations associated with both acute and chronic muscle contraction.
Despite sustained severe obesity (BMI > 40 kg /m2) and insulin resistance (HOMA > 2.5) (Table 1), muscle cells from patients following RYGB were more responsive to the combined effects of contraction and insulin, as indicated by enhanced insulin-stimulated glycogen synthesis (Fig. 1B). The present data suggest that a mechanism by which electrical stimulation improved insulin action after RYGB may involve enhanced insulin-stimulated phosphorylation of AS160 (Fig. 3). AS160 is considered a major target for improving insulin sensitivity, as muscle contraction can enhance insulin-stimulated AS160 phosphorylation at the Thr642 site for up to 27 h postexercise (2, 21). While exercise signals, such as AMPK, may be insufficient to phosphorylate the Thr642 site (40, 41), recent data suggest that phosphorylation of Ser704 (Ser711 in mouse muscle) allows the Thr642 site to be more accessible (24). Furthermore, Ser704 is responsive to insulin, with a greater phosphorylation after exercise in the presence of insulin (40, 41). In agreement with these data, we observed enhanced insulin-stimulated phosphorylation of the Ser704 and Thr642 sites following muscle contraction in cells derived from patients after RYGB surgery (Fig. 3, B and D). These findings suggest improved insulin-stimulated glucose metabolism following muscle contraction in myotubes derived from RYGB patients is potentially due to a synergistic role of enhanced phosphorylation of AS160. Akt phosphorylation at the Ser473 location was not potentiated with RYGB (Fig. 2). Akt Thr308 phosphorylation (which was not determined) may have, however, responded differently and, in turn, modulated downstream signal transduction and metabolism in a manner similar to AS160 (10, 43).
Along with positive adaptations in insulin action, the combined effects of RYGB and electrical stimulation also led to an increase in glucose oxidation (Fig. 4A). This finding is in agreement with previous work that showed an increase in respiration of carbohydrate-derived substrates in permeabilized muscle fibers derived from RYGB patients who underwent 6 mo of exercise training (14). In terms of possible mechanisms, there was a decline in ACC2 phosphorylation with electrical stimulation + RYGB (Fig. 6), which is possibly indicative of reduced AMPK signaling. This is analogous to observations after a short period of exercise training where AMPK and ACC phosphorylation were dampened in response to acute exercise (29). When unphosphorylated, ACC2 is active, resulting in increased cellular concentrations of malonyl-CoA, a prominent inhibitor of lipid oxidation (35). Although not determined in the present study, a greater suppression in lipid oxidation concomitant with increased ACC2 activity following electrical stimulation would, in theory, result in enhanced reliance on glucose as a fuel source for high-intensity muscle contraction (Fig. 4A).
In agreement with others using a similar electrical stimulation protocol (30), we did not observe a change in indexes of mitochondrial content (citrate synthase) and MHC I slow isoform composition (Fig. 5). It is possible that RYGB, in combination with contractile activity, induces a metabolic profile in which mitochondria are more efficient in oxidizing fuels under increased energetic supply and/or demand. In addition, glucose oxidation could have increased due to elevated glucose uptake. However, any hypothesis is speculative, and the mechanism(s) linked with the enhanced ability for glucose oxidation in response to contractile activity with RYGB remains to be investigated.
A divergent and counterintuitive downregulation of genes associated with substrate utilization and mitochondrial biogenesis has been reported in some individuals in response to exercise (39). This downregulation may stem from alterations in the epigenetic profile of skeletal muscle, which, in turn, evokes exercise resistance (38). It has been shown that RYGB surgery induces epigenetic modifications in skeletal muscle, likely mediated through weight loss (4). In the present study, we observed that the improved ability of the myotubes to respond to contractile activity occurred when weight loss (Table 1) was evident. These data suggest that RYGB surgery may alter the inherent profile of skeletal muscle in a manner that results in a more robust response to contractile activity; however, the role of possible epigenetic alterations is not evident. The potential involvement of circulating/secreted factors from skeletal muscle could also be involved (26). The differences noted with RYGB do not appear to be due to changes in muscle differentiation characteristics with the intervention (i.e., no differences in GLUT-4 pre- and post-RYGB surgery (Fig. 5).
In conclusion, the results of the present study suggest that skeletal muscle cells derived from severely obese patients following RYGB surgery are more responsive to electrical stimulation in terms of enhancing insulin action and glucose oxidation than before the surgery. While the mechanism for increased basal glucose oxidation is unclear, improved insulin action following electrical stimulation and RYGB surgery may be linked with enhanced insulin-stimulated AS160 phosphorylation. Collectively, our results, along with those from others (14, 15), suggest that an exercise program should be implemented following RYGB (1–3 mo) to potentiate muscle-specific improvements in metabolism.
GRANTS
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-56112; J. A. Hinkley) and an American Heart Association Postdoctoral Fellowship (15POST25080003; K. Zou).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.M.H. and J.A.H. conceived and designed research; J.M.H., K.Z., S.P., K.T., and D.Z. performed experiments; J.M.H., K.Z., and S.P. analyzed data; J.M.H., K.Z., S.P., K.T., D.Z., and J.A.H. interpreted results of experiments; J.M.H. prepared figures; J.M.H. drafted manuscript; J.M.H., K.Z., S.P., K.T., D.Z., and J.A.H. edited and revised manuscript; J.M.H., K.Z., S.P., K.T., D.Z., and J.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Gabe Dubis and Angela Clark (East Carolina University) for assisting in specimen collection, and Dr. Jonas T. Treebak (Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen) for providing the phospho-AS160 (Ser704) primary antibody.
Present addresses: J. M. Hinkley, Dept. of Applied Physiology and Kinesiology, University of Florida, Gainesville, FL; K. Zou, Dept. of Exercise and Health Sciences, University of Massachusetts Boston, Boston, MA.
REFERENCES
- 1.Al-Khalili L, Chibalin AV, Kannisto K, Zhang BB, Permert J, Holman GD, Ehrenborg E, Ding VDH, Zierath JR, Krook A. Insulin action in cultured human skeletal muscle cells during differentiation: assessment of cell surface GLUT4 and GLUT1 content. Cell Mol Life Sci 60: 991–998, 2003. doi: 10.1007/s00018-003-3001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007. doi: 10.1152/ajpendo.00602.2006. [DOI] [PubMed] [Google Scholar]
- 4.Barres R, Kirchner H, Rasmussen M, Yan J, Kantor FR, Krook A, Näslund E, Zierath JR. Weight loss after gastric bypass surgery in human obesity remodels promoter methylation. Cell Rep 3: 1020–1027, 2013. [Erratum. Cell Rep 3: 1755, 2013.] doi: 10.1016/j.celrep.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Bell JA, Reed MA, Consitt LA, Martin OJ, Haynie KR, Hulver MW, Muoio DM, Dohm GL. Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J Clin Endocrinol Metab 95: 3400–3410, 2010. doi: 10.1210/jc.2009-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berggren JR, Boyle KE, Chapman WH, Houmard JA. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am J Physiol Endocrinol Metab 294: E726–E732, 2008. doi: 10.1152/ajpendo.00354.2007. [DOI] [PubMed] [Google Scholar]
- 7.Berggren JR, Tanner CJ, Houmard JA. Primary cell cultures in the study of human muscle metabolism. Exerc Sport Sci Rev 35: 56–61, 2007. doi: 10.1249/JES.0b013e31803eae63. [DOI] [PubMed] [Google Scholar]
- 8.Bikman BT, Zheng D, Reed MA, Hickner RC, Houmard JA, Dohm GL. Lipid-induced insulin resistance is prevented in lean and obese myotubes by AICAR treatment. Am J Physiol Regul Integr Comp Physiol 298: R1692–R1699, 2010. doi: 10.1152/ajpregu.00190.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchard C, Blair SN, Church TS, Earnest CP, Hagberg JM, Häkkinen K, Jenkins NT, Karavirta L, Kraus WE, Leon AS, Rao DC, Sarzynski MA, Skinner JS, Slentz CA, Rankinen T. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One 7: e37887, 2012. doi: 10.1371/journal.pone.0037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouzakri K, Zachrisson A, Al-Khalili L, Zhang BB, Koistinen HA, Krook A, Zierath JR. siRNA-based gene silencing reveals specialized roles of IRS-1/Akt2 and IRS-2/Akt1 in glucose and lipid metabolism in human skeletal muscle. Cell Metab 4: 89–96, 2006. doi: 10.1016/j.cmet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Boyle KE, Zheng D, Anderson EJ, Neufer PD, Houmard JA. Mitochondrial lipid oxidation is impaired in cultured myotubes from obese humans. Int J Obes 36: 1025–1031, 2012. doi: 10.1038/ijo.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartee GD, Funai K. Exercise and insulin: Convergence or divergence at AS160 and TBC1D1? Exerc Sport Sci Rev 37: 188–195, 2009. doi: 10.1097/JES.0b013e3181b7b7c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coen PM, Menshikova EV, Distefano G, Zheng D, Tanner CJ, Standley RA, Helbling NL, Dubis GS, Ritov VB, Xie H, Desimone ME, Smith SR, Stefanovic-Racic M, Toledo FGS, Houmard JA, Goodpaster BH. Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes 64: 3737–3750, 2015. doi: 10.2337/db15-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coen PM, Tanner CJ, Helbling NL, Dubis GS, Hames KC, Xie H, Eid GM, Stefanovic-Racic M, Toledo FGS, Jakicic JM, Houmard JA, Goodpaster BH. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. J Clin Invest 125: 248–257, 2015. doi: 10.1172/JCI78016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B; American College of Sports Medicine; American Diabetes Association . Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care 33: e147–e167, 2010. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Consitt LA, Bell JA, Koves TR, Muoio DM, Hulver MW, Haynie KR, Dohm GL, Houmard JA. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha overexpression increases lipid oxidation in myocytes from extremely obese individuals. Diabetes 59: 1407–1415, 2010. doi: 10.2337/db09-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohm GL, Tapscott EB, Pories WJ, Dabbs DJ, Flickinger EG, Meelheim D, Fushiki T, Atkinson SM, Elton CW, Caro JF. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest 82: 486–494, 1988. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 20.De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab 294: E607–E614, 2008. doi: 10.1152/ajpendo.00729.2007. [DOI] [PubMed] [Google Scholar]
- 21.Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab 297: E242–E251, 2009. doi: 10.1152/ajpendo.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haskell WL, Lee I-M, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39: 1423–1434, 2007. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 23.Houmard JA, Pories WJ, Dohm GL. Is there a metabolic program in the skeletal muscle of obese individuals? J Obes 2011: 250496, 2011. doi: 10.1155/2011/250496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279: E1039–E1044, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Lambernd S, Taube A, Schober A, Platzbecker B, Görgens SW, Schlich R, Jeruschke K, Weiss J, Eckardt K, Eckel J. Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia 55: 1128–1139, 2012. doi: 10.1007/s00125-012-2454-z. [DOI] [PubMed] [Google Scholar]
- 27.Lira VA, Benton CR, Yan Z, Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab 299: E145–E161, 2010. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manabe Y, Miyatake S, Takagi M, Nakamura M, Okeda A, Nakano T, Hirshman MF, Goodyear LJ, Fujii NL. Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS One 7: e52592, 2012. doi: 10.1371/journal.pone.0052592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McConell GK, Lee-Young RS, Chen ZP, Stepto NK, Huynh NN, Stephens TJ, Canny BJ, Kemp BE. Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J Physiol 568: 665–676, 2005. doi: 10.1113/jphysiol.2005.089839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muoio DM, Way JM, Tanner CJ, Winegar DA, Kliewer SA, Houmard JA, Kraus WE, Dohm GL. Peroxisome proliferator-activated receptor-alpha regulates fatty acid utilization in primary human skeletal muscle cells. Diabetes 51: 901–909, 2002. doi: 10.2337/diabetes.51.4.901. [DOI] [PubMed] [Google Scholar]
- 31.Nikolić N, Bakke SS, Kase ET, Rudberg I, Flo Halle I, Rustan AC, Thoresen GH, Aas V. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS One 7: e33203, 2012. [Erratum. PLoS One 8: 2013.] doi: 10.1371/journal.pone.0033203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pories WJ, Card JF, Flickinger EG, Meelheim HD, Swanson MS. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg 206: 316–323, 1987. doi: 10.1097/00000658-198709000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pories WJ, MacDonald KG Jr, Flickinger EG, Dohm GL, Sinha MK, Barakat HA, May HJ, Khazanie P, Swanson MS, Morgan E, Leggett-Frazier N, Long SD, Brown BM, OʼBrien K, Card JF. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg 215: 633–642, 1992. doi: 10.1097/00000658-199206000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raschke S, Eckardt K, Bjørklund Holven K, Jensen J, Eckel J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS One 8: e62008, 2013. doi: 10.1371/journal.pone.0062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen BB, Holmbäck UC, Volpi E, Morio-Liondore B, Paddon-Jones D, Wolfe RR. Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase-1 activity and fat oxidation in human skeletal muscle. J Clin Invest 110: 1687–1693, 2002. doi: 10.1172/JCI0215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes 56: 836–848, 2007. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephens NA, Sparks LM. Resistance to the beneficial effects of exercise in type 2 diabetes: are some individuals programmed to fail? J Clin Endocrinol Metab 100: 43–52, 2015. doi: 10.1210/jc.2014-2545. [DOI] [PubMed] [Google Scholar]
- 39.Stephens NA, Xie H, Johannsen NM, Church TS, Smith SR, Sparks LM. A transcriptional signature of “exercise resistance” in skeletal muscle of individuals with type 2 diabetes mellitus. Metabolism 64: 999–1004, 2015. doi: 10.1016/j.metabol.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treebak JT, Pehmøller C, Kristensen JM, Kjøbsted R, Birk JB, Schjerling P, Richter EA, Goodyear LJ, Wojtaszewski JFP. Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle. J Physiol 592: 351–375, 2014. doi: 10.1113/jphysiol.2013.266338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treebak JT, Taylor EB, Witczak CA, An D, Toyoda T, Koh H-J, Xie J, Feener EP, Wojtaszewski JFP, Hirshman MF, Goodyear LJ. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am J Physiol Cell Physiol 298: C377–C385, 2010. doi: 10.1152/ajpcell.00297.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wojtaszewski JFP, Richter EA. Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays Biochem 42: 31–46, 2006. doi: 10.1042/bse0420031. [DOI] [PubMed] [Google Scholar]
- 43.Yung HW, Charnock-Jones DS, Burton GJ. Regulation of AKT phosphorylation at Ser473 and Thr308 by endoplasmic reticulum stress modulates substrate specificity in a severity dependent manner. PLoS One 6: e17894, 2011. doi: 10.1371/journal.pone.0017894. [DOI] [PMC free article] [PubMed] [Google Scholar]