Abstract
Hypertension is growing in epidemic proportions worldwide and is now the leading preventable cause of premature death. For over a century, we have known that the kidney plays a critical role in blood pressure regulation. Specifically, abnormalities in renal sodium transport appear to be a final common pathway that gives rise to elevated blood pressure regardless of the nature of the initial hypertensive stimulus. However, it is only in the past decade that we have come to realize that inflammatory cytokines secreted by innate and adaptive immune cells, as well as renal epithelial cells, can modulate the expression and activity of sodium transporters all along the nephron, leading to alterations in pressure natriuresis, sodium and water balance, and ultimately hypertension. This mini-review highlights specific cytokines and the transporters that they regulate and discusses why inflammatory cytokines may have evolved to serve this function.
Keywords: interleukin-6, interleukin-17, interferon-γ, angiotensinogen
hypertension afflicts one in three adults worldwide, and this figure is growing rapidly. In 2015, hypertension was ranked as the single leading risk factor for global burden of disease in both economically developed and developing countries (4). Renal and vascular dysfunction are important causes and consequences of this disease and lead to the development of heart failure, stroke, myocardial infarction, and chronic kidney disease, resulting in substantial morbidity and mortality (16, 32). Alarmingly, ~50% of patients with hypertension have uncontrolled blood pressures, and, even when blood pressures are reasonably controlled, these patients have an elevated risk of cardiovascular disease (3, 21, 29). Unfortunately, we do not fully understand the pathophysiology of essential hypertension, but alterations in renal sodium transport appear to be a final common pathway in most forms of this disease as discussed below. Thus, it is imperative that we explore the factors that regulate renal sodium transport in physiological and pathophysiological conditions.
The kidney in hypertension.
The kidney has long been implicated in the pathogenesis of hypertension. In 1934, Dr. Harry Goldblatt and colleagues demonstrated that constriction of the renal arteries leads to persistent hypertension in dogs (6). Decades later, based on experimental results and computational modeling, Dr. Arthur Guyton and colleagues put forth what is now called Guyton’s paradigm. This paradigm offers a reasonable framework for understanding the physiological control of blood pressure and the development of hypertension by renal regulation of fluid volume. Blood pressure is the product of cardiac output (CO) and total peripheral resistance (TPR). Briefly, according to Guyton’s paradigm, hypertension develops initially from salt and water retention that results in increased CO. Subsequently, through incompletely understood mechanisms, the increased CO induces whole body autoregulation and an increase in TPR that sustains the hypertensive response. Central to Guyton’s paradigm is the concept of pressure-natriuresis in which an increase in blood pressure results in an increase in sodium and water excretion to lower blood pressure back to its set point. Thus, in chronic hypertension, alterations in the kidney must occur such that the pressure-natriuresis curve is shifted to maintain sodium balance at an elevated blood pressure set point (5, 7, 8).
The immune system in hypertension.
So what controls resetting of the pressure-natriuresis curve? One possibility is inflammatory cytokines, products of the innate and adaptive immune system, and, in some cases, the renal epithelial cells themselves. Immune cells have long been observed in the kidneys of hypertensive animals and humans, but only in recent years has our understanding of the role of these cells and their products grown at a rapid pace (18). We and others have shown that cytokines such as interleukin-6 (IL-6), interleukin-1 (IL-1), interferon-γ (IFN-γ), interleukin-17A (IL-17A), and tumor necrosis factor-α (TNF-α) play critical roles in hypertension and that inhibition or genetic deletion of these cytokines or their receptors blunts experimental hypertension and reduces the associated end-organ damage (18, 20, 36). Below, we discuss how these cytokines can affect sodium handling through alterations in nitric oxide bioavailability, the intrarenal renin angiotensin system, and the expression of renal sodium transporters, thus impacting blood pressure control.
Cytokines and renal transporter function in hypertension.
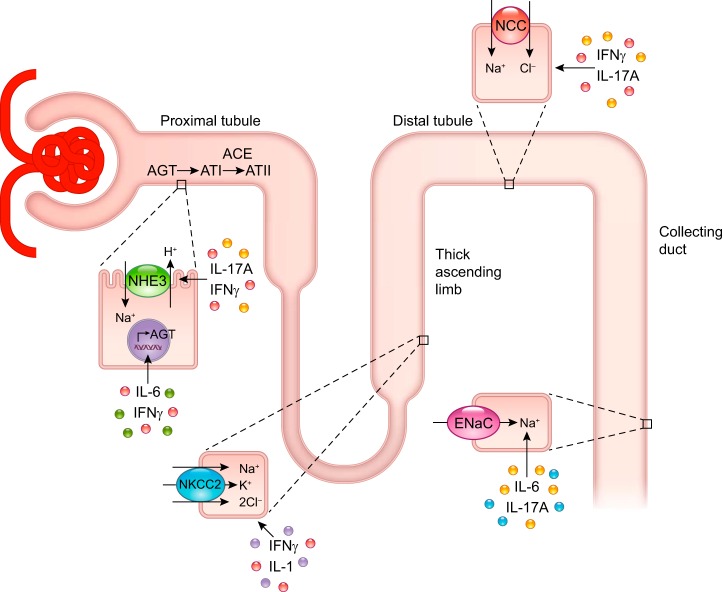
IL-6 is a prototypical proinflammatory cytokine produced by hematopoietic and nonhematopoietic cells. In 2006, Lee et al. demonstrated that genetic deletion of IL-6 in mice results in blunted hypertension and significantly reduced albuminuria in response to angiotensin II (ANG II) infusion compared with wild-type (WT) mice (14). Recently, Hashmat et al. corroborated these findings in the Dahl salt-sensitive rat model using a neutralizing antibody to IL-6 (9). In cultured cortical collecting duct cells, IL-6 was shown to increase the expression and activity of the epithelial sodium channel (ENaC) (15). Of note, mutations in ENaC that increase sodium reabsorption lead to the development of high blood pressure in Liddle’s syndrome, a rare inherited form of hypertension. Mutations that inactivate ENaC have also been identified in humans, and these mutations lead to low blood pressure (1). IL-6 can also indirectly increase sodium reabsorption through activation of the intrarenal renin-angiotensin system (RAS). In this system [reviewed by Navar et al. (19)], angiotensinogen produced by the proximal tubule is converted to angiotensin I and subsequently to ANG II through intrarenal angiotensin-converting enzymes. ANG II can then directly stimulate proximal and distal sodium transporters via its effect on angiotensin type 1 receptors (18, 23). Satou et al. showed that the combination of IL-6 and ANG II induces a modest increase in angiotensinogen production from a human proximal tubule cell line (26). More recently, Satou and colleagues reported that IL-6 derived from ANG II-treated macrophages increases angiotensinogen expression in a rat proximal tubule cell line (22). Although the effects of IL-6 on sodium transporters have not been tested in vivo, taken together, these cell culture studies provide potential direct and indirect mechanisms for IL-6-induced increases in sodium reabsorption and therefore hypertension (Fig. 1).
Fig. 1.
Summary of inflammatory cytokines and the renal transporters that they have been shown to activate either directly or indirectly. AGT, angiotensinogen; ATI, angiotensin I; ATII, angiotensin II; ACE, angiotensin-converting enzyme; NHE3, sodium/hydrogen exchanger 3; NKCC2, sodium-potassium-two chloride cotransporter; NCC, sodium-chloride cotransporter; ENaC, epithelial sodium channel; IL-17A, interleukin-17A; IFN-γ, interferon-γ; IL-6, interleukin-6; IL-1, interleukin-1.
IL-1 is a marker of acute and chronic inflammation and a primary inducer of the innate immune response. IL-1 can be produced by hematopoietic cells and several types of intrinsic kidney cells in normal and diseased states (28, 30). Early studies by Kohan and colleagues demonstrated that IL-1 administration to rats resulted in a marked increase in sodium excretion, in part through stimulation of prostaglandin E2 production by collecting duct cells with resultant inhibition of sodium-potassium-ATPase activity that was not accompanied by changes in systemic blood pressure (13, 34). The two isoforms of IL-1, IL-1α and IL-1β, both act on the type 1 IL-1 receptor (IL-1R1). Recently, Crowley and colleagues demonstrated that IL-1R1 deficiency or blockade limits blood pressure elevation in response to ANG II infusion and that this occurs by modulation of sodium reabsorption via the sodium-potassium-two chloride cotransporter (NKCC2) cotransporter located in the thick ascending limb of the loop of Henle (Fig. 1). Through an elegant series of experiments, they showed that IL-1R1 signaling within intrarenal myeloid cells prevents their maturation into Ly6C+Ly6G− macrophages capable of producing nitric oxide (NO) (36). NO in turn suppresses NKCC2 activity. Thus, they identified a pathway by which activation of IL-1R1 signaling within renal myeloid cells blunts NO production, thus enhancing sodium reabsorption through NKCC2 and increasing blood pressure (36).
IFN-γ is a proinflammatory cytokine produced by innate and adaptive immune cells that plays a critical role in infectious and autoimmune disorders. We showed that T cell production of IFN-γ is increased in ANG II-induced hypertension and that mice deficient in IFN-γ have a blunted blood pressure response to ANG II infusion (25). In collaboration with McDonough and colleagues, we showed that, following ANG II infusion, IFN-γ-deficient mice maintained baseline diuretic and natriuretic responses to a saline challenge unlike WT mice in which ANG II infusion resulted in impaired sodium and water excretion. Renal transporter profiling in these mice following 2 wk of ANG II-induced hypertension revealed that IFN-γ positively regulates sodium/hydrogen exchanger 3 (NHE3) in the proximal tubule and NKCC2 and the sodium chloride cotransporter (NCC) in the distal tubule (11). Whether IFN-γ directly modulates these sodium transporters or acts through downstream mediators is unknown. Similar to IL-6, IFN-γ has been shown to increase angiotensinogen production from cultured renal proximal tubule cells (27), thus suggesting that IFN-γ may regulate sodium reabsorption through activation of the intrarenal renin-angiotensin system (RAS) (Fig. 1). However, ENaC abundance was not affected by IFN-γ deficiency, demonstrating that there is some specificity to the effect of IFN-γ on sodium transporters beyond simply increasing intrarenal RAS.
IL-17A is a relatively newly discovered cytokine produced by T-helper 17 cells and other specialized immune cell subsets as well as proximal and distal renal epithelial cells (20). IL-17A plays a central role in many autoimmune disorders. We first showed that mice deficient in IL-17A exhibit blunted hypertension and preserved vascular function in response to ANG II infusion compared with WT mice (17). Like IFN-γ-deficient mice, IL-17A-deficient mice also maintain baseline diuretic and natriuretic responses to a saline challenge following ANG II infusion (11). Interestingly, IL-17A deficiency results in a biphasic effect on renal sodium transporters with a decrease in NHE3 following 2 wk of ANG II infusion (11) and a blunting of distal transporter activation, NCC and ENaC, following 4 wk of ANG II infusion. Using cultured proximal and distal convoluted tubule cell lines, we showed that IL-17A treatment increased NHE3 expression and NCC activity, respectively. In addition, we found that IL-17A induced phosphorylation of serum- and glucocorticoid-regulated kinase 1 (SGK1) at serine-78 and that treatment with an SGK1 inhibitor blocked the effects of IL-17A on NHE3 and NCC. These effects on blood pressure and sodium transporter abundance were largely absent in mice deficient in the related isoform, IL-17F. Unlike IL-6 and IFN-γ, IL-17A did not increase angiotensinogen production from cultured proximal tubule cells (20). Thus, IL-17A directly modulates proximal and distal sodium transporters through an SGK1-dependent pathway, leading to sodium and water reabsorption and increased blood pressure (Fig. 1).
Perspectives and future directions.
The studies described above demonstrate that inflammatory cytokines released from immune cells and intrinsic renal cells regulate sodium transporter abundance and activity. A major question is whether there are other cytokines that also regulate sodium transporters either directly or indirectly (for example, through modulation of the intrarenal RAS or renal NO bioavailability). For example, TNF-α has been shown to reduce endothelial nitric oxide synthase (eNOS) expression in the thick ascending limb, and mice deficient in TNF-α only in the kidney exhibit enhanced renal eNOS expression and blunted hypertensive responses to ANG II infusion (24, 35). Thus, TNF-α signaling would be predicted to increase NKCC2 activity through its effects on NO production, similar to that observed with IL-1 signaling. However, Battula et al. showed that TNF may actually inhibit NKCC2 protein expression and function in the thick ascending limb (2). Thus, further studies are needed to determine the precise mechanisms by which TNF signaling regulates renal sodium reabsorption and blood pressure. Another area of future investigation is whether anti-inflammatory cytokines, such as IL-10, elicit a decrease in sodium transporter activity. One limitation of the aforementioned studies is that they were primarily performed in male mice; therefore, future studies are necessary to determine whether inflammatory cytokines have similar effects on sodium handling in female animals.
These findings raise the interesting teleological question of why inflammatory cytokines evolved to control sodium transport in the kidney. The answer may lie in recent observations by Titze that, contrary to the classical concept of sodium equilibration between tissues and extracellular fluid, sodium can actually accumulate in the skin and muscle of mice and humans without commensurate water retention (31). Increased sodium has recently been shown to promote pathogenic activity and inhibit anti-inflammatory functions of innate and adaptive immune cells (10, 12, 33, 37). Thus, it is possible that the immune system and inflammatory cytokines evolved to enhance sodium reabsorption at times of need to boost its own response, creating a feed-forward amplification mechanism to ward off pathogens. In the case of autoimmune disorders and hypertension, activation of the immune system by neo-antigens or oxidative stress may maladaptively increase sodium reabsorption, thereby worsening the disease and contributing to inflammatory end-organ damage. In conclusion, understanding how, where, and why cytokines control sodium transport is a new and exciting area that will likely lead to novel immunotherapies for hypertension and renal dysfunction.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Training Grant T32-HL-069765–11A1 to A. E. Norlander, NHLBI NRSA Award F31-HL-27986 to A. E. Norlander, NHLBI K08 award HL-21671 to M. S. Madhur, and a Gilead Sciences grant to M. S. Madhur.
DISCLOSURES
M.S.M. received a research grant from Gilead Sciences, Inc.
AUTHOR CONTRIBUTIONS
A.E.N. and M.S.M. prepared figures; A.E.N. and M.S.M. drafted manuscript; A.E.N. and M.S.M. approved final version of manuscript; M.S.M. edited and revised manuscript.
REFERENCES
- 1.Baker EH. Ion channels and the control of blood pressure. Br J Clin Pharmacol 49: 185–198, 2000. doi: 10.1046/j.1365-2125.2000.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battula S, Hao S, Pedraza PL, Stier CT, Ferreri NR. Tumor necrosis factor-α is an endogenous inhibitor of Na+-K+-2Cl− cotransporter isoform A in the thick ascending limb. Am J Physiol Renal Physiol 301: F94–F100, 2011. doi: 10.1152/ajprenal.00650.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blacher J, Evans A, Arveiler D, Amouyel P, Ferrières J, Bingham A, Yarnell J, Haas B, Montaye M, Ruidavets JB, Ducimetière P, Group PS; PRIME Study Group . Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME Study. J Hum Hypertens 24: 19–26, 2010. doi: 10.1038/jhh.2009.34. [DOI] [PubMed] [Google Scholar]
- 4.Collaborators GBDRF; GBD 2015 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1659–1724, 2016. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans RG, Bie P. Role of the kidney in the pathogenesis of hypertension: time for a neo-Guytonian paradigm or a paradigm shift? Am J Physiol Regul Integr Comp Physiol 310: R217–R229, 2016. doi: 10.1152/ajpregu.00254.2015. [DOI] [PubMed] [Google Scholar]
- 6.Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension: i. the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59: 347–379, 1934. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyton AC. Blood pressure control—special role of the kidneys and body fluids. Science 252: 1813–1816, 1991. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 8.Guyton AC, Coleman TG, Cowley AV Jr, Scheel KW, Manning RD Jr, Norman RA Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 9.Hashmat S, Rudemiller NP, Lund H, Abais-Battad JM, Van Why SK, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 311: F555–F561, 2016. doi: 10.1152/ajprenal.00594.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M, Hafler DA. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest 125: 4212–4222, 2015. doi: 10.1172/JCI81151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ−/− and interleukin-17A−/− mice. Hypertension 65: 569–576, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522, 2013. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohan DE, Merli CA, Simon EE. Micropuncture localization of the natriuretic effect of interleukin 1. Am J Physiol Renal Fluid Electrolyte Physiol 256: F810–F813, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Lee DL, Sturgis LC, Labazi H, Osborne JB Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol 290: H935–H940, 2006. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 15.Li K, Guo D, Zhu H, Hering-Smith KS, Hamm LL, Ouyang J, Dong Y. Interleukin-6 stimulates epithelial sodium channels in mouse cortical collecting duct cells. Am J Physiol Regul Integr Comp Physiol 299: R590–R595, 2010. doi: 10.1152/ajpregu.00207.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lionakis N, Mendrinos D, Sanidas E, Favatas G, Georgopoulou M. Hypertension in the elderly. World J Cardiol 4: 135–147, 2012. doi: 10.4330/wjc.v4.i5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, Dale BL, Iwakura Y, Hoover RS, McDonough AA, Madhur MS. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin ii-induced hypertension. Hypertension 68: 167–174, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nwankwo T, Yoon SS, Burt V, and Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. 1–8, 2013. https://www.cdc.gov/nchs/data/databriefs/db133.pdf. [PubMed]
- 22.O’Leary R, Penrose H, Miyata K, Satou R. Macrophage-derived IL-6 contributes to ANG II-mediated angiotensinogen stimulation in renal proximal tubular cells. Am J Physiol Renal Physiol 310: F1000–F1007, 2016. doi: 10.1152/ajprenal.00482.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol 13: 1131–1135, 2002. doi: 10.1097/01.ASN.0000013292.78621.FD. [DOI] [PubMed] [Google Scholar]
- 24.Ramseyer VD, Hong NJ, Garvin JL. Tumor necrosis factor α decreases nitric oxide synthase type 3 expression primarily via Rho/Rho kinase in the thick ascending limb. Hypertension 59: 1145–1150, 2012. doi: 10.1161/HYPERTENSIONAHA.111.189761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest 125: 1189–1202, 2015. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Katsurada A, Navar LG, Kobori H. Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am J Physiol Renal Physiol 295: F283–F289, 2008. doi: 10.1152/ajprenal.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satou R, Miyata K, Gonzalez-Villalobos RA, Ingelfinger JR, Navar LG, Kobori H. Interferon-γ biphasically regulates angiotensinogen expression via a JAK-STAT pathway and suppressor of cytokine signaling 1 (SOCS1) in renal proximal tubular cells. FASEB J 26: 1821–1830, 2012. doi: 10.1096/fj.11-195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol 10: 89–102, 2010. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 29.Struthers AD. A new approach to residual risk in treated hypertension--3P screening. Hypertension 62: 236–239, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01586. [DOI] [PubMed] [Google Scholar]
- 30.Tesch GH, Yang N, Yu H, Lan HY, Foti R, Chadban SJ, Atkins RC, Nikolic-Paterson DJ. Intrinsic renal cells are the major source of interleukin-1 beta synthesis in normal and diseased rat kidney. Nephrol Dial Transplant 12: 1109–1115, 1997. doi: 10.1093/ndt/12.6.1109. [DOI] [PubMed] [Google Scholar]
- 31.Titze J. Sodium balance is not just a renal affair. Curr Opin Nephrol Hypertens 23: 101–105, 2014. doi: 10.1097/01.mnh.0000441151.55320.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization A Global Brief on Hypertension. Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 33.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496: 513–517, 2013. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeidel ML, Brady HR, Kohan DE. Interleukin-1 inhibition of Na+-K+-ATPase in inner medullary collecting duct cells: role of PGE2. Am J Physiol Renal Physiol 261: F1013–F1016, 1991. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD. Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension 64: 1275–1281, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD, Crowley SD. Interleukin-1 receptor activation potentiates salt reabsorption in angiotensin ii-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab 23: 360–368, 2016. doi: 10.1016/j.cmet.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang WC, Zheng XJ, Du LJ, Sun JY, Shen ZX, Shi C, Sun S, Zhang Z, Chen XQ, Qin M, Liu X, Tao J, Jia L, Fan HY, Zhou B, Yu Y, Ying H, Hui L, Liu X, Yi X, Liu X, Zhang L, Duan SZ. High salt primes a specific activation state of macrophages, M(Na). Cell Res 25: 893–910, 2015. doi: 10.1038/cr.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]