Abstract
Sepsis outcomes are heavily dependent on the development of septic organ injury, but no interventions exist to interrupt or reverse this process. microRNA-223 (miR-223) is known to be involved in both inflammatory gene regulation and host-pathogen interactions key to the pathogenesis of sepsis. The goal of this study was to determine the role of miR-223 as a mediator of septic kidney injury. Using miR-223 knockout mice and multiple models of experimental sepsis, we found that miR-223 differentially influences acute kidney injury (AKI) based on the model used. In the absence of miR-223, mice demonstrated exaggerated AKI in sterile models of sepsis (LPS injection) and attenuated AKI in a live-infection model of sepsis (cecal ligation and puncture). We demonstrated that miR-223 expression is induced in kidney homogenate after cecal ligation and puncture, but not after LPS or fecal slurry injection. We investigated additional potential mechanistic explanations including differences in peritoneal bacterial clearance and host stool virulence. Our findings highlight the complex role of miR-223 in the pathogenesis of septic kidney injury, as well as the importance of differences in experimental sepsis models and their consequent translational applicability.
Keywords: sepsis, acute kidney injury, microRNA-223
sepsis is a clinical syndrome of acute infection and organ injury that causes substantial morbidity and mortality. Large epidemiological studies estimate that ~750,000 hospitalizations for sepsis occur yearly in the United States, with a 20–30% in-hospital mortality rate (2, 16). It is well established that organ failure during sepsis leads to worsened outcomes (15), the kidneys are the most commonly affected organ system, and that the development of septic kidney injury is an independent predictor of mortality (17, 18, 21). Despite extensive research, no therapies attempting to interrupt or reverse organ failure have proven to be successful in humans (1, 5). To make impactful changes to the clinical management of sepsis, the pathogenesis of septic organ injury needs to be more completely understood for the development of therapeutic strategies.
Given its role in both host-pathogen interactions and inflammatory responses, microRNA-223 (miR-223) is a promising potential mediator of septic kidney injury. microRNAs are small RNA molecules involved in posttranscriptional regulation of gene expression by selectively inhibiting or degrading target mRNA. miR-223 is a highly conserved microRNA induced via Toll-Like Receptor (TLR) stimulation during infection and thought to decrease proinflammatory cytokine expression (4). It also plays a role in host-pathogen interactions as evidenced by neutrophils from miR-223 knockout mice demonstrating increased oxidative burst and Candida killing compared with neutrophils from wild-type mice (11). Multiple cross-sectional human studies have investigated miR-223 as a biomarker for sepsis, further highlighting its clinical relevance (7).
In this study, we sought to determine the role of miR-223 as a mediator of sepsis-induced kidney injury. Based on prior work showing elevated blood urea nitrogen (BUN) in miR-223 knockout mice with lipopolysaccharide injection (11), we hypothesized that the absence of miR-223 would similarly lead to exaggerated kidney injury in a live-infection model of sepsis. Contrary to our hypothesis, we now demonstrate that the role of miR-223 in the development of septic kidney injury is model specific.
MATERIALS AND METHODS
Animals
C57BL/6 mice.
We purchased male C57BL/6 mice from The Jackson Laboratory (Bar Harbor, ME). Animals were maintained in standard housing at the University of Colorado Anschutz Medical Campus. Male mice were used for experiments between the ages of 8 and 12 wk.
miR-223 knockout mice.
miR-223X-/Y mice (on a C57BL/6 background) were generously provided to us by Dr. Valina Dawson (Johns Hopkins Univ.). Male mice were used for experiments between the ages of 8 and 12 wk.
Animal protocols and care.
All animal protocols were submitted to, and approved by, the Institutional Animal Care and Use Committee of the University of Colorado. The National Institutes of Health guidelines for ethical treatment of animals were followed for standard care and all experimental protocols.
Septic AKI Models
Cecal ligation and puncture.
We performed cecal ligation and puncture (CLP) as previously described (20). Briefly, under isoflurane anesthesia, we exposed the cecum via laparatomy. We ligated 50% of the cecum with 2–0 sutures followed by through-and-through puncture with a 23-gauge needle. A small amount of stool was expressed from the cecum before return to the abdominal cavity and incision closure with 4–0 sutures. Sham surgery was identical to CLP with the exception of no ligation or puncture of the cecum. All animals received subcutaneous volume resuscitation (500 μl sterile saline) immediately after the procedure.
Intraperitoneal LPS injection.
We used body weight-based dosing of 2.5, 5, and 10 µg/g of LPS (cat. no. L2880, Sigma). The appropriate dose of LPS was delivered with 500 μl of sterile saline via the intraperitoneal route. Saline control animals received 500 μl of sterile saline intraperitoneal injection.
Fecal slurry intraperitoneal injection.
Dry fecal pellets were collected 1 day before experiments from animal cages. We suspended the pellets in sterile saline at either a dose of 180 mg/ml of saline or 90 mg/ml of saline and vortexed for 5 min. After storage overnight at 4°C, the sample was centrifuged for 5 min at 100 rpm, and the supernatant was removed for injection. At the time of injection, we also plated the slurry on trypticase soy agar plates and demonstrated growth of viable bacteria. We injected a dose of 0.4 ml via the intraperitoneal route to induce sepsis, as per a previously published protocol (9).
Methicillin-resistant Staphylococcus aureus intraperitoneal injection.
A single colony of USA300 strain methicillin-resistant Staphylococcus aureus (from frozen stock) was grown overnight in sterile Casein, Citrate, Yeast (CCY) (8) broth at 37°C with agitation. The culture was then diluted 1:100 in sterile CCY broth and grown to an optical density of 0.3–0.35 ( = 1 × 109 CFU/ml). Samples were diluted to the appropriate concentration and injected in 200 μl of sterile PBS as described previously (19). PBS control animals received 200 μl of sterile PBS via intraperitoneal injection.
Plasma Assays
Blood urea nitrogen (BUN).
We measured plasma BUN using the BioAssay Systems Quantichrom Urea Assay Kit (cat. no. DIUR-500, Hayward, CA). Briefly, 5 μl of blank, standard, or sample were pipetted in duplicate into a 96-well plate. Two-hundred microliters of working reagent were added to each well and incubated for 20 min. We read the optical density at 520 nm.
Kidney Homogenate Assays
miR-223 quantification.
We isolated microRNA from frozen kidney samples by adding 700 μl of Qiazol (cat. no. 79306, Qiagen) lysis reagent to the tissue followed by isolation using the RNEasy Kit (cat. no. 74106, Qiagen). We quantified miR-223 using qRT-PCR with primers from Qiagen for miR-223 (cat. no. MS00032592) and housekeeping gene RNU-6 (cat. no. MS00033740).
mRNA analysis.
We isolated microRNA from frozen kidney samples by adding 700 μl of Qiazol (cat. no. 79306, Qiagen) lysis reagent to the tissue followed by isolation using the RNEasy Kit (cat. no. 74106, Qiagen). We quantified our kidney injury markers using qRT-PCR with primers from Qiagen for IL-6 (cat. no. QT00098875), KIM-1 (cat. no. QT02378467), NGAL (cat. no. QT00113407), and housekeeping gene actin (cat. no. QT00095242).
Peritoneal Bacterial Clearance
Twenty-four hours after surgery (CLP or sham), we injected 5 ml of sterile saline via the intraperitoneal route under anesthesia. The lavage fluid was aspirated using an additional injection site to ensure mixing of the peritoneal contents. Fifty microliters of peritoneal lavage fluid were serially diluted by a factor of 10 in sterile saline. We plated 5 μl of each dilution on a trypticase soy agar plate. The plate was incubated for 18–24 h at 37°C. Colonies were counted within the 10- to-100 range, and colony-forming units were calculated based on the dilution used.
Statistical Analysis
Kidney injury, peritoneal bacterial growth, and miR-223 expression were represented in scatterplots with horizontal lines representing the mean of each group. Nonnormal data were log transformed before analysis. Differences are noted as statistically significant if P < 0.05 using Student’s two-tailed t-test. Significant differences between groups of interest were confirmed using Tukey’s post hoc method for multiple comparisons. All calculations were performed using GraphPad Prism version 7.0 (La Jolla, CA).
RESULTS
Impact of miR-223 on Lipopolysaccharide-Induced Acute Kidney Injury
Consistent with previous reports (11), miR-223 knockout mice experience worsened kidney injury compared with wild-type mice 24 h after intraperitoneal LPS injection. We characterized the degree of kidney injury by measuring plasma BUN as a marker of glomerular filtration rate (Fig. 1A). Additionally, we quantified tubular injury via mRNA analysis of kidney homogenate for neutrophil gelatinase associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) (Fig. 1, B and C). Last, we used kidney homogenate mRNA quantification of multiple inflammatory markers [interleukin-6 (IL-6), interleukin-1B (IL-1Β), tumor necrosis factor-α (TNFα), and the chemokine CXCL1] as surrogates for degree of inflammation. The IL-6 data are shown in Fig. 1D, whereas no significant differences were found between wild-type LPS and miR-223 knockout LPS groups in the other three inflammatory markers: IL-1Β (WT LPS fold change: mean = 1.81, SE = 0.33; KO LPS fold change: mean = 1.10, SE = 0.14; P = 0.051); TNFα (WT LPS fold change: mean = 2.03, SE = 0.69; KO LPS fold change: mean = 1.49, SE = 0.51; P = 0.53); and CXCL1 (WT LPS fold change: mean = 175.28, SE = 93.45; KO LPS fold change: mean = 233.99, SE = 131.83; P = 0.72). Saline control mice exhibited no kidney injury.
Fig. 1.
Kidney injury is exaggerated in miR-223X-/Y mice after intraperitoneal LPS. A: plasma blood urea nitrogen (BUN). B: kidney homogenate neutrophil gelatinase-associated lipocalin (NGAL) quantified by RT-PCR. C: kidney homogenate kidney injury molecule-1 (KIM-1) quantified by RT-PCR. D: kidney homogenate interleukin-6 (IL-6) quantified by RT-PCR. *P < 0.05 by Student’s 2-tailed t-test, ANOVA. n > 3 replicates per group.
Impact of miR-223 on CLP-Induced Acute Kidney Injury
In contrast to the LPS model, miR-223 knockout mice were protected from kidney injury compared with wild-type 24 h after CLP. Indexes of GFR (BUN, Fig. 2A), tubular injury (NGAL/KIM-1, Fig. 2, B and C), and inflammation (IL-6, Fig. 2D) were reduced in the miR-223 knockout mice vs. wild type. Similar to the LPS experiments, there were no significant differences found between wild-type CLP and miR-223 knockout CLP in the other three inflammatory markers: IL-1Β (WT CLP fold change: mean = 2.78, SE = 0.47; KO CLP fold change: mean = 4.59, SE = 1.69; P = 0.33), TNFα (WT CLP fold change: mean = 2.48, SE = 0.95; KO CLP fold change: mean = 5.86, SE = 3.40; P = 0.40), and CXCL1 (WT CLP fold change: mean = 45.06, SE = 28.66; KO CLP fold change: mean = 50.12, SE = 25.62; P = 0.90). Sham control mice exhibited no kidney injury.
Fig. 2.
Kidney injury is attenuated in miR-223X-/Y mice after CLP. A: plasma BUN. B: kidney homogenate NGAL quantified by RT-PCR. C: kidney homogenate KIM-1 quantified by RT-PCR. D: kidney homogenate IL-6 quantified by RT-PCR. *P < 0.05 by Student’s 2-tailed t-test, ANOVA. n > 3 replicates per group.
Peritoneal Bacterial Clearance in miR-223 Knockout or Wild-Type Mice
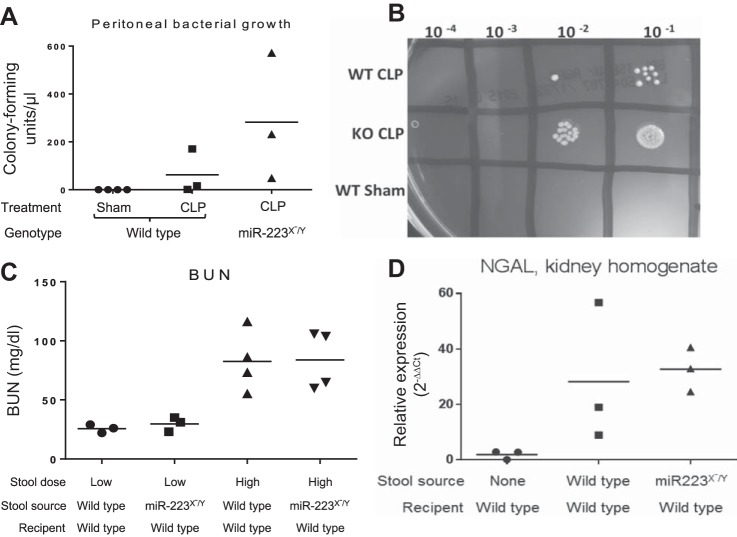
Given the known differences in oxidative burst, reactive oxygen species production, and Candida killing in miR-223 knockout mice (11), we sought to determine if the surprisingly divergent kidney outcomes between LPS injection and CLP were due to enhanced bacterial killing in the miR-223 knockout mice. Twenty-four hours after CLP or sham surgery, peritoneal lavage fluid was diluted and plated for quantification of bacterial growth. There was no peritoneal bacterial growth after sham surgery and no significant difference between wild-type and miR-223 knockout growth after CLP (Fig. 3A). There was a trend toward higher peritoneal bacterial colony counts in the miR-223 knockout mice as demonstrated by a representative plate (Fig. 3B). These data suggest that the attenuated kidney injury after CLP in miR-223 knockout mice is not due to enhanced bacterial killing in this genotype.
Fig. 3.
Bacterial growth and virulence are not impacted by miR-223. A: peritoneal bacterial growth quantified by peritoneal lavage fluid culture 24 h after CLP. B: representative trypticase soy agar plate demonstrating quantification of peritoneal lavage fluid. C: plasma BUN. D: kidney homogenate NGAL quantified by RT-PCR. n > 3 replicates per group.
Determination of Relative Stool Virulence of miR-223 Knockout and Wild-Type Mice
The experimental sepsis model of CLP causes peritonitis by exposing the animal to its own stool. As previous reports have noted alterations in the stool microbiome of knockout mice lacking key inflammatory regulators, it is possible that differences in CLP severity may not reflect altered host responses but rather differences in stool virulence. We therefore sought to determine if the improved outcomes of CLP-exposed miR-223 mice reflected less-virulent stool in comparison to wild-type mice. We made stool slurry from either wild-type mice or miR-223 knockout mice, and injected stool slurry from both genotypes into wild-type mice. Using both “high”- and “low”-dose stool slurry, we saw no difference in kidney injury outcomes between stool slurry types, as measured by BUN (Fig. 3C) and NGAL (Fig. 3D).
Impact of miR-223 on Intraperitoneal MRSA
The experimental sepsis models of LPS, CLP, and fecal slurry all model infection originating from stool organisms (mostly gram negative and anaerobic organisms). To determine if the role of miR-223 is bacterial type specific, we developed a gram positive (MRSA) model of peritonitis. However, we found that the experimental window of MRSA peritonitis as an inducer of septic AKI is narrow. Intraperitoneal injection of MRSA (107 CFU) into wild-type mice caused 0% mortality at 24 h, but induced no kidney injury. Higher doses led to rapid, complete mortality before the 24 h time point (Fig. 4A). Although this narrow experimental window limits the value of intraperitoneal MRSA as a model of septic kidney injury, this technique allows for determination if miR223X-/Y mice demonstrate increased renal susceptibility to an otherwise subinjurious intraperitoneal gram positive infection, similar to the increased renal susceptibility of these mice to intraperitoneal LPS. We observed that intraperitoneal injection of 107 CFU of MRSA into miR-223 knockout mice did not increase mortality or kidney injury (as measured by BUN) at 24 h (Fig. 4B), contrasting with the increased renal susceptibility of these mice to LPS.
Fig. 4.

miR-223X-/Y mice do not demonstrate increased renal susceptibility to intraperitoneal MRSA. A: 24-h mortality in wild-type mice after intraperitoneal injection of various doses of MRSA. B: plasma BUN after intraperitoneal injection of 107 CFU of MRSA. *P < 0.05 by Student’s 2-tailed t-test, ANOVA. n > 3 replicates per group.
Kidney miR-223 Expression in Different Models of Septic Kidney Injury
Twenty-four hour after onset of experimental sepsis, which is coincident with kidney injury in all three models, there is a difference in renal miR-223 induction based on the model used. Three different weight-based doses of LPS and fecal slurry show no increase in renal miR-223 compared with saline control (Fig. 5, A and C). In contrast to this finding, kidney homogenates from wild-type mice receiving CLP demonstrate approximately a fivefold increase in miR-223 expression (Fig. 5B). As expected, miR-223 knockout mice have no kidney miR-223 expression (Fig. 5D).
Fig. 5.
miR-223 expression differs based on injury model. Kidney homogenate miR-223 expression quantified by RT-PCR in multiple models of experimental sepsis: intraperitoneal LPS injection (A), CLP (B), and intraperitoneal fecal slurry injection (C). D demonstrates the absence of miR-223 in miR-223X-/Y mouse kidney homogenate. *P < 0.05 by Student’s 2-tailed t-test, ANOVA. n > 3 replicates per group.
DISCUSSION
Our data demonstrate that miR-223 has a dramatically different impact on septic kidney injury severity based on the experimental model used. To our knowledge, this study demonstrates novel divergent renal end points dependent on the choice of sepsis model. Although often used interchangeably in the literature, live-infection sepsis models (i.e., CLP) and models which stimulate TLRs in a sterile fashion (i.e., LPS) are fundamentally different. Although still an experimental murine model, the polymicrobial nature and crescendo onset of CLP strongly resembles human sepsis, leading to the widespread acceptance of this model as the gold standard for experimental sepsis (6, 10). Operator variability with the CLP procedure is recognized as a limitation of this model. LPS injection is extremely reproducible, but differs from human sepsis with regard to its abrupt onset and sterile nature (12). The third model of sepsis we employed, fecal slurry injection, shares certain strengths and weaknesses of CLP and LPS: although fecal slurry is a reproducible, live infection model, its single-dose bolus fails to capture the progressive infection characteristic of human sepsis. Last, the MRSA injection model further highlights the differential impact of miR-223 on different types of infection-induced kidney injury. It is a bolus, reproducible, live infection model of sepsis, but in contrast to CLP and fecal slurry, is not polymicrobial. Interestingly, unlike the other models, we were unable to find a dose of MRSA that induced kidney injury at 24 h without causing mortality.
In addition to model-specific differences, there is also substantial nuance to the quantification of kidney injury during experimental sepsis. Our group has previously extensively studied plasma, urine, and histology changes associated with septic kidney injury in multiple experimental models (3, 14). Similar to others’ findings in animal and human studies (13, 22), septic kidney injury often occurs without consistent evidence of histological changes or proteinuria early after the infectious insult. We have found that sepsis-induced changes in BUN occur before global loss of renal blood flow, suggesting that this marker is not simply a measure of a “pre-renal” state, but rather reflects glomerular microvascular dysfunction. We have previously confirmed the validity of BUN as a measure of glomerular filtration rate by inulin clearance (3, 14). For the purposes of this manuscript, we also sought to quantify validated markers of tubular injury (NGAL, KIM-1) and inflammation (IL-6, IL-1Β, TNFα, and CXCL1) for a more complete characterization of the role of miR-223 in septic AKI. We concluded that the inflammatory response in these experimental models was predominantly driven by IL-6.
The history of sepsis research is full of promising preclinical mechanistic interventions that have not translated into successful clinical therapies (1, 5). Part of the explanation for this disconnect is the considerable medical complexity and heterogeneity inherent to clinical research in critically ill patients. But, in addition to these factors, our data suggest that the experimental sepsis model used plays a large role in the clinical applicability of the findings. Understanding the subtleties of various sepsis models may improve our ability to translate successful basic science interventions into real-world clinical impact.
From this work, we conclude that renal miR-223 induction is coincident with, and contributes to, kidney injury during physiological sepsis (CLP). Genetically miR-223-deficient mice are protected from septic kidney injury in this model. Sterile models of sepsis such as LPS injection do not induce renal expression of miR-223 and in this case the absence of miR-223 is harmful. We investigated multiple potential explanations for this contrast including differences in bacterial killing/clearance and stool virulence without a definite answer. The mechanism by which renal miR-223 expression contributes to septic kidney injury remains unclear. It is possible that the renal miR-223 quantified in this study is simply a marker of upstream host-pathogen interactions either at the local (renal) level or being shuttled by host innate immune cells.
Our study is limited by the inability to parse out the time course of miR-223-related effects. The miR-223 knockout mice used in these experiments have been genetically without this particular microRNA the entirety of their lives. This strain’s protection from kidney injury with CLP and exaggerated kidney injury with LPS could be due to either short-term changes in host response during acute infection, or long-term differences due to maturation without miR-223. Potential promising clinical interventions could be developed if due to changes during acute infection, but differences due to long-term absence of miR-223 are unlikely to be clinically relevant. Future studies including the use of a miR-223 inhibitor in wild-type mice or bone marrow chimera experiments between the two genotypes could further delineate this issue.
Conclusion
The role of miR-223 in the development and severity of septic kidney injury is model specific. The absence of miR-223 leads to attenuated kidney injury after CLP and exaggerated kidney injury after LPS injection. Our data highlight an important and fundamental difference between models of experimental sepsis, which has implications for future translational sepsis research.
GRANTS
This study was supported by National Institute of Healths (NIH) Grant T32-AG-000279 and John A. Hartford Center of Excellence Pilot Grant to J. F. Colbert; NIH Grants R01-DK-097075, R01-HL-092188, R01-HL-098294, POI-HL-114457, and R01-HL-119837 to H. K. Eltzschig; and NIH Grant R01-HL-125371 to E. P. Schmidt.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.F.C., E.P.S., and A.A.G. conceived and designed research; J.F.C., J.A.F., S.M.H., Y.Y., K.L.D., K.C.A., V.L.R., and K.B. performed experiments; J.F.C., K.B., E.P.S., and A.A.G. analyzed data; J.F.C., J.A.F., S.M.H., Y.Y., K.L.D., K.C.A., V.N., C.M.E., V.L.R., K.B., S.F., H.K.E., E.P.S., and A.A.G. interpreted results of experiments; J.F.C. and E.P.S. prepared figures; J.F.C. drafted manuscript; J.F.C., J.A.F., S.M.H., Y.Y., K.L.D., K.C.A., V.N., C.M.E., V.L.R., K.B., S.F., H.K.E., E.P.S., and A.A.G. edited and revised manuscript; J.F.C., J.A.F., S.M.H., Y.Y., K.L.D., K.C.A., V.N., C.M.E., V.L.R., K.B., S.F., H.K.E., E.P.S., and A.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Valina Dawson (Johns Hopkins University) for providing the miR-223 knockout mice necessary for these experiments.
Present address of K. C. Allison: Dept. of Pediatrics, Univ. of Colorado, Aurora, Colorado.
Present address of K. S. Brodsky: Dept. of Medicine, Univ. of Colorado, Aurora, Colorado.
Present address of H. K. Eltzschig: Dept. of Anesthesiology, Univ. of Texas Health Sciences Center at Houston, McGovern Medical School, Houston, Texas.
REFERENCES
- 1.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA 306: 2614–2615, 2011. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bhargava R, Altmann CJ, Andres-Hernando A, Webb RG, Okamura K, Yang Y, Falk S, Schmidt EP, Faubel S. Acute lung injury and acute kidney injury are established by four hours in experimental sepsis and are improved with pre, but not post, sepsis administration of TNF-α antibodies. PLoS One 8: e79037, 2013. doi: 10.1371/journal.pone.0079037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q, Wang H, Liu Y, Song Y, Lai L, Han Q, Cao X, Wang Q. Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1β production in macrophages by targeting STAT3. PLoS One 7: e42971, 2012. doi: 10.1371/journal.pone.0042971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen J, Opal S, Calandra T. Sepsis studies need new direction. Lancet Infect Dis 12: 503–505, 2012. doi: 10.1016/S1473-3099(12)70136-6. [DOI] [PubMed] [Google Scholar]
- 6.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol 19: 198–208, 2011. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Essandoh K, Fan GC. Role of extracellular and intracellular microRNAs in sepsis. Biochim Biophys Acta 1842: 2155–2162, 2014. doi: 10.1016/j.bbadis.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finck-Barbançon V, Prévost G, Piémont Y. Improved purification of leukocidin from Staphylococcus aureus and toxin distribution among hospital strains. Res Microbiol 142: 75–85, 1991. doi: 10.1016/0923-2508(91)90099-V. [DOI] [PubMed] [Google Scholar]
- 9.Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Ward KR, Voelkel NF, Fowler AA III, Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am J Physiol Lung Cell Mol Physiol 303: L20–L32, 2012. doi: 10.1152/ajplung.00300.2011. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW III, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock 24, Suppl 1: 52–57, 2005. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 11.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451: 1125–1129, 2008. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- 12.Kingsley SM, Bhat BV. Differential paradigms in animal models of sepsis. Curr Infect Dis Rep 18: 26, 2016. doi: 10.1007/s11908-016-0535-8. [DOI] [PubMed] [Google Scholar]
- 13.Langenberg C, Bagshaw SM, May CN, Bellomo R. The histopathology of septic acute kidney injury: a systematic review. Crit Care 12: R38, 2008. doi: 10.1186/cc6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lygizos MI, Yang Y, Altmann CJ, Okamura K, Hernando AA, Perez MJ, Smith LP, Koyanagi DE, Gandjeva A, Bhargava R, Tuder RM, Faubel S, Schmidt EP. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol Rep 1: e00153, 2013. doi: 10.1002/phy2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackenzie I, Lever A. Management of sepsis. BMJ 335: 929–932, 2007. doi: 10.1136/bmj.39346.696620.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554, 2003. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 17.Oppert M, Engel C, Brunkhorst FM, Bogatsch H, Reinhart K, Frei U, Eckardt KU, Loeffler M, John S; German Competence Network Sepsis (Sepnet) . Acute renal failure in patients with severe sepsis and septic shock--a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrol Dial Transplant 23: 904–909, 2008. doi: 10.1093/ndt/gfm610. [DOI] [PubMed] [Google Scholar]
- 18.Plataki M, Kashani K, Cabello-Garza J, Maldonado F, Kashyap R, Kor DJ, Gajic O, Cartin-Ceba R. Predictors of acute kidney injury in septic shock patients: an observational cohort study. Clin J Am Soc Nephrol 6: 1744–1751, 2011. doi: 10.2215/CJN.05480610. [DOI] [PubMed] [Google Scholar]
- 19.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature 505: 412–416, 2014. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, Zemans RL, Bowman JC, Koyanagi DE, Yunt ZX, Smith LP, Cheng SS, Overdier KH, Thompson KR, Geraci MW, Douglas IS, Pearse DB, Tuder RM. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 18: 1217–1223, 2012. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810, 2016. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187: 509–517, 2013. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]