Abstract
We studied gender differences in Na+-Cl− cotransporter (NCC) activity and expression in wild-type (WT) and AT1a receptor knockout (KO) mice. In renal clearance experiments, urine volume (UV), glomerular filtration rate, absolute Na+ (ENa) and K+ (EK), and fractional Na+ (FENa) and K+ excretion were measured and compared at peak changes after bolus intravenous injection of hydrochlorothiazide (HCTZ; 30 mg/kg). In WT, females responded more strongly than males to HCTZ, with larger fractional increases of UV (7.8- vs. 3.4-fold), ENa (11.7- vs. 5.7-fold), FENa (7.9- vs. 4.9-fold), and EK (2.8- vs. 1.4-fold). In contrast, there were no gender differences in the responses to the diuretic in KO mice; HCTZ produced greater effects on male KO than on WT but similar effects on females. In WT, total (tNCC) and phosphorylated (pNCC) NCC protein expressions were 1.8- and 4.6-fold higher in females compared with males (P < 0.05), consistent with the larger response to HCTZ. In KO mice, tNCC and pNCC increased significantly in males to levels not different from those in females. There were no gender differences in the expression of the Na+/H+ exchanger (NHE3) in WT; NHE3 protein decreased to similar extents in male and female KO animals, suggesting AT1a-mediated NHE3 expression in proximal tubules. The resulting increase in delivery of NaCl to the distal nephron may underlie increased NCC expression and activity in mice lacking the AT1a receptor.
Keywords: angiotensin II, angiotensin type 1a receptor, urinary sodium and potassium excretion, sodium-chloride cotransporter, gender differences, wild-type, knockout
the thiazide-sensitive Na+-Cl− cotransporter NCC, encoded by the SLC12A3 gene (14), is expressed primarily in kidney (27), is located in the apical membrane of distal convoluted tubule (DCT), and mediates the major Na+ reabsorption in that segment (3). Alteration of NCC activity directly affects electrolyte homeostasis and blood pressure, as demonstrated in the genetic disorders Gitelman’s syndrome and Gordon’s syndrome (28). Lack of NCC activity in Gitelman’s syndrome is characterized by hypotension with hypomagnesemia, hypocalciuria, and hypokalemic metabolic alkalosis (41). Conversely Gordon’s syndrome, thought to involve activation of NCC, features hypertension with hyperkalemia and metabolic acidosis (46). NCC expression and activity are gender dependent. In rats and mice, females have higher NCC expression and phosphorylation and stronger diuretic and natriuretic responses to thiazides than males (6, 35, 44a). NCC content decreased with ovariectomy in female rats and was partially rescued by estradiol replacement (35, 44a), suggesting that female reproductive hormones control cotransporter expression. In humans, females had a higher content of NCC protein in urinary exosomes excretion than males (35). Clinical trials of antihypertension treatment show that women received diuretics more frequently than men for initiation of antihypertensive treatment (20) and had more efficient blood pressure reduction with a combination of an angiotensin-converting enzyme (ACE) inhibitor and diuretics than men (13).
Angiotensin II (ANG II) is a multifunctional hormone that plays an important role in regulation of blood pressure, vascular resistance, and salt and water balance (7). ANG II acutely stimulates Na+ reabsorption in the proximal tubule through activation of the Na+/H+ exchanger NHE3 (33). Chronic infusion of the hormone increases the expression of NHE3 protein (10), as well as that of NCC (52). There are two major categories of ANG II receptors, type 1 receptor (AT1R) and type 2 receptors (29, 30). In rodents, AT1R has two subtypes, AT1a and AT1B, with different expression patterns and regulation (2, 24, 26). AT1a receptor is detected throughout the kidney, including the vasculature of the renal cortex and the proximal tubules (26), and is thought to mediate the major renal effects of ANG II (2, 24). NHE3 expression was reduced in AT1a KO mouse kidney (11, 15) and in proximal tubule cells with knocking down the AT1a receptor by small-interfering RNA (21).
Gender differences in ANG II regulation of blood pressure have been reported. Male mice were more sensitive than females to ANG II-induced hypertension (48). In humans, diuretic therapy for hypertension was comparable to ACE inhibition in females, but less effective in males (46a). However, whether there is any gender difference of AT1a receptor-mediated NHE3 and NCC regulation is still unknown. In this study, we compare NCC activity and the expression of NCC and NHE3 protein in male and female WT and AT1a receptor knockout mice. Our goal was to elucidate the roles of ANG II and its receptor in the gender differences in the cotransporter.
MATERIALS AND METHODS
Animals.
All work with animals was conducted according to an Institutional Animal Care and Use Committee-approved protocol at the Yale School of Medicine. The breeding pairs of AT1a knockout mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and both WT and mice were bred and housed at the Yale University Animal Care facility in New Haven, CT. Animals were maintained on a normal diet and tap water until the day of the experiment. Experiments were performed on adult wild-type (WT) and AT1a knockout (KO) mice weighing 25–35 g. Ages (16–20 wk), genders, and genetic background (C57BL/6J) were matched among control and all experimental groups. Animals were anesthetized with an intraperitoneal injection (100–150 mg/kg body wt) of Inactin (thiobutabarbital sodium salt hydrate; Sigma, St. Louis, MO) before the experiment.
Renal clearance and measurements.
Renal clearance experiments were performed by using methods developed previously in our laboratory (49). Anesthetized mice were placed on a thermostatically controlled surgical table to maintain body temperature at 37°C. A tracheostomy was performed, and the left carotid artery was exposed and cannulated with PE-10 polyethylene tubing (Becton Dickinson, Franklin Lakes, NY) for arterial blood collections. The baseline mean blood pressure was measured from the cannulated carotid artery by a pressure transducer in anesthetized mice (32). The left jugular vein was catheterized with PE-10 tubing for intravenous infusion. The bladder was catheterized with PE-50 connected to PE-10 tubing for timed urine collections. After surgical preparation, 0.05 ml of isotonic saline was administered intravenously to replace surgical fluid loss. Subsequently, a priming dose of 0.1 mg inulin-fluorescein isothiocyanate (Sigma) in 0.05 ml isotonic saline was given, and a maintenance dose of isotonic solution containing 2 mg/ml inulin-fluorescein isothiocyanate was infused at a rate of 0.41 ml/h throughout the experiment. After a 30-min equilibration period, urine collections were made every 30 min. The first two collections (0–60 min) were used as the control period (baseline) and then hydrochlorothiazide (HCTZ; Sigma) was administered by bolus injection (30 mg/kg) followed by four additional 30-min urine collections. Blood samples (30 μl) were taken between two urine collections. Urine and plasma Na+ and K+ concentrations were measured by flame photometry (type 480 Flame Photometer; Corning Medical and Scientific, Corning, NY). Urine volume (UV), glomerular filtration rate (GFR), absolute Na+ (ENa) and K+ (EK), and fractional Na+ (FENa) and K+ (FEK) were calculated by standard methods (49).
Western blotting analysis of NHE3 and NCC protein level.
Kidney samples frozen in liquid nitrogen were homogenized and suspended in lysis buffer with protease inhibitor cocktail (Sigma-Aldrich) at pH 7.4. Equal amounts of total protein (50 μg) were separated on 10% polyacrylamide gels using SDS-PAGE and immunoblotted with antibodies against NHE3 (3H3, 1:1,000, gift from Dr. Daniel Biemesderfer, Yale University) (11), NCC (1:5,000, a gift from Dr. Alicia McDonough, University of Southern California) (50), or NCC-pT53 (1:1,000, gift from Dr. David Ellison, Oregon Health Sciences University) (25), with-no-lysine kinase (WNK4, 1:1,000; Novus Biologicals), and phospho-sterile20-related proline-alanine-rich kinase (SPAK, 1:1,000, pS373)/oxidative stress response kinase1 (OSR1, oS325, a gift from Dr. Wenhui Wang, New York Medical College) (8). Subsequently, the membrane was probed with secondary antibodies (anti-rabbit IgG conjugated with alkaline phosphatase). Bound antibody was visualized on autoradiography film (HyBlot CL; Denville Scientific) using a chemiluminescence substrate (Western Breeze; Invitrogen), followed by incubation with an ECL Western blot reagent. Films were scanned, and semiquantitative densitometry of protein bands was carried out using a program designed to resolve closely spaced bands (50), with β-actin used as a loading control. Bands were quantified by densitometry, and transporter protein expression was normalized to β-actin level. All comparisons were made relative to the control condition (12).
Real-time RT-PCR analysis of NCC and NHE3 mRNA level.
Kidneys were harvested, and total RNA was extracted using an RNeasy kit (Qiagen) according to the manufacturer’s instructions. Residual genomic DNA was removed by DNase I digestion, using a DNA-free kit (Ambion, Austin, TX). cDNA synthesis from total RNA was carried out using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA), and mRNA expression was quantified by real-time RT-PCR using Power SYBR Green PCR Master Mix (Applied Biosystems). The cycling conditions for all genes were as follows: 95°C for 10 min, followed by 40 cycles at 94°C for 15 s and 60°C for 60 s. The final mRNA abundance of each gene was normalized to the abundance of the housekeeping gene cyclophilin A. The following primers were used in this study: NCC, gggtttgtgtcatgaggatg (forward) and NCC, agatggtggtggcctgctc (reverse), NHE3, gctccccaagtacggacaatat (forward) and NHE3, acagcactgacattttccctcaa (reverse), cyclophilin A, ttgcagacaaagttccaaagaca (forward) and cyclophilin A, aagtcaccaccctggcacat (reverse).
Statistics.
All experimental data are presented as means ± SE. At least six animals were contained in each group of renal clearance experiments, and four kidneys from four mice were contained in Western blotting experiments. Student’s t-test was used to compare control and experimental groups. One-way ANOVA was used for comparison of multiple experimental groups with a control group following by Dunnett’s test. The difference between the mean values of an experimental group and a control group was considered significant if P < 0.05.
RESULTS
General phenotype and gender difference of AT1a KO compared with the WT mice.
The general phenotypes of male and female WT and AT1a KO mice, including body weight, blood pressure, and plasma electrolytes, are summarized in Table 1. In WT mice, body weight of females was slightly lower than males, but the difference was not statistically significant. In KO mice, both males and females were smaller than WT. These results are similar to those previously reported by Kaneko et al. (19). In addition, the body weight was significantly lower in female KO than male KO mice. Mean arterial blood pressure was lower in AT1a KO mice compared with the WT for both males and females. There were no gender differences in blood pressure for either genotype. There were no significant differences in plasma Na+ and K+ or hematocrit among groups. These values were all within normal ranges, indicating there was no overall electrolyte imbalance in AT1a KO mice. We found no significant differences in baseline GFR or absolute and fractional urinary Na+ and K+ excretions between WT and AT1a KO mice. These findings confirm a previous report from Navar’s group by using the same method (5) but differ from another study claiming elevated UV and Na+ excretion measured by 24-h urine collection from the metabolic cage (22).
Table 1.
Basal conditions of wild-type and AT1a knockout mice
| Genotype | Group | n | Body Wt, g | BP, mmHg | PNa, meq/l | PK, meq/l | HCT, % |
|---|---|---|---|---|---|---|---|
| Male | 6 | 33.8 ± 1.5 | 101.9 ± 3.4 | 143.0 ± 1.9 | 4.2 ± 0.3 | 45.9 ± 1.3 | |
| Female | 6 | 29.0 ± 2.4 | 103.6 ± 3.7 | 145.3 ± 3.5 | 4.0 ± 0.2 | 48.6 ± 0.7 | |
| Male | 6 | 29.1 ± 0.9# | 83.1 ± 1.1# | 142.0 ± 3.7 | 4.1 ± 0.3 | 43.7 ± 1.1 | |
| Female | 6 | 21.3 ± 0.5†# | 84.0 ± 4.7# | 140.1 ± 2.5 | 3.7 ± 0.2 | 44.5 ± 0.5 |
Data are presented as means ± SE; n, no. of animals. BP, mean blood pressure; PNa, plasma Na+, PK, plasma K+; HCT, hematocrit; AT1a, angiotensin type 1a.
P < 0.05, significant difference between males and females (†) and
WT and KO in the same gender (#).
HCTZ produced diuretic, natriuretic, and kaliuretic effects in male and female WT mice.
We compared the effects of HCTZ, a specific inhibitor of NCC (34), on UV, GFR, ENa, EK, FENa, and FEK in male and female WT and AT1a KO mice using renal clearance measurements (Fig. 1 and Table 2). After two 30-min baseline measurements, HCTZ (30 mg/kg) was given by intravenous bolus injection followed by four additional 30-min urine collections. Changes in UV, GFR, Na+, and K+ excretion were recorded and compared among groups. We first examined the time-dependent effects of HCTZ, obtaining the peak change of UV, Na+, and K+ excretion. We then calculated the fractional (fold) change from baseline for the maximal effects produced by HCTZ in each group.
Fig. 1.
Time-dependent effects of hydrochlorothiazide (HCTZ) on urine volume (UV, A), glomerular filtration rate (GFR, B), absolute Na+ excretion (ENa, C), and absolute K+ excretion (EK, D) in male (blue) and female (red) wild-type (WT) and knockout (KO) mice. HCTZ (30 mg/kg) was given by bolus iv injection as indicated by arrows. n = 6 Animals in each group. P < 0.05 between gender (*) and WT and KO (#).
Table 2.
Effects of HCTZ on UV, GFR, ENa, EK, FENa, and FEK
| Time, min | UV, µl/min | GFR, ml/min | ENa, µeq/min | EK, µeq/min | FENa, % | FEK, % | |
|---|---|---|---|---|---|---|---|
| Male | Baseline | 3.6 ± 0.3 | 0.67 ± 0.08 | 0.41 ± 0.1 | 0.38 ± 0.05 | 0.54 ± 0.2 | 23.5 ± 5.0 |
| 0–30 | 15.8 ± 1.8* | 0.72 ± 0.05 | 2.62 ± 0.5* | 0.90 ± 0.10* | 3.05 ± 0.8* | 45.0 ± 5.1* | |
| 30–60 | 9.7 ± 2.3* | 0.46 ± 0.08 | 1.88 ± 0.5* | 0.53 ± 0.09 | 2.88 ± 0.7* | 40.4 ± 4.4* | |
| 60–90 | 5.8 ± 1.3 | 0.35 ± 0.07* | 1.23 ± 0.3* | 0.35 ± 0.06 | 2.06 ± 0.5* | 37.1 ± 3.3 | |
| 90–120 | 4.2 ± 1.1 | 0.33 ± 0.01* | 0.97 ± 0.3 | 0.31 ± 0.04 | 1.95 ± 0.5* | 30.9 ± 3.3 | |
| Female | Baseline | 3.2 ± 0.2 | 0.83 ± 0.07 | 0.46 ± 0.1 | 0.40 ± 0.07 | 0.44 ± 0.1 | 16.6 ± 3.0 |
| 0–30 | 27.7 ± 3.9*† | 1.07 ± 0.10† | 5.90 ± 1.1*† | 1.31 ± 0.12*† | 3.91 ± 0.5* | 44.8 ± 6.9* | |
| 30–60 | 11.3 ± 2.0* | 0.65 ± 0.06 | 2.78 ± 0.7* | 0.70 ± 0.05* | 3.01 ± 0.6* | 39.3 ± 4.3* | |
| 60–90 | 8.2 ± 1.8* | 0.55 ± 0.06* | 1.45 ± 0.1* | 0.61 ± 0.08 | 2.18 ± 0.6* | 40.2 ± 6.5* | |
| 90–120 | 5.5 ± 1.6 | 0.46 ± 0.09* | 1.26 ± 0.5 | 0.52 ± 0.07 | 1.26 ± 0.5 | 41.2 ± 6.4* | |
| Male | Baseline | 3.4 ± 0.4 | 0.67 ± 0.11 | 0.45 ± 0.1 | 0.48 ± 0.10 | 0.55 ± 0.2 | 23.8 ± 4.8 |
| 0–30 | 26.9 ± 1.7*# | 0.64 ± 0.06 | 4.38 ± 0.6*# | 2.30 ± 0.23*# | 4.99 ± 1.0* | 106.3 ± 10.8*# | |
| 30–60 | 15.6 ± 4.1* | 0.45 ± 0.04 | 3.26 ± 1.0* | 0.98 ± 0.15*# | 2.38 ± 0.7* | 65.6 ± 14.8* | |
| 60–90 | 10.5 ± 2.6* | 0.44 ± 0.02 | 2.00 ± 0.7* | 0.80 ± 0.12# | 1.63 ± 0.8 | 39.6 ± 8.4 | |
| 90–120 | 7.3 ± 2.0 | 0.42 ± 0.02 | 1.43 ± 0.5 | 0.59 ± 0.14 | 1.15 ± 0.5 | 36.9 ± 6.3 | |
| Female | Baseline | 3.1 ± 0.4 | 0.69 ± 0.12 | 0.50 ± 0.1 | 0.44 ± 0.15 | 0.66 ± 0.2 | 21.5 ± 6.8 |
| 0–30 | 32.5 ± 4.5* | 0.78 ± 0.12 | 5.75 ± 1.7* | 1.46 ± 0.27* | 5.36 ± 1.5* | 65.1 ± 11.6*† | |
| 30–60 | 15.8 ± 2.7* | 0.61 ± 0.09 | 3.56 ± 0.8* | 1.00 ± 0.16* | 4.57 ± 1.3* | 52.3 ± 9.7 | |
| 60–90 | 12.4 ± 1.8* | 0.40 ± 0.03 | 2.20 ± 0.5* | 0.76 ± 0.15 | 3.73 ± 0.9* | 54.0 ± 9.8 | |
| 90–120 | 9.5 ± 1.1 | 0.44 ± 0.02 | 2.01 ± 0.6* | 0.65 ± 0.16 | 2.66 ± 0.6* | 50.9 ± 12.3 |
Data are presented as means ± SE; n = 6 in each group. HCTZ, hydrochlorothiazide; UV, urine volume; GFR, glomerular filtration rate; ENa, absolute excretion of Na+; EK, absolute excretion of K+; FENa, fractional excretion of Na+; FEK, fractional excretion of K+.
Significant difference from the same group of animals in the control periods (*),
between genders in the same genotype (†), and
between wild-type and knockout mice in the same gender (#).
As shown in Fig. 1, HCTZ (by iv bolus injection) produced robust diuretic, natriuretic, and kaliuretic effects both in male and female WT and KO mice. These effects were maximal at 30 min after administration and then declined, presumably because of reduced concentrations of the diuretic. In WT mice, females had twofold greater diuretic and natriuretic responses than males (Fig. 1C). The HCTZ also produced a stronger kaliuresis in females than in males (Fig. 1D). The larger responses in females also result in significantly greater fractional changes in UV, ENa, and FENa (Fig. 2A). The fractional change in EK was also higher in females than males (Fig. 2A), although FEK was not significantly different between genders (Fig. 2A). GFR increased during the first 30 min after injection, especially in females, and subsequently fell (Fig. 1B). Males had lower GFR levels than females, with statistically significant differences at 30 and 90 min.
Fig. 2.
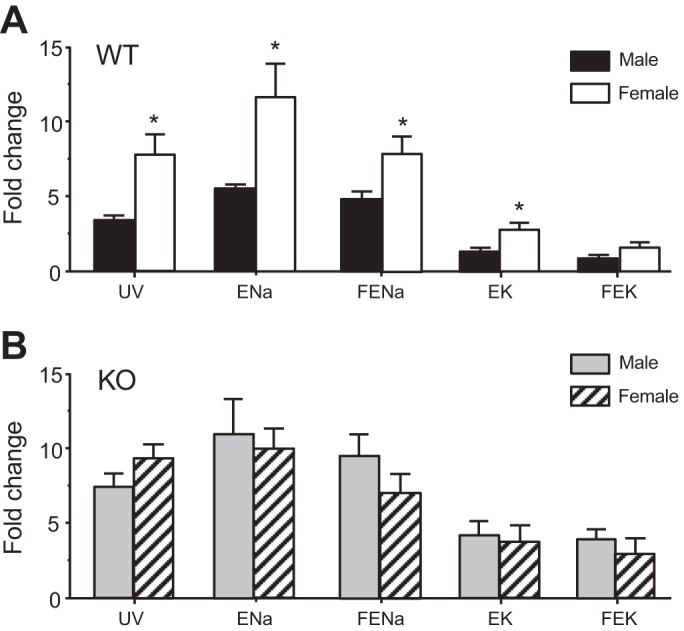
Comparison of fractional increases of UV, ENa, fractional excretion of Na+ (FENa), EK, and fractional excretion of K+ (FEK) at the peak effect of HCTZ (30-min iv injection) between WT and angiotensin type 1a (AT1a) KO male and female mice. Data are expressed as fold changes from baseline. A: male vs. female WT mice. B: male vs. female KO mice. *P < 0.05, significant difference between males and females.
Diuretic, natriuretic, and kaliuretic responses to HCTZ in male and female AT1a KO mice.
In contrast to the WT, in AT1a KO mice HCTZ affected males and females similarly. As shown in Fig. 1, HCTZ produced robust but similar diuretic, natriuretic, and kaliuretic responses in both genders. There were no significant differences in peak UV, ENa, or EK (Fig. 1, A, C, and D) nor were there gender differences in the fold increases of these parameters (Fig. 2B). Baseline GFR was similar in male and female KO mice. In contrast to WT, there was no significant change in GFR after HCTZ injection (Fig. 1B), consistent with the impaired transforming growth factor in mice (38).
Figure 3 compares fractional changes in UV, Na+, and K+ excretion in WT and KO animals. In males, HCTZ-dependent increases in UV, ENa, EK, FENa, and FEK are all significantly larger in KO compared with the WT (Fig. 3A). In contrast, in female mice, the responses to the diuretic were not significantly different in the two genotypes (Fig. 3B). These results indicate that the absence of gender difference of NCC activity in AT1a KO resulted from the elevation of NCC activity in males, with no change in females.
Fig. 3.
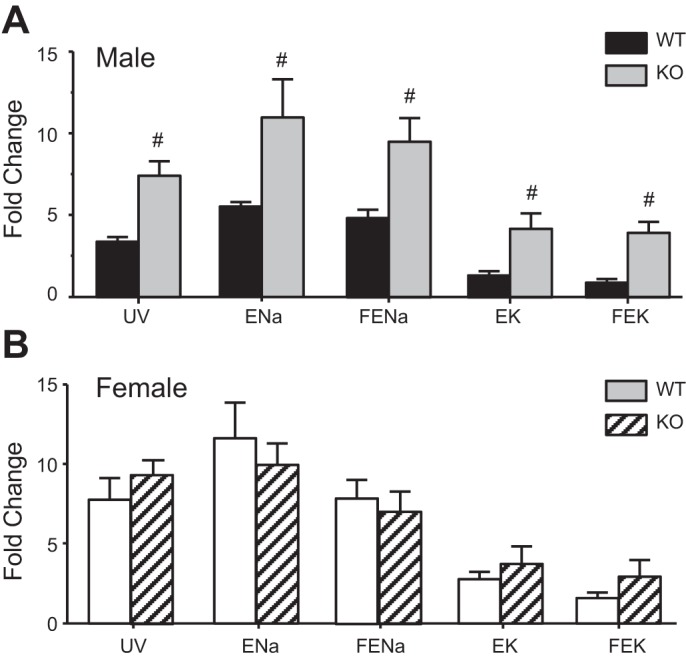
Comparison of fractional increase of UV, ENa, FENa, EK, and FEK at the peak effect of HCTZ between male and female WT and AT1a KO mice. A: WT vs. AT1a KO male mice. B: WT vs. AT1a KO female mice. #Significant difference between WT and AT1a KO mice (P < 0.05).
NCC expression in male and female WT and AT1a KO mice.
Because the relationship of NCC activity and expression is well correlated (27), we measured NCC mRNA and protein levels in male and female WT and KO mice. In WT, mRNA expression assessed by Q-PCR (Fig. 4D) was 1.6-fold higher in females compared with males (P < 0.05), consistent with the higher NCC activity and with a previous report (6, 35, 44a). In KO, NCC mRNA levels were similar to those in WT, and the increased expression in females relative to males was conserved. Total (tNCC) and phosphorylated (pNCC) protein expressions were measured by Western blotting. Figure 4A shows the result from three sets of animals in the same blot. Figure 4, B and C, summarizes densitometric measurements of NCC-to-β-actin ratios from four sets of mice. In WT mice, both tNCC and pNCC abundances were significantly higher in females than males, consistent with our functional measurements. Both tNCC and pNCC protein were significantly increased in male KO compared with the WT. Although the levels also increased in female KO vs. WT, these changes did not reach statistical significance. Furthermore, the protein expressions in male and female KO were not significantly different, again consistent with our functional measurements.
Fig. 4.
Na+-Cl− cotransporter (NCC) expression in WT and AT1a KO male and female mice kidneys. Total and phosphorylated NCC protein expression in WT and AT1a KO male and female mouse kidneys. A: representative Western blots for total (tNCC) and phosphorylated (pNCC) protein expression. tNCC and pNCC with 3 samples in each group loaded in the same blot. β-Actin expression served as a loading control. B and C: densitometric analysis of tNCC (B) and pNCC (C) expression, represented as the NCC-to-β-actin ratio (n = 4 in each group). D: NCC mRNA expression measured by Q-PCR using whole kidney preparations. P < 0.05, significant difference between male and female (*) and WT and AT1a KO (#).
NHE3 expression in male and female WT and AT1a KO mice.
It was previously reported that NHE3 expression was reduced in AT1a KO mouse kidney (11, 15) and in proximal tubule cells in which the AT1a receptor expression was knocked down by small-interfering RNA (21). We therefore examined whether there is any gender difference in AT1a receptor-mediated NHE3 expression at the mRNA and protein levels. Figure 5D shows that the NHE3 mRNA expression was similar in all four groups of mice, indicating that it does not depend on gender or on AT1R. In contrast, the abundance of NHE3 protein was significantly reduced in both male and female AT1a KO mice (Fig. 5, A and B), consistent with previous reports (11). As shown in Fig. 5C, the NHE3 abundance showed slightly more reduction in male than in female KO mouse kidneys. However, the difference did not reach statistical significance.
Fig. 5.
Na+/H+ exchanger (NHE3) expression in WT and AT1a KO male and female mice kidneys. A: representative blot for NHE3 protein with 3 samples for each group in the same blot. B: densitometric analysis of NHE3 protein expression represented as the NHE3/β-actin ratio (n = 4 in each group). C: reduction of NHE3 abundance in AT1a KO mice kidneys. D: NHE3 mRNA expression measured by Q-PCR using kidney cortex preparations. #P < 0.05, significant difference between WT and AT1a KO mice kidneys.
WNK4 and pSPAK/OSR1 expression in male and female WT and AT1a KO mice.
Because WNK4 and SPAK/OSR1 comprise a major pathway for regulation of NCC (16), we examined WNK4 and pSPAK/OSR1 expression in male and female WT and AT1a KO mice by Western blotting (Fig. 6). In WT, both WNK4 and pSPAK/OSR1 expressions are higher in males than females, opposite to the difference in NCC expression. In KO males, WNK4 expression was significantly reduced relative to WT, again opposite to the change of NCC. There was no significant difference in WNK4 expression in female KO vs. WT such that in the KO the gender difference was again abolished. Similar to WNK4, the pSPAK/OSR1 level is lower in female than male WT mice. However, the pSPAK/OSR1 expression was slightly reduced in male and significantly increased in female KO mice, resulting in a higher level of pSPAK/OSR1 expression in female than male KO mice. We did not measure the total SPAK expression, but a previous study found no difference of SPAK between male and female mice (51).
Fig. 6.
With-no-lysine kinase (WNK4) and phosphorylated (p)-sterile20-related proline-alanine-rich kinase (SPAK)/oxidative stress response kinase1 (OSR1) expression in male and female WT and AT1a KO mice kidneys. A: representative blot for WNK4 and pSPAK/OSR1 protein with 3 samples for each group. B: densitometric analysis of WNK4 and pSPAK/OSR1 protein expression represented as the WNK4/β-actin ratio and pSPAK/OSR1/β-actin ratio (n = 3 in each group). P < 0.05, significant difference between males and females (*) and WT and AT1a KO (#).
DISCUSSION
ANG II is critical to cell growth, salt and water balance, and blood pressure regulation (7, 17). In vitro and in vivo studies show that 1) in heterologous expression systems ANG II can acutely stimulate NCC activity through a WNK4/SPAK pathway (35) 2) acute infusion of ANG II in rats promoted NCC trafficking to apical membrane in DCT (37), and significantly increased SPAK-dependent NCC phosphorylation (31), and 3) chronic infusion of ANG II enhanced Na+ absorption in the distal tubule (52) and increased NCC protein expression independent of aldosterone (44). These studies strongly indicate that ANG II upregulates NCC and suggest that cotransporter activity would be reduced in the AT1a receptor KO mouse. Contrary to that expectation, we found that NCC activity and expression either increased (in males) or remained unchanged (in females).
One possible explanation is that NCC is upregulated through increased NaCl delivery to the distal nephron in the KO animals. Consistent with this idea, the expression of NHE3, a major Na+ transporter in the proximal tubule that normally reabsorbs a large fraction of filtered Na+, was diminished in the absence of AT1R. A greater reduction of NHE3 in males, although not statistically significant, could contribute to the more pronounced upregulation of NCC in males. One caveat to this interpretation is that NHE3 expression also decreased in female KO, but this did not result in upregulation of NCC. It is possible that NCC expression and activity is already maximal in female WT mice and unable to respond to increased NaCl delivery.
WNK4 is a positive regulator of NCC in the kidney (4, 43). WNK4 acts at least in part through the phosphorylation of SPAK and OSR1, kinases thought to directly phosphorylate the cotransporter (16). WNK4 KO mice exhibited almost complete absence of tNCC and pNCC, with little (43) or no (4) effect on total SPAK. Our data show that gender- and AT1a-dependent changes in WNK4 expressions are opposite to those of NCC, with higher NCC but lower WNK4 in female WT mice and increased NCC but reduced WNK4 in male AT1a KO mice. These results suggest that WNK4 does not drive the observed differences in NCC expression. Instead, changes in WNK4 and SPAK may reflect partial compensation for altered NCC levels.
The larger effect of AT1a receptor deletion on NCC in male mice could also be related to gender differences in angiotensin receptor expression. Using immunofluorescence, Armando et al. (1) and Rogers et al. (36) found that AT1R binding in glomeruli was significantly higher in male than female rats. In addition, in a hypertensive rat model, AT1a receptor protein expression was higher in male than female renal cortex (42). Estrogen can decrease AT1 receptor expression in the kidneys of ovariectomized rats (9, 36) and downregulate its protein translation in adrenal gland (47). In addition to AT1R, AT2R is also widely expressed in the kidney, and activation of AT2 receptor produces an effect opposite to that of AT1a in the regulation of NHE3 in the proximal tubule, as we reported previously (11). A higher ratio of AT2R/AT1R in females relative to males has also been reported (40). Further study will be needed to clarify what role these differences may have in the gender dependence of the NCC responses to AT1a receptor knockout.
Our results confirm gender differences in NCC expression and activity reported previously. Chen et al. showed a greater natriuresis in response to thiazide administration in female vs. male rats (6). This correlated with a larger density of thiazide-binding sites in females. Subsequently Rojas-Vega et al. reported that ovariectomy decreased expression of tNCC and pNCC in mice and pNCC (but not tNCC) in rats (35). The effects appear to be due at least in part to differences in circulating female hormones. Ovariectomy reduced, and estradiol replacement increased, apical membrane NCC expression and NCC phosphorylation in rat kidney (35, 44a). Progesterone and prolactin also upregulated pNCC (35). Increased tNCC and pNCC in females was also observed in human kidneys (35). This could underlie the increased use of diuretics in women for initiation of antihypertension treatment (20) and the increased efficacy of diuretics vs. ACE inhibition in females vs. males (13, 39).
Our results suggest that increased mRNA coding for NCC underlies, at least in part, the greater expression and activity of the cotransporter in WT female mice. The fractional increase in mRNA abundance (60%) was similar to that of tNCC protein (70%). However, changes at the mRNA level do not appear to account for all of the differences observed. In WT, pNCC protein increased substantially more than tNCC (6.6- vs. 1.7-fold). Furthermore, the KO animals showed a similar gender dependence of mRNA expression, but there was no difference in tNCC protein content. Wang et al. also reported no significant change in NCC protein expression in female AT1a KO mice compared with WT (45). Levels in males were not compared in this study. Thus posttranscriptional events are also important in determining activity. Overall, the expression of both tNCC and pNCC protein correlates with function. These levels were higher in WT females than males, higher in KO males than WT males, and similar in KO females and males (Fig. 4). Further work will be required to determine whether these AT1R-dependent differences in NCC processing depend on ANG II signaling in the DCT cells themselves or are secondary to effects on the proximal tubule.
GRANTS
This investigation was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants RO1-DK-099284 (L. Palmer and T. Wang) and P01-DK-17433, Core E (T. Wang), and the George M. O'Brien Kidney Center at Yale.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L., R.H., S.X., L.W., L.Y., and T.W. performed experiments; J.L., R.H., S.X., L.W., L.Y., and T.W. analyzed data; J.L., R.H., and T.W. interpreted results of experiments; J.L., S.X., and T.W. prepared figures; J.L. and T.W. drafted manuscript; J.L., R.H., S.X., A.M.W., L.G.P., and T.W. edited and revised manuscript; J.L., R.H., S.X., L.W., L.Y., A.M.W., L.G.P., and T.W. approved final version of manuscript; A.M.W., L.G.P., and T.W. conceived and designed research.
ACKNOWLEDGMENTS
We thank Dr. Thomas Coffman for providing the original AT1a breeding mice and Drs. David Ellison, Alicia McDonough, Daniel Biemesderfer, and Wen-Hui Wang for providing antibodies.
REFERENCES
- 1.Armando I, Jezova M, Juorio AV, Terrón JA, Falcón-Neri A, Semino-Mora C, Imboden H, Saavedra JM. Estrogen upregulates renal angiotensin II AT2 receptors. Am J Physiol Renal Physiol 283: F934–F943, 2002. doi: 10.1152/ajprenal.00145.2002. [DOI] [PubMed] [Google Scholar]
- 2.Burson JM, Aguilera G, Gross KW, Sigmund CD. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol Endocrinol Metab 267: E260–E267, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Câmpean V, Kricke J, Ellison D, Luft FC, Bachmann S. Localization of thiazide-sensitive Na+-Cl− cotransport and associated gene products in mouse DCT. Am J Physiol Renal Physiol 281: F1028–F1035, 2001. doi: 10.1152/ajprenal.0148.2001. [DOI] [PubMed] [Google Scholar]
- 4.Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA 109: 7929–7934, 2012. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervenka L, Mitchell KD, Oliverio MI, Coffman TM, Navar LG. Renal function in the AT1A receptor knockout mouse during normal and volume-expanded conditions. Kidney Int 56: 1855–1862, 1999. doi: 10.1046/j.1523-1755.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Vaughn DA, Fanestil DD. Influence of gender on renal thiazide diuretic receptor density and response. J Am Soc Nephrol 5: 1112–1119, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Crowley SD, Coffman TM. Recent advances involving the renin-angiotensin system. Exp Cell Res 318: 1049–1056, 2012. doi: 10.1016/j.yexcr.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, Yang CL, Ellison DH, Wang WH. Potassium sensing by renal distal tubules requires Kir4.1. J Am Soc Nephrol 28: 1814–1825, 2017. doi: 10.1681/ASN.2016090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean SA, Tan J, O’Brien ER, Leenen FH. 17β-Estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am J Physiol Regul Integr Comp Physiol 288: R759–R766, 2005. doi: 10.1152/ajpregu.00595.2004. [DOI] [PubMed] [Google Scholar]
- 10.Dixit MP, Xu L, Xu H, Bai L, Collins JF, Ghishan FK. Effect of angiotensin-II on renal Na+/H+ exchanger-NHE3 and NHE2. Biochim Biophys Acta 1664: 38–44, 2004. doi: 10.1016/j.bbamem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Du Z, Wan L, Yan Q, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance. II: Impact of angiotensin II on flow-dependent transport. Am J Physiol Renal Physiol 303: F1507–F1516, 2012. doi: 10.1152/ajprenal.00277.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan Y, Weinstein AM, Weinbaum S, Wang T. Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc Natl Acad Sci USA 107: 21860–21865, 2010. doi: 10.1073/pnas.1015751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everett BM, Glynn RJ, Danielson E, Ridker PM; Val-MARC Investigators . Combination therapy versus monotherapy as initial treatment for stage 2 hypertension: a prespecified subgroup analysis of a community-based, randomized, open-label trial. Clin Ther 30: 661–672, 2008. doi: 10.1016/j.clinthera.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Gamba G. The thiazide-sensitive Na+-Cl− cotransporter: molecular biology, functional properties, and regulation by WNKs. Am J Physiol Renal Physiol 297: F838–F848, 2009. doi: 10.1152/ajprenal.00159.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadchouel J, Ellison DH, Gamba G. Regulation of renal electrolyte transport by WNK and SPAK-OSR1 kinases. Annu Rev Physiol 78: 367–389, 2016. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- 17.Huckle WR, Earp HS. Regulation of cell proliferation and growth by angiotensin II. Prog Growth Factor Res 5: 177–194, 1994. doi: 10.1016/0955-2235(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko K, Ito M, Fumoto T, Fukuhara R, Ishida J, Fukamizu A, Ikeda K. Physiological function of the angiotensin AT1a receptor in bone remodeling. J Bone Miner Res 26: 2959–2966, 2011. doi: 10.1002/jbmr.501. [DOI] [PubMed] [Google Scholar]
- 20.Keyhani S, Scobie JV, Hebert PL, McLaughlin MA. Gender disparities in blood pressure control and cardiovascular care in a national sample of ambulatory care visits. Hypertension 51: 1149–1155, 2008. doi: 10.1161/HYPERTENSIONAHA.107.107342. [DOI] [PubMed] [Google Scholar]
- 21.Li XC, Hopfer U, Zhuo JL. AT1 receptor-mediated uptake of angiotensin II and NHE-3 expression in proximal tubule cells through a microtubule-dependent endocytic pathway. Am J Physiol Renal Physiol 297: F1342–F1352, 2009. doi: 10.1152/ajprenal.90734.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li XC, Shao Y, Zhuo JL. AT1a receptor signaling is required for basal and water deprivation-induced urine concentration in AT1a receptor-deficient mice. Am J Physiol Renal Physiol 303: F746–F756, 2012. doi: 10.1152/ajprenal.00644.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension 24: 538–548, 1994. doi: 10.1161/01.HYP.24.5.538. [DOI] [PubMed] [Google Scholar]
- 25.McCormick JA, Nelson JH, Yang CL, Curry JN, Ellison DH. Overexpression of the sodium chloride cotransporter is not sufficient to cause familial hyperkalemic hypertension. Hypertension 58: 888–894, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyata N, Park F, Li XF, Cowley AW Jr. Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol Renal Physiol 277: F437–F446, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Moes AD, van der Lubbe N, Zietse R, Loffing J, Hoorn EJ. The sodium chloride cotransporter SLC12A3: new roles in sodium, potassium, and blood pressure regulation. Pflügers Arch 466: 107–118, 2014. doi: 10.1007/s00424-013-1407-9. [DOI] [PubMed] [Google Scholar]
- 28.Morla L, Edwards A, Crambert G. New insights into sodium transport regulation in the distal nephron: role of G-protein coupled receptors. World J Biol Chem 7: 44–63, 2016. doi: 10.4331/wjbc.v7.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 351: 233–236, 1991. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima M, Mukoyama M, Pratt RE, Horiuchi M, Dzau VJ. Cloning of cDNA and analysis of the gene for mouse angiotensin II type 2 receptor. Biochem Biophys Res Commun 197: 393–399, 1993. doi: 10.1006/bbrc.1993.2492. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen MT, Han J, Ralph DL, Veiras LC, McDonough AA. Short-term nonpressor angiotensin II infusion stimulates sodium transporters in proximal tubule and distal nephron. Physiol Rep 3: 3, 2015. doi: 10.14814/phy2.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riquier-Brison AD, Leong PK, Pihakaski-Maunsbach K, McDonough AA. Angiotensin II stimulates trafficking of NHE3, NaPi2, and associated proteins into the proximal tubule microvilli. Am J Physiol Renal Physiol 298: F177–F186, 2010. doi: 10.1152/ajprenal.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojas-Vega L, Gamba G. Mini-review: regulation of the renal NaCl cotransporter by hormones. Am J Physiol Renal Physiol 310: F10–F14, 2016. doi: 10.1152/ajprenal.00354.2015. [DOI] [PubMed] [Google Scholar]
- 35.Rojas-Vega L, Reyes-Castro LA, Ramírez V, Bautista-Pérez R, Rafael C, Castañeda-Bueno M, Meade P, de Los Heros P, Arroyo-Garza I, Bernard V, Binart N, Bobadilla NA, Hadchouel J, Zambrano E, Gamba G. Ovarian hormones and prolactin increase renal NaCl cotransporter phosphorylation. Am J Physiol Renal Physiol 308: F799–F808, 2015. doi: 10.1152/ajprenal.00447.2014. [DOI] [PubMed] [Google Scholar]
- 36.Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol 292: R794–R799, 2007. doi: 10.1152/ajpregu.00424.2006. [DOI] [PubMed] [Google Scholar]
- 37.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl− cotransporter to apical membrane. Am J Physiol Renal Physiol 293: F662–F669, 2007. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 38.Schnermann JB, Traynor T, Yang T, Huang YG, Oliverio MI, Coffman T, Briggs JP. Absence of tubuloglomerular feedback responses in AT1a receptor-deficient mice. Am J Physiol Renal Physiol 273: F315–F320, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Schoofs MW, van der Klift M, Hofman A, de Laet CE, Herings RM, Stijnen T, Pols HA, Stricker BH. Thiazide diuretics and the risk for hip fracture. Ann Intern Med 139: 476–482, 2003. doi: 10.7326/0003-4819-139-6-200309160-00010. [DOI] [PubMed] [Google Scholar]
- 40.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res 62: 587–593, 2004. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 42.Sullivan JC, Semprun-Prieto L, Boesen EI, Pollock DM, Pollock JS. Sex and sex hormones influence the development of albuminuria and renal macrophage infiltration in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 293: R1573–R1579, 2007. doi: 10.1152/ajpregu.00429.2007. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi D, Mori T, Nomura N, Khan MZ, Araki Y, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S. WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep 34: 34, 2014. doi: 10.1042/BSR20140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int 79: 66–76, 2011. doi: 10.1038/ki.2010.290. [DOI] [PubMed] [Google Scholar]
- 44a.Verlander JW, Tran TM, Zhang L, Kaplan MR, Hebert SC. Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J Clin Invest 101: 1661–1669, 1998. doi: 10.1172/JCI601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Armando I, Mangrum A, Knepper M, Jose P. P-363: Protein abundance of renal Na transporters in femal mice lacking type1A angiotensin II receptor. Am J Hypertens 18: A137, 2005. doi: 10.1016/j.amjhyper.2005.03.381. [DOI] [Google Scholar]
- 46.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 46a.Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Marley JE, Morgan TO, West MJ; Second Australian National Blood Pressure Study Group . A comparison of outcomes with angiotensin-converting--enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med 348: 583–592, 2003. doi: 10.1056/NEJMoa021716. [DOI] [PubMed] [Google Scholar]
- 47.Wu Z, Maric C, Roesch DM, Zheng W, Verbalis JG, Sandberg K. Estrogen regulates adrenal angiotensin AT1 receptors by modulating AT1 receptor translation. Endocrinology 144: 3251–3261, 2003. doi: 10.1210/en.2003-0015. [DOI] [PubMed] [Google Scholar]
- 48.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288: H2177–H2184, 2005. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 49.Yan Q, Yang X, Cantone A, Giebisch G, Hebert S, Wang T. Female ROMK null mice manifest more severe Bartter II phenotype on renal function and higher PGE2 production. Am J Physiol Regul Integr Comp Physiol 295: R997–R1004, 2008. doi: 10.1152/ajpregu.00051.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang L, Frindt G, Lang F, Kuhl D, Vallon V, Palmer LG. SGK1-dependent ENaC processing and trafficking in mice with high dietary K intake and elevated aldosterone. Am J Physiol Renal Physiol 312: F65–F76, 2017. doi: 10.1152/ajprenal.00257.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Siew K, Macartney T, O’Shaughnessy KM, Alessi DR. Critical role of the SPAK protein kinase CCT domain in controlling blood pressure. Hum Mol Genet 24: 4545–4558, 2015. doi: 10.1093/hmg/ddv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension 54: 120–126, 2009. doi: 10.1161/HYPERTENSIONAHA.109.133785. [DOI] [PMC free article] [PubMed] [Google Scholar]






