Abstract
To test the hypothesis that epoxyeicosatrienoic acids (EETs) facilitate pulmonary responses to hypoxia, male wild-type (WT) and soluble-epoxide hydrolase knockout (sEH-KO) mice, and WT mice chronically fed a sEH inhibitor (t-TUCB; 1 mg·kg−1·day−1) were used. Right ventricular systolic pressure (RVSP) was recorded under control and hypoxic conditions. The control RVSP was comparable among all groups. However, hypoxia elicited increases in RVSP in all groups with predominance in sEH-KO and t-TUCB-treated mice. 14,15-EEZE (an EET antagonist) attenuated the hypoxia-induced greater elevation of RVSP in sEH-deficient mice, suggesting an EET-mediated increment. Exogenous 5,6-; 8,9-, or 14,15-EET (0.05 ng/g body wt) did not change RVSP in any conditions, but 11,12-EET enhanced RVSP under hypoxia. Isometric tension was recorded from pulmonary arteries isolated from WT and sEH-KO mice, vessels that behaved identically in their responsiveness to vasoactive agents and vessel stretch. Hypoxic pulmonary vasoconstriction (HPV, expressed as increases in hypoxic force) was significantly greater in vessels of sEH-KO than WT vessels; the enhanced component was inhibited by EEZE. Treatment of WT vessels with 11,12-EET enhanced HPV to the same level as sEH-KO vessels, confirming EETs as primary players. Inhibition of cyclooxygenases (COXs) significantly enhanced HPV in WT vessels, but attenuated HPV in sEH-KO vessels. Blocking/inhibiting COX-1, prostaglandin H2 (PGH2)/thromboxane A2 (TXA2) receptors and TXA synthase prevented the enhanced HPV in sEH-KO vessels but had no effects on WT vessels. In conclusion, an EET-dependent alteration in PG metabolism that favors the action of vasoconstrictor PGH2 and TXA2 potentiates HPV and hypoxia-induced elevation of RVSP in sEH-deficient mice.
Keywords: epoxyeicosatrienoic acids, soluble epoxide hydrolase, hypoxic pulmonary vasoconstriction, right ventricular systolic pressure, prostaglandins
in the pulmonary circulation, a reduction in oxygen tension causes contraction of pulmonary vascular smooth muscle, a phenomenon that is defined as hypoxic pulmonary vasoconstriction (HPV). HPV, in vivo, is manifested as an increase in right ventricular systolic pressure (RVSP), which indicates the enhanced pulmonary artery pressure and, therefore, may play a role in the pathogenesis of pulmonary hypertension. HPV is physiologically relevant, as it acutely optimizes gas exchange by shunting blood flow from hypoxic alveoli toward better ventilated areas. Indeed, in response to LPS challenge, preservation of HPV, as a function of soluble epoxide hydrolase (sEH) deficiency, is accompanied by an increase in the level of systemic arterial oxygenation (44). However, a prolonged exposure of hypoxia followed by sustained HPV initiates pathological consequences of vascular remodeling and progressive development of pulmonary hypertension. Several decades have passed since the first description of HPV, but its mechanism remains elusive. Within the literature, the mechanistic nature of HPV varies with different species and sexes, in vivo or in vitro preparations, the duration for exposure of hypoxia (acute or chronic), and vessel localization/size and cell type (endothelium-dependent or independent), etc., all of which may specifically determine different cellular mediators and channels involved, as well as the characteristics of modulating the response (13, 27, 32, 38, 41).
Even though specific mechanisms responsible for HPV are currently unknown, an alteration in the metabolism of arachidonic acid (AA) seems to be a promising target, since each of the three major pathways responsible for AA metabolism by lipoxygenases (33, 34), cyclooxygenases (COXs) (1, 3, 22), and cytochrome P-450s (CYPs) (21, 23, 24), has been reported to be involved in potentiating HPV via either sensing changes in O2 or participating in mediation of the signaling (41). Among the three pathways, the CYP pathway has attracted considerable attention based on its capability to serve as O2 sensor and to cause a hypoxia-induced reduction in CYP ω-hydrolase production of 20-hydroxyeicosatetraenoic acid (20-HETE), leading to the promotion of HPV (40, 46). Moreover, crucial roles of CYPs, enzymes responsible for the synthesis of epoxyeicosatrienoic acids (EETs), in HPV have emerged. As reported by Kandhi et al. (22) and Pokreisz et al. (35), HPV in mice was significantly attenuated by the inhibition of CYP2C9 and potentiated by overexpression of CYP2C9 or downregulation or blockage of sEH, a downstream enzyme that is responsible for EET metabolism and degradation in the CYP pathway. Indeed, in the pulmonary vasculature, CYP metabolism of AA provides the majority of vasoactive compounds, including 20-HETE and EETs (19). As we reported previously (21), administration of exogenous EETs to mice increased RVSP in a dose-dependent manner, suggesting that EETs elicit direct pulmonary vasoconstriction (29, 47). Moreover, EET-induced increases in RVSP became more predominant under hypoxia, revealing a positive feedback between EETs and hypoxia during the process of HPV (21).
Alternatively, we have also found that increased bioavailability of pulmonary EETs by sEH gene deletion did not affect basal pulmonary artery pressure, but dramatically enhanced U46619 (a thromboxane analog)-induced elevation of RVSP (22), indicating that an EET-potentiation of constrictor prostaglandins appears to be involved in pulmonary vasoconstriction. Thus, the question was raised as to whether CYP-derived EETs solely facilitate HPV, or take part in an AA cascade pathway that links the O2 sensor/hypoxia to vasoconstriction. With respect to interactions among EET, hypoxia, and cyclooxygenases, we designed experiments aimed to test the hypothesis that the nature of EET-dependent contribution to HPV is a facilitating response via a constrictor prostanoid-mediated mechanism. To this end, both in vivo and in vitro studies were conducted on sEH-knockout (sEH-KO) and wild-type (WT) mice to determine whether physiological presence of EETs participates in the pathological process of HPV, and if so, what specific pathways and/or mediators are involved.
MATERIAL AND METHODS
Animals
Twelve- to fifteen-week-old male C57BL/6J mice served as WT controls, and Ephx2−/− (sEH-KO) mice were used. As described previously (39), cryorecovered heterozygous (Ephx2+/−, B6.129XEphx2tm1Gonz/J) and WT mice were obtained from the Jackson Laboratory (Bar Harbor, ME), and the homozygous (sEH-KO) mice were generated in the Department of Comparative Medicine at the New York Medical College. Another group of WT mice was fed t-TUCB (a sEH inhibitor, 1 mg·kg−1·day−1, oral gavage) for 4 wk. The efficiency of t-TUCB treatment of mice was evidenced by a significant decrease in blood pressure, which was monitored twice a week by tail-cuff measurement during the course of treatment, as described previously (36). All protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College and conformed to the guidelines of the National Institutes of Health and the American Physiological Society for the use and care of laboratory animals.
Surgery
Right ventricular catheterization was performed as described previously (21, 22). Briefly, mice were anesthetized by inhalation of isoflurane and kept on a heating plate to maintain the body temperature at 37°C. After the neck area was shaved, a middle incision was made to expose the right and left external jugular veins. A polyethylene tube (PE-10) was placed in the left jugular vein for infusion of agents such as EETs or 14,15-EEZE (a putative inhibitor of EET action).
Another fluid-filled catheter was inserted into the right jugular vein and was advanced into the right ventricle for monitoring RVSP. Yielding a stable ventricular pressure wave was indicative of the catheter being successfully localized in the right ventricle. RVSP was recorded on PowerLab (ADInstruments, Colorado Springs, CO) and analyzed with LabChart V8 software (ADInstruments).
RVSP Measurement In Vivo
Protocol 1 aimed to assess RVSP in WT sEH-KO mice and WT mice treated with t-TUCB under the control condition (which was defined as breathing oxygen (100% O2)-enriched isoflurane vapor through a nose-cone mask) and hypoxia, respectively. The isoflurane vaporizer and oxygen flow were adjusted to 2% and 200 ml/min, respectively (22). Hypoxia was generated by replacing the oxygen tank with a tank containing 10% oxygen balanced with nitrogen, while gas flow and isoflurane vaporizer settings remained unchanged. After stable RVSP was recorded under control conditions, hypoxia was induced and continued for 20 min, during which, RVSP was continuously recorded. Mice were recovered under the control condition for 30 min, and 14,15-EEZE (0.3 µg/g of BW) was then infused via the left jugular vein catheter. After perfusion of 14,15-EEZE for 30 min, hypoxia-induced changes in RVSP was once more recorded.
Protocol 2 was designed to identify the action of each regioisomer of EETs in elevating RVSP, if any, as a function of hypoxia. Particularly, specific EETs that potentiate hypoxia-induced increases in RVSP were independent of its direct pulmonary vasoconstriction. Toward this end, a low dose (0.05 ng/g body wt) of each EET regioisomer (5,6-, 8,9-, 11,12-, or 14,15-EET) was infused into the right ventricle of WT mice under either a control or hypoxic condition, and RVSP was then recorded. The selected dose has no effect on systemic blood pressure (21).
Isometric Tension Experiments In Vitro
The studies were conducted on pulmonary arteries isolated from WT and sEH-KO mice to identify possible mechanism(s) responsible for the EET-dependent potentiation of HPV. Mice were anesthetized by isoflurane, and lungs were then removed. Intralobar pulmonary arteries were isolated and cut into rings of 1.46 ± 0.05 and 1.48 ± 0.06 mm of length in WT and KO mice, respectively. The rings were mounted on a Danish myograph (DMT620M; Danish Myo Technology, Aarhus, Denmark) using 40-µm stainless-steel wires and perfused with Krebs-bicarbonate solution containing (in mM) 118 NaCl, 4.7 KCl, 1.5 CaCl2, 25 NaHCO3, 1.1 MgSO4, 1.2 KH2PO4, and 5.6 glucose, and they were gassed with 95% air-5% CO2 at 37°C. The internal circumference of each ring was originally determined by the width of the ring and the known geometry of wires when the ring was stretched to a level that generated the least stretching force. On the basis of the circumference, rings were further stretched in a stepwise manner to establish a length-tension relationship. With the use of the length-tension curve, a baseline force that was equivalent to a wall tension generated under 20 mmHg of intravascular pressure was calculated and applied to the rings. The average baseline force (2.28 ± 0.15 mN and 2.24 ± 0.21 mN) and corresponding diameter of rings (301.9 ± 18.9 µm and 326.4 ± 18.5 µm) were obtained from WT and sEH-KO mice, respectively.
All rings were equilibrated under baseline force in Krebs solution for 1 h. Repeated exposures of vessel rings to the 120 mM KCl-Krebs (high K+) solution three times aimed to stabilize the reactivity of rings. The normal endothelial function of vessel rings was evaluated by a dose-dependent relaxation to ACh; 10 nM–10 µM) under a phenylephrine (PE, 10 nM)-induced precontraction. After reequilibration of the vessels in Krebs solution for an additional 30 min, vessel rings were precontracted with PE to the level equivalent to ~40% contraction induced by high K+, and basal/nonhypoxic force was recorded. This PE-induced force level (40% contraction induced by high K+) was then maintained to exclude any changes in hypoxic responses attributed to an altered basal force. Hypoxia was subsequently induced by gassing the Krebs solution with 95% nitrogen-5% CO2 for 20 min, during which changes in hypoxic force were continuously recorded. Hypoxic force was expressed as the difference between control force (PE precontraction), and peak force induced by hypoxia for each single vessel.
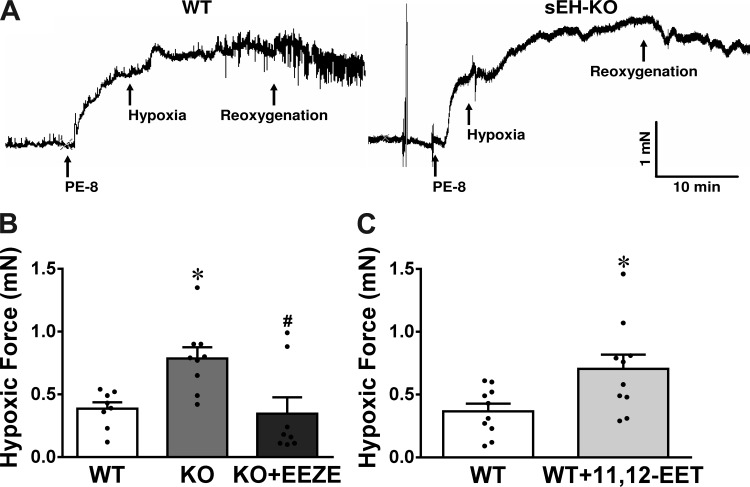
To determine whether the enhanced endogenous EETs as a function sEH deficiency would affect HPV, hypoxia-induced force changes were assessed before and after treatment of vessels of sEH-KO mice with 14,15-EEZE (10 µM). In separate experiments, hypoxic force changes in vessels of WT mice were also evaluated before and after exposure of vessels to a low dose of 11,12-EET (10 nM).
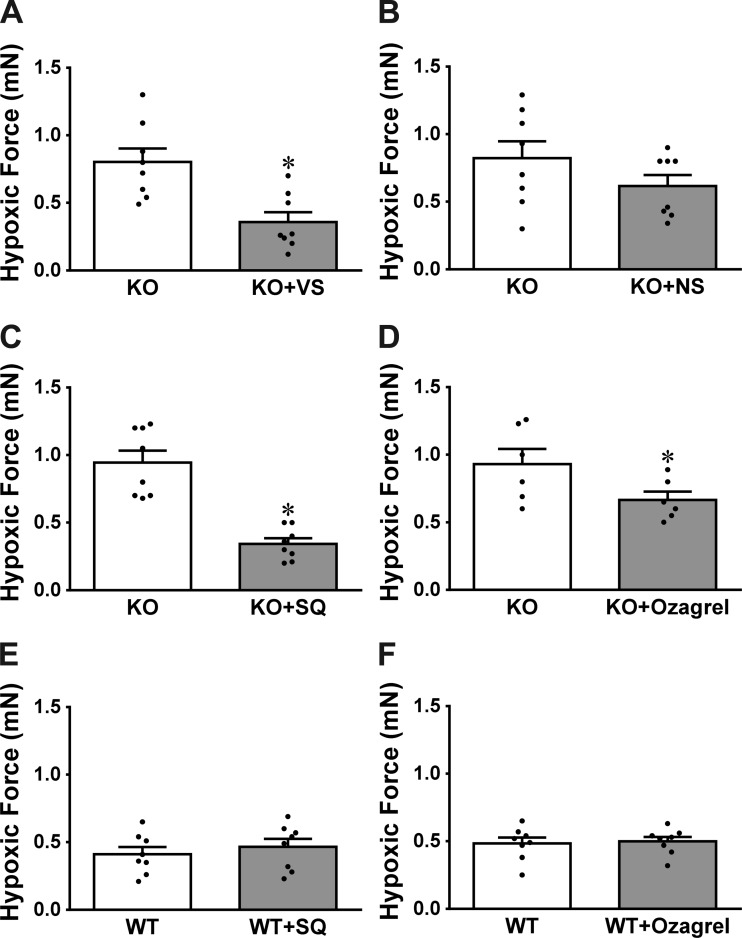
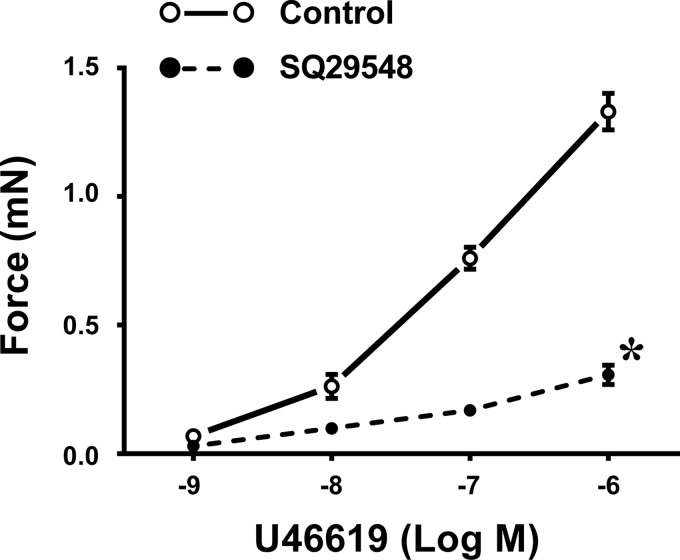
The endothelial mediators responsible for EET-dependent potentiation of HPV were characterized in separate experiments in which hypoxic responses of vessels in the absence and presence of Nω-nitro-l-arginine methyl ester (l-NAME, a nitric oxide synthase inhibitor; 300 µM) or indomethacin (Indo, a COX inhibitor; 10 µM) were determined. Moreover, in additional experiments, roles of prostaglandin H2 (PGH2) and thromboxane A2 (TXA2) in the mediation of greater HPV in sEH-KO vessels were evaluated by treatment of the hypoxia-exposed vessels with valeryl salicylate (VS, a selective COX-1 inhibitor; 1 mM), NS-398 (NS, a specific COX-2 inhibitor; 10 µM), SQ29548 (SQ, a PGH2/TXA2 receptor antagonist; 10 nM), or ozagrel (a TXA synthase inhibitor; 100 nM). The efficiency of SQ on the TXA2 receptor was validated by assessing its specific inhibitory effects on U46619 (TXA2 receptor analog)-induced vasoconstriction.
Chemicals and Statistics
All chemicals, unless specified otherwise, were obtained from Sigma (St. Louis, MO). The four EET regioisomers (5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET), 14,15-EEZE, valeryl salicylate, NS-398, SQ29548, and ozagrel were purchased from Cayman Chemical (Ann Arbor, MI). t-TUCB was synthesized by and obtained from Dr. Hammock’s laboratory.
Data are represented as means ± SE, and n refers to the number of mice. Statistical analyses were performed using GraphPad Prism 6 software. One-way ANOVA followed by the Tukey-Kramer post hoc test was used to compare the difference among multiple groups. Student’s t-test was used to compare the difference between the two groups. Statistical significance was accepted at a level of P < 0.05.
RESULTS
sEH Deficiency Potentiates Hypoxia-Initiated Increase in RVSP
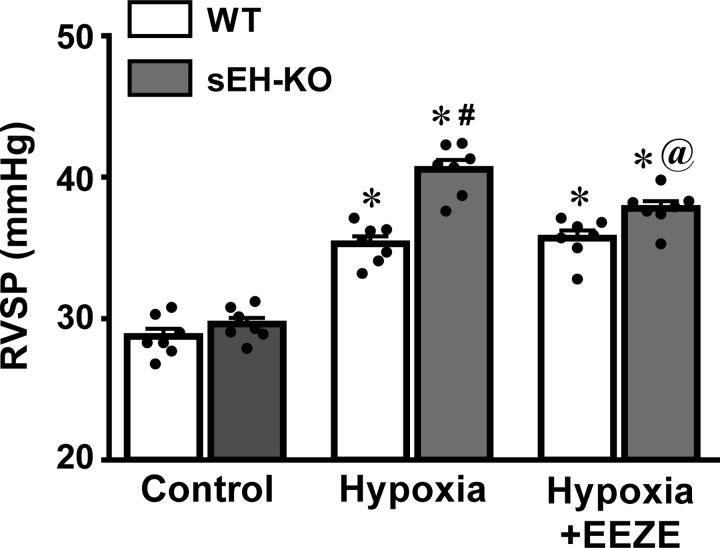
RVSP in WT and sEH-KO mice under the basal/control and hypoxia were summarized in Fig. 1. Basal RVSP was comparable between the two strains of mice, suggesting that sEH-deficiency does not affect RVSP under normal conditions, as we previously reported (22). However, when mice were exposed to the hypoxic environment, RVSP was significantly increased in both mouse strains. Hypoxia-initiated elevation of RVSP was significantly greater in sEH-KO than WT mice, implying that the extra-increment of RVSP could be dependent on a greater tissue level of EETs due to sEH gene deletion. To test this hypothesis, the hypoxia-initiated increase in RVSP was assessed in the presence of 14,15-EEZE. Indeed, 14,15-EEZE had no effect on hypoxia-induced elevation of RVSP in WT mice, but it prevented the greater increment of RVSP in sEH-KO mice back to a level comparable with WT controls, eliminating the difference between the two groups of mice. Thus elevated EETs are not responsible for the initiation of hypoxia-induced increase in RVSP, but they are capable of facilitating the hypoxic responses, as evidenced by the result that 14,15-EEZE specifically inhibited the additional increment in hypoxic augmentation of RVSP, which is observed only in sEH-KO mice. In time-course experiments conducted on WT mice, changes in RVSP were assessed during two separate episodes of hypoxia. Data show that hypoxia-induced increases in RVSP were not significantly affected by the frequency of hypoxic episodes, as evidenced by the comparable RVSP (34.88 ± 0.68 and 35.21 ± 0.81 mmHg) in a sequential exposure of mice to hypoxia (n = 4).
Fig. 1.
Right ventricular systolic pressure (RVSP) of male wild-type (WT; n = 7) and soluble epoxide hydrolase knockout (sEH-KO; n = 7) mice under control condition and exposure of mice to hypoxia in the absence and presence of 10 µM 14,15-EEZE [an epoxyeicosatrienoic acid (EET) antagonist], respectively. *Significant difference from their controls, P < 0.05. #Significant difference from WT mice under hypoxia, P < 0.05. @Significant difference from sEH-KO mice under hypoxia, P < 0.05.
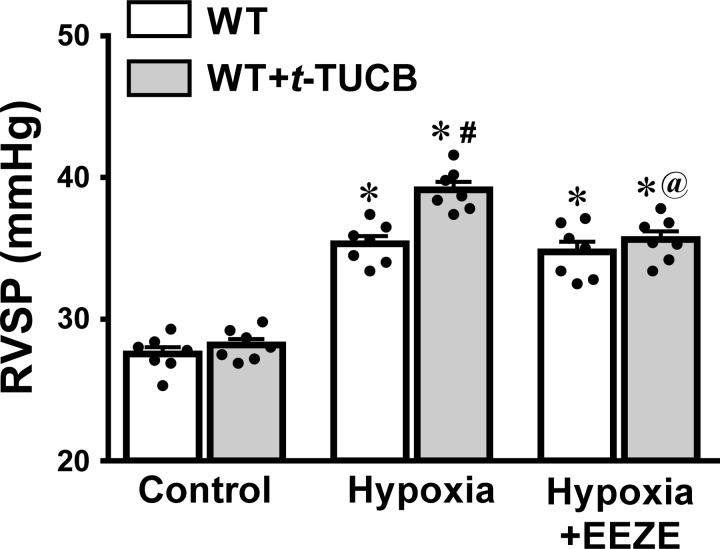
To validate the role of EETs/sEH deficiency in the facilitation of increase in RVSP by hypoxia, similar experiments were conducted on WT mice that had been treated with t-TUCB for 4 wk. The results depicted in Fig. 2 show that pharmacological inhibition of sEH elicited the same phenotype of hypoxic augmentation of RVSP as those induced by genetic deletion of the sEH gene (Fig. 1), which further confirms the contribution of EETs to hypoxic responses.
Fig. 2.
RVSP of WT mice (n = 9) and WT mice treated chronically with t-TUCB (n = 7), a sEH inhibitor, under control conditions and exposure of mice to hypoxia in the absence and presence of 14,15-EEZE respectively. *Significant difference from their controls, P < 0.05. #Significant difference from WT mice under hypoxia, P < 0.05. @Significant difference from sEH-KO mice under hypoxia, P < 0.05.
Effects of Exogenous EETs on RVSP
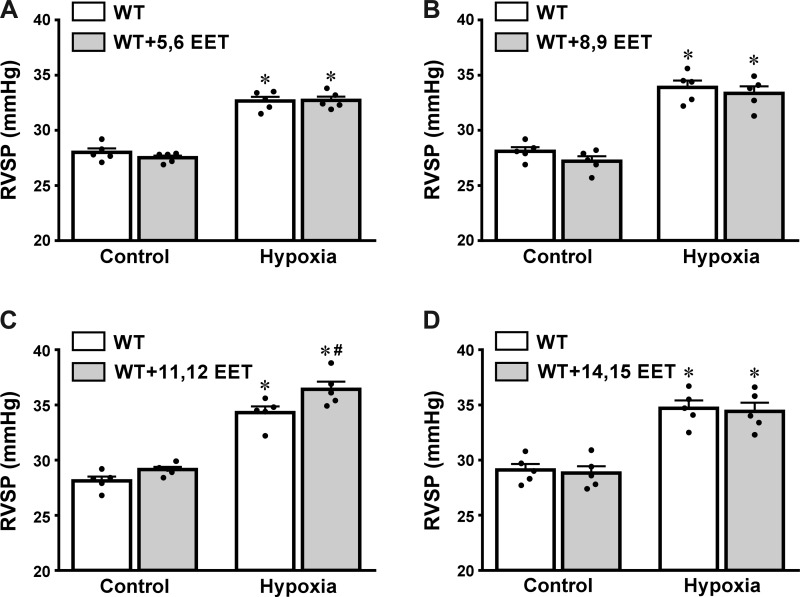
Changes in RVSP were recorded during the infusion of low doses of each EET regioisomer into the right ventricle of WT mice to determine the contribution of each EET to hypoxic responses. As shown in Fig. 3, A, B, and D, the three EET regioisomers (5,6-; 8,9-, and 14,15-EET) did not affect RVSP at any conditions. In contrast, 11,12-EET significantly enhanced hypoxia-induced elevation of RVSP (Fig. 3C), suggesting that this EET regioisomer is a primary contributor to the promotion of hypoxic elevation of RVSP.
Fig. 3.
Changes in RVSP of WT mice (n = 5) in response to exogenous administration of a low dose (0.05 ng/g body wt) of 5,6-EET (A), 8,9-EET (B), 11,12-EET (C), and 14,15-EET (D), respectively, in both control and hypoxic conditions. *Significant difference from their normal controls, P < 0.05. #Significant difference from hypoxic controls, P < 0.05.
EET-Dependent Potentiation of Hypoxic Pulmonary Vasoconstriction
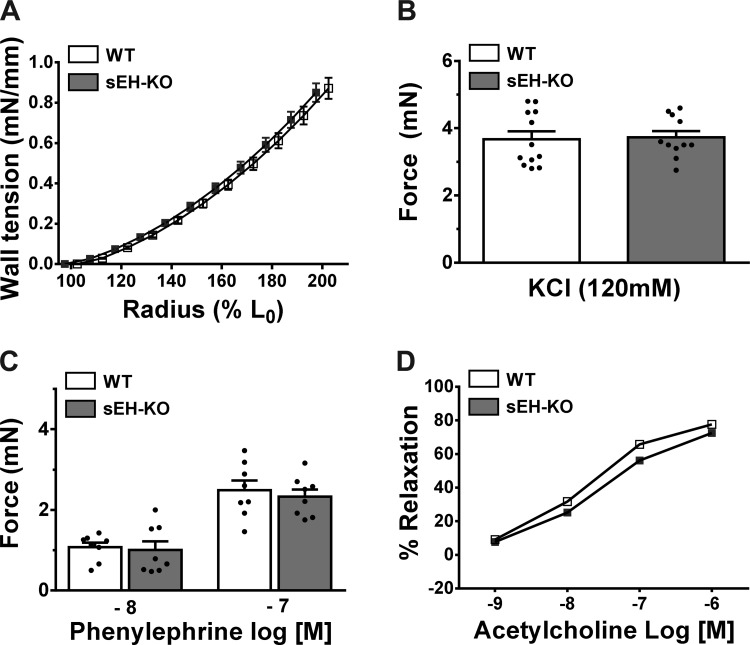
To clarify the notion that in vivo increases in RVSP by hypoxia are HPV-based in nature, changes in pulmonary artery force in response to hypoxia were determined in WT and sEH-KO mice. As shown in Fig. 4, the wall tension developed as a consequence of an increase in radius (Fig. 4A), the force generated in response to high-K+ (Fig. 4B) and PE (Fig. 4C), and the relaxation to ACh (Fig. 4D) were comparable in vessel rings of WT and sEH-KO mice. These findings suggest that both strains of mice displayed identical pulmonary arterial reactivity in response to the endothelium-dependent vasodilator (ACh) and vasoconstrictors (high-K+ and PE) that contract vascular smooth muscle via different mechanisms. In addition, they have similar wall mechanical properties in response to stretch, the response that was not significantly affected by l-NAME or Indo (data not shown). Under a stable vessel force maintained by PE for 20 min, switching vessels from a normoxic to a hypoxic environment elicited a significant increase in vessel force (as an indicative of vasoconstriction) in both strains of mice (Fig. 5A); this augmentation, however, was twofold greater in sEH-KO than those of WT mice (Fig. 5B), suggesting that HPV was enhanced by sEH deficiency. This finding is highly consistent with the greater elevation of RVSP in response to hypoxia in sEH-KO mice observed in vivo (Figs. 1 and 2). To further confirm that the greater HPV in sEH-KO mice is mediated by EETs, hypoxia-induced increases in vessel force were assessed in the presence of 14,15-EEZE. Similar to observations in in vivo conditions (Fig. 1), 14,15-EEZE reversed the greater HPV in vessels of sEH-KO to the level comparable with that of WT mice (Fig. 5B). Additionally, exogenous administration of 10 nM 11,12-EET to WT vessels augmented their hypoxia-induced increases in force (Fig. 5C) to the same level as sEH-KO vessels (Fig. 5B). Collectively, these results strongly confirm the significant contribution of EETs to the potentiation of HPV.
Fig. 4.
Changes in wall tension in response to percentage changes in vessel radius (A; n = 5), and vessel force in response to high K+ (B; n = 11), phenylephrine (C; n = 8), and acetylcholine (D; n = 6) in WT and sEH-KO mice. L0 stands for a zero tension.
Fig. 5.
A: original tracing of control and hypoxic force of pulmonary arterial ring isolated from WT and sEH-KO mice. B: hypoxia-induced increases in force of pulmonary arterial rings isolated from WT (n = 8), sEH-KO mice (KO; n = 9), and sEH-KO vessels treated with 14,15-EEZE (n = 8). C: WT vessel rings (n = 10) in the absence and presence of exogenous 11,12-EET (10−8 M). *Significant difference from WT, P < 0.05. #Significant difference from sEH-KO, P < 0.05.
Roles of Nitric Oxide and Prostaglandins in HPV
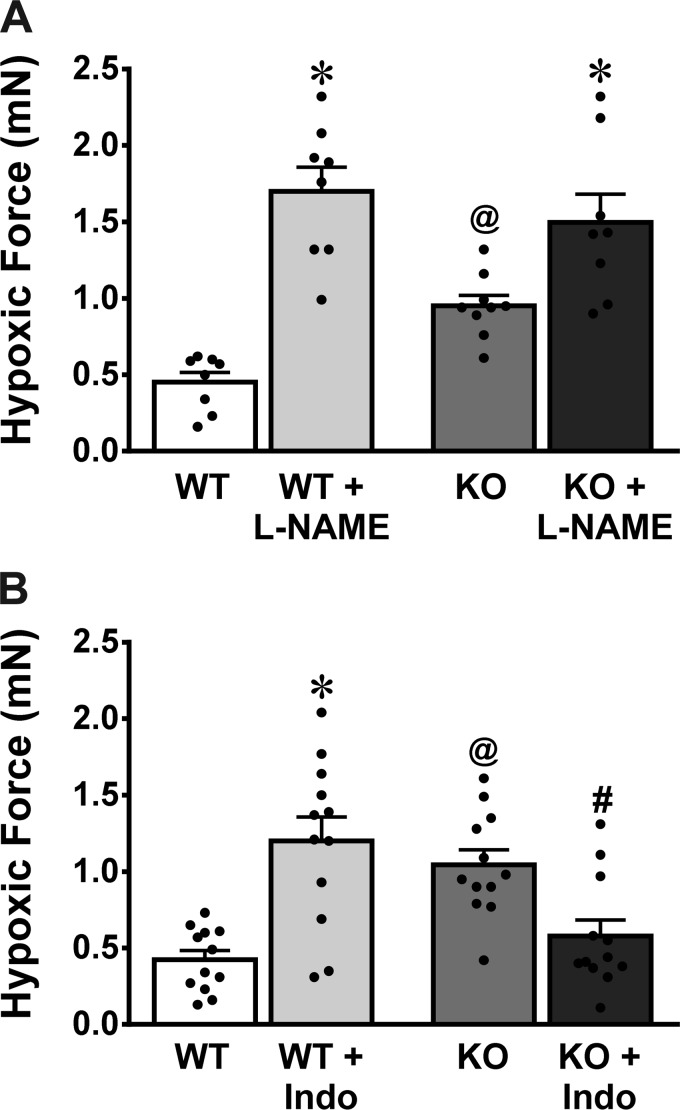
Figure 6 summarizes the effects of l-NAME (Fig. 6A) and Indo (Fig. 6B) on HPV of both WT and sEH-KO mice. In the presence of l-NAME, both groups of vessels exhibited the augmentation of hypoxic force, revealing a universal characteristic of nitric oxide (NO)-dependent alleviation of pulmonary vasoconstriction (Fig. 6A). Intriguingly, in the presence of Indo, WT vessels displayed a similar enhancement of hypoxic force to that elicited by l-NAME, suggesting that vasodilator PGs compromise HPV. In contrast, increased HPV in sEH-KO vessels were significantly attenuated by Indo, negating, therefore, their differences from WT controls (Fig. 6B). This response highlights constrictor prostanoids as responsible players in the mediation of greater HPV and higher RVSP in response to hypoxia in sEH-KO mice.
Fig. 6.
Hypoxia-induced increases in force of pulmonary arterial rings isolated from WT (n = 12) and sEH-KO mice (n = 12) in the absence and presence of Nω-nitro-l-arginine methyl ester (l-NAME; A) or indomethacin (Indo; B). *Significant difference from their corresponding controls without inhibitors, P < 0.05. @Significant difference from WT, P < 0.05. #Significant difference from sEH-KO without Indo, P < 0.05.
The identity of constrictor prostanoids responsible for the greater HPV in sEH-KO mice was characterized in Fig. 7. As shown in Fig. 7, A and B, exposure of sEH-KO vessels to COX-1 inhibitor VS significantly attenuated the greater hypoxic force to levels that are comparable with those caused by Indo treatment (Fig. 6B), whereas the COX-2 inhibitor NS initiated a reduction that was not statistically significant (P = 0.0697), suggesting a key role of COX-1 pathway in greater hypoxic responses. Specific roles of PGH2/TXA2 in the responses were clarified by the results, which show that similar to inhibition of COX-1, blocking PGH2/TXA2 receptors (Fig. 7C) or inhibiting TXA synthesis (Fig. 7D) normalizes hypoxic responses of sEH-KO vessels. This further confirms that it is COX-1-sourced PGH2/TXA2 that contributes significantly to the potentiation of hypoxic responses in pulmonary arteries deficient in the sEH gene. Conversely, neither inhibition of PGH2 nor TXA2 elicited significant changes in HPV of WT mice (Fig. 7, E and F), arguing against constrictor prostanoids as major players in pulmonary hypoxic responses of WT mice. Also, efficiency of SQ (10 nM) on the TXA2 receptor was validated by the abolishment of U46619-induced increases in vessel force (Fig. 8).
Fig. 7.
Hypoxia-induced increases in force of pulmonary arterial rings in control conditions and in the presence of valeryl salicylate (VS; n = 8; A), NS-398 (NS; n = 8; B), SQ29548 (SQ; n = 8; C and E) or ozagrel (n = 6–8; D and F) in sEH-KO (A–D) and WT (E and F) mice. *Significant difference from their controls, P < 0.05.
Fig. 8.
Dose-dependent changes in force of pulmonary arterial rings isolated from WT mice (n = 6) in response to U46619, in control conditions and in the presence of SQ29548. *Significant difference from controls, P < 0.05.
DISCUSSION
The present study addresses two important issues: first, EETs are not major contributors to the “initiation” of hypoxic responses characterized as hypoxia-induced increase in RVSP (in vivo) and HPV (in vitro), but they are potentially capable of “promoting” hypoxic responses. Second, EET-facilitated hypoxic responses in the pulmonary circulation are dependent, at least in part, on COX-1-metabolized constrictor PGH2/TXA2. This conclusion is drawn on the basis of the following evidence. First, the impact of physiological concentrations of EETs on the control of RVSP under normal conditions can be negligible, as demonstrated by the comparable basal RVSP in WT and sEH-KO mice, even though sEH-KO mice exist with high pulmonary EETs. Second, inhibition of EET action did not affect the hypoxic responses in WT mice (Figs. 1 and 2), suggesting that EETs are unlikely indispensable for the generation of hypoxic responses in the pulmonary circulation. Third, sEH-deficient mice exhibited significantly greater hypoxic responses than WT controls, and EEZE specifically eliminated the additional elevated component without having an impact on the remaining portion of hypoxic responses (Figs. 1, 2, and 5), thereby indicating that only the potentiated response is EET-dependent. Taken together, EETs seem to encourage the potential of hypoxic responses rather than produce it. Fourth, 10 nM 11,12-EET, which behaved similarly to other EET regioisomers in normal conditions, was the only regioisomer that promoted hypoxic responses (Figs. 3 and 5C), confirming its specificity in the response. Finally, Indo evoked opposite actions in hypoxic force changes in vessels of WT (increase) and sEH-KO mice (decrease) (Fig. 6B), a response that was also elicited by VS, SQ29548, and ozagrel (Fig. 7), respectively, highlighting an altered COX-1 signaling in a PGH2/TXA2-favorable manner, which is responsible for mediating EET-dependent potentiation of hypoxic responses.
EET-Dependent Promotion of Hypoxic Responses
With respect to the action of hypoxia in the CYP/EETs/sEH signal pathway, it has been demonstrated that hypoxia per se suppresses activity of the CYP enzymes, such as ω-hydrolase (20-HETE synthase), which is extremely sensitive to changes in Po2 (14) and EET synthase, which requires O2 for synthesis of EETs (41). Therefore, it may be speculated that the downstream signaling would be achieved by a decrease in CYP products in hypoxia. Our previous studies demonstrated a significant increase in total tissue levels of EETs in the lung, heart, and vasculature of sEH-KO mice, and this increment of tissue EETs resulted mainly from a reduction in EET degradation, rather than an increase in EET synthesis (9, 22, 36, 39). This clearly explains our results, which show that exposure of mice deficient in the sEH gene (sEH-KO mice; Fig. 1) or sEH activity (sEH inhibitor-treated WT mice; Fig. 2) to hypoxia promoted the hypoxia-induced increase in RVSP in an EEZE-reversible manner. In alignment with the in vivo findings, the in vitro studies also provided evidence that indicated the greater component of HPV observed in vessels of sEH-KO mice was eradicated by the inhibition of EET activity (Fig. 5B). As such, the high pulmonary EETs in sEH-KO mice, as a function of reduction in EET degradation, evoke the potentiation of hypoxic responses. Indeed, HPV was not affected by blockage of EETs in vessels of WT mice, whereas it enhanced to the same magnitude as those observed in sEH-KO vessels when exogenous 11,12-EET was administered (Fig. 5C), supporting further that it is the EET that promotes HPV. Our results provide supporting evidence for the enhancement of HPV by overexpression of CYP2C9 or inhibition of sEH (23, 24). Although EETs cause direct pulmonary vasoconstriction (21, 23, 29, 47), their responses appear to be region-selective in a dose-dependent manner. In the present study, none of EET regioisomers except for 11,12-EET changed RVSP under both control and hypoxia (Fig. 3), and neither did the inhibition of endogenous EETs affect basal RVSP in WT and sEH-KO mice (Figs. 1 and 2), excluding the possibility of direct vasoconstriction by EETs. Moreover, both strains of mice had an identical vascular responsiveness to divergent vasoactive agents and mechanical stretch (Fig. 4), but administration of exogenous 11,12-EET or increases in endogenous EETs by genetic and pharmacological inhibition of sEH elicited greater elevation of RVSP and enhanced HPV in response to only hypoxia (Figs. 1, 2, 3C, and 5–7), indicating that EETs potentiate the elevation of RVSP and force development under hypoxic, but not normal, conditions. As such, an aim to identify responsible mediators that bridge the linkage between EETs and hypoxia formed the basis of our in vitro studies. On the other hand, although we (Figs. 3C and 5C), as well as others (29), found a specificity and high efficiency of 11,12-EET in processing hypoxic responses, it does not necessarily mean that any responsibility from other EET regioisomers can be ignored, because there, indeed, exists synergistic, additive, or even counteracting effects among EETs in vivo.
Altered COX/PGs Signaling Mediates EET-Dependent Potentiation of HPV
NO mediation.
In the pulmonary circulation, protective actions of NO in the modulation of hypoxic responses have been well documented. Studies conducted on whole animals or perfused lungs almost universally demonstrate that inhibition of NO production (2, 15, 26) or blocking NO target guanylate cyclase (8, 12), and genetic knockout of eNOS (7, 28) enhances HPV. Conversely, transfection of the gene for eNOS to the lung suppresses HPV (4, 20). Consistent with previous findings, we demonstrated that inhibition of NOS significantly augmented the hypoxia-induced increase in vessel force in both WT and sEH-KO mice, which eliminated original differences in hypoxic force between the two strains of mice (Fig. 6A), suggesting protective effects of NO on compromising HPV in both strains of mice.
PG mediation.
In physiological conditions, the balance between vasoactive prostaglandins (dilators such as PGI2 and PGE2 vs. constrictors such as PGH2 and TXA2) maintains basal vascular tone. In pathological conditions, this balance can be tipped to ultimately favor vasoconstriction. In this context, dysregulation of the PG metabolic pathways has been reported in patients with pulmonary arterial hypertension (42) and was also considered to serve as a trigger in HPV (41). In addition, we have also demonstrated that an imbalance that favors actions of constrictor prostanoids contributes significantly to enhanced arteriolar tone in systemic hypertension (17). Studies have reached a general consensus arguing against the hypothesis that decreased production of vasodilator prostaglandins (5, 6) or increased action of vasoconstrictor prostanoids accounts for HPV (16), as supported by the observation that inhibition of COXs fails to suppress HPV but enhances it (25–27, 41, 43, 45). Moreover, hypoxia was reported to stimulate production of PGI2 in perfused lungs and pulmonary vascular endothelium (18, 30). In parallel with the aforementioned studies, we found that treatment of pulmonary arteries of WT mice with Indo elicited a threefold increase in hypoxic force compared with their control vessels (Fig. 6B), confirming that HPV was alleviated by dilator prostaglandins physiologically. In contrast to the responses observed in the WT vessel, Indo significantly attenuated the greater hypoxic force in sEH-KO vessels to the level comparable to that of WT controls (WT without Indo, Fig. 6B), elucidating crucial roles of vasoconstrictor prostanoids in potentiating greater HPV in sEH-KO mice. The identity of constrictor prostanoids was functionally clarified by results showing that either blocking PGH2/TXA2 receptors (Fig. 7C) or inhibiting TXA synthesis (Fig. 7D) elicits the same responses as those induced by VS (Fig. 7A), Indo (Fig. 6B), and 14,15-EEZE as well (Fig. 5B). Alternatively, inhibition of PGH2/TXA2 failed to significantly reduce HPV in WT vessels (Fig. 7, E and F), revealing the negligible contribution of constrictor prostanoids to the hypoxic responses in normal mice. In AA metabolic cascades, PGH2 is an intermediator that causes vasoconstriction via binding with the PGH2 receptor and is rapidly catalyzed via TXA synthases, into TXAs with predominance in TXA2, which constricts vessels in a TXA2 receptor-dependent manner. As such, blocking each of the upstream or downstream located mediators is able to prevent the constrictor responses (Fig. 7, C and D). Thus EETs are original initiators/players that are responsible for the alteration of PG metabolism to a point that intensifies PGH2/TXA2 actions. In regard to how EETs tip the balance toward the constrictor axis, we have no specific explanations at this moment. More likely, however, through a currently unknown mechanism, EETs may activate and/or sensitize PGH2/TXA2 receptors to strengthen their actions. Such a hypothesis is supported by our previous studies, which indicate that U46619 (TXA2 receptor agonist) elicited a significantly greater elevation of RVSP in sEH-KO than WT mice in the presence of comparable expression of the TXA2 receptor, a response that was sensitive to EEZE (22). In particular, a currently published paper clarified EETs to be substrates for COX (37), providing evidence indicating that COX can serve as a target for EETs. In the study by Keseru et al. (23), divergent mechanisms were revealed, indicating that transferring TRPC6 channels to the cell membrane and activating Rho kinase were responsible for the potentiation of HPV in sEH deficiency. Regarding their findings, we believed that EET-potentiating pulmonary responsiveness to hypoxia is a multiple mechanism-engaged pathological process, during which each unique signaling/pathway may be mechanistically connected with or regulated by the other(s) in a pattern of integral complexity of networks. Indeed, the present study showed a different Indo-induced magnitude of HPV in WT and sEH-KO mice (Fig. 6B), which implies the presence of alterations that are independent of, but may work in concert with, COX-mediated pathways to promote the development of hypoxic responses. Moreover, vessel localization, size, and cell type may particularly generate different cellular mediators and channels in a tissue- and cell-specific manner.
In conclusion, increases in endogenous EETs as a function of sEH deficiency or administration of exogenous EETs in physiological concentrations significantly potentiate hypoxic responses in the pulmonary circulation. The EET-induced propellant of hypoxic responses is independent of the property of direct vasoconstrictions, but it is attributed to the altered cyclooxygenase pathway, which is characterized as a shift of prostaglandin metabolic signaling away from functions of dilator prostaglandins toward actions of constrictor PGH2 and TXA2. Findings from the present study are clinically applicable by shedding light on the significance of low doses of EETs in facilitating pathological process of hypoxia-induced pulmonary injury/hypertension. In this context, females with a downregulation of sEH, which imitates the action of sEH deficiency and consequentially associated with high pulmonary levels of EETs (11, 22), may not necessarily develop pulmonary hypertension under physiological conditions, but their susceptibility to be pulmonary hypertensive when exposed to suitable environments, such as hypoxia, might significantly increase. In this regard, our results provide a mechanistically based explanation for categorizing idiopathic pulmonary arterial hypertension as a disease with female-specific prevalence (10, 31).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-070653, HL-129797, and HL-115124, as well as partially supported by the National Institute of Environmental Health Sciences (NIEHS) R01 ESRO2710 and NIEHS Superfund Research Program P42 ES04699.
DISCLOSURES
B.D.H. and S.H.H are authors of University of California patents on the synthesis and use of sEH inhibitors. These patents are licensed to EicOsis, LLC. The other authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
S.K., B.Z., A.H., and D.S. conceived and designed research; S.K., B.Z., G.F., J.Q., N.A., Y.L., Y.-M.Y., A.H., and D.S. performed experiments; S.K., B.Z., A.H., and D.S. analyzed data; S.K., B.Z., A.H., and D.S. interpreted results of experiments; S.K., B.Z., A.H., and D.S. prepared figures; S.K., B.Z., A.H., and D.S. drafted manuscript; S.K., B.Z., G.F., J.Q., N.A., Y.L., Y.-M.Y., S.H.H., B.D.H., M.S.W., A.H., and D.S. edited and revised manuscript; S.K., B.Z., G.F., J.Q., N.A., Y.L., Y.-M.Y., S.H.H., B.D.H., M.S.W., A.H., and D.S. approved final version of manuscript.
REFERENCES
- 1.Baber SR, Deng W, Rodriguez J, Master RG, Bivalacqua TJ, Hyman AL, Kadowitz PJ. Vasoactive prostanoids are generated from arachidonic acid by COX-1 and COX-2 in the mouse. Am J Physiol Heart Circ Physiol 289: H1476–H1487, 2005. doi: 10.1152/ajpheart.00195.2005. [DOI] [PubMed] [Google Scholar]
- 2.Blitzer ML, Loh E, Roddy MA, Stamler JS, Creager MA. Endothelium-derived nitric oxide regulates systemic and pulmonary vascular resistance during acute hypoxia in humans. J Am Coll Cardiol 28: 591–596, 1996. doi: 10.1016/0735-1097(96)00218-5. [DOI] [PubMed] [Google Scholar]
- 3.Buzzard CJ, Pfister SL, Campbell WB. Endothelium-dependent contractions in rabbit pulmonary artery are mediated by thromboxane A2. Circ Res 72: 1023–1034, 1993. doi: 10.1161/01.RES.72.5.1023. [DOI] [PubMed] [Google Scholar]
- 4.Champion HC, Bivalacqua TJ, D’Souza FM, Ortiz LA, Jeter JR, Toyoda K, Heistad DD, Hyman AL, Kadowitz PJ. Gene transfer of endothelial nitric oxide synthase to the lung of the mouse in vivo. Effect on agonist-induced and flow-mediated vascular responses. Circ Res 84: 1422–1432, 1999. doi: 10.1161/01.RES.84.12.1422. [DOI] [PubMed] [Google Scholar]
- 5.Demiryurek AT, Wadsworth RM, Kane KA. Pharmacological evidence for the role of mediators in hypoxia-induced vasoconstriction in sheep isolated intrapulmonary artery rings. Eur J Pharmacol 203: 1–8, 1991. doi: 10.1016/0014-2999(91)90783-M. [DOI] [PubMed] [Google Scholar]
- 6.Demiryurek AT, Wadsworth RM, Kane KA, Peacock AJ. The role of endothelium in hypoxic constriction of human pulmonary artery rings. Am Rev Respir Dis 147: 283–290, 1993. doi: 10.1164/ajrccm/147.2.283. [DOI] [PubMed] [Google Scholar]
- 7.Fagan KA, Fouty BW, Tyler RC, Morris KG Jr, Hepler LK, Sato K, LeCras TD, Abman SH, Weinberger HD, Huang PL, McMurtry IF, Rodman DM. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 103: 291–299, 1999. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouty B, Komalavilas P, Muramatsu M, Cohen A, McMurtry IF, Lincoln TM, Rodman DM. Protein kinase G is not essential to NO-cGMP modulation of basal tone in rat pulmonary circulation. Am J Physiol Heart Circ Physiol 274: H672–H678, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Froogh G, Qin J, Kandhi S, Le Y, Jiang H, Luo M, Sun D, Huang A. Female-favorable attenuation of coronary myogenic constriction via reciprocal activations of epoxyeicosatrienoic acids and nitric oxide. Am J Physiol Heart Circ Physiol 310: H1448–H1454, 2016. doi: 10.1152/ajpheart.00906.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, Krichman A, Liou TG, Raskob GE, Wason P, Feldkircher K, Turner M, McGoon MD. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest 139: 128–137, 2011. doi: 10.1378/chest.10-0075. [DOI] [PubMed] [Google Scholar]
- 11.Gill SS, Hammock BD. Distribution and properties of a mammalian soluble epoxide hydrase. Biochem Pharmacol 29: 389–395, 1980. doi: 10.1016/0006-2952(80)90518-3. [DOI] [PubMed] [Google Scholar]
- 12.Gupte SA, Okada T, McMurtry IF, Oka M. Role of pentose phosphate pathway-derived NADPH in hypoxic pulmonary vasoconstriction. Pulm Pharmacol Ther 19: 303–309, 2006. doi: 10.1016/j.pupt.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Gurney AM. Multiple sites of oxygen sensing and their contributions to hypoxic pulmonary vasoconstriction. Respir Physiol Neurobiol 132: 43–53, 2002. doi: 10.1016/S1569-9048(02)00048-4. [DOI] [PubMed] [Google Scholar]
- 14.Harder DR, Narayanan J, Birks EK, Liard JF, Imig JD, Lombard JH, Lange AR, Roman RJ. Identification of a putative microvascular oxygen sensor. Circ Res 79: 54–61, 1996. doi: 10.1161/01.RES.79.1.54. [DOI] [PubMed] [Google Scholar]
- 15.Hasunuma K, Yamaguchi T, Rodman DM, O’Brien RF, McMurtry IF. Effects of inhibitors of EDRF and EDHF on vasoreactivity of perfused rat lungs. Am J Physiol Lung Cell Mol Physiol 260: L97–L104, 1991. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino Y, Morrison KJ, Vanhoutte PM. Mechanisms of hypoxic vasoconstriction in the canine isolated pulmonary artery: role of endothelium and sodium pump. Am J Physiol Lung Cell Mol Physiol 267: L120–L127, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Huang A, Sun D, Koller A. Endothelial dysfunction augments myogenic arteriolar constriction in hypertension. Hypertension 22: 913–921, 1993. doi: 10.1161/01.HYP.22.6.913. [DOI] [PubMed] [Google Scholar]
- 18.Ibe BO, Isenberg WB, Raj JU. Endogenous arachidonic acid metabolism by calcium ionophore A23187-stimulated lamb lungs: effect of hypoxia. Am J Respir Cell Mol Biol 4: 379–385, 1991. doi: 10.1165/ajrcmb/4.4.379. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs ER, Zeldin DC. The lung HETEs (and EETs) up. Am J Physiol Heart Circ Physiol 280: H1–H10, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Janssens SP, Bloch KD, Nong Z, Gerard RD, Zoldhelyi P, Collen D. Adenoviral-mediated transfer of the human endothelial nitric oxide synthase gene reduces acute hypoxic pulmonary vasoconstriction in rats. J Clin Invest 98: 317–324, 1996. doi: 10.1172/JCI118795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kandhi S, Froogh G, Qin J, Luo M, Wolin MS, Huang A, Sun D. EETs elicit direct increases in pulmonary arterial pressure in mice. Am J Hypertens 29: 598-604, 2016. doi: 10.1093/ajh/hpv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandhi S, Qin J, Froogh G, Jiang H, Luo M, Wolin MS, Huang A, Sun D. EET-dependent potentiation of pulmonary arterial pressure: sex-different regulation of soluble epoxide hydrolase. Am J Physiol Lung Cell Mol Physiol 309: L1478–L1486, 2015. doi: 10.1152/ajplung.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keserü B, Barbosa-Sicard E, Popp R, Fisslthaler B, Dietrich A, Gudermann T, Hammock BD, Falck JR, Weissmann N, Busse R, Fleming I. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J 22: 4306–4315, 2008. doi: 10.1096/fj.08-112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keserü B, Barbosa-Sicard E, Schermuly RT, Tanaka H, Hammock BD, Weissmann N, Fisslthaler B, Fleming I. Hypoxia-induced pulmonary hypertension: comparison of soluble epoxide hydrolase deletion vs. inhibition. Cardiovasc Res 85: 232–240, 2010. doi: 10.1093/cvr/cvp281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovitz KL, Aleskowitch TD, Sylvester JT, Flavahan NA. Endothelium-derived contracting and relaxing factors contribute to hypoxic responses of pulmonary arteries. Am J Physiol Heart Circ Physiol 265: H1139–H1148, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Leeman M, de Beyl VZ, Biarent D, Maggiorini M, Mélot C, Naeije R. Inhibition of cyclooxygenase and nitric oxide synthase in hypoxic vasoconstriction and oleic acid-induced lung injury. Am J Respir Crit Care Med 159: 1383–1390, 1999. doi: 10.1164/ajrccm.159.5.9807114. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Sham JS, Shimoda LA, Sylvester JT. Hypoxic constriction of porcine distal pulmonary arteries: endothelium and endothelin dependence. Am J Physiol Lung Cell Mol Physiol 280: L856–L865, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Liu R, Evgenov OV, Ichinose F. NOS3 deficiency augments hypoxic pulmonary vasoconstriction and enhances systemic oxygenation during one-lung ventilation in mice. J Appl Physiol (1985) 98: 748–752, 2005. doi: 10.1152/japplphysiol.00820.2004. [DOI] [PubMed] [Google Scholar]
- 29.Loot AE, Moneke I, Keserü B, Oelze M, Syzonenko T, Daiber A, Fleming I. 11,12-EET stimulates the association of BK channel α and β(1) subunits in mitochondria to induce pulmonary vasoconstriction. PLoS One 7: e46065, 2012. doi: 10.1371/journal.pone.0046065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin LD, Barnes SD, Wetzel RC. Acute hypoxia alters eicosanoid production of perfused pulmonary artery endothelial cells in culture. Prostaglandins 43: 371–382, 1992. doi: 10.1016/0090-6980(92)90037-T. [DOI] [PubMed] [Google Scholar]
- 31.Miller VM. Why are sex and gender important to basic physiology and translational and individualized medicine? Am J Physiol Heart Circ Physiol 306: H781–H788, 2014. doi: 10.1152/ajpheart.00994.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26, 1977. doi: 10.1161/01.RES.41.1.19. [DOI] [PubMed] [Google Scholar]
- 33.Pfister SL. Role of lipoxygenase metabolites of arachidonic acid in enhanced pulmonary artery contractions of female rabbits. Hypertension 57: 825–832, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfister SL, Campbell WB. Role of endothelium-derived metabolites of arachidonic acid in enhanced pulmonary artery contractions in female rabbits. Hypertension 27: 43–48, 1996. doi: 10.1161/01.HYP.27.1.43. [DOI] [PubMed] [Google Scholar]
- 35.Pokreisz P, Fleming I, Kiss L, Barbosa-Sicard E, Fisslthaler B, Falck JR, Hammock BD, Kim IH, Szelid Z, Vermeersch P, Gillijns H, Pellens M, Grimminger F, van Zonneveld AJ, Collen D, Busse R, Janssens S. Cytochrome P450 epoxygenase gene function in hypoxic pulmonary vasoconstriction and pulmonary vascular remodeling. Hypertension 47: 762–770, 2006. doi: 10.1161/01.HYP.0000208299.62535.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin J, Sun D, Jiang H, Kandhi S, Froogh G, Hwang SH, Hammock BD, Wolin MS, Thompson CI, Hintze TH, Huang A. Inhibition of soluble epoxide hydrolase increases coronary perfusion in mice. Physiol Rep 3: E12427, 2015. doi: 10.14814/phy2.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rand AA, Barnych B, Morisseau C, Cajka T, Lee KSS, Panigrahy D, Hammock BD. Cyclooxygenase-derived proangiogenic metabolites of epoxyeicosatrienoic acids. Proc Natl Acad Sci USA 114: 4370–4375, 2017. doi: 10.1073/pnas.1616893114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimoda LA, Sham JS, Liu Q, Sylvester JT. Acute and chronic hypoxic pulmonary vasoconstriction: a central role for endothelin-1? Respir Physiol Neurobiol 132: 93–106, 2002. doi: 10.1016/S1569-9048(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 39.Sun D, Cuevas AJ, Gotlinger K, Hwang SH, Hammock BD, Schwartzman ML, Huang A. Soluble epoxide hydrolase-dependent regulation of myogenic response and blood pressure. Am J Physiol Heart Circ Physiol 306: H1146–H1153, 2014. doi: 10.1152/ajpheart.00920.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sylvester JT, McGowan C. The effects of agents that bind to cytochrome P-450 on hypoxic pulmonary vasoconstriction. Circ Res 43: 429–437, 1978. doi: 10.1161/01.RES.43.3.429. [DOI] [PubMed] [Google Scholar]
- 41.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 92: 367–520, 2012. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch D, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 159: 1925–1932, 1999. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 43.Weissmann N, Seeger W, Conzen J, Kiss L, Grimminger F. Effects of arachidonic acid metabolism on hypoxic vasoconstriction in rabbit lungs. Eur J Pharmacol 356: 231–237, 1998. doi: 10.1016/S0014-2999(98)00563-9. [DOI] [PubMed] [Google Scholar]
- 44.Wepler M, Beloiartsev A, Buswell MD, Panigrahy D, Malhotra R, Buys ES, Radermacher P, Ichinose F, Bloch DB, Zapol WM. Soluble epoxide hydrolase deficiency or inhibition enhances murine hypoxic pulmonary vasoconstriction after lipopolysaccharide challenge. Am J Physiol Lung Cell Mol Physiol 311: L1213–L1221, 2016. doi: 10.1152/ajplung.00394.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang BC, Mehta JL. Alterations in pulmonary artery tone during repeated episodes of hypoxia. Am J Physiol Lung Cell Mol Physiol 269: L293–L298, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Zhu D, Birks EK, Dawson CA, Patel M, Falck JR, Presberg K, Roman RJ, Jacobs ER. Hypoxic pulmonary vasoconstriction is modified by P-450 metabolites. Am J Physiol Heart Circ Physiol 279: H1526–H1533, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Zhu D, Bousamra M II, Zeldin DC, Falck JR, Townsley M, Harder DR, Roman RJ, Jacobs ER. Epoxyeicosatrienoic acids constrict isolated pressurized rabbit pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 278: L335–L343, 2000. [DOI] [PubMed] [Google Scholar]