Abstract
The role and mechanism of renal protein disulfide isomerase (PDI) in blood pressure regulation has not been tested before. Here, we test this possibility in Sprague-Dawley rats. Rats were treated with PDI inhibitor bacitracin (100 mg·kg−1 ip·day−1 for 14 days), and then blood pressure and renal angiotensin II type 1 (AT1) receptor function were determined in anesthetized rats. Renal AT1 receptor function was determined as the ability of candesartan (an AT1 receptor blocker) to increase diuresis and natriuresis. A second set of vehicle- and bacitracin-treated rats was used to determine biochemical parameters. Systolic blood pressure as well as diastolic blood pressure increased in bacitracin-treated compared with vehicle-treated rats. Compared with vehicle, bacitracin-treated rats showed increased diuresis and natriuresis in response to candesartan (10-µg iv bolus dose) suggesting higher AT1 receptor function in these rats. These were associated with higher renin activities in the plasma and renal tissues. Furthermore, urinary 8-isoprostane and kidney injury molecule-1 levels were higher and urinary antioxidant capacity was lower in bacitracin-treated rats. Renal protein carbonyl and nitrotyrosine levels also were higher in bacitracin- compared with vehicle-treated rats, suggesting oxidative stress burden in bacitracin-treated rats. Moreover, PDI activity decreased and its protein levels increased in renal tissues of bacitracin-treated rats. Also, nuclear levels of Nrf2 transcription factor, which regulates redox homeostasis, were decreased in bacitracin-treated rats. Furthermore, tissue levels of Keap1, an Nrf2 inhibitory molecule, and tyrosine 216-phosphorylated GSK3β protein, an Nrf2 nuclear export protein, were increased in bacitracin-treated rats. These results suggest that renal PDI by regulating Keap1-Nrf2 pathway acts as an antioxidant, maintaining redox balance, renal AT1 receptor function, and blood pressure in rats.
Keywords: protein disulfide isomerase, Nrf2, Keap1, AT1 receptor, oxidative stress
renal angiotensin ii type 1 (AT1) receptor plays a vital role in maintaining sodium homeostasis and subsequently control of blood pressure (4, 7). Hypertensive animal models including genetic, obesity, and aging animal models have been shown to be associated with higher AT1 receptor function, blood pressure, and oxidative stress (5, 13, 33). Studies demonstrate that reducing oxidative stress with antioxidant treatment restores AT1 receptor function and blood pressure in these animals (7). This suggests that redox balance is crucial for maintaining normal AT1 receptor function and blood pressure.
Erythroid 2-related factor 2 (Nrf2) transcription factor is a master regulator of cellular antioxidant defense genes and helps maintain cellular redox balance (15, 16). Nrf2 interacts with Kelch-like ECH-associated protein 1 (Keap1), an Nrf2 inhibitory molecule that degrades Nrf2 and reduces its nuclear translocation (17, 23). Impairment in Nrf2 activation is linked to chronic kidney diseases, whereas its stimulation has been attributed to reducing blood pressure in hypertension (14, 28, 29). However, the role of renal PDI in this process has not been investigated.
Protein disulfide isomerase (PDI) is a multifunctional protein that catalyzes many reactions such as oxidation, reduction, and isomerization (2, 35). It is mainly localized in the endoplasmic reticulum; however, it is also found in other cellular compartments including cell surface, cytosol, mitochondria, and nuclear envelopes (1, 36). In resistance arteries, PDI via Nox1 mechanism has been linked to hypertension, glutathione depletion-induced cytotoxicity in neuronal cells, and inflammation in immune cells (3, 12, 26). However, the role and mechanism of renal PDI in regulation of blood pressure is not known. Therefore, the present study was designed in Sprague-Dawley (SD) rats to test the role of renal PDI in regulation of blood pressure. SD rats were treated with vehicle or PDI inhibitor bacitracin followed by determination of blood pressure, renal AT1 receptor function, and plasma and renal tissue levels of renin. Markers of oxidative stress, PDI activity and its protein levels, tissue levels of Keap1 and tyrosine 216-phosphorylated GSK3β protein (GSK3β-pY216), nuclear Nrf2 levels, and the activities of certain enzymes that Nrf2 regulates were also determined.
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats (200–250 g) were purchased from Harlan (Indianapolis, IN) and acclimatized for a week before any treatment. Animal experiments were performed per National Institutes of Health guidelines and a protocol approved by the Institutional Animal Care and Use Committee. Animals were divided into control and bacitracin-treated groups. Control group was treated with vehicle saline. Bacitracin, a PDI inhibitor (100 mg/kg ip), was administered in the treated group for 14 days. Bacitracin was dissolved in sterile saline, and 200 µl (final volume) was administered to rats. Control rats were administered with the same volume of sterile saline. Food and water were provided to rats ad libitum.
Measurements of blood pressure and AT1 receptor function.
Rats were anesthetized with Inactin (100 mg/kg), and then blood pressure and AT1 receptor function were determined using our (7) published method. Briefly, under deep anesthesia, tracheotomy was performed to facilitate spontaneous breathing in rats. To measure blood pressure, left carotid artery was catheterized with a pressure catheter transducer (Mikro-Tip model PVR-1045) connected to a computer with PowerLab data acquisition system and LabChart Pro software (Millar, Houston, TX). Blood pressure was recorded for 1 h. Thereafter, the left jugular vein was catheterized with PE-50 tubing for saline/candesartan infusion. A midline abdominal incision was made, and the bladder was cannulated with PE-50 tubing to collect urine. Normal saline (1% body wt/h) was continuously infused throughout the experiment period to maintain stable urine output and to prevent dehydration. Two urine samples (C1 and C2 each for 15 min) during saline infusion were collected. Thereafter, candesartan was administered (10 µg/kg body wt bolus dose), and two urine samples (D1 and D2 each for 15 min) were collected. Urine volumes for C1, C2, D1, and D2 were recorded and used to measure sodium by atomic absorption spectroscopy (PerkinElmer, Waltham, MA). Values for urine volumes and sodium from C1, C2, D1, and D2 were averaged out and reported as urine output and sodium excretion, respectively.
Measurement of biochemical parameters.
A second set of vehicle- and bacitracin-treated rats were anesthetized as above, a midline abdominal incision was made, bladder urine was collected with 1-ml syringe, and blood was collected through heart puncture. Blood samples were centrifuged at 1,000 g at 4°C, and plasma was obtained.
Plasma and renal tissue levels of renin.
Plasma and renal tissue renin activity was determined using the SensoLyte 520 Rat Renin Assay Kit *Fluorimetric* (AnaSpec, Fremont, CA). Briefly, 10 μl of plasma samples and 5 µg of cortical tissue homogenates were incubated with 40 μl of renin substrate solution for 60 min at 37°C, and the fluorescence intensity was measured using excitation (490 nm) and emission (520 nm) wavelengths.
Oxidative stress markers.
8-Isoprostane (Cayman Chemical, Ann Arbor, MI), kidney injury molecule-1 (R&D Systems, Minneapolis, MN), and antioxidant capacity (Cayman Chemical) were determined in urine samples using kit-based assays as markers of systemic oxidative stress. Superficial cortical tissues were used to measure protein carbonyls (Cayman Chemical) and protein nitrotyrosine (Cayman Chemical) as markers of oxidative stress in the kidney.
Western blotting.
Nuclear protein extraction from superficial kidney cortex was prepared using a commercially available kit (Thermo Fisher Scientific, Rockford, IL). Superficial cortex homogenate (20 µg) and nuclear fraction (20 µg) were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA). Specific antibodies for PDI (rabbit polyclonal antibody; Abcam, Cambridge, MA), Keap1 (rabbit polyclonal; Santa Cruz Biotechnology), GAPDH (mouse monoclonal antibody; Millipore, Temecula, CA), Nrf2 (rabbit polyclonal antibody; Abcam), histone deacetylase (HDAC; mouse monoclonal; Santa Cruz Biotechnology), GSK3β-pY216 (mouse monoclonal antibody; BD Biosciences, San Jose, CA), and GSK3β (mouse monoclonal antibody; BD Biosciences) were used to determine respective proteins by Western blotting. Anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies as appropriate (Santa Cruz Biotechnology) were used to probe respective primary antibodies.
PDI activity assay.
PDI activity was measured by using PROTEOSTAT PDI assay kit (ENZ-51024-KP002; Enzo Life Sciences, Farmingdale, NY). The assay was performed following the instructions provided by the manufacturer with slight modifications. Briefly, 20 µg of proteins were incubated with insulin in the presence of 0.02% sodium azide for 3 h at room temperature in the dark. Thereafter, stop solution and detection reagent were added, incubated for 15 min at room temperature in the dark, and fluorescence was read (excitation 500 nm; emission 603-nm filters).
Phase II antioxidant enzymes assay.
Superoxide dismutase (SOD; Cayman Chemical) and glutathione S-transferase (GST; Cayman Chemical) were determined in superficial cortical tissues using kit-based assays as per manufacturer’s protocol.
Statistics.
Two-way ANOVA with Newman-Keuls post hoc tests or unpaired Student's t-test was used to evaluate the statistical significance of difference between the means. A P < 0.05 value was considered as significant.
RESULTS
Effects of bacitracin on blood pressure and renal AT1 receptor function.
Systolic and diastolic blood pressure (Fig. 1, A and B) were significantly higher in bacitracin-treated rats than vehicle-treated control rats. Compared with saline, candesartan infusion increased urine output and sodium excretion in both vehicle control and bacitracin-treated rats (Fig. 2, A and B). However, magnitude of diuresis and natriuresis were more pronounced in bacitracin-treated than vehicle control rats (Fig. 2, A and B).
Fig. 1.
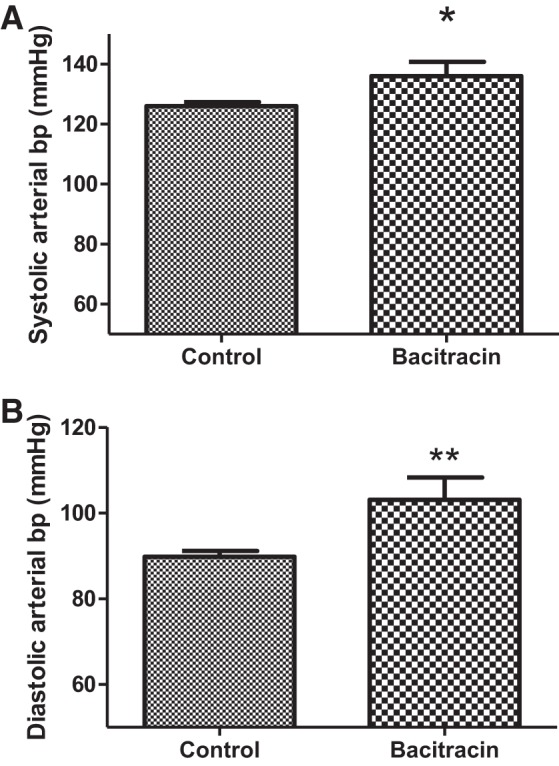
Bacitracin treatment increases systolic (A; Control vs. Bacitracin: 126.1 ± 1.349 vs. 136.1 ± 4.721 mmHg) and diastolic blood pressures (bp; B; Control vs. Bacitracin: 80.82 ± 1.334 vs. 103.1 ± 5.184 mmHg) in rats. Blood pressures were measured in control and bacitracin-treated anesthetized rats (details in materials and methods). *P < 0.05, **P < 0.01 from controls; n = 10–15 animals.
Fig. 2.
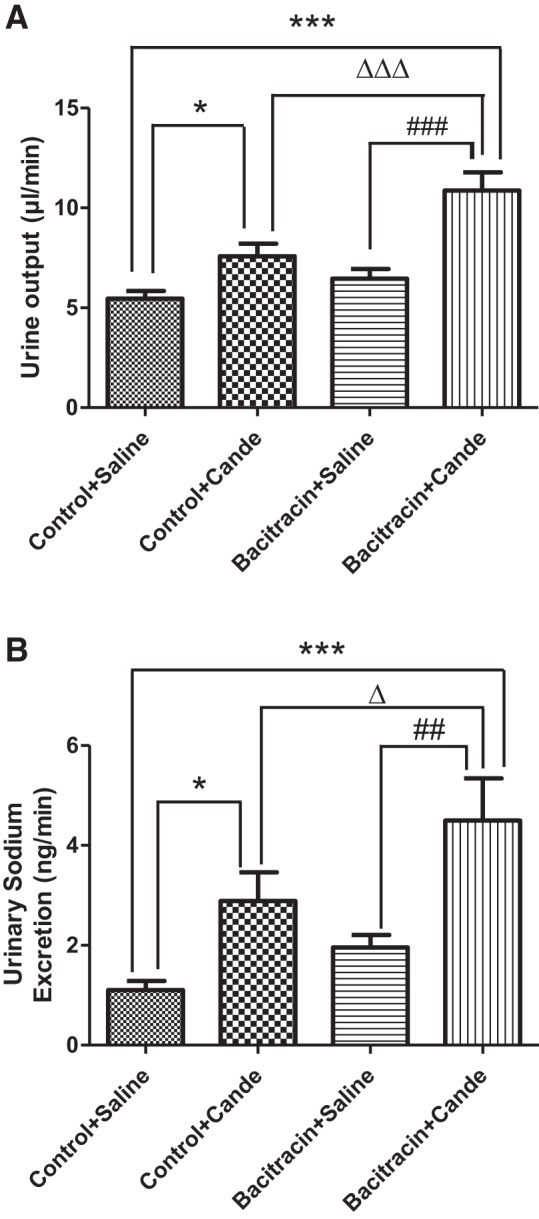
Bacitracin treatment increases diuresis and natriuresis in response to candesartan in rats. Diuresis (A) and natriuresis (B), indices of renal AT1 receptor function, were measured in response to candesartan (cande) in control and bacitracin-treated anesthetized rats (details in materials and methods). *P < 0.05 from control+saline, ***P < 0.001 from control+saline, ∆P < 0.05 from control+cande, ∆∆∆P < 0.001 from control+cande, ##P < 0.01 from bacitracin+saline, ###P < 0.001 from bacitracin+saline; n = 10–12 animals.
Effects of bacitracin on plasma and renal levels of renin.
Plasma and renal levels of renin activities were higher in bacitracin-treated than in vehicle-treated control rats (Fig. 3, A and B).
Fig. 3.
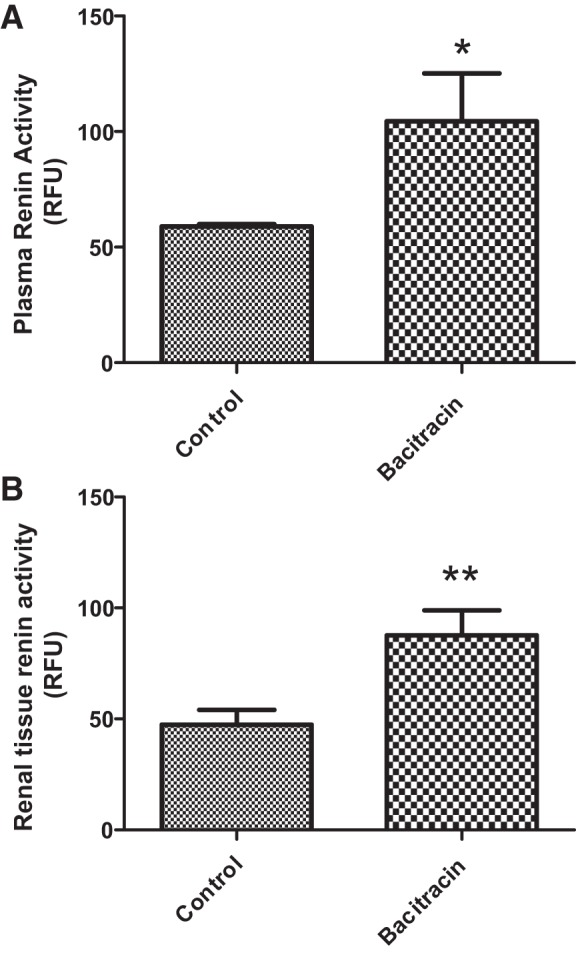
Bacitracin treatment increases the plasma and renal tissue renin activities in rats. Plasma (A) and renal tissue (B) renin activities (in relative fluorescence units, RFU) were measured using a kit-based method (details in materials and methods). *P < 0.05, **P < 0.01 from control; n = 4–6 animals.
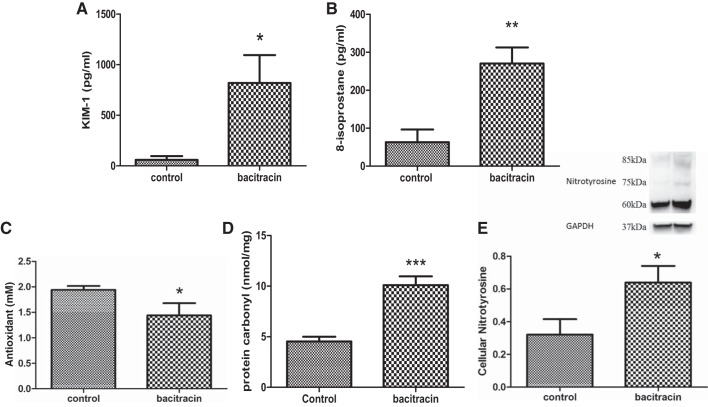
Effects of bacitracin on kidney injury and oxidative stress markers.
Urinary levels of kidney injury molecule-1 (Fig. 4A) and 8-isoprostane (Fig. 4B) were higher and antioxidant capacity (Fig. 4C) was lower in bacitracin-treated compared with vehicle-treated control rats. Also, protein carbonyls and protein nitrotyrosine levels in renal tissues were higher in bacitracin-treated compared with vehicle-treated control rats (Fig. 4, D and E).
Fig. 4.
Bacitracin treatment increases the levels of kidney injury molecule-1 (KIM-1) and oxidative stress markers in rats. KIM-1 (A) and 8-isoprostane (B) levels and antioxidant capacity (C) were measured in the urine. Oxidative stress markers protein carbonyls (D) and protein nitrotyrosine levels (E) were measured in renal tissues. E, top: representative blots for protein nitrotyrosine and loading control GAPDH. Below are ratios of the densities between protein nitrotyrosine and GAPDH. *P < 0.05, **P < 0.01, ***P < 0.01 from control; n = 4–6 animals.
Effects of bacitracin on PDI activity and expression.
PDI activity in renal tissues decreased with bacitracin treatment compared with vehicle treatment of rats (Fig. 5A). However, the levels of PDI proteins increased in bacitracin-treated rats compared with the vehicle-treated control rats (Fig. 5B).
Fig. 5.
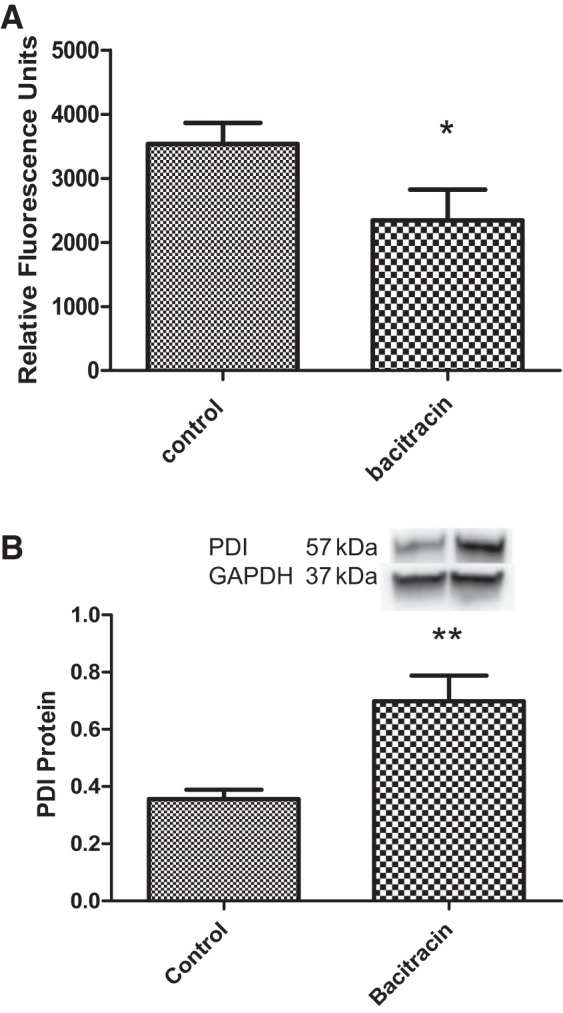
Bacitracin treatment reduces PDI activity but increases its protein levels in renal tissues. A: PDI activity was measured using a kit-based method (details in materials and methods). B: PDI protein levels were determined by Western botting. Top: representative blots for PDI and loading control GAPDH. Below are ratios of the densities between PDI and GAPDH. *P < 0.05, **P < 0.01 from controls; n = 4–6 animals.
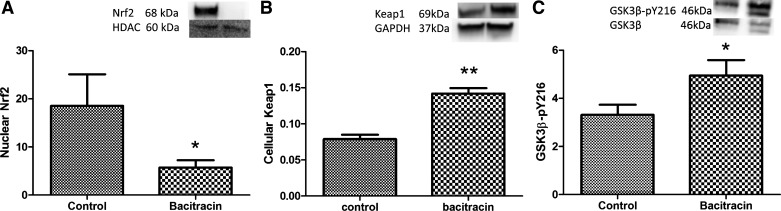
Effects of bacitracin on Nrf2, Keap1, GSK3β-pY216, SOD, and GST.
Nuclear levels of Nrf2 were absent in bacitracin-treated compared with vehicle-treated rats (Fig. 6A), whereas the levels of Keap1, an Nrf2 repressor, increased in bacitracin- compared with vehicle-treated rats (Fig. 6B). Furthermore, GSK3β-pY216, which causes Nrf2 export from the nucleus, increased in bacitracin- compared with vehicle-treated rats (Fig. 6C).
Fig. 6.
Bacitracin treatment decreases Nrf2 activity in renal tissues. Nuclear Nrf2 protein levels (A), Keap1 protein levels (B), and GSK3β-pY216 protein levels (C) were determined by Western blotting. Top: representative blots for Nrf2 (A), Keap1 (B), and GSK3β-pY216 (C) and protein loading controls HDAC (A), GAPDH (B), and GSK3β (C). Below are ratios of the densities between Nrf2 and HDAC (A), Keap1 and GAPDH (B), and GSK3β-pY216 and GSK3β. *P < 0.05, **P < 0.01 from controls; n = 4–6 animals.
The activities of SOD and GST, the antioxidant enzymes regulated by Nrf2, were decreased in the renal tissues of bacitracin-treated rats compared with vehicle control rats (Fig. 7, A and B).
Fig. 7.
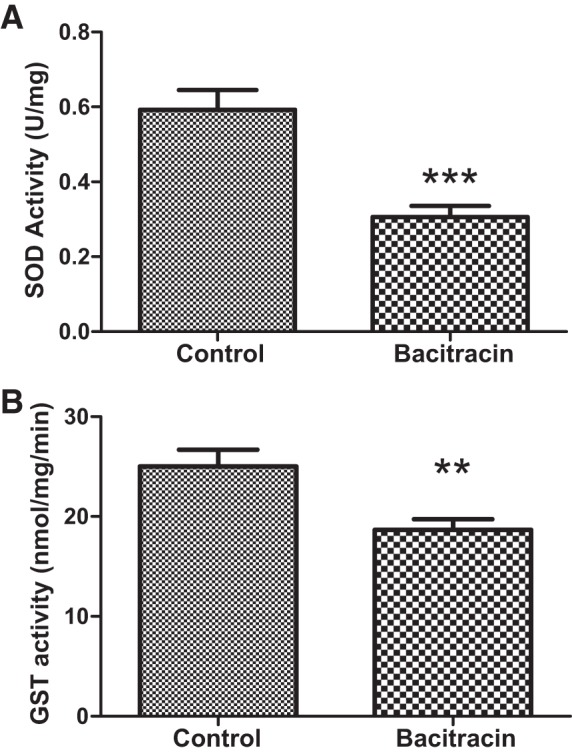
Bacitracin treatment decreases Nrf2-regulated phase II antioxidant enzyme activities of renal superoxide dismutase (SOD) and glutathione S-transferase (GST) in rats. SOD (A) and GST (B) activities were determined in renal tissues (details in materials and methods). **P < 0.01, ***P < 0.001 from control; n = 4–6 animals.
DISCUSSION
In this study, we have shown that bacitracin treatment in rats causes inhibition of PDI activity, increased expressions of Keap1, increased GSK3β-pY216, and impaired accumulation of nuclear Nrf2 in renal tissues. Also, activities of SOD and GST, which are target genes for Nrf2 (14, 37), were reduced and oxidative stress burden was higher in bacitracin-treated (PDI-inhibited) rats. Furthermore, we found that bacitracin-treated (PDI-inhibited) rats had higher plasma and renal levels of renin activities, which was associated with increased renal AT1 receptor function and blood pressure in these rats.
Renal AT1 receptor is an important component of renin-angiotensin system that plays a critical role in regulation of blood pressure (8, 20, 38, 39). There is evidence showing that oxidative stress is one of the most important factors that contributes to hyper AT1 receptor function and hypertension (24, 25). Overwhelming evidence also has linked oxidative stress to hypertension, in genetic, obesity, and aging hypertensive animal models as well as in hypertensive patients, exhibiting oxidative stress burden and hyper renal AT1 receptor function (9, 13, 34). We also have found high blood pressure in bacitracin-treated (PDI-inhibited) rats. This was associated with increased plasma and renal levels of renin, oxidative stress, and AT1 receptor function in these rats, suggesting renal PDI plays a key role in these phenomena. Although increase in blood pressure in these rats was moderate (+10–20 mmHg), it may potentially cause adverse effects on cardiovascular outcomes. A small decrease in blood pressure (~5 mmHg) has been linked to better cardiovascular outcomes in patients (19, 27). Taken together, these studies suggest that redox balance is critical for normal AT1 receptor function and blood pressure.
Redox balance is achieved as a function of cross talk between cellular oxidative stress-generating and antioxidant defense system. Altered functioning of antioxidant defenses leads to increase in oxidative stress. Nrf2 system is one of the master regulators of the antioxidant defense systems (21). Nrf2 interacts with Keap1, which directs Nrf2 for proteasome degradation (17, 18, 23). Nrf2, when not bound to Keap1, translocates to the nucleus and causes transcription of its target antioxidant genes, including heme oxygenase-1, superoxide dismutase, catalase, and glutathione S-transferase (16). We found that Nrf2 was absent in the nucleus of renal tissues of bacitracin-treated rats. This was associated with decreased antioxidant enzymes activities (SOD and GST) and increased oxidative stress burden in bacitracin-treated rats. These data suggest that Nrf2 is an important cellular factor that maintains redox homeostasis. Also, these data point out a critical role of PDI in the regulation of Nrf2 and redox balance, as we found PDI inhibition with bacitracin was associated with absence of Nrf2 in the nuclei and increased oxidative stress in rats.
The absence of Nrf2 from the nuclei in the bacitracin-treated rats could be due to increased expression of its inhibitor, Keap1, and hyper GSK3β tyrosine 216 phosphorylation. Both Keap1 (17, 23) and GSK3β-pY216 (6) have been linked to regulate Nrf2 levels. Keap1 is an Nrf2 repressor protein that binds Nrf2 in the cytosol and directs its proteasomal degradation (14, 19). GSK3β-pY216 is linked to Nrf2 export signal from the nuclei (31). We found increased expression of Keap1 and GSK3β-pY216 with PDI inhibition (bacitracin-treated rats). These factors likely play a significant role in compromised nuclear accumulation of Nrf2 in PDI-inhibited (bacitracin-treated) rats.
Bacitracin is not a specific PDI inhibitor. However, it is the only known PDI inhibitor used in many important animal studies (3, 10, 22, 30). Interestingly, we found that PDI inhibition caused an increased expression of PDI in renal tissues of bacitracin-treated rats. This is most likely due to an existence of a feedback compensatory mechanism in bacitracin-treated rats. Bacitracin has a systemic long half-life (32) and is an irreversible inhibitor of PDI (11). It is possible that PDI activity remains inhibited despite its overexpression as seen in this study.
Perspective.
Taken together, the present results demonstrate a novel role of renal PDI, as an antioxidant, in regulation of blood pressure. The mechanism for this seems to be PDI-mediated regulation of Keap1-GSK3β-pY216-Nrf2 pathway, redox balance, renin-angiotensin system, and renal AT1 receptor function. As hypertension is linked to redox imbalance and impaired renal AT1 receptor function, renal PDI may be a potential future therapeutic target to restore redox balance, renal AT1 receptor function, and blood pressure in hypertensive patients.
GRANTS
Financial assistance from National Institute on Aging Grant AG-039836 to M. Asghar was helpful to carry out this study.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
X.W. and M.A. conceived and designed research; X.W. performed experiments; X.W. analyzed data; X.W. and M.A. interpreted results of experiments; X.W. prepared figures; X.W. drafted manuscript; X.W. and M.A. edited and revised manuscript; M.A. approved final version of manuscript.
REFERENCES
- 1.Akagi S, Yamamoto A, Yoshimori T, Masaki R, Ogawa R, Tashiro Y. Distribution of protein disulfide isomerase in rat hepatocytes. J Histochem Cytochem 36: 1533–1542, 1988. doi: 10.1177/36.12.3192937. [DOI] [PubMed] [Google Scholar]
- 2.Ali Khan H, Mutus B. Protein disulfide isomerase a multifunctional protein with multiple physiological roles. Front Chem 2: 70, 2014. doi: 10.3389/fchem.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Androwiki AC, Camargo LL, Sartoretto S, Couto GK, Ribeiro IM, Veríssimo-Filho S, Rossoni LV, Lopes LR. Protein disulfide isomerase expression increases in resistance arteries during hypertension development. Effects on Nox1 NADPH oxidase signaling. Front Chem 3: 24, 2015. doi: 10.3389/fchem.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banday AA, Lokhandwala MF. Oxidative stress-induced renal angiotensin AT1 receptor upregulation causes increased stimulation of sodium transporters and hypertension. Am J Physiol Renal Physiol 295: F698–F706, 2008. doi: 10.1152/ajprenal.90308.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep 12: 135–142, 2010. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 32: 3765–3781, 2013. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chugh G, Lokhandwala MF, Asghar M. Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats. Hypertension 59: 1029–1036, 2012. doi: 10.1161/HYPERTENSIONAHA.112.192302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contreras F, de la Parte MA, Cabrera J, Ospino N, Israili ZH, Velasco M. Role of angiotensin II AT1 receptor blockers in the treatment of arterial hypertension. Am J Ther 10: 401–408, 2003. doi: 10.1097/00045391-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, Prabhala A, Afzal A, Garg R. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 86: 355–362, 2001. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 10.Descamps E, Petrault-Laprais M, Maurois P, Pages N, Bac P, Bordet R, Vamecq J. Experimental stroke protection induced by 4-hydroxybenzyl alcohol is cancelled by bacitracin. Neurosci Res 64: 137–142, 2009. doi: 10.1016/j.neures.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Dickerhof N, Kleffmann T, Jack R, McCormick S. Bacitracin inhibits the reductive activity of protein disulfide isomerase by disulfide bond formation with free cysteines in the substrate-binding domain. FEBS J 278: 2034–2043, 2011. doi: 10.1111/j.1742-4658.2011.08119.x. [DOI] [PubMed] [Google Scholar]
- 12.Hahm E, Li J, Kim K, Huh S, Rogelj S, Cho J. Extracellular protein disulfide isomerase regulates ligand-binding activity of αMβ2 integrin and neutrophil recruitment during vascular inflammation. Blood 121: 3789–3800, 2013. doi: 10.1182/blood-2012-11-467985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison DG, Gongora MC. Oxidative stress and hypertension. Med Clin North Am 93: 621–635, 2009. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Javkhedkar AA, Quiroz Y, Rodriguez-Iturbe B, Vaziri ND, Lokhandwala MF, Banday AA. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 308: R840–R846, 2015. doi: 10.1152/ajpregu.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol 1: 45–49, 2013. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47: 89–116, 2007. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24: 7130–7139, 2004. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol 26: 221–229, 2006. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess 7: 1–94, 2003. doi: 10.3310/hta7310. [DOI] [PubMed] [Google Scholar]
- 20.Li LD, Tian M, Liao YH, Zhou ZH, Wei F, Zhu F, Wang M, Wang B, Wei YM. Effect of active immunization against angiotensin II type 1 (AT1) receptor on hypertension & arterial remodelling in spontaneously hypertensive rats (SHR). Indian J Med Res 139: 619–624, 2014. [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53: 401–426, 2013. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandel R, Ryser HJ, Ghani F, Wu M, Peak D. Inhibition of a reductive function of the plasma membrane by bacitracin and antibodies against protein disulfide-isomerase. Proc Natl Acad Sci USA 90: 4112–4116, 1993. doi: 10.1073/pnas.90.9.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 278: 21592–21600, 2003. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 24.Montezano AC, Touyz RM. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Antioxid Redox Signal 20: 164–182, 2014. doi: 10.1089/ars.2013.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: Part II: AT(1) receptor regulation. Circulation 105: 530–536, 2002. doi: 10.1161/hc0402.102619. [DOI] [PubMed] [Google Scholar]
- 26.Okada K, Fukui M, Zhu BT. Protein disulfide isomerase mediates glutathione depletion-induced cytotoxicity. Biochem Biophys Res Commun 477: 495–502, 2016. doi: 10.1016/j.bbrc.2016.06.066. [DOI] [PubMed] [Google Scholar]
- 27.Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens 29: 1253–1269, 2011. doi: 10.1097/HJH.0b013e3283469976. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz S, Pergola PE, Zager RA, Vaziri ND. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83: 1029–1041, 2013. doi: 10.1038/ki.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito H. Toxico-pharmacological perspective of the Nrf2-Keap1 defense system against oxidative stress in kidney diseases. Biochem Pharmacol 85: 865–872, 2013. doi: 10.1016/j.bcp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Täger M, Kröning H, Thiel U, Ansorge S. Membrane-bound proteindisulfide isomerase (PDI) is involved in regulation of surface expression of thiols and drug sensitivity of B-CLL cells. Exp Hematol 25: 601–607, 1997. [PubMed] [Google Scholar]
- 31.Tomobe K, Shinozuka T, Kuroiwa M, Nomura Y. Age-related changes of Nrf2 and phosphorylated GSK-3β in a mouse model of accelerated aging (SAMP8). Arch Gerontol Geriatr 54: e1–e7, 2012. doi: 10.1016/j.archger.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Van Leuven F, Marynen P, Cassiman JJ, Van den Berghe H. Receptor-mediated endocytosis of α2macroglobulin-protease complexes by fibroblasts in culture. Competitive inhibition by bacitracin. FEBS Lett 134: 83–87, 1981. doi: 10.1016/0014-5793(81)80556-X. [DOI] [PubMed] [Google Scholar]
- 33.Vaziri ND. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr Opin Nephrol Hypertens 13: 93–99, 2004. doi: 10.1097/00041552-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2: 582–593, 2006. doi: 10.1038/ncpneph0283. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta 1699: 35–44, 2004. doi: 10.1016/S1570-9639(04)00063-9. [DOI] [PubMed] [Google Scholar]
- 36.Xu S, Sankar S, Neamati N. Protein disulfide isomerase: a promising target for cancer therapy. Drug Discov Today 19: 222–240, 2014. doi: 10.1016/j.drudis.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 37.Yan D, Dong J, Sulik KK, Chen SY. Induction of the Nrf2-driven antioxidant response by tert-butylhydroquinone prevents ethanol-induced apoptosis in cranial neural crest cells. Biochem Pharmacol 80: 144–149, 2010. doi: 10.1016/j.bcp.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zelezná B, Velek J, Veselský L, Zicha J, Dobesová Z, Kunes J. Blockade of AT1 receptors by specific antibody attenuated hypertension development in young spontaneously hypertensive rats. Physiol Res 45: 475–477, 1996. [PubMed] [Google Scholar]
- 39.Železná B, Veselský L, Velek J, Zicha J, Kunes J. Angiotensin AT1 receptor blockade by specific antibody prevented two-kidney, one-clip renal hypertension in the rat. Eur J Pharmacol 260: 95–98, 1994. doi: 10.1016/0014-2999(94)90015-9. [DOI] [PubMed] [Google Scholar]