Acute vagus nerve stimulation elevated activity in neurons located in the medial nucleus of the solitary tract. Such stimuli directly activated only myelinated vagal afferents but indirectly activated a subpopulation of second- and higher-order neurons, suggesting that afferent mechanisms and central neuron activation may be responsible for vagus nerve stimulation efficacy.
Keywords: nucleus of the solitary tract, baroreceptors, vagal primary afferents, vagus nerve stimulation, blood pressure, echocardiography, rats
Abstract
Vagus nerve stimulation (VNS) currently treats patients with drug-resistant epilepsy, depression, and heart failure. The mild intensities used in chronic VNS suggest that primary visceral afferents and central nervous system activation are involved. Here, we measured the activity of neurons in the nucleus of the solitary tract (NTS) in anesthetized rats using clinically styled VNS. Our chief findings indicate that VNS at threshold bradycardic intensity activated NTS neuron discharge in one-third of NTS neurons. This VNS directly activated only myelinated vagal afferents projecting to second-order NTS neurons. Most VNS-induced activity in NTS, however, was unsynchronized to vagal stimuli. Thus, VNS activated unsynchronized activity in NTS neurons that were second order to vagal afferent C-fibers as well as higher-order NTS neurons only polysynaptically activated by the vagus. Overall, cardiovascular-sensitive and -insensitive NTS neurons were similarly activated by VNS: 3/4 neurons with monosynaptic vagal A-fiber afferents, 6/42 neurons with monosynaptic vagal C-fiber afferents, and 16/21 polysynaptic NTS neurons. Provocatively, vagal A-fibers indirectly activated C-fiber neurons during VNS. Elevated spontaneous spiking was quantitatively much higher than synchronized activity and extended well into the periods of nonstimulation. Surprisingly, many polysynaptic NTS neurons responded to half the bradycardic intensity used in clinical studies, indicating that a subset of myelinated vagal afferents is sufficient to evoke VNS indirect activation. Our study uncovered a myelinated vagal afferent drive that indirectly activates NTS neurons and thus central pathways beyond NTS and support reconsideration of brain contributions of vagal afferents underpinning of therapeutic impacts.
NEW & NOTEWORTHY Acute vagus nerve stimulation elevated activity in neurons located in the medial nucleus of the solitary tract. Such stimuli directly activated only myelinated vagal afferents but indirectly activated a subpopulation of second- and higher-order neurons, suggesting that afferent mechanisms and central neuron activation may be responsible for vagus nerve stimulation efficacy.
cervical vagus nerve stimulation (VNS) is currently used as a therapy for intractable epilepsy and for treatment-resistant depression. Studies of VNS for cardiac diseases improved cardiac dysrhythmias in heart failure patients (42). In subsets of these patients, surprisingly small acute heart rate reductions are observed even at the strongest intensity averaging 2.2 beats/min for right-sided VNS and 0.6 beats/min for left-sided VNS (42). Despite considerable preclinical and clinical trial work in patients indicating beneficial VNS treatment for heart disease (10–12, 17, 34, 42, 47, 48), many questions remain regarding the mechanistic basis for VNS benefits. In clinical VNS, protocols recommend increasing stimulus intensity to the highest level tolerated by the individual (26, 36). These comfort/tolerability limitations include the development of side effects such as voice alteration, hoarseness, coughing, pain, and other discomforts during increasing intensity settings (“uptitration”) and yet, in a neural sense, clinical VNS stimuli are weak currents. In cardiovascular protocols, a slight bradycardia [i.e., minimal bradycardic intensity (BI)] is commonly used to set stimulus intensity level (12). The therapeutic spectrum of VNS is surprisingly broad with approved treatments for central nervous system (CNS) disorders and new trials for heart failure (12, 51), which suggests that VNS may broadly target a variety of systemic processes. Together, the clinical observations suggest that both efferent and afferent axons are activated during weak-intensity VNS, although the source of therapeutic efficacy remains poorly understood.
Varied intensities of electrical shocks to the cervical vagus nerve can activate action potentials in vagal primary afferent neurons, which are conducted centrally to trigger action potentials in second-order neurons within the nucleus of the solitary tract (NTS) (21). An important implication of NTS activation is that these neurons project axons to different sites across the CNS, and thus VNS activation of vagal afferents has the potential to broadly impact diverse central neuron pathways: a process about which very little is understood (2). Clinical practice features weak stimulus intensities that are thought to be selective for myelinated axons (32). In conscious rats, hours of low-intensity VNS prominently increased the activity marker c-Fos in the NTS, paraventricular nucleus of the hypothalamus, parabrachial nucleus, ventral bed nucleus of the stria terminalis, and locus coeruleus without altering systemic hemodynamics or heart rate (15). Thus weak VNS stimuli clearly activate vagal afferents that directly and indirectly activate neurons distributed widely across the brain. One goal of the present study was to identify which vagal afferents are activated by weak VNS stimuli and which NTS neurons are activated during VNS conditioning.
The cervical vagus nerve trunk contains a mixture of afferent and efferent axons that differ in myelination and axon diameter (1). Our study targeted vagal afferent-responsive neurons from the caudal part of the medial NTS (21, 22, 25, 39). NTS neurons were activated by maximal vagal shocks to test for monosynaptic or polysynaptic vagal connections and were additionally tested for cardiovascular responsiveness [Cardio+ or without cardiovascular responsiveness (Cardio−)] with vasoactive-drug-induced changes in blood pressure (3, 33, 38, 40). Clinically styled weak VNS intensities were determined in each animal as BI. BI VNS evoked action potentials arriving at a fixed time from the vagal stimulus, i.e., synchronized, consistent with myelinated conduction but only in a very limited group of NTS neurons. In most Cardio+ and Cardio− NTS neurons, however, VNS activated only unsynchronized action potentials considered spontaneous. VNS increased spontaneous activity to the greatest extent in higher-order NTS neurons: those without direct vagal excitation. Since even half-BI stimuli increased spontaneous activity, our study suggests that modest numbers of the most highly myelinated vagal afferents effectively enhance network activity indirectly in a subset of C-fiber second-order as well as higher-order neurons within the NTS.
MATERIALS AND METHODS
All procedures were approved by the University Committee on Animal Care of East Tennessee State University and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed.). Male Sprague-Dawley rats weighing ~400 g (n = 12) were housed under a 12:12-h light-dark cycle with food and water provided ad libitum.
Surgical Preparation
Anesthesia was induced with 3% isoflurane using an induction chamber (VetEquip, Pleasanton, CA) and maintained by a nose cone. Sufficiency of anesthesia was then monitored by absence of both hindlimb toe-pinch responses and electromyography artifacts in the electrocardiogram. The femoral vein was cannulated for the administration of vasoactive drugs (40 µg/kg nitroglycerine and 20 µg/kg phenylephrine), and the jugular vein was cannulated for the administration of α-chloralose anesthetic. Blood pressure was recorded from the femoral artery using a Millar solid state device (2F). The ECG monitored heart function (model P511, Grass Instrument, Quincy, MA). The trachea was cannulated, and mechanical ventilation was maintained with a positive pressure of 12-16 mm of H2O using 100% O2 at a tidal volume of 2–3 ml and 60 breaths/min (SAR-830/P ventilator, IITC, Woodland Hills, CA). The expired CO2 level was maintained at 3% by adjusting the inspiration pressure (CWE, CapStar-100). Rectal temperature was maintained at 37°C using a feedback-controlled heating blanket (Harvard Apparatus).
VNS Preparation
The bipolar electrodes consisted of two sets of coiled contacts (multiple platinum/iridium alloy or nonmagnetic nickel-cobalt-chromium-molybdenum alloy contacts along coil length, 1 cm each) separated by 1 cm along the vagus nerve trunk. The twin coils were wrapped around the left cervical vagus nerve, aortic depressor nerve, sympathetic nerve, and adjacent carotid artery using a ventral approach. Our setup in rats mimicked the clinical arena, since cervical VNS in patients targets the vagosympathetic trunk, which includes baroreceptor afferents and sympathetic fibers (53). The lead cables were secured to neck muscles with sutures to minimize movement. Our precoiled leads were specifically built for rodents and were insulated except for contact areas inside the coil that directly touched the vagus, with the cathode placed rostrally. The externalized lead wires were connected to a stimulus generator (Demipulse model 103, Cyberonics, Houston, TX; firmware modified to increase range of stimulation parameters).
Stereotactic Preparations
After ventral wound closure, the animal was flipped and fixed in a stereotaxic frame for the dorsal approach to the NTS. The head of the rat was fixed and tilted forward at ~45° to facilitate access to the dorsal surface of the medulla oblongata without the need to move the cerebellum. The NTS region was exposed rostrocaudally from the calamus scriptorius to the rostral aspect of the area postrema. The exposed brain area was covered with mineral oil to prevent drying. When all surgical procedures were completed, anesthesia was switched to maintenance with α-chloralose initiated with a slow bolus injection (50 mg/kg iv) given over 20 min. After completion of the slow bolus of α-chloralose, isoflurane administration was ceased and a low-dose, continuous infusion of α-chloralose commenced to maintain the anesthetic state (30 mg·kg−1·hr1 iv). Thirty minutes were allowed for the animal to stabilize before neural recordings or VNS interventions.
NTS Recording
Extracellular neural activity was recorded using high-impedance (3 MΩ) tungsten electrodes (FHC) positioned stereotaxically and driven with a piezoelectric microdrive (Burleigh, Thorlabs, NJ). The tungsten electrode was lowered stepwise (1–5 µm) into the caudal portion of the left medial NTS, at the caudal tip of the area postrema, 0.2–1.5 mm laterally from midline and 0.5–1.3 mm ventral to the dorsal surface following published coordinates (43, 44). In a differential recording against a remote ground reference placed in posterior neck muscles, the neural signal was amplified, passed through a filter (bandwidth setting: 0.1–3 kHz, A-M Systems model 1800), and processed to remove 50- to 60-Hz noise along with its harmonics using a Hum-Bug filter (Quest Scientific). Filtered signals were sampled using a CED acquisition system using Spike 2 version 7.17 (CED, Cambridge, UK) at the rate of 10 kHz for neural signals and 1 kHz for blood pressure and ECG.
NTS Unit Neuron Identification
Vagus nerve stimulus artifacts were blanked before analysis. Principal component analysis (PCA) identified unique, single neuron signals (see Fig. 1) within the NTS neural recordings using a script in Spike 2. Single neuron units were characterized by three orthogonal components: shape, amplitude, and length. Templates created for each neuron unit were visually inspected across the entire experiment to exclude potential contamination artifacts from activity counts. This procedure reliably identified consistent waveforms attributed to individual neurons (9). PCA-identified units were used to calculate activity frequencies, temporal distributions, and patterned relations to blood pressure and VNS phases. In some cases, rigorously unique PCA profiles could simultaneously discriminate multiple neurons but such analyses were pragmatically limited to three unique neurons per NTS recording site.
Fig. 1.

Basic identification of single neuron action potentials and definition of A- and C-fiber conduction and monosynaptic/polysynaptic pathways. Recorded electroneurograms (ENGs) were submitted to sorting analysis of extracellular action potentials based on principal component analysis (PCA; Spike 2 software). For PCA, two examples are given of a monosynaptic arrival analysis and calculation of latency, conduction velocity, and synaptic jitter-measured variation in latency (jitter = SD of latency across 10 iterative vagal stimuli). Vagal pulse stimulus (downward arrow) marks stimulus delivery to the left vagus. The break in the ENG represents blanking of the stimulus artifact. A: single supramaximal vagal stimulus (3.5 mA, 100-µs duration) triggered an action potential at the filled circle in neuron 1. Successive stimulus trials (traces not shown) evoked action potential arrivals (small filled circles) in neuron 1 with an average latency of 27 ms (ranging from 23 and 34 ms over 10 consecutive trials). The latency was measured as the time between the onset of the vagus nerve stimulus artifact to the half-height of the unit action potential. PCA created statistical match templates that recognized unique spikes based on the shape, width, and amplitude within set upper and lower limits (green lines in PCA templates). For an action potential profile to be categorized as PCA compliant, at least 80% of the trace must be contained within these limits. Neuron 2 had an average latency of 44 ms (ranging between 30 and 55 ms). NTS, nucleus of the solitary tract. B: responses to paired pulses (interval of 10 ms) discriminated monosynaptic from polysynaptic vagal paths. Neuron 1 successfully followed both stimuli (arrows) to generate two successful action potentials (filled circles) and demonstrated that the vagal input was monosynaptically connected to a C-fiber vagal afferent. Neuron 2 showed a failed action potential (unfilled circle) consistent with a polysynaptic path from the vagus. Note that in A, neuron 2 displayed highly variable arrival times and a high jitter consistent with a polysynaptic path.
Experimental Protocols
To search for vagus-responsive neurons, each advance of the NTS electrode was followed by delivery of a single, high-intensity stimulus to the ipsilateral vagus, i.e., Search Test stimuli (3.5 mA, pulse width of 100 µs at 0.2 Hz). The Search Test stimulus parameters were based on pilot studies indicating that this supramaximal intensity activated all myelinated and unmyelinated vagal afferent axons (not shown). Depth adjustments helped to optimize the signal by positioning the electrode to maximize the evoked spike signal (signal to noise ratio > 3/1 S/N > 3/1), thereby facilitating the creation of PCA templates for unique, action potential profiles with Spike 2. After optimization, the electrode position was then held fixed for the remaining tests. Once an action potential waveform of a single NTS neuron had been identified (Fig. 1), a series of additional experimental protocols were performed beginning with defining the latency characteristics in response to vagus nerve stimuli.
Testing protocol 1.
Ten iterative trials of Search Test stimuli (3.5 mA, pulse width of 100 µs at 0.2 Hz) were used to assess the characteristic latency and jitter (SD of latency) of the neuronal unit response to vagal input activation and estimate the conduction velocity of the vagal afferent. Calculations of conduction velocity were based on estimated conduction distance (3 cm) and a cutoff of a maximum of 2 m/s for C-fibers with all faster conduction times considered arising from myelinated vagal afferents.
Testing protocol 2.
Paired-pulse vagus nerve stimuli (3.5 mA, pulse width of 100 µs) with an interstimulus interval of 10 ms (100 Hz) were delivered to evaluate the likely synaptic path characteristics of the vagal afferent input to the recorded NTS neuron. Vagal afferents making direct contact (i.e., monosynaptic) successfully activate NTS neurons after such paired stimuli (Fig. 1), whereas polysynaptic inputs fail with the second vagus nerve stimulus of the paired pulse (50).
Testing protocol 3.
To test for cardiovascular responsiveness, we recorded NTS activity while briefly altering arterial pressure by intravenous administration of vasoactive compounds. Brief reductions in pressure induced by nitroglycerine (40 µg/kg in a 20-µl bolus) rapidly plateaued to steady minimal values (at ~5 s). These were followed by phenylephrine-induced increases (20 µg/kg in a 40-µl bolus) to above the prevailing pressures (Fig. 2). Baseline cardiovascular values were restored by 10 min of recovery from pharmacological tests.
Fig. 2.
Cardiovascular responsiveness was judged by monitoring NTS neuron activity during induced blood pressure changes (Cardio+). A bolus of nitroglycerin injected intravenously decreased pressure and was followed by intravenous phenylephrine to raise blood pressure (BP). Note that such bolus injections only briefly altered pressure (4-6 s). A, top trace: NTS ENG and PCA identified neuron 1 (same neuron 1 as Fig. 1) summed in the histogram plot during BP manipulations. The histogram plot displays binned spike counts of neuronal activity for each cardiac cycle. Original pulsatile BP and ECG traces are included at bottom. B: neuronal activity of neuron 1 was converted to Hz by dividing spike counts by each cardiac period and analyzed by plotting neuron 1 activity against systolic BP. Note that lowering pressure from rest did not affect neuronal activity. Neuron 1 had a null slope relationship between systolic BPs and discharge below systolic pressure of 155 mmHg (horizontal line). Above pressure threshold, neuron 1 increased activity linearly with increases in systolic BP and was well described by the linear regression fit. The intersection of the subthreshold and suprathreshold linear regressions defined the threshold for discharge (155 mmHg) for neuron 1. The response to vagus nerve stimuli indicated that this NTS neuron received a monosynaptic C-fiber input conducting at 1 m/s with a jitter of 4.5 ms. The x-axes in A are broken (eliminating 20 s) for clarity. C: mean BP and heart rate responses showing that lowering BP with nitroglycerin minimally affected heart rate but increasing BP with phenylephrine induced bradycardia. Nitro, nitroglycerin; PE, phenylephrine. *P < 0.05, significantly different compared with baseline.
Testing protocol 4.
To test for potential effects of VNS conditioning, we selected stimulation parameters fashioned after those used in chronic VNS therapy routines for the treatment of heart disease in animals (10, 11) and patients (17, 34, 42, 48). VNS pulse duration and frequency were fixed at 250 µs and 20 Hz, respectively. VNS conditioning routines followed the clinical protocols and consisted of five repeated cycles [VNS/off phase (OP)] consisting of an 18-s phase of 20-Hz stimulation followed by an OP of 44 s. Unlike the Search Test, the VNS conditioning intensity followed the clinical protocol and was based on a physiological response in each animal. As with the clinical protocol, the minimum current needed to evoke a 5% bradycardia was determined in each animal and termed BI (1.0 ± 0.2 mA, n = 12). In every VNS conditioning cycle, the stimulator ramped the intensity from zero up to the BI over the initial 2 s. After BI was achieved, the next 14 s of stimulation used a constant BI before ramping down to zero over the final 2 s of stimulation. Thus, the intensity pattern was a trapezoidal step in current intensity. In all experiments, we similarly tested a second, lower pulse intensity set at 0.5 BI, which used a similar trapezoidal step pattern in current intensity. After completion of these protocols, the search routine was reinitiated and the microelectrode was advanced to find another cardiovagal neuron from the same track or from another adjacent track. After data collection, rats were euthanized by addition of 3% isoflurane during mechanical ventilation until pinch reflexes were eliminated followed by exsanguination.
Statistical Analyses
To analyze cardiovascular responsiveness, plots of systolic blood pressure against neuronal discharge were submitted to a linear regression analysis. Two regressions, one from the highest pressures downward and another from the lowest pressures upward, were calculated. The low-pressure range in these neuronal responses was generally a zero slope relation suggesting that basal activity was unaffected by pressure. The high-pressure range was generally linear with no evidence of an upper plateau but at the low pressure end intersected the low pressure relation. The intersection was considered the threshold systolic pressure value. The two regressions were optimized for the maximal coefficient of determination (r2) by transferring experimental (values – pressure, activity pairs) from the low pressure relation to the high pressure relation until maximum r2 was achieved. The slope of the upper regression was the cardiovascular sensitivity expressed as action potential rate per unit of systolic blood pressure (in Hz/mmHg). Differences in neuronal gain and activation threshold during blood pressure manipulations were compared between groups of NTS neurons receiving either monosynaptic A-fiber, monosynaptic C-fiber, or polysynaptic inputs using ANOVA with the Student-Newman-Keuls method for post hoc comparisons. During VNS, action potentials occurring within the range of arrival times with Search Test, high-intensity vagal shocks were considered directly evoked or synchronous responses to VNS stimuli while all other spikes satisfying the PCA template were considered not synchronous or spontaneous action potentials. The variations in total, evoked (synchronous), or spontaneous neuronal activity between baseline and five consecutive cycles (VNS/OP) of VNS were compared between groups of NTS neurons using repeated-measures ANOVA with Student-Newman-Keuls post hoc comparisons. Note that only neurons that increased their activity level by ≥20% above baseline were considered responsive to VNS (VNS+). For each comparison, the normality of the distribution was first tested with a Shapiro-Wilk test and, when the normality failed, ANOVA on rank was used. Statistically significant differences are indicated by P < 0.05. Summary data are presented as means ± SE.
RESULTS
Identification of NTS Neurons by Vagal Electrical Pulses
Single, high-intensity stimuli to the cervical trunk of the vagus activated action potentials in 67 medial NTS neurons. In all of these vagally activated neurons, examination of the latency of these electrically stimulated spikes as well as responses to closely timed paired pulses identified neurons as directly or indirectly connected to the vagus. Such responses also determined whether the afferent vagal axon was myelinated. Each neuron was additionally tested for activity changes during alterations in blood pressure to determine whether they were cardiovagal (Cardio+). As expected, over half of these vagally activated neurons responded to changes in blood pressure (41/67 neurons). As observed by others, no neuron showed obvious cardiac rhythmicity in their basal action potential activity (50). These Cardio+ NTS neurons (n = 41) as well as Cardio− NTS neurons (n = 26) were located at depths of 300–1,105 µm from the dorsal surface of the brain stem in 12 rats. In all terminal experiments, four to eight neurons were recorded per animal.
Forty-six of these NTS cells were considered monosynaptically activated by vagal afferents based on their consistent spike timing and successful activation of action potentials in response to both stimuli in the paired-pulse test (Fig. 1). However, 21 neurons failed the paired-pulse test and were considered to be activated by a polysynaptic pathway from the vagal afferent input. The underlying PCA profiles collected highly consistent action potential waveforms that were used to calculate spike rates. The arrival times for monosynaptic inputs fell into two groups: early and late arriving spikes. The early arriving action potentials averaged an onset vagal latency of 6.1 ms (jitter or SD of latency averaged 1.6 ms, range: 4–8 ms, n = 4) and corresponded to myelinated A-fiber axon conduction velocities of >3.5 m/s. Late arriving spikes averaged an onset latency of 25.4 ms (jitter averaged 5.2 ms, range: 15–37 ms, n = 42) and corresponded to unmyelinated C-fiber axon conduction velocities of <2 m/s. Such calculations underestimate myelinated conduction velocity in particular since they are based on action potentials triggered by excitatory postsynaptic potentials, a translation process that has an intrinsic lag plus the unknown amount of slowing that occurs centrally with the loss of myelin at branch points within NTS (31). NTS neurons with myelinated vagal afferents had higher spontaneous activity (13.2 ± 3.2 Hz) than their C-fiber-receiving counterparts (6.1 ± 0.7 Hz, P = 0.02). Medial NTS neurons activated by polysynaptic vagal afferents failed the paired-pulse test and averaged an onset latency of 34.5 ms (jitter averaged 18.7 ms, range: 21–64 ms, n = 21) with low spontaneous neuronal activity of 2.9 ± 0.9 Hz. It should be noted that despite the very high-intensity for Search Test stimuli, a level that activates all axons, electrical stimuli never triggered more than one synchronous spike. Thus, NTS second-order neurons were either activated by an early monosynaptically activated spike or a slowly conducted monosynaptic input with no evidence for both within a single NTS neuron. Such results suggest that medial NTS second-order neurons received either A-fiber inputs or C-fiber inputs from the cervical vagus but not both and that C-fiber-activated neurons greatly outnumbered A-fiber neurons.
Response Patterns to Blood Pressure
In each neuron, the electrical characterization identified the nature of the vagal afferent with regards to pathway and afferent myelination. The PCA analytic profile defined a unique action potential as the signature of each NTS neuron for discrimination from all other activity in our recordings. After this electrical characterization, tests using blood pressure examined the activity of PCA-segregated action potentials to these physiological stimuli. All NTS neurons in which action potential activity was positively correlated with changes in blood pressure were designated Cardio+. Note that the PCA signal segregation assured that the physiological responses (blood pressure) were from the same neurons that were activated by the vagal Search Test pulses. In blood pressure-responsive neurons, the action potential firing rate often changed little during pressure decreases (nitroglycerin) from rest but increased with pressure increases (phenylephrine) above resting levels (Fig. 2A). Separate linear regressions of pressure versus activity analyzed responses to pressure decreases and to pressure increases. These regression slopes represent the systolic pressure sensitivity or gain for neuronal activity (in Hz/mmHg). The intersection between the null slope and gain slope was considered the pressure threshold for each neuron (Fig. 2B). On average (Fig. 2C), decreases in systolic blood pressure (nitroglycerin) failed to alter heart rate, whereas increased systolic blood pressure (phenylephrine) significantly decreased heart rate (P < 0.05). The latency analysis to electrical vagal stimulation shows that NTS neurons with monosynaptic vagal A-fiber inputs were greatly outnumbered by neurons with monosynaptic vagal C-fiber inputs. Polysynaptically conducting vagal inputs activated about a third of the vagal NTS neurons, and these proportions subdivided by conduction class were similar between Cardio+ and Cardio− neurons (Fig. 3A). The average sensitivity (gain) for Cardio+ cells receiving monosynaptic A-fiber afferents was more than double (0.71 ± 0.11 Hz/mmHg, n = 4) that of neurons receiving monosynaptic C-fiber inputs (0.27 ± 0.04 Hz/mmHg, n = 11, P < 0.05). NTS neurons connected to the vagus via a polysynaptic path were positively correlated to systolic pressure but relatively insensitive to changes in blood pressure (0.16 ± 0.04 Hz/mmHg, n = 6, P < 0.05; Fig. 3B). The mean systolic pressure threshold for neurons receiving monosynaptic A-fiber inputs was lower (138 ± 4.3 mmHg, n = 4) and less variable than neurons with C-fiber (160 ± 6.9 mmHg, n = 9) or polysynaptic (155 ± 7.1 mmHg, n = 6) vagal inputs (P < 0.05; Fig. 3C). In Cardio– NTS neurons, pressure changes had no effect on activity (result not shown).
Fig. 3.
Summaries comparing neuron pathway type of NTS neurons for both Cardio+ and Cardio− neurons. A: C-fiber conducting inputs from the vagus activated the largest proportion of second-order neurons regardless of cardiovascular responsiveness. A-fiber vagal second-order neurons were the rarest subset, with none being found in the Cardio– group. B and C: pressure sensitivity (in Hz/mmHg; B) and pressure thresholds (in mmHg; C) in cardiovascular responsive NTS neurons. Values were determined by regression fits, as described in Fig. 2. Monosynaptic A-type vagal afferents were associated with significantly higher pressure sensitivity and lower pressure thresholds compared with C-type monosynaptic or polysynaptic pathways. Polysynaptic pathway responses to pressure manipulations were not different from C-type pathways. *P < 0.05, significantly different.
VNS on NTS Neurons
Once Search Test maximal stimuli had identified NTS neurons receiving vagal afferent inputs and pressure challenges confirmed pressure responsiveness or not, we initiated studies of the effects of acute VNS conditioning on these Cardio+ and Cardio− NTS neurons. This protocol (Fig. 4) was fixed across all neurons. Note that within the protocol at the onset of VNS, the intensity ramped up to the target intensity and we used this ramp up to determine the consistency of the electrical threshold for the vagal afferent activated. VNS conditioning began with 0.5 BI for five complete cycles (VNS/OPs). After an ~10-min pause in all electrical stimulation to allow for recovery, VNS conditioning was stepped up to 1.0 BI for another five cycles. On closer examination, action potentials occurring during VNS could be classified as evoked (eAP), that is, they were synchronized to each vagal pulse within the Search Test range of arrival times, or spontaneous and unsynchronized (sAP) (Fig. 4B). Distributions of eAP and sAP in relation to each vagal stimulus generated at 20 Hz (50-ms interval) during the first VNS cycle (VNS 1) showed that generally ~25% of vagal stimuli resulted in successful eAPs (5 Hz evoked rather than 20 Hz), and this neuron was representative of responses of A-fiber innervated second-order NTS neuron (Fig. 4C).
Fig. 4.
Vagal nerve stimulation (VNS) allowed the determination of vagal afferent intensity thresholds as well as discriminating evoked action potentials (eAP) from spontaneous action potentials (sAP). A: cycles of VNS began with progressive increases in stimulation intensity [relative to 1.0 bradycardic intensity (BI)] (ramp up) over 2 s with each pulse separated by 50 ms (20-Hz stimulation) followed by a constant current output for 14 s and terminated by a ramp down of 2 s. In this example, the plateau current level was set at the 1.0 BI and evoked a decrease in BP of 5% during VNS. BP promptly recovered in the off phases [off phase (OP) 1] of 44 s that completes the first cycle. B: by monitoring whether the NTS neuron responded with an eAP during the initial ramp up, we noted the electrical recruitment intensity thresholds. Open circles indicate spike failures based on the anticipated arrival time. Filled circles mark successfully evoked APs. Bottom: relative shock amplitude (compared with 1.0 BI) during the first VNS cycle (VNS 1). Two subthreshold pulses (P1 and P2) failed to evoke APs, but P3 successfully evoked an action potential (eAP) with a 0.6-mA threshold value and a latency of 6.2 ± 0.1 ms. Note that despite higher intensity, P4 failed to evoke an AP, whereas P5 successfully evoked an action potential. The action potential latencies indicated an A-fiber type with a calculated conduction velocity of >3.5 m/s. C: histogram of VNS activity relative to the stimulus onset. The earliest bin collected the eAP with a success rate of ~25% from 5 Hz to a rate of 20-Hz vagal stimuli. Note that sAPs also occurred that were not temporally synched to vagal stimuli and arrived between 10 and 50 ms.
The unique action potential profiles calculated by PCA templates allowed us to recognize the discharge of separate NTS neurons recorded at a single electrode site. Interestingly, such analyses detected the simultaneous responses of two distinct neurons to identical test protocols. Such paired recordings show that physically adjacent neurons (a single electrode location) had unique response profiles with respect to cardio class, conduction class, connectivity to the vagus (monosynaptic vs. polysynaptic), and responsiveness to VNS (Fig. 5). In a representative paired NTS recording (Fig. 5), one NTS neuron showed second-order neuron characteristics of a monosynaptic, vagal A-fiber and increased its discharge robustly during 1.0 BI VNS (VNS+, Fig. 5, top). Partitioning the action potentials of this Cardio+ neuron into eAPs and sAPs showed that a large portion of the increased discharge during VNS was not synchronized to the VNS stimuli. Note that in this case (Fig. 5, top), 1.0 BI appears to be suprathreshold for this vagal input and successfully activated the incoming vagal fiber. The adjacent NTS neuron (Fig. 5, bottom) was clearly polysynaptically coupled to the vagus as it increased its activity during Search Test intense stimuli and failed the paired-pulse test, yet neither VNS at 1.0 BI (VNS−) nor cardiovascular stimuli affected the discharge of this NTS neuron (Cardio−). As expected in this VNS– neuron, all of the action potentials were not synchronized to vagal stimuli and the sAP rate was constant throughout VNS conditioning. Note that 1.0 BI VNS was subthreshold in intensity for the vagal input detected with the high-intensity Search Test.
Fig. 5.
Simultaneously recorded pairs of NTS neurons allowed comparison of neurons lying in close proximity within the NTS using a single electrode position. The example shows the activity of a monosynaptic A-fiber second-order NTS neuron together with a polysynaptic vagal higher-order NTS neuron. VNS conditioning augmented the activity in the second-order neuron (top, VNS+) that other test not shown also increased to pressure changes (Cardio+), whereas the adjacent higher-order vagal neuron was unaffected by VNS (VNS−) or pressure (Cardio−). The spontaneous (sAP) and evoked (eAP) activity during VNS for each neuron is indicated at the bottom of each VNS cycle. Note that no eAPs were found in the polysynaptic neuron, as expected.
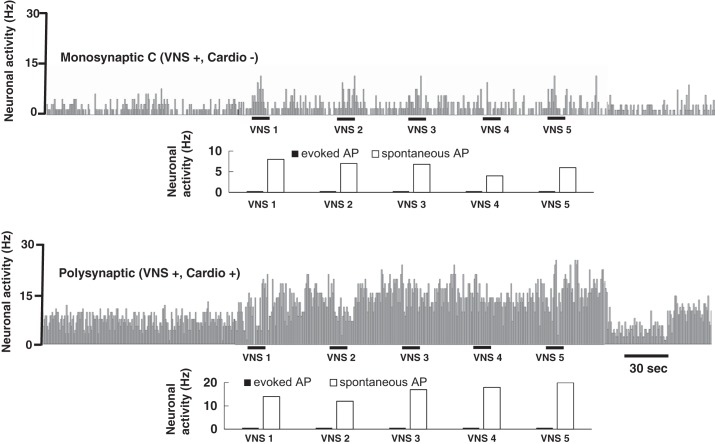
In some paired recordings of NTS neurons, VNS conditioning increased activity in both neurons but with greatly differing response patterns (Fig. 6). Monosynaptic C-fiber second-order neurons that responded to VNS increased only their spontaneous rate of action potentials (Fig. 6, top). Since no synchronous evoked action potentials were detected, 1.0 BI VNS was subthreshold for activation of all monosynaptic C-fiber vagal afferents that we detected. In contrast, many polysynaptic vagal NTS neurons responded very robustly to VNS conditioning and this increase in vagally unsynchronized activity (sAPs) persisted well into the OPs of the VNS protocol (no vagal stimuli) to greatly exceed baseline activity levels. Thus, 0.5 and 1.0 BI VNS appear to activate only myelinated vagal afferents, but nonetheless these myelinated vagal inputs act indirectly as many NTS neurons and are capable of substantially activating second-order neurons with only C-fiber direct vagal inputs as well as polysynaptic, higher-order NTS neurons.
Fig. 6.
The major effect of VNS was to increase in NTS neuron activity that was not synchronized to the VNS stimuli. Paired recordings show that only sAP activity increased both in a C-fiber second-order, Cardio− NTS neuron (top) as well as a polysynaptically coupled Cardio+ vagal higher-order neuron (bottom). In neither neuron did 1.0 BI VNS activate eAPs. However, VNS augmented spontaneous (sAP) activity to a much greater extent in the higher-order neuron, which extended into the OPs.
VNS on Monosynaptic A-Fibers
Across our recorded NTS neurons with myelinated vagal inputs, 0.5 BI VNS did not increase activity (Fig. 7A). When intensity was raised to BI, VNS increased activity levels during every iteration of the active phase of stimulation (VNS 1–5) as well as the OPs (OP 1–5) compared with pre-VNS conditioning. At BI VNS, three of four neurons significantly increased (P < 0.05) their activity level during the VNS periods compared with OP (VNS+, Fig. 7B). One neuron failed to respond to even BI (VNS−). On average (Fig. 7C), sAP exceeded evoked activity during VNS and that did not change throughout VNS. eAP increased between VNS 1 and VNS 5 (P < 0.05, Fig. 7C). When the vagal monosynaptic threshold was monitored using the ramp up in intensity in the first VNS cycle (Fig. 4), distinct electrical thresholds were stable, suggesting that peripheral vagal excitability was unaltered by VNS conditioning.
Fig. 7.

Summary of VNS responses in A-fiber second-order NTS neurons (n = 4). Note all these were Cardio+ and no Cardio– A-fiber neurons were detected. VNS consisted of cycles with active stimulation at 20 Hz (250-µs pulse duration) lasting 18 s followed by 44 s of no stimulation (OP). Five cycles of VNS (VNS 1, VNS 2, etc.) were performed, first at 0.5 BI and then at 1.0 BI. Basal activity was measured as activity for 6 s preceding VNS (BASE). Neurons whose activity during VNS increased were considered VNS+ (squares), whereas other neurons were VNS – (circles) with ±SE. BASE is plotted as a shaded gray band of activity ± SE. A: the weakest stimuli, 0.5 BI VNS, failed to activate any activity response in these A-fiber second-order NTS neurons. B: at 1.0 BI, VNS activated 3 of 4 NTS neurons during the active phase of VNS compared with the OP (P < 0.05). The single VNS– A-fiber second-order neuron was unaffected by 1.0 BI VNS. C: partitioning evoked from spontaneous action potentials showed greater increases in sAPs than eAPs from NTS neurons receiving monosynaptic A-fiber inputs: *P < 0.05, significant main effect of VNS compared with OP using repeated-measures ANOVA on ranks; #P < 0.05, significantly higher compared with the evoked action potential at VNS 1.
VNS on Monosynaptic C-Fibers
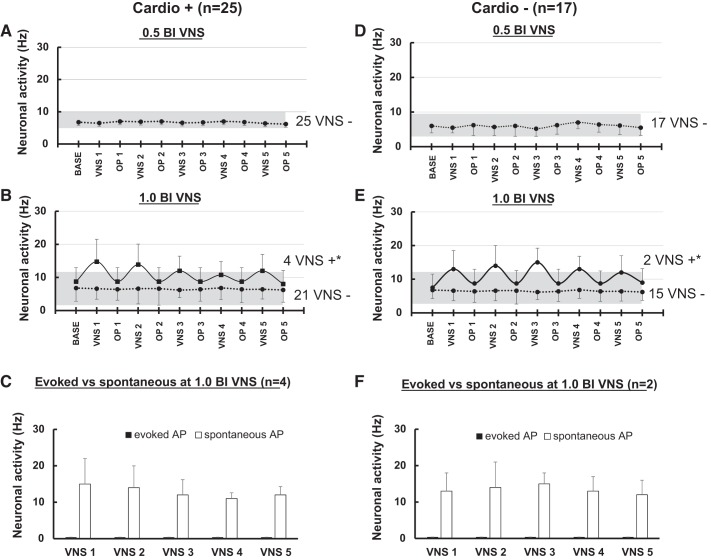
Identical analyses of activity performed in NTS second-order neurons receiving vagal C-fibers (n = 42) suggested that Cardio+ (Fig. 8, A–C) and Cardio− neurons (Fig. 8, D–F) responded similarly. For all NTS neurons receiving C-fiber input, VNS conditioning with 0.5 BI intensities had no effect on activity. Increasing intensity to 1.0 BI VNS significantly increased activity during the active phase of VNS stimulation in a small subset of these neurons compared with OP (P < 0.05): four VNS+/Cardio+ (Fig. 8B) and two VNS+/Cardio− (Fig. 8E). The majority of second-order NTS neurons with C-fibers were unaltered by even 1.0 BI VNS (VNS−). Close examination of VNS+ second-order C-fiber NTS neurons indicated that VNS increased spontaneously (sAP) by a constant amount across all cycles of VNS (Fig. 8, C and F) and no synchronized spikes were detected within the arrival times established in the initial Search Test protocols.
Fig. 8.
Summary of VNS conditioning of C-fiber second-order NTS neurons. Cardio+ neurons (n = 25) and Cardio− neurons (n = 17) showed similar response patterning to VNS. A and D: weak 0.5 BI VNS failed to alter action potentials in these neurons. All activity was spontaneous and equaled basal rates (gray banding). B and E: the increase to 1.0 BI VNS activated a small subset (VNS+) of neurons, 4 Cardio+ and 2 Cardio−. C and F: all increased action potential activity in response to VNS was spontaneous (sAP) in VNS+ neurons. *P < 0.05, significant main effect of VNS compared with nonstimulating phases using repeated-measures ANOVA on ranks.
VNS on Polysynaptic Fibers
Surprisingly, across higher-order NTS neurons (n = 21), two-thirds of these neurons responded to VNS conditioning (VNS+, Fig. 9). Interestingly, half of these neurons showed increased sAP activity at the very weakest intensity tested (0.5 BI, P < 0.05; Fig. 9, A and C, red lines) during the active stimulation phases (VNS) and activity promptly declined to basal levels during the OPs. Raising intensity to 1.0 BI VNS recruited more neurons, and this pattern was similar whether Cardio+ or Cardio− NTS neurons (9/12 neurons for Cardio+ and 7/9 for Cardio−) and the magnitude of the VNS-induced sAP activity levels increased as well. NTS neurons that were activated at 0.5 BI (red lines) were also activated at 1.0 BI (red lines). Five additional Cardio+ neurons and four Cardio− neurons were activated at 1.0 BI (green lines). Unlike second-order NTS neurons, the higher-order neuron activity steadily increased with prolonged VNS conditioning (VNS 5 > VNS 1). At 1.0 BI VNS, VNS+ neurons increased their spontaneous activity compared with OP with (P < 0.05), and a Student-Newman-Keuls post hoc analysis revealed that neuronal activities during OP 4 and 5 were higher (P < 0.05) than baseline or OP 1 for both Cardio+ and Cardio− (Fig. 9, B and D) neurons (green lines). This overall result suggests that most higher-order NTS neurons are activated by VNS conditioning that on average raises their action potential rate by more than double during acute bouts of uptitrated intensities of activation.
Fig. 9.
Summary of VNS conditioning of vagal higher-order NTS neurons. Cardio+ and Cardio− neurons responded to VNS with the distinct that weak (0.5 BI) as well as therapeutic 1.0 BI VNS increased activity. A: weak VNS (0.5 BI) modestly increased sAP activity similarly in 4 Cardio+ and 3 Cardio− NTS neurons. B: when there was an increase to 1.0 BI, VNS increased activity in most higher-order NTS neurons, 9 Cardio+ and 7 Cardio−. Red lines indicate the responses of neurons activated at 0.5 BI to 1.0 BI VNS. Green lines indicate the activity of neurons that responded only at 1.0 BI VNS. Additionally, post hoc analysis showed that the VNS+ neurons that were activated exclusively at 1.0 BI (green lines) in Cardio+ and in Cardio− neurons maintained increased activity during OP 4 and OP 5. *P < 0.05, significant main effect of VNS compared with nonstimulating phases using repeated-measures ANOVA on ranks; #post hoc Student-Newman-Keuls tests showing higher activity level compared with baseline (BASE) and OP 1.
VNS-Induced Bradycardia
Conditioning with the higher, 1.0 BI level of VNS produced significant if modest bradycardia only during the active stimulation phase of VNS (P > 0.05, Fig. 10) but failed to alter heart rate during the OP. There was no evidence of increasing bradycardia progressing from VNS 1 through VNS 5 (Fig. 10).
Fig. 10.

Summary of heart rate changes associated with VNS conditioning at 1.0 BI. VNS significantly induced bradycardia during active VNS but recovered to baseline (BASE) during OP. Data shown are relative changes compared with baseline heart rate ± SE. *P < 0.05, significant effect of VNS compared with both baseline and OP.
DISCUSSION
Activation of the cervical vagus nerve trunk is the focus of multiple VNS clinical trials and preclinical testing for beneficial outcomes. The spectrum of diseases is surprisingly broad, with approved treatments of pharmacologically resistant epilepsy and depression and new trials on heart failure (12, 51). The present studies focused on the impact of delivering acute VNS-conditioning stimuli shaped by clinical protocol recommendations on the action potentials of NTS neurons. Therapeutic VNS regimes dictate relatively mild-intensity shocks limited by side effects and delivered at constant frequency followed by a lengthy pause (12). In mimicking the clinical stimuli, our chief findings on NTS neurons in anesthetized rats include the following listed here. First, relatively weak-intensity VNS stimuli increased activity in subsets of all NTS neuron subtypes recorded: both vagal afferent second-order neuron classes, monosynaptic A-fiber or monosynaptic C-fiber, as well as higher-order vagal NTS neurons contacted only by polysynaptic vagal inputs. Second, both cardiovascular responsive and modality unidentified (Cardio−) NTS neurons were affected in similar proportions. Third, VNS intensities activated only myelinated vagal afferents as evident by monosynaptic drive of synchronized action potentials despite modest success rates (~25% of VNS stimuli successfully triggered synchronous action potentials). Fourth, the strongest VNS intensity (1.0 BI) was subthreshold and failed to activate any synchronized spikes in NTS second-order neurons with unmyelinated vagal afferent inputs. Fifth, most VNS-enhanced action potentials were unsynchronized (spontaneous activity) in both Cardio+ and Cardio– neurons. Sixth, subclinical intensity of VNS (0.5 BI) successfully augmented activity and 1.0 BI recruited additional neurons only in vagal higher-order (polysynaptic) NTS neurons. Together, such results indicate that afferent excitation during VNS relies likely solely via myelinated afferent activation but that augmentation of indirect or network activation broadly impacts the activity of NTS neurons with potentially important ramifications for better understanding therapeutic efficacy and invite a comprehensive reconsideration of the operational rules of therapeutic regimes.
Myelinated Versus Unmyelinated Vagal Afferents
In general, the medial NTS neurons that we encountered were typical of other reports in rats and other species (19). In cat NTS neurons, high-intensity vagal stimuli triggered single action potentials or two in a close time cluster after the stimulus (i.e., synchronous evoked responses), and the calculated conduction velocities were either long or short and never both (i.e., they either received a myelinated, fast conducting input or a late arriving excitation that was consistent with a C-fiber afferent axon) (7, 14, 19). All the studied NTS neurons had spontaneous activity at their prevailing baseline blood pressure similar to NTS recordings in other anesthetized animals (18, 41, 52, 54). Overtly cardiovascular rhythms in NTS neuron activity were not readily apparent as variably reported by others (49, 50). In the present study in rats, 90% of the medial NTS, second-order neurons received a single, unmyelinated vagal afferent input to supramaximal cervical stimulation. Ten percent of our second-order NTS neurons received one A-fiber afferent input, a result that is consistent with estimates from brain slice assays of medial NTS work in the rat and mouse (1). While A- and C-fiber cranial afferents synaptically terminate in similar regions of the medial NTS, convergence of A/C-fiber inputs is limited and these neural circuits are “sorted” to either A-fiber or C-fiber neural circuits (4, 30, 46), which can remain separated even through projections to the forebrain (37). C-fibers activate strong reflexes even at frequencies as low as 2–5 Hz, which fail to activate selective A-fiber cardiovascular reflex responses (25).
VNS Activates NTS Neurons
Here, we focused on directly addressing the relative participation of myelinated and unmyelinated vagal afferents in response to clinically styled VNS. We measured NTS neuron action potentials and used the temporal relationship between vagal activation and spike arrival to determine conduction velocity and the pathway from vagal primary afferents (second or higher order). This focus on timing as the key to understanding the relationship between afferent type and the path of excitation led us to realize that VNS conditioning predominantly activates action potentials indirectly. The maximal Search Test intensity facilitated localization of individual neurons and determined the number of vagal inputs along with the organizational order of the pathways connecting from the vagus to each NTS neuron. For conditioning stimuli, we set VNS intensity to emulate 1.0 BI used in therapeutic practice but also tested 0.5 BI to gauge a potential dosing effect of VNS intensity. Our tests with induced changes in blood pressure allowed us to understand broadly whether cardiovascular neurons were distinct from cardiovascular unresponsive neurons that were near neighbors. This latter tests suggests that impact of VNS appears to be rather global on activity among vagally responsive NTS neurons regardless of visceral system.
VNS as practiced clinically elicits minor bradycardia: a finding replicated in our animal model. The origin of the bradycardia seems unresolved even in animals. Activation of cardiac parasympathetic fibers might occur by reflex activation of central pathways or by direct VNS activation of cardiac parasympathetic axons within the vagus nerve trunk.
Afferent Bias of Weak-Intensity VNS
In tests designed to emulate clinical style stimulation, relatively weak-intensity BI VNS directly activated only myelinated vagal afferents to evoke synchronized action potentials (eAP) in a small subset of second-order NTS neurons. No VNS synchronized action potentials were found in second-order neurons with C-fiber vagal afferents indicating that full 1.0 BI VNS was sufficiently intense to recruit only myelinated vagal afferents. Indeed, many of the NTS neurons studied here did not respond to VNS conditioning at all (VNS−). Most surprisingly, however, VNS significantly augmented spontaneous discharge in both second-order as well as higher-order NTS neurons. We found no evidence of anodal block of vagal afferents that is often suggested to “steer” VNS to favor vagal efferent activation (15, 16, 45). Interestingly, even weaker VNS (0.5 BI) nonetheless produced substantial increases in unsynchronized action potentials, particularly in higher-order neurons. Many of these higher-order NTS neurons augmented their spontaneous action potential rate further in the transition from 0.5 to 1.0 BI VNS, a finding consistent with integration of vagal-derived information in these higher-order neurons.
By definition, this excitatory drive initiated by VNS arrived at higher-order neurons indirectly (unsynchronized) by an unknown intermediary neuron that must have included a second-order NTS neuron. The low intensity of the stimulus can only be accounted for by activation in the peripheral vagus of a very sensitive sensory axon-highly myelinated axon. Such pathway activity might be of therapeutic impact and warrants explicit studies to detect the influence of graded VNS “doses” (12). Elevated network activity indicated by the increased presence of unsynchronized activity may represent sensitization or sustained activation of neurons within NTS and was strongest in higher-order neurons, which were proportionally most sensitive to VNS conditioning. Long-lasting changes in activity are often associated with phenomena such as the induction of long-term potentiation (LTP). Unlike the present results, LTP phenomena generally induce changes lasting >10–20 min, well beyond anything observed in our study. Acute lumbar spinal nerve stimulation causes LTP in the somatosensory cortex (27), and vagal nerve stimulation might induce a similar sensitized state impacting NTS neurons and raising the possibility of bridging from acute to chronic therapy (8).
The Case for Contributions From Potentially Longer Paths of Vagal Excitation to the NTS
Given that 0.5 BI VNS successfully activated multisynapse excitatory inputs, this finding suggests that augmentation observed in higher-order NTS neurons is initiated by the very largest and therefore most sensitive vagal myelinated axons. While these delayed responses (unsynchronized action potentials) could represent intra-NTS network activation, it is conceivable that myelinated vagal afferents might activate alternative paths to higher-order NTS neurons. We clearly cannot rule out the contribution of supra-NTS reciprocal activation, long pathways from the brain stem and higher pathways (13, 35). The timing of unsynchronized activity suggests arrival times that are physically within the realm of well-known reciprocal connections between NTS and, for example, supramedullary neurons in the forebrain. In contrast to second-order NTS neurons, higher-order NTS neurons showed elevated activities that progressively augmented activity across successive VNS cycles. Such polysynaptically afferent-linked NTS neurons are rarer in general than second-order neurons (20) and are disproportionately represented in the NTS neurons that project axons to the forebrain including the paraventricular nucleus of the hypothalamus (6) and central nucleus of the amygdala (37). Activation of such extra-NTS neurons might supply reverberant excitation that is initiated by VNS and returns to excite NTS targets. We speculate based on the timing and the known anatomical connections that such extra-NTS neurons including forebrain neurons might well supply effector connections to other therapeutic targets within the CNS or provide impact on therapeutic efficacy of VNS (29) and VNS-activated c-Fos in such key regions (15).
Together, our NTS neuron experiments demonstrate that simulated therapeutic VNS reliably and selectively activates myelinated vagal afferents to augment both vagally synchronized activity as well as spontaneous or unsynchronized activity. Our findings suggest that the approach to therapeutic VNS might benefit from a greater focus on the potential for selective activation of myelinated vagal afferents as well as the CNS pathways activated to optimize clinical benefits. Our observations support the interesting potential prospect of “downtitrating” VNS stimulus intensity while retaining or perhaps even improving therapeutic outcomes. This latter might come about because of the sparse, highly segregated network properties of autonomic pathways activated by vagal afferents (37).
Perspectives
This new study suggests key contributions of CNS processing in response to VNS. Our study identified the direct activation of myelinated vagal afferents to drive NTS neuron activity directly but indicate a quantitatively major increment in action potential indirectly coupled to vagal afferent activation that appears more stochastic and sensitized by VNS therapy. Because of their disproportional representation in highly integrative projection neuron pathways to key autonomic centers, the identification of vagally coupled NTS higher-order neurons has important implications for better understanding the CNS mechanisms contributing to the therapeutic benefit of VNS. Future investigations need to be directed explicitly to reveal the involvement of higher CNS pathways. Basic science studies have clearly indicated that A- and C-fiber vagal afferents activate different CNS pathways, which might provide different benefits depending on the VNS dose. It remains possible that noncardiovagal pathways are important to therapeutic benefits and might help to better address optimal stimulation across cardiac as well as other diseases like epilepsy and depression.
GRANTS
This work was supported by Cyberonics (Houston, TX) (to E. Beaumont, Principal Investigator), Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Merit Review Award BX002332 (to K. Singh), and National Heart, Lung, and Blood Institute Grants R15-HL-129140 (to K. Singh) and R01-HL-133505 (to M. C. Andresen).
DISCLOSURES
B. KenKnight and I. Libbus are employees of Cyberonics, Inc./LivaNova PLC.
AUTHOR CONTRIBUTIONS
E.B., M.C.A., K.S., and B.H.K. conceived and designed research; E.B., R.P.C., and S.L.S. performed experiments; E.B., R.P.C., S.L.S., L.S., and N.C. analyzed data; E.B., R.P.C., M.C.A., S.L.S., and K.S. interpreted results of experiments; E.B. and R.P.C. prepared figures; E.B. and M.C.A. drafted manuscript; E.B., R.P.C., M.C.A., S.L.S., K.S., I.L., B.H.K., L.S., and N.C. edited and revised manuscript; E.B., R.P.C., M.C.A., S.L.S., K.S., I.L., B.H.K., L.S., and N.C. approved final version of manuscript.
REFERENCES
- 1.Andresen MC, Hofmann ME, Fawley JA. The unsilent majority-TRPV1 drives “spontaneous” transmission of unmyelinated primary afferents within cardiorespiratory NTS. Am J Physiol Regul Integr Comp Physiol 303: R1207–R1216, 2012. doi: 10.1152/ajpregu.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 3.Andresen MC, Kunze DL, Mendelowitz D. Central nervous system regulation of the heart. In: Basic and Clinical Neurocardiology, edited by Armour JA, Ardell JL. New York: Oxford Univ. Press, 2004. [Google Scholar]
- 4.Andresen MC, Peters JH. Comparison of baroreceptive to other afferent synaptic transmission to the medial solitary tract nucleus. Am J Physiol Heart Circ Physiol 295: H2032–H2042, 2008. doi: 10.1152/ajpheart.00568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardell JL, Rajendran PS, Nier HA, KenKnight BH, Armour JA. Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am J Physiol Heart Circ Physiol 309: H1740–H1752, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey TW, Hermes SM, Andresen MC, Aicher SA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. J Neurosci 26: 11893–11902, 2006. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber WD, Yuan CS. Brain stem responses to electrical stimulation of ventral vagal gastric fibers. Am J Physiol Gatrointest Physiol 257: G24–G29, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Beaumont E, Guevara E, Dubeau S, Lesage F, Nagai M, Popovic M. Functional electrical stimulation post-spinal cord injury improves locomotion and increases afferent input into the central nervous system in rats. J Spinal Cord Med 37: 93−100, 2014. doi: 10.1179/2045772313Y.0000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaumont E, Salavatian S, Southerland EM, Vinet A, Jacquemet V, Armour JA, Ardell JL. Network interactions within the canine intrinsic cardiac nervous system: implications for reflex control of regional cardiac function. J Physiol 591: 4515–4533, 2013. doi: 10.1113/jphysiol.2013.259382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaumont E, Southerland EM, Hardwick JC, Wright GL, Ryan S, Li Y, KenKnight BH, Armour JA, Ardell JL. Vagus nerve stimulation mitigates intrinsic cardiac neuronal and adverse myocyte remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol 309: H1198–H1206, 2015. doi: 10.1152/ajpheart.00393.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaumont E, Wright GL, Southerland EM, Li Y, Chui R, KenKnight BH, Armour JA, Ardell JL. Vagus nerve stimulation mitigates intrinsic cardiac neuronal remodeling and cardiac hypertrophy induced by chronic pressure overload in guinea pig. Am J Physiol Heart Circ Physiol 310: H1349–H1359, 2016. doi: 10.1152/ajpheart.00939.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byku M, Mann DL. Neuromodulation of the failing heart: lost in translation? JACC Basic Transl Sci 1: 95–106, 2016. doi: 10.1016/j.jacbts.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Card JP, Sved AF. Central autonomic pathways. In: Central Regulation of Autonomic Functions, edited by Llewellyn-Smith IJ, Verberne HJ. New York: Oxford Univ. Press, 2011. doi: 10.1093/acprof:oso/9780195306637.003.0001. [DOI] [Google Scholar]
- 14.Chambert G, Kobashi M, Adachi A. Convergence of gastric and hepatic information in brain stem neurons of the rat. Brain Res Bull 32: 525–529, 1993. doi: 10.1016/0361-9230(93)90302-R. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham JT, Mifflin SW, Gould GG, Frazer A. Induction of c-Fos and ΔFosB immunoreactivity in rat brain by vagal nerve stimulation. Neuropsychopharmacology 33: 1884–1895, 2008. doi: 10.1038/sj.npp.1301570. [DOI] [PubMed] [Google Scholar]
- 16.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ; CardioFit Multicenter Trial Investigators . Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 32: 847–855, 2011. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 17.Dicarlo L, Libbus I, Amurthur B, Kenknight BH, Anand IS. Autonomic regulation therapy for the improvement of left ventricular function and heart failure symptoms: the ANTHEM-HF study. J Card Fail 19: 655–660, 2013. doi: 10.1016/j.cardfail.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Donoghue S, Felder RB, Gilbey MP, Jordan D, Spyer KM. Post-synaptic activity evoked in the nucleus tractus solitarius by carotid sinus and aortic nerve afferents in the cat. J Physiol 360: 261–273, 1985. doi: 10.1113/jphysiol.1985.sp015616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donoghue S, Fox RE, Kidd C, Koley BN. The distribution in the cat brain stem of neurones activated by vagal nonmyelinated fibres from the heart and lungs. Q J Exp Physiol 66: 391–404, 1981. doi: 10.1113/expphysiol.1981.sp002582. [DOI] [PubMed] [Google Scholar]
- 20.Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol 85: 2213–2223, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Ezure K, Tanaka I. Contralateral projections of barosensitive neurons of the nucleus tractus solitarii. Neurosci Lett 219: 37–40, 1996. doi: 10.1016/S0304-3940(96)13169-4. [DOI] [PubMed] [Google Scholar]
- 22.Ezure K, Tanaka I. Pump neurons of the nucleus of the solitary tract project widely to the medulla. Neurosci Lett 215: 123–126, 1996. doi: 10.1016/0304-3940(96)12968-2. [DOI] [PubMed] [Google Scholar]
- 23.Fan W, Andresen MC. Differential frequency-dependent reflex integration of myelinated and nonmyelinated rat aortic baroreceptors. Am J Physiol Heart Circ Physiol 275: H632–H640, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Fan W, Reynolds PJ, Andresen MC. Baroreflex frequency-response characteristics to aortic depressor and carotid sinus nerve stimulation in rats. Am J Physiol Am J Physiol Heart Circ Physiol 271: H2218–H2227, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Fan W, Schild JH, Andresen MC. Graded and dynamic reflex summation of myelinated and unmyelinated rat aortic baroreceptors. Am J Physiol Regul Integr Comp Physiol 277: R748–R756, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Fisher RS, Afra P, Macken M, Minecan DN, Bagić A, Benbadis SR, Helmers SL, Sinha SR, Slater J, Treiman D, Begnaud J, Raman P, Najimipour B. Automatic vagus nerve stimulation triggered by ictal tachycardia: clinical outcomes and device performance–the U.S. E-37 trial. Neuromodulation 19: 188–195, 2016. doi: 10.1111/ner.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffin KM, Pickering M, O’Herlihy C, O’Connell PR, Jones JF. Sacral nerve stimulation increases activation of the primary somatosensory cortex by anal canal stimulation in an experimental model. Br J Surg 98: 1160–1169, 2011. doi: 10.1002/bjs.7536. [DOI] [PubMed] [Google Scholar]
- 28.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 29.Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. Invited Review EB 2012: C1 neurons: the body’s EMTs. Am J Physiol Regul Integr Comp Physiol 305: R187–R204, 2013. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 24: 4709–4717, 2004. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalia M, Richter D. Morphology of physiologically identified slowly adapting lung stretch receptor afferents stained with intra-axonal horseradish peroxidase in the nucleus of the tractus solitarius of the cat. II. An ultrastructural analysis. J Comp Neurol 241: 521–535, 1985. doi: 10.1002/cne.902410410. [DOI] [PubMed] [Google Scholar]
- 32.Krahl SE, Senanayake SS, Handforth A. Destruction of peripheral C-fibers does not alter subsequent vagus nerve stimulation-induced seizure suppression in rats. Epilepsia 42: 586–589, 2001. doi: 10.1046/j.1528-1157.2001.09700.x. [DOI] [PubMed] [Google Scholar]
- 33.Kunze DL. Reflex discharge patterns of cardiac vagal efferent fibres. J Physiol 222: 1–15, 1972. doi: 10.1113/jphysiol.1972.sp009784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libbus I, Nearing BD, Amurthur B, KenKnight BH, Verrier RL. Autonomic regulation therapy suppresses quantitative T-wave alternans and improves baroreflex sensitivity in patients with heart failure enrolled in the ANTHEM-HF study. Heart Rhythm 13: 721–728, 2016. doi: 10.1016/j.hrthm.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Loewy AD. Central autonomic pathways. In: Central Regulation of Autonomic Functions, edited by Loewy AD, Spyer KM. New York: Oxford Univ. Press, 1990. [Google Scholar]
- 36.Lundgren J, Amark P, Blennow G, Strömblad LG, Wallstedt L. Vagus nerve stimulation in 16 children with refractory epilepsy. Epilepsia 39: 809–813, 1998. doi: 10.1111/j.1528-1157.1998.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 37.McDougall SJ, Guo H, Andresen MC. Dedicated C-fibre viscerosensory pathways to central nucleus of the amygdala. J Physiol 595: 901−917, 2017. doi: 10.1113/JP272898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendelowitz D. Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci 14: 155–161, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labeled aortic baroreceptor terminals on neurons from the nucleus tractus solitarius. Brain Res 581: 339–343, 1992. doi: 10.1016/0006-8993(92)90729-S. [DOI] [PubMed] [Google Scholar]
- 40.Mifflin SW, Spyer KM, Withington-Wray DJ. Baroreceptor inputs to the nucleus tractus solitarius in the cat: modulation by the hypothalamus. J Physiol 399: 369–387, 1988. doi: 10.1113/jphysiol.1988.sp017086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mifflin SW, Spyer KM, Withington-Wray DJ. Baroreceptor inputs to the nucleus tractus solitarius in the cat: postsynaptic actions and the influence of respiration. J Physiol 399: 349–367, 1988. doi: 10.1113/jphysiol.1988.sp017085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nearing BD, Libbus I, Amurthur B, Kenknight BH, Verrier RL, KenKnight BH, Verrier RL. Acute autonomic engagement assessed by heart rate dynamics during vagus nerve stimulation in patients with heart failure in the ANTHEM-HF trial. J Cardiovasc Electrophysiol 27: 1072–1077, 2016. doi: 10.1111/jce.13017. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (2nd ed.). New York: Academic, 1986. [Google Scholar]
- 44.Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods 13: 139–143, 1985. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 45.Perez SM, Carreno FR, Frazer A, Lodge DJ. Vagal nerve stimulation reverses aberrant dopamine system function in the methylazoxymethanol acetate rodent model of schizophrenia. J Neurosci 34: 9261–9267, 2014. doi: 10.1523/JNEUROSCI.0588-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters JH, McDougall SJ, Fawley JA, Andresen MC. TRPV1 marks synaptic segregation of multiple convergent afferents at the rat medial solitary tract nucleus. PLoS One 6: e25015, 2011. doi: 10.1371/journal.pone.0025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH, Anand IS. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 20: 808–816, 2014. doi: 10.1016/j.cardfail.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH, Anand IS, KenKnight BH, Anand IS. Extended follow-up of patients with heart failure receiving autonomic regulation therapy in the ANTHEM-HF study. J Card Fail 22: 639–642, 2016. doi: 10.1016/j.cardfail.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Rogers RF, Paton JF, Schwaber JS. NTS neuronal responses to arterial pressure and pressure changes in the rat. Am J Physiol Regul Integr Comp Physiol 265: R1355–R1368, 1993. [DOI] [PubMed] [Google Scholar]
- 50.Scheuer DA, Zhang J, Toney GM, Mifflin SW. Temporal processing of aortic nerve evoked activity in the nucleus of the solitary tract. J Neurophysiol 76: 3750–3757, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz PJ, La Rovere MT, De Ferrari GM, Mann DL. Autonomic modulation for the management of patients with chronic heart failure. Circ Heart Fail 8: 619–628, 2015. doi: 10.1161/CIRCHEARTFAILURE.114.001964. [DOI] [PubMed] [Google Scholar]
- 52.Seagard JL, van Brederode JF, Dean C, Hopp FA, Gallenberg LA, Kampine JP. Firing characteristics of single-fiber carotid sinus baroreceptors. Circ Res 66: 1499–1509, 1990. doi: 10.1161/01.RES.66.6.1499. [DOI] [PubMed] [Google Scholar]
- 53.Seki A, Green HR, Lee TD, Hong L, Tan J, Vinters HV, Chen PS, Fishbein MC. Sympathetic nerve fibers in human cervical and thoracic vagus nerves. Heart Rhythm 11: 1411–1417, 2014. doi: 10.1016/j.hrthm.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spyer KM, Donoghue S, Felder RB, Jordan D. Processing of afferent inputs in cardiovascular control. Clin Exp Hypertens A 6: 173–184, 1984. [DOI] [PubMed] [Google Scholar]