In the present study, we demonstrate that left coronary artery diversity in mice is one of the primary causes of variable myocardial infarction size and cardiac functional parameters in the left coronary artery ligation model. Recognition of anatomic diversity is essential to improve reliability and reproducibility in heart failure research.
Keywords: coronary artery, myocardial infarction, diversity, anatomy, ejection fraction
Abstract
Low reliability and reproducibility in heart failure models are well established. The purpose of the present study is to explore factors that affect model consistency of myocardial infarction (MI) in mice. MI was induced by left coronary artery (LCA) ligation. The coronary artery was casted with resin and visualized with fluorescent imaging ex vivo. LCA characteristics and MI size were analyzed individually in each animal, and MI size was correlated with left ventricular (LV) function by echocardiography. Coronary anatomy varies widely in mice, posing challenges for surgical ligation and resulting in inconsistent MI size postligation. The length of coronary arterial trunk, level of bifurcation, number of branches, and territory supplied by these branches are unique in each animal. When the main LCA trunk is ligated, this results in a large MI, but when a single branch is ligated, MI size is variable due to differing levels of LCA ligation and area supplied by the branches. During the ligation procedure, nearly 40% of LCAs are not grossly visible to the surgeon. In these situations, the surgeon blindly sutures a wider and deeper area of tissue in an attempt to catch the LCA. Paradoxically, these situations have greater odds of resulting in smaller MIs. In conclusion, variation in MI size and LV function after LCA ligation in mice is difficult to avoid. Anatomic diversity of the LCA in mice leads to inconsistency in MI size and functional parameters, and this is independent of potential technical modifications made by the operator.
NEW & NOTEWORTHY In the present study, we demonstrate that left coronary artery diversity in mice is one of the primary causes of variable myocardial infarction size and cardiac functional parameters in the left coronary artery ligation model. Recognition of anatomic diversity is essential to improve reliability and reproducibility in heart failure research.
heart failure (HF) remains a major challenge in both research and clinical practice with regards to pathophysiology and treatment (2, 14). Animal models are critical to understanding HF and developing new treatments (12). Mice are the most widely used animals in biomedical research, with myocardial infarction (MI) being the most extensively used model for HF development (21). Echocardiography and hemodynamic pressure-volume loops are routine methods that provide essential confirmation of HF in murine models (15, 16, 20). Most studies that examine molecular mechanisms or potential treatments for HF assess left ventricular (LV) function through these methods.
Reliability and reproducibility in biomedical research have been an increasing concern in recent years (13, 18). The predictivity of animal models of human disease is a crucial issue to address if reproducibility rates in laboratory experiments and clinical trials are to be improved (24). In a previous article (5), we showed that there was inherent variability in functional cardiac parameters after induction of HF by MI in mice. Indicators of cardiac structure and function, such as LV ejection fraction (EF), fractional shortening (FS), contractility (±dP/dt), and MI size, are impacted by the specific pathophysiological condition induced and experience of the researcher in data acquisition and analysis (3, 25). Pathological changes in the coronary artery during HF have been well studied by our laboratory and others (6, 8, 17). However, the physiological reason for variability in cardiac function after MI induction is unknown (5). This variation could easily be attributed to individual diversity, a normal distribution in statistical terms. We also acknowledge that this functional data inconsistency may be due to the variation in size of MI induced by left coronary artery (LCA) ligation (4), but the reason for this is unknown.
In the present report, we studied the association among anatomic variation in the LCA, inconsistency in MI size, and cardiac function in mice. Here, we offer a descriptive experience to overcome one of major obstacles in creating a consistent HF model by LCA ligation in mice.
METHODS
All procedures were performed according to the recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 78-23, 1996) and were approved by the Icahn School of Medicine at Mount Sinai Animal Care and Use Committee.
Animal protocol.
C57BL6J male mice were used, ranging in age from 10 to 12 wk and in weight from 25 to 30 g. Mice underwent LCA ligation to induce MI as previously described (5, 7). Briefly, animals were anesthetized intraperitoneally with 0.06 ml mixture of ketamine (1 ml × 100 mg/ml), acepromazine (0.1 ml × 10 mg/ml), and xylazine (0.1 ml × 20 mg/ml) and 0.9% normal saline (1 ml) per mouse. After thoracotomy, ligation of the LCA was performed with a 7-0 silk suture. The successful performance of LCA ligation was verified by visual inspection of the apex color. The chest was closed with 6-0 silk suture, and the skin was closed with 4-0 silk sutures. Postsurgical monitoring was continued until the animal became conscious in warm cage (37°C, with O2). Antibiotics were not given, but no apparent infection developed in surviving animals during the course of the study or at the time of autopsy. All mice were housed under identical conditions and were given water and food ad libitum.
Serial echocardiography.
Echocardiograms were obtained 1 mo after MI. Sham-operated (sham) control mice were investigated at same time. Animals were sedated by intraperitoneal injection of ketamine (40 mg/kg). Echocardiograms were performed with a GE Healthcare Echocardiography VIVID 7 system equipped with i13L-14-MHz probe. EF data were obtained using M-mode and two-dimensional (2-D) mode echocardiography following the vendor’s instructions as previously described (5). From LV parasternal 2-D long-axis view, LV end-diastolic and LV end-systolic volumes were derived from the area-length method using a prolate-ellipsoid formula (volume = 8 × [A2/(L × 3 × π)], where A is LV area and L is LV length, Simpson’s method). MI size (in %) was acquired by measuring the LV apical infarct area divided by the LV area.
Coronary artery resin casting.
After all other methods were completed, hearts were perfused with resin polymer using Batson’s no. 17 Plastic Replica and Corrosion Kit (catalog no. 07349, Polysciences, www.polysciences.com) as previously described (6). Proper fluorescent dye (www.blacklightworld.com) was mixed with red pigment (1:4 in weight) and added into the polymer casting mixture. Briefly, while mice were under ketamine anesthesia (90 mg/kg ip), the chest was opened, the right atrium was cut, and red latex (8 ml) was infused into the LV after blood was flushed out with 10 ml PBS (pH 7.4, room temperature) containing heparin (0.03 ml, 1.000 USP U/ml) with a 20-gauge catheter. Fifteen minutes later, the heart was removed, washed in PBS, and photographed.
Fluorescent imaging and MI measurement.
After the resin casting, the coronary arteries were imaged under fluorescent microscope. Hearts were then cut from the root of the pulmonary artery to the ventricular apex and photographed. MI size (%area) was the average of the infarct area of the left half and right half of the LV only and quantified using Image-Pro software (Media Cybernetics, Bethesda, MD) (4).
Visualization of the LCA with Evans blue injection.
Before LCA ligation, 100 µl of 1% Evans blue in PBS was injected into the LV. In vivo video was conducted to visualize the LCA.
Statistics.
Variables are expressed as means ± SD. One-way ANOVA and Student’s t-test were performed to compare experimental groups using GraphPad Prism software. P < 0.05 was considered statistically significant.
RESULTS
Deviation in MI size and association with cardiac function.
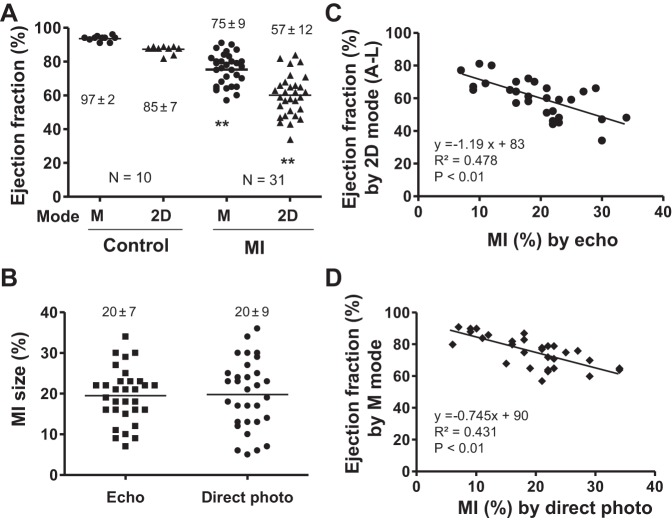
In control animals, EF by echocardiography had a very small SD. However, post-MI, the SD was notably higher while the average EF was significantly decreased, both in M mode and 2-D mode (Fig. 1A). MI size also had a wide range when measured by both echocardiography and direct photo of an opened heart (Fig. 1B). Cardiac function inversely correlated with MI size, but even among animals with similarly sized MIs, there was still a large deviation in EF in both M and 2-D mode, as demonstrated by the midrange R values of 0.4–0.5 (Fig. 1, C and D).
Fig. 1.
Deviation in myocardial infarction (MI) size and cardiac function by echocardiography. A: ejection fraction (EF) as measured by echocardiography [M mode or 2-dimensional (2-D) mode] in control and MI mice. Data are means ± SD. **P < 0.01 compared with control. B: MI size as measured by echocardiography or direct photo. C and D: EF (measured by 2-D or M mode) was reversely correlated with MI size as measured by echo or direct photo.
Variability in LCAs in control mice.
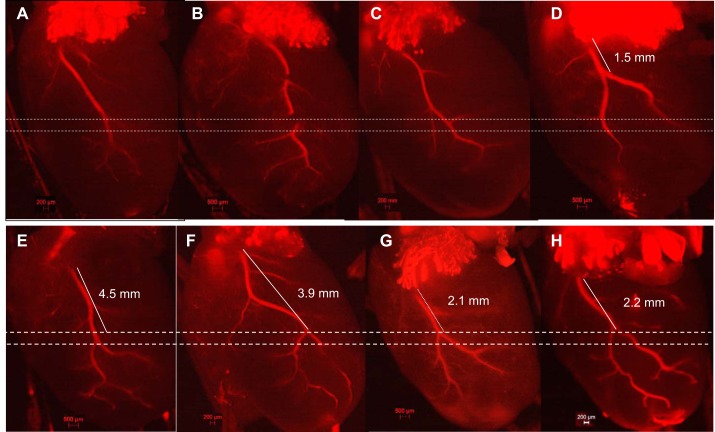
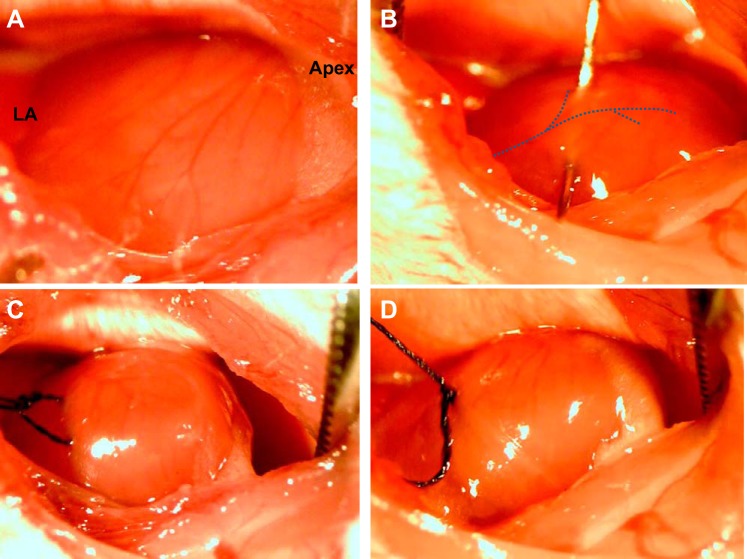
Every coronary artery is unique: the LCA courses toward the apex of the heart from the border of the left atrium, has one main trunk (2–3 mm in length), and then divides into two or three branches. The length and path of the main trunk and location of its branches form a unique pattern that makes every LCA different. It is not only the level of LCA blockage that impacts MI size but also the exact location of ischemia within the three-dimensional heart (Fig. 2). When performing LCA ligation surgery, the LCA in mice is often invisible under the microscope (Fig. 3A). The LCA was assumed to course from the border of the left atrium/LV to the apex of the heart. The level, width, and depth of suture ligation have a very prominent effect on MI size (Fig. 3B). The LV apex becomes visibly pale if LAD ligation is successful (Fig. 3, C and D). However, the assessment based on apical pallor does not guarantee proper establishment of MI as further measured by echo and direct photo.
Fig. 2.
Diversity of left coronary arteries (LCAs) in mice. A–C: major singular trunk of the LCA. D: two bifurcations divided early (1.5 mm) from the left atrium (LA). E: two bifurcations divided later from the LA (4.5 mm). F–H: the arterial trunk length and branch style are apparently different from each other. Dashed lines indicate that 0.5 mm in the placement of ligation could result in a significant variation in ischemic area.
Fig. 3.
Illustration of the LCA ligation procedure. A: in many cases, LCAs are invisible in mice during the procedure. B: placement of needle and suture under the LCA. C: the ischemic area can be checked by slightly pulling on the suture. D: completion of the ligation of the LCA.
Diversity of LCAs and MIs.
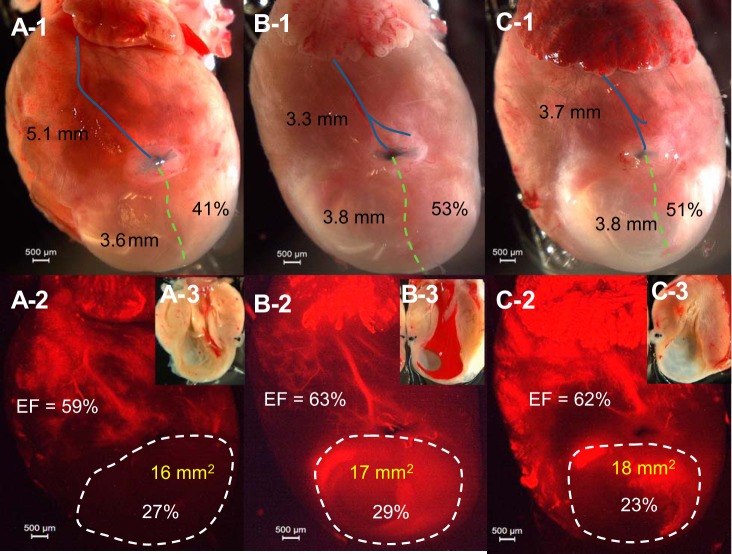
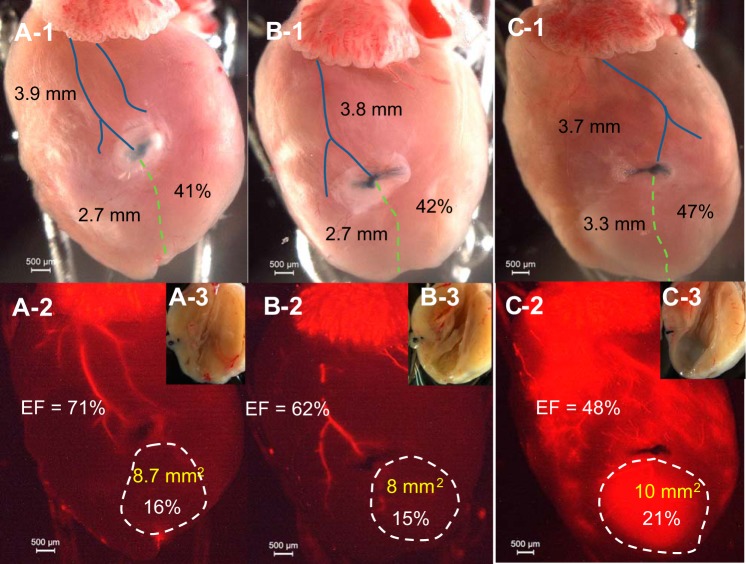
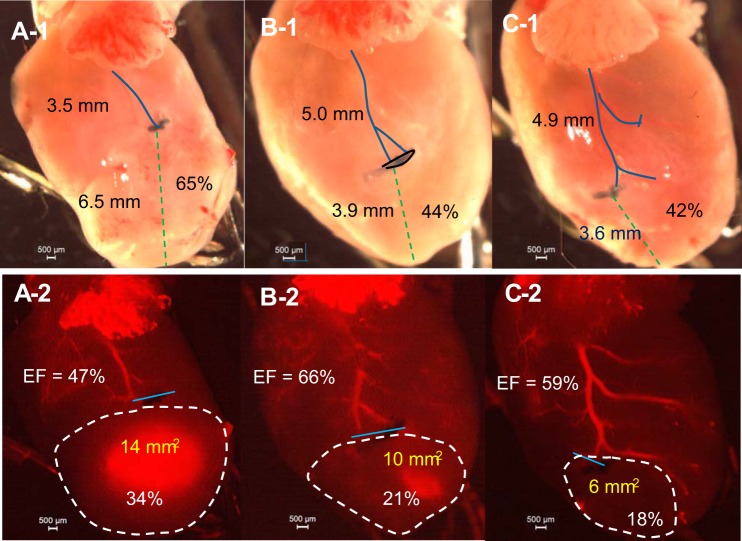
Contrasting regular digital and fluorescent imaging, LCA length, ligature level (in %), MI absolute value with percentage, and cardiac EF are shown simultaneously. Ligation at different levels of the LCA (41% ~53%) could result in a similar MI size and depressed EF (Fig. 4). Ligation at a similar level (3.7 ~3.9 mm from low edge of the left atria) could result in different sizes of MI and EF values (Fig. 5). The dominance of the ligated artery also plays a large role in MI formation. Ligation of the main trunk of the LCA results in a large MI (34%), whereas ligation of a secondary branch results in a moderate MI (21%). Ligation of a minor branch results in a much smaller MI (18%; Fig. 6). However, ligation of the right branch of the LCA usually has a bigger impact on LV function because its occlusion more easily leads to atrioventricular block (Fig. 6C,1 and 2).
Fig. 4.
Ligation on LCA main trunk results in a larger MI. A,1, B,1, and C,1: direct photos showing that ligature level is identical but corresponds to different percentages of length of the LCA (41–53%). The blue line shows the distance from ligation to atria (in mm), and the green dashed line shows the distance from ligation to the apex. (Percentage = distance from ligation to apex/total length of the LCA). A,2, B,2, and C,2: fluorescent imaging of the heart. The MI area is shown by white dashed line (area and percentage of heart shown) and EF (in %) of that heart is shown. EF was obtained by 2-D mode, and MI area was obtained by echo. A,3, B,3, and C,3: direct photos of MI in the left ventricle (LV). The same heart is shown in A,1–3 (same for B,1–3, and C,1–3).
Fig. 5.
Ligation on LCA branch results in a moderate MI. A,1, B,1, and C,1: direct photos showing that ligature level is similar and corresponds to similar percentages of length of the LCA (41–47%). The blue line shows the distance from ligation to the atria (and distance in mm), and the green dashed line shows the distance from ligation to the apex. (Percentage = distance from ligation to apex/total length of the LCA). A,2, B,2, and C,2: fluorescent imaging showing that MI size is moderate. The MI area is shown by the white dashed line (area and percentage of heart given) and EF (%) of that heart is shown. EF was obtained by 2-D mode, and MI area was obtained by echo. A,3, B,3, and C,3: direct photos of MI in the LV. The same heart is pictured in A,1–3 (same for B,1–3, and C,1–3).
Fig. 6.
MI variability due to dominance of the ligated artery. A,1 and A,2: ligation of the LCA trunk results larger MI and a greater reduction in EF. B,1 and B,2: ligation of two branches of the LCA. C,1 and C,2: ligation of the right LCA branch results in small MI but lower EF. A,1, B,1, and C,1: hearts by direct photo. A,2, B,2, and C,2: fluorescent images of the same heart. EF was obtained by 2-D mode, and MI area was obtained by echo. The blue line in A,1, B,1, and C,1 shows the distance from ligation to atria (and distance in mm), and the green dashed line shows the distance from ligation to apex. (Percentage = distance from ligation to apex/total length of the LCA). The blue line in A,2, B,2, and C,2 is the placement of the ligature. The MI area is shown by the white dashed line (area and percentage of heart given) and EF (%) of that heart is shown. The same heart is pictured in A,1 and A,2 (same for B,1 and B,2 and for C,1 and C,2).
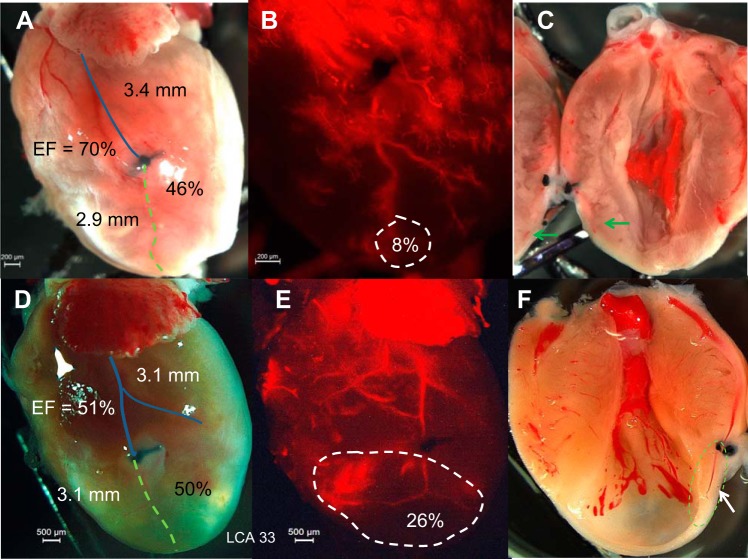
Residual arterial vessels in the ischemic area.
In most cases, the infarcted area had no residual arterial vessels postligation. The LCA could survive if the ligature was not complete, which results in a small MI or no MI at all (Fig. 7, A and B). In some cases, residual arterial vessels could be seen in the LV infarcted wall (Fig. 7, D−F), but microcapillaries were nearly absent compared with the noninfarcted zone (Fig. 7C). The residual arterial vessels in the infarcted wall were not associated with preserved LV function (Fig. 7F).
Fig. 7.
Residual arterial vessels in the infarct area. A: direct photo of the heart showing LCA ligation and a large ischemic area. B: fluorescent imaging of the same heart showing that the ligated artery could survive as the occlusion is not complete, which results in a small MI. C: confirmation by direct photo of the opened heart. Green arrows indicate the surviving residual artery. D: direct photo showing LCA ligation and MI. E and F: residual arterial vessels could be seen in the LV transmural infarction in fluorescent imaging and confirmed by direct photo of the opened heart as indicated by the green dashed circle. The blue line shows the distance from ligation to atria (and distance in mm), and the green dashed line shows the distance from ligation to the LA. (Percentage = distance from the ligation to apex/total length of the LCA). The MI area is shown by the white dashed circle (area and percentage of heart given) and EF (in %) of that heart is shown.
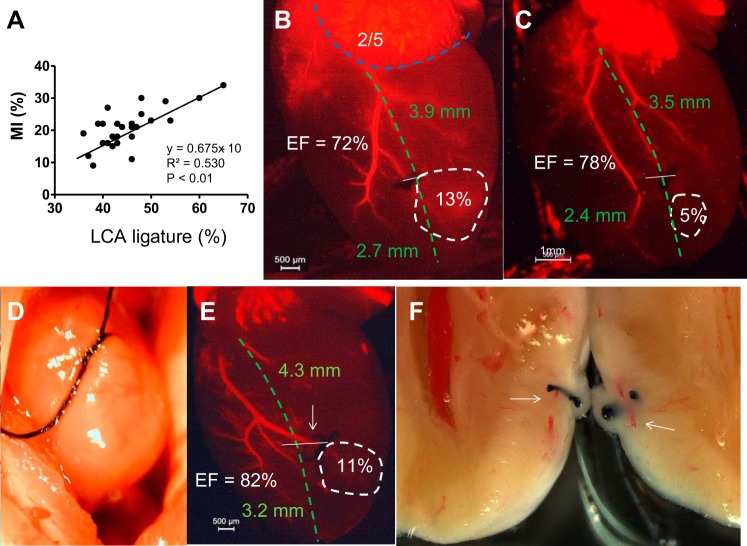
Primary causes of small MIs.
In general, higher ligature of the LCA (meaning further from the apex) leads to a larger MI, but (distance between apex and atria) mortality is very high, and lower than 50% (distance between apex and atria) MI size variation is remarkable (Fig. 8A). The main cause is that the LCA is not visible to the surgical operator under the microscope. The operator usually judges the path of the LCA to be from the anterior point (1/3 of the left atrial low edge) to the apex, roughly 3−4 mm (Fig. 8, B and C, green dashed line). Some LCA may deviate from this trend, which leads to smaller MIs (Fig. 8, B and C, white dashed line). In the cases of invisible LCA, the operator is likely to make a wider and deeper suture in the heart tissue to ensure the LCA is ligated (Fig. 8D). When ligated, the apex was pale, but later it was found that the occlusion was not complete and the MI was small (Fig. 8, E and F). In addition, excessive ligation can lead to cutting of the heart muscle, which induces severe bleeding and death.
Fig. 8.
The primary causes of small MIs. A: correlation of MI size (in %) and LCA ligature level from the apex (in %). B and C: fluorescent imaging showing ligation of the small LCA branch results in small MI. The blue dashed line indicates the length of the low edge of the LA. 2/5: proposed original point of the LCA. The white dashed line indicated MI size. D: example of when placement of the needle and suture is too wide and deep in the heart muscle. E: ligation of the LCA branch was not complete, as indicated by the arrow. F: the artery was not completely occluded and the ischemic myocardium survived. The white arrow indicates the surviving artery. EF was determined by 2-D mode echocardiography. The MI area (white dashed circle) was determined by echocardiography. In B, C, and E, the green dashed line represents the assumed LCA path. The white line represents the site of ligation.
DISCUSSION
In the present study, we explored how the diversity of LCAs affects MI size and LV EF in mice. The LCA architecture is not complicated but varies between individual mice (9). The LCA consists of a main trunk and two or three secondary branches. Technically speaking, LCA ligation is not very complicated: time to completion is generally ~30 min, and some surgeons can perform it in under 2 min (10). However, it is very difficult to obtain uniform MIs in mice, independent of the expertise of the surgical operator (5, 22, 23). An additional confounding factor is the variability that exists between the LCA architecture in different mouse strains (19, 26). This phenomenon is well known and has been attributed to SD. The question is if the SD is inevitable or if something can be done to reduce it.
In our study, 39% of LCAs (65 of 168 mice) were invisible to the surgeon under the surgical microscope. This percentage varies slightly between strains and male/female mice (6). Even when the LCA is visible, it is difficult to get uniform MIs because every arterial path and the level of the branches are different in each mouse. Ligation of the LCA trunk (Fig. 2, A–C) results in a relatively large and consistent MI (Fig. 5), but ligation performed at a slightly higher level (0.5 mm, <50% of LCA) often results in a larger MI and higher mortality. LCA bifurcations and branching occur at varying distances from the atria in mice, even within the same strain (Fig. 2, D and E). This variability results in a concomitant variation in MI size and cardiac function. In the hearts where the LCA branches near the midpoint between the left atrium and apex (Fig. 2, F–H), ligation above the point of bifurcation typically results in animal mortality; ligation after the bifurcation is ideal but technically challenging. Ligation of the right LCA branch can often induce arrhythmia (atrioventricular block and premature ventricular contractions) and death, whereas ligature of the left LCA branch often results in smaller MIs (Fig. 8, B and C). When the LCA is invisible, the surgeon typically assumes the path of LCA is from the front third of the left atrial edge to the ventricle apex, but 25% of LCAs do not follow this path (Fig. 8, B and E). If multiple branches of the LCA are closer to each other, occlusion of one branch usually results in a small MI because the ischemic area could receive blood supply from nearby arteries (Fig. 8C). Moreover, when the LCA was invisible, the operator tends to place the needle wider and deeper to capture the LCA, but this method often results in large MI variation. If the suture knot is too tight, the myocardium could be cut off and the animal will die due to severe bleeding. If the suture knot is not tied tightly enough, the residual LCA will survive, resulting in either a small or no MI (Fig. 8, D–F). The level of LCA ligation is critical: higher-level ligation (>50% of distance between atria and apex) usually results in a larger MI accompanied by higher mortality, whereas lower-level ligation (<40% of distance between atria and apex) usually results in a smaller MI, but even ligation at same level can result in different MI sizes between mice (Fig. 8A). To complicate matters further, the same MI size can result in variable cardiac function (Fig. 1, C and D), although this is more often observed with larger MIs. Indeed, there are factors beyond LCA diversity that can play a role in variable MI size and LV function, such as degree of ligation, type of anesthesia used, echo probe position, scar tissue on LV, myocardial inflammation, etc. Previously, we reported that the small vessels in the infarcted area 1 mo post-MI in rats were likely small veins (6). Based on current data, it is clear that the small vessels in the infarcted area are arteries (Fig. 5F). It is unclear as to why some arterial vessels remain in the infarcted area and if it is possible to regenerate capillaries from the residual vessels (Fig. 7, E and F).
There are studies that have examined the pattern of LCA branches by digestion of casted heart tissue (1, 17); however, these studies lack precise correlation between LCA branching and MI size. With fluorescent imaging, we were able to describe the association between LCA ligation and MI ex vivo. For future studies, a surgical microscope with near-infrared laser Doppler would improve identification LCA in vivo, which would also improve the consistency of the MI model. Real-time vascular imaging that provides both anatomic and hemodynamic information could greatly facilitate the judgment of LCA ligation and provide a more accurate assessment of the ischemic area (11). Higher animal numbers and larger data sets are required to obtain a more accurate correlation between cardiac histological and functional changes after LCA ligation. A number of approaches can be taken to decrease the variability when creating an MI. One would be to inject a visual agent such as Evans blue to opacify the LCA and obtain a better sense of the anatomy before ligation (see Supplemental Video S1 in the Supplemental Material; Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website). A second approach is to assess EF 1 mo post-MI and include in the studies only those that have EF < 40%. A guideline or standardization for the LCA ligation operation and diagnostic criteria to define HF by echocardiography and hemodynamics is necessary in research models involving rodent cardiac failure.
In summary, this report studied how the diversity of the LCA affects MI size and LV function in mice by resin casting and fluorescent imaging in situ. The LCA usually has one major trunk and descends into two to three branches. Ligation of the main arterial trunk results in larger MIs but comes with higher rates of mortality. Ligation at a low level of the LCA (<40% of distance between the atria and apex) or one of the branches often results in moderate or small MIs. MI variation is inevitable if the LCA is invisible. Apex color is not an accurate indicator of MI size because physiological and technical factors, such as the degree of ligature, blood pressure, and vascular ischemic tolerance, would impact the MI and LV function. However, variation in MI is still inevitable even if the LCA is visible in mice.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants P50-HL-112324, R01-HL-119046, R01-HL-117505, R01-HL-128099, R01-HL-129814, R01-HL-131404, and T32-HL-007824 (to R. J. Hajjar) and the Transatlantic Leducq Foundation (to R. J. Hajjar). D. K. Ceholski is supported by American Heart Association Fellowship 15POST25090116.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.C. conceived and designed research; J.C. and L.L. performed experiments; J.C., D.K.C., and L.L. analyzed data; J.C. and D.K.C. interpreted results of experiments; J.C. and D.K.C. prepared figures; J.C. and D.K.C. drafted manuscript; D.K.C., K.F., and R.J.H. edited and revised manuscript; R.J.H. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Ahn D, Cheng L, Moon C, Spurgeon H, Lakatta EG, Talan MI. Induction of myocardial infarcts of a predictable size and location by branch pattern probability-assisted coronary ligation in C57BL/6 mice. Am J Physiol Heart Circ Physiol 286: H1201–H1207, 2004. doi: 10.1152/ajpheart.00862.2003. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. Heart failure. JACC Heart Fail 1: 1–20, 2013. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Chemaly ER, Liang LF, LaRocca TJ, Yaniz-Galende E, Hajjar RJ. A new model of congestive heart failure in rats. Am J Physiol Heart Circ Physiol 301: H994–H1003, 2011. doi: 10.1152/ajpheart.00245.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Hammoudi N, Benard L, Ceholski DK, Zhang S, Lebeche D, Hajjar RJ. The probability of inconstancy in assessment of cardiac function post-myocardial infarction in mice. Cardiovasc Pharmacol Open Access 5: 195, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Petrov A, Yaniz-Galende E, Liang L, de Haas HJ, Narula J, Hajjar RJ. The impact of pressure overload on coronary vascular changes following myocardial infarction in rats. Am J Physiol Heart Circ Physiol 304: H719–H728, 2013. doi: 10.1152/ajpheart.00793.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation 111: 1800–1805, 2005. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Yaniz-Galende E, Kagan HJ, Liang L, Hekmaty S, Giannarelli C, Hajjar R. Abnormalities of capillary microarchitecture in a rat model of coronary ischemic congestive heart failure. Am J Physiol Heart Circ Physiol 308: H830–H840, 2015. doi: 10.1152/ajpheart.00583.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doevendans PA, Daemen MJ, de Muinck ED, Smits JF. Cardiovascular phenotyping in mice. Cardiovasc Res 39: 34–49, 1998. doi: 10.1016/S0008-6363(98)00073-X. [DOI] [PubMed] [Google Scholar]
- 10.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res 107: 1445–1453, 2010. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong G, Lee JC, Jha A, Diao S, Nakayama KH, Hou L, Doyle TC, Robinson JT, Antaris AL, Dai H, Cooke JP, Huang NF. Near-infrared II fluorescence for imaging hindlimb vessel regeneration with dynamic tissue perfusion measurement. Circ Cardiovasc Imaging 7: 517–525, 2014. doi: 10.1161/CIRCIMAGING.113.000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ; American Heart Association Council on Basic Cardiovascular Sciences, Council on Clinical Cardiology, and Council on Functional Genomics and Translational Biology . Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111: 131–150, 2012. doi: 10.1161/RES.0b013e3182582523. [DOI] [PubMed] [Google Scholar]
- 13.Ioannidis JP. Why most published research findings are false. PLoS Med 2: e124, 2005. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krum H, Abraham WT. Heart failure. Lancet 373: 941–955, 2009. doi: 10.1016/S0140-6736(09)60236-1. [DOI] [PubMed] [Google Scholar]
- 15.Leri A, Anversa P. Stem cells: bone-marrow-derived cells and heart failure--the debate goes on. Nat Rev Cardiol 10: 372–373, 2013. doi: 10.1038/nrcardio.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makarewich CA, Zhang H, Davis J, Correll RN, Trappanese DM, Hoffman NE, Troupes CD, Berretta RM, Kubo H, Madesh M, Chen X, Gao E, Molkentin JD, Houser SR. Transient receptor potential channels contribute to pathological structural and functional remodeling after myocardial infarction. Circ Res 115: 567–580, 2014. doi: 10.1161/CIRCRESAHA.115.303831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol 269: H2147–H2154, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Mobley A, Linder SK, Braeuer R, Ellis LM, Zwelling L. A survey on data reproducibility in cancer research provides insights into our limited ability to translate findings from the laboratory to the clinic. PLoS One 8: e63221, 2013. doi: 10.1371/journal.pone.0063221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthuramu I, Lox M, Jacobs F, De Geest B. Permanent ligation of the left anterior descending coronary artery in mice: a model of post-myocardial infarction remodelling and heart failure. J Vis Exp 2014: 52206, 2014. doi: 10.3791/52206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature 410: 701–705, 2001. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 21.Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail 2: 138–144, 2009. doi: 10.1161/CIRCHEARTFAILURE.108.839761. [DOI] [PubMed] [Google Scholar]
- 22.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172, 1990. doi: 10.1161/01.CIR.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 23.Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadaro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ Res 44: 503–512, 1979. doi: 10.1161/01.RES.44.4.503. [DOI] [PubMed] [Google Scholar]
- 24.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 10: 712, 2011. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- 25.Thomas JD, Popović ZB. Assessment of left ventricular function by cardiac ultrasound. J Am Coll Cardiol 48: 2012–2025, 2006. doi: 10.1016/j.jacc.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 26.Yoldas A, Ozmen E, Ozdemir V. Macroscopic description of the coronary arteries in Swiss albino mice (Mus musculus). J S Afr Vet Assoc 81: 247–252, 2010. doi: 10.4102/jsava.v81i4.156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.