Abstract
Salt-sensitive hypertension is associated with renal and vascular dysfunctions, which lead to impaired fluid excretion, increased cardiac output, and total peripheral resistance. It is commonly accepted that increased renal sodium handling and plasma volume expansion are necessary factors for the development of salt-induced hypertension. The epithelial sodium channel (ENaC) is a trimeric ion channel expressed in the distal nephron that plays a critical role in the regulation of sodium reabsorption in both normal and pathological conditions. In this mini-review, we summarize recent studies investigating the role of ENaC in the development of salt-sensitive hypertension. On the basis of experimental data obtained from the Dahl salt-sensitive rats, we and others have demonstrated that abnormal ENaC activation in response to a dietary NaCl load contributes to the development of high blood pressure in this model. The role of different humoral factors, such as the components of the renin-angiotensin-aldosterone system, members of the epidermal growth factors family, arginine vasopressin, and oxidative stress mediating the effects of dietary salt on ENaC are discussed in this review to highlight future research directions and to determine potential molecular targets for drug development.
salt-sensitive hypertension is a widespread disorder associated with increased cardiovascular events and reduced survival (94). Clinical tests for salt sensitivity vary in the literature, but most of them generally define this phenomenon as blood pressure alteration in response to a change in NaCl intake (22). The pathogenesis of salt-sensitive (SS) hypertension includes intrarenal and extrarenal factors affecting water-sodium balance leading to cardiorenal dysfunction; high-salt intake causes sodium retention and plasma volume expansion which, together with an increased total peripheral resistance, are responsible for blood pressure elevation in salt-sensitive subjects (21, 43, 49, 72). In this mini-review, we focus on Na+ reabsorption in the distal nephron, which is mainly mediated by the epithelium sodium channel (ENaC). Amiloride (trade name: Midamor), a selective ENaC blocker, is used in the treatment of such monogenic forms of hypertension as Liddle’s syndrome and T594M polymorphism of ENaC (both causing overactivation of the channel), apparent mineralocorticoid excess, and familial hyperaldosteronism-1 (6, 28). Therefore, ENaC is an important therapeutic target involved in the body fluid volume expansion which, in combination with other factors, underlies the genesis of hypertension, especially its salt-induced form (26, 28).
ENaC is a classic effector of the renin-angiotensin-aldosterone system (RAAS); in addition, it is regulated by other neurohumoral factors, such as arginine vasopressin (AVP) and atrial natriuretic peptide, which are involved in modulation of water-salt balance and blood pressure (27, 52, 74, 87, 93). Although aldosterone-sensitive distal parts of nephron and collecting ducts (CDs) account for less than 5–10% of Na+ reabsorption, the significance of these segments for total sodium handling is very high (79). First, ENaC is expressed downstream from the macula densa. Therefore, reabsorption in these segments occurs after tubuloglomerular feedback interaction and is critical in determining final urinary sodium concentration (12, 29, 58). As it was recently proposed, ENaC in the connecting tubules serves as the central component of connecting tubule glomerular feedback (CTGF) mechanism, sensing Na+ delivery from proximal parts of nephron and regulating afferent arteriole resistance (69–71). Second, ENaC-mediated sodium handling can affect the activity of other sodium transporters. For example, it was shown that suppression of ENaC can lead to a low-sodium chloride symporter function, which strengthens the natriuretic effect of amiloride (85, 89).
Dahl SS Rat as a Model for Studies of Salt-Induced Hypertension
Sufficient data about involvement of ENaC in salt-sensitive hypertension came from studies using the Dahl SS rat strain. This model recapitulates many traits of the human form of salt-sensitive hypertension, such as suppressed plasma renin activity, lower circulating aldosterone concentration, increased vascular resistance, impaired pressure-natriuresis, and decreased venous compliance (13, 64, 65, 86). A hallmark of Dahl SS rats is the rapid progression of hypertension. Typical experimental protocols use 4% or 8% NaCl chows that cause a fast rise in blood pressure, becoming statistically significant just 2 days after the change of diet (15, 17, 30). Additionally, hypertension in Dahl SS rats is accompanied by the marked progression of renal injury. Clinically, there is also evidence that salt-sensitive patients are more susceptible to renal injury (11). Furthermore, Dahl SS rats are characterized by an increased CTGF, severe glomerular damage, and progressive proteinuria (13, 73, 83, 91).
High-Salt Diet Causes Abnormal Renal Hyperactivation of ENaC in Dahl SS Rats
In salt-resistant animal models, ENaC-driven reabsorption is diminished in response to salt load and exaggerated during water deprivation (10, 24, 25, 57, 63, 82); however, in salt-sensitive hypertension, the situation changes. For instance, a large set of data obtained from this strain indicates that ENaC in the central nervous system serves as a sensor of salt load and, thus, contributes to the development of hypertension via RAAS activation (2, 88, 92). However, because of the brief nature of this review, we will only focus on the regulation of ENaC in the kidney. Aoi et al. (3) reported that renal α-ENaC and β-ENaC mRNA levels were increased in SS rats fed an 8% diet for 4 wk compared with control animals fed a 0.3% diet. Later, abnormal upregulation of all three ENaC mRNA subunits, as well as mRNA for SGK1 (serum/glucocorticoid-regulated kinase 1, known to play an essential role in stimulation of ENaC activity), was reported in the SS rats fed a high (8% NaCl) salt diet by the same group of authors (4). Furthermore, Western blot analysis revealed that increased β- and γ-subunits (but not the α-subunit) protein abundance accompanies the development of hypertension on an 8% NaCl diet. Expression of cleaved γ-subunit was also found to be elevated (36). Our recent studies have confirmed these observations; we found higher β-ENaC and γ-ENaC protein abundance (including cleaved form of γ-subunit) in the cortex of SS rats fed a high-salt diet (4%) compared with the low-salt diet (0.4%). This effect was not found in a salt-resistant consomic SS.BN13 strain generated by introgression of chromosome 13 from Brown Norway rats into the Dahl SS background. Importantly, we also assessed β-ENaC abundance in a servo-controlled model, where the left kidney was protected from high renal perfusion pressure. Servo-controlled studies allowed us to separate effects of changes in blood pressure from system factors (circulating hormones and other blood components, innervation, diet) since a surgically installed cuff automatically occludes the aorta if BP exceeds 120 mmHg; this procedure protects the left kidney from mechanistic effects of increases in blood pressure, but keeps it in the same neurohumoral environment as the nonprotected right kidney (34, 54). Histological evaluation demonstrated that the right kidneys, which were exposed to high systemic blood pressure, had higher β-ENaC abundance than the left kidneys, in which perfusion pressure was controlled at the normal level (61). These data allow us to conclude that high renal perfusion pressure and/or associated tissue damage increase cortical ENaC abundance during high-salt load in the SS rats.
It is known that neither ENaC mRNA nor protein levels reflect electrical activity of the functional channels in the cell membrane. Therefore, we used patch-clamp experiments on split-open cortical collecting duct (CCD)/connecting tubule (CNT) segments to directly study channel activity in the tubules isolated from 11-wk-old SS animals fed a normal 0.4%, or a 4% diet for 3 wk. The studies revealed that a high-salt diet increases activity of ENaC via the elevation channel number in the apical membrane. Importantly, ENaC activity measured with electrophysiology did not change in salt-resistant SS.BN13 rats when animals were challenged with a high-salt diet. Additionally, pharmacological blockade of ENaC with amiloride or benzamil significantly precluded development of hypertension in Dahl SS rats (36, 61). Together, these findings indicate that abnormal high cortical ENaC activity and abundance contribute to the development of salt-sensitive hypertension in Dahl SS rats.
Another study tested ENaC expression in inner medullary collecting duct (1). Comparison of the SS rats with a control salt-resistant (SR) strain on a regular (0.3%) salt diet revealed a greater abundance of basal α-ENaC in SS rats. 8% NaCl diet increased the apical membrane staining for β-ENaC and γ-ENaC but did not affect the apical localization of α-ENaC (1). These observations suggest that the persistent high expression of α-ENaC, increased apical localization of β-ENaC and γ-ENaC, and high channel activity in SS rats on a high-salt diet may contribute to retention of sodium and total body fluid expansion and, therefore, result in elevated blood pressure.
Renin-Angiotensin-Aldosterone System in the Regulation of ENaC in SS Rats
The exact mechanism(s) that mediates effects of a high-salt diet on ENaC in SS rats remains unclear. SS rats are considered to be a systemic low-renin model, and high-salt consumption decreases plasma renin activity even further (5). However, hypertension in these animals is accompanied with activation of the local paracrine RAAS system. Kidney transplantation from SS to SR rats dramatically increases salt susceptibility in the latter strain (16) that illustrates the importance of intrarenal factors for the development of hypertension. Although plasma renin activity and concentration of angiotensinogen, ANG II, and aldosterone are suppressed by changing dietary NaCl from 0.3% to 8%, intrarenal levels of these endocrine factors can be significantly elevated (9, 41, 42). A paradoxical activation of renal mineralocorticoid receptors (MR) was also discovered in salt-sensitive hypertension (77). Furthermore, adrenalectomized SS rats do not develop hypertension on a high-salt diet, whereas exogenous aldosterone supplement reverses this phenomenon (77). The critical role of the RAAS components as a key factor of salt sensitivity and positive regulation of ENaC may be illustrated by experiments on the renin (encoded by Ren gene) knockout Dahl SS rats. These rats are unable to synthetize functional renin and are characterized by loss of urine-concentrating ability and low blood pressure (~60 mmHg) (53, 60). Single-channel patch-clamp analysis revealed decreased ENaC activity in Ren−/− rats, which was mediated via changes in the channel open probability (60).
The paradoxical MR activation mentioned above can occur independent of aldosterone through activation of a small GTPase Rac1 (38). Rac1 cycles between an inactive guanosine diphosphate and active guanosine triphosphate-bound forms. In its active form, Rac1 regulates ubiquitination, cytoskeleton rearrangements, and membrane trafficking and might be involved in control of ion channel activity. In addition, it serves as a structural unit of NADPH oxidases. Shibata et al. (78) investigated activation of MR and Rac1 in Dahl SS and SR rats and found that an 8% salt diet elevated the abundance of active Rac1, SGK1, and MR in SS rats, but suppressed them in SR rats (78). This aberrant Rac1-MR activation in response to sodium load led to severe albuminuria, high blood pressure, and renal damage, which could be prevented by pharmacological Rac1 inhibition. The authors also demonstrated, using a mouse model in which mice lack RhoGDIα, an adaptor protein keeping Rac1 in the inactive form, which causes excessive renal Rac1 activity and makes this knockout strain salt-sensitive. Hyperactivity of Rac1 has been described earlier to upregulate ENaC-mediated current density in cell cultures and in cortical CDs isolated from Sprague-Dawley rats (37, 59, 80). We also investigated the involvement of Rac1 in 4% salt-induced excessive ENaC activity and found that a high-salt diet decreased the abundance of RhoGDIα in CDs of SS rats. Ex vivo studies revealed that low RhoGDIα levels augmented the bioavailability of Rac1 and activated ENaC-mediated Na+ reabsorption (37, 62). These findings indicate that a high-salt diet leads to renal MR activation and RhoGDIα downregulation, which increase ENaC-mediated sodium reabsorption via higher Rac1 activity.
Other Hormones Involved in ENaC Control in Salt-Induced Hypertension
Humoral factors involved in regulation of ENaC in salt-sensitive hypertension spread beyond the components of the RAAS. We have observed that 4% salt diet decreases the level of the EGF in the cortex of SS rats. EGF is a peptide involved in cell migration, differentiation, and proliferation and was also shown to regulate the activity of ion channels. Earlier, we and others demonstrated that chronic EGF treatment decreases ENaC activity (45, 46), and EGF family members are involved in the development of hypertension (81). We found that restoration of EGF levels via an intravenous supplementation decreased ENaC activity in SS rats on an 8% salt diet and prevented the increase in blood pressure and kidney injury. Thus, we conclude that EGF deficiency contributes to the development of salt-sensitive hypertension via ENaC overactivation (61). The low RhoGDIα level caused by MR activation may be a permissive factor for activation of Rac1 during EGF deficiency, but further investigation is needed to test this hypothesis.
Another hormone that is critical for regulation of sodium reabsorption is AVP (7, 8, 67, 68, 90). In the kidney, AVP participates in maintaining body fluid homeostasis by regulating water, urea and ion transport, glomerular filtration rate, and renal blood flow. It is well known that AVP exerts its antidiuretic effect by regulating sodium and water transport via the V2 receptor, which is expressed in the basolateral membrane of the thick ascending limb of Henle's loop, distal tubules, and the CDs (40). In mice, AVP has a prominent stimulatory effect on ENaCʼs open probability, as well as the number of active channels and is involved in the regulation of plasma tonicity (10, 51, 52, 84). Moreover, in adrenalectomized mice, plasma AVP is increased, and this was shown to upregulate ENaC activity and compensate for the aldosterone deficiency. There are no studies on the involvement of AVP in ENaC regulation during development of SS hypertension, but the observations reported above strongly suggest a possible role for AVP in salt sensitivity. It is well characterized in Sprague-Dawley rats that vasopressin infusion (or water deprivation) increases the mRNA (55) and protein (19, 20) abundance of the β- and γ-subunits of ENaC (39, 50). This effect is similar to the ramifications of a high-salt diet challenge in Dahl SS rats.
Reactive Oxygen Species Production as the Pivotal Mechanism of ENaC Regulation in SS Hypertension
Increased levels of oxidative stress have been many times reported in Dahl SS rats (14, 34, 56) and hypertensive patients (44, 66); this appears to be an attractive signaling mechanism linking a high-salt diet and elevated ENaC activity. Major sources of reactive oxygen species (ROS) production in the kidney include tubular NADPH oxidase enzymatic complexes and infiltration of lymphocytes, both of which are increased by a high-salt diet in SS rats (17, 18, 31, 33, 35). A panel of studies indicates that knockout of NADPH oxidase subunits p67 and Nox4, or proteins responsible for T- and B-lymphocyte maturation, have renoprotective effects and may preclude development of high blood pressure in Dahl SS rats (15, 23, 76, 95). Principal cells of the CCDs are capable of producing and secreting ROS (75). We demonstrated that ROS increases ENaC activity in immortalized CCD cells (32), and activation of ENaC by hydrogen peroxide was reported earlier in amphibian renal cells (48). A direct link between Nox4 activity and ENaC-driven sodium reabsorption using immortalized mouse CCD principal cell line was shown as the mechanism mediating stimulation of ENaC by prorenin (47). However, the role NADPH oxidase produced ROS in ENaC hyperactivity in SS hypertension requires further investigation.
Conclusion
It still remains unclear which intracellular mechanisms activate ENaC in the CNT/CCD segment in response to a salt load, and how they affect the number of channels in the apical membrane, the open probability of individual channels, and their cleavage and modifications. The role of ENaC in the increased CTGF interaction shown in Dahl SS rats as a cause of glomerular barotrauma (91) also remains to be studied. Therefore, while we have reviewed the important role of ENaC-mediated sodium reabsorption in the development of salt-sensitive hypertension, there is still much to be learned about the involvement of different regulatory pathways (illustrated in Fig. 1), and the specific mechanism that results in the susceptibility of an individual to salt-induced hypertension.
Fig. 1.
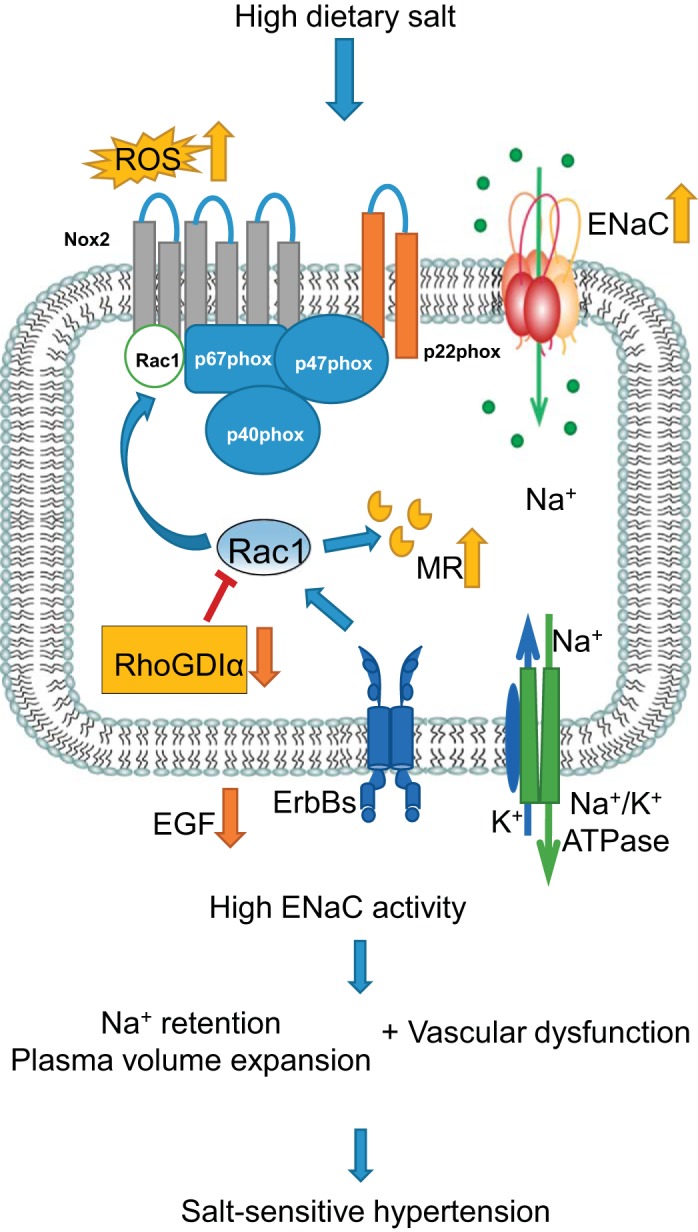
Schematic illustration of epithelial sodium channel (ENaC) involvement in the development of salt-sensitive (SS) hypertension. High salt intake leads to lack of epidermal growth factor (EGF) in cortical tissue, low abundance of Rho GDP-dissociation inhibitor α (RhoGDIα), and abnormal activation of mineralocorticoid receptors (MR) via high activity of Rac1, which also serves as a structural unit of NADPH oxidase. Production of reactive oxygen species (ROS; as well as some other mechanisms not shown here) increases ENaC activity, which, in turn, contributes to the body fluid volume expansion required for the development of SS hypertension.
GRANTS
This article is supported by National Heart, Lung, and Blood Institute Grants HL-116603 (to T. S. Pavlov), HL-108880, and HL-122662 (to A. Staruschenko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.S.P. prepared figures; T.S.P. drafted manuscript; T.S.P. and A.S. edited and revised manuscript; T.S.P. and A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. William Beierwaltes (Henry Ford Hospital) for a critical reading of the manuscript.
REFERENCES
- 1.Amin MS, Reza E, El-Shahat E, Wang HW, Tesson F, Leenen FH. Enhanced expression of epithelial sodium channels in the renal medulla of Dahl S rats. Can J Physiol Pharmacol 89: 159–168, 2011. doi: 10.1139/Y11-005. [DOI] [PubMed] [Google Scholar]
- 2.Amin MS, Reza E, Wang H, Leenen FHH. Sodium transport in the choroid plexus and salt-sensitive hypertension. Hypertension 54: 860–867, 2009. doi: 10.1161/HYPERTENSIONAHA.108.125807. [DOI] [PubMed] [Google Scholar]
- 3.Aoi W, Niisato N, Miyazaki H, Marunaka Y. Flavonoid-induced reduction of ENaC expression in the kidney of Dahl salt-sensitive hypertensive rat. Biochem Biophys Res Commun 315: 892–896, 2004. doi: 10.1016/j.bbrc.2004.01.150. [DOI] [PubMed] [Google Scholar]
- 4.Aoi W, Niisato N, Sawabe Y, Miyazaki H, Tokuda S, Nishio K, Yoshikawa T, Marunaka Y. Abnormal expression of ENaC and SGK1 mRNA induced by dietary sodium in Dahl salt-sensitively hypertensive rats. Cell Biol Int 31: 1288–1291, 2007. doi: 10.1016/j.cellbi.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Baba K, Mulrow PJ, Franco-Saenz R, Rapp JP. Suppression of adrenal renin in Dahl salt-sensitive rats. Hypertension 8: 1149–1153, 1986. doi: 10.1161/01.HYP.8.12.1149. [DOI] [PubMed] [Google Scholar]
- 6.Baker EH, Duggal A, Dong Y, Ireson NJ, Wood M, Markandu ND, MacGregor GA. Amiloride, a specific drug for hypertension in black people with T594M variant? Hypertension 40: 13–17, 2002. doi: 10.1161/01.HYP.0000022570.02119.75. [DOI] [PubMed] [Google Scholar]
- 7.Bankir L, Bichet DG, Bouby N. Vasopressin V2 receptors, ENaC, and sodium reabsorption: a risk factor for hypertension? Am J Physiol Renal Physiol 299: F917–F928, 2010. doi: 10.1152/ajprenal.00413.2010. [DOI] [PubMed] [Google Scholar]
- 8.Bankir L, Fernandes S, Bardoux P, Bouby N, Bichet DG. Vasopressin-V2 receptor stimulation reduces sodium excretion in healthy humans. J Am Soc Nephrol 16: 1920–1928, 2005. doi: 10.1681/ASN.2004121079. [DOI] [PubMed] [Google Scholar]
- 9.Bayorh MA, Ganafa AA, Emmett N, Socci RR, Eatman D, Fridie IL. Alterations in aldosterone and angiotensin II levels in salt-induced hypertension. Clin Exp Hypertens 27: 355–367, 2005. doi: 10.1081/CEH-57423. [DOI] [PubMed] [Google Scholar]
- 10.Bugaj V, Pochynyuk O, Stockand JD. Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol 297: F1411–F1418, 2009. doi: 10.1152/ajprenal.00371.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension 23: 531–550, 1994. doi: 10.1161/01.HYP.23.4.531. [DOI] [PubMed] [Google Scholar]
- 12.Costanzo LS. Comparison of calcium and sodium transport in early and late rat distal tubules: effect of amiloride. Am J Physiol 246: F937–F945, 1984. [DOI] [PubMed] [Google Scholar]
- 13.Cowley AW., Jr Genetic and nongenetic determinants of salt sensitivity and blood pressure. Am J Clin Nutr 65, Suppl: 587S–593S, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Cowley AW., Jr Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786, 2008. doi: 10.1161/HYPERTENSIONAHA.107.092858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowley AW Jr, Yang C, Zheleznova NN, Staruschenko A, Kurth T, Rein L, Kumar V, Sadovnikov K, Dayton A, Hoffman M, Ryan RP, Skelton MM, Salehpour F, Ranji M, Geurts A. Evidence of the importance of Nox4 in production of hypertension in Dahl salt-sensitive rats. Hypertension 67: 440–450, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahl LK, Heine M. Primary role of renal homografts in setting chronic blood pressure levels in rats. Circ Res 36: 692–696, 1975. doi: 10.1161/01.RES.36.6.692. [DOI] [PubMed] [Google Scholar]
- 17.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ecelbarger CA, Kim G-H, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Ecelbarger CA, Kim G-H, Wade JB, Knepper MA. Regulation of the abundance of renal sodium transporters and channels by vasopressin. Exp Neurol 171: 227–234, 2001. doi: 10.1006/exnr.2001.7775. [DOI] [PubMed] [Google Scholar]
- 21.Evans RG, Bie P. Role of the kidney in the pathogenesis of hypertension: time for a neo-Guytonian paradigm or a paradigm shift? Am J Physiol Regul Integr Comp Physiol 310: R217–R229, 2016. doi: 10.1152/ajpregu.00254.2015. [DOI] [PubMed] [Google Scholar]
- 22.Felder RA, White MJ, Williams SM, Jose PA. Diagnostic tools for hypertension and salt sensitivity testing. Curr Opin Nephrol Hypertens 22: 65–76, 2013. doi: 10.1097/MNH.0b013e32835b3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O’Connor PM, Cowley AW Jr. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15: 201–208, 2012. doi: 10.1016/j.cmet.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frindt G, Ergonul Z, Palmer LG. Na channel expression and activity in the medullary collecting duct of rat kidney. Am J Physiol Renal Physiol 292: F1190–F1196, 2007. doi: 10.1152/ajprenal.00399.2006. [DOI] [PubMed] [Google Scholar]
- 25.Frindt G, Masilamani S, Knepper MA, Palmer LG. Activation of epithelial Na channels during short-term Na deprivation. Am J Physiol Renal Physiol 280: F112–F118, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Greene AS, Yu ZY, Roman RJ, Cowley AW Jr. Role of blood volume expansion in Dahl rat model of hypertension. Am J Physiol 258: H508–H514, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Guo L-J, Alli AA, Eaton DC, Bao H-F. ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling. Am J Physiol Renal Physiol 304: F930–F937, 2013. doi: 10.1152/ajprenal.00638.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall JE. Renal dysfunction, rather than nonrenal vascular dysfunction, mediates salt-induced hypertension. Circulation 133: 894–906, 2016. doi: 10.1161/CIRCULATIONAHA.115.018526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hierholzer K, Wiederholt M. Some aspects of distal tubular solute and water transport. Kidney Int 9: 198–213, 1976. doi: 10.1038/ki.1976.21. [DOI] [PubMed] [Google Scholar]
- 30.Huang BS, White RA, Jeng AY, Leenen FHH. Role of central nervous system aldosterone synthase and mineralocorticoid receptors in salt-induced hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 296: R994–R1000, 2009. doi: 10.1152/ajpregu.90903.2008. [DOI] [PubMed] [Google Scholar]
- 31.Hye Khan MA, Neckár J, Manthati V, Errabelli R, Pavlov TS, Staruschenko A, Falck JR, Imig JD. Orally active epoxyeicosatrienoic acid analog attenuates kidney injury in hypertensive Dahl salt-sensitive rat. Hypertension 62: 905–913, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilatovskaya DV, Pavlov TS, Levchenko V, Staruschenko A. ROS production as a common mechanism of ENaC regulation by EGF, insulin, and IGF-1. Am J Physiol Cell Physiol 304: C102–C111, 2013. doi: 10.1152/ajpcell.00231.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa M, Kobayashi N, Sugiyama F, Onoda S, Ishimitsu T. Renoprotective effect of vasopressin v2 receptor antagonist tolvaptan in Dahl rats with end-stage heart failure. Int Heart J 54: 98–106, 2013. doi: 10.1536/ihj.54.98. [DOI] [PubMed] [Google Scholar]
- 34.Jin C, Hu C, Polichnowski A, Mori T, Skelton M, Ito S, Cowley AW Jr. Effects of renal perfusion pressure on renal medullary hydrogen peroxide and nitric oxide production. Hypertension 53: 1048–1053, 2009. doi: 10.1161/HYPERTENSIONAHA.109.128827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin C, Sun J, Stilphen CA, Smith SME, Ocasio H, Bermingham B, Darji S, Guha A, Patel R, Geurts AM, Jacob HJ, Lambert NA, O’Connor PM. HV1 acts as a sodium sensor and promotes superoxide production in medullary thick ascending limb of Dahl salt-sensitive rats. Hypertension 64: 541–550, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakizoe Y, Kitamura K, Ko T, Wakida N, Maekawa A, Miyoshi T, Shiraishi N, Adachi M, Zhang Z, Masilamani S, Tomita K. Aberrant ENaC activation in Dahl salt-sensitive rats. J Hypertens 27: 1679–1689, 2009. doi: 10.1097/HJH.0b013e32832c7d23. [DOI] [PubMed] [Google Scholar]
- 37.Karpushev AV, Levchenko V, Ilatovskaya DV, Pavlov TS, Staruschenko A. Novel role of Rac1/WAVE signaling mechanism in regulation of the epithelial Na+ channel. Hypertension 57: 996–1002, 2011. doi: 10.1161/HYPERTENSIONAHA.110.157784. [DOI] [PubMed] [Google Scholar]
- 38.Kawarazaki W, Fujita T. Aberrant Rac1-mineralocorticoid receptor pathways in salt-sensitive hypertension. Clin Exp Pharmacol Physiol 40: 929–936, 2013. doi: 10.1111/1440-1681.12177. [DOI] [PubMed] [Google Scholar]
- 39.Kishore BK, Ecelbarger CM. Lithium: a versatile tool for understanding renal physiology. Am J Physiol Renal Physiol 304: F1139–F1149, 2013. doi: 10.1152/ajprenal.00718.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knepper MA, Kwon T-H, Nielsen S. Molecular physiology of water balance. N Engl J Med 372: 1349–1358, 2015. doi: 10.1056/NEJMra1404726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 42.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension 41: 592–597, 2003. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurtz TW, DiCarlo SE, Pravenec M, Schmidlin O, Tanaka M, Morris RC Jr. An alternative hypothesis to the widely held view that renal excretion of sodium accounts for resistance to salt-induced hypertension. Kidney Int 90: 965–973, 2016. doi: 10.1016/j.kint.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lacy F, Kailasam MT, O’Connor DT, Schmid-Schönbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36: 878–884, 2000. doi: 10.1161/01.HYP.36.5.878. [DOI] [PubMed] [Google Scholar]
- 45.Levchenko V, Zheleznova NN, Pavlov TS, Vandewalle A, Wilson PD, Staruschenko A. EGF and its related growth factors mediate sodium transport in mpkCCDc14 cells via ErbB2 (neu/HER-2) receptor. J Cell Physiol 223: 252–259, 2010. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Duke BJ, Malik B, Yue Q, Eaton DC. Biphasic regulation of ENaC by TGF-alpha and EGF in renal epithelial cells. Am J Physiol Renal Physiol 296: F1417–F1427, 2009. doi: 10.1152/ajprenal.90337.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu X, Wang F, Liu M, Yang KT, Nau A, Kohan DE, Reese V, Richardson RS, Yang T. Activation of ENaC in collecting duct cells by prorenin and its receptor PRR: involvement of Nox4-derived hydrogen peroxide. Am J Physiol Renal Physiol 310: F1243–F1250, 2016. doi: 10.1152/ajprenal.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma HP. Hydrogen peroxide stimulates the epithelial sodium channel through a phosphatidylinositide 3-kinase-dependent pathway. J Biol Chem 286: 32444–32453, 2011. doi: 10.1074/jbc.M111.254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Majid DSA, Prieto MC, Navar LG. Salt-sensitive hypertension: perspectives on intrarenal mechanisms. Curr Hypertens Rev 11: 38–48, 2015. doi: 10.2174/1573402111666150530203858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mironova E, Bugaj V, Roos KP, Kohan DE, Stockand JD. Aldosterone-independent regulation of the epithelial Na+ channel (ENaC) by vasopressin in adrenalectomized mice. Proc Natl Acad Sci USA 109: 10095–10100, 2012. doi: 10.1073/pnas.1201978109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mironova E, Chen Y, Pao AC, Roos KP, Kohan DE, Bugaj V, Stockand JD. Activation of ENaC by AVP contributes to the urinary concentrating mechanism and dilution of plasma. Am J Physiol Renal Physiol 308: F237–F243, 2015. doi: 10.1152/ajprenal.00246.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreno C, Hoffman M, Stodola TJ, Didier DN, Lazar J, Geurts AM, North PE, Jacob HJ, Greene AS. Creation and characterization of a renin knockout rat. Hypertension 57: 614–619, 2011. doi: 10.1161/HYPERTENSIONAHA.110.163840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mori T, Polichnowski A, Glocka P, Kaldunski M, Ohsaki Y, Liang M, Cowley AW Jr. High perfusion pressure accelerates renal injury in salt-sensitive hypertension. J Am Soc Nephrol 19: 1472–1482, 2008. doi: 10.1681/ASN.2007121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicco C, Wittner M, DiStefano A, Jounier S, Bankir L, Bouby N. Chronic exposure to vasopressin upregulates ENaC and sodium transport in the rat renal collecting duct and lung. Hypertension 38: 1143–1149, 2001. doi: 10.1161/hy1001.092641. [DOI] [PubMed] [Google Scholar]
- 56.O’Connor PM, Cowley AW Jr. Modulation of pressure-natriuresis by renal medullary reactive oxygen species and nitric oxide. Curr Hypertens Rep 12: 86–92, 2010. doi: 10.1007/s11906-010-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palmer LG, Sackin H, Frindt G. Regulation of Na+ channels by luminal Na+ in rat cortical collecting tubule. J Physiol 509: 151–162, 1998. doi: 10.1111/j.1469-7793.1998.151bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol 10: 676-687, 2015. doi: 10.2215/CJN.12391213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14-3-3/Nedd4-2. J Am Soc Nephrol 21: 833–843, 2010. doi: 10.1681/ASN.2009080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pavlov TS, Levchenko V, Ilatovskaya DV, Moreno C, Staruschenko A. Renal sodium transport in renin-deficient Dahl salt-sensitive rats. J Renin Angiotensin Aldosterone Syst 17: pii: 1470320316653858, 2016. doi: 10.1177/1470320316653858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pavlov TS, Levchenko V, O’Connor PM, Ilatovskaya DV, Palygin O, Mori T, Mattson DL, Sorokin A, Lombard JH, Cowley AW Jr, Staruschenko A. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J Am Soc Nephrol 24: 1053–1062, 2013. doi: 10.1681/ASN.2012080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavlov TS, Levchenko V, Staruschenko A. Role of Rho GDP dissociation inhibitor α in control of epithelial sodium channel (ENaC)-mediated sodium reabsorption. J Biol Chem 289: 28651–28659, 2014. doi: 10.1074/jbc.M114.558262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pochynyuk O, Rieg T, Bugaj V, Schroth J, Fridman A, Boss GR, Insel PA, Stockand JD, Vallon V. Dietary Na+ inhibits the open probability of the epithelial sodium channel in the kidney by enhancing apical P2Y2-receptor tone. FASEB J 24: 2056–2065, 2010. doi: 10.1096/fj.09-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rapp JP, Wang SM, Dene H. A genetic polymorphism in the renin gene of Dahl rats cosegregates with blood pressure. Science 243: 542–544, 1989. doi: 10.1126/science.2563177. [DOI] [PubMed] [Google Scholar]
- 65.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 4: 753–763, 1982. doi: 10.1161/01.HYP.4.6.753. [DOI] [PubMed] [Google Scholar]
- 66.Redón J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, Sáez GT. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 41: 1096–1101, 2003. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- 67.Reif MC, Troutman SL, Schafer JA. Sodium transport by rat cortical collecting tubule. Effects of vasopressin and desoxycorticosterone. J Clin Invest 77: 1291–1298, 1986. doi: 10.1172/JCI112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reif MC, Troutman SL, Schafer JA. Sustained response to vasopressin in isolated rat cortical collecting tubule. Kidney Int 26: 725–732, 1984. doi: 10.1038/ki.1984.208. [DOI] [PubMed] [Google Scholar]
- 69.Ren Y, D’Ambrosio MA, Garvin JL, Wang H, Carretero OA. Prostaglandin E2 mediates connecting tubule glomerular feedback. Hypertension 62: 1123–1128, 2013. doi: 10.1161/HYPERTENSIONAHA.113.02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren Y, Garvin JL, Liu R, Carretero OA. Cross-talk between arterioles and tubules in the kidney. Pediatr Nephrol 24: 31–35, 2009. doi: 10.1007/s00467-008-0852-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int 71: 1116–1121, 2007. doi: 10.1038/sj.ki.5002190. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez-Iturbe B, Romero F, Johnson RJ. Pathophysiological mechanisms of salt-dependent hypertension. Am J Kidney Dis 50: 655–672, 2007. doi: 10.1053/j.ajkd.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 73.Roman RJ. Abnormal renal hemodynamics and pressure-natriuresis relationship in Dahl salt-sensitive rats. Am J Physiol 251: F57–F65, 1986. [DOI] [PubMed] [Google Scholar]
- 74.Rossier BC, Baker ME, Studer RA. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev 95: 297–340, 2015. doi: 10.1152/physrev.00011.2014. [DOI] [PubMed] [Google Scholar]
- 75.Rovin BH, Wurst E, Kohan DE. Production of reactive oxygen species by tubular epithelial cells in culture. Kidney Int 37: 1509–1514, 1990. doi: 10.1038/ki.1990.142. [DOI] [PubMed] [Google Scholar]
- 76.Salehpour F, Ghanian Z, Yang C, Zheleznova NN, Kurth T, Dash RK, Cowley AW Jr, Ranji M. Effects of p67phox on the mitochondrial oxidative state in the kidney of Dahl salt-sensitive rats: optical fluorescence 3-D cryoimaging. Am J Physiol Renal Physiol 309: F377–F382, 2015. doi: 10.1152/ajprenal.00098.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest 121: 3233–3243, 2011. doi: 10.1172/JCI43124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008. doi: 10.1038/nm.1879. [DOI] [PubMed] [Google Scholar]
- 79.Staruschenko A. Regulation of transport in the connecting tubule and cortical collecting duct. Compr Physiol 2: 1541–1584, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Staruschenko A, Nichols A, Medina JL, Camacho P, Zheleznova NN, Stockand JD. Rho small GTPases activate the epithelial Na(+) channel. J Biol Chem 279: 49989–49994, 2004. doi: 10.1074/jbc.M409812200. [DOI] [PubMed] [Google Scholar]
- 81.Staruschenko A, Palygin O, Ilatovskaya DV, Pavlov TS. Epidermal growth factors in the kidney and relationship to hypertension. Am J Physiol Renal Physiol 305: F12–F20, 2013. doi: 10.1152/ajprenal.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Staruschenko A, Pochynyuk O, Vandewalle A, Bugaj V, Stockand JD. Acute regulation of the epithelial Na+ channel by phosphatidylinositide 3-OH kinase signaling in native collecting duct principal cells. J Am Soc Nephrol 18: 1652–1661, 2007. doi: 10.1681/ASN.2007010020. [DOI] [PubMed] [Google Scholar]
- 83.Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, Ganten D, Waldherr R, Schnabel E, Kriz W. Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int 33: 1119–1129, 1988. doi: 10.1038/ki.1988.120. [DOI] [PubMed] [Google Scholar]
- 84.Stockand JD. Vasopressin regulation of renal sodium excretion. Kidney Int 78: 849–856, 2010. doi: 10.1038/ki.2010.276. [DOI] [PubMed] [Google Scholar]
- 85.Subramanya AR, Ellison DH. Distal convoluted tubule. Clin J Am Soc Nephrol 9: 2147–2163, 2014. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension 17, Suppl: I61–I68, 1991. doi: 10.1161/01.HYP.17.1_Suppl.I61. [DOI] [PubMed] [Google Scholar]
- 87.Sun Y, Zhang JN, Zhao D, Wang QS, Gu YC, Ma HP, Zhang ZR. Role of the epithelial sodium channel in salt-sensitive hypertension. Acta Pharmacol Sin 32: 789–797, 2011. doi: 10.1038/aps.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takahashi H, Yoshika M, Komiyama Y, Nishimura M. The central mechanism underlying hypertension: a review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens Res 34: 1147–1160, 2011. doi: 10.1038/hr.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, Park HJ, McCormick JA, Yang C-L, Ellison DH. Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436-2445, 2016. doi: 10.1681/ASN.2015070815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomita K, Pisano JJ, Knepper MA. Control of sodium and potassium transport in the cortical collecting duct of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest 76: 132–136, 1985. doi: 10.1172/JCI111935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang H, D’Ambrosio MA, Garvin JL, Ren Y, Carretero OA. Connecting tubule glomerular feedback in hypertension. Hypertension 62: 738–745, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang HW, Huang BS, Chen A, Ahmad M, White RA, Leenen FHH. Role of brain aldosterone and mineralocorticoid receptors in aldosterone-salt hypertension in rats. Neuroscience 314: 90–105, 2016. doi: 10.1016/j.neuroscience.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 93.Wang W, Li C, Nejsum LN, Li H, Kim SW, Kwon TH, Jonassen TE, Knepper MA, Thomsen K, Frøkiaer J, Nielsen S. Biphasic effects of ANP infusion in conscious, euvolumic rats: roles of AQP2 and ENaC trafficking. Am J Physiol Renal Physiol 290: F530–F541, 2006. doi: 10.1152/ajprenal.00070.2005. [DOI] [PubMed] [Google Scholar]
- 94.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001. doi: 10.1161/01.HYP.37.2.429. [DOI] [PubMed] [Google Scholar]
- 95.Zheleznova NN, Yang C, Cowley AW Jr. Role of Nox4 and p67phox subunit of Nox2 in ROS production in response to increased tubular flow in the mTAL of Dahl salt-sensitive rats. Am J Physiol Renal Physiol 311: F450–F458, 2016. doi: 10.1152/ajprenal.00187.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


