Abstract
Even with advances in the care of preterm infants, chronic lung disease or bronchopulmonary dysplasia (BPD) continues to be a significant pulmonary complication. Among those diagnosed with BPD, a subset of infants develop severe BPD with disproportionate pulmonary morbidities. In addition to decreased alveolarization, these infants develop obstructive and/or restrictive lung function due to increases in or dysregulation of extracellular matrix proteins. Analyses of plasma obtained from preterm infants during the first week of life indicate that circulating miR-29b is suppressed in infants that subsequently develop BPD and that decreased circulating miR-29b is inversely correlated with BPD severity. Our mouse model mimics the pathophysiology observed in infants with severe BPD, and we have previously reported decreased pulmonary miR-29b expression in this model. The current studies tested the hypothesis that adeno-associated 9 (AAV9)-mediated restoration of miR-29b in the developing lung will improve lung alveolarization and minimize the deleterious changes in matrix deposition. Pregnant C3H/HeN mice received an intraperitoneal LPS injection on embryonic day 16 and newborn pups were exposed to 85% oxygen from birth to 14 days of life. On postnatal day 3, AAV9-miR-29b or AAV9-control was administered intranasally. Mouse lung tissues were then analyzed for changes in miR-29 expression, alveolarization, and matrix protein levels and localization. Although only modest improvements in alveolarization were detected in the AAV9-miR29b-treated mice at postnatal day 28, treatment completely attenuated defects in matrix protein expression and localization. Our data suggest that miR-29b restoration may be one component of a novel therapeutic strategy to treat or prevent severe BPD in prematurely born infants.
Keywords: bronchopulmonary dysplasia, microRNA, fibrosis, adeno-associated virus, maternal inflammation, hyperoxia
although advances in neonatal care have increased the survival of extremely preterm infants, this population remains at great risk for gestational age (GA)-specific morbidities. Most prevalent is the need for respiratory support, which often includes mechanical ventilation and/or oxygen therapy. Moreover, a subset of these infants remains significantly affected throughout their lifetime, developing severe lung disease associated with stunted growth and neurodevelopmental deficiencies during childhood. These chronically ill children consume a disproportionate amount of clinical and financial resources (3, 12, 33). Infants that develop severe chronic lung disease or bronchopulmonary dysplasia (BPD) have distinctive and multifaceted clinical pathophysiology and are the greatest challenge for the clinicians providing their care.
The severity of chronic lung disease in this population suggests that the mechanisms that underlie the clinical spectrum of disease are not straightforward and most likely involve a “multiple hit” scenario associated with but not limited to maternal inflammation, prematurity, and hyperoxia exposure, which contribute additive or synergistic roles. Our overall working hypothesis is that fetal responses to an adverse maternal environment create epigenetic alterations in the developing lung that affect developmental pathways and/or prime the infant for more severe disease in response to necessary life-saving interventions after birth (14, 39). Our laboratory has developed and extensively characterized a novel model that combines systemic maternal inflammation (induced by intraperitoneal LPS injection) followed by 14 days of neonatal hyperoxia exposure (85% ) (35–38). The maternal inflammatory response is minimal and similar to that observed in pregnant women with subacute systemic inflammation such as periodontitis, pneumonia, or urinary tract infection (12, 39). The offspring develop a phenotype that closely mimics the pathophysiology of severe BPD observed in the most critically affected preterm infants and is greater than that observed with either maternal LPS or neonatal hyperoxia exposure alone (35, 37). These pups have decreased alveolar number, increased lung density with diffuse interstitial fibrosis (thickening of the interstitial wall), and restrictive lung disease (by pulmonary function tests). Additional characterizations of older mice exposed to LPS in utero and postnatal hyperoxia are similar to reported pathophysiology in adult survivors of infant BPD (11, 12, 43). Thus we propose that our model is a useful tool to better understand the mechanisms responsible for the complex pathophysiology of severe BPD.
MicroRNAs (miRs), small noncoding RNAs (~22 nucleotides), are epigenetic regulators of both normal physiologic and abnormal pathologic processes (23). miRs regulate growth, development, and repair processes, and dysregulation of miR expression is associated with pulmonary disease. Specifically, miR-29b-3p expression has been linked to pulmonary cancers, fibrosis, and BPD (9, 10, 20, 46, 48). miR-29b is expressed from two distinct locations in the mouse, one on chromosome 1 (coding for miR-29b2 and -29c) and one on chromosome 6 (coding for miR-29a and 29b1), and each cluster is regulated by distinct promoter sequences. miR-29b-3p is expressed by both transcripts, and while the pri(pre)-miR sequences are different, the mature microRNA sequences are identical.
We have previously reported that murine neonatal lung miR-29b levels are suppressed in response to maternal LPS treatment followed by subsequent neonatal hyperoxic exposure (35). Many of the extracellular matrix proteins misexpressed in our model are derived from transcripts known or predicted to be regulated by miR-29b-3p. Specifically, suppression of miR-29b-3p expression is associated with increased elastin, transforming growth factor-β (TGFβ), SMAD2/3, collagen, α-smooth muscle actin (α-SMA), and vimentin protein levels, all of which contribute to changes in matrix composition and structure (24, 42). Our reported increases in TGFβ and SMAD2/3 protein levels and collagen deposition in LPS/O2-exposed neonatal lungs are similar to other reports of fibrotic responses to oxygen exposure alone (1, 35). We hypothesize that decreases in pulmonary miR-29b levels are, in part, responsible for the observed increases in matrix proteins and that adeno-associated virus (AAV)-mediated restoration of miR-29b in the developing lung will attenuate LPS/O2-induced pulmonary deficits. The current studies explored the effects of AAV-mediated restoration of miR-29b in the lungs of LPS/O2-exposed newborn mouse pups. Our results indicated that normalization of pulmonary miR-29b levels reduced aberrant increases in matrix proteins and attenuated alveolarization defects caused by exposure to maternal LPS and neonatal hyperoxia.
METHODS
miR-29b measurements in human plasma using molecular beacon technology.
After Institutional Review Board approval (Nationwide Children’s Hospital Institutional Review Board: IRB05-00338) and informed consent of the parents, blood samples were obtained during 3–5 days of life from preterm infants born <32 wk gestation. miR-29b expression was measured in the same cohort of infant plasma using molecular beacon (MB) technology as previously described (29). Briefly, tethered cationic lipoplex nanoparticles containing MBs for miR-29b were prepared by injecting the MB/lipid mixture into PBS and using mixed thiol self-assembled monolayers as an anchoring membrane (18, 19, 47). Samples were added on a tethered cationic lipoplex nanoparticles biochip and incubated at 37°C for 2 h. Total internal reflection fluorescence microscopy was used to detect the fluorescence signals and images were analyzed (47).
In situ hybridization of miR-29b in human autopsy tissues.
In situ hybridization for miR-29b expression was performed on human autopsy tissues as previously described (25). In brief, locked nucleic acid-modified and 5′-digoxigenin-tagged probes specific for miR-29b were utilized. The probe/target complex was visualized after reaction of the alkaline phosphatase-linked conjugate with the chromogen (nitroblue tetrazolium and bromochloroindolyl phosphate), using a nuclear fast red counterstain. Negative controls included omission of the probe and the use of a scrambled probe.
Animal model.
Animal studies were approved by The Research Institute at Nationwide Children’s Hospital’s Institutional Animal Care and Use Committee (AR07-00028). Mice were exposed to maternal LPS and neonatal hyperoxia as previously described (35, 37). Pregnant C3H/HeN mice were injected on embryonic day (E) 16 with LPS (80 µg/kg) or an equal volume of saline. Each litter of newborn mice was paired and mixed with a litter born to a dam receiving the same E16 treatment. One of the paired litters was exposed to 85% for 14 days (LPS/O2) and subsequently returned to room air (RA), while the corresponding group was maintained in RA (saline/RA). For this study, mice were euthanized at postnatal days (PN) 6, 7, 10, 14, or 28, and five to eight mice per treatment group were included with no more than two mice from any specific litter used for any given analysis.
AAV9-miR-29b vector.
The development of self-complementary AAV vectors and the availability of AAV serotypes for improved transduction have made AAV a viable option for therapeutic gene delivery (21, 22, 41). We utilized AAV9 to deliver miR-29b to the lungs of newborn mouse pups. AAV9 uses NH2-linked terminal galactose residues as a receptor and as a result transduces distal lung cells with relatively high efficiency compared with bronchiolar epithelium, allowing targeted delivery of miR-29b to the distal lung (4). The vector includes enhanced green fluorescent protein (eGFP) driven by the ubiquitously expressed elongation factor 1α (EF1α) promoter (40). Since miRs are frequently embedded within introns of primary transcripts, we ligated miR-29b into the short intron that is part of the EF1α promoter unit, thus allowing simultaneous production of eGFP and miR-29b from a single transcript (scAAV.miR29b.eGFP). A 388-bp region of mouse chromosome 6 (corresponding to bp 28,894–29,281 of BAC clone 402k12 RPCI-23; NCBI Sequence ID AC024913.33) carrying the miR-29b-1 sequence (AGAACACTGATTTCAAATGGTGCTAGACAATCACTATTTAAATCTAAACCA CCATATGAAACCAGCTTCCT) was amplified from mouse genomic DNA using primers with FseI restriction sites (underlined) as follows: forward, 5′-GATAGGCCGGCCAATTCTAACGGTGGTTGACTAATCAGCTATCC-3′ and reverse, 5′-GATAGGCCGGCCATTTGGAGGGAAGAATGCCATGAGTGACC-3′ and ligated to the FseI site of the AAV9 vector.
Administration of AAV9 vector.
Newborn mice exposed to maternal saline or LPS and assigned to breathe RA or 85% were treated with PBS containing either scAAV9.miR29b.eGFP [AAV9-miR29b(+)] or scAAV9.eGFP [AAV9-miR29b(−)] virus at a dose of 1 × 109 DNAse-resistant particles on PN3. The head of the neonatal mouse was stabilized by holding the nape of the neck, and the virus was released into the nostrils with a micropipette, 1 to 2 μl at a time (6). This induced rapid breathing in the pups, which should increase inhalation of the liquid. When necessary, the abdomens of the pups were rubbed to potentiate deep breaths. The pups were held in an upright position for several minutes and then returned to their assigned treatment group.
Immunofluorescence.
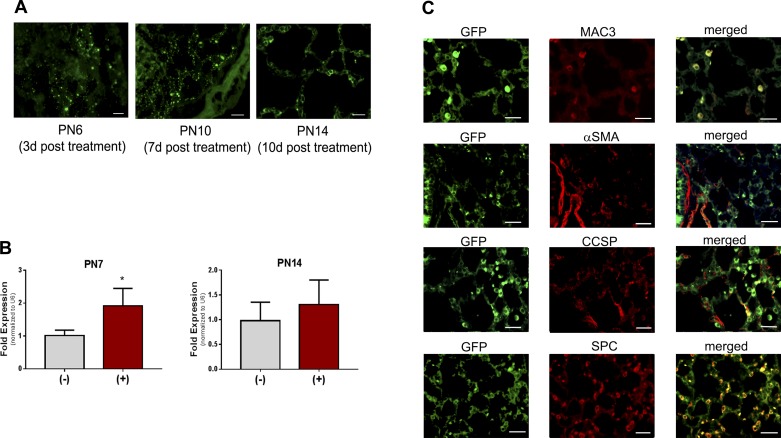
At PN6 or PN10, pups were anesthetized and the lungs were perfused, harvested and fixed in 10% buffered formalin, At PN14, pups were anesthetized and the lung tissues were perfused and inflation fixed with 10% buffered formalin at a pressure of 25 cmH2O for 15 min. Following paraffin embedding, tissue sections (6 µm) were cut and sections were baked at 65°C for 20 min and washed in xylene and ethanol to deparaffinize. After antigen retrieval and blocking, tissue sections were treated with the appropriate antibodies and dilutions as indicated in Table 1. Tissues sections were probed for GFP alone (all time points, see Fig. 2A) or GFP and cell type specific antibodies (PN14, see Fig. 2C).
Table 1.
Antibodies
| Cat. No. | Dilution | Source | |
|---|---|---|---|
| Primary antibodies | |||
| Anti-GFP | ab290 | 1:1000 | Abcam |
| Anti-CCSP | SC-9772 | 1:250 | Santa Cruz Biotechnology |
| Anti-aSMA | M0851 | 1:250 | Dako |
| Anti-MAC3 (cd107b) | 108512 | 1:250 | Biolegend |
| Anti-SPC | SC-7706 | 1:50 | Santa Cruz Biotechnology |
| Secondary antibodies | |||
| Donkey anti-rabbit 488 | A-21206 | 1:700 | Invitrogen |
| Donkey anti-goat 546 | A-11046 | 1:700 | Molecular Probes |
| Goat anti-mouse 568 | A-11004 | 1:700 | Invitrogen |
Fig. 2.
Viral expression in lung tissues. Pups were exposed as described, treated with a single dose of adeno-associated 9 (AAV9)-miR29b(+) or control(−) at postnatal day (PN) 3, euthanized at PN6, PN10, or PN14, and tissues were fixed (PN6 and PN10 lungs were not inflated because of pup size). A: sections were probed with anti-GFP antibody and immunofluorescence was visualized. Bars = 100 μm; photos were taken at ×200. B: additional lung tissues were harvested and analyzed by quantitative PCR analysis of miR-29b-3p at PN7 or PN14. Data were analyzed by t-test; n = 4 per group; *P < 0.05 different than control(−). C: coimmunofluorescence was assessed in tissues obtained at PN14. Bars = 100 μm in all images; photos were taken at ×400.
miR-29b analyses.
Total RNA was isolated using Trizol and RNEasy Mini Columns (Qiagen). miR-29b-3p and U6 gene-specific RT primers from obtained from TaqMan MicroRNA Assays and 100 ng total RNA were used for reverse transcription (TaqMan MicroRNA Reverse Transcription Kit; Life Technologies). PCR was performed using gene-specific primers from TaqMan MicroRNA Assays and TaqMan Universal PCR Master Mix II (Life Technologies).
For the pre-miRs, equivalent amounts of RNA from each sample were reverse-transcribed using the Thermo Scientific First Strand cDNA Synthesis Kit and amplified using Thermo Scientific Maxima Sybr Green/ROX PCR mix (Thermo Fisher, Waltham, MA). The mouse miR-29 sequences expressed from chromosome 1 (miR-29b-2 and miR-29c) and from chromosome 6 (miR-29b-1 and miR-29a) are contained within long noncoding RNA (lncRNA) transcripts, although the chromosome 6 transcript is largely uncharacterized. PCR amplification primers were directed to regions in these lncRNAs immediately upstream and downstream of each miR-29 isoform. The primers used and amplified regions detected are as follows: miR-29b-2 primers were 5′-GTGAGATCCTCTTCTTCTGGAAGCTG-3′ and 5′-CTGCTGTGCTGCAATTCTTACTCC-3′, and miR-29c primers were 5′-CACCAGTCGGTCCATCTCTTACAC-3′ and 5′-CTGAGGCTGGTGCTCTTCC-3′, amplifying bp 4,314–4,427 and 4,821–4,937, respectively, of the chromosome 1 lncRNA A330023F24 (NCBI Accession No. NR_015566.2); miR-29a primers were 5′-GCTGAACGGTGCTCTTCCC-3′ and 5′-GTGCACATGACCTCTTGTGACC-3′, and miR-29b-1 primers were 5′-CAGCGAAGTAGTTCTCCACCA-3′ and 5′-CAGACGACAAAGCTTCTTCAG-3′, amplifying bp 28,673-28,794 and 29,030-29,140, respectively, of the chromosome 6 BAC clone 402k12 RPCI-23 (NCBI Sequence ID AC024913.33). For relative RNA expression analysis, ΔΔCt analysis was performed for each target using the ribosomal transcript Rpl13a (NCBI Accession No. NM_009438.4) as a primary normalizer (2) and saline/RA AAV9-miR29b(−) mice as the secondary normalizer.
Morphometric analysis of lung tissue sections.
At PN14 or 28, mice were euthanized and the left lung was inflation fixed as described above. Following paraffin embedding, tissue sections (5 µm) were cut and slides were stained with hematoxylin-eosin for morphometric measurements as previously described (27). Five images per animal were analyzed and averaged using digital image analysis software (Image-Pro Plus 6.3; Media Cybernetics, Silver Spring, MD). Additionally, the air space septal wall thickness was measured randomly in five individually intact alveoli per image by manually identifying the septal wall edges and measuring the width.
Histochemical analysis of matrix proteins.
At PN28, mice were euthanized and the left lung was inflation fixed as described above. Following paraffin embedding, tissue sections (5 µm) were cut and slides were stained with Hart’s stain for elastin and picrosirius red for collagen using standard protocols. Additional slides were processed at PN28 for immunohistochemical analyses for α-SMA using standard immunohistochemistry procedures (α-SMA antibody; M0851m; Dako, Carpinteria, CA) and at PN14 for primary antibodies listed in Table 1.
Immunoblot analysis of matrix proteins.
Lung homogenates obtained from mice at PN28 were separated on SDS-polyacrylamide gels and transferred to polyvinylidene fluoride membranes. Membranes were probed with antibodies to phospho-SMAD2 (Cat. No. 3108; Cell Signaling, Danvers, MA), total SMAD 2/3 (Cat. No. 3102; Cell Signaling), α-SMA (Cat. No. A2547; Sigma, St. Louis, MO), vimentin (Cat. No. V5255; Sigma), and matrix metalloproteinase-9 (Cat. No. ab119906; Abcam, Cambridge, MA). Blots were developed using enhanced chemiluminescence (ECL Western Blotting Detection; GE Healthcare, UK) and protein levels were quantified by densitometry using Image Quant software, version 5.0 (Molecular Dynamics, Sunnydale, CA). For each target, the density of the band for the protein of interest was normalized to the density of β-actin (Cat. No. ab6276; Abcam) or α-tubulin (Cat. No. ab15246; Abcam).
Statistics.
Statistical analyses were performed using GraphPad Prism 5 (La Jolla, CA). Human data were analyzed by one-way ANOVA (Fig. 1A) or by Pearson correlation coefficient and multivariate regression analyses to control for GA (Fig. 1B). All animal data were analyzed by either t-test, one-way ANOVA, or two-way ANOVA with exposure (saline/RA or LPS/O2) and treatment [miR29b(+) or (−)] as variables and analyzed for effects or interactions. Post hoc analyses were performed to identify individual differences between groups. Dunnett’s was performed if all data were compared with a control group. Tukey’s was performed if all data groups were compared with each other. Effects or interactions and P values identified by ANOVA are indicated in the figures, and the results of the post hoc analyses are indicated by the symbols as follows: *different than saline/RA(−); or #different than LPS/O2(−). Data are expressed as means ± SE.
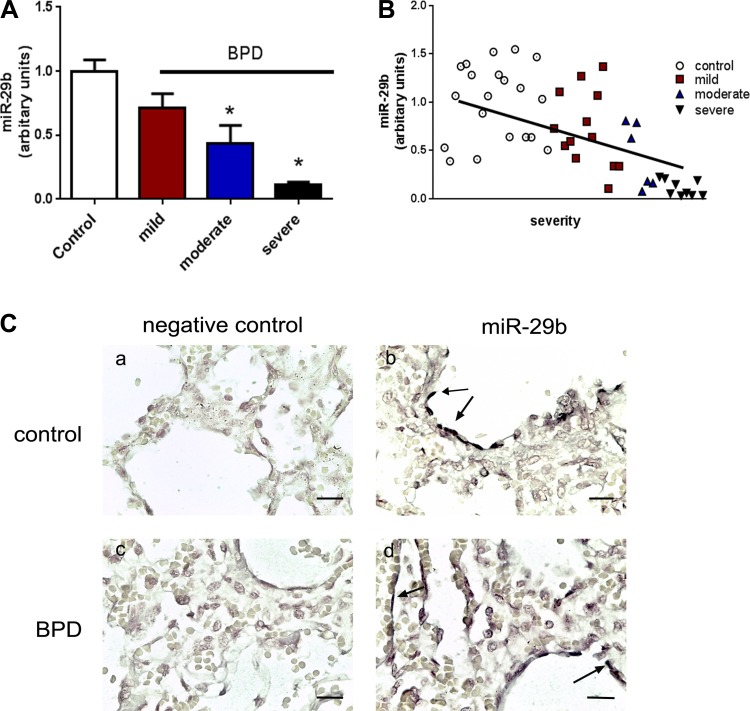
Fig. 1.
miR-29b expression in human infants. A: plasma samples were obtained during the first week of life from infants born <32 wk gestation. miR-29b levels were measured using molecular beacon technology (see methods), and subsequent diagnosis and severity of bronchopulmonary dysplasia (BPD) were noted at 36-wk corrected gestational age. Data were analyzed by one-way ANOVA with Dunnett’s multiple comparisons post hoc test; n = 6–20 per group; *P < 0.05 different than control. B: correlations between plasma miR-29b levels and severity of BPD were plotted and analyzed using Pearson’s correlation; P < 0.0001. In addition, multivariate linear regression analysis was performed to control for gestational age at birth. Adjusted values indicated P = 0.008; r2 = 0.497. C: autopsy lung tissue sections were obtained from infants who died from nonpulmonary causes (a and b) or from BPD (c and d). Bars = 50 μm in all images, and photos were taken at ×400. Expression of miR-29 was determined by in situ hybridization. miR-29 expression was localized to flat cells lining the airways or vessels (arrows).
RESULTS
miR-29b in human plasma and tissue samples.
Plasma was obtained from premature infants during the first week of life, and medical records were subsequently abstracted for diagnosis of BPD at 36-wk corrected gestation. Clinical characteristics of the infants providing these samples have been previously reported (30). Samples were analyzed for miR-29b levels using MB technology (29, 47). We observed a decrease in plasma miR-29b levels relative to infants of similar clinical characteristics but that were not diagnosed with BPD, and miR-29b levels further decreased with increasing severity of BPD (using National Institutes of Health/National Institute of Child Health and Human Development Workshop criteria) (16) (Fig. 1A). Pearson correlation coefficient was performed to determine the relative correlation between miR-29b expression and BPD (P < 0.0001, Fig. 1B). Furthermore, multivariate regression analysis was performed and indicated a significant correlation between miR-29b levels and disease severity even after controlling for GA (P = 0.008, adjusted r2 = 0.497). Tissues obtained from an additional cohort of infants that died of BPD or of nonpulmonary causes were analyzed by in situ hybridization. Tissue sections from infants that died of nonpulmonary causes (Fig. 1, C,a and C,b) demonstrated miR-29b staining in flat cells lining the alveolar and vessel walls (arrows). In tissues from infants that died with BPD (Fig. 1, C, c and C,d), less robust staining for miR-29b was observed.
Assessment of AAV9-miR-29b expression in lung tissues.
Expression of eGFP fluorescence from AAV9-miR-29b treatment was visible in lung tissue sections at 3, 7, and 11 days posttreatment (Fig. 2A). In addition, RT-PCR was performed on pups at PN7 (4 days posttreatment) and PN14 (11 days posttreatment). At PN7, increases in miR-29b-3p expression were evident in whole lung RNA preparations from pups treated with AAV9-miR29b vs. those treated with empty vector (Fig. 2B). Interestingly, the increased expression was no longer statistically significant by PN14 indicating that increase growth and cell number of the postnatal lung likely diluted the detectable miR-29b expression in the transduced cells. Coimmunofluorescence localizing eGFP with known cellular protein markers was employed to identify the cell type transduced by the AAV9 vector. In tissues obtained at PN14, obvious coimmunofluorescence was observed in macrophages (MAC3) and in type 2 cells (SPC) but not in club cells (CCSP) or in α-SMA-expressing cells (Fig. 2C).
Pre-miR-29 expression in the lung.
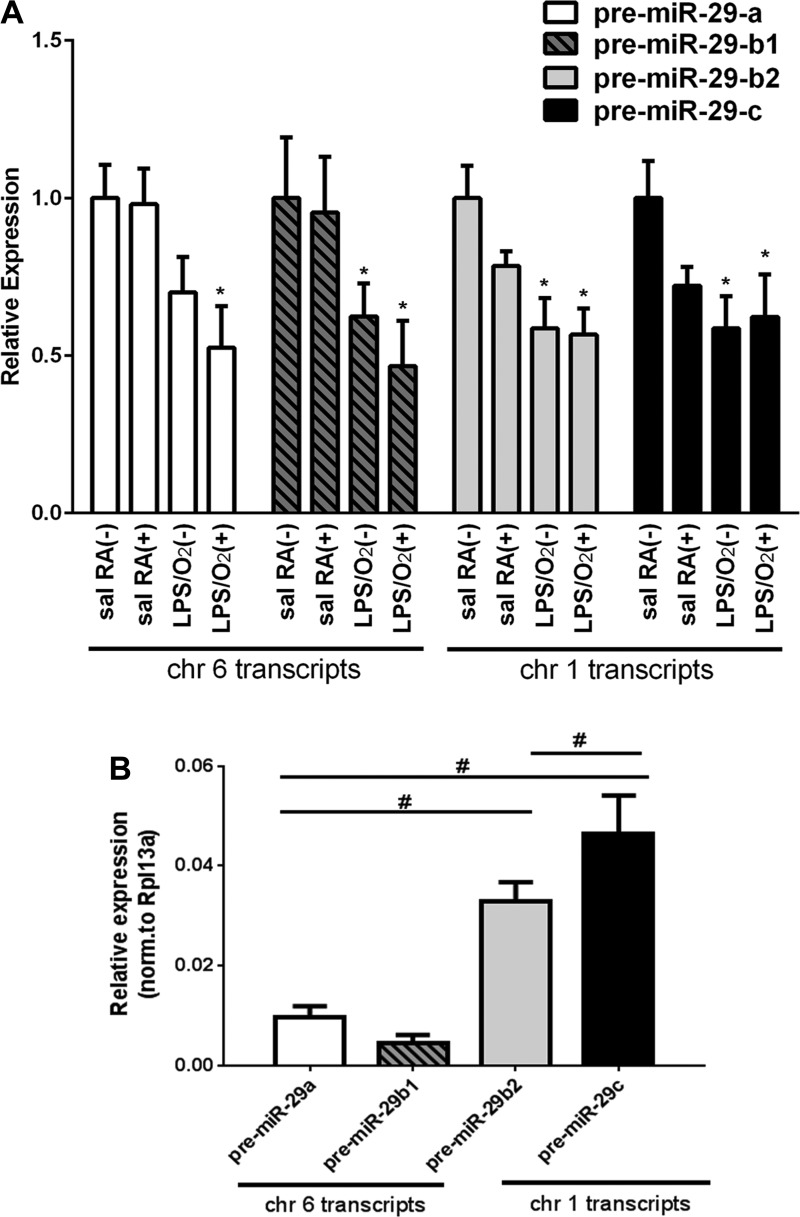
Additional PCR analyses were performed to test whether viral-mediated miR-29b supplementation affected transcription of other miR-29 isoforms. Saline/RA and LPS/O2 mice were treated with either AAV9-miR29b(+) or AAV9-miR29b(−) at PN3 and LPS/O2 mice and remained in their assigned group until death at PN14. At PN14, we measured overall lung levels of the pre-miRs for miR-29a, -b1, -b2, and -c by quantitative PCR. Expression of each miR-29 isoform in the lung tissues was normalized to saline/RA AAV9-miR-29b(−)-treated lungs. Our data indicate that lung miR-29b expression was attenuated by perinatal LPS/O2 exposure (Fig. 3A). We also detected decreased miR-29a and miR-29c expression in LPS/O2-exposed mice. We observed no additional changes in any of the pre-miR-29 isoforms at PN14 attributable to our single dosing at PN3 with AAV-miR29b, suggesting that the viral treatment had no adverse global effects on miR-29 expression. Interestingly, absolute expression levels of miR-29b2 and miR-29c were approximately eight times higher (ΔCt, ~3) than those of miR-29a and miR-29b1 (Fig. 3B).
Fig. 3.
Pre-miR analysis of miR-29 transcripts. The expression of pre-miR-29a, pre-miR-29b1, pre-miR-29b2, and pre-miR-29c was measured in RNA extracted from lung tissues at PN14 of mice treated with a single dose of AAV9-miR-29b(+) or control(−) at PN3. A: suppression of all pre-miR-29 isoforms was observed in LPS/O2-treated mice (RA, room air). Expression of each pre-miR was normalized to the housekeeping gene Rpl13a and to levels seen in each saline/RA(−) sample by ΔΔCt analysis. Average Ct values for saline/RA(−) pre-miR-29 transcripts: b1 = 26.4, a = 25.2, b2 = 23.2, c = 22.6. Data were analyzed by one-way ANOVA; miR29a, P = 0.032; miR29b1, P = 0.032; miR29b2, P = 0.014; miR-29c, P = 0.016 (n = 4 each group). *Dunnett’s post hoc analyses indicated differences between saline/RA and other treatment groups. B: relative expression of each pre-miR-29 was measured in RNA samples extracted from lung tissues at PN14 from AAV9-control(−) mice. Data were analyzed by one-way ANOVA; P = 0.029; n = 4 per group. #Tukey’s post hoc analysis indicated differences between group and the corresponding bars are shown.
Morphometric analyses of lungs.
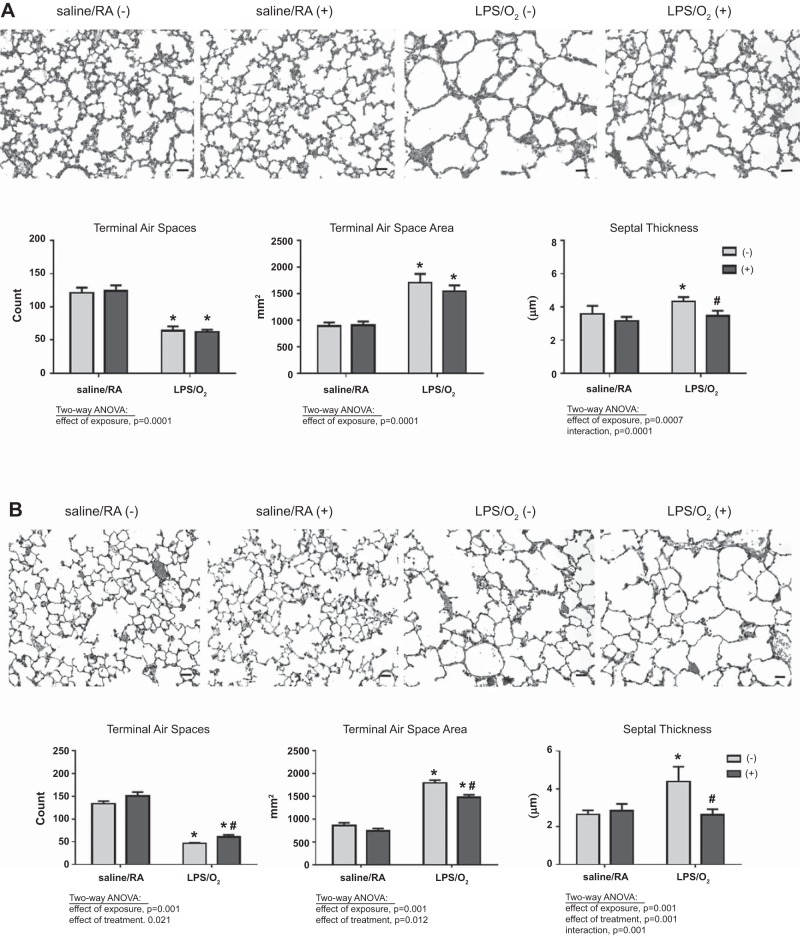
Hematoxylin-eosin-stained histological sections of PN14 or PN28 lungs were obtained following AAV9-miR29b(+) or AAV9-control(−) treatment to determine the effects of miR-29b supplementation on lung alveolarization. Consistent with our previous data, we detected a substantial decrease in alveolarization (terminal air space number and area) and increases in septal thickness at PN14 in LPS/O2-exposed pups compared with saline/RA and these differences were more profound at PN28 (Fig. 4). Administration of miR-29b did not alter the effects of LPS/O2 on alveolarization at PN14 but did significantly attenuate the increases in septal thickness in observed in the LPS/O2(−) mice. At PN28, AAV9-miR29b administration improved alveolarization as assessed by terminal air space number and area in the LPS/O2-exposed mice; however, treatment did not restore these parameters to control saline/RA levels. Administration of miR-29b completely normalized the septal thickness to control levels at PN28 (Fig. 4B).
Fig. 4.
Morphometric analysis of lungs from AAV9-miR29b or control-treated mice. Mice were exposed to LPS/O2 and treated with a single dose of AAV9-miR29b(+) or control virus(−) at PN3. Pups were euthanized at PN14 (A) or PN28 (B). Lung tissues were stained with hematoxylin-eosin, and morphometric analyses were performed using Image Pro software. Photomicrographs were taken at ×100; bars = 100 μm. Data were analyzed by two-way ANOVA to identify effects of saline/RA or LPS/O2 exposure and miR-29b(−) or miR-29b(+) treatment as indicated on the graph; n = 4–6. Tukey’s post hoc analysis indicated significant differences between groups: *different than saline/RA(−); #different than LPS/O2(−).
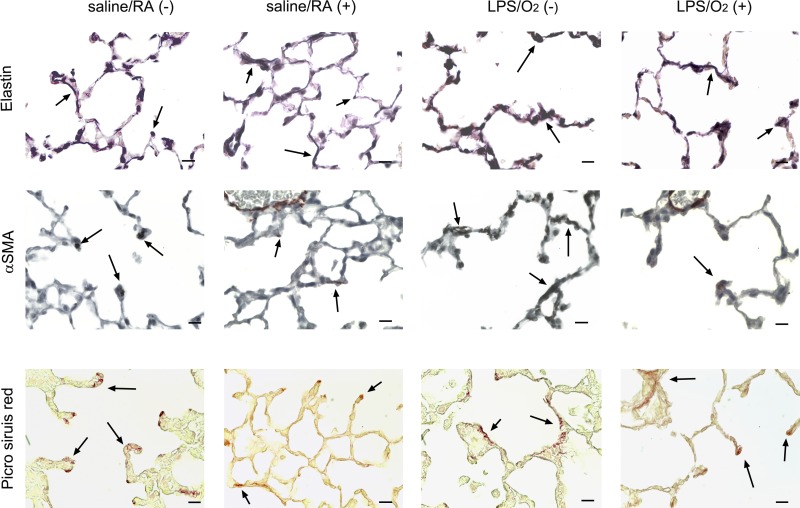
Immunohistochemistry of matrix proteins.
Lung tissue sections obtained at PN28 were stained for elastin (Hart’s stain, Fig. 5, top), α-SMA (immunohistochemistry, Fig. 5, middle), or collagen (picrosirius red, Fig. 5, bottom). Photomicrographs were obtained at ×400 (Fig. 5). We observed disorganized elastin fibers at the ends of the forming septae and in the interstitial area in lung tissues from LPS/O2(−) mice. These effects were attenuated in AAV9-miR29b-treated mice (Fig. 5, top). Assessment of elastin by digital quantification of histological sections and by immunoblot indicated no changes in total elastin contents (data not shown). α-SMA was localized to the tips of the forming septae in the saline/RA(−) controls but was highly expressed in the interstitial region and absent in the forming septal buds in LPS/O2(−) mice. This misexpression was corrected in AAV9-miR29b-treated mice. Increased collagen deposition within the interstitial space and in the areas of septal formation was observed in LPS/O2(−) mice, and excess collagen deposition was prevented in the mice that received AAV-miR29b (Fig. 5, bottom).
Fig. 5.
Immunohistochemical assessment of matrix proteins. Lung tissue from mice exposed to saline/RA or LPS/O2 and treated with a single dose of AAV9-miR-29b(+) or control virus(−) at PN3 were isolated and formalin fixed at PN28. Sections were stained with Hart’s stain for elastin (A), immunohistochemistry for α-smooth muscle actin (α-SMA; B), and Picrosirius red for collagen (C). Photomicrographs were taken at ×400; bars = 50 μm.
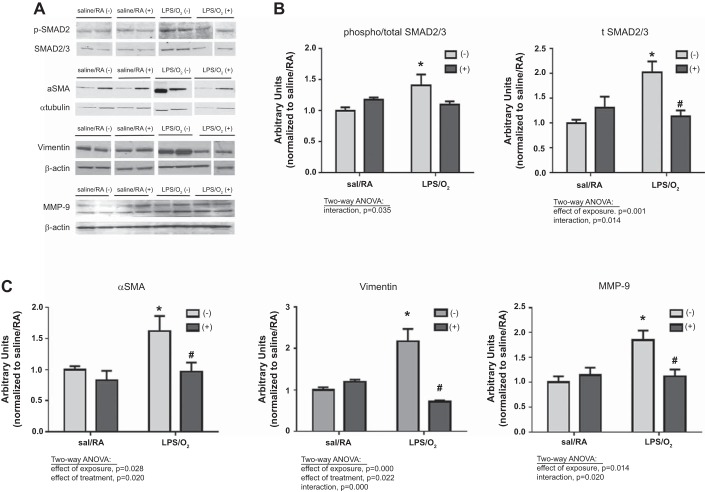
Immunoblot analysis of matrix protein.
The observed normalization of septal thickness in LPS/O2-exposed mice treated with AAV-miR29b(+) coincided with normalization of phospho-SMAD2 and total SMAD2/3 protein levels at PN28 (Fig. 6, A and B). These data suggest TGFβ signaling was attenuated following AAV9-miR29b treatment. In addition, the elevations in pulmonary protein levels of α-SMA, vimentin, and matrix metalloproteinase-9 observed in mice exposed to LPS/O2 were attenuated with AAV-miR29(+) treatment (Fig. 6, A and C). Taken together, these data suggest that supplementing the levels of miR-29b in the lungs of LPS/O2-exposed mice attenuated pathological fibrotic signaling.
Fig. 6.
Immunoblot analysis of matrix proteins. A: matrix protein levels were measured by immunoblot analysis of lung tissue homogenates of PN28 mice exposed to saline/RA or LPS/O2 and treated with a single dose of AAV9-miR29b(+) or control virus(−) at PN3. Levels of phospho-SMAD 2/3 and total SMAD 2/3 (B), as well as α-SMA, vimentin, and matrix metalloproteinase (MMP)-9 (C), were quantified by densitometry and were analyzed by two-way ANOVA to identify effects of saline/RA or LPS/O2 exposure and AAV9-miR29b(+) or control virus(−) treatment as indicated on the graph; n = 4–6. Tukey’s post hoc analysis indicated differences between groups: *different than saline/RA(−); #different than LPS/O2(−).
DISCUSSION
Decades of research have failed to fully define the etiology of neonatal chronic lung disease or BPD, creating a significant barrier to the development of effective therapeutics. This failure likely reflects the complexity of disease pathogenesis given the vulnerability of prematurely born infants who are born when lung development is in the saccular stage (7, 13). Infants born prematurely are especially susceptible to epigenetic modifications that likely contribute to persistent life-long effects of prematurity (17). Similar to infants with mild or moderate BPD, infants with severe BPD exhibit decreased alveolarization. Importantly, infants with severe BPD also develop restrictive and/or obstructive interstitial lung disease, have permanently altered pulmonary function, and disproportionately suffer from long-term pulmonary and nonpulmonary morbidities (11, 31, 32, 43). With the use of plasma obtained from prematurely born infants in the first week of life, our data revealed decreased circulating miR-29b levels in infants that were subsequently diagnosed with BPD at 36-wk corrected gestational age (Fig. 1A). Our finding of a significant inverse association between the BPD severity and miR-29b expression suggests that decreased miR-29b shortly after birth may be an early predictor of or contributor to disease severity (Fig. 1B).
Our well-developed and extensively characterized mouse model of maternal inflammation combined with neonatal hyperoxia mimics the pathophysiology of infants with severe BPD (35, 37) and is a useful model to investigate epigenetic modifications associated with severe disease. The pulmonary pathophysiology of LPS/O2 exposure is characterized by aberrant matrix proteins levels, which manifest as a stiff, noncompliant lung. We subsequently noted dramatic suppression of miR-29b in the lungs of LPS/O2-exposed mice compared with control saline/RA mice (35). While we acknowledge that changes in plasma and lung tissue levels of miR-29b may not be equivalent, the limited plasma available from our neonatal mice precludes a direct measurement of plasma miR-29b levels in our model. Nevertheless, our observation of significant decreases in miR-29b levels in LSP/O2-exposed mouse lung, as well as the apparent decrease in miR-29b levels in human BPD lungs (Fig. 1C), supports the hypothesis that decreases in miR-29b levels contribute to the pathophysiology of severe BPD and may be a potential therapeutic target.
Suppression of mature miR-29b expression in the LPS/O2-exposed mice is seen at E18 and PN14 (35), and suppression of pre-miR-29a, -29b, and -29c in LPS/O2-exposed mice is seen at PN14 (Fig. 2). However, suppression of mature miR-29b expression is no longer evident at PN28 (data not shown) suggesting that the observed pathological changes in our model are likely due to aberrant miR-29b expression during key periods of alveolar development. In addition, overall miR-29 expression increases during lung development, which may mask the decreases evident in early life (9). miR-29 is one of the most highly expressed microRNAs in the lung and is most abundantly found in cells that coexpress α-SMA (8). Mice with each of the miR-29 transcripts knocked out independently (miR-29a and -b1 or miR-29b2 and -c) exhibited no pathological phenotype, but mice with both miR-29 transcripts simultaneously knocked out were small, had respiratory insufficiency, and died before reproductive age (8). Immunofluorescence and quantitative PCR of tissues obtained from pups treated with AAV9-miR29b indicated increases in expression of mature miR-29b-3p for at least 7 days post-AAV9 treatment (Fig. 2, A and B). Furthermore, AAV9-mediated miR-29b appears to be expressed primarily in macrophages and type 2 cells (Fig. 2C). Pre-miRs were measured in all treatment groups at PN14 (11 days post-AAV9 treatment) to identify any persistent global effects of AAV9-miR29b administration on all isoforms of miR29. As observed previously for mature miR-29b (35), the combination of maternal LPS and neonatal hyperoxia suppressed endogenous expression of pre-miR-29b. Exogenous AAV9-miR-29b treatment led to further suppression of pre-miR29a (Fig. 3) but did not alter expression of the other pre-miR-29 isoforms. The relative differences in miR-29b expression in viral-treated mice at PN14 (Figs. 2B and 3A) are not straightforward but are likely due to differences in measurement of endogenous verses viral mediated expression.
Modest improvements in alveolarization were observed at PN28 in mice treated with a single dose of AAV9-miR29b at day 3 of life (Fig. 4). Furthermore, our findings suggest that exogenously administered AAV9-miR29b reaches the distal lung as AAV9-miR29b is expressed in cell types responsible for fibrotic responses (Fig. 2C). We observed thinning of the septal walls (Fig. 4) and complete normalization of phospho-SMAD2, total SMAD2/3, α-SMA, and vimentin levels (Fig. 6) in AAV9-miR29b-treated mice. Histochemical analyses demonstrated that exposure to LPS/O2 caused disorganization of elastin fibers; a finding that has been linked to disruption of matrix deposition (Fig. 5) (5, 15). Altered α-SMA localization away from the tips of the protruding septa and into alveolar walls was also observed (Fig. 5). Furthermore, increases in picrosirius red staining were clearly evident in the interstitial areas of the lung architecture reflecting excessive collagen deposition. Misexpression of these matrix proteins collectively results in a stiffer and less compliant lung that will negatively impact alveolarization. Importantly, each of these histological abnormalities was attenuated by AAV9-miR29b treatment (Fig. 5).
We selected AAV9 as a vehicle because it expresses in the distal lung with minimal immunological responses (4, 34). Multiple studies have described AAV9-mediated delivery of genes to the distal lung epithelium (44, 45) or to heart myofibroblasts (28). Using current techniques, we were able to identify GFP fluorescence in lung tissue sections at PN6, PN10, and PN14 and miR-29b-3p expression using PCR-based approaches at PN7 in response to our therapy (Fig. 2). We speculate that early delivery of the AAV9-miR29b, at PN3, takes place at a time when the lung is rapidly proliferating and that AAV9-transduced cells are likely a small percentage of the total lung population at later time points.
The complex pathophysiology of BPD necessitates equally complex therapeutic approaches. Our investigations have identified miR-29b as a potential target in treating BPD, more specifically the matrix disruption associated with severe BPD. Modest improvements in alveolarization were observed following AAV9-miR29b therapy, and we speculate that additional studies that include time and dose-dependent effects could enhance overall efficacy in our model. Other authors have identified miRs that are altered in the course of BPD development and found that restoration of expression dramatically improved septation and alveolarization (26). The collective data on miR expression and BPD suggest that combination miR-based therapies, including miR-29b, may hold promise for restoring normal lung structure in preterm infants at risk of developing severe BPD or in infants with established severe BPD.
GRANTS
This work was supported by the National Institutes of Health, National Center for Complementary and Alternative Medicine/Office of Dietary Supplements Grant R01-AT-006880 (to L. K. Rogers) and National Institute for Child Health and Disease Grant R01-HD-088033 (to L. K. Rogers).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
L.K.R., M.J.C., and T.E.T. conceived and designed research; S.D.-K., C.A.P., A.G., K.M.H., M.J.C., L.J.L., and Z.Y. performed experiments; S.D.-K., C.A.P., A.G., K.M.H., M.J.C., L.J.L., Z.Y., T.E.T., and L.K.R. analyzed data; S.D.-K., T.E.T., and L.K.R. drafted manuscript; S.D.-K., C.A.P., A.G., K.M.H., M.J.C., L.J.L., Z.Y., T.E.T., and L.K.R. edited and revised manuscript; C.A.P., A.G., K.M.H., G.S.P., T.E.T., and L.K.R. interpreted results of experiments; K.M.H., M.J.C., G.S.P., L.J.L., Z.Y., T.E.T., and L.K.R. approved final version of manuscript; M.J.C. and L.K.R. prepared figures.
ACKNOWLEDGMENTS
We thank Xiaojin Zhang for excellent technical help with the immunofluorescence experiments and Cate Warnement for contributions to these studies.
REFERENCES
- 1.Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007. doi: 10.1152/ajplung.00050.2006. [DOI] [PubMed] [Google Scholar]
- 2.Ali M, Heyob KM, Velten M, Tipple TE, Rogers LK. DHA suppresses chronic apoptosis in the lung caused by perinatal inflammation. Am J Physiol Lung Cell Mol Physiol 309: L441–L448, 2015. doi: 10.1152/ajplung.00137.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baraldi E, Carraro S, Filippone M. Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early Hum Dev 85, Suppl: S1–S3, 2009. doi: 10.1016/j.earlhumdev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Bell CL, Gurda BL, Van Vliet K, Agbandje-McKenna M, Wilson JM. Identification of the galactose binding domain of the adeno-associated virus serotype 9 capsid. J Virol 86: 7326–7333, 2012. doi: 10.1128/JVI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, Alvira CM, Rabinovitch M, Shinwell ES, Dixit A. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice. Prelude to defective alveolar septation during lung development? Am J Physiol Lung Cell Mol Physiol 294: L3–L14, 2008. doi: 10.1152/ajplung.00362.2007. [DOI] [PubMed] [Google Scholar]
- 6.Buckley SM, Howe SJ, Rahim AA, Buning H, McIntosh J, Wong SP, Baker AH, Nathwani A, Thrasher AJ, Coutelle C, McKay TR, Waddington SN. Luciferin detection after intranasal vector delivery is improved by intranasal rather than intraperitoneal luciferin administration. Hum Gene Ther 19: 1050–1056, 2008. doi: 10.1089/hum.2008.023. [DOI] [PubMed] [Google Scholar]
- 7.Clark RH, Gerstmann DR, Jobe AH, Moffitt ST, Slutsky AS, Yoder BA. Lung injury in neonates: causes, strategies for prevention, and long-term consequences. J Pediatr 139: 478–486, 2001. doi: 10.1067/mpd.2001.118201. [DOI] [PubMed] [Google Scholar]
- 8.Cushing L, Costinean S, Xu W, Jiang Z, Madden L, Kuang P, Huang J, Weisman A, Hata A, Croce CM, Lü J. Disruption of miR-29 leads to aberrant differentiation of smooth muscle cells selectively associated with distal lung vasculature. PLoS Genet 11: e1005238, 2015. doi: 10.1371/journal.pgen.1005238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lü J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol 45: 287–294, 2011. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong J, Carey WA, Abel S, Collura C, Jiang G, Tomaszek S, Sutor S, Roden AC, Asmann YW, Prakash YS, Wigle DA. MicroRNA-mRNA interactions in a murine model of hyperoxia-induced bronchopulmonary dysplasia. BMC Genomics 13: 204, 2012. doi: 10.1186/1471-2164-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. Am J Respir Crit Care Med 182: 237–245, 2010. doi: 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenough A. Long term respiratory outcomes of very premature birth (<32 weeks). Semin Fetal Neonatal Med 17: 73–76, 2012. doi: 10.1016/j.siny.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Groneck P, Götze-Speer B, Oppermann M, Eiffert H, Speer CP. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics 93: 712–718, 1994. [PubMed] [Google Scholar]
- 14.Harding R, Maritz G. Maternal and fetal origins of lung disease in adulthood. Semin Fetal Neonatal Med 17: 67–72, 2012. doi: 10.1016/j.siny.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Hilgendorff A, Parai K, Ertsey R, Juliana Rey-Parra G, Thébaud B, Tamosiuniene R, Jain N, Navarro EF, Starcher BC, Nicolls MR, Rabinovitch M, Bland RD. Neonatal mice genetically modified to express the elastase inhibitor elafin are protected against the adverse effects of mechanical ventilation on lung growth. Am J Physiol Lung Cell Mol Physiol 303: L215–L227, 2012. doi: 10.1152/ajplung.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729, 2001. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 17.Joss-Moore LA, Albertine KH, Lane RH. Epigenetics and the developmental origins of lung disease. Mol Genet Metab 104: 61–66, 2011. doi: 10.1016/j.ymgme.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwak KJ, Valincius G, Liao WC, Hu X, Wen X, Lee A, Yu B, Vanderah DJ, Lu W, Lee LJ. Formation and finite element analysis of tethered bilayer lipid structures. Langmuir 26: 18199–18208, 2010. doi: 10.1021/la1021802. [DOI] [PubMed] [Google Scholar]
- 19.Lee LJ, Yang Z, Rahman M, Ma J, Kwak KJ, McElroy J, Shilo K, Goparaju C, Yu L, Rom W, Kim TK, Wu X, He Y, Wang K, Pass HI, Nana-Sinkam SP. Extracellular mRNA detected by tethered lipoplex nanoparticle biochip for lung adenocarcinoma detection. Am J Respir Crit Care Med 193: 1431–1433, 2016. doi: 10.1164/rccm.201511-2129LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Wang B, Lin J, Zhao L. microRNA-29b: an emerging player in human cancer. Asian Pac J Cancer Prev 15: 9059–9064, 2014. doi: 10.7314/APJCP.2014.15.21.9059. [DOI] [PubMed] [Google Scholar]
- 21.McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther 16: 1648–1656, 2008. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 22.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther 10: 2112–2118, 2003. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 23.Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell 133: 217–222, 2008. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem 110: 1155–1164, 2010. doi: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuovo GJ, Garofalo M, Valeri N, Roulstone V, Volinia S, Cohn DE, Phelps M, Harrington KJ, Vile R, Melcher A, Galanis E, Sehl S, Adair R, Scott K, Rose A, Toogood G, Coffey MC. Reovirus-associated reduction of microRNA-let-7d is related to the increased apoptotic death of cancer cells in clinical samples. Mod Pathol 25: 1333–1344, 2012. doi: 10.1038/modpathol.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olave N, Lal CV, Halloran B, Pandit K, Cuna AC, Faye-Petersen OM, Kelly DR, Nicola T, Benos PV, Kaminski N, Ambalavanan N. Regulation of alveolar septation by microRNA-489. Am J Physiol Lung Cell Mol Physiol 310: L476–L487, 2016. doi: 10.1152/ajplung.00145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park MS, Rieger-Fackeldey E, Schanbacher BL, Cook AC, Bauer JA, Rogers LK, Hansen TN, Welty SE, Smith CV. Altered expressions of fibroblast growth factor receptors and alveolarization in neonatal mice exposed to 85% oxygen. Pediatr Res 62: 652–657, 2007. doi: 10.1203/PDR.0b013e318159af61. [DOI] [PubMed] [Google Scholar]
- 28.Piras BA, Tian Y, Xu Y, Thomas NA, O’Connor DM, French BA. Systemic injection of AAV9 carrying a periostin promoter targets gene expression to a myofibroblast-like lineage in mouse hearts after reperfused myocardial infarction. Gene Ther 23: 469–478, 2016. doi: 10.1038/gt.2016.20. [DOI] [PubMed] [Google Scholar]
- 29.Rogers LK, Robbins M, Dakhlallah D, Yang Z, Lee LJ, Mikhail M, Nuovo G, Pryhuber GS, McGwin G, Marsh CB, Tipple TE. Attenuation of miR-17∼92 cluster in bronchopulmonary dysplasia. Ann Am Thorac Soc 12: 1506–1513, 2015. doi: 10.1513/AnnalsATS.201501-058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers LK, Young CM, Pennell ML, Tipple TE, Leonhart KL, Welty SE. Plasma lipid metabolites are associated with gestational age but not bronchopulmonary dysplasia. Acta Paediatr 101: e321–e326, 2012. doi: 10.1111/j.1651-2227.2012.02694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest 132: 651–656, 2007. doi: 10.1378/chest.06-2663. [DOI] [PubMed] [Google Scholar]
- 32.Shi W, Warburton D. Is COPD in adulthood really so far removed from early development? Eur Respir J 35: 12–13, 2010. doi: 10.1183/09031936.00145809. [DOI] [PubMed] [Google Scholar]
- 33.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sánchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID III, Watterberg KL, Saha S, Das A, Higgins RD; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126: 443–456, 2010. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tai DS, Hu C, Kim EH, Lipshutz GS. Augmentation of transgene-encoded protein after neonatal injection of adeno-associated virus improves hepatic copy number without immune responses. Pediatr Res 78: 239–246, 2015. doi: 10.1038/pr.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velten M, Britt RD Jr, Heyob KM, Welty SE, Eiberger B, Tipple TE, Rogers LK. Prenatal inflammation exacerbates hyperoxia-induced functional and structural changes in adult mice. Am J Physiol Regul Integr Comp Physiol 303: R279–R290, 2012. doi: 10.1152/ajpregu.00029.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velten M, Gorr MW, Youtz DJ, Velten C, Rogers LK, Wold LE. Adverse perinatal environment contributes to altered cardiac development and function. Am J Physiol Heart Circ Physiol 306: H1334–H1340, 2014. doi: 10.1152/ajpheart.00056.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velten M, Heyob KM, Rogers LK, Welty SE. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J Appl Physiol (1985) 108: 1347–1356, 2010. doi: 10.1152/japplphysiol.01392.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velten M, Hutchinson KR, Gorr MW, Wold LE, Lucchesi PA, Rogers LK. Systemic maternal inflammation and neonatal hyperoxia induces remodeling and left ventricular dysfunction in mice. PLoS One 6: e24544, 2011. doi: 10.1371/journal.pone.0024544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viscardi RM. Perinatal inflammation and lung injury. Semin Fetal Neonatal Med 17: 30–35, 2011. doi: 10.1016/j.siny.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wakabayashi-Ito N, Nagata S. Characterization of the regulatory elements in the promoter of the human elongation factor-1 alpha gene. J Biol Chem 269: 29831–29837, 1994. [PubMed] [Google Scholar]
- 41.Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther 10: 2105–2111, 2003. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 42.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 293: L525–L534, 2007. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 43.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J 32: 321–328, 2008. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 44.Wu CJ, Chen LC, Huang WC, Chuang CL, Kuo ML. Alleviation of lung inflammatory responses by adeno-associated virus 2/9 vector carrying CC10 in OVA-sensitized mice. Hum Gene Ther 24: 48–57, 2013. doi: 10.1089/hum.2012.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu CJ, Huang WC, Chen LC, Shen CR, Kuo ML. Pseudotyped adeno-associated virus 2/9-delivered CCL11 shRNA alleviates lung inflammation in an allergen-sensitized mouse model. Hum Gene Ther 23: 1156–1165, 2012. doi: 10.1089/hum.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu DW, Hsu NY, Wang YC, Lee MC, Cheng YW, Chen CY, Lee H. c-Myc suppresses microRNA-29b to promote tumor aggressiveness and poor outcomes in non-small cell lung cancer by targeting FHIT. Oncogene 34: 2072–2082, 2015. doi: 10.1038/onc.2014.152. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, Kwak KJ, Agarwal K, Marras A, Wang C, Mao Y, Huang X, Ma J, Yu B, Lee R, Vachani A, Marcucci G, Byrd JC, Muthusamy N, Otterson G, Huang K, Castro CE, Paulaitis M, Nana-Sinkam SP, Lee LJ. Detection of extracellular RNAs in cancer and viral infection via tethered cationic lipoplex nanoparticles containing molecular beacons. Anal Chem 85: 11265–11274, 2013. doi: 10.1021/ac401983w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao J, Meng XM, Huang XR, Chung AC, Feng YL, Hui DS, Yu CM, Sung JJ, Lan HY. miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol Ther 20: 1251–1260, 2012. doi: 10.1038/mt.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]