Abstract
The pathways implicated in diabetic kidney disease (DKD) are largely derived from animal models. To examine if alterations in renin-angiotensin system (RAS) in humans are concordant with those in rodent models, we measured concentration of angiotensinogen (AOG), cathepsin D (CTSD), angiotensin-converting enzyme (ACE), and ACE2 and enzymatic activities of ACE, ACE2, and aminopeptidase-A in FVB mice 13–20 wk after treatment with streptozotocin (n = 9) or vehicle (n = 15) and people with long-standing type 1 diabetes, with (n = 37) or without (n = 81) DKD. In streptozotocin-treated mice, urine AOG and CTSD were 10.4- and 3.0-fold higher than in controls, respectively (P < 0.001). Enzymatic activities of ACE, ACE2, and APA were 6.2-, 3.2-, and 18.8-fold higher, respectively, in diabetic animals (P < 0.001). Angiotensin II was 2.4-fold higher in diabetic animals (P = 0.017). Compared with people without DKD, those with DKD had higher urine AOG (170 vs. 15 μg/g) and CTSD (147 vs. 31 μg/g). In people with DKD, urine ACE concentration was 1.8-fold higher (1.4 vs. 0.8 μg/g in those without DKD), while its enzymatic activity was 0.6-fold lower (1.0 vs. 1.6 × 109 RFU/g in those without DKD). Lower ACE activity, but not ACE protein concentration, was associated with ACE inhibitor (ACEI) treatment. After adjustment for clinical covariates, AOG, CTSD, ACE concentration, and ACE activity remained associated with DKD. In conclusion, in mice with streptozotocin-induced diabetes and in humans with DKD, urine concentrations and enzymatic activities of several RAS components are concordantly increased, consistent with enhanced RAS activity and greater angiotensin II formation. ACEI use was associated with a specific reduction in urine ACE activity, not ACE protein concentration, suggesting that it may be a marker of exposure to this widely-used therapy.
Keywords: diabetic kidney disease, renin-angiotensin system, angiotensinogen, cathepsin D, angiotensin-converting enzyme, angiotensin-converting enzyme 2, aminopeptidase-A
diabetic kidney disease (DKD) is the leading cause of chronic and end-stage kidney disease in the United States and worldwide (2). Several biological pathways are implicated in the pathogenesis of DKD. This information is largely obtained from animal models of DKD. However, animal models only partially recapitulate the pathology and pathophysiology of human DKD (5, 50). As such, the timing and extent of activity of these pathways in the course of human DKD are not known. Given the dearth of research biopsies in human DKD, information on pathway activity in human kidney biopsies obtained throughout the course of DKD is not currently available.
Urine is a readily available, noninvasive biofluid, which is extensively used in clinical medicine and now increasingly utilized for biomarker discovery (16). Although formed initially as a filtrate of plasma, urine protein composition is subsequently influenced by secretory and reabsorptive activities of the cells lining the length of the nephron. Urine albumin and total urine protein quantity is a powerful clinical risk stratifier not only for progression of kidney disease but also heart disease and mortality (36). More recent evidence suggests that urine protein composition may be able to inform on the underlying intrarenal pathogenic mechanisms (13, 18, 21, 57, 59). This potential would open up new diagnostic avenues for the use of urine protein composition as a personalized assay for tailoring treatment of kidney disease to the pathways active in each individual at a given time.
We sought to comprehensively examine urine concentrations, and when possible enzymatic activities, of key renin-angiotensin system (RAS) components in an animal model of DKD, the FVB mice treated with streptozotocin, as well as people with type 1 diabetes and DKD, compared with those with long-standing type 1 diabetes and no DKD. The examined RAS components included angiotensinogen, angiotensin-converting enzyme (ACE), and ACE2, as well as cathepsin D (CTSD). The last is a lysosomal protein capable of cleaving angiotensinogen to angiotensin I, enabling ACE-independent angiotensin II generation (24). In addition, we examined enzymatic activity of aminopeptidase A (APA), which regulates angiotensin II concentration by catalyzing its degradation to angiotensin III (56). The RAS was selected as an exemplary pathway because it is presumed to be overactive in DKD kidneys and its pharmacological suppression is part of current standards of care. Our findings show concordance between the urine concentration and enzymatic activity of RAS pathway proteins and the known intrarenal pathway activity and between rodent DKD models and human DKD. Furthermore, in humans with DKD, urine ACE activity shows a negative correlation with use of ACE inhibitors that may reflect suppression of ACE activity at the kidney level by these agents.
MATERIALS AND METHODS
Study cohort.
People with type 1 diabetes, who were seen in the Diabetes Care Center (University of Washington) for outpatient endocrinology care, were approached for participation in the Kidney Research Institute Diabetic Kidney Disease Repository (University of Washington) and were enrolled into the repository after providing informed consent. Demographic and clinical information was obtained from the electronic medical records and a questionnaire filled by the participants. DKD was defined as either a urine ACR ≥300 mg/g or an estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2 and ACR ≥30 mg/g. People with longstanding diabetes but no evidence of overt DKD were those with ≥30 yr of type 1 diabetes, eGFR >90 ml/min per 1.73 m2, and ACR <300 mg/g. Demographic and clinical data (age, race, sex, diabetes duration and RAS inhibitor use) were obtained from the electronic medical records and confirmed by patient questionnaires (race, diabetes duration and RAS inhibitor use). Diabetes type was extracted from the clinical notes, as ascertained by the endocrinologists caring for the patients. Hemoglobin A1c and serum creatinine were obtained from the electronic medical records at a time closest to the date of urine sample collection. The glomerular filtration rate was calculated from serum creatinine using a CKD-EPI formula (26).
A random clean-catch, midstream urine sample was collected and stored at 4°C after collection until processed. Urine samples were centrifuged at 4,700 g for 15 min at 4°C, and the supernatant was collected, aliquoted, and stored at −80°C. The mean (SD) time from sample collection to storage at −80°C was 5.7 (2.0) hours. The use of human samples and data was approved by the Institutional Review Board of the University of Washington.
Experimental animals.
Female FVB mice were acquired from Jackson Laboratories (Bar Harbor, ME). Animals were housed at the Center for Comparative Medicine at Northwestern University Feinberg School of Medicine. Animal care and procedures were approved by the Institutional Animal Care and Use Committee at Northwestern University and in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the institutional, state, and federal guidelines. Streptozotocin (150 mg/kg; Sigma Chemical, St. Louis, MO) dissolved in sodium citrate buffer pH 4.5 (streptozotocin-treated mice) or sodium citrate buffer pH 4.5 alone (nondiabetic vehicle controls) was injected in two intraperitoneal injections to female FVB mice at 19 and 20 wk of age. Spot urines were collected 13 and 20 wk after the last streptozotocin or vehicle injection (at 32 and 40 wk of age, respectively) and stored at −80°C.
Laboratory measurements in mouse urine samples.
Albumin was measured with a quantitative solid-phase sandwich enzyme-linked immunosorbent assay (ELISA; Albuwell M, Exocell, Philadelphia, PA). Creatinine was measured using the Jaffe method (Creatinine Companion, Exocell). AOG was measured using a quantitative solid-phase sandwich ELISA (IBL-America, Minneapolis, MN). CTSD was measured using a sandwich ELISA (OriGene Technologies, Rockville, MD). ACE activity was measured in a high-throughput fluorometric assay that uses the substrate hippuryl-l-histidyl-l-leucine, as previously described (48). The concentration of affinity purified recombinant testis ACE showed a direct linear relationship to the production of l-histidyl-l-leucine within the range of 0–4 × 10−4 nmol ACE (48). ACE2 activity was determined following incubation with the intramolecularly quenched synthetic ACE2-specific substrate Mca-APK-Dnp (Anaspec, Fremont, CA), using a method that was validated by spiking recombinant ACE2 protein into ACE2 knockout urines, as described previously (63). Briefly, urine was added to wells containing a buffer (50 mmol/l 4-morpholineethanesulfonic acid, 300 mmol/l NaCl, 10 μmol/l ZnCl2, and 0.01% Triton X-100, pH 6.5), EDTA-free tablets (Roche, Applied Science, Mannheim, Germany), and 10 μmol/l substrate. Reactions were in duplicate, with one of two wells constituting a negative control. Negative control wells contained the same components, in addition to 10 μmol/l of a specific ACE2 inhibitor, MLN-4760 (Millenium Pharmaceuticals, Cambridge, MA). ACE2 activity was calculated by subtracting negative control values from fluorescence obtained in wells without the specific ACE2 inhibitor (63).
APA activity was measured as previously described (12) and the fluorescence corrected with addition of APA inhibitor amastatin (Sigma-Aldrich), at 10−5 M concentration to negative control wells. For angiotensin II studies, an aliquot of 100 μl of freshly collected urine was transferred into tubes kept on ice at 4°C containing 10× concentrated cocktail of peptidase inhibitors: 25 mM EDTA, 0.44 mM o-phenanthroline, 1 mM chloromercuribenzoic acid (PCMB), and 120 μM pepstatin A in PBS mixed thoroughly. The urines with inhibitors were then stored at −80°C until the extraction. Angiotensin peptides were extracted from urines using reverse-phase phenyl silica columns (Thermo Scientific cat. no. 60108-386, 100 mg) according to the manufacturer’s instructions. Angiotensin II levels were measured using an EIA kit from Cayman Chemical (Ann Arbor, MI). This assay had less than 0.001% cross-reactivity with Ang (1–7) and 4% cross-reactivity with Ang I(1–10). Cross-reactivity with AngIII (2–8) and Ang (3–8) were 33 and 36%, respectively, which compares favorably with the corresponding competitive immunoassay (58). Within-assay coefficients of variation (CV%) were 6.9, 2, 5.6, and 10.2 for 100, 20, 5, and 2 pg/ml Ang II, respectively; between-assay CVs were 6.9, 4.8, 10, and 14.7 for 100, 20, 5, and 2 pg/ml Ang II, respectively (58).
Laboratory measurements in human urine samples.
Albumin and creatinine were measured in urine samples using an immunoturbidometric assay and the modified rate Jaffe reaction, respectively, using a DXC 600 clinical chemistry analyzer (Beckman-Coulter, Indianapolis, IN). Angiotensinogen was measured using a quantitative solid-phase sandwich ELISA distributed by IBL-America with a minimum detection limit of 30 pg/ml and <0.1% cross-reactivity with human angiotensin I, II, III, or IV, angiotensin (1–9), or angiotensin (1–7). CTSD was measured using a quantitative solid-phase sandwich ELISA distributed by Abcam (Cambridge, MA) with a minimum detection limit of 10 pg/ml and no detectable cross-reactivity with other capthepsins. ACE concentration was measured using a quantitative solid-phase sandwich ELISA distributed by R&D Systems with a minimum detection limit of 50 pg/ml and <0.5% cross-reactivity with human ACE2, neprilysin, or ECE-2. ACE2 concentration was measured using a quantitative solid-phase sandwich ELISA distributed by IBL-America with a minimum detection limit of 10 pg/ml and reported by the manufacturer to have no detectable cross-reactivity with other relevant proteins. ACE, ACE2, and APA activities were measured using the same assays as for mouse urine samples (details in previous section).
Statistical analysis.
Distribution of the protein concentrations and enzymatic activities were described using median and interquartile range (IQR). Concentration and enzymatic activity were log2 transformed, and logistic regression was used to assess the association between concentration or enzymatic activity of individual RAS protein components with the DKD status as outcome, after adjusting for age (continuous), sex, race (binary), and diabetes duration (continuous) as covariates. The analysis was performed for urine proteins with and without normalization to urine creatinine. To assess the difference in ACE, ACE2, and CTSD concentration and ACE and ACE2 activity in people with DKD who were or were not on ACE inhibitors, linear regression was used, with the concentration or enzymatic activity of each protein (e.g., ACE or ACE2) as the outcome and ACE inhibitor use as the exposure, after adjustment for age (continuous), sex, race (binary), and diabetes duration (continuous) as covariates. To examine relative associations between RAS proteins and DKD status, logistic regression was again used with the DKD status as outcome, and the exposure consisting of all RAS protein concentrations or activities that were significantly associated with the outcome in individual analyses as above (AOG, CTSD, ACE activity, ACE2 activity, and APA activity). This model was also adjusted for age, sex, race, and diabetes duration. The nominal significance threshold was set as a two-sided P value of <0.05. All analyses were performed with SAS 9.4 (Carey, NC).
RESULTS
Urine RAS proteins in experimental DKD.
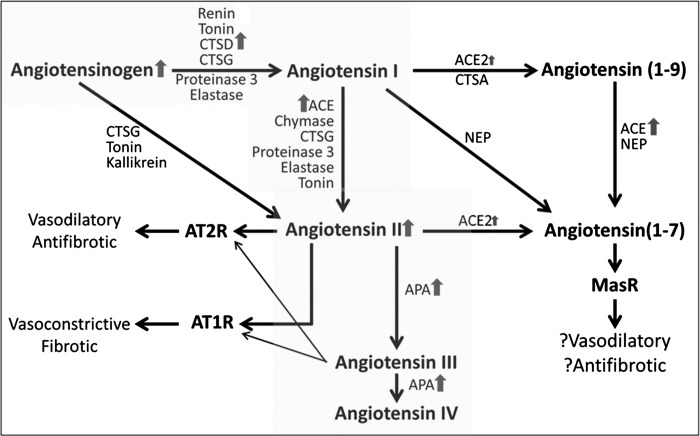
Treatment with streptozotocin elicited an increase in glucose and caused mild albuminuria (Table 1). These animals also developed some of the pathological features of DKD, including glomerular hypertrophy and mesangial matrix expansion, as previously reported (64). Median urine angiotensin (AOG) concentration normalized for creatinine concentration was significantly higher in mice treated with streptozotocin vs. those treated with vehicle (27.1 vs. 2.6 μg/g Cr, P < 0.001). CTSD concentration, normalized to creatinine, was also significantly higher in streptozotocin-treated mice than in controls (96.5 vs 32.0 μg/g Cr, P < 0.001; Table 1 and Fig. 1).
Table 1.
RAS pathway components in urine of streptozotocin-treated mice
|
n |
Vehicle | STZ | P Value, Mann-Whitney | ||
|---|---|---|---|---|---|
| Vehicle | STZ | ||||
| Albumin/Cr, mg/g | 9 | 15 | 18.3 (10.8, 39.4) | 71.6 (36.0, 104.1) | 0.015 |
| Angiotensinogen/Cr, μg/g | 9 | 15 | 2.6 (1.8, 5.5) | 27.1 (17.5, 46.4) | 0.0001 |
| Cathepsin D, μg/g | 9 | 15 | 32.0 (24.4, 36.9) | 96.5 (67.3, 122.4) | <0.001 |
| ACE activity/Cr, 109 RFU/g | 9 | 15 | 7.1 (6.5, 9.2) | 44.2 (39.5, 64.0) | <0.0001 |
| ACE2 activity/Cr, 106 RFU/h/g | 9 | 15 | 5.2 (4.7, 6.3) | 16.5 (12.9, 21.5) | <0.0001 |
| APA activity/Cr, 106 RFU/h/g | 9 | 15 | 2.5 (1.9, 5.6) | 47.1 (26.3, 74.6) | 0.0001 |
| Angiotensin II/Cr, ng/g | 7 | 15 | 30.8 (15.3, 49.6) | 74.1 (53.5, 103.9) | 0.017 |
Values are medians and interquartile ratio (IQR). STZ, streptozotocin-treated mice; Vehicle, control mice treated with vehicle; Cr, creatinine; ACE, angiotensin-converting enzyme; APA, aminopeptidase A.
Fig. 1.
Renin-angiotensin system (RAS) pathway status in experimental diabetic kidney disease (DKD), based on urine concentration and enzymatic activity of RAS components.
Urine RAS enzymatic activities in experimental DKD.
The enzymatic activities of three enzymes involved in the formation of angiotensin II (ACE) and its degradation (APA and ACE2) were assessed in urine. Median creatinine-normalized enzymatic activities of ACE, ACE2, and APA were all significantly higher in urine of diabetic mice vs. controls (6.2-, 3.2-, and 18.8-fold, respectively). Urine angiotensin II-to-creatinine concentration was also higher in diabetic mice vs. controls (2.4-fold, P = 0.017; Table 1 and Fig. 1).
Characteristics of the cohort of patients with type 1 diabetes.
Compared with people with long-standing type 1 diabetes and no DKD (controls), those with DKD were slightly younger (49 vs. 53 yr old), and less likely to be white (78 vs. 98%) and female (24 vs. 54%; Table 2). Diabetic retinopathy was more common in those with DKD than in those without DKD (73 vs. 64%, respectively), as was hypertension (97 vs. 47%, respectively). As expected from selection criteria, eGFR was markedly lower (median 39 vs. 92 ml/min per 1.73 m2) and urine albumin/creatinine ratio (ACR) markedly higher (median 497 vs. 7 mg/g) in people with DKD compared with controls. RAS inhibitor use was more prevalent in people with DKD than in those without (87 vs. 54%). Diabetes duration was shorter in people with DKD than in the controls (mean 34 vs. 39 yr) (Table 2).
Table 2.
Characteristics of the study cohort
| No DKD | DKD | P Value | |
|---|---|---|---|
| n | 81 | 37 | |
| Age, yr | 53 (9) | 49 (12) | 0.077 |
| Caucasian, n (%) | 75 (93) | 29 (78) | 0.035 |
| Female, n (%) | 44 (54) | 9 (24) | 0.003 |
| Diabetes duration, yr | 39 (6) | 34 (12) | 0.009 |
| RAS inhibitor use, n (%) | 46 (57) | 32 (87) | 0.002 |
| Diabetic retinopathy, b (%) | |||
| Yes | 52 (65) | 27 (73) | <0.0001 |
| No | 22 (27) | 0 (0) | |
| Unknown | 6 (7) | 10 (27) | |
| Hypertension, n (%) | |||
| Yes | 38 (47) | 36 (97) | <0.0001 |
| No | 42 (52) | 0 (0) | |
| Unknown | 1 (1) | 1 (3) | |
| Hemoglobin A1c, % | 7.5 (7.0, 8.1) | 8.3 (7.3, 9.6) | 0.003 |
| GFR, ml/min/1.73m2 | 92 (82, 102) | 39 (33, 53) | <0.0001 |
| Urine albumin/Cr, mg/g | 7 (5, 12) | 497 (122, 1260) | <0.0001 |
Values are means (SD), medians (interquartile range, IQR) or numbers (%). DKD, diabetic kidney disease. Glomerular filtration rate (GFR) was calculated from serum creatinine using the CKD-EPI formula. P values were obtained using Fisher’s exact test.
Urine RAS proteins in human DKD.
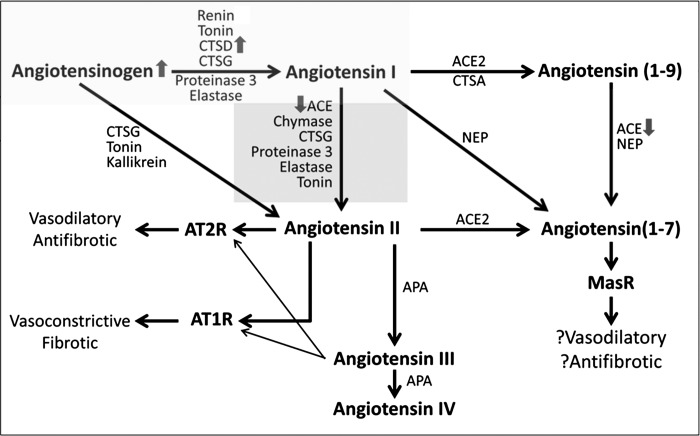
Compared with controls, people with DKD had higher median creatinine-normalized urine AOG (170 vs.15 μg/g Cr). Median creatinine-normalized urine CTSD concentrations were also markedly higher in people with DKD vs. those without (147 vs. 31 μg/g Cr), as was median creatinine-normalized urine ACE concentration (1.4 vs. 0.8 μg/g Cr) (Table 3 and Fig. 2). Creatinine-normalized urine ACE2 concentration was higher in people with than without DKD (medians 4.1 vs. 2.7 μg/g Cr), but this difference was not statistically significant.
Table 3.
Association of creatinine-adjusted urine concentration or activity of each RAS pathway component with DKD in people with type 1 diabetes
|
n |
No DKD | DKD | OR (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| No DKD | DKD | |||||
| Angiotensinogen/Cr, μg/g | 76 | 37 | 15 (8, 24) | 170 (18, 597) | 2.2 (1.6, 3.3) | 9.6 × 10−10 |
| Cathepsin D/Cr, μg/g | 73 | 37 | 31 (22, 46) | 147 (85, 219) | 6.5 (3.5, 14.6) | 2.3 × 10−14 |
| ACE/Cr, μg/g | 73 | 37 | 0.8 (0.4, 1.4) | 1.4 (0.9, 2.5) | 2.2 (1.4, 3.9) | 3.8 × 10−4 |
| ACE activity/Cr, 109 RFU/g | 81 | 37 | 1.6 (1.0, 2.1) | 1.0 (0.8, 1.2) | 0.3 (0.1, 0.6) | 4.6 × 10−4 |
| ACE2/Cr, μg/g | 73 | 37 | 2.7 (1.7, 4.2) | 4.1 (1.6, 5.7) | 1.1 (0.8, 1.7) | 0.54 |
| ACE2 activity/Cr, 106 RFU/h/g | 81 | 37 | 0.3 (0.1, 0.9) | 0.4 (0.2, 0.9) | 1.2 (1.0, 1.4) | 0.03 |
| APA activity/Cr, 106 RFU/h/g | 81 | 37 | 22 (4, 89) | 40 (11, 210) | 1.2 (1.0, 1.3) | 0.05 |
Values are medians (IQR). People with no DKD had eGFR ≥90 ml/min/1.73m2 and ACR <300 mg/g after ≥30 yr of type 1 diabetes. Those with DKD had either an ACR ≥300 mg/g or both eGFR <60 ml/min/1.73m2 and ACR ≥30 mg/g. Odds ratios (OR) were obtained from logistic regression models with case-control status as outcome and urine concentration or activity of a single RAS component as exposure. Models were adjusted for age, sex, race, and diabetes duration.
Fig. 2.
RAS pathway status in human DKD, based on urine concentration and enzymatic activity of RAS components.
Urine RAS enzyme activities in human DKD.
In addition to evaluation of ACE and ACE2 protein concentrations in urine, their enzymatic activities were also quantified in urine. ACE2 and APA activities were higher in people with DKD than in those without DKD (Table 3). In contrast, ACE activity was significantly decreased in patients with DKD compared with those without it. This was associated with the use of ACE inhibitors in this group (see below).
Adjusted associations of urine RAS components with DKD in people with type 1 diabetes.
After adjustment for age, sex, race, and diabetes duration, each twofold increase in creatinine-normalized urine AOG, CTSD, and ACE protein concentrations was significantly associated with 2.7-, 6.5-, and 2.2-fold higher odds of having DKD, respectively (Table 3). In contrast to urine ACE protein concentration, higher urine ACE activity was significantly associated with lower (0.3-fold) odds of having DKD, most likely due to its association with ACE inhibitor use, as described below.
Association between urine ACE2 concentration and DKD status did not withstand adjustment for covariates, whereas enzymatic activities of urine ACE2 and APA showed weaker associations (ORs 1.2 for both) with DKD status (Table 3). The same associations were observed when urine concentrations and activities of these RAS components were not normalized for urine creatinine (Table 4). In a combined model including demographic covariates and those RAS protein concentrations or enzymatic activities individually associated with DKD, only urine CTSD protein and urine ACE activity remained significantly associated with DKD status (Table 5).
Table 4.
Association of urine concentration or activity of each RAS pathway component with DKD in people with type 1 diabetes
|
n |
No DKD | DKD | OR (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| No DKD | DKD | |||||
| Angiotensinogen, ng/ml | 59 | 27 | 9 (5, 16) | 155 (27, 538) | 2.1 (1.6, 2.9) | 2.0 × 10−9 |
| CTSD, ng/ml | 73 | 37 | 18 (11, 35) | 99 (63, 135) | 3.5 (2.2, 6.4) | 1.0 × 10−10 |
| ACE, ng/ml | 73 | 37 | 0.5 (0.3, 0.7) | 1.1 (0.7, 2.2) | 1.9 (1.3, 3.0) | 0.001 |
| ACE activity, RFU/μl | 81 | 37 | 950 (637, 1446) | 736 (585, 872) | 0.4 (0.2, 0.7) | 0.001 |
| ACE2, pg/ml | 73 | 37 | 1631 (723, 3394) | 2810 (1196, 3637) | 1.0 (0.8, 1.3) | 0.93 |
| ACE2 activity, RFU/h/μl | 81 | 37 | 0.19 (0.02, 0.56) | 0.21 (0.13, 0.50) | 1.0 (0.8, 1.3) | 0.83 |
| APA activity, 1,000 RFU/h/μl | 81 | 37 | 13 (2, 47) | 24 (6, 116) | 1.1 (1.0, 1.3) | 0.09 |
Values are medians (IQR). Presence and absence of DKD are defined as in Table 3. ORs were obtained from logistic regression models with case-control status as outcome and urine concentration or activity of a single RAS component as exposure. Models were adjusted for age, sex, race, and diabetes duration.
Table 5.
Association of urine concentration or activity of all RAS pathway components with DKD in people with type 1 diabetes
| OR (95% CI) | P Value | |
|---|---|---|
| Age, yr | 0.9 (0.8, 1.0) | 0.16 |
| Non-white | 1.8 (0.2, 14.8) | 0.59 |
| Female | 0.5 (0.1, 2.6) | 0.41 |
| Diabetes duration, yr | 1.0 (0.9, 1.0) | 0.31 |
| Angiotensinogen/Cr, μg/g | 1.5 (0.9, 2.8) | 0.11 |
| Cathepsin D/Cr, μg/g | 6.5 (2.7, 22.4) | 1.5 × 106 |
| ACE activity/Cr, 106 RFU/gCr | 0.2 (0.1, 0.6) | 0.003 |
| ACE2 activity/Cr, 1,000 RFU/h/gCr | 1.0 (0.8, 1.4) | 0.98 |
| APA activity/Cr, 106 RFU/h/gCr | 0.9 (0.7, 1.2) | 0.36 |
Controls and cases are defined as in Table 3. ORs were obtained from logistic regression models with case-control status as outcome and the 6 listed urine RAS component concentrations or activities as exposures. All urine protein concentrations or activities are represented as continuous variables except urine renin, which is binary (detected, not detected). Models were adjusted for age, sex, race, and diabetes duration.
Change in RAS proteins and enzymatic activities with ACE inhibitor use.
Among people with DKD, urine ACE activity was significantly lower in people who were on ACE inhibitors than in those who were not (−335 RFU/h/mg Cr, P value 0.014) after adjustment for age, sex, race, and diabetes duration. In contrast, urine ACE concentration did not vary by ACE inhibitor use (P value 0.6). Neither did urine CTSD, and ACE2 concentrations or ACE2 activity (P values 0.6, 0.5, and 0.3, respectively).
DISCUSSION
This study shows a concordant increase in key urinary RAS components in an experimental model of DKD and in human DKD associated with type 1 diabetes, providing support for activation of the kidney RAS pathway in DKD. Diabetic animals showed increases in urine AOG and CTSD concentrations and ACE activity, all upstream components of the RAS pathway that lead to increased angiotensin II formation. This was shown more directly from the finding of increased urine angiotensin II in the streptozotocin-treated mice. Similarly, people with type 1 diabetes and DKD had higher urine AOG, CTSD, and ACE concentrations than people with long-standing diabetes and no DKD, suggesting a greater capacity for angiotensin II formation, even though this peptide could not be measured directly in human samples. Also of interest was the finding that people with DKD who were treated with ACE inhibitors had lower urine ACE activity.
RAS activation is generally accepted as an important mechanism of DKD progression (3, 27, 39). Diabetes per se is associated with an increase in urinary concentration and enzymatic activities of several RAS components (6). Furthermore, an increase in kidney expression and urine concentration of some RAS components has been reported in experimental DKD. Our report adds to the existing work in several ways. First, it supports RAS overactivity in DKD kidneys by providing a uniquely comprehensive assessment of key RAS components in terms of their urine concentrations and enzymatic activities in carefully phenotyped patients with type 1 diabetes with and without DKD. Moreover, we observe an overall congruent pattern of change in the pathway proteins and their activities between experimental and human DKD. Combined assessment of RAS components in animal models and human DKD provides a more complete picture than either setting alone. For example, while experimental DKD arguably presents an earlier disease stage with minimal albuminuria, the human DKD phenotype studied here is a later stage with proteinuria and/or reduced GFR. The observation that urine RAS components are increased in both models makes it less likely that these increases are merely the consequence of cofiltration with albumin through a damaged glomerular sieve. Another example of the strength of combined animal-human analysis is that the experimental DKD provides a glimpse of DKD pathophysiology, which is not modified by treatment and as such may help decipher the changes treatment makes in the pathophysiology of human DKD. An example of this is the observation that whereas both urine ACE concentration and activity are increased in untreated animal DKD, urine ACE concentration is increased, while its activity is reduced in people with DKD under ACEI treatment.
AOG is the substrate for the formation of angiotensin peptides, and its overproduction, particularly at the kidney level, may promote progression of kidney disease (21, 28, 49). AOG is increased in kidneys of people with DKD (23) and in urine samples from people with type 1 or type 2 diabetes and kidney disease (1, 20, 22). Urine AOG is strongly correlated with renal angiotensin II and RAS activity (37, 67). As such, our observed increase in urine AOG in both experimental and human DKD is consistent with increased renal RAS activity.
ACE catalyzes conversion of angiotensin I to angiotensin II. ACE expression is increased in renal glomeruli (34, 51, 69) and reduced in tubules of most DKD animal models (51, 65, 68, 69), where ACE protein quantity correlates tightly with its enzymatic activity (65). In human DKD, however, ACE protein is increased in both glomerular and tubular compartments in kidney biopsies (17, 30, 33, 41) and also increased in urine samples from people with DKD (6, 15, 32). We found increased urine ACE activity in streptozotocin-treated mice and, consistent with ACE overexpression in human kidney biopsies, we found higher urine ACE protein concentration. In contrast, urinary ACE activity was lower in people with DKD than in those without. This reduction in ACE activity was associated with treatment with ACE inhibitors. ACE inhibitors are expected to reduce renal ACE activity without influencing ACE protein expression (47). Consistently, treatment with ACE inhibitors was associated with reduced urine ACE activity without any change in urine ACE concentration. Furthermore, the ACE inhibitor-associated reduction in ACE activity was specific to ACE in that it did not affect concentration or enzymatic activity of other RAS proteins, such as ACE2 or CTSD. This observation suggests that urine ACE activity may inform on dynamic changes in the kidney ACE activity and as such may be useful for monitoring compliance with, and possibly adequacy of therapeutic response to, ACE inhibitor treatment.
Another novel finding of this study is the increase in urine CTSD protein concentration observed in both mice with streptozotocin-induced diabetes and people with DKD. Cathepsins are a family of lysosomal proteases, several members of which (cathepsins D, G, and A) can catalyze cleavage of AOG and angiotensin I (Figs. 1 and 2), presenting a mechanism for ACE-independent angiotensin II generation (31, 38, 40, 42, 54, 61). Several pieces of evidence suggest that ACE-independent angiotensin II production occurs and is relevant to renal pathophysiology. First, angiotensin II is only partially blocked with ACE inhibition (10, 55). In addition, the renal hemodynamic response to angiotensin receptor blockers (ARBs) exceeds the response to ACE inhibitors, suggesting that non-ACE pathways contribute to intrarenal angiotensin II generation (14). Furthermore, ARBs augment the effect of ACE inhibitors on blood pressure and proteinuria reduction in experimental DKD models (7, 19, 60), as well as in human DKD (29, 44). There is evidence that cathepsins may contribute to ACE-independent RAS activation in the kidneys. In porcine kidney extracts, cathepsin G-mediated angiotensin-II production is comparable to that of ACE (45). CTSD expression is induced in experimental CKD models, and inhibition of CTSD (but not other cathepsins) improves fibrosis in these models (9, 11). CTSD is also expressed in human kidneys in both glomerular and tubular compartments, and its expression is increased in damaged tubules in DKD (9). Of interest is the recent finding that podocyte-specific CTSD deficiency leads to proteinuria and ESRD in mice (66). This, however, is proposed to be due to the nonredundant role of CTSD in autophagy (66). While the increase in urinary cathepsin may reflect renal processes unrelated to the RAS pathway activity, the presence of large quantities of enzymes that can promote ACE-independent angiotensin II generation suggests the possibility of an ACE-independent mechanism for RAS activation that is novel and worthy of further investigation. If so, urine CTSD concentration may present a biomarker for potential RAS resistance to ACE inhibition and signal the need for additional therapies targeting cathepsin activity.
ACE2 is thought to counteract RAS pathway activity by degrading angiotensin I and II to Ang (1–9) and Ang (1–7), respectively, which have opposing biological functions to angiotensin II (53). In DKD animal models, ACE2 protein and enzymatic activity are increased in kidney tubules (8, 25, 46, 63, 65, 68, 69) as well as in urine (6, 8, 43, 63). Pharmacological inhibition (51, 69) or genetic deletion of ACE2 worsens experimental DKD (62), whereas ACE2 overexpression within podocytes (35) ameliorates DKD, suggesting that the compensatory ACE2 increase in DKD may act to counter RAS pathway activation by helping dispose of angiotensin II. We find an increase in urine ACE2 activity in streptozotocin-treated mice and in people with DKD, consistent with a compensatory increase in kidney ACE2 activity in response to RAS activation in DKD. However, unlike mice, humans with DKD show only a trend toward higher urine ACE2 concentration, which does not attain significance. It is unclear whether this reflects the differences in pathophysiology between animal and human DKD or if it is the result of alterations in the human DKD pathophysiology by treatment.
APA, which degrades angiotensin II to angiotensin III, is the primary mechanism of degradation for circulating angiotensin II (Fig. 1) (56). As such, APA and ACE2 present different mechanisms of countering RAS pathway activation by reducing angiotensin II concentration. Unlike ACE2, APA is abundantly expressed both in tubules and the glomerular compartments of animals with DKD (52). Thus, urine APA could originate from both glomerular and tubular sources. Here, we report for the first time a concordant increase in urine APA activity in experimental and human DKD. The increase in enzymatic activities of ACE2 and APA in experimental and human DKD can be viewed as compensatory in an effort to enhance angiotensin II degradation and thus attenuate the accumulation of this peptide, which is driven by enhanced formation. However, alternative explanations exist, including shedding of these enzymes, which are plasma membrane proteins, in the urine as a result of kidney damage in DKD (63).
The strengths of this study include a comprehensive analysis of the protein concentrations and enzymatic activities for several RAS pathway components in urine samples from a well-characterized DKD animal model and an accurately ascertained human case-control cohort with DKD, where findings were carefully adjusted for potential confounders. The cross-sectional nature of the study precludes assessing association of the RAS proteins with relevant future outcomes. Also, since DKD was not confirmed by a kidney biopsy, inclusion of nondiabetic CKD among our cases is possible. Another limitation is absence of parallel protein quantification in serum or kidney tissue. Finally, it is worth noting that, although widely used and accepted, none of the animal models of DKD, including the STZ-induced model, fully recapitulate the human disease (4, 5).
In summary, in humans with type 1 diabetes and DKD and in the widely-used streptozotocin model of DKD, urine concentrations and enzymatic activities of several RAS components are concordantly increased, consistent with the increased intrarenal RAS activity in DKD. Furthermore, the ACE inhibitor-induced reduction in urine ACE activity suggests its utility as a measurement of suppressed intrarenal ACE activity. Last, increased urine CTSD concentration in both mice and humans with DKD suggests cathepsins as both a potential mechanism for, and a marker of, ACE-independent RAS pathway activation in DKD.
GRANTS
M. Afkarian was supported by Grants K23 DK-089017 and R01 DK-104706 from the National Institute of Diabetes, Digestive, and Kidney Disease (NIDDK) and the Norman S. Coplon Extramural Grant from Satellite Healthcare. D. Batlle was supported by NIDDK Grants R01 DK-104785 and R01 DK-080089.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W., A.G., M.B., and J.R. performed experiments; J.W., A.G., and K.W. analyzed data; J.W., D.B., and M.A. interpreted results of experiments; J.W., D.B., and M.A. edited and revised manuscript; D.B. and M.A. conceived and designed research; M.A. prepared figures; M.A. drafted manuscript; J.W., A.G., M.B., K.W., J.R., D.B., and M.A. approved final version of manuscript.
REFERENCES
- 1.Afkarian M, Hirsch IB, Tuttle KR, Greenbaum C, Himmelfarb J, de Boer IH. Urinary excretion of RAS, BMP, and WNT pathway components in diabetic kidney disease. Physiol Rep 2: e12010, 2014. doi: 10.14814/phy2.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH. Clinical manifestations of kidney disease among us adults with diabetes, 1988–2014. JAMA 316: 602–610, 2016. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 4.Breyer MD, Böttinger E, Brosius FC III, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2004. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 5.Brosius FC III, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T. Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns KD, Lytvyn Y, Mahmud FH, Daneman D, Deda L, Dunger DB, Deanfield JE, Dalton RN, Elia Y, Har R, Van JA, Bradley TJ, Slorach C, Hui W, Xiao F, Zimpelmann J, Mertens L, Moineddin R, Reich HN, Sochett EB, Scholey JW, Cherney DZ. The relationship between urinary renin angiotensin system markers, renal and vascular function in adolescents with type 1 diabetes. Am J Physiol Renal Physiol 312: F335–F342, 2016. doi: 10.1152/ajprenal.00438.2016. [DOI] [PubMed] [Google Scholar]
- 7.Cao Z, Bonnet F, Davis B, Allen TJ, Cooper ME. Additive hypotensive and anti-albuminuric effects of angiotensin-converting enzyme inhibition and angiotensin receptor antagonism in diabetic spontaneously hypertensive rats. Clin Sci (Lond) 100: 591–599, 2001. doi: 10.1042/cs1000591. [DOI] [PubMed] [Google Scholar]
- 8.Chodavarapu H, Grobe N, Somineni HK, Salem ES, Madhu M, Elased KM. Rosiglitazone treatment of type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion. PLoS One 8: e62833, 2013. doi: 10.1371/journal.pone.0062833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox C, Cocchiaro P, Oakley F, Howarth R, Callaghan K, Leslie J, Luli S, Wood KM, Genovese F, Sheerin NS, Moles A. Inhibition of lysosomal protease cathepsin D reduces renal fibrosis in murine chronic kidney disease. Sci Rep 6: 20101, 2016. doi: 10.1038/srep20101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox J, Guan S, Hymel AA, Navar LG. Dietary Na and ACE inhibition effects on renal tissue angiotensin I and II and ACE activity in rats. Am J Physiol Renal Physiol 262: F902–F909, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Graciano ML, Cavaglieri RC, Dellê H, Dominguez WV, Casarini DE, Malheiros DM, Noronha IL. Intrarenal Renin-Angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol 15: 1805–1815, 2004. doi: 10.1097/01.ASN.0000131528.00773.A9. [DOI] [PubMed] [Google Scholar]
- 12.Haber PK, Ye M, Wysocki J, Maier C, Haque SK, Batlle D. Angiotensin-converting enzyme 2-independent action of presumed angiotensin-converting enzyme 2 activators: novelty and significance. Hypertension 63: 774–782, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF, Liu Y. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol 23: 294–304, 2012. doi: 10.1681/ASN.2011050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollenberg NK, Osei SY, Lansang MC, Price DA, Fisher ND. Salt intake and non-ACE pathways for intrarenal angiotensin II generation in man. J Renin Angiotensin Aldosterone Syst 2: 14–18, 2001. doi: 10.3317/jraas.2001.002. [DOI] [PubMed] [Google Scholar]
- 15.Hosojima H, Miyauchi E, Morimoto S. Urinary excretion of angiotensin-converting enzyme in NIDDM patients with nephropathy. Diabetes Care 12: 580–582, 1989. doi: 10.2337/diacare.12.8.580. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CY, Ballard S, Batlle D, Bonventre JV, Böttinger EP, Feldman HI, Klein JB, Coresh J, Eckfeldt JH, Inker LA, Kimmel PL, Kusek JW, Liu KD, Mauer M, Mifflin TE, Molitch ME, Nelsestuen GL, Rebholz CM, Rovin BH, Sabbisetti VS, Van Eyk JE, Vasan RS, Waikar SS, Whitehead KM, Nelson RG. Cross-Disciplinary Biomarkers Research: Lessons Learned by the CKD Biomarkers Consortium. Clin J Am Soc Nephrol 10: 894–902, 2015. doi: 10.2215/CJN.11541114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang XR, Chen WY, Truong LD, Lan HY. Chymase is upregulated in diabetic nephropathy: implications for an alternative pathway of angiotensin II-mediated diabetic renal and vascular disease. J Am Soc Nephrol 14: 1738–1747, 2003. doi: 10.1097/01.ASN.0000071512.93927.4E. [DOI] [PubMed] [Google Scholar]
- 18.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PX, Mariani LH, Eichinger FH, Berthier CC, Randolph A, Lai JY, Zhou Y, Hawkins JJ, Bitzer M, Sampson MG, Thier M, Solier C, Duran-Pacheco GC, Duchateau-Nguyen G, Essioux L, Schott B, Formentini I, Magnone MC, Bobadilla M, Cohen CD, Bagnasco SM, Barisoni L, Lv J, Zhang H, Wang HY, Brosius FC, Gadegbeku CA, Kretzler M. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 7: 316ra193, 2015. doi: 10.1126/scitranslmed.aac7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalender B, Oztürk M, Tunçdemir M, Uysal O, Dagistanli FK, Yegenaga I, Erek E. Renoprotective effects of valsartan and enalapril in STZ-induced diabetes in rats. Acta Histochem 104: 123–130, 2002. doi: 10.1078/0065-1281-00643. [DOI] [PubMed] [Google Scholar]
- 20.Kim SS, Song SH, Kim IJ, Yang JY, Lee JG, Kwak IS, Kim YK. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract 97: 251–257, 2012. doi: 10.1016/j.diabres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int 61: 579–585, 2002. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobori H, Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Yamamoto T. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens 2: 349–354, 2008. doi: 10.1016/j.jash.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai KN, Leung JC, Lai KB, To WY, Yeung VT, Lai FM. Gene expression of the renin-angiotensin system in human kidney. J Hypertens 16: 91–102, 1998. doi: 10.1097/00004872-199816010-00014. [DOI] [PubMed] [Google Scholar]
- 24.Lavrentyev EN, Estes AM, Malik KU. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ Res 101: 455–464, 2007. doi: 10.1161/CIRCRESAHA.107.151852. [DOI] [PubMed] [Google Scholar]
- 25.Leehey DJ, Singh AK, Bast JP, Sethupathi P, Singh R. Glomerular renin angiotensin system in streptozotocin diabetic and Zucker diabetic fatty rats. Transl Res 151: 208–216, 2008. doi: 10.1016/j.trsl.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, Brezniceanu ML, Wei CC, Chénier I, Sachetelli S, Zhang SL, Filep JG, Ingelfinger JR, Chan JS. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol 19: 269–280, 2008. doi: 10.1681/ASN.2007010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S; ONTARGET investigators . Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 30.Mezzano S, Droguett A, Burgos ME, Ardiles LG, Flores CA, Aros CA, Caorsi I, Vío CP, Ruiz-Ortega M, Egido J. Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int Suppl 64: S64–S70, 2003. doi: 10.1046/j.1523-1755.64.s86.12.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller JJ, Changaris DG, Levy RS. Conversion of angiotensin I to angiotensin II by cathepsin A isoenzymes of porcine kidney. Biochem Biophys Res Commun 154: 1122–1129, 1988. doi: 10.1016/0006-291X(88)90257-4. [DOI] [PubMed] [Google Scholar]
- 32.Miyauchi E, Hosojima H, Morimoto S. Urinary angiotensin-converting enzyme activity in type 2 diabetes mellitus: its relationship to diabetic nephropathy. Acta Diabetol 32: 193–197, 1995. doi: 10.1007/BF00838491. [DOI] [PubMed] [Google Scholar]
- 33.Mizuiri S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis 51: 613–623, 2008. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Moon JY, Jeong KH, Lee SH, Lee TW, Ihm CG, Lim SJ. Renal ACE and ACE2 expression in early diabetic rats. Nephron, Exp Nephrol 110: e8–e16, 2008. doi: 10.1159/000149586. [DOI] [PubMed] [Google Scholar]
- 35.Nadarajah R, Milagres R, Dilauro M, Gutsol A, Xiao F, Zimpelmann J, Kennedy C, Wysocki J, Batlle D, Burns KD. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int 82: 292–303, 2012. doi: 10.1038/ki.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 20: 1813–1821, 2009. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, Hamada M, Kishida M, Hitomi H, Shirahashi N, Kobori H, Imanishi M. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant 26: 170–177, 2011. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen CA, Campbell EJ. Angiotensin II generation at the cell surface of activated neutrophils: novel cathepsin G-mediated catalytic activity that is resistant to inhibition. J Immunol 160: 1436–1443, 1998. [PubMed] [Google Scholar]
- 39.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870–878, 2001. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 40.Ramaha A, Patston PA. Release and degradation of angiotensin I and angiotensin II from angiotensinogen by neutrophil serine proteinases. Arch Biochem Biophys 397: 77–83, 2002. doi: 10.1006/abbi.2001.2687. [DOI] [PubMed] [Google Scholar]
- 41.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int 74: 1610–1616, 2008. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- 42.Reilly CF, Tewksbury DA, Schechter NM, Travis J. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. J Biol Chem 257: 8619–8622, 1982. [PubMed] [Google Scholar]
- 43.Riera M, Márquez E, Clotet S, Gimeno J, Roca-Ho H, Lloreta J, Juanpere N, Batlle D, Pascual J, Soler MJ. Effect of insulin on ACE2 activity and kidney function in the non-obese diabetic mouse. PLoS One 9: e84683, 2014. doi: 10.1371/journal.pone.0084683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossing K, Jacobsen P, Pietraszek L, Parving HH. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: a randomized double-blind crossover trial. Diabetes Care 26: 2268–2274, 2003. doi: 10.2337/diacare.26.8.2268. [DOI] [PubMed] [Google Scholar]
- 45.Rykl J, Thiemann J, Kurzawski S, Pohl T, Gobom J, Zidek W, Schlüter H. Renal cathepsin G and angiotensin II generation. J Hypertens 24: 1797–1807, 2006. doi: 10.1097/01.hjh.0000242404.91332.be. [DOI] [PubMed] [Google Scholar]
- 46.Salem ES, Grobe N, Elased KM. Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice. Am J Physiol Renal Physiol 306: F629–F639, 2014. doi: 10.1152/ajprenal.00516.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlueter W, Keilani T, Batlle DC. Tissue renin angiotensin systems: theoretical implications for the development of hyperkalemia using angiotensin-converting enzyme inhibitors. Am J Med Sci 307, Suppl 1: S81–S86, 1994. [PubMed] [Google Scholar]
- 48.Schwager SL, Carmona AK, Sturrock ED. A high-throughput fluorimetric assay for angiotensin I-converting enzyme. Nat Protoc 1: 1961–1964, 2006. doi: 10.1038/nprot.2006.305. [DOI] [PubMed] [Google Scholar]
- 49.Singh R, Singh AK, Leehey DJ. A novel mechanism for angiotensin II formation in streptozotocin-diabetic rat glomeruli. Am J Physiol Renal Physiol 288: F1183–F1190, 2005. doi: 10.1152/ajprenal.00159.2003. [DOI] [PubMed] [Google Scholar]
- 50.Soler MJ, Riera M, Batlle D. New experimental models of diabetic nephropathy in mice models of type 2 diabetes: efforts to replicate human nephropathy. Exp Diabetes Res 2012: 1, 2012. doi: 10.1155/2012/616313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int 72: 614–623, 2007. doi: 10.1038/sj.ki.5002373. [DOI] [PubMed] [Google Scholar]
- 52.Song L, Healy DP. Kidney aminopeptidase A and hypertension, part II: effects of angiotensin II. Hypertension 33: 746–752, 1999. doi: 10.1161/01.HYP.33.2.746. [DOI] [PubMed] [Google Scholar]
- 53.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 54.Tonnesen MG, Klempner MS, Austen KF, Wintroub BU. Identification of a human neutrophil angiotension II-generating protease as cathepsin G. J Clin Invest 69: 25–30, 1982. doi: 10.1172/JCI110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Kats JP, Schalekamp MA, Verdouw PD, Duncker DJ, Danser AH. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int 60: 2311–2317, 2001. doi: 10.1046/j.1523-1755.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 56.Velez JC. The importance of the intrarenal renin-angiotensin system. Nat Clin Pract Nephrol 5: 89–100, 2009. doi: 10.1038/ncpneph1015. [DOI] [PubMed] [Google Scholar]
- 57.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest 120: 4065–4076, 2010. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volland H, Pradelles P, Ronco P, Azizi M, Simon D, Créminon C, Grassi J. A solid-phase immobilized epitope immunoassay (SPIE-IA) permitting very sensitive and specific measurement of angiotensin II in plasma. J Immunol Methods 228: 37–47, 1999. doi: 10.1016/S0022-1759(99)00097-6. [DOI] [PubMed] [Google Scholar]
- 59.Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, Takeda SI, Takasawa K, Yoshimura M, Kida H, Kobayashi KI, Mukaida N, Naito T, Matsushima K, Yokoyama H. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 58: 1492–1499, 2000. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- 60.Wilkinson-Berka JL, Gibbs NJ, Cooper ME, Skinner SL, Kelly DJ. Renoprotective and anti-hypertensive effects of combined valsartan and perindopril in progressive diabetic nephropathy in the transgenic (mRen-2)27 rat. Nephrol Dial Transplant 16: 1343–1349, 2001. doi: 10.1093/ndt/16.7.1343. [DOI] [PubMed] [Google Scholar]
- 61.Wintroub BU, Klickstein LB, Dzau VJ, Watt KW. Granulocyte-angiotensin system. Identification of angiotensinogen as the plasma protein substrate of leukocyte cathepsin G. Biochemistry 23: 227–232, 1984. doi: 10.1021/bi00297a009. [DOI] [PubMed] [Google Scholar]
- 62.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol 171: 438–451, 2007. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wysocki J, Garcia-Halpin L, Ye M, Maier C, Sowers K, Burns KD, Batlle D. Regulation of urinary ACE2 in diabetic mice. Am J Physiol Renal Physiol 305: F600–F611, 2013. doi: 10.1152/ajprenal.00600.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wysocki J, Ye M, Khattab AM, Fogo A, Martin A, David NV, Kanwar Y, Osborn M, Batlle D. Angiotensin-converting enzyme 2 amplification limited to the circulation does not protect mice from development of diabetic nephropathy. Kidney Int 91: 1336–1346, 2017. doi: 10.1016/j.kint.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139, 2006. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto-Nonaka K, Koike M, Asanuma K, Takagi M, Oliva Trejo JA, Seki T, Hidaka T, Ichimura K, Sakai T, Tada N, Ueno T, Uchiyama Y, Tomino Y. Cathepsin D in Podocytes Is Important in the Pathogenesis of Proteinuria and CKD. J 2016. doi: 10.1681/ASN.2015040366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 18: 1558–1565, 2007. doi: 10.1681/ASN.2006060554. [DOI] [PubMed] [Google Scholar]
- 68.Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension 43: 1120–1125, 2004. doi: 10.1161/01.HYP.0000126192.27644.76. [DOI] [PubMed] [Google Scholar]
- 69.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D. Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]