We identified a novel, de novo PRKAG2 mutation (K475E) in the cystathionine β-synthase 3 repeat, a region critical for AMP binding but with no previous reported mutation. Our data suggest the mutation affects AMP-activated protein kinase activity, activates cell growth pathways, and results in cardiac hypertrophy, which can be reversed with rapamycin.
Keywords: AMP-activated protein kinase, cardiac hypertrophy, eukaryotic translation initiation factor 4E-binding protein, H9c2 cells, PRKAG2 gene mutation, p70S6 kinase, rapamycin
Abstract
PRKAG2 encodes the γ2-subunit isoform of 5′-AMP-activated protein kinase (AMPK), a heterotrimeric enzyme with major roles in the regulation of energy metabolism in response to cellular stress. Mutations in PRKAG2 have been implicated in a unique hypertrophic cardiomyopathy (HCM) characterized by cardiac glycogen overload, ventricular preexcitation, and hypertrophy. We identified a novel, de novo PRKAG2 mutation (K475E) in a neonate with prenatal onset of HCM. We aimed to investigate the cellular impact, signaling pathways involved, and therapeutic options for K475E mutation using cells stably expressing human wild-type (WT) or the K475E mutant. In human embryonic kidney-293 cells, the K475E mutation induced a marked increase in the basal phosphorylation of T172 and AMPK activity, reduced sensitivity to AMP in allosteric activation, and a loss of response to phenformin. In H9c2 cardiomyocytes, the K475E mutation induced inhibition of AMPK and reduced the response to phenformin and increases in the phosphorylation of p70S6 kinase (p70S6K) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1). Primary fibroblasts from the patient with the K475E mutation also showed marked increases in the phosphorylation of p70S6K and 4E-BP1 compared with those from age-matched, nondiseased controls. Moreover, overexpression of K475E induced hypertrophy in H9c2 cells, which was effectively reversed by treatment with rapamycin. Taken together, we have identified a novel, de novo infantile-onset PRKAG2 mutation causing HCM. Our study suggests the K475E mutation induces alteration in basal AMPK activity and results in a hypertrophy phenotype involving the mechanistic target of rapamycin signaling pathway, which can be reversed with rapamycin.
NEW & NOTEWORTHY We identified a novel, de novo PRKAG2 mutation (K475E) in the cystathionine β-synthase 3 repeat, a region critical for AMP binding but with no previous reported mutation. Our data suggest the mutation affects AMP-activated protein kinase activity, activates cell growth pathways, and results in cardiac hypertrophy, which can be reversed with rapamycin.
mutations in the PRKAG2 gene, which encodes the γ2-regulatory subunit isoform of AMP-activated protein kinase (AMPK), cause a wide spectrum of cardiac phenotypes, including Wolff-Parkinson-White (WPW) syndrome (ventricular preexcitation), hypertrophic cardiomyopathy (HCM), conduction system disease, significant glycogen accumulation in myocytes, and sudden death (2, 6, 10, 18, 21). AMPK is known as a cellular energy sensor and a major regulator of whole body energy homeostasis (15). The γ-subunit of AMPK is the regulatory subunit and contains four tandem repeats of a sequence called cystathionine β-synthase (CBS) motif. These motifs act in pairs to form two “Bateman domains”: binding sites for AMP and ATP (4, 29). During cellular energy deficiency (increased ratio of AMP to ATP), binding of AMP to the Bateman domains activates AMPK by inducing phosphorylation of T172 in the α-subunit (kinase domain) (12, 13). Activation of AMPK in many tissues, including the heart, results in the inhibition of ATP-consuming processes and activation of catabolic processes that favor ATP generation.
To date, many PRKAG2 mutations have been reported. The majority of these mutations are heterozygous missense mutations within one of the four CBS domains, but mutations can happen near the NH2 terminal, near the COOH terminal, or in a linker region between two CBS domains (Table 1).
Table 1.
Known human PRKAG2 mutations and their cardiac phenotypes
| NH2 |
CBS1 |
L1 |
CBS2 |
CBS3 |
L3 |
CBS4 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G100S | R302Q | M335T | H344P | H383R | R384T | T400N | L2 | K475E | K485E | Y487H | N488I | E506K | E506K* | H530R | R531G† | R531Q | S548P | COOH | |
| Early onset | X | X | X | X | X | X | X | ||||||||||||
| AMP-activated protein kinase activity | ↓ | ↑ | ↓ | ↑ | ↑ | ||||||||||||||
| Myofibrilla disarray | ? | X | X | ||||||||||||||||
| Reference(s) | 34, 35 | 2, 10 | 27 | 6 | 1 | 2 | 21 | 3 | 2, 22 | 5 | 16 | 23 | 11 | 8 | 20 | ||||
CBS, cystathionine β-synthase; L, linker region.
No significant glycogen accumulation;
no hypertrophy.
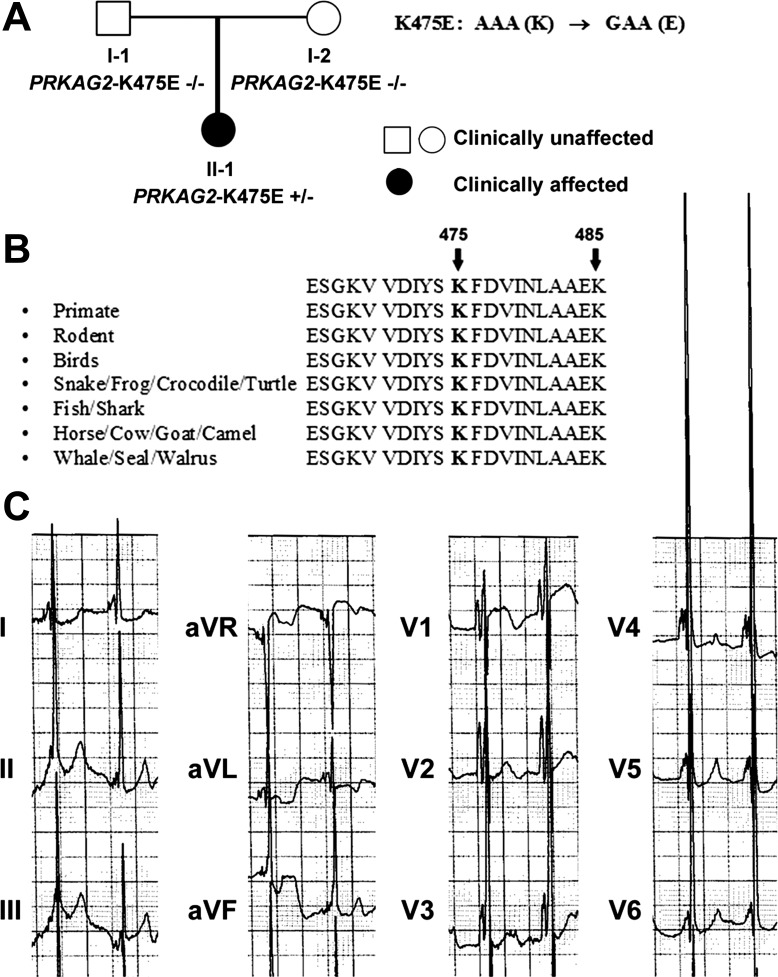
For the case presentation, we encountered a child who presented with an abnormal 27-wk prenatal ultrasound, consistent with HCM. At birth, the child was noted to have significant murmur. Echocardiograms confirmed the diagnosis. Electrocardiograms revealed an extremely short PR interval and combined ventricular hypertrophy (Fig. 1C). Molecular testing for a hypertrophic cardiomyopathy panel revealed a novel, de novo likely pathogenic variant in the PRKAG2 gene [AAA (Lys475) to GAA (glutamic acid) or K475E]. This variant is located in the CBS3 repeat, an area that has no previous report of mutation and is conserved in all species (Table 1 and Fig. 1B). At 3 yr of age, the child has modest HCM and is on supportive medication. She is otherwise developing normally. For the family investigation, there was no family history of sudden cardiac death. Neither parent had abnormal cardiac echocardiograms or electrocardiograms. Molecular testing for PRKAG2 in both parents was normal (Fig. 1A).
Fig. 1.
Amino acid alignment of the γ -subunit of AMP-activated protein kinase (AMPK) and echocardiogram of the patient with the K475E mutation. A: family pedigree of the PRKAG2 K475E carrier. B: lysine at the 475 position in the cystathionine β-synthase 3 (CBS3) repeat of the γ2-isoform of AMPK is conserved among all species. A K485E mutation at the linker region between CBS3 and CBS4 was recently reported (21). C: the echocardiogram was taken at 7 mo old, when the patient was being treated for hypertrophic cardiomyopathy (HCM).
To investigate the significance of the de novo K475E mutation on the AMPK complex, we stably expressed human PRKAG2 wild-type (WT) and K475E in human embryonic kidney (HEK)-293 cells and H9c2 cardiomyocytes to examine AMPK activity, biochemical function, and the signaling pathways involved that resulted from the K475E mutation. Primary fibroblasts from the patient with the K475E mutation were obtained for clinical purposes, and Institutional Review Board approval from the Women & Infant’s Hospital of Rhode Island was obtained for further research study. Primary fibroblasts obtained from commercial sources and age- and sex-matched individuals were obtained and used as controls. The potential of mechanistic target of rapamycin (mTOR) inhibition as a targeted therapeutic approach for mutation-induced HCM was also investigated using rat H9c2 embryonic cardiomyocytes. We demonstrated that the K475E mutation induced changes in the AMPK complex in a way very different from other PRKAG2 mutations. The K475E mutation in H9c2 cells results in the activation of cell growth pathways and hypertrophy, which can be attenuated by inhibition of mTOR.
METHODS
Genetic testing.
HCM next-generation sequencing panel testing was sent to a Clinical Laboratory Improvement Amendments-certified laboratory (GeneDx, Gaithersburg, MD) in December 2010. Genomic DNA was extracted, amplified, and sequenced by a solid-state sequencing-by-synthesis process. The DNA sequence was assembled and analyzed compared with the published genomic reference sequences. This panel included the complete coding and splice region of the following 18 genes known to be associated with HCM: myosin heavy chain 7 (MYH7), myosin-binding protein-C3 (MYBPC3), troponin T2 (TNNT2), troponin I3 (TNNI3), tropomyosin 1 (TPM1), actin-α, cardiac muscle 1 (ACTC), myosin light chain 3 (MYL3), myosin light chain 2 (MYL2), lysosomal associated membrane protein 2 (LAMP2), PRKAG2, α-galactosidase A (GLA), caveolin 3 (CAV3), mitochondrially encoded tRNA glycine (MTTG), mitochondrially encoded tRNA isoleucine (MTTI), mitochondrially encoded tRNA lysine (MTTK), mitochondrially encoded tRNA glutamine (MTTQ), transthyretin (TTR), and troponin C1 (TNNC1). The presence of potentially disease-associated sequence variant was confirmed by conventional dideoxy DNA sequence analysis.
Cell culture.
HEK-293 and H9c2 rat fetal cardiomyocytes were grown in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% (vol/vol) FBS (Atlanta Biologicals, Flowery Branch, GA) and 1% (vol/vol) penicillin-streptomycin (Sigma) in a humidified atmosphere containing 5% CO2 at 37°C. Cells were grown to 70% confluence and synchronized overnight in serum-free medium before experiments (33).
Institutional Research Board approval from the Women & Infant’s Hospital of Rhode Island was obtained to use patients’ dermal fibroblasts. Multiple age-matched control neonatal fibroblasts were obtained from commercial sources, and an age- and sex-matched controls were obtained from the Cytogenetics Laboratory, Women & Infants Hospital of Rhode Island. Fibroblasts were cultured in medium 106 (GIBCO) supplemented with 2% (vol/vol) FBS, 2% (vol/vol) low serum growth supplement, and 1% (vol/vol) penicillin-streptomycin (Sigma). The final concentrations of the components in the low serum growth supplement were as follows: hydrocortisone (1 µg/ml), human EGF (10 ng/ml), basic FGF (3 ng/ml), and heparin (10 µg/ml). Cells were used between passages 2 and 6; 80–100% confluent cells were used for Western blot experiments. Media were changed once daily starting at day 2 and until the termination of the experiment.
Plasmids.
Full-length human WT and K475E mutant PRKAG2 (FLAG tagged from a tetracycline-inducible promoter) were generated as previously described (14) and transfected into HEK-293 or H9c2 cells using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen). To establish multiple clones, individual single cells were isolated and screened for neomycin resistance.
Immunoprecipitation kinase assays of AMPK.
AMPK activity was assayed using AMARA peptide on immunoprecipitated HEK-293 cell lysates as previously described (14).
Western blot analysis.
Protein levels were evaluated by Western blot analysis as previously described (33) using phospho-specific antibodies against p70S6 kinase 1 (p70S6K1; T389, catalog no. 9234, and T421/S424, catalog no. 9204), Akt (S473, catalog no. 9271, and T308, catalog no. 9275), AMPKα (T172, catalog no. 5832), and eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1; T37/46, catalog no. 2855, S65, catalog no. 9451, and T70, catalog no. 9455), and antibodies against total p70S6K1 (catalog no. 2708), Akt (catalog no. 9272), AMPKα (catalog no. 2531), and 4E-BP1 (catalog no. 9452) (Cell Signaling Technology, Danvers, MA). The intensity of bands from Western blots was scanned with densitometry and analyzed digitally with ImageJ software (National Institutes of Health).
H9c2 cell hypertrophy and cell size measurement.
H9c2 embryonic cardiomyocytes (1,000 cells per 60 × 15-mm plate) were treated with vehicle, 200 nM ANG II [ANG II type 2 (AT2) receptor; Tocris, Bristol, UK], 50 nM rapamycin (Selleckchem, Houston, TX), or AT2 receptor in combination with rapamycin for 24 h. Cells were washed three times with Dulbecco's-PBS and then incubated with green-fluorescent calcein AM (1 µg/ml, Life Technologies) for 20 min at 37°C. Cells were washed five times with Dulbecco's-PBS. Cell size was assessed by fluorescence microscopy using an inverted microscope with a ×20 objective (Nikon Eclipse TE2000-E, charge-coupled device camera). Sixty to eighty random cell images were taken per plate, and cell areas were determined by ImageJ software. In another series of related experiments, H9c2 cells were treated with or without rapamycin for 3.5, 4, or 5 days. At each time point, cells were stripped and replated exactly 24 h before being harvested and stained for fluorescence microscopy. Cell hypertrophy was also confirmed using flow cytometry. Cells were trypsinized and analyzed by flow cytometry using a FACSCanto system (BD Biosciences, Franklin Lakes, NJ). Forward scatter was used to measure cell volume. For each condition, 10,000 cells were counted. Forward scatter detector gain was kept constant between samples to allow direct comparison of samples.
Statistics.
Statistical significance of the differences between groups was analyzed using a paired Student’s t-test or ANOVA followed by a Newman-Keuls test. All data are expressed as means ± SE based on results derived from three to four independent experiments. A probability of P < 0.05 was considered to represent a significant difference.
RESULTS
Genetic testing result.
The proband was found to carry a heterozygous novel missense likely pathogenic variant in the PRKAG2 gene. The nucleic acid substitution at the cDNA level was c.1423 A>G, resulting in an amino acid alteration from lysine to glutamic acid (K475E). This change in PRKAG2 has not been published and is not a known benign polymorphism. K475E represents a nonsynonymous substitution of negatively charged glutamic acid for positively charged lysine residue in a highly conserved across species throughout evolution and gene isoforms. In addition, in silico analysis (PolyPhen2) predicts this change to be damaging to the structure/function of the protein. Mutation affecting nearby codons (485, 487, and 488) has been published in association with cardiac hypertrophy, further supporting the functional importance of this region of the protein (2, 3, 21). Furthermore, K475E was not observed in 704 control chromosomes of varying ethnic backgrounds tested at the laboratory, indicating it is not a common benign variant. In addition, the parents, who are both clinically asymptomatic, had normal ECG and cardiac echo and were not found to carry this variant. Based on this information, we concluded that the K475E in PRKAG2 is most consistent with a de novo pathogenic variant.
The K475E mutation markedly increases basal AMPK activity and reduces response to phenformin in HEK-293 cells.
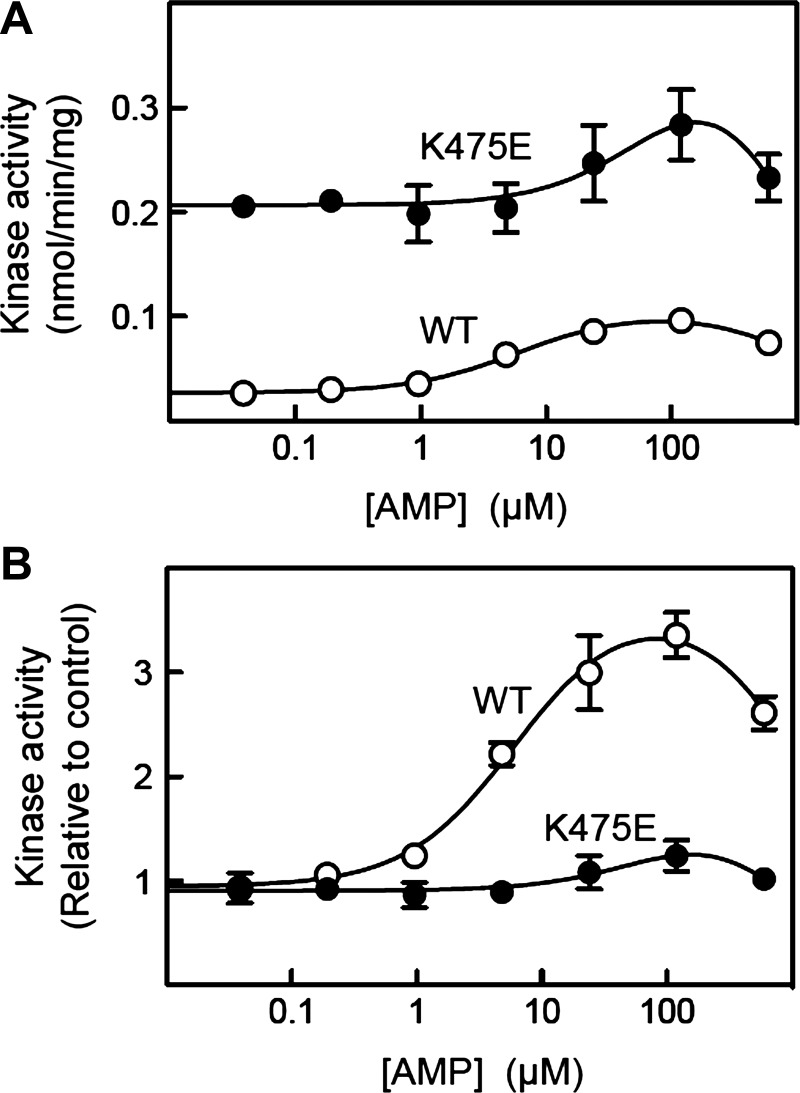
To investigate the molecular effects of the K475E mutation on the function of the AMPK complex, we generated HEK-293 cells stably expressing either human WT or K475E mutant FLAG-tagged γ2 from a tetracycline-inducible promoter. We have previously shown using this system that the expressed γ2-subunit binds to endogenous α- and β-subunits (primarily α1 and β1 in HEK-293 cells), partially replacing the endogenous γ1-subunit (14). After induction with tetracycline, AMPK complexes containing WT or K475E γ2-subunits were immunoprecipitated from unstressed cells using anti-FLAG antibodies, and their activities were determined at varying AMP concentrations. When assayed in the absence of AMP, the activity of the K475E complex was eightfold higher than that of the WT complex (Fig. 2A). However, while the WT complex was allosterically activated almost fourfold by low concentrations of AMP (half-maximal effect = 6 µM), the K475E mutant was only modestly activated (~50%) and then only at much higher concentrations (EC50 ≈ 90 µM). The different degrees of allosteric activation of the WT and K475E mutant by AMP were particularly evident when the activities were expressed relative to the activities measured in the absence of AMP (Fig. 2B).
Fig. 2.
Effects of the K475E mutation on the activity of AMPK in human embryonic kidney (HEK)-293 cells. A: effect of AMP on absolute AMPK activity. HEK-293 cells stably expressing FLAG-tagged wild-type (WT) γ2 or the K475E mutant were lysed. AMPK was immunoprecipitated using anti-FLAG antibodies and kinase activity and assayed at different AMP concentrations. Data (means ± SE, triplicate assays) were fitted to the following equation: Y = basal + [(activation × basal − basal) × X)/(EC50 + X)] − [(activation × basal) × X)/(IC50 + X)], where Y is activity, X is AMP concentration, EC50 is the concentration of AMP giving half-maximal activation, and IC50 is the concentration of AMP giving half-maximal inhibition (AMP inhibits at high concentration due to competition with ATP at the catalytic site). Curves were generated by the following best-fit parameters: WT, basal = 0.027 ± 0.002 nmol·min−1·mg−1, activation = 3.9 ± 0.4-fold, EC50 = 6.2 ± 2.0 µM, and IC50 = 1.5 ± 0.5 mM; and K475E, basal = 0.21 ± 0.01 nmol·min−1·mg−1, activation ≈2-fold, EC50 ≈100 µM, and IC50 ≈1 mM. B: effect of AMP on relative AMPK activity. Data are as in A but with expression of kinase activity relative to activity in the absence of AMP.
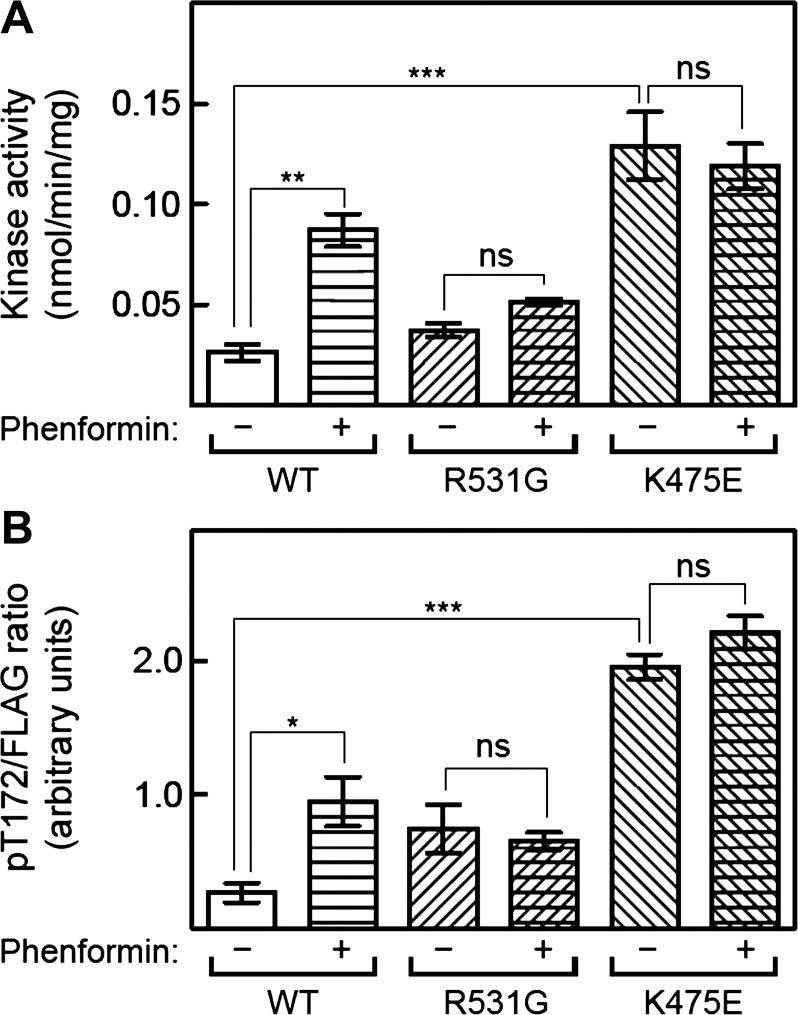
We next compared the effects of metabolic stress induced by the drug phenformin on AMPK in HEK-293 cells stably expressing WT γ2, the K475E mutation, or another γ2-mutation (R531G), which is associated with an inherited form of HCM with childhood onset (11). WT, K475E, or R531G complexes were immunoprecipitated using anti-FLAG antibodies, and their activities were measured at optimal AMP concentration (200 µM). As expected, phenformin caused a fourfold increase in WT AMPK activity that was associated with a similar increase in T172 phosphorylation (Fig. 3). As previously reported (14), complexes containing the R531G mutation exhibited modest (1.5- to 2-fold) increases in basal activity and T172 phosphorylation compared with WT (although these differences were not statistically significant in this data set), but there were no further increases in these parameters in response to phenformin. In contrast, complexes containing the K475E mutation exhibited large increases in basal activity and T172 phosphorylation (5- and 7-fold, respectively) compared with WT, although once again there were no further increases in these parameters in response to phenformin (Fig. 3). Thus, the K475E mutation yields a much larger increase in basal and kinase activity and T172 phosphorylation than the R531G mutation but, like the latter, has a greatly reduced sensitivity to AMP and/or ADP, both for allosteric activation and for enhanced T172 phosphorylation.
Fig. 3.
Effects of the K475E mutation on response to phenformin treatment in HEK-293 cells. Cells stably expressing FLAG-tagged wild-type (WT) γ2, R531G mutant, or K475E mutant were treated with or without phenformin (1.25 mM) for 1 h. AMPK was immunoprecipitated using anti-FLAG antibody and assayed at 200 µM AMP (A). B: signal obtained using anti-phospho-T172 antibody by Western blot analysis of immunoprecipitates and expressed relative to the signal obtained using anti-FLAG antibody. Data are means ± SE (n = 3). Statistical significance was determined by one-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001). ns, Not significant.
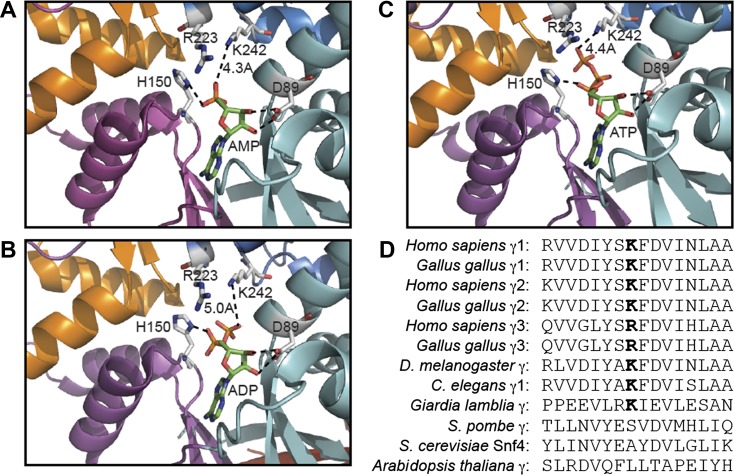
We also used atomic coordinates from crystal structures of partial AMPK complexes with bound AMP, ADP, or ATP (9, 31) to examine the location of K475 with respect to these regulatory nucleotides. The available structures are of complexes containing γ1 rather than γ2, but K475 is conserved in rat γ1 as K242. Interestingly, the nitrogen atom on the K242 side chain is within 4–5 Å of free oxygen atoms of the α-, β-, or γ-phosphate groups of AMP, ADP, or ATP bound in site 1 (Fig. 4, A–C), suggesting that K242 forms electrostatic interaction with phosphate groups of all three nucleotides. We also noted that K242 was located close to R223, a residue within the CBS3 repeat that is conserved in all vertebrate γ-subunit sequences. Although the side chain of R223 points away from K242 (and the nucleotide phosphate groups) in the structures determined, if K475 was replaced with glutamate, it seems feasible that the side chain of R223 could rotate to form a salt bridge with it.
Fig. 4.
Structural analysis of the role of the equivalent K242 residue in rat γ1 and sequence conservation around K475 in AMPK isoforms. A−C: models of rat γ1 showing the relationship of D89, H150, R223, and K242 with bound AMP (A), ADP (B), and ATP (C) using RCSB Protein Data Bank ID 2V8Q, 2Y8L, and 2V9J, respectively (24, 30) (http://www.rcsb.org/pdb/home/home.do). Models were constructed using the MacPyMol molecular visualization system with CBS1 (cyan), CBS2 (magenta), CBS3 (blue), and CBS4 (orange) in “cartoon” representation and nucleotides and highlighted residues in stick representation (C atoms in grey in side chains and green in nucleotides, O atoms in blue, N atoms in red, and P atoms in orange). D: alignment of sequences around K475 in human γ2 and equivalent residues in other species and isoforms.
Database searches showed that a lysine equivalent to K242 or K475 is conserved in all vertebrate γ1- and γ2-isoforms (illustrated by the human and chicken sequences) and in Drosophila melanogaster, Caenorhabditis elegans (γ1-isoform), and Giardia lamblia (Figs. 1B and 4D). In vertebrate γ3-isoforms and in some other invertebrate orthologs, there is another basic residue (arginine) at this position. Figure 4D also shows that a basic residue is not conserved at this position in fungi (Schizosaccharomyces pombe and Saccharomyces cerevisiae) or higher plants (Arabidopsis thaliana); interestingly, the orthologs in these species are not allosterically activated by AMP.
The K475E mutation decreases AMPK activity, reduces the response to phenformin, and induces hypertrophy in H9c2 cells.
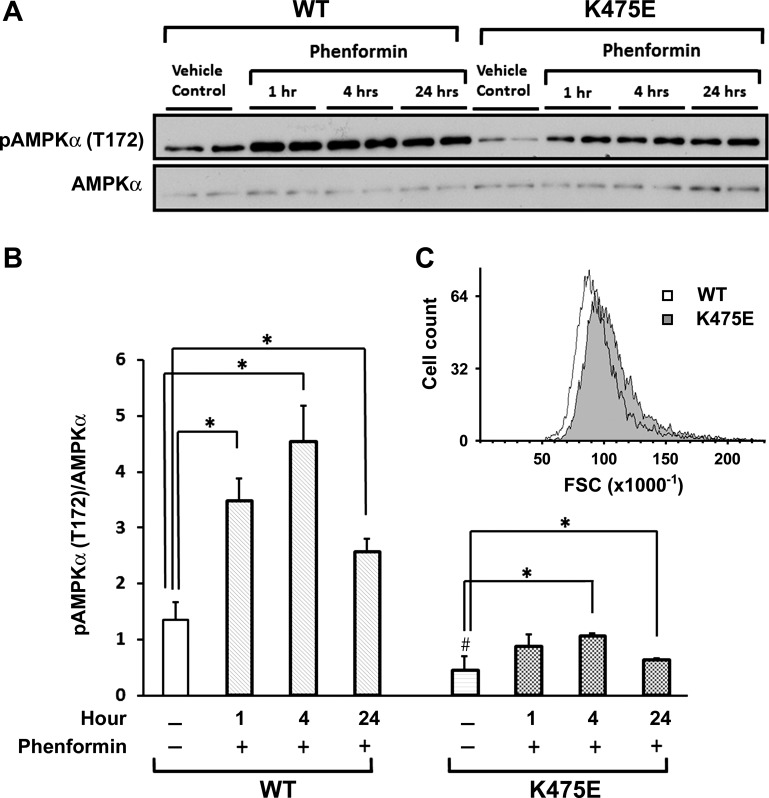
Since we are studying a human gene mutation causing HCM, it would be informative to examine changes in AMPK induced by the K475E mutation in a cardiac context. Multiple individual clones of H9c2 cells stably expressing either the WT or K475E mutant were established, and Western blots were performed on cell lysates. WT- and K475E-expressing H9c2 cardiomyocytes were treated with or without phenformin for 1, 4, or 24 h. In vehicle controls, K475E-expressing cells had significantly reduced phosphorylation of AMPKα (T172) compared with WT-expressing cells (Fig. 5, A and B). Phenformin induced significant increases in AMPKα phosphorylation at all the time points tested in both WT- and K475E-expressing cells. The effect of phenformin, however, was significantly reduced in K475E-expressing cells compared with WT-expressing cells (Fig. 5, A and B). The cell size difference between WT- and K475E-expressing H9c2 cells was analyzed using flow cytometry. We found a small but noticeable increase in the cell size of K475E mutant-expressing cells compared with WT-expressing cells (Fig. 5C).
Fig. 5.
Overexpression of the K475E mutant in H9c2 cells results in AMPK inhibition and hypertrophy. H9c2 cells were stably transfected with human PRKAG2 WT or the K475E mutant construct. A: cells were treated with vehicle or phenformin (1.25 mM) for 1, 4, or 24 h. Representative Western blots with antibody against phospho-AMPKα (T172) or AMPKα are shown. B: densitometric scanning results of the Western blots shown in A. Each bar represents measurement of phospho-AMPKα normalized with AMPKα from 3−4 separate experiments. *P < 0.05 vs. vehicle control within each gene expression group; #P < 0.05 vs. vehicle control of the WT group. C: cells were trypsinized and analyzed by flow cytometry. Forward scatter (FSC) was used to determine the relative cell volume. Ten thousand cells each were analyzed for WT (white histogram)- and K475E mutant (shaded histogram)-expressing cells.
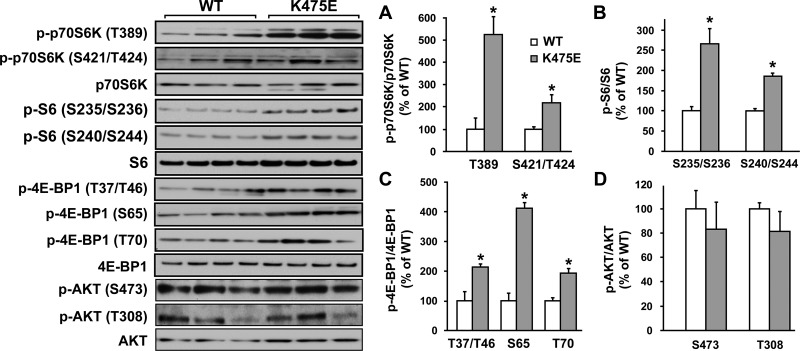
Effects of the K475E mutation on p70S6K, Akt, and 4E-BP1 in H9c2 cardiomyocytes and primary fibroblasts.
Western blots were performed on cell lysates from H9c2 cells expressing WT or K475E. As shown in Fig. 6, K475E-expressing cells had significantly increased phosphorylation of p70S6K and 4E-BP1 compared with the WT-expressing cells. Phosphorylation at T389 is critical for p70S6K activation and is considered to be rapamycin sensitive (7). 4E-BP1, a translation repressor protein, inhibits cap-dependent translation by binding to eIF4E. Phosphorylation of 4E-BP1 at multiple residues leads to its dissociation from eIF4E and thus results in the activation of cap-dependent mRNA translation (25). Therefore, phosphorylation of p70S6K and 4E-BP1 was used as a readout for cellular growth. Phosphorylation of 4E-BP1 at T37/46, S65, and T70 was significantly increased in K475E-expressing cells compared with WT-expressing cells (Fig. 6). In contrast, Akt phosphorylation (S473 or T308) was not changed.
Fig. 6.
Left: overexpression of the K475E mutant in H9c2 cells induces activation of the cell growth signaling pathways. H9c2 cells were stably transfected with human PRKAG2 WT or the K475E mutant construct. Representative Western blots were performed on cell lysates with an antibody against phospho-p70S6 kinase (p70S6K; T389), phospho-p70S6K (T421/S424), phospho-S6 (S235/S236), phospho-S6 (S240/S244), phospho-eukaryotic translation initiation factor 4E-binding protein1 (4E-BP1; T37/T46), phospho-4E-BP1 (S65), phospho-4E-BP1 (T70), phospho-Akt (S473), or phospho-Akt (T308). Blots of each individual total protein (p70S6K, S6, 4E-BP1, and Akt) were also included. A–D: densitometric scanning results of Western blots of H9c2 cells overexpressing human WT or K475E mutant PRKAG2. Each bar represents the measurement of each phospho-specific antibody normalized with its individual total antibody from 3−4 separate experiments. *P < 0.05 vs. individual WT.
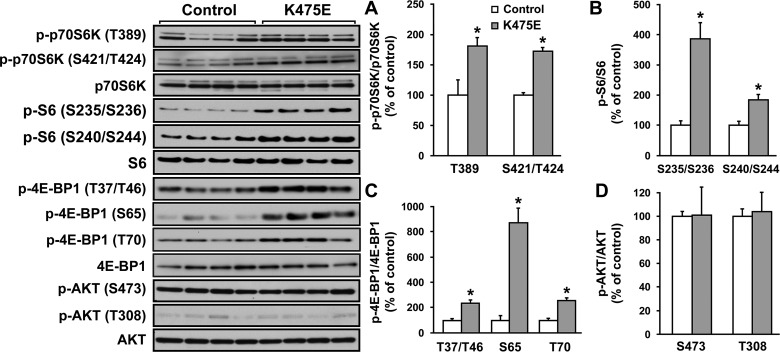
The Western blot experiments described above were repeated using primary fibroblasts from the patient with the K475E mutation and multiple age- and sex-matched controls. Similarly, fibroblasts carrying the K475E mutation had significant increases in the phosphorylation of p70S6K and 4E-BP1 compared with the controls (Fig. 7). Again, phosphorylation of Akt was not changed.
Fig. 7.
Left: primary fibroblasts from the patient with the K475E mutation showed activated cell growth signaling pathways. Fibroblasts from the patient with the K475E mutation and multiple controls were cultured. Western blots were performed on cell lysates with an antibody against phospho-p70S6K (T389), phospho-p70S6K (T421/S424), phospho-S6 (S235/S236), phospho-S6 (S240/S244), phospho-4E-BP1 (T37/T46), phospho-4E-BP1 (S65), phospho-4E-BP1 (T70), phospho-Akt (S473), or phospho-Akt (T308). Blots of each individual total protein (p70S6K, S6, 4E-BP1, and Akt) were also included. A–D, right: densitometric scanning results of Western blots of primary fibroblasts from the patient with the K475E mutation and multiple age- and/or sex-matched controls. Each bar represents the measurement of each phospho-specific antibody normalized with its individual total antibody from 3−4 separate experiments. *P < 0.05 vs. individual WT.
Taken together, these results suggest the K475E mutation results in an Akt-independent activation of cell growth pathway.
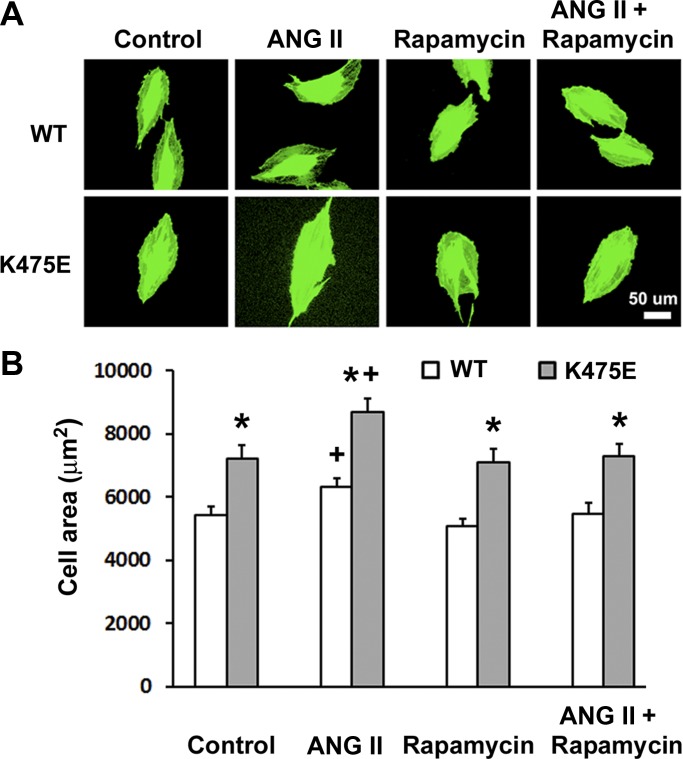
Inhibition of mTOR by rapamycin reversed ANG II-induced H9c2 cell hypertrophy and the K475E cardiac phenotype in vitro.
Induction of H9c2 cell hypertrophic responses by ANG II has been demonstrated to be similar to that in primary neonatal cardiomyocytes (30). To examine if inhibition of mTOR can reverse ANG II-induced hypertrophy, WT- and K475E-expressing cells were treated with vehicle, ANG II alone, rapamycin alone, or ANG II + rapamycin for 24 h. Cells were immunofluorescence stained, and cell areas were assessed by microscopy. In all treatment groups, including the vehicle control group, K475E-expressing H9c2 cells had a significant increase in cell size compared with WT-expressing cells (Fig. 8). ANG II treatment induced hypertrophy in both WT- and K475E-expressing cells. Rapamycin treatment alone did not change cell size but effectively abrogated cell hypertrophy induced by ANG II. These results demonstrate that mTOR inhibition by rapamycin can reverse ANG II-induced cell hypertrophy in both WT- and K475E-expressing H9c2 cells but fails to “rescue” the K475E phenotype as the K475E-expressing cell size remained significantly increased compared with WT-expressing cells in all treatment groups.
Fig. 8.
ANG II induces H9c2 cell hypertrophy, which can be reversed by rapamycin treatment. A: H9c2 cells overexpressing human PRKAG2 WT or the K475E mutant were treated with vehicle (control), 200 nM ANG II, and 50 nM rapamycin or ANG II in combination with rapamycin for 24 h before being stained for green fluorescent calcein AM for the microscopic assessment of cell area. B: bar graphs showing quantification results (n = 60–80 cells) from four individual experiments. *P < 0.05 vs. WT within the same treatment group; +P < 0.05 vs. vehicle control.
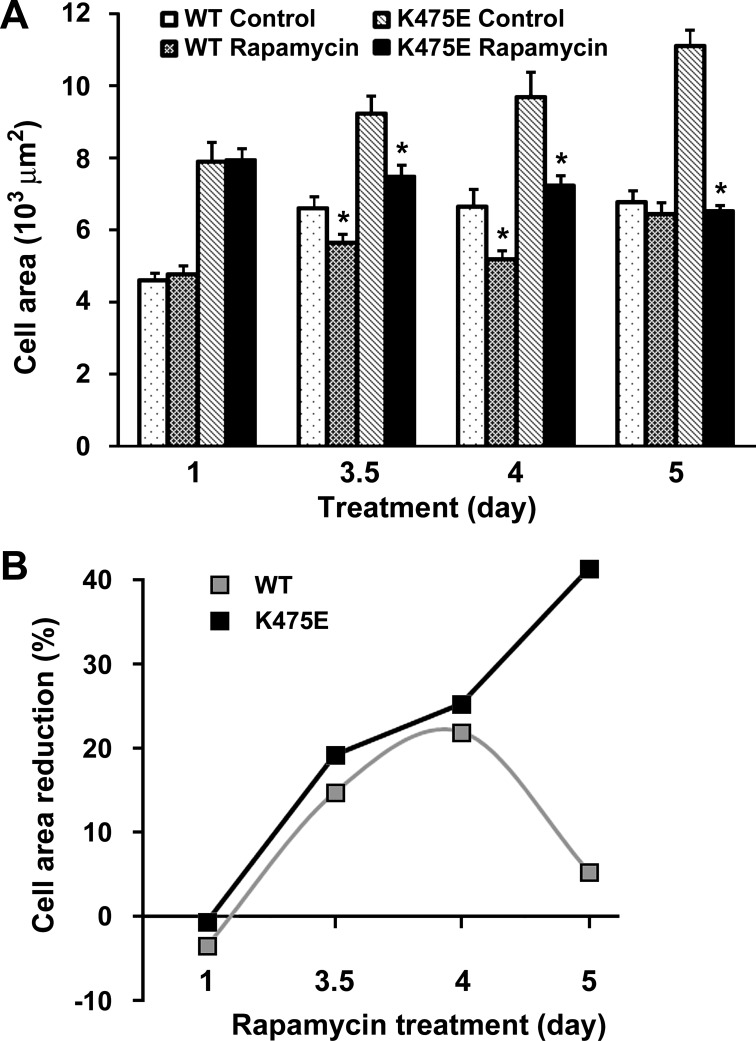
One possibility for the lack of phenotype “rescue” after rapamycin treatment is that a 24-h treatment regimen may not be sufficient. We therefore examined the time course effect of rapamycin on H9c2 cell hypertrophy. WT- and K475E-expressing cells were treated with or without rapamycin for 1, 3.5, 4, or 5 days. In both cell groups, cell size increased significantly after longer incubation time (over 3.5 days; Fig. 9A). While the cell size of WT-expressing cells reached a plateau at day 3.5, cell sizes of K475E-expressing cells continued to increase. In WT-expressing cells, treatment with rapamycin effectively reduced the increases in cell size on days 3.5 and 4, but the effect seemed to taper off on day 5. In contrast, the effect of rapamycin remained effective in reducing the size of K475E-expressing cells with the effect becoming even more obvious at day 5, when the cell sizes of rapamycin-treated K475E-expressing cells were comparable with those of WT-expressing cells at day 5 (Fig. 9B). Taken together, these data demonstrate that mTOR inhibition by rapamycin can be an effective therapeutic approach for K475E mutation-induced hypertrophy.
Fig. 9.
Hypertrophy phenotype induced by overexpression of the K475E mutant in H9c2 cells can be corrected by rapamycin treatment. A: H9c2 cells overexpressing human PRKAG2 WT or the K475E mutant were treated with vehicle (control) or 50 nM rapamycin for the indicated periods of time. Cell area was assessed as described in Fig. 8. Each bar represents the measurement of cell area from 3−4 separate experiments (n = 60–80 cells). *P < 0.05 vs. individual control of the same genotype and treatment day group. B: time course effect of rapamycin treatment presented as cell area reduction (in %) based on the data in A.
DISCUSSION
Mutations in PRKAG2 induce a wide range of cardiac phenotypes, including glycogen overload, ventricular preexcitation, conduction diseases, and hypertrophy. Many mutations have an early onset cardiac phenotype, as is the case for K475E. Given that identification of this disease can be challenging (patient with an infantile-onset mutations may die in utero or during early postnatal life) and misdiagnosis frequently happens (for example, confusion with Pompe disease, a glycogen storage disease), it is likely that the incidence of PRKAG2-induced HCM is underreported. Our study revealed a mutation located in the CBS3 repeat, which has no previous reported mutation and behaved quite differently in terms of changes in AMPK activity and allosteric activation from other PRKAG2 mutations. Moreover, our data suggest that targeted therapy of PRKAG2-induced HCM using mTOR inhibition should be considered.
Our molecular experiments in HEK-293 cells showed that the K475E mutation has three effects on the regulation of the AMPK complex: 1) markedly increasing the basal phosphorylation of T172 and hence basal kinase activity; 2) reducing the sensitivity to AMP in allosteric activation; and 3) preventing the increase in T172 phosphorylation observed in response to phenformin, which increases cellular ADP-to-ATP and AMP-to-ATP ratios. The three nucleotide-binding sites (sites 1, 3, and 4) in the AMPK-γ subunit are numbered by convention according to the number of the CBS repeat that carries an aspartate side chain interacting with the 2′- and 3′-hydroxyls of the nucleotide ribose ring (17). The structural analysis shown in Fig. 4 suggests that K242 in γ1 (equivalent to K475 in γ2) is involved in forming electrostatic interactions with phosphate groups of all three adenine nucleotides, AMP, ADP, and ATP, in site 1. Interestingly, a lysine at this position is conserved in all vertebrate γ1- and γ2-isoforms and also in some invertebrate orthologs such as that from D. melanogaster; in vertebrate γ3-isoforms and many other invertebrate orthologs, it is replaced by another basic residue, arginine. A basic residue at this position is not, however, conserved in the fungal and plant A. thaliana, where allosteric activation by AMP is not observed (Fig. 4D). The K475E mutation replaces the positively charged amine of lysine with the negatively charged carboxyl group of glutamate, potentially disrupting electrostatic interactions with all three nucleotides. Although it is difficult to predict the relative effects this might have on binding of the three nucleotides, if it had a larger effect on the binding of AMP than ATP, it would be expected that higher concentrations of AMP would be required to displace ATP from site 1. Consistent with this, the EC50 for allosteric activation of the K475E mutant by AMP was around 15-fold higher than that of WT (Fig. 2). The maximal degree of activation by AMP also appeared to be reduced, although analysis of this was hindered by the fact AMP starts to inhibit AMPK at concentrations above 200 µM, due to competition with ATP at the catalytic site.
Xiao et al. (32) originally proposed that the effects of AMP on allosteric activation were entirely due to binding at site 1, whereas the effects of AMP and/or ADP on T172 dephosphorylation were due to binding at site 3. Since K242 in γ1 only seems to interact with nucleotides in site 1, the model of Xiao et al. (32) is consistent with our data showing that the K475E mutation markedly affects allosteric activation by AMP (Fig. 2B). However, other major effects of the K475E mutation were to increase the basal T172 phosphorylation and hence basal activity while preventing further phosphorylation induced by phenformin-triggered metabolic stress in intact cells. These effects observed in HEK-293 cells are less easy to explain using the simple model of Xiao et al. (32). The three nucleotide-binding sites in the γ-subunit lie very close together, and the side chains of some conserved basic residues (e.g., H150 and H297 in rat γ1) make interactions with the phosphate groups of more than one nucleotide (31). It is therefore not surprising that mutations affecting binding of a nucleotide at one site can also affect binding and function at other sites. For example, mutation to alanine of any one of the three aspartate residues that define sites 1, 3, and 4 (D89 in CBS1, D244 in CBS3, and D316 in CBS4 in rat γ1) affects not only allosteric activation but also T172 phosphorylation (24). That the model of Xiao et al. (32) is an oversimplification is also suggested by a recent study in which a core AMPK complex was crystallized in the presence of ATP rather than AMP. In this structure, ATP was found in sites 1 and 4, with site 3 being empty; it was also suggested that the mode of ATP binding in site 4 would preclude binding of any nucleotide at site 3 (9). Xiao et al. (31) had previously proposed that site 4 was a “nonexchangeable” site at which AMP bound tightly and irreversibly. It is clear that the roles of the three γ-subunit nucleotide-binding sites on AMPK function are complex and remain incompletely understood.
Of the three effects of the K475E mutation on AMPK function, which is the most likely to cause the clinical problems for the patient? The K475E mutation causes both loss-of-function and gain-of-function effects. The loss-of-function effects are the reduced allosteric activation by AMP and the reduced ability of cellular stresses such as phenformin to increase T172 phosphorylation as seen in both HEK-293 and H9c2 cells. We suspect that these effects could be compensated for by the γ1-isoform, which is expressed along with γ2 in the developing and adult mouse heart and adult human heart (19, 26) and appears to account for ~80% of total AMPK activity in the adult rat heart (13). Both de novo and inherited mutations in the PRKAG2 gene are dominant in effect, and increased basal activity and T172 phosphorylation have been previously reported for R531G, R531Q, and R384T mutations, with both of the latter being de novo mutations that caused severe disease leading to death in early life (1, 8). Our study in H9c2 cells showed a significant reduction in T172 phosphorylation and a reduced response to phenformin. Thus, the cell environment appears to be important in studying this gene mutation. The K475E mutation-induced inhibition of AMPK observed in H9c2 cells is in agreement with the structural modeling results where the mutation causes disruption of electrostatic interactions with adenine nucleotides. Activation of AMPK is well known to inhibit downstream signaling pathway, including mTOR, which plays a critical roles in the regulation of cell growth. Since we observed marked increase in the phosphorylation of p70S6K, S6, and 4E-BP1 in K475E-expressing H9c2 cells and the patient with the mutation, a loss-of-function K475E phenotype seems logical.
mTOR functions by interacting with several adaptor proteins to form two distinct multiprotein complexes: mTOR complex (mTORC)1 and mTORC2. mTORC1 is a critical regulator of protein synthesis, cell growth and proliferation, and many other metabolic processes (28). p70S6K is required for G1 cell cycle progression and cell growth. Phosphorylation of p70S6K is required for its activation, and T389 is the principal regulatory phosphorylation site. 4E-BP1 is a translation repressor protein. It functions by binding to eIF4E. Only when being hyperphosphorylated would 4E-BP1 stop interaction with eIF4E, which results in the activation of cap-dependent translation (25). Since both p70S6K and 4E-BP1 are considered downstream of mTORC1 and can be phosphorylated by mTOR, we examined phosphorylation of these two critical signaling factors to determine changes in the cell growth response by the K475E mutation. We observed significant increases in p70S6K phosphorylation at T389 as well as S421/T424 sites with the K475E mutation. Not surprisingly, ribosomal protein S6 phosphorylation (S235/S236 and S240/S244) was also significantly increased. A noted hyperphosphorylation was also found with the K475E mutation. These changes were consistent in H9c2 cells as well as in patient fibroblasts. These observations suggest that the K475E mutation induces activation of a cell growth pathway downstream of mTOR. Our next step, logically, was to test the therapeutic potential of mTOR inhibition in ameliorating PRKAG2 mutation-induced changes in vivo. Using H9c2 cells, we demonstrated that cell hypertrophy induced by the K475E mutation can be reversed by rapamycin. This is encouraging, as, to date, there is no targeted therapy for PRKAG2 HCM. Hence, the next step would be to develop transgenic mouse models to demonstrate the effectiveness of the mTOR inhibition strategy in treating PRKAG2 mutation-induced HCM.
Overall, our data show a novel, de novo K475E PRKAG2 mutation causing HCM. The K475E mutation induces changes in the AMPK complex that are not shared by the other PRKAG2 mutations. The K475E mutation also induces activation of cell growth pathways and hypertrophy, which can be effectively reversed by rapamycin treatment.
GRANTS
This work was supported by National Institute of General Medical Sciences Grant P30-GM-114750 [to A. Uzen., J. Padbury (Director), S. Shaw, and Y.-T. Tseng] and the Oh-Zopfi Research Award, Women & Infants Hospital (to C. Phornphutkul and Y.-T. Tseng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.G.H., S.S., J.F.P., C.P., and Y.-T.T. conceived and designed research; Y.X., A.G., and S.S. performed experiments; Y.X., A.G., A.U., and S.S. analyzed data; Y.X., A.G., D.G.H., A.U., S.S., J.F.P., C.P., and Y.-T.T. interpreted results of experiments; Y.X., A.G., and S.S. prepared figures; Y.X. and A.G. drafted manuscript; Y.X., J.F.P., C.P., and Y.-T.T. approved final version of manuscript; J.F.P., C.P., and Y.-T.T. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank the COBRE in Perinatal Biology Core and Kilguss Core Facility for the excellent flow cytometry and microscopy support. We thank Dr. Umadevi Tantravahi (Director of Medical Genetics, Women & Infants Hospital) for providing the age- and sex-matched fibroblasts.
REFERENCES
- 1.Akman HO, Sampayo JN, Ross FA, Scott JW, Wilson G, Benson L, Bruno C, Shanske S, Hardie DG, Dimauro S. Fatal infantile cardiac glycogenosis with phosphorylase kinase deficiency and a mutation in the gamma2-subunit of AMP-activated protein kinase. Pediatr Res 62: 499–504, 2007. doi: 10.1203/PDR.0b013e3181462b86. [DOI] [PubMed] [Google Scholar]
- 2.Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest 109: 357–362, 2002. doi: 10.1172/JCI0214571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arad M, Maron BJ, Gorham JM, Johnson WH Jr, Saul JP, Perez-Atayde AR, Spirito P, Wright GB, Kanter RJ, Seidman CE, Seidman JG. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med 352: 362–372, 2005. doi: 10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- 4.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci 22: 12–13, 1997. doi: 10.1016/S0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 5.Bayrak F, Komurcu-Bayrak E, Mutlu B, Kahveci G, Basaran Y, Erginel-Unaltuna N. Ventricular pre-excitation and cardiac hypertrophy mimicking hypertrophic cardiomyopathy in a Turkish family with a novel PRKAG2 mutation. Eur J Heart Fail 8: 712–715, 2006. doi: 10.1016/j.ejheart.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, Salmon A, Ostman-Smith I, Watkins H. Mutations in the γ2 subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet 10: 1215–1220, 2001. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 7.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA 95: 1432–1437, 1998. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burwinkel B, Scott JW, Bührer C, van Landeghem FK, Cox GF, Wilson CJ, Grahame Hardie D, Kilimann MW. Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Am J Hum Genet 76: 1034–1049, 2005. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Wang J, Zhang YY, Yan SF, Neumann D, Schlattner U, Wang ZX, Wu JW. AMP-activated protein kinase undergoes nucleotide-dependent conformational changes. Nat Struct Mol Biol 19: 716–718, 2012. doi: 10.1038/nsmb.2319. [DOI] [PubMed] [Google Scholar]
- 10.Gollob MH, Green MS, Tang AS, Gollob T, Karibe A, Ali Hassan AS, Ahmad F, Lozado R, Shah G, Fananapazir L, Bachinski LL, Roberts R. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med 344: 1823–1831, 2001. doi: 10.1056/NEJM200106143442403. [DOI] [PubMed] [Google Scholar]
- 11.Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, Bachinski L, Roberts R. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation 104: 3030–3033, 2001. doi: 10.1161/hc5001.102111. [DOI] [PubMed] [Google Scholar]
- 12.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. BioEssays 23: 1112–1119, 2001. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 13.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271: 27879–27887, 1996. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 14.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 11: 554–565, 2010. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15–25, 2005. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Kelly BP, Russell MW, Hennessy JR, Ensing GJ. Severe hypertrophic cardiomyopathy in an infant with a novel PRKAG2 gene mutation: potential differences between infantile and adult onset presentation. Pediatr Cardiol 30: 1176–1179, 2009. doi: 10.1007/s00246-009-9521-3. [DOI] [PubMed] [Google Scholar]
- 17.Kemp BE, Oakhill JS, Scott JW. AMPK structure and regulation from three angles. Structure 15: 1161–1163, 2007. doi: 10.1016/j.str.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Kim M, Hunter RW, Garcia-Menendez L, Gong G, Yang YY, Kolwicz SC Jr, Xu J, Sakamoto K, Wang W, Tian R. Mutation in the γ2-subunit of AMP-activated protein kinase stimulates cardiomyocyte proliferation and hypertrophy independent of glycogen storage. Circ Res 114: 966–975, 2014. doi: 10.1161/CIRCRESAHA.114.302364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim M, Shen M, Ngoy S, Karamanlidis G, Liao R, Tian R. AMPK isoform expression in the normal and failing hearts. J Mol Cell Cardiol 52: 1066–1073, 2012. doi: 10.1016/j.yjmcc.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laforêt P, Richard P, Said MA, Romero NB, Lacene E, Leroy JP, Baussan C, Hogrel JY, Lavergne T, Wahbi K, Hainque B, Duboc D. A new mutation in PRKAG2 gene causing hypertrophic cardiomyopathy with conduction system disease and muscular glycogenosis. Neuromuscul Disord 16: 178–182, 2006. doi: 10.1016/j.nmd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Bai R, Wang L, Zhang C, Zhao R, Wan D, Chen X, Caceres G, Barr D, Barajas-Martinez H, Antzelevitch C, Hu D. Identification of a novel de novo mutation associated with PRKAG2 cardiac syndrome and early onset of heart failure. PLoS One 8: e64603, 2013. doi: 10.1371/journal.pone.0064603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luptak I, Shen M, He H, Hirshman MF, Musi N, Goodyear LJ, Yan J, Wakimoto H, Morita H, Arad M, Seidman CE, Seidman JG, Ingwall JS, Balschi JA, Tian R. Aberrant activation of AMP-activated protein kinase remodels metabolic network in favor of cardiac glycogen storage. J Clin Invest 117: 1432–1439, 2007. doi: 10.1172/JCI30658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morita H, Rehm HL, Menesses A, McDonough B, Roberts AE, Kucherlapati R, Towbin JA, Seidman JG, Seidman CE. Shared genetic causes of cardiac hypertrophy in children and adults. N Engl J Med 358: 1899–1908, 2008. doi: 10.1056/NEJMoa075463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oakhill JS, Chen ZP, Scott JW, Steel R, Castelli LA, Ling N, Macaulay SL, Kemp BE. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc Natl Acad Sci USA 107: 19237–19241, 2010. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pause A, Belsham GJ, Gingras AC, Donzé O, Lin TA, Lawrence JC Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371: 762–767, 1994. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- 26.Pinter K, Grignani RT, Czibik G, Farza H, Watkins H, Redwood C. Embryonic expression of AMPK γ subunits and the identification of a novel γ2 transcript variant in adult heart. J Mol Cell Cardiol 53: 342–349, 2012. doi: 10.1016/j.yjmcc.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pöyhönen P, Hiippala A, Ollila L, Kaasalainen T, Hänninen H, Heliö T, Tallila J, Vasilescu C, Kivistö S, Ojala T, Holmström M. Cardiovascular magnetic resonance findings in patients with PRKAG2 gene mutations. J Cardiovasc Magn Reson 17: 89, 2015. doi: 10.1186/s12968-015-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res 114: 549–564, 2014. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 113: 274–284, 2004. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim 47: 125–131, 2011. doi: 10.1007/s11626-010-9368-1. [DOI] [PubMed] [Google Scholar]
- 31.Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, Carling D, Gamblin SJ. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 449: 496–500, 2007. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 32.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ. Structure of mammalian AMPK and its regulation by ADP. Nature 472: 230–233, 2011. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yano N, Ianus V, Zhao TC, Tseng A, Padbury JF, Tseng YT. A novel signaling pathway for β-adrenergic receptor-mediated activation of phosphoinositide 3-kinase in H9c2 cardiomyocytes. Am J Physiol Heart Circ Physiol 293: H385–H393, 2007. doi: 10.1152/ajpheart.01318.2006. [DOI] [PubMed] [Google Scholar]
- 34.Zhang BL, Xu RL, Zhang J, Zhao XX, Wu H, Ma LP, Hu JQ, Zhang JL, Ye Z, Zheng X, Qin YW. Identification and functional analysis of a novel PRKAG2 mutation responsible for Chinese PRKAG2 cardiac syndrome reveal an important role of non-CBS domains in regulating the AMPK pathway. J Cardiol 62: 241–248, 2013. doi: 10.1016/j.jjcc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Zhang BL, Ye Z, Xu RL, You XH, Qin YW, Wu H, Cao J, Zhang JL, Zheng X, Zhao XX. Overexpression of G100S mutation in PRKAG2 causes Wolff-Parkinson-White syndrome in zebrafish. Clin Genet 86: 287–291, 2014. doi: 10.1111/cge.12267. [DOI] [PubMed] [Google Scholar]