pulmonary hypertension (PH) is a progressive disease characterized by elevated pulmonary arterial pressure and pulmonary vascular resistance (19). According to the latest estimations, the PH incidence accounts for 1% of the total global population (up to 5 million people), and its rate increases up to 10% in individuals above 65 yr of age (24). The median survival rate for patients diagnosed with PH is predicted to be 2.8 yr (8).
PH develops as a result of vasoconstriction and remodeling in the intra-alveolar pulmonary arteries and leads to progressive right ventricular hypertrophy and cardiac failure (1, 11, 27, 29, 31, 34, 37). Pulmonary vasoconstriction is thought to be an early component of PH and has been primarily attributed to vascular endothelial dysfunction that leads to impaired production of vasodilators such as nitric oxide and prostacyclin as well as overexpression of vasoconstrictors such as endothelin-1 (25). According to recently published data, there are currently 10 drugs from 5 different substance classes available and licensed for the treatment of PH, including endothelin receptor antagonists, phosphodiesterase-5 inhibitors, stimulators of soluble guanylate cyclase, and arachidonic acid pathway-targeted drugs such as prostacyclin analogs and prostacyclin receptor agonists (23). These medications are typically prescribed singly or in combination, and their primary focus is to induce vasodilation of the pulmonary arteries. A recent randomized trial that used a combination of these classes of medications has reported a 40% success rate in patients with PH (18). In addition, the use of some medications, such as endothelin-1 receptor antagonists, remains limited due to their known hepatotoxicity, and several drugs from this class have been already withdrawn from clinical practice (6). These data suggest that other options should be investigated to develop new therapeutic targets to improve outcomes in patients with PH.
While the cyclooxygenase and lipoxygenase metabolites of arachidonic acid metabolism have been well studied and clinically implicated in the prevention of endothelium-targeted vasoconstrictor effects during PH, the cytochrome P-450 (CYP) pathway of arachidonic acid metabolism remains understudied. Because CYP proteins are sensitive to changes in O2 tension and are widely expressed in lung tissues (38), there is an increasing need to further study the effects of CYP on the progression and development of PH. For example, an intriguing recent study (26) by Joshi et al. reported the importance of the Cyp2c44 gene in the progression of PH and showed that Cyp2c44 gene deletion results in increased hypoxia-induced pulmonary artery remodeling and hypertension. The Cyp2c44 gene is broadly expressed, and studies have shown that it may be found in the kidney, liver, and adrenal glands (12, 26, 35). In addition, original studies on arachidonic acid metabolism proposed a primary role for CYP in the generation of EETs; however, more recent studies have reported that Cyp2c44, in particular, is capable of generating 15-hydroxyeicosatetraenoic acid (15-HETE) and that the amount of 15-HETE produced by Cyp2c44 accounts for up to 30% of total 15-HETE production (12).
In a recent study in the American Journal of Physiology-Heart and Circulatory Physiology, Hashimoto et al. (21) proposed a new role for the effects of CYP family proteins on hematopoetic progenitor stem cells in mice. The authors showed that Cyp2c44 gene deletion leads to a decrease in 15-HETE production and to an increase in the numbers of CD133-positive hematopoetic progenitor stem cells. The evidence of the relationship between PH and CD133-positive progenitor cells has been accumulating for the past decade, although the reported relationship was only correlative. As such, increased numbers of circulating and tissue levels of CD133-positive cells have been reported in both preclinical models and patients with PH (2, 14, 28, 30, 32, 36). In addition, transplantation of bone marrow CD133-positive cells from patients with PH into mice resulted in angioproliferative pulmonary vascular remodeling, right ventricular failure, and death, suggesting a pathophysiological mechanism for CD133-positive cells (3). In this study, Hashimoto et al. provided the mechanistic link between the CYP pathway of arachidonic acid metabolism and showed a decrease in 15-HETE production and an increase in th eaccumulation of CD133-positive cells in the adventitia surrounding the medial layer of the remodeled pulmonary arteries in the lungs of Cyp2c44-null mice with PH. These data suggest that Cyp2c44 gene deletion and the subsequent reduction in 15-HETE levels further worsen and actively participate in the remodeling processes during PH. The data produced by Hashimoto et al. support previously published data of the involvement of CD133-positive cells in the development of plexiform lesions in the arteries of patients with PH (7, 33). These findings indicate a possible beneficial effect of 15-HETE in the remodeling of arteries during PH. However, the existing knowledge of the diverse biological actions of 15-HETE (10) indicates a necessity for future studies on the compensatory mechanisms that are activated due to Cyp2c44 gene deletion.
Hashimoto et al. showed that 15-HETE is capable of directly affecting the CD117-positive precursor of endothelial cells. In the in vitro experiments, 15-HETE significantly reduced numbers of CD117-positive cells when they were incubated with bone marrow cells. The authors linked the increased circulating levels of CD117-positive cells and increased expression of von Willibrand factor-positive cells in the lungs of Cyp2c44-null mice, indicating that in addition to active remodeling of the arteries during PH, 15-HETE may also have a strong effect on the formation of neointimal lesions. These data suggest that 15-HETE also plays an important role in altering the proangiogenic responses during PH. In addition, the presence of CD117-positive cells in hypertrophied hearts of Cyp2c44-null mice also indicates possible roles of 15-HETE in heart failure during PH. In 2015, the Global Burden of Disease Study reported >60 million heart failure patients worldwide, and it has been predicted that up to 50% of these patients could develop PH (20). Thus, finding a pathway that plays a role in both the pathology of failing hearts and PH might serve as a potentially effective therapeutic target. However, further studies to dissect the specific roles of 15-HETE in the failing heart and during the development of PH are warranted.
Inflammation is associated with most forms of PH (4, 22). Mononuclear cells activate fibroblasts in the adventitia and pulmonary artery smooth muscle cells in the media, leading to remodeling of the pulmonary arteries in both preclinical models and patients with PH (2, 3, 9, 13–17). The Hashimoto et al. study (21) also showed that Cyp2c44 gene deletion, and the subsequent decrease in 15-HETE production, resulted in an increase of monocytes and macrophages in both the bone marrow and circulation. Following previously published material that indicated that CD133-positive cells can give rise to all hematopoietic lineages (including erythroid cells, myeloid cells, monocytes/macrophages, and megakaryocytes) and the data that granulocyte/monocyte colony formation is increased in PH patients (3, 14), the Hashimoto et al. study suggests a new role for 15-HETE in the mediation of inflammatory responses, the subsequent arteriole wall thickening, and the progression of PH.
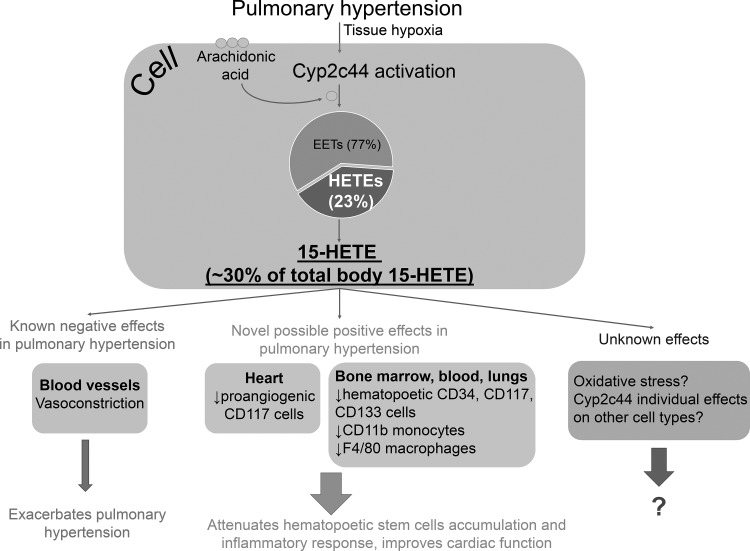
Collectively, these findings suggest that the Cyp2c44 pathway, particularly through regulation of the production of 15-HETE, may have strong implications for the amelioration of PH pathophysiology (Fig. 1). These findings also suggest that targeting the Cyp2c44 pathway may be beneficial for the development of new therapeutic targets to improve the success rate of the currently available treatments for PH.
Fig. 1.
Schematic representation of Cyp2c44 and 15-HETE roles in pulmonary hypertension (PH). Upon activation, Cyp2c44 produces 15-HETE, which accounts for ~30% of total 15-HETE production. During PH, 15-HETE is known to induce vasoconstriction; however, recent findings suggest that 15-HETE may also have beneficial effects in the pathophysiology of PH through the attenuation of hematopoetic stem cell accumulation and the inflammatory response. Future studies are needed to investigate the effects of 15-HETE on oxidative stress and vascular remodeling in PH.
Among future studies to investigate the effects of the Cyp2c44 and 15-HETE in the pathophysiology of PH, special focus should be paid to oxidative stress. A previous study (5) has demonstrated that lung tissue from patients with severe PH exhibits significant oxidative stress and is associated with increased pulmonary production of several HETEs, including 12- and 15-HETE, as well as 5-oxo-eicosatetraenoic acid, suggesting possible roles in Cyp2c44 gene signaling. It is also important to consider how different cells within the vessel wall use Cyp2c44-dependent pathways and how genetic depletion of these pathways affects these cell types individually. Finally, it is important to further study how vascular remodeling processes (i.e., smooth muscle proliferation as well as the synthesis and deposition of extracellular matrix components) and inflammatory processes are controlled at a cellular level by Cyp2c44-dependent pathways.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-AT-006526, R01-AG047879, R01-AG038747, and R01-NS056218, the Geroscience Training Program in Oklahoma (NIH Grant T32-AG-052363), the Oklahoma Nathan Shock Center (NIH Grant 3-P30-AG050911-02S1), Oklahoma Shared Clinical and Translational Resources (NIH Grant U54-GM-104938, to A. Yabluchanskiy), the Oklahoma Center for the Advancement of Science and Technology (to A. Yabluchanskiy), the Reynolds Foundation (to A. Yabluchanskiy), and the Presbyterian Health Foundation (to A. Yabluchanskiy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.Á.F. and A.Y. interpreted results of experiments; G.Á.F. and A.Y. drafted manuscript; G.Á.F. and A.Y. edited and revised manuscript; A.Y. approved final version of manuscript.
REFERENCES
- 1.Alaa M, Abdellatif M, Tavares-Silva M, Oliveira-Pinto J, Lopes L, Leite S, Leite-Moreira AF, Lourenço AP. Right ventricular end-diastolic stiffness heralds right ventricular failure in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 311: H1004–H1013, 2016. doi: 10.1152/ajpheart.00202.2016. [DOI] [PubMed] [Google Scholar]
- 2.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, Anand-Apte B, Yoder MC, Tuder RM, Erzurum SC. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol 172: 615–627, 2008. doi: 10.2353/ajpath.2008.070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asosingh K, Farha S, Lichtin A, Graham B, George D, Aldred M, Hazen SL, Loyd J, Tuder R, Erzurum SC. Pulmonary vascular disease in mice xenografted with human BM progenitors from patients with pulmonary arterial hypertension. Blood 120: 1218–1227, 2012. doi: 10.1182/blood-2012-03-419275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awad KS, West JD, de Jesus Perez V, MacLean M. Novel signaling pathways in pulmonary arterial hypertension (2015 Grover Conference Series). Pulm Circ 6: 285–294, 2016. doi: 10.1086/688034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med 169: 764–769, 2004. doi: 10.1164/rccm.200301-147OC. [DOI] [PubMed] [Google Scholar]
- 6.Chester AH, Yacoub MH. The role of endothelin-1 in pulmonary arterial hypertension. Glob Cardiol Sci Pract 2014: 62–78, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chettimada S, Joshi SR, Alzoubi A, Gebb SA, McMurtry IF, Gupte R, Gupte SA. Glucose-6-phosphate dehydrogenase plays a critical role in hypoxia-induced CD133+ progenitor cells self-renewal and stimulates their accumulation in the lungs of pulmonary hypertensive rats. Am J Physiol Lung Cell Mol Physiol 307: L545–L556, 2014. doi: 10.1152/ajplung.00303.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 115: 343–349, 1991. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 9.Davie NJ, Crossno JT Jr, Frid MG, Hofmeister SE, Reeves JT, Hyde DM, Carpenter TC, Brunetti JA, McNiece IK, Stenmark KR. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol Lung Cell Mol Physiol 286: L668–L678, 2004. doi: 10.1152/ajplung.00108.2003. [DOI] [PubMed] [Google Scholar]
- 10.Davis CM, Ammi AY, Alkayed NJ, Kaul S. Ultrasound stimulates formation and release of vasoactive compounds in brain endothelial cells. Am J Physiol Heart Circ Physiol 309: H583–H591, 2015. doi: 10.1152/ajpheart.00690.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Raaf MA, Herrmann FE, Schalij I, de Man FS, Vonk-Noordegraaf A, Guignabert C, Wollin L, Bogaard HJ. Tyrosine kinase inhibitor BIBF1000 does not hamper right ventricular pressure adaptation in rats. Am J Physiol Heart Circ Physiol 311: H604–H612, 2016. [DOI] [PubMed] [Google Scholar]
- 12.DeLozier TC, Tsao CC, Coulter SJ, Foley J, Bradbury JA, Zeldin DC, Goldstein JA. CYP2C44, a new murine CYP2C that metabolizes arachidonic acid to unique stereospecific products. J Pharmacol Exp Ther 310: 845–854, 2004. doi: 10.1124/jpet.104.067819. [DOI] [PubMed] [Google Scholar]
- 13.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, Fini MA, Graham BB, Tuder RM, Friedman JE, Eltzschig HK, Sokol RJ, Stenmark KR. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol 193: 597–609, 2014. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farha S, Asosingh K, Xu W, Sharp J, George D, Comhair S, Park M, Tang WH, Loyd JE, Theil K, Tubbs R, Hsi E, Lichtin A, Erzurum SC. Hypoxia-inducible factors in human pulmonary arterial hypertension: a link to the intrinsic myeloid abnormalities. Blood 117: 3485–3493, 2011. doi: 10.1182/blood-2010-09-306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farha S, Sharp J, Asosingh K, Park M, Comhair SA, Tang WH, Thomas J, Farver C, Hsieh F, Loyd JE, Erzurum SC. Mast cell number, phenotype, and function in human pulmonary arterial hypertension. Pulm Circ 2: 220–228, 2012. doi: 10.4103/2045-8932.97609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, Sylman JL, Stenmark KR. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol 297: L1059–L1072, 2009. doi: 10.1152/ajplung.90611.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galiè N, Barberà JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, Peacock AJ, Simonneau G, Vachiery JL, Grünig E, Oudiz RJ, Vonk-Noordegraaf A, White RJ, Blair C, Gillies H, Miller KL, Harris JH, Langley J, Rubin LJ; AMBITION Investigators . Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 373: 834–844, 2015. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 19.Galiè N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T, Olschewski H, Peacock A, Pietra G, Rubin LJ, Simonneau G, Priori SG, Garcia MA, Blanc JJ, Budaj A, Cowie M, Dean V, Deckers J, Burgos EF, Lekakis J, Lindahl B, Mazzotta G, McGregor K, Morais J, Oto A, Smiseth OA, Barbera JA, Gibbs S, Hoeper M, Humbert M, Naeije R, Pepke-Zaba J; Task Force . Guidelines on diagnosis and treatment of pulmonary arterial hypertension. Eur Heart J 25: 2243–2278, 2004. doi: 10.1016/j.ehj.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhardt L, Dicker D, Duan L, Erskine H, Feigin VL, Ferrari AJ, Fitzmaurice C, Fleming T, Graetz N, Guinovart C, Haagsma J, Hansen GM, Hanson SW, Heuton KR, Higashi H, Kassebaum N, Kyu H, Laurie E, Liang X, Lofgren K, Lozano R, MacIntyre MF, Moradi-Lakeh M, Naghavi M, Nguyen G, Odell S, Ortblad K, Roberts DA, Roth GA, Sandar L, Serina PT, Stanaway JD, Steiner C, Thomas B, Vollset SE, Whiteford H, Wolock TM, Ye P, Zhou M, Ãvila MA, Aasvang GM, Abbafati C, Ozgoren AA, Abd-Allah F, Aziz MI, Abera SF, Aboyans V, Abraham JP, Abraham B, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Aburto TC, Achoki T, Ackerman IN, Adelekan A, Ademi Z, Adou AK, Adsuar JC, Arnlov J, Agardh EE, Al Khabouri MJ, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allebeck P, Allen PJ, Al-Mazroa MA, Alsharif U, Alvarez E, Alvis-Guzman N, Ameli O, Amini H, Ammar W, Anderson BO, Anderson HR, Antonio CA, Anwari P, Apfel H, Arsenijevic VS, Artaman A, Asghar RJ, Assadi R, Atkins LS, Atkinson C, Badawi A, Bahit MC, Bakfalouni T, Balakrishnan K, Balalla S, Banerjee A, Barker-Collo SL, Barquera S, Barregard L, Barrero LH, Basu S, Basu A, Baxter A, Beardsley J, Bedi N, Beghi E, Bekele T, Bell ML, Benjet C, Bennett DA, Bensenor IM, Benzian H, Bernabe E, Beyene TJ, Bhala N, Bhalla A, Bhutta Z, Bienhoff K, Bikbov B, Abdulhak AB, Blore JD, Blyth FM, Bohensky MA, Basara BB, Borges G, Bornstein NM, Bose D, Boufous S, Bourne RR, Boyers LN, Brainin M, Brauer M, Brayne CE, Brazinova A, Breitborde NJ, Brenner H, Briggs AD, Brooks PM, Brown J, Brugha TS, Buchbinder R, Buckle GC, Bukhman G, Bulloch AG, Burch M, Burnett R, Cardenas R, Cabral NL, Nonato IR, Campuzano JC, Carapetis JR, Carpenter DO, Caso V, Castaneda-Orjuela CA, Catala-Lopez F, Chadha VK, Chang J-C, Chen H, Chen W, Chiang PP, Chimed-Ochir O, Chowdhury R, Christensen H, Christophi CA, Chugh SS, Cirillo M, Coggeshall M, Cohen A, Colistro V, Colquhoun SM, Contreras AG, Cooper LT, Cooper C, Cooperrider K, Coresh J, Cortinovis M, Criqui MH, Crump JA, Cuevas-Nasu L, Dandona R, Dandona L, Dansereau E, Dantes HG, Dargan PI, Davey G, Davitoiu DV, Dayama A, De la Cruz-Gongora V, de la Vega SF, De Leo D, del Pozo-Cruz B, Dellavalle RP, Deribe K, Derrett S, Des Jarlais DC, Dessalegn M, deVeber GA, Dharmaratne SD, Diaz-Torne C, Ding EL, Dokova K, Dorsey ER, Driscoll TR, Duber H, Durrani AM, Edmond KM, Ellenbogen RG, Endres M, Ermakov SP, Eshrati B, Esteghamati A, Estep K, Fahimi S, Farzadfar F, Fay DFJ, Felson DT, Fereshtehnejad S-M, Fernandes JG, Ferri CP, Flaxman A, Foigt N, Foreman KJ, Fowkes FGR, Franklin RC, Furst T, Futran ND, Gabbe BJ, Gankpe FG, Garcia-Guerra FA, Geleijnse JM, Gessner BD, Gibney KB, Gillum RF, Ginawi IA, Giroud M, Giussani G, Goenka S, Goginashvili K, Gona P, de Cosio TG, Gosselin RA, Gotay CC, Goto A, Gouda HN, Guerrant R, Gugnani HC, Gunnell D, Gupta R, Gupta R, Gutierrez RA, Hafezi-Nejad N, Hagan H, Halasa Y, Hamadeh RR, Hamavid H, Hammami M, Hankey GJ, Hao Y, Harb HL, Haro JM, Havmoeller R, Hay RJ, Hay S, Hedayati MT, Pi IB, Heydarpour P, Hijar M, Hoek HW, Hoffman HJ, Hornberger JC, Hosgood HD, Hossain M, Hotez PJ, Hoy DG, Hsairi M, Hu H, Hu G, Huang JJ, Huang C, Huiart L, Husseini A, Iannarone M, Iburg KM, Innos K, Inoue M, Jacobsen KH, Jassal SK, Jeemon P, Jensen PN, Jha V, Jiang G, Jiang Y, Jonas JB, Joseph J, Juel K, Kan H, Karch A, Karimkhani C, Karthikeyan G, Katz R, Kaul A, Kawakami N, Kazi DS, Kemp AH, Kengne AP, Khader YS, Khalifa SE, Khan EA, Khan G, Khang Y-H, Khonelidze I, Kieling C, Kim D, Kim S, Kimokoti RW, Kinfu Y, Kinge JM, Kissela BM, Kivipelto M, Knibbs L, Knudsen AK, Kokubo Y, Kosen S, Kramer A, Kravchenko M, Krishnamurthi RV, Krishnaswami S, Defo BK, Bicer BK, Kuipers EJ, Kulkarni VS, Kumar K, Kumar GA, Kwan GF, Lai T, Lalloo R, Lam H, Lan Q, Lansingh VC, Larson H, Larsson A, Lawrynowicz AE, Leasher JL, Lee J-T, Leigh J, Leung R, Levi M, Li B, Li Y, Li Y, liang J, Lim S, Lin H-H, Lind M, Lindsay MP, Lipshultz SE, Liu S, Lloyd BK, Ohno SL, Logroscino G, Looker KJ, Lopez AD, Lopez-Olmedo N, Lortet-Tieulent J, Lotufo PA, Low N, Lucas RM, Lunevicius R, Lyons RA, Ma J, Ma S, Mackay MT, Majdan M, Malekzadeh R, Mapoma CC, Marcenes W, March LM, Margono C, Marks GB, Marzan MB, Masci JR, Mason-Jones AJ, Matzopoulos RG, Mayosi BM, Mazorodze TT, McGill NW, McGrath JJ, McKee M, McLain A, McMahon BJ, Meaney PA, Mehndiratta MM, Mejia-Rodriguez F, Mekonnen W, Melaku YA, Meltzer M, Memish ZA, Mensah G, Meretoja A, Mhimbira FA, Micha R, Miller TR, Mills EJ, Mitchell PB, Mock CN, Moffitt TE, Ibrahim NM, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montico M, Montine TJ, Moore AR, Moran AE, Morawska L, Mori R, Moschandreas J, Moturi WN, Moyer M, Mozaffarian D, Mueller UO, Mukaigawara M, Murdoch ME, Murray J, Murthy KS, Naghavi P, Nahas Z, Naheed A, Naidoo KS, Naldi L, Nand D, Nangia V, Narayan KM, Nash D, Nejjari C, Neupane SP, Newman LM, Newton CR, Ng M, Ngalesoni FN, Nhung NT, Nisar MI, Nolte S, Norheim OF, Norman RE, Norrving B, Nyakarahuka L, Oh IH, Ohkubo T, Omer SB, Opio JN, Ortiz A, Pandian JD, Panelo CIA, Papachristou C, Park E-K, Parry CD, Caicedo AJP, Patten SB, Paul VK, Pavlin BI, Pearce N, Pedraza LS, Pellegrini CA, Pereira DM, Perez-Ruiz FP, Perico N, Pervaiz A, Pesudovs K, Peterson CB, Petzold M, Phillips MR, Phillips D, Phillips B, Piel FB, Plass D, Poenaru D, Polanczyk GV, Polinder S, Pope CA, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Prasad NM, Qato D, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman S, Raju M, Rakovac I, Rana SM, Razavi H, Refaat A, Rehm J, Remuzzi G, Resnikoff S, Ribeiro AL, Riccio PM, Richardson L, Richardus JH, Riederer AM, Robinson M, Roca A, Rodriguez A, Rojas-Rueda D, Ronfani L, Rothenbacher D, Roy N, Ruhago GM, Sabin N, Sacco RL, Ksoreide K, Saha S, Sahathevan R, Sahraian MA, Sampson U, Sanabria JR, Sanchez-Riera L, Santos IS, Satpathy M, Saunders JE, Sawhney M, Saylan MI, Scarborough P, Schoettker B, Schneider IJ, Schwebel DC, Scott JG, Seedat S, Sepanlou SG, Serdar B, Servan-Mori EE, Shackelford K, Shaheen A, Shahraz S, Levy TS, Shangguan S, She J, Sheikhbahaei S, Shepard DS, Shi P, Shibuya K, Shinohara Y, Shiri R, Shishani K, Shiue I, Shrime MG, Sigfusdottir ID, Silberberg DH, Simard EP, Sindi S, Singh JA, Singh L, Skirbekk V, Sliwa K, Soljak M, Soneji S, Soshnikov SS, Speyer P, Sposato LA, Sreeramareddy CT, Stoeckl H, Stathopoulou VK, Steckling N, Stein MB, Stein DJ, Steiner TJ, Stewart A, Stork E, Stovner LJ, Stroumpoulis K, Sturua L, Sunguya BF, Swaroop M, Sykes BL, Tabb KM, Takahashi K, Tan F, Tandon N, Tanne D, Tanner M, Tavakkoli M, Taylor HR, Te Ao BJ, Temesgen AM, Have MT, Tenkorang EY, Terkawi AS, Theadom AM, Thomas E, Thorne-Lyman AL, Thrift AG, Tleyjeh IM, Tonelli M, Topouzis F, Towbin JA, Toyoshima H, Traebert J, Tran BX, Trasande L, Trillini M, Truelsen T, Trujillo U, Tsilimbaris M, Tuzcu EM, Ukwaja KN, Undurraga EA, Uzun SB, van Brakel WH, van de Vijver S, Dingenen RV, van Gool CH, Varakin YY, Vasankari TJ, Vavilala MS, Veerman LJ, Velasquez-Melendez G, Venketasubramanian N, Vijayakumar L, Villalpando S, Violante FS, Vlassov VV, Waller S, Wallin MT, Wan X, Wang L, Wang JL, Wang Y, Warouw TS, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Wessells KR, Westerman R, Wilkinson JD, Williams HC, Williams TN, Woldeyohannes SM, Wolfe CD, Wong JQ, Wong H, Woolf AD, Wright JL, Wurtz B, Xu G, Yang G, Yano Y, Yenesew MA, Yentur GK, Yip P, Yonemoto N, Yoon S-J, Younis M, Yu C, Kim KY, Zaki MES, Zhang Y, Zhao Z, Zhao Y, Zhu J, Zonies D, Zunt JR, Salomon JA, Murray CJ; Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990−2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386: 743–800, 2015. 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto R, Joshi SR, Jiang H, Capdevila JH, McMurtry IF, Schwartzman ML, Gupte SA. Cyp2c44 gene disruption is associated with increased hematopoietic stem cells: Implication in chronic hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00785.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54, Suppl: S10–S19, 2009. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Hoeper MM, Ghofrani HA, Grünig E, Klose H, Olschewski H, Rosenkranz S. Pulmonary Hypertension. Dtsch Arztebl Int 114: 73–84, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K, Jing ZC, Gibbs JS. A global view of pulmonary hypertension. Lancet Respir Med 4: 306–322, 2016. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 25.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43, Suppl S: 13S–24S, 2004. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Joshi SR, Lakhkar A, Dhagia V, Zias AL, Soldatos V, Oshima K, Jiang H, Gotlinger K, Capdevila JH, Schwartzman ML, McMurtry IF, Gupte SA. Cyp2c44 gene disruption exacerbated pulmonary hypertension and heart failure in female but not male mice. Pulm Circ 6: 360–368, 2016. doi: 10.1086/688060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakhkar A, Dhagia V, Joshi SR, Gotlinger K, Patel D, Sun D, Wolin MS, Schwartzman ML, Gupte SA. 20-HETE-induced mitochondrial superoxide production and inflammatory phenotype in vascular smooth muscle is prevented by glucose-6-phosphate dehydrogenase inhibition. Am J Physiol Heart Circ Physiol 310: H1107–H1117, 2016. doi: 10.1152/ajpheart.00961.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majka SM, Skokan M, Wheeler L, Harral J, Gladson S, Burnham E, Loyd JE, Stenmark KR, Varella-Garcia M, West J. Evidence for cell fusion is absent in vascular lesions associated with pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 295: L1028–L1039, 2008. doi: 10.1152/ajplung.90449.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin YN, Pabelick CM. Sex differences in the pulmonary circulation: implications for pulmonary hypertension. Am J Physiol Heart Circ Physiol 306: H1253–H1264, 2014. doi: 10.1152/ajpheart.00857.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montani D, Perros F, Gambaryan N, Girerd B, Dorfmuller P, Price LC, Huertas A, Hammad H, Lambrecht B, Simonneau G, Launay JM, Cohen-Kaminsky S, Humbert M. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 184: 116–123, 2011. doi: 10.1164/rccm.201006-0905OC. [DOI] [PubMed] [Google Scholar]
- 31.Patel D, Alhawaj R, Kelly MR, Accarino JJ, Lakhkar A, Gupte SA, Sun D, Wolin MS. Potential role of mitochondrial superoxide decreasing ferrochelatase and heme in coronary artery soluble guanylate cyclase depletion by angiotensin II. Am J Physiol Heart Circ Physiol 310: H1439–H1447, 2016. doi: 10.1152/ajpheart.00859.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizarro S, García-Lucio J, Peinado VI, Tura-Ceide O, Díez M, Blanco I, Sitges M, Petriz J, Torralba Y, Marín P, Roca J, Barberà JA. Circulating progenitor cells and vascular dysfunction in chronic obstructive pulmonary disease. PLoS One 9: e106163, 2014. doi: 10.1371/journal.pone.0106163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, Stewart S, Hecker M, Zhu Z, Gehling U, Seeger W, Pepke-Zaba J, Morrell NW. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med 180: 780–787, 2009. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visovatti SH, Hyman MC, Goonewardena SN, Anyanwu AC, Kanthi Y, Robichaud P, Wang J, Petrovic-Djergovic D, Rattan R, Burant CF, Pinsky DJ. Purinergic dysregulation in pulmonary hypertension. Am J Physiol Heart Circ Physiol 311: H286–H298, 2016. doi: 10.1152/ajpheart.00572.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang WH, Zhang C, Lin DH, Wang L, Graves JP, Zeldin DC, Capdevila JH. Cyp2c44 epoxygenase in the collecting duct is essential for the high K+ intake-induced antihypertensive effect. Am J Physiol Renal Physiol 307: F453–F460, 2014. doi: 10.1152/ajprenal.00123.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia L, Zhu JH, Qiu FY, Yang Y, Xie XD, Wang XX, Chen JZ, Fu GS. Senescent endothelial progenitor cells from dogs with pulmonary arterial hypertension: a before-after self-controlled study. J Physiol Sci 59: 429–437, 2009. doi: 10.1007/s12576-009-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B, Naik JS, Jernigan NL, Walker BR, Resta TC. Reduced membrane cholesterol limits pulmonary endothelial Ca2+ entry after chronic hypoxia. Am J Physiol Heart Circ Physiol 312: H1176–H1184, 2017. doi: 10.1152/ajpheart.00097.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou GL, Beloiartsev A, Yu B, Baron DM, Zhou W, Niedra R, Lu N, Tainsh LT, Zapol WM, Seed B, Bloch KD. Deletion of the murine cytochrome P450 Cyp2j locus by fused BAC-mediated recombination identifies a role for Cyp2j in the pulmonary vascular response to hypoxia. PLoS Genet 9: e1003950, 2013. doi: 10.1371/journal.pgen.1003950. [DOI] [PMC free article] [PubMed] [Google Scholar]