Abstract
Kidney transplantation (KTX) is a life-saving procedure for patients with end-stage renal disease. Expression levels of many genes in the kidney vary between males and females, which may play an essential role in the sex differences in graft function. However, whether these differences are affected after cross-sex-KTX is unknown. In the present study, we assessed postoperative changes in genotype, function, and inflammatory responses of the grafts in same-sex- and cross-sex-KTX. Single kidney transplants were performed between same and different sex C57BL/6 mice paired into four combination groups: female donor/female recipient (F/F); male donor/male recipient (M/M); female donor/male recipient (F/M); and male donor/female recipient (M/F). The remnant native kidney was removed 4 days posttransplant. Expression levels of genes related to the contractility of the afferent arteriole and tubular sodium reabsorption were assessed. Same-sex-KTX did not significantly alter the magnitude or sex difference pattern of gene expression in male or female grafts. Cross-sex-KTX showed an attenuated sex difference in gene expressions. The measurements of endothelin 1, endothelin ETA receptor, Na+-K−-2Cl cotransporter 2 (NKCC2), and epithelial Na+ channels (ENaC) subunits exhibited decreases in M/F compared with M/M and increases in F/M compared with F/F. There were no significant differences in hemodynamics or sodium excretion in response to acute volume expansion for any sex combinations. Cross-sex-KTX stimulated more robust inflammatory responses than same-sex-KTX. IL-6 and KC mRNA levels elevated 5- to 20-fold in cross-sex-KTX compared with same-sex-KTX. In conclusion, cross-sex-KTX alters gene expression levels and induces inflammatory responses, which might play an important role in long-term graft function.
Keywords: mouse kidney transplantation, sex difference, receptors and sodium transporter
kidney transplantation (KTX) is not only a life-saving procedure, but it also improves the quality of life of patients with end-stage kidney disease (13, 15, 21, 45). However, long-term allograft function is still limited. The gender of donors and recipients is involved in the organ donation and transplant surgery. Gender has been considered one of the factors that affect short- and long-term graft outcomes and survival for kidney transplant patients (39, 47). Sexual dimorphism exists in renal genotype and renal function. Expression levels of many genes in kidneys are different between males and females (2, 6, 37, 40). These differences may be closely linked to the sex differences in renal response to salt loading, hypertension, and kidney injury (23). It is known that premenopausal women are generally protected from developing many diseases including hypertension and chronic kidney disease (14, 16). However, kidneys from female donors have been demonstrated with poor outcomes in graft function in some studies (7, 9, 20). Early and late graft dysfunction occur more frequently with female donors than male donors (4, 9, 22, 42). However, the mechanisms have not been fully elucidated.
Our goal was to determine whether the genotype of the donor’s kidney itself or the recipient’s systemic environment determines the sex difference of the gene expression levels in renal grafts and thus the kidney graft outcomes. In the present study, we performed cross-sex- and same-sex-KTX. We measured gene expression levels and inflammatory response of the transplanted grafts and characterized the hemodynamics and renal clearance function in response to an acute volume expansion (AVE). We hope that the present study can provide insight into the mechanisms and potential targets for improvement of graft function in clinical practice.
MATERIALS AND METHODS
All procedures and experiments were approved by the Institutional Animal Care and Use Committee at the University of South Florida College of Medicine. All chemicals were purchased from Sigma (St. Louis, MO) except as indicated.
Animals
Male C57BL/6J mice, aged 10–12 wk (22–25 g), and female mice, aged 13–14 wk (19–22 g), were obtained from The Jackson Laboratory. After arrival, the mice were allowed to equilibrate in a temperature-controlled environment with a 12:12-h light-dark cycle for 1 wk with ad libitum access to mouse chow and tap water.
Fifty-four male and female mice were randomly divided into four groups based on donor/recipient sex combinations of male (M) and female (F) mice: M/M, F/F, M/F, and F/M. Male and female mice with unilateral nephrectomy of right kidney as controls (MC and FC) for male- and female-transplanted kidneys, respectively.
Kidney Transplants
Kidney transplants were performed using the new knotless technique with five steps: donor nephrectomy, recipient preparation, kidney implantation, ureteral implantation, and contralateral nephrectomy (32).
Donor nephrectomy and perfusion.
The donor mice were anesthetized via inhalation with isoflurane (2% in the air; flow 200 ml/min). A midline abdominal incision was utilized to fully expose the left kidney, aorta, and inferior vena cava (IVC). The left ureter was isolated and cut close to the bladder. The aortic region between the left and right renal arteries was mobilized. The infrarenal IVC and aorta were separated. Moreover, a piece of 6-0 silk suture was tied loosely around the infrarenal aorta. The aorta below the right renal artery, the distal aorta, and the IVC were cross clamped with 5-mm microvascular clamps. The left renal vein was transected at the vena cava. One milliliter of cold saline (4°C) was perfused through the aorta, followed by tightening of the ligature around the infrarenal aorta. The aorta was cut below the ligature and below the proximal clamp. The left kidney and associated vessels were removed and stored in saline at 4°C. Each donor nephrectomy was finished in 8–10 min. The donor mouse was then euthanized by cervical dislocation under isoflurane anesthesia.
Recipient preparation.
The recipient mice were anesthetized with isoflurane as described above. A midline abdominal incision was made, and the bowel was mobilized to the right abdomen. The left ureter was cauterized and cut. The left renal artery and vein were ligated, and the left kidney was nephrectomized. Complete mobilization and full dissection of the aorta and vena cava inferior to the renal artery and vein were then conducted. A section of aorta and IVC was dissected and then cross clamped with two microvascular clamps. An aortotomy was generated by cutting a 1-mm oval patch. The IVC was cut longitudinally with a 1.5-mm patch in similar fashion as the aortotomy. The blood clots were thoroughly flushed from the vessels with saline. The recipient preparation was finished within 7–9 min.
Kidney graft implantation.
The donor kidney was transferred from cold saline into the right flank of the recipient mouse. The arterial anastomosis was performed distal to the native right renal artery in an end-to-side manner between the donor and recipient aorta with 10-0 Ethilon sutures. Two stay sutures were tied at the proximal and distal apexes of the recipient’s arteriotomy with the donor’s aortic cuff. The left wall of the anastomosis was sewn continuously with two to three stitches. The donor kidney was rotated to the left flank of the recipient to facilitate completion of the suture line. A length of ~2–3 mm of the suture was left freely at the pole of the arteriotomy. The venous anastomosis was achieved in similar fashion as the arterial anastomosis. The arterial and the venous anastomoses were finished within 16–20 min (Table 3). The inferior and the superior clamps were released sequentially to reperfuse the transplanted kidney.
Table 3.
Procedure timeline
| Donor Prep | Recipient Prep | Arterial Anastomosis | Venous Anastomosis | Ureteral Implantation | |
|---|---|---|---|---|---|
| Time, min | 8–10 | 7–9 | 8–10 | 8–10 | 6 |
Recipient ureteral implantation.
The donor kidney ureter was isolated from the surrounding fat and advanced by a forceps that punctured through the bladder. Upon gentle exit of the bladder with the forceps, the end of the ureter was clipped with a microvascular clamp. The ureter was secured to the exterior wall of the bladder dome using a 10-0 Ethilon suture. The end of the ureter was incised and the punctured bladder wall was closed with a “figure of eight” stitch using a 10-0 Ethilon suture. Finally, the abdomen was closed with a continuous 4-0 vicryl suture. Warm saline (0.4 ml) was administrated subcutaneously after the operation. The ureteral implantation and incision closure lasted ~6 min. The mouse was placed in an incubator at 32°C until fully awake. Buprenorphine (0.2 mg/kg) was administrated intramuscularly on day 0 and day 1 postoperation. The mouse was then returned to its regular housing with free access to food and water.
Recipient contralateral nephrectomy.
Four days after transplantation, the mice were anesthetized as described above and the remaining contralateral native kidneys were removed.
Renal Hemodynamics and Sodium Excretion in Response to an AVE
Experiments were performed 4 wk after kidney transplantation. Similar methods were used as we described previously (18, 43). The mice were anesthetized with ketamine (30 µg/g body wt) and inactin (50 µg/g body wt), and catheters were placed in the femoral artery for measurement of the mean arterial pressure (MAP) and in the femoral vein for an intravenous infusion of 2% BSA and FITC-inulin (2 mg/ml) in a 0.9% NaCl solution at a rate of 0.5 ml/h. A third catheter was inserted into the ureter for the collection of urine. After surgery, urine and plasma were collected over a 30-min period following a 30-min equilibration phase. The mice were then infused with saline (3% body wt) in a bolus followed by the infusion of FITC-inulin at 0.5 ml/h. Urine and plasma were collected during a 60-min period and a 60- to 90-min period after AVE. The concentrations of Na+ and inulin in the urine and plasma sample were measured systematically (Medica EasyElectrolytes Analyzer, Bedford, MA). The glomerular filtration rate (GFR) was calculated with the inulin concentration of urine and plasma and the urinary flow rate.
Real-Time PCR
At the end of the experiment, we measured the mRNA levels of genes related to the vascular contractility and sodium reabsorption: angiotensin II (ANG II) receptors (AT1R and AT2R) and endothelin system (ET-1, ETA, and ETB receptors), Na+/H+ exchanger 3 (NHE3), Na+-K−-2Cl cotransporter 2 (NKCC2), Na+-Cl− cotransporter (NCC) and epithelial Na+ channels (ENaC), and inflammatory responses: proinflammation cytokines tumor necrosis factor α (TNF-α), interleukin (IL)-6, interleukin (IL)-17, transforming growth factor-β (TGF-β), chemokine (C-C motif) ligand 2 and 5 (CCL2 and CCL5), interferon (INF)-γ, and keratinocyte-derived chemokine (KC) mRNA. The total RNA was extracted from kidney tissue homogenate using TRIzol. One microgram of total RNA was digested with RNase-free DNase (Promega, WI), and the cDNAs were synthesized with reverse transcription system using corresponding primer sets. β-Actin was used as a housekeeping gene as the reference for internal standardization. The quantitative RT-PCR primers were designed using the primer3Plus based on the sequences deposited in the GenBank (Table 1). After qualification of the cDNA template, quantitative PCR analysis was performed using iQ SYBR Green Supermix (iTaq SYRB; Bio-Rad, CA) and CFX96 Real-Time Detection System (Chromo4; Bio-Rad) according to the manufacturer’s protocol. The reaction conditions were set as follows: 95°C for 1 min, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s. Reaction of each sample was performed in triplicate. Dissociation analysis was performed at the end of each PCR reaction to confirm the amplification specificity. After the PCR program, data were analyzed and quantified with the comparative Ct method (2−ΔΔCt) based on Ct values for complement genes and β-actin to calculate the relative mRNA expression level.
Table 1.
Primers
| Primer Sequence | |
|---|---|
| β-Actin | |
| Forward | 5′-GTCCCTCACCCTCCCAAAAG-3′ |
| Reverse | 5′-GCTGCCTCAACACCTCAACCC-3′ |
| AT1 | |
| Forward | 5′-ATCGCTACCTGGCCATTGTC-3′ |
| Reverse | 5′-GGAAGCCCAGGATGTTCTTG-3′ |
| AT2 | |
| Forward | F 5′-TTACCAGCAGCCGTCCTTTT-3′ |
| Reverse | 5′-GTCAGCCAAGGCCAGATTGA-3′ |
| ET-1 | |
| Forward | 5′-TCTTCCAGGTCCAAGCGTTC-3′ |
| Reverse | 5′-TGCTATTGCTGATGGCCTCC-3′ |
| ETA | |
| Forward | 5′-TTGACCTCCCCATCAACGTG-3′ |
| Reverse | 5′-AGCACAGAGGTTCAAGACGG-3′ |
| ETB | |
| Forward | 5′-CAAGGTCGCTCAGAAAACGC-3′ |
| Reverse | 5′-CCTAAACAGGCCTCTCGCAA-3′ |
| NHE3 | |
| Forward | 5′-GCCTTCATTCGCTCCCCAAGT-3′, |
| Reverse | 5′-GAGATGCTTGTACTCCTGCCGA-3′. |
| NCC | |
| Forward | 5′-CCATTGGAAGGAAGGGGAAGTGC-3′ |
| Reverse | 5′-GTGCGTTCTGACTCTATCCCA-3′. |
| NKCC2 | |
| Forward | 5′-GATGCAGAACTGGAAGCAGTCAA-3′ |
| Reverse | 5′-TCAGACACCAAGGCACAACATTT-3′ |
| α-ENaC | |
| Forward | 5′-CGGGAAACGACCAAACGAAC-3′ |
| Reverse | 5′-CTTACTCTAGCCCCCACCCT-3′ |
| β-ENaC | |
| Forward | 5′-TTGATGAGCGGAACCCTGAC3′ |
| Reverse | 5′-GGATTATGCGATCAGGGGCA3′ |
| γ-ENaC | |
| Forward | 5′-CCTGGAGAGAAGATCAAAGCCA-3′ |
| Reverse | 5′-CCAAGTCAGTCAGGAGGTCAC-3′ |
| IL-6 | |
| Forward | 5′-CTCTGGGAAATCGTGGAAAT-3′, |
| Reverse | 5′-CCAGTTTGGTAGCATCCATC-3′. |
| KC | |
| Forward | 5′-GCTGGGATTCACCTCAAGAA-3′, |
| Reverse | 5′-TGGGGACACCTTTTAGCATC-3′. |
| TGF-β | |
| Forward | 5′-CCCTATATTTGGAGCCTGGA-3′, |
| Reverse | 5′-CTTGCGACCCACGTAGTAGA-3′ |
| TNF-α | |
| Forward | 5′-ATGAGAAGTTCCCAAATGGC-3′, |
| Reverse | 5′-CTCCACTTGGTGGTTTGCTA-3′ |
| INF-γ | |
| Forward | 5′-CGCTACACACTGCATCTTGG-3′, |
| Reverse | 5′-GGCTGGATTCCGGCAACA-3′. |
| IL-17 | |
| Forward | 5′-ATCAGGACGCGCAAACATG-3′, |
| Reverse | 5′-TGATCGCTGCTGCCTTCAC-3′. |
| CCL2 | |
| Forward | 5′-AGTTAACGCCCCACTCACCT-3′, |
| Reverse | 5′-CCATTCCTTCTTGGGGTCAGC-3′ |
| CCL5 | |
| Forward | 5′-AGATCTCTGCAGCTGCCCTCA-3′, |
| Reverse | 5′-GGAGCACTTGCTGCTGGTGTAG-3′ |
AT1 and AT2, angiotensin II receptors; ET, endothellin; NHE3, Na+/H+ exchanger 3; NCC, Na+-Cl− cotransporter; NKCC2, Na+-K−-2Cl cotransporter 2; ENaC, epithelial Na+ channels; TGF-β, transforming growth factor-β; KC, keratinocyte-derived chemokine; CCL, chemokine (C-C motif) ligand.
Western Blotting
Proteins were extracted from the kidney grafts at the end of experiment. Protein extracts (30 μg/lane) were subjected to 12% SDS-PAGE separation. After blocking for 30 min at room temperature with 5% skim milk, the membranes were incubated overnight at 4°C with NHE3, NKCC-2, TNF-α, and IL-6 primary antibodies (Abcam, Cambridge, MA). After incubation of the membranes with horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit, IgG; 1:20,000; Bio-Rad), the antibody-reactive bands were revealed by enhanced chemiluminescence detection on Hyperfilm (Amersham Pharmacia Biotech, Piscataway, NJ). β-Actin was used as an internal loading control and for normalization of protein quantification. Immunoblots were scanned and quantified using ImageJ densitometry software.
Histology
Histologic examination of all kidney grafts was performed at the end of experiments by an experienced renal pathologist blinded to the experimental procedures. Kidneys were removed and dissected in the longitudinal axis. Kidney samples were preserved and fixed in 4% paraformaldehyde solution for at least 24 h and then embedded in paraffin wax, cut into 3-µm sections, and stained with periodic acid-Schiff. Tubular injury, luminal inflammatory cells, tubular atrophy and interstitial fibrosis were analyzed and compared for all the grafts.
Statistical Analysis
All data are presented as mean values ± SE. The significance of differences in mean values between and within groups was determined by ANOVA for repeated measures and a post hoc Fisher’s least significant difference test or a paired t-test where appropriate. P < 0.05 was considered to be statistically significant.
RESULTS
Receptors and Sodium Cotransporters
Components of the renin-angiotensin system (RAS), the endothelin system, and the sodium transporters were examined.
ANG II receptors.
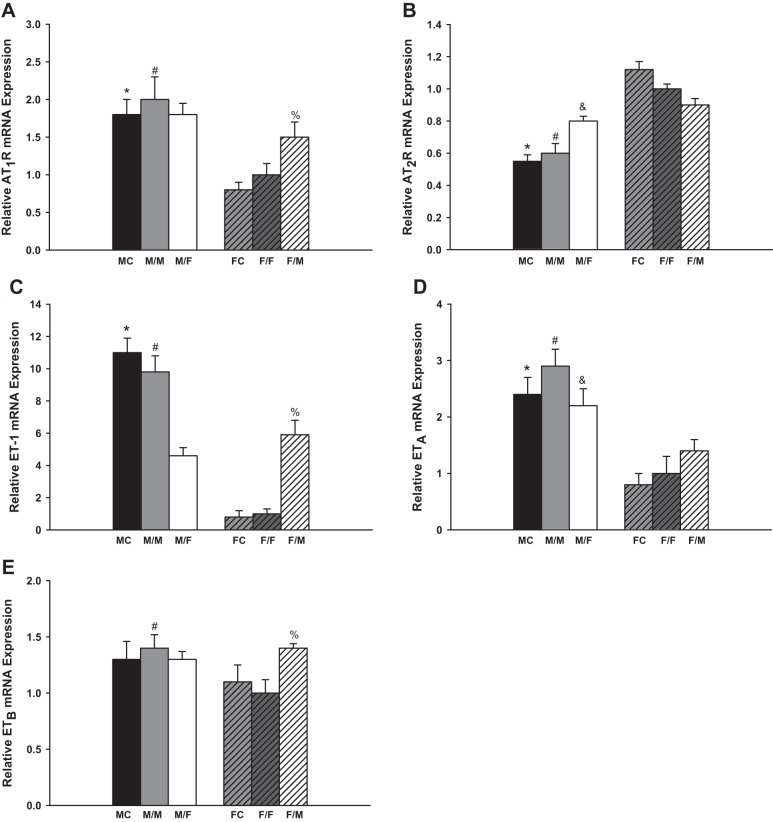
Figure 1A summarized the observed changes of the AT1R mRNA expression. The same-sex-KTX groups showed similar amount of AT1R mRNA and similar sex difference patterns compared with sham-operated groups for both males and females. The AT1R mRNA levels in the male sham-operated mice (MC) and transplanted kidneys from male donors to male recipients (M/M) were approximately onefold higher than in female sham-operated mice (FC) and female-transplanted kidneys to female recipients (F/F), respectively (P < 0.01 MC vs. FC and M/M vs. F/F; n = 6). In the cross-sex-KTX groups, the grafts from male donors to female recipients (M/F) showed similar expression levels of AT1R mRNA compared with M/M mice, but the transplants from female donors to male recipients (F/M) exhibited a 50% increase in AT1R mRNA compared with F/F mice (P < 0.05 F/M vs. F/F; n = 6). There were no significant differences in AT1R mRNA expression in the grafts between male and female recipients after cross-sex-KTX.
Fig. 1.
The relative renal gene expression of the components in renin-angiotensin system (RAS) and endothelin (ET) system. M, male; F, female; FC, female control; MC, male control; AT1R and AT2R, angiotensin II receptors. Renal gene expression of AT1R (A; *P < 0.01 vs. FC; #P < 0.01 vs. F/F; %P < 0.05 vs. F/F); AT2R (B; *P < 0.01 vs. FC; #P < 0.01 vs. F/F; &P < 0.05 vs. M/M); ET-1 (C; *P < 0.01 vs. FC; #P < 0.01 vs. F/F and M/F; %P < 0.01 vs. F/F); ETA (D; *P < 0.01 vs. FC; #P < 0.01 vs. F/F; &P < 0.05 vs. F/M); and ETB (E; #P < 0.05 vs. F/F; %P < 0.05 vs. F/F) of the transplanted kidneys at 4 wk after transplantation (donor/recipient; M, male, F, female; n = 6).
The AT2R amounts were similar between sham-operated mice and same-sex-KTX mice for both males and females. The AT2R expression levels were ~100% higher in the FC than in MC and 40% higher in grafts of F/F than in grafts of M/M mice (P < 0.01 MC vs. FC and M/M vs. F/F; Fig. 1B; n = 6). The AT2R levels were enhanced in the grafts from male donors to female recipients (M/F) compared with M/M mice (P < 0.05 M/F vs M/M; Fig. 1B; n = 6), but the levels were slightly reduced in the grafts from female donors to male recipients (F/M) compared with F/F mice. There were no significant differences in the AT2R mRNA expression in the transplanted grafts between male and female recipients after cross-sex-KTX.
Endothelin system.
There were no significant differences in the mRNA expression of ET-1, ETA, and ETB between the sham-operated groups and same-sex-KTX groups for both males and females.
The ET-1 mRNA levels in the male sham-operated mice (MC) and grafts from male donors to male recipients (M/M) were ~11- and 10-fold higher than in female sham-operated mice (FC) and F/F mice (P < 0.01 MC vs. FC and M/M vs. F/F; Fig. 1C; n = 6), respectively. After cross-sex-KTX, the ET-1 mRNA in the grafts from male donors to female recipients (M/F) significantly decreased compared with M/M mice (P < 0.01 M/F vs. M/M; Fig. 1C; n = 6) and, on the contrary, the ET-1 mRNA measurements significantly increased in the grafts from female donors to male recipients (F/M) compared with the F/F mice (P < 0.01 F/M vs. F/F; Fig. 1C; n = 6). The sex difference in ET-1 expression was dramatically attenuated in cross-sex-KTX compared with same-sex-KTX.
The ETA mRNA level in the male sham-operated mice was ~150% higher than in female sham-operated mice (P < 0.01 MC vs. FC; Fig. 1D; n = 6). There was no significant differences in the expression of ETB mRNA between male and female sham-operated mice. In the same-sex-KTX groups, the sex differences followed the similar pattern as sham-operated mice. Grafts from male donors to male recipients (M/M) exhibited a 200 and 30% higher expression levels than F/F mice for the ETA mRNA and ETB mRNA, respectively (P < 0.01 M/M vs. F/F; Fig. 1, D and E; n = 6). After cross-sex-KTX, the ETA mRNA was decreased in the grafts from male donors to female recipients (M/F) compared with M/M mice and increased in the grafts from female donors to male recipients (F/M) compared with F/F mice. The levels of ETA by sex were much attenuated (P < 0.05 F/M vs. M/F; Fig. 1D; n = 6). There was no significant change in the expression level of ETB in grafts from male donors to female recipients (M/F) compared with M/M-transplanted mice; however higher expression levels of ETB mRNA were evident in grafts from female donors to male recipients (F/M) compared with F/F mice (P < 0.05 F/M vs. F/F; Fig. 1E; n = 6). The sex differences were nonexistent for the ETB mRNA expression in the transplanted grafts after cross-sex-KTX.
Sodium Transporters
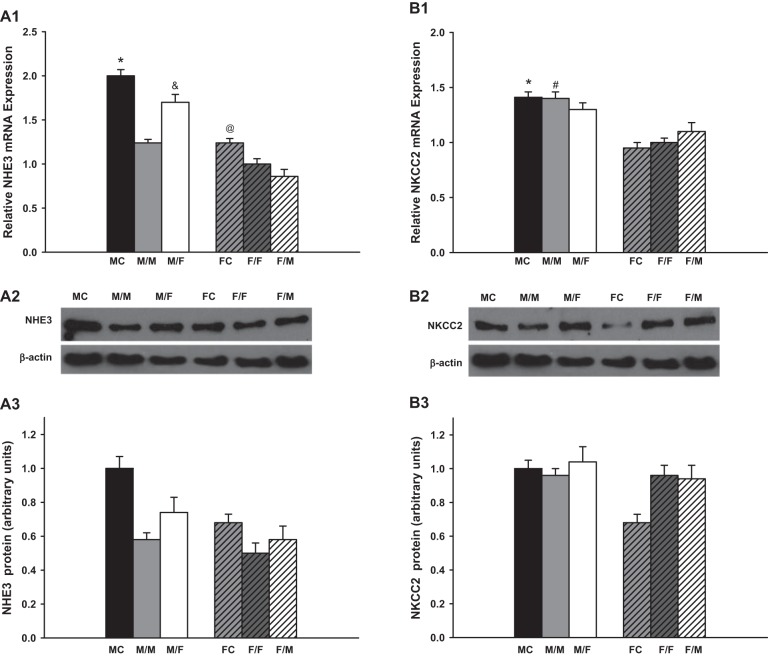
NHE3.
The expression of the NHE3 was suppressed ~35 and 20% after same-sex-KTX compared with sham-operated mice for males and females, respectively (P < 0.01 MC vs. M/M and FC vs. F/F; Fig. 2A; n = 6). There were no sex differences in the expression levels of NHE3 in the same-sex-KTX (M/M and F/F). The NHE3 levels increased 30% in the grafts from male donors to female recipients (M/F) compared with M/M mice (P < 0.01 M/F vs. M/M; Fig. 2A1; n = 6), while there were no significant changes in the expression of NHE3 in the grafts from female donors to male recipients (F/M) compared with F/F mice. A distinct difference in the expression of NHE3 in the grafts between males and females appeared after cross-sex-KTX (P < 0.01 M/F vs. F/M; Fig. 2A; n = 6).
Fig. 2.
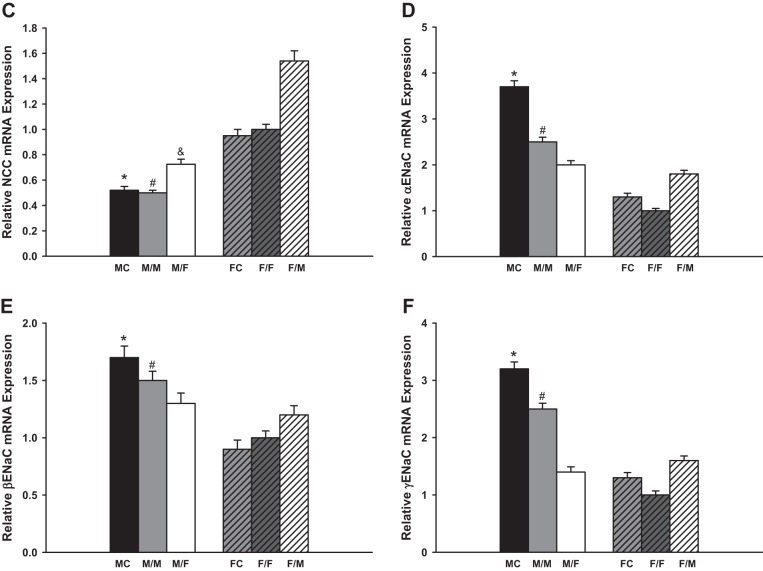
The relative renal gene expression of the sodium transporters. Renal gene expression of Na+/H+ exchanger 3 (NHE3; A; *P < 0.01 vs. M/M; &P < 0.01 vs. M/M and F/M; @P < 0.05 vs. F/F). Protein levels showed the similar pattern as mRNA expression. Na+-K−-2Cl cotransporter 2 (NKCC2; B; *P < 0.01 vs. FC; #P < 0.01 vs. F/F); Na+-Cl− cotransporter (NCC; C; *P < 0.01 vs. FC; #P < 0.01 vs. F/F; &P < 0.01 vs. F/M); and α (E)-, β (D)- and γ (F)-epithelial Na+ channels (ENaC) (*P < 0.01 vs. FC; #P < 0.01 vs. F/F;) of the transplanted kidneys at 4 wk after transplantation. Protein levels of NHE3 and NKCC2 showed the similar sex difference pattern as mRNA expression, respectively (donor/recipient; M, male, F, female; n = 6).
The protein levels of NHE3 for all the groups were assessed by Western blot. The sex differences of the protein expression showed similar patterns as did the amounts of mRNA.
NKCC2.
No significant changes were observed in the expression in NKCC2 mRNA between sham-operated mice and same-sex-KTX mice for either males or females. The NKCC2 mRNA expression measurements in the kidneys of MC and the grafts of M/M mice were 50% higher than in the kidneys of FC and in the grafts of F/F mice, respectively (P < 0.01 MC vs. FC and M/M vs. F/F; Fig. 2B; n = 6). After cross-sex-KTX, the NKCC2 levels slightly declined in grafts from male donors to female recipients (M/F) compared with M/M-transplanted mice and slightly increased in the grafts from female donors to male recipients (F/M) compared with F/F mice. There was no significant difference in NKCC2 mRNA levels in the grafts between males and females after cross-sex-KTX.
Western blot measurement showed the similar protein level of NKCC2 with mRNA expression for almost all the groups except that the mice in FC exhibited a lower protein expression than other groups.
NCC.
There are no differences in the mRNA expression of NCC mRNA between the sham-operated groups and same-sex-KTX groups for both males and females. The NCC mRNA levels in FC and in the grafts of F/F were approximately onefold higher than in MC and in the grafts of M/M mice (P < 0.01 MC vs. FC and M/M vs. F/F; Fig. 2C; n = 6). Cross-sex-KTX enhanced the expression of NCC mRNA levels in both sexes. The NCC levels increased by 40% in the grafts from male donors to female recipients (M/F) compared with the M/M-transplanted mice, and they increased by 80% in the grafts from female donors to male recipients (F/M) compared with the F/F mice. The sex differences in NCC levels in the grafts were exaggerated after cross-sex-KTX (P < 0.01 F/M vs. M/F; Fig. 2C; n = 6).
ENaC.
The mRNA expression of α- and γ-ENaC were downregulated ~30 and 20% in transplanted kidneys compared with sham-operated mice, respectively. There was no significant difference in the expression of β-ENaC between the sham-operated groups and the same-sex-KTX groups for both males and females. The changes of the three subunits of ENaC, α-, β-, and γ- ENaC, occurred with similar patterns. These mRNA levels were higher in the grafts of M/M than in F/F mice with same-sex-KTX. Cross-sex-KTX decreased the levels of the three subunits in the grafts from male donors to female recipients (M/F) compared with M/M mice; however levels of the three subunits increased in the grafts from female donor to male recipients (F/M) compared with F/F mice. The sex differences in the expression levels of all three subunits of ENaC were diminished after cross-sex-KTX (P < 0.01 MC vs. FC and M/M vs. F/F; P < 0.05 F/M vs. F/F; Fig. 2, D– F; n = 6).
Inflammatory Response
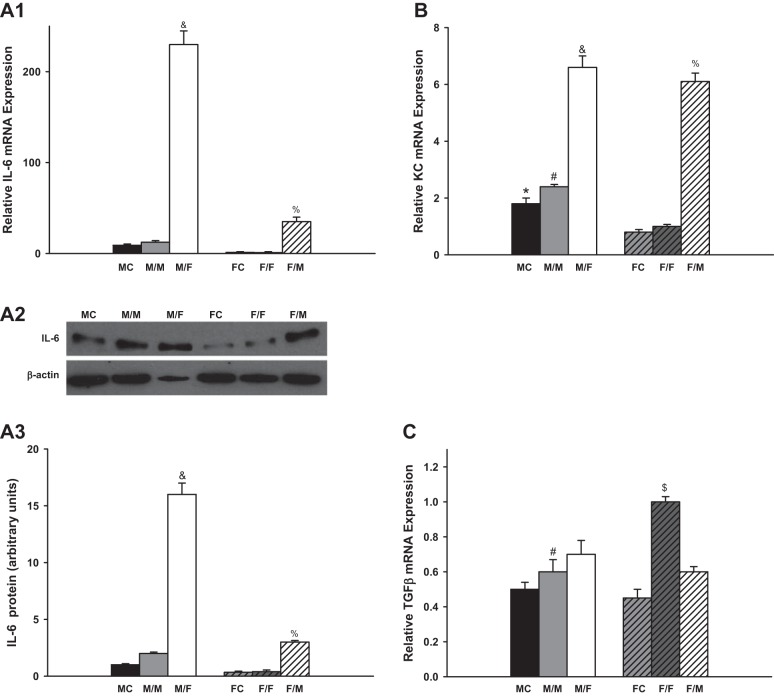
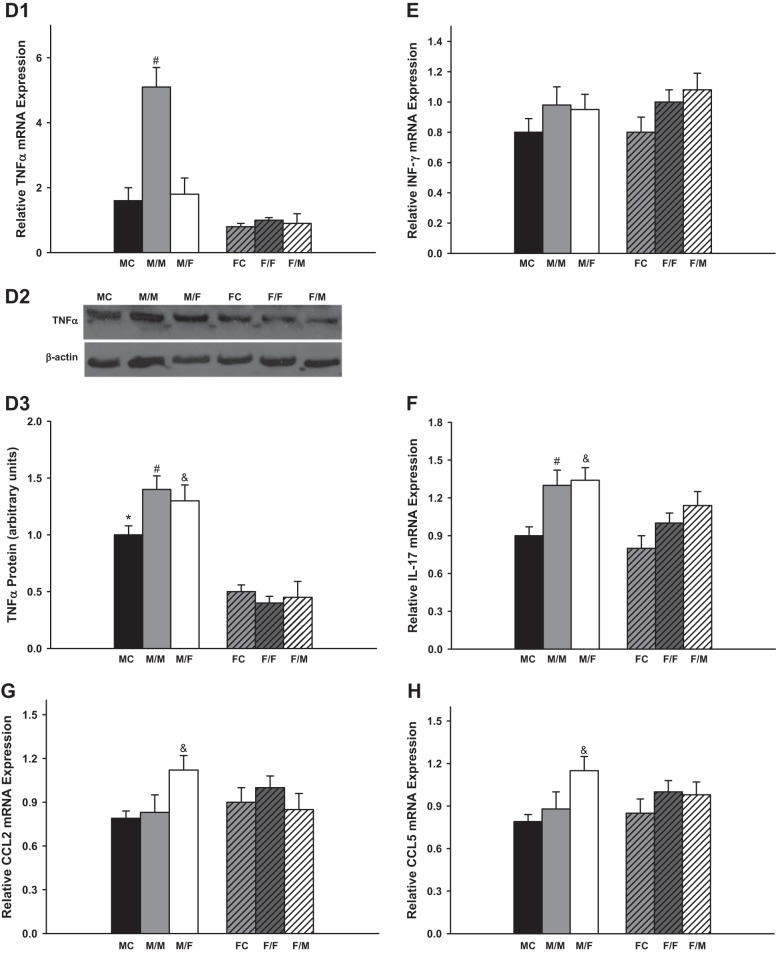
Several typical inflammation markers including IL-6, KC, TGF-β, TNF-α, INF-γ, IL-17, CCL2, and CCL5 were measured 4 wk after kidney transplantation.
There were no significant difference in the mRNA expression of IL-6, KC, TGF-β, CCL2, and CCL5 between sham-operated groups and same-sex-KTX groups for both males and females. However, the male recipients with male kidneys showed the highest mRNA expression of TNF-α.
IL-6 levels were very low in the grafts with same-sex-KTX, and there were no significant differences between males and females. The IL-6 levels increased more than 20-fold in the grafts from male donors to female recipients (M/F) compared with M/M mice (P < 0.01 M/F vs M/M; Fig. 3A; n = 6) and ~40-fold higher in the grafts from female donors to male recipients (F/M) compared with F/F mice (P < 0.01 F/M vs. F/F; Fig. 3A; n = 6). There was a distinct sex difference in the abundance of IL-6 after cross-sex-KTX, and the M/F showed fivefold higher in IL-6 mRNA than F/M group (P < 0.01 M/F vs. F/M; Fig. 3A; n = 6). The protein levels of the IL-6 exhibited similar sex difference patterns with mRNA expression for all the groups.
Fig. 3.
The relative renal gene expression of the inflammation factors. Renal gene expression of the inflammation factors: IL-6 (A; &P < 0.01 vs. M/M and F/M; % P < 0.01 vs. F/F); keratinocyte-derived chemokine (KC; B; *P < 0.01 vs FC; #P < 0.01 vs. F/F; &P < 0.01 vs. M/M and %P < 0.01 vs. F/F); TGF-β (C; #P < 0.01 vs. F/F; $P < 0.01 vs. F/M); TNF-α (D; #P < 0.01 vs. F/F in D1; *P < 0.01 vs. FC; #P < 0.01 vs. F/F; &P < 0.01 vs. F/M in D3); INF-γ (E); IL-17 (F; #P < 0.01 vs. MC and F/F; &P < 0.01 vs. MC); CCL2 (G; &P < 0.01 vs. M/M and F/M); and CCL5 (H; &P < 0.01 vs. M/M and F/M) of the transplanted kidneys at 4 wk after transplantation. (donor/recipient; M, male, F, female; n = 6).
The KC mRNA levels were approximately onefold higher in male kidneys than in female kidneys in both the sham-operated groups and same-sex-KTX groups (P < 0.05 MC vs. FC and M/M vs. F/F; Fig. 3B; n = 6). In the cross-sex-transplanted grafts, the KC levels elevated roughly two- and fivefold in M/F- and F/M-transplanted mice compared with same-sex-KTX groups, respectively (P < 0.01 M/F vs. M/M; P < 0.01 F/M vs. F/F; Fig. 3B; n = 6). No sex difference was found in the expression of KC after cross-sex-KTX.
The mRNA levels of TGF-β were 40% higher in the grafts of F/F than in M/M mice with same-sex-KTX (P < 0.01 F/F vs. M/M; Fig. 3C; n = 6). There was no difference in the expression of TGF-β in the grafts of M/F compared with M/M mice. The level of TGF-β was ~40% lower in grafts of F/M than in the grafts of F/F mice (P < 0.01 F/M vs. F/F; Fig. 3C; n = 6). The sex difference in the expression of TGF-β in the grafts was abolished after cross-sex-KTX.
The mRNA levels of TNF-α were fourfold higher in the grafts of M/M than in F/F mice. The TNF-α levels were significantly reduced in the grafts from male donors to female recipients (M/F) compared with M/M mice. There was no difference in the expression of TNF-α in the grafts from female donors to male recipients (F/M) compared with F/F mice. The sex difference in the expression of TNF-α was greatly attenuated after cross-sex-KTX (P < 0.01 M/M vs. F/F and M/F; Fig. 3D; n = 6). Figure 3D3 demonstrates protein expression of TNF-α as determined by Western blot analysis. The differences of mRNA abundance were paralleled by the differences of protein for most groups except for M/M group, in which only a slight increase in TNF-α protein expression was observed compared with other groups.
Kidney transplantation stimulated the mRNA expression of INF-γ and IL-17 in both same and cross-sex-KTX groups compared with control groups (P < 0.01 M/M vs. MC and F/F vs. FC; Fig. 3, E and F; n = 6). The mRNA expression of INF-γ and IL-17 did not differ between males and females in either same or cross-sex-KTX. Female grafts retained similar amount of CCL2 and CCL5 mRNA no matter they were housed in male or female recipients. However, male grafts in female recipients exhibited greatly enhanced expression for both CCL2 and CCL5. Thus, after cross-sex-KTX, female mice with male kidneys showed higher expression of CCL2 and CCL5 than male mice with female kidneys (P < 0.01 M/F vs. M/F; Fig. 3, G and H; n = 6).
Histology
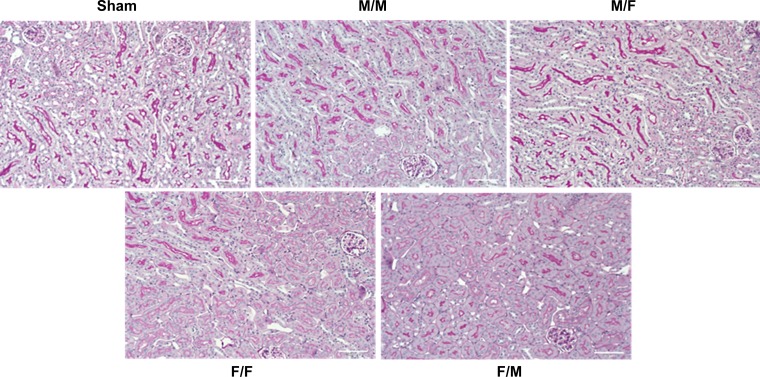
Kidney graft histopathological analysis were carried out by light microscopy. The kidney grafts in all groups demonstrated preserved architecture, normal intrarenal arteries. No significant tubular injuries, interstitial fibrosis, or vascular abnormalities were evident in the kidney grafts (Fig. 4).
Fig. 4.
Histology The kidney grafts in all groups demonstrated preserved architecture, normal intrarenal arteries. No significant tubular injuries, interstitial fibrosis, or vascular abnormalities were evident in the kidney grafts.
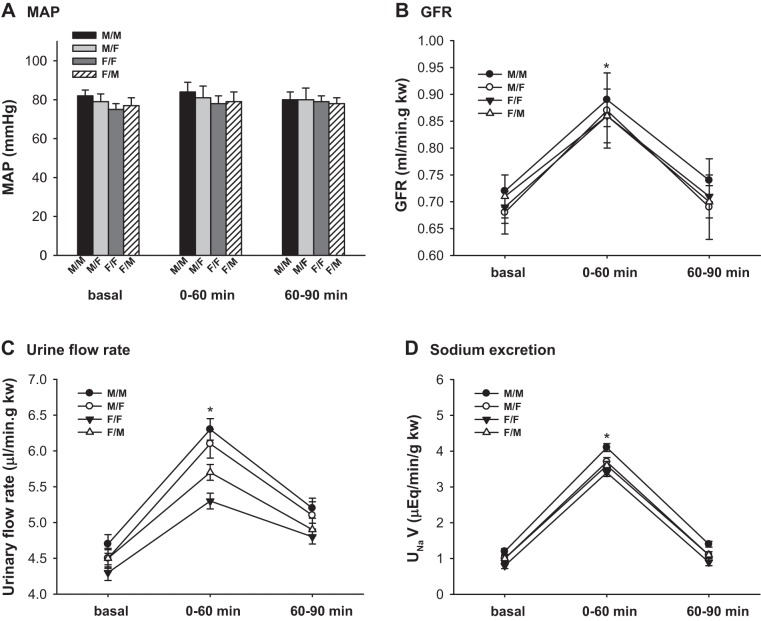
Renal Hemodynamics and Sodium Excretion in Response to AVE
To evaluate the renal hemodynamics and sodium excretion, we measured kidney clearance function in response to an AVE by intravenous infusion of saline in all groups of mice at the end of the experiments.
MAP.
The MAP was constant in all groups of mice before and during AVE. There were no significant differences in MAP among the four groups. (Fig. 5A; n = 6)
Fig. 5.
Renal clearance function of transplanted kidneys in response to acute volume expansion. Renal clearance function was measured at basal and during 0–60 and 60–90 min after an acute volume expansion (AVE) by infusion of 3% body wt of 0.9% NaCl saline. A: mean arterial pressure (MAP); B: glomerular filtration rate (GFR); C: urinary flow rate; D: Na+ excretion. GFR rose by ≈30% during 60 min after AVE and returned almost to baseline 90 min after AVE. The urinary flow rates and sodium excretion rates increased ~25–35% in all groups of animals within 60 min after AVE (*P < 0.01 vs. basal and 60–90 min, n = 6).
GFR.
The GFR of all groups rose by ≈30% during 60 min after AVE and returned almost to baseline 90 min after AVE. There was no significant difference in the GFR/kw among the different groups of mice. (P < 0.01 vs. basal and 60–90 min; Fig. 5B; n = 6).
Urinary flow rate and sodium excretion.
The urinary flow rates and sodium excretion rates increased ~25–35% in all groups of animals within 60 min after AVE. M/M mice had a higher urinary flow rate than F/F mice. The sex differences in the urinary flow rates were diminished after cross-sex-KTX (P < 0.01 vs. basal; P < 0.01 vs. F/F at basal and 0–60 and 60–90 min; Fig. 5C; n = 6).
There were no significant differences in the sodium excretion rates among different groups of mice in response to AVE (P < 0.01 vs. basal; Fig. 5D; n = 6).
Body Weight, Kidney Weight, and Survival Rate
Survival rate, body weight, and kidney weight were studied in all transplant recipients. After kidney transplantation, the body weight of all the mice dropped ~10% in the first 3 days and gradually recovered in 2 wk. At the end of the experiment, the body weight of all the groups was comparable to the mice without any operation for both sexes, respectively. The kidney weight of the M/M mice was ~1.5 times heavier than F/F mice. The kidney weights between the M/F- and the F/M-transplanted mice were similar. (Table 2.) The survival rate over 4 wk was ~90%, and the mice constantly gained body weight, indicating normal growth and well-being.
Table 2.
Body weight and kidney weight
| M/M | M/F | F/F | F/M | |
|---|---|---|---|---|
| BW, g | 26.6 ± 0.12 | 23.2 ± 0.13 | 22.8 ± 0.09 | 26.4 ± 0.14 |
| KW, g | 0.3198 ± 0.02* | 0.2327 ± 0.04 | 0.1967 ± 0.03 | 0.2253 ± 0.02 |
Values are means ± SE. M, male; F, female; BW, body weight; KW, kidney weight.
P < 0.05 vs. other groups in KW.
DISCUSSION
We performed same-sex- and cross-sex-KTX in mice and measured the gene expression levels in the transplanted kidneys. We found that same-sex-KTX did not alter the sex difference patterns of gene expression in the grafts. However, cross-sex-KTX significantly reduced or diminished the sex differences in most of the genes we measured. Cross-sex-KTX dramatically stimulated the inflammatory responses, which were reflected by the mRNA and protein expression of typical inflammation factors compared with the same-sex transplants. The kidney clearance function in response to AVE exhibited no significant differences between male and female mice at 4 wk after cross-sex-KTX.
Kidney transplantation often leads to disturbances of solute and volume maintenance in humans (11, 20), which is more complicated by the fact of immunosuppression therapies. We have chosen animal models that allowed us to eliminate the effects of transplantation with major rejection. Using these animal models, we first investigated if there were any changes of the expression of the key components of RAS, endothelin system, and tubular sodium transporters following same-sex-KTX compared with sham-operated mice. We observed small reduction in mRNA expression for NHE3 and ENaC. Other transporters (NKCC2 and NCC) and hormone receptors (AT1R, AT2R, ET-1, ETA, and ETB) showed unaltered expression. ANG II regulates transepithelial Na+ reabsorption via Na+/H+-exchange mediated by NHE3. No changes of AT1 expression were found, indicating that cellular components of the RAS were unaltered. It has been shown by Wang et al. (44) that 30 min of renal artery occlusion caused ~70% decrease in NHE3 mRNA and NHE3 activity. This small decrease in NHE3 and ENaC expression probably reflects the early ischemia-induced effect. Females have been demonstrated with relative protection from AKI (19, 25), which may explain the unaltered or less reduction of the expression of the receptors and transporters in female grafts in the same-sex-KTX group compared with sham-operated females.
Sex differences exist in the expression of the components of RAS, endothelin system, and tubular sodium transporters. In our present study, we found that same-sex-KTX did not alter the sex differences of the mentioned markers in the transplanted grafts. ANG II, ET-1, and their receptors are widely expressed in the renal vasculature and tubules and play critical roles in renal hemodynamics and sodium excretion. A higher ratio of AT1/AT2 receptors of ANG II has been found in males than in females (2, 34, 46). Male animals have higher renal ET-1 mRNA expression and greater ETA-mediated responses than females (17, 38). ENaC is a major sodium reabsorption transporter in the aldosterone-sensitive distal convoluted tubule and collecting duct. ENaC is assembled as a heterotrimer composed of three homologous subunits, α, β, and γ. Male mice have been found higher expression levels of all three ENaC subunits, α, β, and γ, than female mice (8, 26). We observed that the same-sex-transplanted kidneys exhibited the same pattern of sex difference in the expression of the components of RAS, endothelin system, and ENaC subunits in current study. Compared with F/F group, grafts in M/M mice showed nearly a 1-fold higher expression level of AT1; about ½-fold lower expression level of AT2; 11-fold higher expression level in ET-1; 2-fold higher expression level in ETA level; and significantly higher mRNA levels for all the 3 subunits of ENaC. However, after cross-sex-KTX, the sex differences in ANG II and endothelin receptors and ENaC subunits were dramatically decreased or diminished, and their expression levels demonstrated no significant difference between the M/F and F/M groups. Cross-sex-KTX altered the extrarenal environment of the grafts including the immunological and hormonal factors, which might explain at least partially the decreased sex differences. These results suggest that both donor’s genotype and recipient’s internal environment modulate these gene expression levels.
However, the changes in expression levels of NHE3, NKCC2, and NCC were not consistent with known expression levels by the respective sexes after KTX. NHE3 is highly expressed in the apical membranes of proximal tubule (PT), thick ascending loop of Henle (TALH), and macula densa. Male mice have been reported with either a similar (41) or a lower level of NHE3 at the outer medulla region compared with female mice (40). We found that the NHE3 mRNA expression levels were similar between males and females in the same-sex-KTX. The NHE3 level significantly increased in the grafts from male donors transplanted to female recipients (M/F) compared with M/M mice. There was no significant change in the expression of NHE3 in the grafts from female donors to male recipients (F/M) compared with F/F mice. The sex differences in protein levels of NHE3 paralleled the sex differences in mRNA expression. These results suggested that the recipient environment of females may play a major role in upregulation of NHE3. NKCC2 is abundant in the apical membrane of the epithelial cells of the TALH, which facilitates ∼20–30% of the reuptake of the total filtered NaCl. NKCC2 in the macula densa mediates tubuloglomerular feedback and control of renin release (27, 35). The NKCC2 level was reported to be higher in male than female mice (41). In our present study, we also found that NKCC2 mRNA was higher in males (M/M) than in females (F/F) after the same-sex-KTX. In cross-sex-KTX groups, however, the sex differences in NKCC2 expression were diminished. NCC is expressed in the distal convoluted tubule. Females have a higher expression level of NCC than age-matched males (31, 40). The same-sex-KTX in the present study showed a consistent pattern of sex difference in NCC expression, in which the NCC expression was approximately onefold higher in F/F compared with M/M mice. Cross-sex-KTX enhanced the NCC expression in both F/M and M/F groups. The reasons for these unexpected results are not clear. However, the ratio of the amount of the NCC between male and female grafts did not change, which may suggest the trifling effect of the recipient extrarenal environment on the expression of NCC.
Inflammatory response has been demonstrated to play an essential role in the regulation of renal hemodynamics and kidney injury (1). The infiltration of T cells into the kidney in response to renal insult resulted in augmented renal inflammation, including increased renal cytokine and chemokine (10). Sex-specific modulation of the immune system (33) and magnitude of T-cell infiltration of the kidney (28) have been reported. In the present study, we measured several typical inflammatory factors, including IL-6, TNF-α, TGF-β, and KC after KTX. We found that cross-sex-KTX significantly enhanced the inflammatory response in both males and females. The most significant change was a more than 20-fold increase in IL-6 for male kidneys implanted into females. Minor histocompatibility antigen rejection, in which female recipients reject grafts from the male donors due to incompatibility with the male H-Y antigen (5, 24), may be a factor caused the obvious stimulation of the inflammation response in M/F group compared with other paired groups. We realized that we measured the inflammatory responses at the end of the experiments, which was 4 wk after transplantation. We may have missed the peak period of inflammatory response, which is probably one of the reasons why we did not detect the difference in the levels of KC and TNF-α between M/F and F/M. Also, only mRNA levels were measured for TGF-β and KC in the present study, which may or may not be consistent with or representative of the protein and activity levels.
Kidney clearance function in response to AVE was examined in all groups of animals. Even though there were significant differences in gene expression levels in the kidney grafts, and in the inflammatory responses, there was no significant difference in the kidney clearance function. The MAP, GFR, and sodium excretion rates were similar in response to AVE among all groups of mice, which may reflect an efficient systemic compensatory effect. However, even though the kidney clearance function was similar at 4 wk after KTX among the various groups of mice in the present study, the differences in gene expression levels and inflammatory responses may play an important role in long-term graft function or future rejection episodes.
Sex difference and sex steroids in modulating the gene expression levels and activities of the renal vascular and tubular sodium transporters have been extensively studied. Ovariectomy, orchiectomy, central blockade, or total knockout of estrogen or androgen receptors and administration of steroid have been applied to study the mechanisms of sex difference (3, 12, 30). While these studies have provided valuable information and advanced our understanding about sex difference in many areas, all these strategies did not exactly mimic the physiological conditions. One example is the failure of the estrogen replacement clinical trial (29, 36). The sex difference is not as simple as addition or removal of a specific hormone. Cross-sex-KTX makes it possible to put a male’s kidney in a real female’s environment and vice versa, which may provide an additional approach to study sex difference in renal functions.
In summary, we performed same-sex- and cross-sex-KTX in mice. Gene expression levels, inflammatory responses, and kidney clearance function were evaluated. We found that the same-sex-KTX did not alter the sex difference in the expression of most of the genes we measured. However, cross-sex-KTX decreased or diminished the sex difference in gene expressions. We conclude that both the genotype of the donor’s kidney and recipient’s environment modulate the gene expressions in the transplanted grafts. Cross-sex-KTX significantly enhanced the inflammatory response as reflected by elevated IL-6 and KC levels. Even though apparent alterations were observed in inflammatory response and gene expression levels in the cross-sex-transplanted grafts, no obvious abnormalities were detected in both histology and renal clearance function. It could be due to the systemic compensatory mechanism or the time after transplantation is short. We will extend the observation time to 3–6 mo after kidney transplantation to determine if the transplanted grafts exhibit any functional abnormality. The present study investigated the sex differences of the main components of RAS, the endothelin system, and tubular sodium transporters following same-sex- and cross sex-KTX. With broader assessment of more receptors, transporters, and inflammatory mediators in the future, we believe that our study will provide insight into and new information toward the improvement of long-term graft function, as well as a new approach for research in to sex difference of renal functions.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-099276 and DK-098582 (both to R. Liu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Lei Wang and R.L. conceived and designed research; Lei Wang, J.S., S.W., and R.C. performed experiments; Lei Wang, J.S., J.B., Liqing Wang, S.R., W.L., J.W., and R.L. analyzed data; Lei Wang, J.B., J.Z., and R.L. interpreted results of experiments; Lei Wang and S.W. prepared figures; Lei Wang drafted manuscript; Lei Wang, J.B., R.C., J.Z., Liqing Wang, S.R., W.L., J.W., and R.L. edited and revised manuscript; R.L. approved final version of manuscript.
REFERENCES
- 1.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009: 137072, 2009. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul Pept 124: 7–17, 2005. doi: 10.1016/j.regpep.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S, Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovasc Res 79: 179–186, 2008. doi: 10.1093/cvr/cvn068. [DOI] [PubMed] [Google Scholar]
- 4.Davis CL. Sex mismatches in kidney transplantation. Lancet 372: 10–11, 2008. doi: 10.1016/S0140-6736(08)60969-1. [DOI] [PubMed] [Google Scholar]
- 5.Dierselhuis M, Goulmy E. The relevance of minor histocompatibility antigens in solid organ transplantation. Curr Opin Organ Transplant 14: 419–425, 2009. doi: 10.1097/MOT.0b013e32832d399c. [DOI] [PubMed] [Google Scholar]
- 6.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 53: 672–677, 2002. doi: 10.1016/S0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]
- 7.Głyda M, Czapiewski W, Karczewski M, Pięta R, Oko A. Influence of donor and recipient gender as well as selected factors on the five-year survival of kidney graft. Pol Przegl Chir 83: 188–195, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Gormley K, Dong Y, Sagnella GA. Regulation of the epithelial sodium channel by accessory proteins. Biochem J 371: 1–14, 2003. doi: 10.1042/bj20021375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gratwohl A, Döhler B, Stern M, Opelz G. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. Lancet 372: 49–53, 2008. doi: 10.1016/S0140-6736(08)60992-7. [DOI] [PubMed] [Google Scholar]
- 10.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heering P, Degenhardt S, Grabensee B. Tubular dysfunction following kidney transplantation. Nephron 74: 501–511, 1996. doi: 10.1159/000189443. [DOI] [PubMed] [Google Scholar]
- 12.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension 35: 484–489, 2000. doi: 10.1161/01.HYP.35.1.484. [DOI] [PubMed] [Google Scholar]
- 13.Hricik DE, Halbert RJ, Barr ML, Helderman JH, Matas AJ, Pirsch JD, Schenkel FA, Siegal B, Ferguson RM. Life satisfaction in renal transplant recipients: preliminary results from the transplant learning center. Am J Kidney Dis 38: 580–587, 2001. doi: 10.1053/ajkd.2001.26884. [DOI] [PubMed] [Google Scholar]
- 14.Iseki K, Iseki C, Ikemiya Y, Fukiyama K. Risk of developing end-stage renal disease in a cohort of mass screening. Kidney Int 49: 800–805, 1996. doi: 10.1038/ki.1996.111. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant 2: 970–974, 2002. doi: 10.1034/j.1600-6143.2002.21015.x. [DOI] [PubMed] [Google Scholar]
- 16.Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl (2011) 3: 368–371, 2013. doi: 10.1038/kisup.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kittikulsuth W, Sullivan JC, Pollock DM. ET-1 actions in the kidney: evidence for sex differences. Br J Pharmacol 168: 318–326, 2013. doi: 10.1111/j.1476-5381.2012.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y. Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, Liu EY, Zhang J, Hansen PB, Fan F, Juncos LA, Wang L, Pollock J, Huang PL, Fu Y, Wang S, Liu R. Macula densa NOS1β protects against salt-sensitive hypertension. J Am Soc Nephrol 27: 2346–2356, 2006. doi: 10.1681/ASN.2015050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller V, Losonczy G, Heemann U, Vannay A, Fekete A, Reusz G, Tulassay T, Szabó AJ. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int 62: 1364–1371, 2002. doi: 10.1111/j.1523-1755.2002.kid590.x. [DOI] [PubMed] [Google Scholar]
- 20.Muller V, Szabo AJ, Erdely A, Tain YL, Baylis C. Sex differences in response to cyclosporine immunosuppression in experimental kidney transplantation. Clin Exp Pharmacol Physiol 35: 574–579, 2008. doi: 10.1111/j.1440-1681.2007.04841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med 268: 1315–1323, 1963. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 22.Øien CM, Reisaeter AV, Leivestad T, Dekker FW, Line PD, Os I. Living donor kidney transplantation: the effects of donor age and gender on short- and long-term outcomes. Transplantation 83: 600–606, 2007. doi: 10.1097/01.tp.0000255583.34329.dd. [DOI] [PubMed] [Google Scholar]
- 23.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension 51: 1142–1148, 2008. doi: 10.1161/HYPERTENSIONAHA.107.105205. [DOI] [PubMed] [Google Scholar]
- 24.Pabón MA, Navarro CE, Martin R, Rodríguez M, Martin I, Gaitán L, Gómez A, Lozano E. Minor histocompatibility antigens as risk factor for poor prognosis in kidney transplantation. Transplant Proc 43: 3319–3323, 2011. doi: 10.1016/j.transproceed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 279: 52282–52292, 2004. doi: 10.1074/jbc.M407629200. [DOI] [PubMed] [Google Scholar]
- 26.Pearce D, Soundararajan R, Trimpert C, Kashlan OB, Deen PM, Kohan DE. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10: 135–146, 2015. doi: 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peti-Peterdi J, Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol 21: 1093–1096, 2010. doi: 10.1681/ASN.2009070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64: 384–390, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pripp U, Hall G, Csemiczky G, Eksborg S, Landgren BM, Schenck-Gustafsson K. A randomized trial on effects of hormone therapy on ambulatory blood pressure and lipoprotein levels in women with coronary artery disease. J Hypertens 17: 1379–1386, 1999. doi: 10.1097/00004872-199917100-00004. [DOI] [PubMed] [Google Scholar]
- 30.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31: 435–439, 1998. doi: 10.1161/01.HYP.31.1.435. [DOI] [PubMed] [Google Scholar]
- 31.Riazi S, Madala-Halagappa VK, Hu X, Ecelbarger CA. Sex and body-type interactions in the regulation of renal sodium transporter levels, urinary excretion, and activity in lean and obese Zucker rats. Gend Med 3: 309–327, 2006. doi: 10.1016/S1550-8579(06)80219-6. [DOI] [PubMed] [Google Scholar]
- 32.Rong S, Lewis AG, Kunter U, Haller H, Gueler F. A knotless technique for kidney transplantation in the mouse. J Transplant 2012: 127215, 2012. doi: 10.1155/2012/127215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandberg K, Ji H, Hay M. Sex-specific immune modulation of primary hypertension. Cell Immunol 294: 95–101, 2015. doi: 10.1016/j.cellimm.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider MP, Wach PF, Durley MK, Pollock JS, Pollock DM. Sex differences in acute ANG II-mediated hemodynamic responses in mice. Am J Physiol Regul Integr Comp Physiol 299: R899–R906, 2010. doi: 10.1152/ajpregu.00638.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnermann J, Traynor T, Yang T, Arend L, Huang YG, Smart A, Briggs JP. Tubuloglomerular feedback: new concepts and developments. Kidney Int Suppl 54, Suppl 67: S40–S45, 1998. doi: 10.1046/j.1523-1755.1998.06708.x. [DOI] [PubMed] [Google Scholar]
- 36.Seely EW, Walsh BW, Gerhard MD, Williams GH. Estradiol with or without progesterone and ambulatory blood pressure in postmenopausal women. Hypertension 33: 1190–1194, 1999. doi: 10.1161/01.HYP.33.5.1190. [DOI] [PubMed] [Google Scholar]
- 37.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 38.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet. Hypertension 41: 657–662, 2003. doi: 10.1161/01.HYP.0000048193.85814.78. [DOI] [PubMed] [Google Scholar]
- 39.Tian YF, Liao CH, Chen MJ. Risk factors among donor characteristics which affect graft outcome in paired kidney transplantation. Transplant Proc 40: 2281–2284, 2008. doi: 10.1016/j.transproceed.2008.07.105. [DOI] [PubMed] [Google Scholar]
- 40.Tiwari S, Li L, Riazi S, Halagappa VK, Ecelbarger CM. Sex and age result in differential regulation of the renal thiazide-sensitive NaCl cotransporter and the epithelial sodium channel in angiotensin II-infused mice. Am J Nephrol 30: 554–562, 2009. doi: 10.1159/000252776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiwari S, Li L, Riazi S, Halagappa VK, Ecelbarger CM. Sex differences in adaptive downregulation of pre-macula densa sodium transporters with ANG II infusion in mice. Am J Physiol Renal Physiol 298: F187–F195, 2010. doi: 10.1152/ajprenal.00088.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vavallo A, Lucarelli G, Spilotros M, Bettocchi C, Palazzo S, Selvaggi FP, Battaglia M, Ditonno P. Impact of donor-recipient gender on kidney graft and patient survival: short- and long-term outcomes. World J Urol 32: 709–714, 2014. doi: 10.1007/s00345-013-1137-9. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Chandrashekar K, Wang L, Lai EY, Wei J, Zhang G, Wang S, Zhang J, Juncos LA, Liu R. Inhibition of nitric oxide synthase 1 induces salt-sensitive hypertension in nitric oxide synthase 1α knockout and wild-type mice. Hypertension 67: 792–799, 2016. doi: 10.1161/HYPERTENSIONAHA.115.07032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Rabb H, Craig T, Burnham C, Shull GE, Soleimani M. Ischemic-reperfusion injury in the kidney: overexpression of colonic H+-K+-ATPase and suppression of NHE-3. Kidney Int 51: 1106–1115, 1997. doi: 10.1038/ki.1997.153. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 46.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Ma LL, Ma BR, Tian Y. [Effects of donor age and gender on early acute rejection episode in living related donor kidney transplantation]. Zhonghua Yi Xue Za Zhi 88: 3407–3410, 2008. [PubMed] [Google Scholar]