Abstract
Electronic cigarettes (e-cigarettes or e-cigs) are designed to heat and aerosolize mixtures of vegetable glycerin, propylene glycol, nicotine, and flavoring additives, thus delivering nicotine by inhalation in the absence of combustion. These devices were originally developed to facilitate smoking cessation and have been available in the United States for over a decade. Since 2010, e-cig use has expanded rapidly, especially among adolescents, despite a paucity of short- and long-term safety data. Patterns of use have shifted to include never smokers and many dual users of e-cigs and combustible tobacco products. Over the last several years, research into the potential toxicities of e-cig aerosols has grown exponentially. In the interim, regulatory policymakers across the world have struggled with how to regulate an increasingly diverse array of suppliers and products, against a backdrop of strong advocacy from users, manufacturers, and tobacco control experts. Herein we provide an updated review of the pulmonary toxicity profile of these devices, summarizing evidence from cell culture, animal models, and human subjects. We highlight the major gaps in our current understanding, emphasize the challenges confronting the scientific and regulatory communities, and identify areas that require more research in this important and rapidly evolving field.
Keywords: electronic cigarette, smoking, bronchial epithelium, alveolar epithelium, lung, toxicity
electronic cigarettes, also known as e-cigarettes or e-cigs, belong to a growing class of electronic nicotine delivery systems (ENDS). E-cigs are devices of variable design that use heat to aerosolize e-liquids, mixtures of nicotine and flavorings, for inhalation. These devices have been marketed as safer alternatives to combustible cigarettes, despite a lack of adequate safety data. Since their introduction to the United States marketplace in 2006, e-cigs have become a multibillion-dollar industry, with $2.5 billion in sales in 2014 (57). If current trends continue, e-cig sales may surpass those of combustible cigarettes by 2023 (58).
In 2015, 3.7% of adults reported regular use of e-cigs, with the highest rates of use in the 18–24 age group (25). Of adult e-cig users in the United States, ~59% are concurrent cigarette smokers (dual users), 30% are former smokers, and ~11% are never smokers (24). In a recent Center for Disease Control study of nearly 35,000 adults in 2014, e-cig users were found more likely to be male, poorer, nonmarried, non-Hispanic white, and younger than those who had never tried e-cigs (136). Perhaps most striking has been the rapid increase in e-cig use among adolescents. In only four years, e-cig use among high school students rose 10-fold, from 1.5% in 2011 to 16% in 2015, with over 5% of middle school students reporting regular use in the most recent survey (116). These usage trends are of major concern in light of the reported association between adolescent e-cig use and risk for the initiation of other tobacco products, including combustible cigarettes (15, 31, 35, 79, 119). Furthermore, there is evidence from human studies and animal models that nicotine may act as a “gateway drug,” sensitizing key brain regions to the reinforcing effects of other drugs of abuse, including cocaine (65).
The rapid increase in e-cig use underscores a lack of knowledge about the acute and chronic effects of e-cig aerosols on pulmonary function. Herein we critically review the basic biological and clinical studies in this rapidly evolving area of scientific investigation, highlighting major gaps in our understanding and areas that need further research.
GENERAL CONSIDERATIONS
Atomizer Design, Function, and Composition
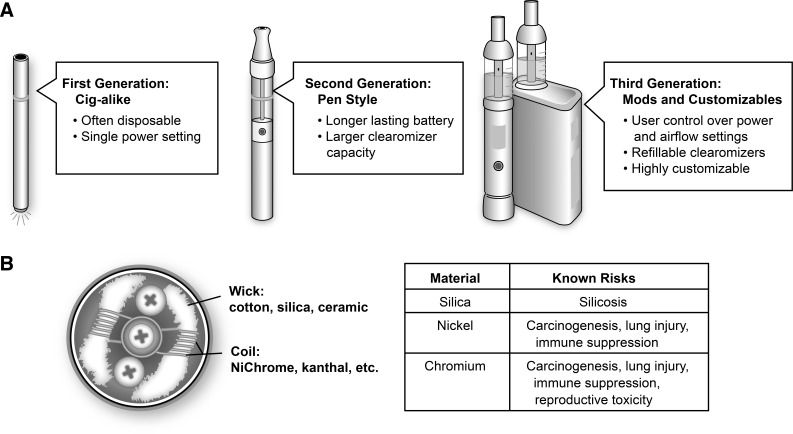
E-cigs are composed of an atomizer that uses electrical current from a battery to heat a metal coil, aerosolizing an “e-liquid” conducted from a reservoir to the coil by a wick typically made of cotton or silica. A touch of a button heats the coil and a plume of droplets are then sucked out of the device (“vaped”) by the user, transmitting aerosol in the oropharynx and respiratory system, resulting in high levels of particle deposition with every puff (84, 85). First-generation e-cig products (“cig-a-likes”) were designed to mimic the look of cigarettes; however, regular users have increasingly shifted from cig-a-likes to second- and third-generation devices (Fig. 1A) that allow users greater control over e-liquid content and the physics of aerosolization (56, 129). According to Ohm’s law, the power (P) in an electrical circuit in watts is equal to the square of the voltage (V) divided by the resistance (R) in ohms: P = V2/R. Thus the power at work in the aerosolization process is dependent on voltage and resistance, both of which may vary with age or even with the temperature of the components. Furthermore, many newer devices allow users to substitute coils of desired resistance and adjust voltage directly. Increased power levels have been shown to result in the production of volatile carbonyl species (48, 51, 117) and increase the mass of aerosol produced (51, 117). As such, there has been concern that resistance values of the coils could play a key role in e-cigarette toxicity (27).
Fig. 1.
A: the three generations of e-cigarettes. Beginning with the early cig-a-like devices, e-cigs introduced to the market more recently incorporate increased user control over the physics of aerosolization and e-liquid composition. B: e-cigarette atomizers contain wicking materials to transfer e-liquids from a storage tank in close proximity to a heating filament made from one of several types of metal alloys. Many of the commonly employed materials have well-described health risks.
The lithium batteries that power e-cig devices are susceptible to explosions and fires, especially while charging (126). Burns to e-cig users from battery-related explosions have become so common that a classification system of five different types of thermal injuries has recently been proposed (103).
Other aspects of atomizer design, including coil and wick material, may also contribute to toxicity (Fig. 1B). Alloys of nickel-chromium (“NiChrome”) and of chromium-aluminum-iron (“Kanthal”) comprise the metal coils of the majority of heating coils in the current generation of atomizers. However, additional metal material, including copper, silver, zinc, and tin, were reported in a recent study on device composition (134). As e-cig metal components undergo repeated cycles of heating and cooling, traces of these metal components can leach into the e-liquid, causing the device to emit metallic nanoparticles (135). One study found traces of chromium, manganese, nickel, and lead in the e-liquids of five brands of cig-a-like devices (59). Levels of chromium and nickel were especially high, and it is likely the nickel-chromium heating coils were the source of this contamination. The potential toxicity of these metal components requires further research for several reasons. Exposure to metal fumes containing a mixture of metals and metal oxides in welders has well-established short-term systemic toxicity (“metal fume fever”), increases the frequency of respiratory tract infections, and may also increase the risk of lung cancer (7). Similarly, animal models of metallic fume exposure, including chromium, nickel, and stainless steel, have produced evidence of lung injury (5) and immune suppression (6). Nickel and chromium are classified by the Food and Drug Administration (FDA) as “harmful and potentially harmful constituents,” with nickel labeled as a carcinogen and respiratory toxicant and chromium as a carcinogen, respiratory toxicant, and reproductive toxicant (42). In addition to these metallic toxicants, silica wicks are commonly used in e-cigarettes, despite silica’s known associations with silicosis, respiratory disease, and autoimmune dysfunction (78, 104). Silicate nanoparticles have also been found in aerosols produced by devices using fiberglass wicks (135).
E-Liquid Composition
E-liquids are composed of varying ratios of vegetable glycerin (VG), propylene glycol (PG), nicotine, and flavoring agents. Nicotine content varies widely; however, concentrations between 16 and 24 mg/ml are most common in premixed e-liquids. Although nicotine can be synthesized artificially from other chemicals, this process is quite expensive, and thus synthetic nicotine is not widely available. The vast majority of commercially available nicotine is extracted from tobacco plants using organic solvents (60, 82). E-liquid consumer websites offer extracted nicotine, typically prepared in VG/PG at concentrations ranging from 12 to 100 mg/ml and at volumes up to 20 liters, although details about the manufacturing process or evidence of quality assurance are frequently absent. For perspective, a typical cigarette delivers ~2 mg of nicotine to its smoker, and the LD50 for an adult is reported to be ~60 mg (92). Thus it is not surprising that ingestion of e-liquid can be fatal to children (102, 112) and adults (28, 138). The most recent data from the U.S. National Poison Control database indicates that in 2015 there were ~3,000 reported exposures to nicotine-containing e-liquids with over 1,000 requiring medical attention, the majority of incidents involving children under 5 yr of age (100).
Both VG and PG have been designated by the FDA as “generally recognized as safe” (GRAS) for oral intake, and, because of their ability to interact with photons in the visible light spectrum when aerosolized, they have long been used in the entertainment industry to create artificial smoke. VG and PG are also frequently employed as humectants in traditional cigarettes (47). Most e-liquids are composed of varying ratios of VG and PG, formulated to maximize the subjective sensation of the carried flavoring agents as well as the appearance of the aerosol. Because there are relatively few components in e-liquids compared with combustible tobacco products, they produce fewer toxic chemical species than do cigarettes (55). Indeed, recent studies sponsored by the tobacco industry have reported simpler chemical profiles than cigarette smoke (87) and less cytotoxicity following short-term in vitro exposure (11).
The FDA GRAS designation does not apply to aerosolization, and there are no controlled long-term studies of the effects of inhaling heated aerosolized VG or PG in humans. Both VG and PG can form toxic aldehydes when heated (117). Acute inhalational exposure to aerosolized PG in humans has been reported to cause throat discomfort, cough, and decreased forced expiratory volume in 1 s (FEV1)/forced vital capacity (133). Rabbits exposed to just 2 h of PG aerosol show evidence of tracheal goblet cell degeneration (70). Although rats are relatively resistant to brief exposures to aerosolized PG, longer term exposures have been shown to increase nasal goblet cell number and mucin content (120), irritate nasal and ocular mucous membranes, and cause laryngeal squamous metaplasia (132). Interestingly, theatrical workers involved in productions using “fogs” generated from VG and PG have been reported to have increased dyspnea and work-related chest tightness and wheezing in proportion to estimated cumulative exposure (128). Thus there is legitimate concern over the health effects of chronically inhaling these substances.
Furthermore, flavoring components, present in most e-liquids, are typically absent from combustible cigarettes. Historically, inhaling food flavorings has been associated with the unexpected development of life-threating respiratory failure. Diacetyl, a buttery flavoring agent used in microwave popcorn, had long been designated by the FDA as GRAS. In 2002, however, one group of investigators reported a cluster of cases of bronchiolitis obliterans in association with workplace exposure to airborne diacetyl (72). The effects of diacetyl inhalation were recapitulated in a subsequent mouse study, which found that subchronic exposures to diacetyl caused lymphocytic bronchitis and bronchiolitis (99). Despite the widespread publicity surrounding “popcorn lung” in both the medical community and the lay press, a recent study reported the presence of diacetyl in 110 out of 159 tested “sweet” e-liquids (38).
Diacetyl inhalation is the most well-known case of artificial flavoring-induced respiratory disease. However, a recent study of 367 workers at a flavoring manufacturing facility that had shifted toward usage of diacetyl substitutes found that time spent in production areas of 1 h or greater per day predicted dyspnea and spirometric and diffusing capacity abnormalities (32). Thus, inhalational exposure to flavoring additives beyond diacetyl may have dose-dependent pulmonary toxicity that may not manifest for many years. Given that there are >7,500 flavors of e-liquids on the market (139), our lack of knowledge regarding the basic safety of inhaling these flavoring additives presents a major challenge to the scientific and regulatory environment (14). It should be noted that the subjective experience of flavor is a critical motivator for e-cigarette use (9, 123), with sweet flavors (e.g., candy, fruit) being especially appealing to youth (1, 73) and young adult users alike (52, 67). Additionally, a recent study reported that flavoring components of e-liquids were the main contributor in the production of toxic carbonyl species (66). Cinnamaldehyde, a reactive organic compound that gives cinnamon its flavor, has been shown to have cytotoxic and mutagenic effects in cell culture assays at concentrations found in cinnamon-flavored and a variety of fruit-, tobacco-, and sweet-flavored commercially available e-liquids (16, 18). Investigators have also reported cytotoxic effects from specific e-cig aerosols with the flavor of coffee (107), cinnamon cookie (39), “swiss dark,” “menthol arctic,” and butterscotch (12). Furthermore, a recent report has shown high levels of the irritant compound benzaldehyde in cherry-flavored e-liquids (71).
Quality Control and Accuracy in Labeling
Multiple analyses of e-liquids have found inconsistencies between actual nicotine concentrations and those indicated by labeling (29, 33, 41, 54, 82). Another study found detectable levels of arsenic, nickel, and other metals in e-liquids, with additional traces of lead in the devices themselves (96). The lack of quality control presents challenges to toxicological investigation, with many published studies providing no information on the chemical content of e-liquids or their aerosols.
E-Cig Usage Patterns
A study of puffing topography found that e-cig users required, on average, 32 puffs over a 20-min interval, with each puff lasting 2.7 s (17). Another recent study reported an average puff duration of 3.5 s, with a mean flow rate of 37 ml/s (106). In general, puff duration for e-cigs lasts longer than those for cigarettes (40). An analysis of changes in puff topography among smokers switching to e-cigs found that average puff duration increased from 2.2 to 3.1 s with a decrease in flow rate from 31 to 25 ml/s, possibly to modulate nicotine delivery (75). In one study, the investigators used a tank system to aerosolize eight types of e-liquids, measured the generated particles, and used modeling to predict that, for each 2-s puff, 6.25 × 1010 particles would be deposited in the respiratory system (84). The results also indicated that most particle deposition would occur in the alveolar region (18th–22nd airway generations), and that after just 10 puffs the thickness of the e-cig liquid deposited on the alveolar epithelium would be comparable to the thickness of the surfactant layer. Taken together, these studies suggest that the average e-cig user inhales a significant volume of e-liquid aerosols on a daily basis, exposing the entire respiratory epithelium to substantial concentrations of aerosol mass.
Regulatory Issues
At present, the regulation of e-cigs varies widely across the world. Several countries such as Argentina, Brazil, and Jordan have simply banned the manufacture and sale of the devices. The U.S. FDA recently increased the regulation on the manufacturing and sales of e-cigs and other ENDS. ENDS have now been deemed by FDA to merit the same regulatory oversight as traditional tobacco products, including enforcement of standards for labeling and restriction of sales to minors (43). This shift in the regulatory environment may address some concerns regarding the possibility of gateway effects among minors, as well as the potential short- and long-term health effects of e-cigs and other ENDS.
In December 2016, the U.S. Surgeon General published a report addressing e-cigarette use in youth and young adults (125). The report summarizes the use patterns, health effects, and current policy strategies associated with e-cig use. It acknowledges the limited data on long-term effects and also concludes that e-cig aerosol is not harmless and “can contain harmful and potentially harmful constituents, including nicotine.” Furthermore, it proposes that actions be taken at all governmental levels to address e-cig use. The local or state-level implementation of clean air laws, further prohibition of sales to youth, and the development of countermarketing campaigns were discussed as possible strategies. Taken together, the FDA deeming rule and the Surgeon General’s report appear to signal a definite shift in the U.S. toward the need for greater regulatory oversight of e-cigarettes. Possible regulatory targets include atomizer design and construction, battery design, VG/PG purity, nicotine concentration, and added flavoring compounds.
There has been additional debate over whether laws banning smoking in public spaces, which currently protect >80% of the American population (2), should also apply to e-cig use. Notably, a recent study found that 60% of American e-cig users reported vaping in public areas where cigarette smoking was disallowed (114). E-cigarettes have been shown to lower indoor air quality (109), and nonsmokers have been found to absorb nicotine from secondhand e-cig aerosol at levels comparable to secondhand tobacco smoke (13). Based on a nationally representative survey from 2012, there was moderate support (38%) for restricting e-cig use in smoke-free areas (83). Recent studies have found positive correlations between perceived health risks of second-hand vape exposure and support for vape-free policy (95). In another study, exposure to e-cig marketing communications was associated with decreased support for restrictive policies (122). Currently, 12 states have laws restricting e-cig use in previously designated smoke-free venues (3).
As the U.S. has moved toward greater regulation of e-cigs, the United Kingdom Royal College of Physicians (RCP) recently endorsed e-cigs as an effective aid to smoking cessation (105). Citing cigarette smoking as the “biggest avoidable cause of death and disability, and social inequality in health, in the UK,” this report acknowledges that “e-cigarettes are not currently made to medicines standards and are probably more hazardous than nicotine replacement therapy.” The report makes a claim that “the hazard to health arising from long-term vapour inhalation from the e-cigarettes available today is unlikely to exceed 5% of the harm from smoking tobacco,” although the body of the report offers no evidence for this numeric estimate. Clearly the overall public health impact of the adoption of e-cigarettes will depend on many factors, including the inherent toxicity of e-cigs and the tendency of e-cig users to increase or decrease their consumption of traditional tobacco products (64). Physicians, policymakers, and regulatory bodies need more research and improved toxicological data on which to base their recommendations and decisions.
Most investigators have focused on the short-term effects of e-cig aerosol exposure, although it is worth reiterating that long-term exposures may well have unpredictable and significant pulmonary toxicity, as has been reported with inhalational exposure to diacetyl. Broadly speaking, the investigations of the acute pulmonary toxicity of e-cigarettes can be divided based on model system: cell culture, animal models, and human volunteers. To date, these studies have several limitations but in the aggregate provide evidence that e-cig aerosols may have toxic effects on the lung epithelium and impair lung immune defenses, as summarized in the next sections. Of note, this review is focused on pulmonary toxicity; excellent reviews of the toxicity of e-cigarettes on the cardiovascular system (20) and oral cavity (63) have recently been published.
IN VITRO MODELS OF TOXICITY
Airway Epithelium, E-Cig Exposure in Submersion Culture
While pulmonary airway epithelial cell cultures lack the complete array of cellular components present in the conducting airways, they do include multiple cell types, including goblet, basal, and ciliated cells, providing a useful reductionist approach to studying the effects of e-cig exposure on individual airway cells. In vitro preparations of airway epithelial cells include both immortalized and primary cell cultures, and researchers have developed e-cig exposure methods suitable for both submersion culture and air-liquid interface (ALI) models (Table 1). The toxicity of e-cig products is likely influenced both by the presence or absence of heat-based aerosolization and by the method of interaction of epithelial cell surface with the resulting chemical species (dissolved in aqueous solution in submersion culture vs. dispersed in aerosol in ALI). Therefore we have elected to group these studies by method of exposure.
Table 1.
In vitro models of e-cigarette pulmonary toxicity
| Study | Cell Type | Exposure Method | Exposure Type | Key Findings |
|---|---|---|---|---|
| Wu et al. (137) | Airway epithelial cells (primary) | Submersion culture | Media diluted e-liquid | No effects on cell viability; increased viral loads after rhinovirus infection; decreased antimicrobial activity |
| Sherwood and Boitano (113) | HBEC (immortalized) | Submersion culture | Media diluted flavoring agents | High concentrations caused cell death; lower concentrations altered membrane ion conductance (via cystic fibrosis transmembrane conductance regulator ion channel, CFTR) |
| Schweitzer et al. (110) | Lung endothelial cells (primary) | Submersion culture | Media diluted e-cig aerosol condensate | Decreased cell proliferation; diminished lung endothelial barrier function; involvement of both nicotine and other e-cig aerosol chemicals |
| Gerloff et al. (50) | Beas2B, H292, HFL1, 16-HBE | Submersion culture | Flavoring compounds and nicotine dissolved in culture media | Many tested compounds increased release of the inflammatory cytokine IL-8, although responses were cell line specific |
| Aug et al. (10) | HBEC (primary) | Air-liquid interface | Media diluted e-liquid | Increased levels of cellular stress; metabolome effects similar to CS exposure |
| Scheffler et al. (108) | HBEC (primary) | Air-liquid interface | E-cig aerosols | Increased oxidative stress; reduced cell viability |
| Garcia-Arcos et al. (46) | HBEC (primary) | Air-liquid interface | E-cig aerosols | No effects on cell viability; nicotine-dependent reduction in ciliary beat frequency and CFTR ion conductance |
| Leigh et al. (76) | H292 | Air-liquid interface | E-cig aerosols | Toxicity measured by viability, metabolic activity and inflammatory cytokine release was device dependent and was increased with higher voltages and flavoring compounds |
| Neilson et al.* (101) | HBEC (primary) | Epi-Airway 3-D model | E-cig aerosols | No effects on cell viability; increased particle deposition compared with CS |
| Hwang et al. (62) | A549 | Submersion culture | Media diluted e-cig aerosol extract; e-cig aerosols | Significant cell death with exposure; decreased antimicrobial activity of macrophages and neutrophils; increased virulence of MRSA |
| Misra et al.* (97) | A549 | Submersion culture | Media diluted e-liquid; pad collected aerosol | Absence of cytotoxicity or inflammatory response; no effects on cell viability; no mutagenic or genotoxic effects |
| Cervellati et al. (26) | A549 | Submersion culture | E-cig aerosols | Increased markers of cytotoxicity; decreased cell viability; changes in cell morphology (vacuolization) |
| Husari et al. (61) | A549 | Submersion culture | Media diluted e-cig aerosol condensate | Increased cell death with high nicotine levels |
HBEC, human bronchial epithelial cells; CS, cigarette smoke; e-cig, electronic cigarette; MRSA, methicillin-resistant Staphylococcus aureus.
Studies reporting fundings from the tobacco industry.
One group conducted an interesting experiment in which human tracheobronchial epithelial cells were grown in submersion culture and exposed to e-liquids diluted with cell culture media (137). Cells were then infected with human rhinovirus to determine the effects of e-cigarettes on immune defenses. Although cell viability was not decreased after a 48-h incubation period, e-liquid-exposed epithelial cells had decreased production of antiviral proteins and increased viral load. Given the key role that respiratory epithelia play in host defense, this study highlights the need to test e-cig aerosols both for direct cellular toxicity and altered susceptibility to and propagation of infectious agents.
Another group of investigators exposed cells directly to media-diluted flavoring agents and found that vanillin and dimethylpyrazine (a component of chocolate flavoring) altered cellular impedance and signaling pathways (113). Higher concentrations of flavoring agents resulted in cell death. Although most pulmonary toxicity studies have been done using epithelial cells, one group cultured rat and human primary lung endothelial cells, finding that nicotine and other soluble components of e-liquids caused a dose-dependent reduction in endothelial barrier function (110).
A recent study exposed human bronchial epithelial cells (Beas2B, a transformed cell line), human mucoepidermoid carcinoma epithelial cells (H292), and primary lung fibroblasts (HFL1) to varying concentrations of e-liquid flavoring compounds for 24 h (acetoin, diacetyl, pentanedione, maltol, o-vannilin, coumarin, and cinnemadlehyde), finding that many of these compounds resulted in release of the inflammatory cytokine IL-8 (50). Remarkably, acetoin (in Beas2B cells) and pentanedione in HFL1 cells caused IL-8 levels to exceed that induced by the potent cytokine TNF-α (50). The same study used electric cell surface impedance sensing (ECIS) to test the effects of flavorants and nicotine on barrier function of 16-HBE cells (a transformed bronchial cell line), finding that all tested compounds caused decreases in barrier function over time.
Airway Epithelium, E-Cig Exposure in ALI
To better model the physiology of human exposures to e-cigs, some investigators have capitalized on the ability of cultured primary human bronchial epithelial cells (HBECs) to form a tight monolayer and generate vectorial ion and fluid transport, creating an ALI. One group exposed both the basolateral and apical surfaces of HBECs grown in ALI to e-liquid, finding that just 7 h of exposure caused a major shift in the cellular metabolome (10). E-liquid-exposed cells had increased amino acid production and ADP levels, both of which are stress responses also observed with cigarette smoke concentrate. Another group exposed HBECs grown in an ALI to e-cig aerosols and cigarette smoke (108). Both treatments resulted in significant decreases in cell viability and increases in oxidative stress compared with air-exposed cells; cigarette smoke exposure was significantly more harmful to cells by all measures. Interestingly, exposure to pure propylene glycol elicited responses similar to exposure to e-liquids containing nicotine and glycerol. Another study exposed HBECs in an ALI to aerosols of either nicotine-containing or nicotine-free e-liquids, finding that nicotine did not reduce cell viability at 4 h but did produce significant reductions in ciliary beat frequency and cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel conductance (46). However, these experiments did not include a control group that lacked exposure to e-cig aerosol, limiting conclusions about potential toxicants other than nicotine.
Investigators from British American Tobacco used a commercially available three-dimensional culture system comprised of human airway epithelial cells, basal cells, and goblet cells (EpiAirway), reporting that a short-term 6-h exposure to e-cig aerosol from cig-a-like devices did not reduce cell viability as cigarette smoke did. The study also reported that the deposited mass of e-cig aerosols was greater than that of cigarette smoke, and, as the authors point out, longer duration or repeated e-cig aerosol exposures might have yielded different results (101).
In a more recent study, researchers exposed H292 bronchial epithelial cells in ALI to a broad range of e-cigarette aerosols from multiple classes of ENDS products, including disposable cig-a-likes, rechargeables, and eGo tank systems, reporting device-dependent reductions in cell viability and metabolic activity and increased cytokine release (76). With the use of the eGo tank system and constant voltage, a number of commercially available flavors of e-liquids were then tested, with menthol-, coffee-, and strawberry-flavored aerosols found to significantly reduce viability and metabolic activity and increase inflammatory cytokine production. Strawberry flavoring in particular elicited a response similar to that seen following cigarette smoke exposure. This group also studied a range of voltages and nicotine concentrations, reporting increased toxicity with higher applied voltages, and increased IL-6 release (although no effect on viability) with the highest level of nicotine (24 mg/ml).
Alveolar Epithelium
In addition to understanding the effects of e-cig aerosols on the conducting airways, it is important to understand how these aerosols affect the alveolar epithelium, which constitutes the vast majority of surface area in the lung and is subject to high levels of particle deposition (84). Injury to the alveolar-capillary barrier can produce severe physiological consequences, including acute respiratory distress syndrome (ARDS). Notably, cigarette smoke exposure increases the risk of developing ARDS in a variety of settings, including following blunt trauma and in nonpulmonary sepsis (22, 23). Similarly, healthy smoking volunteers compared with nonsmokers manifest greater alveolar-capillary barrier permeability to protein after inhaling nebulized lipopolysaccharide (98).
To date, all published studies of e-cigs and in vitro models of the alveolar epithelium have used the A549 epithelial cell line derived from human adenocarcinoma. Unlike primary human alveolar type II cells, A549 cells do not form a tight barrier in culture (68), mandating submersion exposure in toxicity assessments. Primary human ATII cell culture is well described (37, 86), yet no published studies have used this method to evaluate the toxicity of e-cig aerosols. Primary ATII cell cultures replicate many of the in vivo functions of the alveolar epithelium, including production of surfactant and vectorial fluid transport to maintain the dryness of the airspaces. ATII cell cultures can also be used as a model of alveolar epithelial injury, which can be followed with measures of cell migration and repair (8).
One group exposed the apical surface of A549 cells to either e-cigarette aerosol or aerosol extract and found dose-dependent increases in the levels of lactate dehydrogenase (LDH) in the supernatant just 2 h after exposure, consistent with necrotic cell death (62). These researchers also studied the antimicrobial properties of alveolar macrophages and neutrophils following exposure to extracts of vaped VG and PG. All vaped components increased the growth of methicillin-resistant Staphylococcus aureus (MRSA) in coculture, an effect exaggerated by the addition of nicotine. MRSA exposed to e-cigs had increased resistance to the antimicrobial cathelicidin peptide LL-37. Furthermore, MRSA became more hydrophobic and adherent to human epithelial cells following e-cig aerosol exposure. A study published by the tobacco industry reported no evidence of significant cytotoxic, mutagenic, or inflammatory responses following exposure of A549 cells to e-cigarette vapor (97). However, other studies in A549 cells have reported increased cell death (26), and decreased cell viability (61), especially remarkable because this hypotriploid adenocarcinoma cell line is highly proliferative and resistant to cell death (53, 80).
In vitro methods have an important role in high-throughput screening, mechanistic studies (including inflammatory and oxidative stress pathways, barrier function), and in clarifying the effects of e-cigs on individual cell types such as airway and alveolar epithelium. Additionally, coculture studies can provide insight on the cellular interactions with innate and adaptive immune cells. However, animal studies are also needed to determine the effects of e-cigarettes at the whole organ level.
ANIMAL MODELS OF TOXICITY
Broadly speaking, there is increasing evidence from animal models over the last 2 yr that e-cig aerosol exposure 1) may be directly toxic to lung tissues, 2) may impair host defense against respiratory pathogens, and 3) may interfere with normal lung development (Table 2). Experimental methods are variable, and frequently the published details of aerosol generation and exposure are limited. For example, many studies failed to report details of flavoring agents, device design, coil resistance, and applied power.
Table 2.
Animal models of e-cigarette pulmonary toxicity
| Study | Animal Model | Exposure Method | Exposure Duration | E-Liquid | Device | Key Findings |
|---|---|---|---|---|---|---|
| Lerner et al. (77) | Mouse | Whole body | 3 days, 5 h daily | Nicotine: 16 mg/ml + flavoring | Blu e-cig with Teague TE-10 | CS equivalent serum cotinine levels; increased inflammatory cytokine levels in BAL; oxidative stress in lungs |
| Schweitzer et al. (110) | Mouse | Whole Body | Not stated | Not stated | Nebulized e-liquid | Increased markers of oxidative stress in BAL fluid and plasma 24 h after a single exposure |
| Sussan et al. (121) | Mouse | Whole body | 2 wk, 3 h daily | Nicotine: 18 mg/ml + flavoring | NJOY rechargeable | Increased lipid peroxidation; impaired antimicrobial defenses; CS equivalent serum cotinine levels; inflammatory response in airway |
| Garcia-Arcos et al. (46) | Mouse | Whole body | 4 mo, 1 h daily | Nicotine: 18 mg/ml | Compressed air nebulizer | Increased airway hyperreactivity; distal airspace enlargement; increased lung mucin production, inflammatory cytokines, proteases |
| McGrath-Morrow et al. (94) | Neonatal mice | Whole body | 10 days, 20 min daily | Nicotine: 18 mg/ml | Joytech 510-T | Decreased weight gain; elevated plasma and urine cotinine; impaired alveolar development |
| Shivalingappa et al. (115) | Mouse | Whole body | 3 × 1 h daily | Nicotine: 25 mg/ml | Vibrational nebulizer | Increased lung lysate ubiquitin; evidence for impaired autophagy |
| Larcombe et al. (74) | 4-wk old Mice | Whole body | 8 wk of 1 h daily | Nicotine: 0 or 12 mg/ml + American Tobacco Flavor; varied ratio of VG to PG | iTaste MVP 2.0 | Increased responsiveness to methacholine despite no increase in inflammatory cells; lower lung volumes at fixed transmural pressure; more severe impairments with VG predominant excipient |
| Hwang (62) | Mouse | Nose only | 4 wk, 1 h daily | Nicotine: 24 mg/ml | Multiple | Increased BAL inflammatory cytokines; impaired antimicrobial defenses |
| Werley et al. (131) | Rat | Nose only | 28–90 days 15–160 min daily | Nicotine: 20 mg/ml + flavoring | MarkTen | Increased BAL protein, neutrophils, macrophages; BAL changes persisted for 7 wk; morphological changes in nasal epithelium, larynx, and lungs |
| Lim and Kim (81) | Mouse | Intratracheal instillation of diluted e-liquid | 10 wk, 2 times weekly | Nicotine: dosed at 1.3 mg/kg | N/A (intratracheal instillation) | Increased airway hyperresponsiveness to methacholine; increased BAL eosinophils and Th2 cytokines |
VG, vegetable glycerin; PG, propylene glycol; N/A, not applicable; CS, cigarette smoke; BAL, bronchoalveolar lavage.
Whole body exposure methods, which involve exposing the entire animal to e-cig aerosols, are relatively simple to perform and are used by most investigators. However, these studies are potentially confounded by skin exposure and ingestion of aerosol residues that may occur during grooming. Nose-only exposures require some form of restraint, facilitating the study of the direct effects of inhalational exposure, but at the cost of significant stress on the animals (126a).
Direct Lung Toxicity
Whole body exposure.
One group of investigators exposed 8-wk-old mice to e-cig aerosol at 200 mg/m3 total suspended particulate generated by a Teague TE-10 machine and Blu e-cig with classic tobacco flavor e-juice and 16 mg/ml nicotine. Mice were exposed 5 h/day for 3 days, and bronchoalveolar lavage (BAL) was done 24 h after the last exposure. Although aerosol exposure did not measurably alter BAL cell number or cell type, it increased the concentration of inflammatory cytokines in the BAL, including IL-6, MCP-1, IL-1α, and IL-13 (77). Additionally, lung lysate glutathione levels were reduced in aerosol-exposed mice, consistent with increased oxidative stress. A separate group of investigators exposed adult mice to nebulized e-liquid and reported modest increases in BAL 8-hydroxy-2'-deoxyguanosine, a marker of oxidative stress (110). Similarly, another group used a smoking machine to vape NJOY menthol bold (rechargeable cig-a-like, 1.8% nicotine), exposing mice for 1.5 h two times daily for 2 wk, reporting increased lung homogenate thiobarbituric acid reactive substances (a marker of lipid peroxidation) 24 h after the last exposure, along with an increased number of BAL macrophages (121). However, BAL IL-6 with this type of e-cig exposure was decreased relative to control mice, unlike the shorter-duration Blu e-cig experiments described above.
One group of investigators used a vibrational nebulizer to expose C57BL/6 and CD-1 (outbred) mice to 1 h of e-cig aerosol (25 mg/ml nicotine) three times over 24 h (115). E-cig-exposed mice compared with nonexposed controls were found to have increased lung lysate levels of ubiquitin along with evidence of impairment of autophagy, a mechanism previously reported to be important in COPD (124). Another research group (46) used a nebulizer to aerosolize saline or VG/PG with 0 or 18 mg/ml nicotine, exposing 12-wk-old A/J mice 5 days/wk for 4 mo. Notably, this study employed compressed air rather than a heating source (as employed by e-cig devices) to aerosolize the tested solutions. VG/PG/nicotine but not VG/PG alone increased 1) airway resistance and mucin content, 2) lung homogenate inflammatory cytokines, including MCP-1 and IL-6, 3) homogenate collagenase activity, 4) apoptosis of airway and alveolar cells, and 5) airspace enlargement and other histological changes consistent with emphysema. The convincing COPD phenotype generated in these mice after just 4 mo of nicotine-containing e-cig aerosol exposure highlights the importance of studying chronic effects and could portend a future wave of e-cig-related obstructive lung disease.
Finally, in a recent study, 4-wk-old (corresponding to human adolescence, a time during which the lungs are still growing) female BALB/c mice were exposed to cigarette smoke or e-cig aerosol (American Tobacco flavor containing 0 or 12 mg/ml nicotine and differing with respect to ratio of VG to PG) generated by an Innokin iTaste MVP 2.0 e-cig for 1 h/day, 5 days/wk for 8 wk (74). Following the last exposure, lung inflammation, volumes, and mechanics were determined, along with response to methacholine. Although e-cig aerosol exposure did not increase cellular inflammation, it did increase methacholine responsiveness and reduced lung volumes at fixed inflation pressures, suggesting a change in mechanical properties and/or lung size.
Nose-only exposure.
One group of investigators placed 4-mo-old male C57BL6 mice in tubes and exposed them to air, air mixed with cigarette smoke, or air mixed with e-cig aerosol generated by a V4L CoolCart (cig-a-like, strawberry flavor, 18 mg/ml nicotine) every 14 s for 6 h daily for 3 days. E-cig-exposed mice had increased lung wet-to-dry ratio of ~5.5 compared with an average ratio of 3.5 in controls, indicating the development of pulmonary edema. The increased wet-to-dry ratios in e-cig-exposed mice were observed in association with elevated BAL IL-6 mRNA (61). Another group exposed 6- to 8-wk-old female CD-1 mice to e-cig aerosol for 1 h daily for 4 wk, finding increased BAL KC (murine homolog of IL-8) and TREM-1, a proinflammatory cytokine associated with COPD and interstitial lung disease (62). Finally, another group (131) exposed 5- to 7-wk-old Sprague-Dawley rats to aerosol generated from VG/PG, VG/PG/nicotine, or VG/PG/nicotine/flavor by a MarkTen (cig-a-like) for 15, 48, or 160 min/day. After 28 and 90 days, higher aerosol exposures were associated with increased BAL protein (a marker of alveolar-capillary barrier permeability) and BAL neutrophilia, nasal mucus cell hyperplasia, and increased numbers of alveolar macrophages. Changes in the BAL fluid persisted through a 42-day recovery period after the last exposure, suggesting that the inflammatory changes induced by e-cig aerosols may be persistent and long lasting.
Direct intratracheal instillation of e-liquids.
One study induced an asthma-like mouse phenotype through intraperitoneal and intratracheal ovalbumin (OVA) sensitization with or without concurrent diluted intratracheal e-liquid exposure two times weekly for 10 wk (81). E-liquid-exposed mice had spirometric evidence of increased airway hyperresponsiveness to methacholine, in association with increased serum OVA-specific IgE and increased BAL eosinophils and Th2 cytokines IL-4, IL-5, and IL-13. Thus e-cig exposure may potentiate the development of allergic respiratory disease.
Impaired Immune Defenses
In a study of the effects of vaping on host immune defense, mice were exposed to e-cig aerosols (NJOY menthol or traditional bold, 1.8% nicotine, whole body for 1.5 h 2 times/day for 2 wk) before being infected intranasally with Streptococcus pneumonia (121). Compared with air-exposed controls, mice exposed to e-cigs had increased bacterial load in BAL at 24 h. In subsequent experiments, alveolar macrophages were obtained from uninfected air- and e-cig-exposed mice and then incubated with S. pneumonia in vitro. Macrophages obtained from e-cig-exposed mice exhibited decreased bacterial phagocytosis. Similar e-cig whole body exposures before intranasal inoculation of mouse-adapted H1N1 influenza revealed that e-cig-exposed mice had reduced 2-wk survival in association with increased lung viral titers at 4 days. A separate group of investigators exposed murine alveolar macrophages to e-cig vapor extract (EVE) from nicotine-containing e-liquids followed by incubation with MRSA, finding increased levels of MRSA in EVE-exposed cells (62). Taken together, the results suggest that even brief exposures to e-cig aerosol can cause major defects in the innate and adaptive immune defenses against common bacterial and viral respiratory pathogens.
Effects on Development
One group of investigators (94) exposed neonatal C57BL6 mice to aerosol generated by a Joyetech 510-T (cig-a-like, 1.8 or 0% nicotine in PG) for 20 min one or two times daily from postnatal days 1 to 9. Compared with air-exposed control mice, e-cig-exposed mice gained less weight. Neonatal alveolar development, as measured by cell proliferation and mean linear intercept analysis, was impaired in mice exposed to nicotine-containing e-cig aerosols. In the context of prior studies that reported impaired alveolarization following nicotine exposure in models ranging from rats (90) to primates (111), these results suggest that inhalation of nicotine-containing e-cig aerosols by pregnant women or by children and adolescents may impair lung development, a hypothesis advanced in a recent review (118).
EVIDENCE OF TOXICITY IN HUMAN SUBJECTS
Because e-cigs have only been commercially available for the last decade, there is little evidence regarding long-term toxicity. Short-term dangers from e-cig use not related to the pulmonary system include device explosions (69), accidental and intentional fatal nicotine overdose from e-liquids as described in detail above, and nickel contact dermatitis (88). Although a detailed summary of research in e-cig cardiovascular toxicity is beyond the scope of this review, several studies in humans have reported the expected acute hemodynamic changes related to nicotine inhalation (19, 20). Additionally, a recent study reported increased circulating endothelial progenitor cells following just 1 h of e-cig aerosol exposure, suggesting the possibility of acute endothelial injury similar to cigarette smoke (4).
The data from human subjects on pulmonary toxicity (Table 3) can be broadly grouped into respiratory symptoms, effects on respiratory physiology, and altered host defense against infection.
Table 3.
Evidence of e-cigarette pulmonary toxicity in humans
| Study | Subjects | Methods | Exposure Duration | E-Liquid | Key Findings |
|---|---|---|---|---|---|
| McConnell et al. (93) | 2,086 Youth survey participants | Examination of self-reported e-cig use and respiratory symptoms over 12 mo | Increased risk of chronic broncitic symptoms among current and past users | ||
| Vardavas et al. (127) | 30 Healthy smokers | Ad libitum e-cig session and control session | 5 min | Nicotine: 11 mg/ml | Increased airflow resistance; decreased FeNO (marker of oxidative stress) |
| Flouris et al. (45) | 15 Smokers; 15 nonsmokers | Smokers: e-cig and cigarette sessions nonsmokers: passive exposures to e-cig and cigarette | Smokers: 30 min nonsmokers: 1 h | Nicotine: 11 mg/ml | Serum cotinine similar between e-cig and cigarette use; fewer changes in lung function with e-cigarette use |
| Marini et al. (89) | 25 Healthy smokers | Control session, cigarette, e-cig (±nicotine) | 5 min | Decreased FeNO; increased particle deposition compared with cigarette smoke | |
| Dicpinigaitis et al. (34) | 30 Healthy nonsmokers | Capsaicin cough reflex sensitivity: control session, 15 min, 24 h after e-cig exposure | 30 Puffs 30 s apart | Blu classic tobacco flavor (1.5–1.8 mg nicotine in 30 puffs) | Decreased cough sensitivity at 15 min, recovery by 24 h |
| Martin et al. (91) | 13 Nonsmokers; 14 smokers; 12 e-cig users | Nasal scrape biopsies; nasal lavage; urine and serum collection | Suppression of 358 immune response genes in e-cig users (53 in smokers); similar serum cotinine levels between groups |
Respiratory Symptoms
A cross-sectional study surveyed ~45,000 adolescents in Hong Kong from 2012 to 2013 regarding e-cig use in the prior 30 days and respiratory symptoms (130), reporting increased cough or phlegm in e-cig users [odds ratio (OR) 2.1, confidence interval (CI) 1.8–2.5], including the subset of never smokers (OR 2.1, CI 1.3–3.4). Similarly, a cross-sectional study of nearly 40,000 high school students in Korea from 2014 (30) surveyed self-reported physician diagnosis of asthma in the prior 12 mo, school days missed because of asthma, cigarette and e-cig use in the prior 30 days, and a variety of social, physical, and demographic variables, including body mass index (BMI), atopic symptoms, second-hand smoke (SHS) exposure, and economic status. Compared with never e-cig users, current e-cig users had an unadjusted OR for asthma of 2.4 (CI 1.9–2.9); after stratifying by cigarette smoke exposure, the effect remained significant, OR 2.7 (CI 1.3–5.8). The increased prevalence of asthma in e-cig users was also robust to adjustment for other student characteristics, including BMI, city size, and history of allergic rhinitis or atopic dermatitis. Furthermore, current e-cig use increased the odds of school absences because of asthma in never smokers, OR 3.4 (1.8–6.5), which was robust to adjustment for gender, city size, BMI, second-hand smoke exposure, and history of atopic symptoms (30).
Approximately 2,000 adolescents in southern California were surveyed in 2014 regarding e-cig use and wheeze or bronchitic symptoms (daily productive cough), along with the use of other tobacco products, socioeconomic status (SES), and self-reported history of asthma (93). Wheeze was associated with current e-cig use (OR 1.9, CI 1.3–2.7) but not when adjusted for cigarette smoke exposure (OR 1.2, CI 0.8–2.0). However, bronchitic symptoms were associated with current (OR 2.0, CI 1.4–2.9) and past (OR 1.9, CI 1.4–2.5) e-cig use, and these effects were robust to adjustment for SES and only mildly attenuated when adjusted for cigarette smoking and SHS exposure. Furthermore, bronchitic symptoms were more likely with increased number of days of e-cig use (OR 2.5, CI 1.6–4.1 for 3 or more days in the previous 30).
Respiratory Physiology
One group of investigators (127) recruited 30 healthy smokers in Greece, had them abstain from cigarette use for at least 4 h, and then vape ad libitum for 5 min on a Nobacco e-cig (cig-a-like, black line, medium nicotine) or sham e-cigarette as if they were smoking a cigarette. They then performed spirometry and impulse oscillometry, an alternative method of analyzing flow characteristics that is less effort dependent than classic spirometry (21). Compared with sham exposure, e-cig exposure caused an 18% increase in airflow resistance in association with a 16% decrease in exhaled nitric oxide, suggesting an increase in oxidative stress (127). Another group of investigators performed spirometry on 8 never smokers and 24 smokers before and after 10 min of e-cig use, reporting in an abstract that e-cig exposure caused a significant increase in airway resistance (49).
Flouris and colleagues (45) performed spirometry and measured exhaled carbon monoxide and exhaled nitric oxide in 15 healthy smokers under control conditions, after smoking their favorite cigarette and after vaping an e-cig (Nobacco, Giant, 11 mg/ml nicotine). This group also studied 15 never smokers under control conditions and after passive exposure to cigarette smoke and e-cigarette aerosol. No differences in airflow characteristics were reported in smokers or never smokers following e-cig exposure, although unlike Vardavas et al. (127), impulse oscillometry was not employed. Also, exhaled nitric oxide levels were not significantly reduced following e-cig exposure in smokers or never smokers (45). In contrast, a more recent investigation measured exhaled nitric oxide in 25 healthy smokers under 1) control conditions, 2) after smoking a cigarette, or after a 5-min vaping session using a 3) no-nicotine or 4) nicotine-containing e-cig, reporting that cigarette smoke and both types of e-cig exposures reduced exhaled nitric oxide equivalently (89). Finally, an Italian group recently reported in an abstract that ad libitum use of a nicotine-free e-cig (ELIPS C Series, hazelnut flavored e-liquid) for 5 min in 10 healthy smokers significantly reduced FEV1 and 25% forced expiratory flow, although no significant effect of e-cig use was found in the 10 nonsmokers in this small study (44).
A recent study recruited 30 healthy never smokers and carried out baseline cough reflex sensitivity testing with capsaicin challenge, followed the next day by inhaling 30 puffs from a disposable e-cig (Blu, Classic Tobacco flavor, estimated 1.5–1.8 mg nicotine in 30 puffs). At 15 min postvaping, cough reflex sensitivity was significantly reduced, recovering to baseline levels 24 h later (34). Interestingly, the reduction in cough reflex sensitivity appears to be nicotine dependent, since non-nicotine-containing e-cigs had no effect.
Altered Host Defense
In one study (62) neutrophils were isolated from the blood of healthy human volunteers, primed with phorbol myristate acetate, and then incubated with MRSA in control media or media containing EVE. EVE-exposed neutrophils had decreased bacterial killing. Interestingly, this effect appeared to be mediated by nicotine, VG, and PG, since each of these components of EVE recapitulated the decrease in bactericidal neutrophil activity.
Recently another group of researchers collected nasal lavage and scrape-biopsy epithelium samples from 14 smokers, 13 nonsmokers, and 12 e-cig users, analyzing RNA with the nCounter Human Immunology v2 Expression panel (assessing 597 immunology-related genes). Compared with nonsmokers, smokers had decreased expression of 53 genes. Remarkably, e-cig users had decreased expression of 358 genes compared with nonsmokers, including all 53 genes that had shown decreased expression in smokers (91). The functional significance of this reduction in gene expression is not clear, but in the context of the in vitro and animal studies described above, along with the increase in cough and sputum production in e-cig users, these data suggest that e-cig aerosol exposure may have clinically important immunosuppressive effects.
RESEARCH PRIORITIES
The data reviewed in this article indicate the significant potential for serious lung toxicity from e-cig use. However, limitations in research methodology and the wide range of variables involved in studying the in vitro and in vivo effects of e-cigs pose several challenges to the medical, scientific, and regulatory communities. Of the many research needs in this evolving field, we highlight the following areas for improvements in experimental design: 1) modular flexible aerosol generation systems to keep pace with rapidly evolving atomizers, e-liquids, and usage patterns; 2) more complete description of experimental methods, including exposure procedures, specific devices, e-liquid formulations, and device power settings; 3) standardization of research approaches, including e-liquids, e-cig atomizers (analogous to what has been done for research cigarettes by the University of Kentucky), and methods of exposure; 4) analytic chemistry evaluations of e-cigarette aerosols to relate observed toxic phenotypes to classes of chemical species; 5) increased use of repetitive exposures for both in vitro and animal studies that better simulate e-cig usage in the population of e-cig users; 6) better model systems to study the interactions of toxicity between cigarette smoke and e-cig aerosols given the high rates of dual use; 7) improved in vitro models of both distal airway epithelium and alveolar epithelium, where the majority of aerosol mass is deposited; 8) research designs that measure the toxic effects of e-cigs with concentrations of nicotine that match the concentrations in the population of e-cig users; 9) effects on e-cig aerosol on surfactant composition and function; 10) effects of e-cig aerosol on injury and repair of lung epithelial barriers following infectious and inflammatory injury; 11) longitudinal studies of e-cig and dual users with spirometry and diffusing capacity; 12) impact of e-cig use on innate and adaptive immunity; and 13) impact of e-cig use on the frequency and severity of acute viral and bacterial infections.
CONCLUSIONS
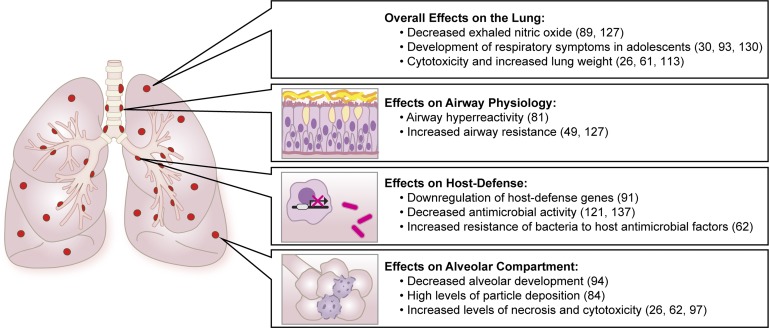
In summary, there is a rapidly growing body of evidence derived from in vitro, animal, and human studies that e-cigarette use may have significant pulmonary toxicity (Fig. 2). However, many more studies are needed to examine the multiple variables involved in e-cig use, including the role of nicotine, added flavorings, the physics of aerosolization, and the impact of chronic repetitive exposure. Similarly, there is a compelling need to evaluate toxicity in more clinically relevant model systems, including primary culture of airway and alveolar epithelium and in vivo animal models that combine e-cig exposure with clinically relevant bacterial and viral infections. Finally, improved longitudinal studies of the pulmonary health of e-cig users are needed, including physiological and microbiological end points. The rapid adoption of e-cigarette use, especially in younger age groups, should prompt more research to inform the public of health risks and guide regulatory standards.
Fig. 2.
E-cigarettes impact multiple regions and functions of the respiratory system, including altering airflow through the conducting airways, increasing oxidative stress, interfering with lung development, and impairing host defense against bacterial and viral pathogens.
GRANTS
Research reported in this publication was supported by National Institutes of Health (NIH) and Food and Drug Administration Center for Tobacco Products Grant No. 1P50-CA-180890 and NIH Grants HL-51856 and HL-133390.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
AUTHOR CONTRIBUTIONS
L.F.C., F.M., C.S.C., M.A.M., and J.E.G. conceived and designed research; L.F.C., F.M., C.S.C., M.A.M., and J.E.G. prepared figures; L.F.C., C.S.C., M.A.M., and J.E.G. drafted manuscript; L.F.C., F.M., C.S.C., M.A.M., and J.E.G. edited and revised manuscript; L.F.C., F.M., C.S.C., M.A.M., and J.E.G. approved final version of manuscript.
REFERENCES
- 1.Ambrose BK, Day HR, Rostron B, Conway KP, Borek N, Hyland A, Villanti AC. Flavored tobacco product use among US youth aged 12-17 years, 2013-2014. JAMA 314: 1871–1873, 2015. doi: 10.1001/jama.2015.13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Nonsmokers’ Rights Foundation (ANRF) Overview List—How many Smokefree Laws? [Online]. 2017. http://www.no-smoke.org/pdf/mediaordlist.pdf [6 Apr. 2017].
- 3.American Nonsmokers’ Rights Foundation (ANRF) States and Municipalities with Laws Regulating Use of Electronic Cigarettes [Online]. 2017. http://no-smoke.org/pdf/ecigslaws.pdf.
- 4.Antoniewicz L, Bosson JA, Kuhl J, Abdel-Halim SM, Kiessling A, Mobarrez F, Lundbäck M. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis 255: 179–185, 2016. doi: 10.1016/j.atherosclerosis.2016.09.064. [DOI] [PubMed] [Google Scholar]
- 5.Antonini JM, Krishna Murthy GG, Brain JD. Responses to welding fumes: lung injury, inflammation, and the release of tumor necrosis factor-alpha and interleukin-1 beta. Exp Lung Res 23: 205–227, 1997. doi: 10.3109/01902149709087368. [DOI] [PubMed] [Google Scholar]
- 6.Antonini JM, Roberts JR. Chromium in stainless steel welding fume suppresses lung defense responses against bacterial infection in rats. J Immunotoxicol 4: 117–127, 2007. doi: 10.1080/15476910701336953. [DOI] [PubMed] [Google Scholar]
- 7.Antonini JM, Taylor MD, Zimmer AT, Roberts JR. Pulmonary responses to welding fumes: role of metal constituents. J Toxicol Environ Health A 67: 233–249, 2004. doi: 10.1080/15287390490266909. [DOI] [PubMed] [Google Scholar]
- 8.Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol 283: L163–L169, 2002. doi: 10.1152/ajplung.00396.2001. [DOI] [PubMed] [Google Scholar]
- 9.Audrain-McGovern J, Strasser AA, Wileyto EP. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug Alcohol Depend 166: 263–267, 2016. doi: 10.1016/j.drugalcdep.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aug A, Altraja S, Kilk K, Porosk R, Soomets U, Altraja A. E-cigarette affects the metabolome of primary normal human bronchial epithelial cells. PLoS One 10: e0142053, 2015. doi: 10.1371/journal.pone.0142053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azzopardi D, Patel K, Jaunky T, Santopietro S, Camacho OM, McAughey J, Gaça M. Electronic cigarette aerosol induces significantly less cytotoxicity than tobacco smoke. Toxicol Mech Methods 26: 477–491, 2016. doi: 10.1080/15376516.2016.1217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol 34: 529–537, 2012. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Ballbè M, Martínez-Sánchez JM, Sureda X, Fu M, Pérez-Ortuño R, Pascual JA, Saltó E, Fernández E. Cigarettes vs. e-cigarettes: passive exposure at home measured by means of airborne marker and biomarkers. Environ Res 135: 76–80, 2014. doi: 10.1016/j.envres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Barrington-Trimis JL, Samet JM, McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? JAMA 312: 2493–2494, 2014. doi: 10.1001/jama.2014.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrington-Trimis JL, Urman R, Berhane K, Unger JB, Cruz TB, Pentz MA, Samet JM, Leventhal AM, McConnell R. E-cigarettes and future cigarette use. Pediatrics 138: e20160379, 2016. doi: 10.1542/peds.2016-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro pii: S0887-2333(13)00261-0, 2013. doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One 10: e0117222, 2015. doi: 10.1371/journal.pone.0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behar RZ, Luo W, Lin SC, Wang Y, Valle J, Pankow JF, Talbot P. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control 25, Suppl 2: ii94–ii102, 2016. doi: 10.1136/tobaccocontrol-2016-053224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc Med 26: 515–523, 2016. doi: 10.1016/j.tcm.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol; advance online publication, 2017. doi: 10.1038/nrcardio.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blonshine S, Goldman MD. Optimizing performance of respiratory airflow resistance measurements. Chest 134: 1304–1309, 2008. doi: 10.1378/chest.06-2898. [DOI] [PubMed] [Google Scholar]
- 22.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet J-F, Cohen MJ. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. Am J Respir Crit Care Med 183: 1660–1665, 2011. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calfee CS, Matthay MA, Kangelaris KN, Siew ED, Janz DR, Bernard GR, May AK, Jacob P, Havel C, Benowitz NL, Ware LB. Cigarette smoke exposure and the acute respiratory distress syndrome. Crit Care Med 43: 1790–1797, 2015. doi: 10.1097/CCM.0000000000001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC QuickStats: cigarette smoking status* among current adult e-cigarette users,(†) by age group—National Health Interview Survey,(§) United States, 2015. MMWR Morb Mortal Wkly Rep 65: 1177, 2016. doi: 10.15585/mmwr.mm6542a7. [DOI] [PubMed] [Google Scholar]
- 25.CDC Electronic Cigarette Use Among Adults: United States, 2014; Products—Data Briefs—Number 217—October 2015 [Online]. http://www.cdc.gov/nchs/products/databriefs/db217.htm [12 Oct. 2016].
- 26.Cervellati F, Muresan XM, Sticozzi C, Gambari R, Montagner G, Forman HJ, Torricelli C, Maioli E, Valacchi G. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol In Vitro 28: 999–1005, 2014. doi: 10.1016/j.tiv.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chausse P, Naughton G, Dutheil F. Electronic cigarettes: the resistance value of the heating filament could be the key to lung toxicity. Chest 148: e29–e30, 2015. doi: 10.1378/chest.15-0497. [DOI] [PubMed] [Google Scholar]
- 28.Chen BC, Bright SB, Trivedi AR, Valento M. Death following intentional ingestion of e-liquid. Clin Toxicol (Phila) 53: 914–916, 2015. doi: 10.3109/15563650.2015.1090579. [DOI] [PubMed] [Google Scholar]
- 29.Cheng T. Chemical evaluation of electronic cigarettes. Tob Control 23, Suppl 2: ii11–ii17, 2014. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho JH, Paik SY. Association between electronic cigarette use and asthma among high school students in South Korea. PLoS One 11: e0151022, 2016. doi: 10.1371/journal.pone.0151022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman BN, Apelberg BJ, Ambrose BK, Green KM, Choiniere CJ, Bunnell R, King BA. Association between electronic cigarette use and openness to cigarette smoking among US young adults. Nicotine Tob Res 17: 212–218, 2015. doi: 10.1093/ntr/ntu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings KJ, Boylstein RJ, Stanton ML, Piacitelli CA, Edwards NT, LeBouf RF, Kreiss K. Respiratory symptoms and lung function abnormalities related to work at a flavouring manufacturing facility. Occup Environ Med 71: 549–554, 2014. doi: 10.1136/oemed-2013-101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res 17: 134–141, 2015. doi: 10.1093/ntr/ntu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dicpinigaitis PV, Lee Chang A, Dicpinigaitis AJ, Negassa A. Effect of e-cigarette use on cough reflex sensitivity. Chest 149: 161–165, 2016. doi: 10.1378/chest.15-0817. [DOI] [PubMed] [Google Scholar]
- 35.Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among U.S. adolescents: a cross-sectional study. JAMA Pediatr 168: 610–617, 2014. doi: 10.1001/jamapediatrics.2013.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang X, Song Y, Hirsch J, Galietta LJV, Pedemonte N, Zemans RL, Dolganov G, Verkman AS, Matthay MA. Contribution of CFTR to apical-basolateral fluid transport in cultured human alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol 290: L242–L249, 2006. doi: 10.1152/ajplung.00178.2005. [DOI] [PubMed] [Google Scholar]
- 38.Farsalinos KE, Kistler KA, Gillman G, Voudris V. Evaluation of electronic cigarette liquids and aerosol for the presence of selected inhalation toxins. Nicotine Tob Res 17: 168–174, 2015. doi: 10.1093/ntr/ntu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farsalinos KE, Romagna G, Allifranchini E, Ripamonti E, Bocchietto E, Todeschi S, Tsiapras D, Kyrzopoulos S, Voudris V. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int J Environ Res Public Health 10: 5146–5162, 2013. doi: 10.3390/ijerph10105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farsalinos KE, Spyrou A, Stefopoulos C, Tsimopoulou K, Kourkoveli P, Tsiapras D, Kyrzopoulos S, Poulas K, Voudris V. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naïve users (smokers). Sci Rep 5: 11269, 2015. doi: 10.1038/srep11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.FDA Public Health Focus—Summary of Results: Laboratory Analysis of Electronic Cigarettes Conducted by FDA [Online]. https://www.fda.gov/NewsEvents/PublicHealthFocus/ucm173146.htm [11 Oct. 2016].
- 42.FDA Center for Tobacco Products Products, Ingredients & Components—Vaporizers, E-Cigarettes, and Other Electronic Nicotine Delivery Systems (ENDS) [Online]. https://www.fda.gov/TobaccoProducts/Labeling/ProductsIngredientsComponents/ucm456610.htm#regulation [7 Nov. 2016].
- 43.FDA Center for Tobacco Products Compliance, Enforcement & Training—Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke: Established List [Online]. https://www.fda.gov/TobaccoProducts/GuidanceComplianceRegulatoryInformation/ucm297786.htm [20 Apr. 2017].
- 44.Ferrari M, Zanasi A, Nardi E, Morselli Labate AM, Ceriana P, Balestrino A, Pisani L, Corcione N, Nava S. Short-term effects of a nicotine-free e-cigarette compared to a traditional cigarette in smokers and non-smokers. BMC Pulm Med 15: 120, 2015. doi: 10.1186/s12890-015-0106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, Wallace Hayes A, Tsatsakis AM, Koutedakis Y. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal Toxicol 25: 91–101, 2013. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, Jundi B, Cummins N, Eden E, Grosche A, Salathe M, Foronjy R. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 71: 1119–1129, 2016. doi: 10.1136/thoraxjnl-2015-208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaworski CL, Oldham MJ, Coggins CRE. Toxicological considerations on the use of propylene glycol as a humectant in cigarettes. Toxicology 269: 54–66, 2010. doi: 10.1016/j.tox.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Geiss O, Bianchi I, Barrero-Moreno J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int J Hyg Environ Health 219: 268–277, 2016. doi: 10.1016/j.ijheh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Gennimata S-A, Palamidas A, Kaltsakas G, Tsikrika S, Vakali S, Gratziou C, Koulouris N. Acute effect of e-cigarette on pulmonary function in healthy subjects and smokers. Eur Respir J 40: 1053, 2012. 23024329 [Google Scholar]
- 50.Gerloff J, Sundar IK, Freter R, Sekera ER, Friedman AE, Robinson R, Pagano T, Rahman I. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography-mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl In Vitro Toxicol 3: 28–40, 2017. doi: 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gillman IG, Kistler KA, Stewart EW, Paolantonio AR. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol 75: 58–65, 2016. doi: 10.1016/j.yrtph.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 52.Goldenson NI, Kirkpatrick MG, Barrington-Trimis JL, Pang RD, McBeth JF, Pentz MA, Samet JM, Leventhal AM. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology. Drug Alcohol Depend 168: 176–180, 2016. doi: 10.1016/j.drugalcdep.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldsmith KT, Listinsky CM, Garver RI Jr. Serum response heterogeneity among nonsmall cell lung cancer cell lines. Am J Pathol 139: 939–947, 1991. [PMC free article] [PubMed] [Google Scholar]
- 54.Goniewicz ML, Gupta R, Lee YH, Reinhardt S, Kim S, Kim B, Kosmider L, Sobczak A. Nicotine levels in electronic cigarette refill solutions: A comparative analysis of products from the U.S., Korea, and Poland. Int J Drug Policy 26: 583–588, 2015. doi: 10.1016/j.drugpo.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P III, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 23: 133–139, 2014. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grana RP, Benowitz NM, Glantz SAP. Background Paper on E-cigarettes (Electronic Nicotine Delivery Systems) [Online]. eScholarship. http://escholarship.org/uc/item/13p2b72n [10 Oct. 2016].
- 57.Herzog B, Gerberi J, Scott A. Equity Research: Tobacco—Nielsen C-store Data—E-cig $ Sales Decline Moderates [Online]. Wells Fargo Securities: 2014. http://www.c-storecanada.com/attachments/article/153/Nielsen%20C-Stores%20-%20Tobacco.pdf [10 Oct. 2016].
- 58.Herzog B, Gerberi J, Scott A. Equity Research, “Tobacco Talk”–Q4 U.S. Vapor Retailer Survey [Online]. Wells Fargo Securities: 2015. http://www.ecigarette-politics.com/files/4q14-wells-fargo.pdf [25 Oct. 2016].
- 59.Hess CA, Olmedo P, Navas-Acien A, Goessler W, Cohen JE, Rule AM. E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ Res 152: 221–225, 2017. doi: 10.1016/j.envres.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu R-S, Wang J, Li H, Ni H, Chen Y-F, Zhang Y-W, Xiang S-P, Li H-H. Simultaneous extraction of nicotine and solanesol from waste tobacco materials by the column chromatographic extraction method and their separation and purification. Separ Purif Tech 146: 1–7, 2015. doi: 10.1016/j.seppur.2015.03.016. [DOI] [Google Scholar]
- 61.Husari A, Shihadeh A, Talih S, Hashem Y, El Sabban M, Zaatari G. Acute exposure to electronic and combustible cigarette aerosols: effects in an animal model and in human alveolar cells. Nicotine Tob Res 18: 613–619, 2016. doi: 10.1093/ntr/ntv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, Das S, Moshensky A, Bapat S, Pride DT, Ongkeko WM, Crotty Alexander LE. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl) 94: 667–679, 2016. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- 63.Javed F, Kellesarian SV, Sundar IK, Romanos GE, Rahman I. Recent updates on electronic cigarette aerosol and inhaled nicotine effects on periodontal and pulmonary tissues. Oral Dis, 2017. doi: 10.1111/odi.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalkhoran S, Glantz SA. Modeling the health effects of expanding e-cigarette sales in the United States and United Kingdom: a Monte Carlo analysis. JAMA Intern Med 175: 1671–1680, 2015. doi: 10.1001/jamainternmed.2015.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kandel ER, Kandel DB. Shattuck Lecture. A molecular basis for nicotine as a gateway drug. N Engl J Med 371: 932–943, 2014. doi: 10.1056/NEJMsa1405092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khlystov A, Samburova V. Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ Sci Technol 50: 13080–13085, 2016. doi: 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- 67.Kim H, Lim J, Buehler SS, Brinkman MC, Johnson NM, Wilson L, Cross KS, Clark PI. Role of sweet and other flavours in liking and disliking of electronic cigarettes. Tob Control 25, Suppl 2: ii55–ii61, 2016. doi: 10.1136/tobaccocontrol-2016-053221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim KJ, Borok Z, Crandall ED. A useful in vitro model for transport studies of alveolar epithelial barrier. Pharm Res 18: 253–255, 2001. doi: 10.1023/A:1011040824988. [DOI] [PubMed] [Google Scholar]
- 69.Kite AC, Le BQ, Cumpston KL, Hieger MA, Feldman MJ, Pozez AL. Blast injuries caused by vape devices: 2 case reports. Ann Plast Surg 77: 620–622, 2016. doi: 10.1097/SAP.0000000000000875. [DOI] [PubMed] [Google Scholar]
- 70.Konrádová V, Vávrová V, Janota J. Effect of the inhalation of a surface tension-reducing substance (propylene glycol) on the ultrastructure of epithelium of the respiratory passages in rabbits. Folia Morphol (Praha) 26: 28–34, 1978. [PubMed] [Google Scholar]
- 71.Kosmider L, Sobczak A, Prokopowicz A, Kurek J, Zaciera M, Knysak J, Smith D, Goniewicz ML. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax 71: 376–377, 2016. doi: 10.1136/thoraxjnl-2015-207895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med 347: 330–338, 2002. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- 73.Krishnan-Sarin S, Morean ME, Camenga DR, Cavallo DA, Kong G. E-cigarette use among high school and middle school adolescents in Connecticut. Nicotine Tob Res 17: 810–818, 2015. doi: 10.1093/ntr/ntu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larcombe AN, Janka MA, Mullins BJ, Berry LJ, Bredin A, Franklin PJ. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. Am J Physiol Lung Cell Mol Physiol 2016, 2017. doi: 10.1152/ajplung.00203.2016. [DOI] [PubMed] [Google Scholar]
- 75.Lee YH, Gawron M, Goniewicz ML. Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes. Addict Behav 48: 1–4, 2015. doi: 10.1016/j.addbeh.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leigh NJ, Lawton RI, Hershberger PA, Goniewicz ML. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tob Control 25, Suppl 2: ii81–ii87, 2016. doi: 10.1136/tobaccocontrol-2016-053205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10: e0116732, 2015. doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leung CC, Yu ITS, Chen W. Silicosis. Lancet 379: 2008–2018, 2012. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 79.Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, Stone MD, Khoddam R, Samet JM, Audrain-McGovern J. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 314: 700–707, 2015. doi: 10.1001/jama.2015.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer 17: 62–70, 1976. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 81.Lim HB, Kim SH. Inhallation of e-cigarette cartridge solution aggravates allergen-induced airway inflammation and hyper-responsiveness in mice. Toxicol Res 30: 13–18, 2014. doi: 10.5487/TR.2014.30.1.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH. Chemical composition and evaluation of nicotine, tobacco alkaloids, pH, and selected flavors in e-cigarette cartridges and refill solutions. Nicotine Tob Res 17: 1270–1278, 2015. doi: 10.1093/ntr/ntu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majeed BA, Dube SR, Sterling K, Whitney C, Eriksen MP. Opinions about electronic cigarette use in smoke-free areas among U.S. Adults, 2012. Nicotine Tob Res 17: 675–681, 2015. doi: 10.1093/ntr/ntu235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manigrasso M, Buonanno G, Fuoco FC, Stabile L, Avino P. Aerosol deposition doses in the human respiratory tree of electronic cigarette smokers. Environ Pollut 196: 257–267, 2015. doi: 10.1016/j.envpol.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Manigrasso M, Buonanno G, Stabile L, Morawska L, Avino P. Particle doses in the pulmonary lobes of electronic and conventional cigarette users. Environ Pollut 202: 24–31, 2015. doi: 10.1016/j.envpol.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Mao P, Wu S, Li J, Fu W, He W, Liu X, Slutsky AS, Zhang H, Li Y. Human alveolar epithelial type II cells in primary culture. Physiol Rep 3: 3, 2015. doi: 10.14814/phy2.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, Proctor C. Chemical composition of aerosol from an e-cigarette: a quantitative comparison with cigarette smoke. Chem Res Toxicol 29: 1662–1678, 2016. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- 88.Maridet C, Atge B, Amici J-M, Taïeb A, Milpied B. The electronic cigarette: the new source of nickel contact allergy of the 21st century? Contact Dermat 73: 49–50, 2015. doi: 10.1111/cod.12373. [DOI] [PubMed] [Google Scholar]
- 89.Marini S, Buonanno G, Stabile L, Ficco G. Short-term effects of electronic and tobacco cigarettes on exhaled nitric oxide. Toxicol Appl Pharmacol 278: 9–15, 2014. doi: 10.1016/j.taap.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 90.Maritz GS. Maternal nicotine exposure during gestation and lactation of rats induce microscopic emphysema in the offspring. Exp Lung Res 28: 391–403, 2002. doi: 10.1080/01902140290092010. [DOI] [PubMed] [Google Scholar]
- 91.Martin EM, Clapp PW, Rebuli ME, Pawlak EA, Glista-Baker E, Benowitz NL, Fry RC, Jaspers I. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 311: L135–L144, 2016. doi: 10.1152/ajplung.00170.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol 88: 5–7, 2014. doi: 10.1007/s00204-013-1127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McConnell R, Barrington-Trimis JL, Wang K, Urman R, Hong H, Unger J, Samet J, Leventhal A, Berhane K. Electronic cigarette use and respiratory symptoms in adolescents. Am J Respir Crit Care Med 195: 1043–1049, 2017. doi: 10.1164/rccm.201604-0804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McGrath-Morrow SA, Hayashi M, Aherrera A, Lopez A, Malinina A, Collaco JM, Neptune E, Klein JD, Winickoff JP, Breysse P, Lazarus P, Chen G. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS One 10: e0118344, 2015. doi: 10.1371/journal.pone.0118344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mello S, Bigman CA, Sanders-Jackson A, Tan ASL. Perceived harm of secondhand electronic cigarette vapors and policy support to restrict public vaping: results from a national survey of US adults. Nicotine Tob Res 18: 686–693, 2016. doi: 10.1093/ntr/ntv232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. Real-time measurement of electronic cigarette aerosol size distribution and metals content analysis. Nicotine Tob Res 18: 1895–1902, 2016. doi: 10.1093/ntr/ntw128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Misra M, Leverette RD, Cooper BT, Bennett MB, Brown SE. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols. Int J Environ Res Public Health 11: 11325–11347, 2014. doi: 10.3390/ijerph111111325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moazed F, Burnham EL, Vandivier RW, O’Kane CM, Shyamsundar M, Hamid U, Abbott J, Thickett DR, Matthay MA, McAuley DF, Calfee CS. Cigarette smokers have exaggerated alveolar barrier disruption in response to lipopolysaccharide inhalation. Thorax 71: 1130–1136, 2016. doi: 10.1136/thoraxjnl-2015-207886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morgan DL, Flake GP, Kirby PJ, Palmer SM. Respiratory toxicity of diacetyl in C57BL/6 mice. Toxicol Sci 103: 169–180, 2008. doi: 10.1093/toxsci/kfn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila) 54: 924–1109, 2016. doi: 10.1080/15563650.2016.1245421. [DOI] [PubMed] [Google Scholar]
- 101.Neilson L, Mankus C, Thorne D, Jackson G, DeBay J, Meredith C. Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicol In Vitro 29: 1952–1962, 2015. doi: 10.1016/j.tiv.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 102.Normandin PA, Benotti SA. Pediatric emergency update: lethality of liquid nicotine in e-cigarettes. J Emerg Nurs 41: 357–359, 2015. doi: 10.1016/j.jen.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 103.Patterson SB, Beckett AR, Lintner A, Leahey C, Greer A, Brevard SB, Simmons JD, Kahn SA. A novel classification system for injuries after electronic cigarette explosions. J Burn Care Res 38: e95–e100, 2017. doi: 10.1097/BCR.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 104.Pollard KM. Silica, silicosis, and autoimmunity. Front Immunol 7: 97, 2016. doi: 10.3389/fimmu.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.RCP London Nicotine Without Smoke: Tobacco Harm Reduction [Online]. https://www.rcplondon.ac.uk/projects/outputs/nicotine-without-smoke-tobacco-harm-reduction-0 [7 Nov. 2016].
- 106.Robinson RJ, Hensel EC, Morabito PN, Roundtree KA. Electronic cigarette topography in the natural environment. PLoS One 10: e0129296, 2015. doi: 10.1371/journal.pone.0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Romagna G, Allifranchini E, Bocchietto E, Todeschi S, Esposito M, Farsalinos KE. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (ClearStream-LIFE): comparison with tobacco cigarette smoke extract. Inhal Toxicol 25: 354–361, 2013. doi: 10.3109/08958378.2013.793439. [DOI] [PubMed] [Google Scholar]
- 108.Scheffler S, Dieken H, Krischenowski O, Förster C, Branscheid D, Aufderheide M. Evaluation of E-cigarette liquid vapor and mainstream cigarette smoke after direct exposure of primary human bronchial epithelial cells. Int J Environ Res Public Health 12: 3915–3925, 2015. doi: 10.3390/ijerph120403915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, Jörres RA, Fromme H. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health 217: 628–637, 2014. doi: 10.1016/j.ijheh.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 110.Schweitzer KS, Chen SX, Law S, Van Demark M, Poirier C, Justice MJ, Hubbard WC, Kim ES, Lai X, Wang M, Kranz WD, Carroll CJ, Ray BD, Bittman R, Goodpaster J, Petrache I. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol 309: L175–L187, 2015. doi: 10.1152/ajplung.00411.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, Lindstrom J, Spindel ER. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest 103: 637–647, 1999. doi: 10.1172/JCI5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seo AD, Kim DC, Yu HJ, Kang MJ. Accidental ingestion of E-cigarette liquid nicotine in a 15-month-old child: an infant mortality case of nicotine intoxication. Korean J Pediatr 59: 490–493, 2016. doi: 10.3345/kjp.2016.59.12.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sherwood CL, Boitano S. Airway epithelial cell exposure to distinct e-cigarette liquid flavorings reveals toxicity thresholds and activation of CFTR by the chocolate flavoring 2,5-dimethypyrazine. Respir Res 17: 57, 2016. doi: 10.1186/s12931-016-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi Y, Cummins SE, Zhu S-H. Use of electronic cigarettes in smoke-free environments. Tob Control 26: e19–e22, 2017. doi: 10.1136/tobaccocontrol-2016-053118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shivalingappa PC, Hole R, Westphal CV, Vij N. Airway exposure to e-cigarette vapors impairs autophagy and induces aggresome formation. Antioxid Redox Signal ars.2015.6367, 2015. doi: 10.1089/ars.2015.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, Homa DM, King BA. Tobacco use among middle and high school students—United States, 2011–2015. MMWR Morb Mortal Wkly Rep 65: 361–367, 2016. doi: 10.15585/mmwr.mm6514a1. [DOI] [PubMed] [Google Scholar]
- 117.Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, Destaillats H. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ Sci Technol 50: 9644–9651, 2016. doi: 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- 118.Spindel ER, McEvoy CT. The role of nicotine in the effects of maternal smoking during pregnancy on lung development and childhood respiratory disease. implications for dangers of e-cigarettes. Am J Respir Crit Care Med 193: 486–494, 2016. doi: 10.1164/rccm.201510-2013PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spindle TR, Hiler MM, Cooke ME, Eissenberg T, Kendler KS, Dick DM. Electronic cigarette use and uptake of cigarette smoking: A longitudinal examination of U.S. college students. Addict Behav 67: 66–72, 2017. doi: 10.1016/j.addbeh.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Suber RL, Deskin R, Nikiforov I, Fouillet X, Coggins CR. Subchronic nose-only inhalation study of propylene glycol in Sprague-Dawley rats. Food Chem Toxicol 27: 573–583, 1989. doi: 10.1016/0278-6915(89)90016-1. [DOI] [PubMed] [Google Scholar]
- 121.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim J-H, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A, Biswal S. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 10: e0116861, 2015. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan ASL, Bigman CA, Sanders-Jackson A. Sociodemographic correlates of self-reported exposure to e-cigarette communications and its association with public support for smoke-free and vape-free policies: results from a national survey of US adults. Tob Control 24: 574–581, 2015. doi: 10.1136/tobaccocontrol-2014-051685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tavolacci M-P, Vasiliu A, Romo L, Kotbagi G, Kern L, Ladner J. Patterns of electronic cigarette use in current and ever users among college students in France: a cross-sectional study. BMJ Open 6: e011344, 2016. doi: 10.1136/bmjopen-2016-011344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tran I, Ji C, Ni I, Min T, Tang D, Vij N. Role of cigarette smoke-induced aggresome formation in chronic obstructive pulmonary disease-emphysema pathogenesis. Am J Respir Cell Mol Biol 53: 159–173, 2015. doi: 10.1165/rcmb.2014-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health E-Cigarette Use Among Youth and Young Adults. A Report From the Surgeon General. Rockville, MD: U.S. Dept of Health and Human Services, 2016. [Google Scholar]
- 126.US Fire Administration Electronic Cigarette Fires and Explosions [Online]. FEMA; 2014. https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf. [Google Scholar]
- 126a.van Eijl S, van Oorschot R, Olivier B, Nijkamp FP, Bloksma N. Stress and hypothermia in mice in a nose-only cigarette smoke exposure system. Inhal Toxicol 18: 911–918, 2006. doi: 10.1080/08958370600822672. [DOI] [PubMed] [Google Scholar]
- 127.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141: 1400–1406, 2012. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- 128.Varughese S, Teschke K, Brauer M, Chow Y, van Netten C, Kennedy SM. Effects of theatrical smokes and fogs on respiratory health in the entertainment industry. Am J Ind Med 47: 411–418, 2005. doi: 10.1002/ajim.20151. [DOI] [PubMed] [Google Scholar]
- 129.Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N, Queimado L. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control 26, e1: e23–e28, 2017. doi: 10.1136/tobaccocontrol-2016-053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang MP, Ho SY, Leung LT, Lam TH. Electronic cigarette use and respiratory symptoms in chinese adolescents in hong kong. JAMA Pediatr 170: 89–91, 2016. doi: 10.1001/jamapediatrics.2015.3024. [DOI] [PubMed] [Google Scholar]
- 131.Werley MS, Kirkpatrick DJ, Oldham MJ, Jerome AM, Langston TB, Lilly PD, Smith DC, Mckinney WJ Jr. Toxicological assessment of a prototype e-cigaret device and three flavor formulations: a 90-day inhalation study in rats. Inhal Toxicol 28: 22–38, 2016. doi: 10.3109/08958378.2015.1130758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Werley MS, McDonald P, Lilly P, Kirkpatrick D, Wallery J, Byron P, Venitz J. Non-clinical safety and pharmacokinetic evaluations of propylene glycol aerosol in Sprague-Dawley rats and beagle dogs. Toxicology 287: 76–90, 2011. doi: 10.1016/j.tox.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 133.Wieslander G, Norbäck D, Lindgren T. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med 58: 649–655, 2001. doi: 10.1136/oem.58.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Williams M, To A, Bozhilov K, Talbot P. Strategies to reduce tin and other metals in electronic cigarette aerosol. PLoS One 10: e0138933, 2015. doi: 10.1371/journal.pone.0138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One 8: e57987, 2013. doi: 10.1371/journal.pone.0057987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wilson FA, Wang Y. Recent findings on the prevalence of e-cigarette use among adults in the U.S. Am J Prev Med 52: 385–390, 2017. doi: 10.1016/j.amepre.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 137.Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One 9: e108342, 2014. doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.You G, Rhee J, Park Y, Park S. Determination of nicotine, cotinine and trans-3′-hydroxycotinine using LC/MS/MS in forensic samples of a nicotine fatal case by oral ingestion of e-cigarette liquid. J Forensic Sci 61: 1149–1154, 2016. doi: 10.1111/1556-4029.13083. [DOI] [PubMed] [Google Scholar]
- 139.Zhu S-H, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 23, Suppl 3: iii3–iii9, 2014. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]