Abstract
Pulmonary arterial hypertension is a complication of methamphetamine use (METH-PAH), but the pathogenic mechanisms are unknown. Given that cytochrome P450 2D6 (CYP2D6) and carboxylesterase 1 (CES1) are involved in metabolism of METH and other amphetamine-like compounds, we postulated that loss of function variants could contribute to METH-PAH. Although no difference in CYP2D6 expression was seen by lung immunofluorescence, CES1 expression was significantly reduced in endothelium of METH-PAH microvessels. Mass spectrometry analysis showed that healthy pulmonary microvascular endothelial cells (PMVECs) have the capacity to both internalize and metabolize METH. Furthermore, whole exome sequencing data from 18 METH-PAH patients revealed that 94.4% of METH-PAH patients were heterozygous carriers of a single nucleotide variant (SNV; rs115629050) predicted to reduce CES1 activity. PMVECs transfected with this CES1 variant demonstrated significantly higher rates of METH-induced apoptosis. METH exposure results in increased formation of reactive oxygen species (ROS) and a compensatory autophagy response. Compared with healthy cells, CES1-deficient PMVECs lack a robust autophagy response despite higher ROS, which correlates with increased apoptosis. We propose that reduced CES1 expression/activity could promote development of METH-PAH by increasing PMVEC apoptosis and small vessel loss.
pulmonary arterial hypertension (PAH) is a life-threatening disease characterized by abnormally elevated pulmonary pressure and right heart failure with a median survival of 3 yr after diagnosis (20). Lung pathology in PAH patients is characterized by marked loss of distal pulmonary microvessels and severe obliterative vasculopathy, which progressively overwhelms the capacity of the right heart to pump venous blood into the lungs (43). Current treatments include vasodilatory drugs that help palliate symptoms but fail to prevent disease progression, leaving lung transplantation as the only therapy for patients with end-stage PAH (56a).
Available evidence supports a key role for interaction between genes and environment in triggering PAH in susceptible individuals. This is the case for PAH associated with exposure to certain drugs and toxins, where patients develop a clinical and pathological picture that is indistinguishable from other forms of PAH (1, 5, 47). The 2015 European Society of Cardiology and European Respiratory Society guidelines now recognize 16 different compounds associated with PAH, which range from FDA-approved therapies (e.g., mitomycin, dasatanib) to illicit stimulants such as methamphetamine (METH) (19). Methamphetamine is a highly addictive compound whose popularity among young and middle-aged adults has steadily increased worldwide in the past decade. In addition to being a potent neurostimulant, METH can also affect other organs, such as the kidneys, brain, and liver, resulting in severe organ dysfunction and premature death (21). Although METH use is associated with a higher incidence of cardiovascular diseases such as ischemic cardiomyopathy, arrhythmias, and myocardial infarction, it is only recently that PAH has been recognized as a life-threatening complication of METH use. The association between inhaled METH use and PAH was first reported by Schaiberger et al.(51) and further supported by a retrospective study by Chin et al. (10) that found significantly higher rates of METH use in patients diagnosed with idiopathic PAH (IPAH) when compared with other PAH groups. PET studies have shown that [(11)C] d-METH administered intravenously localizes primarily in the lung tissue, suggesting that the lung is a primary target for METH-related injury (58). On the basis of these studies, METH use was included in the most recent clinical classification of pulmonary hypertension as a likely risk factor in drug- and toxin-induced PAH (D + T PAH) (20).
Although the true incidence and prevalence of METH-PAH in the US is unknown, we have seen a disturbing increase in the number of METH-PAH cases diagnosed at the Stanford Adult Pulmonary Hypertension Clinic over the last 10 years. At present, 85% of our D + T PAH patients carry a diagnosis of METH-PAH, and their median 5-yr survival is estimated at 35% (Zamanian R, personal communication), which is significantly worse compared with that of IPAH patients. Despite the current clinical evidence, it must be emphasized that not all patients with a history of METH use develop PAH. Similarly to patients with familial and sporadic PAH, it is possible that variations in certain genes may be required to trigger PAH in a subset of METH users, but no gene candidates have been established in any study to date.
Two major liver enzyme families carry out metabolism of most amphetamine derivatives: the cytochrome P450 2D6 (CYP2D6) and carboxylesterase 1 (CES1). CYP2D6 is an isoenzyme belonging to the cytochrome P450 family required for phase 1 metabolism of a wide range of drugs. CES1 is a key enzyme in the detoxification of illicit toxins such as cocaine and heroin as well as FDA-approved drugs such as methylphenidate (Ritalin), an amphetamine-derived drug used in the treatment of attention deficit disorders (28, 62). Polymorphisms that reduce expression and/or activity of either enzyme can affect the rate of drug metabolism and result in chronic organ injury (6, 13, 32, 39, 55, 65), but whether this could also be linked to METH-induced pulmonary vascular injury is unknown. On the basis of these findings, we speculated that loss of function of CYP2D6 and/or CES1 could increase risk of PAH in METH users. Here, we present for the first time evidence that pulmonary endothelial cells can metabolize METH and have identified CES1 as a candidate gene required for protecting the pulmonary endothelium against METH-related injury.
METHODS
Lung Tissue and Cell Culture
Lung tissue from healthy donors and METH-PAH patients was obtained via the Cardiovascular Medical Education and Research Fund-Pulmonary Hypertension Breakthrough Initiative (CMREF-PHBI). Healthy donor pulmonary microvascular endothelial cells (PMVECs) were obtained from the CMREF-PHBI and a commercial source (cat. no. C-12282; Promocell). All cells were grown in EC media (cat. no. 1001; ScienCell, Carlsbad, CA), with growth supplements and used between passages 4 and 8. Methamphetamine was purchased from Sigma-Aldrich (St. Louis, MO; cat. no. M8750). A METH concentration of 5 mM was chosen after dose response studies were conducted on healthy PMVECs. To control for the different growth rates of PMVECs between experimental groups (siCES1/siCYP2D6 vs. siControl), cells were seeded into culture plates 2 h before METH exposure in all in vitro experiments.
Immunofluorescence
Lung tissue from healthy donors, METH-PAH, IPAH, cystic fibrosis (CF), and idiopathic pulmonary fibrosis (IPF) patients was obtained from explanted lungs at the time of transplant or during autopsy and embedded in paraffin blocks or optimal cutting temperature compound. Paraffin-embedded tissue sections were treated with xylene, followed by serial dilutions of ethanol. The sections were then put into a beaker of boiling citrate buffer for 10 min. After the sections had cooled to room temperature, they were washed in PBS buffer. The slides were then incubated with diluted normal goat blocking serum, followed by incubation overnight with anti-CES1 [a kind gift of Dr. Bruce Hammock, as described (67)], CD31 (cat. no. B4737; LSBio, Seattle, WA), or anti-CYP2D6 (cat. no. 185625; Abcam, Boston, MA) primary antibody in a humidity chamber at 4°C. The following day, the sections were washed three times for 5 min each in PBS then incubated for 1 h with Alexa Fluor 488/594-conjugated secondary antibody (Thermo Fisher, Waltham, MA). Following treatment with antifade reagent with DAPI (cat. no. 8961S; Cell Signaling Technology, Danvers, MA), the slides were mounted and sealed.
Liquid Chromatography-Mass Spectrometry
A unified liquid chromatography (LC)-UV-mass spectrometry (MS) method was used for structural elucidation of methamphetamine metabolites, using an 1100 series HPLC-UV (Agilent Technologies) integrated with an LTQ XL ion trap mass spectrometer (Thermo Fisher Scientific).
Extraction procedure.
Three-hundred microliters of ice-cold methanol was added to 100-μl cell suspension. Solutions were sonicated and centrifuged, and the supernatant was removed and evaporated to dryness under nitrogen. The extract was reconstituted in 100 μl of water, and 10 μl was injected onto the HPLC column.
Liquid chromatography.
Chromatography was performed on a 250 × 2.1 mm Polaris 5 C18-A column (Varian), using 0.1% formic acid in acetonitrile (B) and 0.1% formic acid in water (A) and eluting with a linear gradient from 0 to 50% B in 25 min, followed by an increase to 95% B in 7 min. Total run time was 37 min. Flow rate was 250 μl/min, and UV detection was at 214 nm.
Mass spectrometry.
MS2 data for structural elucidation were acquired using heated electrospray ionization with positive/negative ion switching using full-scan acquisition (110–600 m/z mass range) and data-dependent acquisition in dynamic exclusion mode. Collision-induced dissociation (CID) channel was set up for monitoring hydroxymethamphetamine metabolites (m/z = 166.2 Da; isolation width 2 m/z).
Patient Selection and DNA Extraction
Written, informed consent for this study was obtained in agreement with protocols approved by the Institutional Review Boards (IRB no. 5443) at Stanford University. METH-PAH was defined as PAH with no identifiable cause with a mean pulmonary arterial pressure ≥25 mmHg at rest, pulmonary artery wedge pressure (PAWP) ≤15 mmHg, and a pulmonary vascular resistance (PVR) >3 wood units (WU) (2). Significant METH exposure was considered if the patient reported a >3-mo history of weekly METH use and reported no use of other stimulants associated with PAH. Genomic DNA was purified from buffy coat samples obtained from whole blood using the Ficoll extraction method and the Qiagen DNeasy kit, following the manufacturer’s protocol.
Whole Exome Sequencing
Whole exome sequencing (WES) samples were prepared as an Illumina sequencing library, and in the second step, the sequencing libraries were enriched for the desired target using the Illumina Exome Enrichment protocol. The captured libraries were sequenced in an Illumina HiSeq 2000 Sequencer (Illumina, San Diego, CA) using paired-end 75- to 100-bp sequences. Samples were sequenced to ≥125-fold (3125) sequence coverage. Raw sequence reads were aligned to human reference sequence hg19 using the Sequence Alignment Map and Binary Alignment Map. Sequencing data were analyzed using ANNOVAR software (http://annovar.openbioinformatics.org/en/latest/). A list of CYP2D6 and CES1 candidate variants was prepared by selecting variants predicted to result in nonsynonymous protein-coding changes (missense, nonsense) and confirmed using Sanger sequencing. Given that all METH-PAH patients were Caucasians, we used the minor allele frequency (MAF) for European (non-Finnish) whites reported in the latest version of the ExAC browser.
Allelic Discrimination Quantitative PCR
Genomic DNA was isolated from patient lung tissue embedded in paraffin using the QIAmp DNA FFPE Tissue Kit (cat. no. 56404; Qiagen, Valencia, CA). First, three 10-μm sections were cut from paraffin block using a microtome and then placed in 1 ml of xylene, and then DNA extraction was achieved by following the manufacturer’s instructions. Real-time PCR for CES1 SNP rs115629050 was performed using Taqman assays (cat. no. 4331349, assay ID no. AHMSYGO; Applied Biosystems, Foster City, CA) on an ABI7500 PCR machine. Each reaction contains 11.25 μl of isolated gDNA (~20 ng), 12.5 μl of PCR master mix (2×), and 1.25 μl of TaqMan SNP probe (20×). The PCR reaction is 50 cycles of 92°C for 15 s (step 1) and 60°C for 1 min (step 2), with an initial step of 95°C for 10 min. The StepOne Plus software generated the Allelic Discrimination Plot, with the 2-Cluster Calling enabled.
CES1 Activity Assay
CES1 activity in peripheral blood mononuclear cells (PBMCs) was performed using the CES1 Specific Activity Assay Kit (cat. no. 109717Abcam, Boston, MA), following the manufacturer’s protocol. Briefly, cells were pelleted and lysed with RIPA buffer. After protein concentration was measured using the BCA protein assay (Thermo Fisher), samples were loaded on a 96-well plates coated with an antibody against CES1 and incubated for 3 h at room temperature with gentle shaking. After washing, CES1 enzyme activity was measured by adding 200 μl of a 1-mM 4-nitrophenol (4-NP) solution loaded into each well, followed by measurement of absorbance at 402 nm at 15 min using a Promega spectrophotometer. CES1 quantity per well was measured by incubating with an anti-CES1 HRP-tagged antibody for 1 h, followed by addition of DAB and measurement of absorbance at 600 nm via spectrophotometer. Results are expressed as CES1 activity relative to quantity.
Western Immunobloting
PMVECs were washed twice with ice-cold 1× PBS, and lysates were prepared by adding lysis buffer (1× RIPA and 1 mM PMSF), scraping into a 1.5-ml microcentrifuge tube, and vortex homogenization before centrifugation. Supernatants were transferred to fresh microcentrifuge tubes and stored at −80°C. The protein concentration was determined by the BCA assay (Thermo Fisher). Equal amounts of protein were loaded onto each lane of a 4–12% Bis-Tris gel and subjected to electrophoresis under reducing conditions. After blotting, PVDF membranes were blocked for 1 h (5% milk powder in 0.1% PBS-Tween) and incubated with primary antibodies overnight at 4°C. Bands were visualized using ECL (Thermo Fisher), and loading was assessed with α-tubulin (Sigma-Aldrich, St. Louis, MO). Some Western blot images show only the relevant lanes, whereas those not associated with the current study have been removed. However, all grouped samples were run on the same gel.
CES1 and CYP2D6 siRNA Transfection
To achieve gene knockdown, 2 μM siRNA against CES1 (cat. no. L-009051-00GE; Dharmacon), CYP2D6 (siRNA ID no. s3834; Thermo Fisher), or nontargeting siRNA control (cat. no. D-001810-10-05GE; Dharmacon) was transfected into healthy PMVECs (cat. no. C-12282; Promocell). Knockdown efficiency of CES1 and CYP2D6 was evaluated 72 h after nucleofection by measuring protein levels in cell lysates via Western blot. Transfection was performed using a Nucleofector 2b Device (program T-23) with the Basic Endothelial Cell Nucleofection kit (cat. no. VPI-1001; Lonza). All experiments were performed 72 h after nucleofection.
Caspase 3/7 Apoptosis Assay
Fifty microliters of Caspase Glo-3/7 (cat. no. G8091; Promega, Madison, WI) was added into each sample at a 1:1 ratio with culture medium and incubated in the dark for 1 h. The luminescence of each sample was measured in a plate reader, Promega GloMAx luminometer (cat. no. E9032; Promega), following the manufacturer’s protocol, and normalized to media + Glo-3/7 without cells.
CES1 Plasmids and Transfection Methods
Plasmids encoding the full-length CES1 and control blank were purchased from OriGene (Rockville, MD), and a mutant construct containing the CES1 rs115629050 SNV was generated using in situ mutagenesis (Mutagenex, Suwanee, GA). Transfection of plasmids was performed using a Nucleofector 2b Device (program T-23) with the Basic Endothelial Cell Nucleofection kit. All experiments were performed 24 h after nucleofection.
CellROX Assay for Reactive Oxygen Species
PMVECs were plated at 1.0 × 104 cells/well on eight-well EZ chamber slides (Millipore) and cultured overnight. Four hours after the addition of METH, the cells were rinsed with 1× PBS once and treated with 5 µM CellROX Green Reagent (cat. no. C10444; Thermo Fisher) for 30 min at 37°C. After incubation, cells were imaged live on a Leica DMRMII inverted microscope.
Autophagy Flux Analysis
Autophagy flux was assessed by Western blot analysis of P62 (cat. no. H00008878-M01; Novus, Littleton, CO) and LC3-II (cat. no. 12741; Cell Signaling Technology) levels in the absence and presence of lysosomal blockade, which was accomplished specifically by incubating cells with 100 nM bafilomycin A1 (Sigma-Aldrich) for 2 h, as described previously (26).
Statistical Analysis
The number of samples studied per experiment are indicated in the figure legends. Values from multiple experiments are expressed as means ± SE. Statistical significance was determined using unpaired t-test or ordinary one-way ANOVA with Tukey’s multiple comparison tests unless stated otherwise. A value of P < 0.05 was considered significant.
RESULTS
CES1 Exhibits Differential Expression in Pulmonary Microvessels of METH-PAH Patients
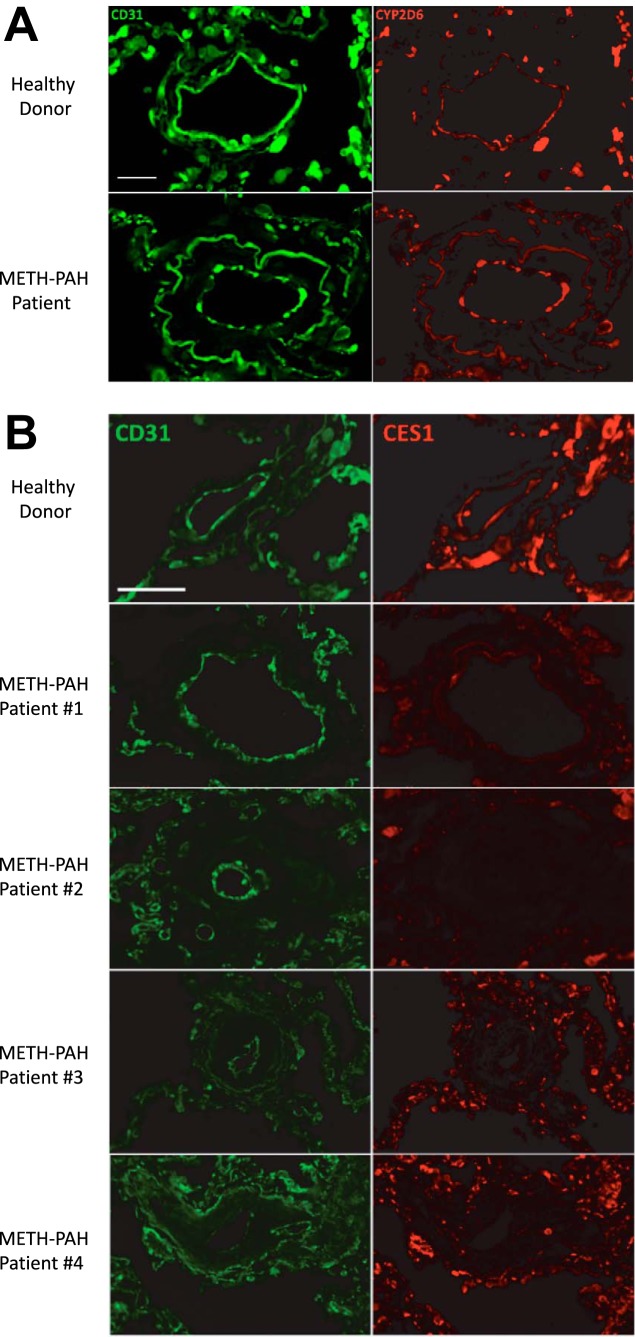
Whereas CES1 and CYP2D6 expression is highest in the liver, BioGPS microarray and Illumina Human BodyMap RNA-seq atlas have shown that mRNA for these two enzymes is also expressed in lung and circulating peripheral blood mononuclear cells (PBMCs) (25, 31). To assess CES1 and CYP2D6 protein expression in pulmonary arteries, we performed immunofluorescence (IF) in lung tissue sections from two healthy donors and four METH-PAH patients obtained at the time of autopsy or transplant. Our studies demonstrated that CYP2D6 is expressed in the endothelium of small microvessels; however, we found no difference in CYP2D6 expression between healthy donor and METH-PAH lungs (Fig. 1A). In contrast, CES1 expression was found to be reduced or absent in remodeled vessels of all four METH-PAH samples compared with healthy patient samples (Fig. 1B).
Fig. 1.
Carboxylesterase 1 (CES1) expression is reduced in vascular lesions of methamphetamine (METH)-pulmonary arterial hypertension (PAH). A: representative immunofluorescence studies of lung sections stained for CYP2D6 (red) obtained from healthy donor and METH-PAH patients. No difference was seen among our 4 METH-PAH patients. B: representative immunofluorescence studies of lung sections stained for CES1 (red) obtained from healthy donor (top) and 4 METH-PAH patients. CD31 (green) stains for endothelial cells. Scale bar, 25 μm.
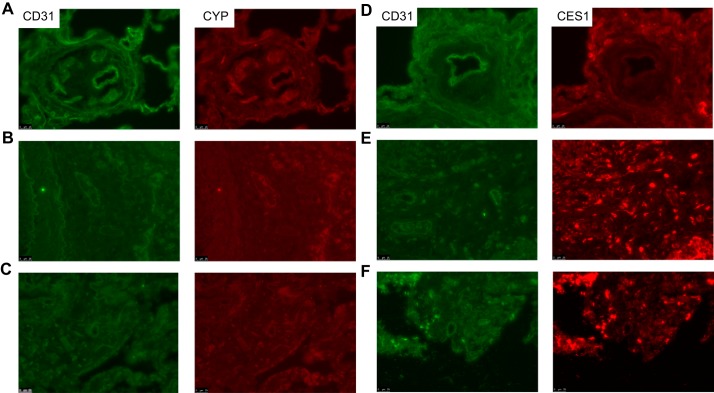
To determine whether our findings were specific to METH-PAH, we also performed IF on lung tissue from patients with IPAH, cystic fibrosis (CF), and idiopathic pulmonary fibrosis (IPF). Again, no difference in CYP2D6 expression was found in IPAH (Fig. 2A) or in CF and IPF lungs (Fig. 2, B and C). In contrast, we found that CES1 expression was substantially reduced in IPAH vascular lesions (Fig. 2D) but not in CF and IPF (Fig. 2, E and F). Our observation of reduced CES1 expression in IPAH lungs is in agreement with the recent description of reduced CES1 expression in endothelial cells derived from IPAH-inducible pluripotent stem cells (49).
Fig. 2.
Expression of CYP2D6 and CES1 in lung sections of idiopathic PAH (IPAH), cystic fibrosis (CF), and idiopathic pulmonary fibrosis (IPF) patients. A–C: representative immunofluorescence studies of IPAH (A), CF (B), and IPF (C) lung sections stained for CYP2D6 (red). D–F: lung sections from IPAH (D), CF (E), and IPF (F) stained for CES1. CD31 (green) stains for endothelial cells. Scale bar, 25 μm.
Taken together, these studies demonstrate that both CES1 and CYP2D6 are expressed in pulmonary vessels, but only the former appears to be differentially expressed in lungs of healthy and METH-PAH patients. For the next step, we sought to determine whether pulmonary microvascular endothelial cells (PMVECs) have the capacity to metabolize METH in vitro.
PMVECs Can Internalize and Metabolize METH
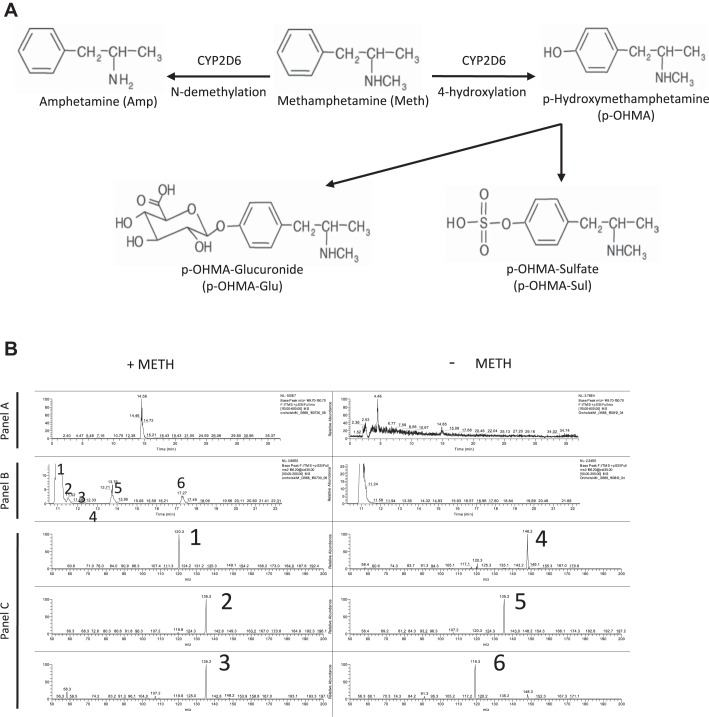
Some cells can metabolize METH into the major metabolites amphetamine and p-hydroxymethamphetamine (p-OHMA; Fig. 3A), as well as other minor metabolites (norephedrine, phenylacetone, benzoic acid, and hippuric acid), and their intracellular accumulation may account for the toxicity of the parent compound (9, 53). To assess whether healthy human PMVECs have the capacity to metabolize METH, we analyzed cell media and lysates of METH-treated PMVECs for metabolites using LC-MS. Analysis of cell media did not identify significant amounts of METH or any known metabolites in either the treated or nontreated group (data not shown), suggesting internalization and/or degradation of METH. However, analysis of cell lysates from METH-treated PMVECs demonstrated at least six hydroxyl isomers of METH, likely including p-OHMA (Fig. 3B). Of note is that no evidence of amphetamine was found.
Fig. 3.
METH is metabolized by pulmonary microvascular endothelial cells (PMVECs) into p-hydroxymethamphetamine (p-OHMA). A: diagram illustrating the phase I metabolism of METH into amphetamine (Amp) and p-OHMA. p-OHMA could be further metabolized into p-OHMA glucuronide (p-OHMA-Glu) and p-OHMA sulfate (p-OHMA-Sul) in the liver to increase solubility and facilitate urinary excretion. B: liquid chromatography (LC)-mass spectrometry (MS) study of PMVECs treated with METH for 4 h. Analysis of cell lysates demonstrated at least 5 OHMA metabolites (peaks 2–6). Panel A represents full-scan LC-MS chromatograms of treated and untreated samples. Panel B represents collision-induced dissociation chromatograms (MS2 m/z 166.2 @CID 35.00) of hydroxylated methamphetamine metabolites in the same samples. Panel C represents MS-MS spectra of METH hydroxymetabolites (peaks 2–6). Peak 1 is a nonspecific endogenous component also found in unstimulated PMVECs.
Although production of p-OHMA by PMVECs is likely driven by CYP2D6, the enzyme responsible for metabolizing METH in the liver (33), the role of CES1 in METH metabolism is unclear. It is important to point out that CES1 is an esterase known to break ester bonds in molecules; since METH does not have any ester bonds, it is unlikely to serve as a substrate for CES1. However, since CES1 was significantly reduced in METH-PAH, we sought to determine whether CES1 knockdown could affect METH metabolism in PMVECs. We reduced CES1 protein levels via siRNA transfection and exposed PMVECs treated with either control (siCt) or CES1 (siCES1) siRNA to METH for 4 h, followed by LC-MS. We found no difference in the METH metabolite profile of CES1 siRNA-treated PMVECs compared with nontransfected or siCt-transfected cells (data not shown).
It is important to note that our LC-MS looked only at direct METH metabolites (phase I reaction) and did not capture information regarding other metabolites (e.g., toxic esters) generated in cells when exposed to METH (36). Thus, although CES1 is not involved in phase I metabolism of METH, it could be required to neutralize toxic metabolites produced by METH metabolism.
METH-PAH Patients Demonstrate High Prevalence of a Potentially Pathogenic Variant in CES1
Patients who carry polymorphisms in CYP2D6 and CES1 that reduce enzymatic activity are at risk of drug-related toxicity due to accumulation of toxic by-products and tissue damage (15, 62, 63, 65), but whether METH-PAH patients are carriers of potential pathogenic polymorphisms in these two genes is unknown. Previously, we published a WES study looking at 12 patients with IPAH, where we found TopBp1 to be a novel gene modifier in PAH (12). Since then, we have expanded our WES analysis to include 18 unrelated METH-PAH patients who had undergone a complete diagnostic workup in our Pulmonary Hypertension Clinic over a 5-yr period, none of whom had any family history of PAH (Table 1). These patients reported an average weekly use of METH for 10 yr (range: 3 mo to 25 yr) and denied use of other illicit stimulants or anorexinogens. Our patient population was composed predominantly of females (n = 11, 62%) with a mean age of 47.8 ± 6.7 yr and a body mass index of 29.5 ± 3.8. Upon presentation, most patients were categorized as New York Heart Association functional class III (44.4%) and had documented a mean 6-min walk distance of 486 ± 145 m. All patients underwent right heart catheterization that showed an average mean right atrial pressure of 10.3 ± 6.3 mmHg, mean PAP of 51.8 ± 15.8 mmHg, mean PAWP of 11.4 ± 4.5 mmHg, mean cardiac output of 4.2 ± 1.0 l/min, and PVR of 10.7 ± 6.0 WU.
Table 1.
Patient characteristics of METH-PAH patients involved in WES
| METH-PAH Cohort (n = 18) | |
|---|---|
| Age, yr | 47.8 ± 6.7 |
| Sex, men/women (%) | 7/11 (62%) |
| BMI, kg/m2 | 29.5 ± 3.8 |
| NYHA symptom class, n (%) | |
| I | 3 (16.7) |
| II | 6 (33.3) |
| III | 8 (44.4) |
| IV | 1 (5.6) |
| 6MWD, m | 486 ± 145 |
| Therapies, n (%) | |
| Prostacyclin | 12 (66.7) |
| ERA | 12 (66.7) |
| PDE-I | 15 (83.3) |
| CCB | 2 (11.1) |
| Hemodynamics | |
| mRA, mmHg | 10.3 ± 6.3 |
| mPAP, mmHg | 51.8 ± 15.8 |
| PCWP, mmHg | 11.4 ± 4.5 |
| CO, l/min | 4.2 ± 1.0 |
| PVR, WU | 10.7 ± 6.0 |
Values represent means ± SD; METH, methamphetamine; PAH, pulmonary arterial hypertension; WES, whole exome sequencing; BMI, body mass index; NYHA, New York Heart Association; 6MWD, 6-min walk distance; ERA, endothelin-1 receptor antagonist; PDE-I , phosphodiesterase inhibitor; CCB, calcium channel blocker; mRA, mean right atrial pressure; mPAP, mean pulmonary artery pressures; PCWP, pulmonary capillary wedge pressure; CO, cardiac output; PVR, pulmonary vascular resistance; WU wood units.
After the WES data set of METH-PAH patients was filtered for synonymous variants, we found a total of 10,072 single nucleotide variants (SNV) and 737 insertions/deletions (i.e., indels) predicted to affect an estimated total of 1,767 genes. Review of WES data from IPAH and METH-PAH patients led to the identification of six potentially pathogenic CYP2D6 SNVs, which were predicted to produce missense variants in the protein (Table 2). Although clinically significant reduction in CYP2D6 enzymatic activity has been documented with rs1135840, rs16947, and rs5030867 (54), only the rs1135840 variant was slightly more prominent in METH-PAH (82.3%) compared with IPAH patients (64%). Regarding CES1, we found five SNVs, two of which were present in both METH-PAH and IPAH patients (Table 3). Although all three CES1 SNVs fall in highly conserved residues, rs115629050 was found exclusively in METH-PAH patients. Interestingly, this variant is predicted to produce a missense variant located within the active site of the enzyme, raising the possibility that it could adversely affect its detoxifying properties.
Table 2.
Predicted AA location and functional impact of CYP2D6 variants found in WES of iPAH and METH-PAH patients
| Allele Frequency, % |
||||||
|---|---|---|---|---|---|---|
| CYP2D6 SNV | Predicted Allele Frequency, % | IPAH | METH-PAH | Function | AA Switch | AA Position |
| rs5030867 | 0.05 | 9 | 0 | Missense | His→Pro | 324 |
| rs1058172 | 16.7 | 63.6 | 58.8 | Missense | Arg→His | 365 |
| rs16947 | 66 | 95 | 95 | Missense | Arg→Cys | 296 |
| rs1135840 | 64 | 64 | 82.3 | Missense | Ser→Thr | 486 |
| rs1135828 | 0.23 | 9.1 | 0 | Missense | Met→Lys | 279 |
| rs28371704 | 17 | 9 | 26.3 | Missense | His→Arg | 94 |
| rs28371703 | 16.3 | 9 | 10.5 | Missense | Leu→Met | 91 |
iPAH, idiopathic PAH; SNV, single nucleotide variant; AA, amino acid.
Table 3.
Predicted amino acid location and functional impact of CES1 variants found in WES of iPAH and METH-PAH patients
| Allele Frequency, % |
||||||
|---|---|---|---|---|---|---|
| CES1 SNV | Predicted Allele Frequency, % | PAH | METH-PAH | Function | AA Switch | AA Position |
| rs115629050 | 3.1 | 0 | 94.4 | Missense | Ala→Ser | 270 |
| rs3826193 | 35.6 | 64 | 94.4 | Missense | Ile→Val | 50 |
| rs3826192 | 32.6 | 64 | 94.4 | Missense | Val→Ile | 39 |
| rs62028647 | 42 | 72.7 | 0 | Missense | Ser→Leu | 82 |
| rs2307240 | 5 | 9 | 0 | Missense | Ser→Asn | 75 |
CES1, carboxylesterase 1.
The CES1 rs115629050 SNV is Associated With Reduced Enzymatic Activity and Susceptibility to METH-Induced Apoptosis
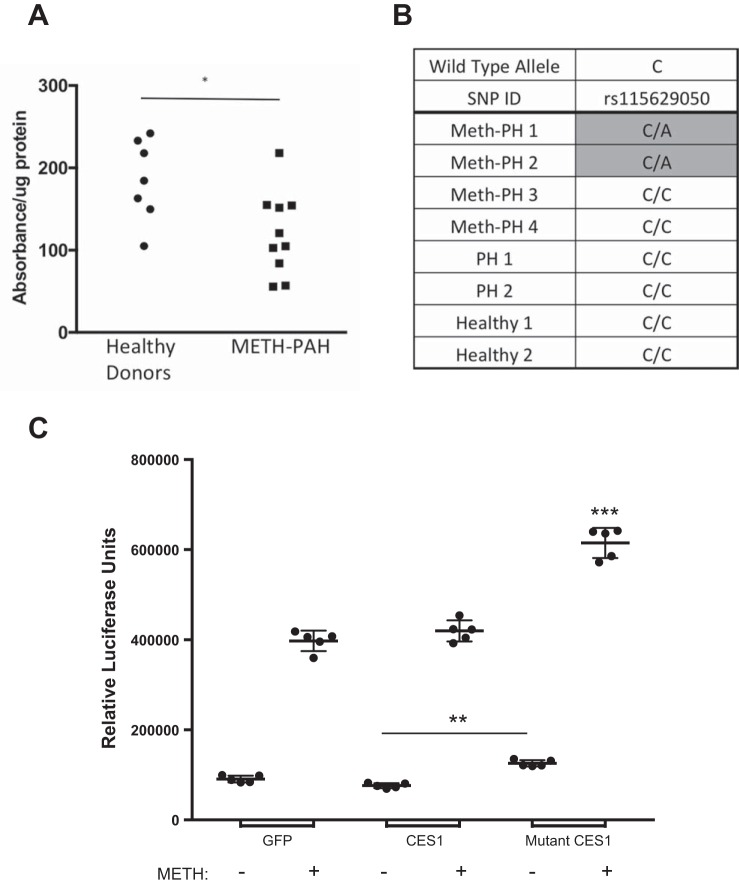
The CES1 SNVs known to reduce enzymatic activity have been associated with cocaine and methylphenidate toxicity, stressing the importance of intact enzymatic activity to protect against toxin-related injury (15, 65). To test whether METH-PAH carriers of the rs115629050 SNV exhibit reduced CES1 enzymatic activity, we isolated CES1 from PBMCs obtained from 10 METH-PAH patients that participated in our WES analysis and measured enzymatic activity by quantifying the rate of 4-NP hydrolysis, a well-established method to quantify CES1 activity in cells and tissues (45, 60). After controlling for protein amount, we found that CES1 activity was significantly reduced in CES1 rs115629050 SNV-positive METH-PAH patients compared with healthy donors (Fig. 4A).
Fig. 4.
CES1 rs115629050 variant is associated with reduced enzymatic activity and increased apoptosis in unstimulated and METH-exposed PMVECs. A: CES1 activity assay of peripheral blood mononuclear cells from healthy donors (wild type) and METH-PAH patients carrying rs115629050. *P < 0.05, unpaired t-test. B: allelic discrimination assay for rs115629050 in gDNA samples extracted from lung tissue paraffin blocks (see methods). C: caspase 3/7 activity assay of PMVECs transfected with either GFP, wild-type (CES1), or rs115629050 mutant (mutant CES1) plasmids. **P < 0.01 and ***P < 0.001 vs. all other groups; ordinary 1-way ANOVA with Tukey’s multiple-comparison test.
Next, we sought to assess whether the rs115629050 CES1 SNV was also present in explanted lung tissues of the four METH-PAH patients presented in Fig. 1. We chose these four patients since they were not part of the WES analysis cohort and would serve as a validation cohort for detection of the CES1 SNV. We performed an allelic discrimination assay to screen for the rs115629050 CES1 SNV in DNA extracted from the lung sections. Our analysis demonstrated that two of these four patients (50%) were positive for the rs115629050, whereas both controls, two IPAH, and the remaining two METH-PAH patients were negative for this SNV (Fig. 4B). With this evidence, we sought to characterize the biological impact of the rs115629050 CES1 mutant allele on PMVEC survival after METH exposure.
Apoptosis is a major consequence of METH exposure and is associated with neuronal cell death and disruption of the endothelial blood-brain barrier (34, 66), but no study has looked at PMVECs. To test this, we obtained a plasmid containing a wild-type (WT) CES1 construct and generated the rs115629050 mutant using site-directed mutagenesis. In the presence of 5 mM METH, PMVECs transfected with the WT CES1 construct demonstrated levels of caspase 3/7 activity similar to cells transfected with the empty vector (Fig. 4C). In contrast, cells transfected with the mutant CES1 plasmid were more vulnerable to METH-induced apoptosis, as evidenced by significantly higher caspase 3/7 activity (Fig. 4C). Interestingly, in the absence of METH, cells transfected with mutant CES1 had a significant increase in caspase 3/7 activity as well as a reduced number of viable cells 24 h after transfection (data not shown), suggesting that either CES1 deficiency or reduced activity can also reduce cell viability. Taken together, these studies support a protective role for CES1 in PMVECs exposed to METH, and the CES1 rs115629050 variant appears to reduce this protection.
CES1 Reduction Leads to Increased ROS Production and Abnormal Autophagy Flux in METH Exposed PMVECs
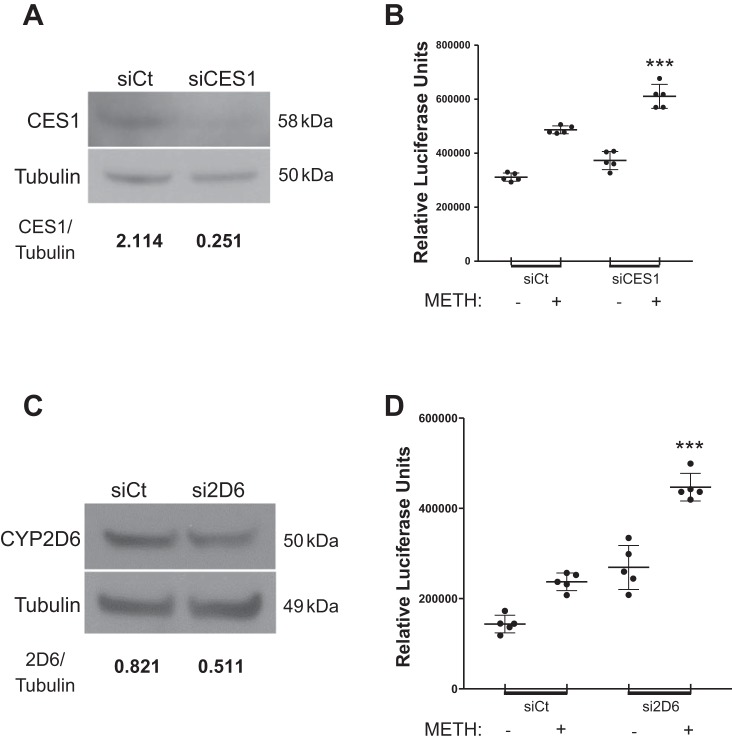
Given reduced expression of CES1 in the endothelium of vascular lesions in all four METH-PAH patients, we decided to characterize the consequences of reduced CES1 expression on PMVEC survival with METH exposure. Similarly to PMVECs transfected with the mutant CES1 plasmid, CES1 siRNA treated PMVECs (Fig. 5A) demonstrate a significant increase in caspase 3/7 activation in response to 5 mM METH as well as a modest increase at baseline (Fig. 5B). CYP2D6 siRNA-treated PMVECs (Fig. 5C) also had significantly more caspase 3/7 activation in both the METH-exposed and nonexposed groups (Fig. 5D). This led us to conclude that reduction in either CES1 expression or activity results in reduced PMVEC viability that is further compounded by METH exposure.
Fig. 5.
CES1- and CYP2D6-deficient PMVECs have increased susceptibility to apoptosis. A: Western blot (WB)for CES1 in control (siCt) or CES siRNA-transfected healthy human PMVECs. B: caspase 3/7 activity assays of siCt and CES1 siRNA-transfected PMVECs at baseline and following METH (5 mM) exposure for 4 h. C and D: WB (C) and caspase 3/7 assay (D) for CYP2D6 in siCt or CYP2D6 (si2D6) siRNA-transfected healthy human PMVECs. Numbers under WB represent densitometry ratio of signal vs. tubulin from 3 independent studies. ***P < 0.001 vs. siCt + METH, ordinary 1-way ANOVA with Tukey’s multiple-comparison test.
We next proceeded to explore the mechanism by which CES1 regulates PMVEC survival against METH. Studies in neurons and brain endothelial cells have shown that METH triggers production of reactive oxygen species (ROS) (11, 50) that results in activation of the autophagy response (3, 42, 44, 64). Autophagy is a process by which the cell degrades and recycles damaged cytoplasmic components in an effort to help the cell cope with stress and restore homeostasis; should these efforts fail, the apoptosis cascade is triggered, and cell death ensues. We sought to document whether these events also take place in PMVECs in response to METH exposure and whether loss of CES1 compromises autophagy.
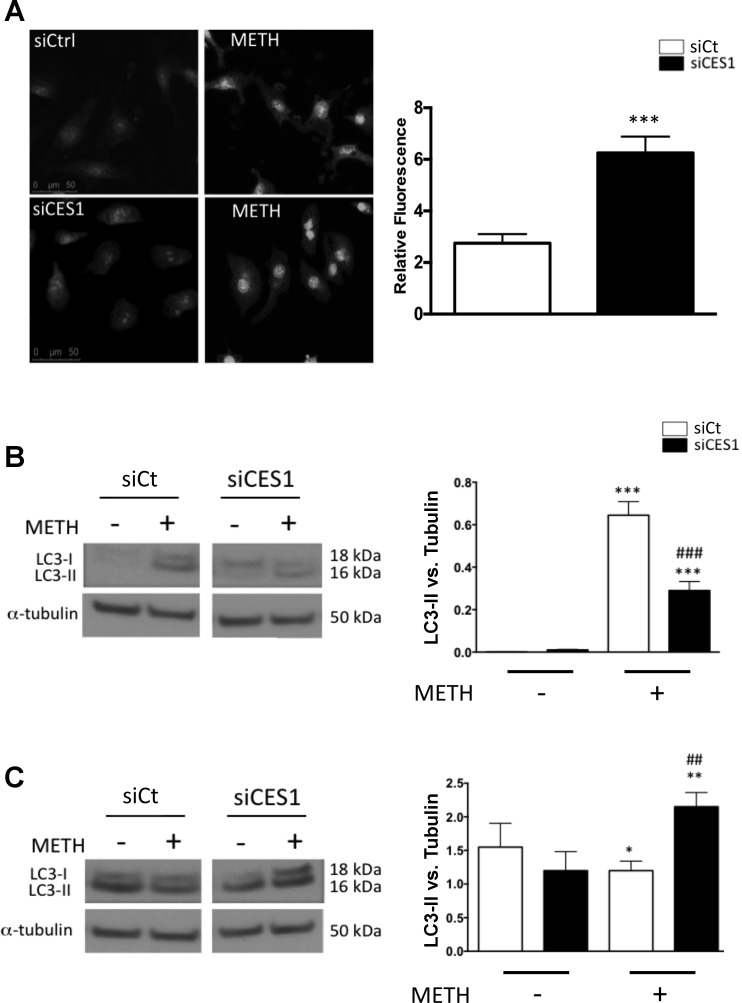
Following METH treatment, we saw an increase in cytoplasmic ROS in both cell groups; however, intensity of ROS signal was significantly stronger in CES1 siRNA-treated PMVECs (Fig. 6A). Interestingly, the cytoplasm of METH-exposed cells exhibited the presence of numerous vacuoles, which appear to be more numerous in CES1 siRNA-treated PMVECs. Vacuolization has been documented previously in neurons and endothelial cells exposed to METH and appears to correlate pathologically to organelle swelling and autophagy. We assessed autophagy by measuring autophagic flux, an approach that captures the entire process of autophagy, including the delivery of cargo to lysosomes and its subsequent breakdown, through changes in cellular levels of LC3, a cytosolic protein that at baseline (LC3-I) exists as an 18-kDa form but, during autophagy, is conjugated to form LC3-II and recruited to autophagosomes. In control cells, METH treatment resulted in an increase of LC3-II (Fig. 6B, left); however, CES1 siRNA-treated PMVECs demonstrated a significant LC3-II reduction (Fig. 6B, right).
Fig. 6.
CES1-deficient PMVECs demonstrate reduced autophagy in response to METH. A: nonexposed and 4-h METH exposed control (siCt) and CES1 (siCES1) siRNA-treated cells were fixed and stained with CellRox green. Fluorescence was measured relative to Ctrl-no METH. ***P < 0.001, unpaired t-test. B: WB of unstimulated and 4-h-METH control (Ctrl) and siCES1-treated cells for LC3. C: WB of bafilomycin-treated unstimulated and 4-h-METH treated Ctrl and siCES1 cells for LC3. Densitometry is measured relative to tubulin as a loading control. In B and C: *P < 0.05, **P < 0.01, and ***P < 0.001 vs. siCt no METH; ##P < 0.01, ###P < 0.001 vs. corresponding control; 1-way ANOVA with Bonferroni posttest (n = 3).
To determine whether the changes in LC3-II are determined by changes in autophagic flux, we treated cells with bafilomycin A1, an antibiotic that prevents degradation of autophagosomes by reducing their fusion with lysosomes (35). In control cells, bafilomycin alone resulted in an increase in LC3-II, whereas the addition of METH resulted in a mild reduction in LC3-II (Fig. 6C, left). In CES1 siRNA-treated PMVECs, we also saw an increase in LC3-II with bafilomycin alone, but concomitant exposure to METH led to a significant increase in LC3-II (Fig. 6C, right).
Taken together, these results support activation of autophagy by METH that is altered in CES1-deficient PMVECs by changes in the autophagic flux. Based on these findings, we conclude that CES1 is required for normal autophagy, which could explain the increased susceptibility of PMVECs to apoptosis in the setting of METH exposure (Fig. 7).
Fig. 7.
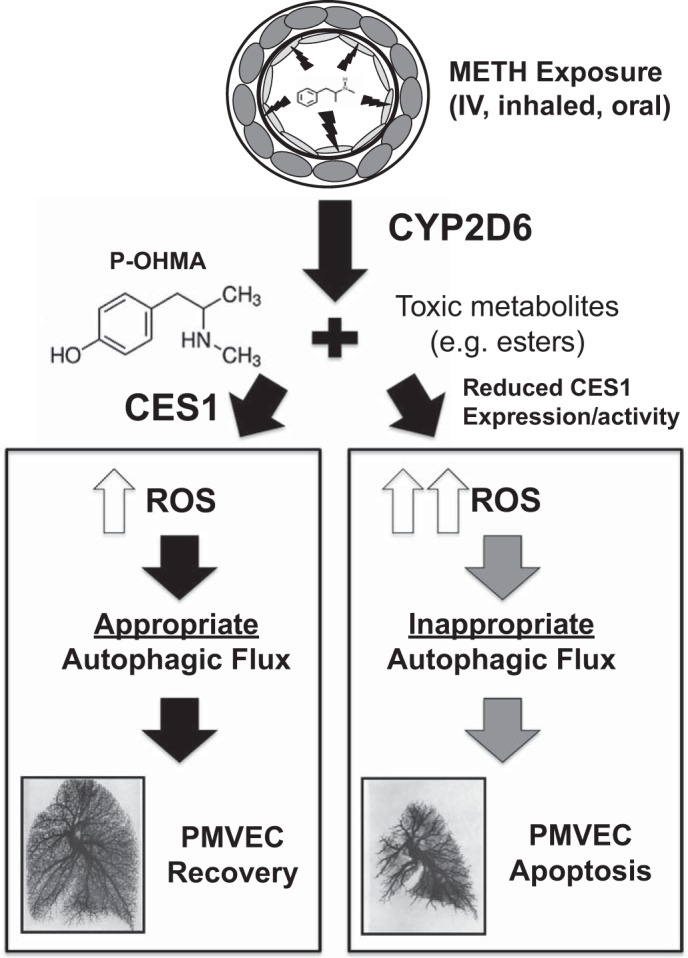
Proposed model. METH enters PMVECs and is metabolized to p-OHMA and other toxic metabolites, which trigger reactive oxygen species (ROS) production and an autophagy response. CES1 (green) is required to reduce ROS production and maintain steady autophagy flux. Reduced CES1 expression and/or activity (red) result in increased ROS production and abnormal autophagy flux, resulting in increased PMVEC apoptosis and loss of pulmonary microvessels.
DISCUSSION
Originally developed as a therapy for a variety of common clinical disorders such as obesity and Parkinson’s disease, illegal manufacture and use of METH has risen worldwide over the past two decades (7, 22, 48). The United Nations Office on Drugs and Crime reported in 2007 that ~24.7 million people worldwide were addicted to METH, making this a serious public health problem. In 2008, the US government reported that 13 million people over the age of 12 have used METH at some point and that 529,000 are estimated to be regular users. The popularity of METH stems from the fact that it can be easily produced from commercially available reagents in local households (a.k.a. METH laboratories or kitchens) and its relatively low costs compared with other illicit stimulants. Because of its highly addictive nature, METH can easily become a drug of abuse with a tendency to ruin the lives and productivity of young adults currently in high school and college. At the cost of experiencing transient euphoria and subjective bliss, METH abusers are at risk of severe health problems such as HIV infection, liver damage, stroke, and premature cardiac diseases. As one of the largest pulmonary hypertension centers in California, we have seen a disturbing rise in the number of patients being diagnosed with METH-PAH (24), forcing us to expand the research efforts dedicated to understanding the clinical history and mechanism of this devastating form of PAH. This study represents, to our knowledge, the first effort to identify candidate genes in METH-PAH and has resulted in three important discoveries: 1) PMVECs are capable of metabolizing METH, 2) CYP2D6 and CES1 are expressed in the lung vessels and appear to be necessary for PMVEC protection and 3) reduced CES1 activity can increase susceptibility to METH-induced apoptosis. Since apoptosis contributes to small vessel loss, it is possible that loss of CES1 expression and/or activity could contribute to the development of METH-PAH in susceptible individuals.
CES1 belongs to a family of esterases expressed predominantly in the liver, where they are engaged in drug and cholesterol metabolism (38). Interest in CES1 biochemistry has piqued considerably in the last 20 yr, as allosteric modulators and/or compounds that enhance CES1 activity could serve as therapies for organophosphate poisoning and to treat victims of chemical warfare (60). In our study, we found that both CES1 expression and enzymatic activity appear reduced in METH-PAH and could play parallel roles in compromising the capacity of this enzyme for protecting the endothelium against environmental toxins such as METH. Although it could be possible that the two variants found in the NH2-terminal domain of CES1 (rs3826193 and rs3826192) could compromise stability or appropriate localization of the translated protein to the ER, another possibility is epigenetic suppression secondary to METH, which has been described to occur in other cells (30, 41). On the other hand, pathogenic variants that reduce CES1 activity are associated with the risk of life-threatening adverse drug reactions, as seen in patients treated with amantadine and users of illicit drugs such as heroin and cocaine (65). Although methylphenidate is a well-established substrate of CES1, this is the first time to our knowledge that METH toxicity has been associated with this enzyme. How CES1 assists in neutralizing METH toxicity in the pulmonary endothelium is unclear at this time. Our LC-MS study argues against direct involvement of CES1 in METH metabolism, which could be predicted on account that the METH molecule does not have esters or amide bonds that could serve as substrate for CES1 (57). That being said, recent metabolomics studies have shown that METH can induce production of many metabolites in brain (36, 61); therefore, it is possible to predict that METH could trigger the generation of ester or amide containing toxic metabolites in PMVECs that, in the absence of CES1 activity, may result in cell injury. In support of this, studies in dopaminergic cells demonstrate that METH triggers production of 4-hydroxy-2-nonelal, an oxidative byproduct of polyunsaturated fatty acids that can trigger production of cytotoxic esters (4, 8). As mentioned in our results, LC-MS provides only qualitative information on phase 1 metabolism; a more comprehensive metabolomics study will be required to determine whether CES1 is required to buffer production of toxic esters.
Whole exome sequencing (WES) is a next-generation sequencing technique that focuses on the coding sequences throughout the genome, which has accelerated the discovery of novel variants associated with both Mendelian and non-Mendelian disorders. It is relevant to point out that we found two METH-PH patients (10.5%) who were carriers of BMPR2 missense variant (rs2228545), which targets a residue in the cytoplasmic tail. This SNV has been associated with increased risk of colorectal cancer and has been documented to occur in IPAH patients (16). In addition, other variants in several genes associated with the BMP and TGFβ signaling pathways were documented across the entire population (Table 4). To date, no study has firmly established an association between BMPR2 variants and D + T PAH, although studies performed on patients with fenfluramine-associated PAH revealed that 9% of patients demonstrated pathogenic variants in BMPR2 (29). It will be interesting to determine whether screening larger patient populations could serve to further establish whether a functional link exists between CES1 and BMPR2 in METH-PAH.
Table 4.
Variants found in genes involved in the BMP signaling pathway via WES
| Gene | SNV | Allele Frequency, % | Function | AA Switch | AA Position | Affected Carriers |
|---|---|---|---|---|---|---|
| BMPR2 | rs2228545 | 3.92 | Missense | Ser→Asn | 775 | 2 |
| BMP3 | rs6831040 | 1.0 | Missense | Leu→Phe | 205 | 18 |
| BMP1 | rs11996036 | 5.7 | Missense | Val→Ile | 719 | 1 |
| BMPR1A | rs3182217 | 35.6 | Missense | Pro→Thr | 657 | 7 |
| BMP4 | rs17563 | 55.6 | Missense | Val→Ala | 152 | 16 |
| SMAD9 | rs111748421 | 0.23 | Missense | Leu→Pro | 22 | 1 |
| SMAD3 | rs35874463 | 5.2 | Missense | Ile→Val | 170 | 1 |
| SMAD7 | rs3764482 | 19.15 | Missense | Ser→Phe | 28 | 4 |
BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; SMAD, S-mother against decapentaplegic.
METH-induced cytotoxicity is linked to oxidative injury by its direct activation of NAPDH oxidase and generation of ROS that cause cellular damage (42). Autophagy is part of an effort to promote cell repair and preserve cell viability in response to oxidative stress; however, if the cells cannot recover, autophagy can initiate proapoptotic signaling cascades. Therefore, activation of autophagy is a double-edged sword that is vulnerable to changes in protein composition of the ER. Our observation of the increased number of vacuoles in CES1-deficient cells raises several interesting mechanistic possibilities that could provide insight into the fundamental mechanism of METH-induced PMVEC apoptosis. Based on our findings, it appears that CES1 deficiency tilts the balance of autophagic flux toward apoptosis in response to METH exposure. One possible explanation is that CES1 could be facilitating the capacity of the ER to contribute to production of autophagosomes and/or alter lysosome function under METH, since CES1 is expressed in both organelles (2, 17, 40, 56).
Our studies demonstrate that the reduction in CES1 expression and activity increases PMVEC apoptosis alone and in response to METH, but several questions remain concerning the mechanism behind METH-induced ROS generation, initiation of ER stress, and possible impairment of mitochondrial bioenergetics, a well-established feature of other forms of PAH (14, 37, 46). It is worth pointing out that mitochondrial toxicity is also a consequence of METH exposure and could be linked to the autophagy response, as these pathways can also interact with the mitochondria to trigger release of cytochrome c and other proapoptotic factors (52, 59). In light of the link between our other gene candidates (HERPUD1, AKAP1) and the mitochondria, we propose to focus future studies on understanding the role played by the mitochondria in integrating the autophagy responses to METH-induced injury. Whether pharmacological agents that restore CES1 or the autophagy response could be clinically relevant in the treatment of METH-PAH remains to be determined.
Although we have focused mainly on the characterization of CES1 variants in this study, it is important to stress that CYP2D6 remains a critical candidate that requires more comprehensive characterization. Besides being the major player in METH metabolism, CYP2D6 is also required for metabolizing anorexinogens linked to PAH, such as dexfenfluramine, and variants that reduce activity could increase toxicity from these compounds (23, 27). Our WES revealed several candidate CYP2D6 variants, some of which occurred with increased frequency in both PAH and METH-PAH patients, but it is likely that these are influenced by our small patient number values and lack of METH users, without evidence of cardiopulmonary disease to serve as the proper reference. Our group has initiated discussions with drug rehabilitation programs in California to start collecting clinical data and blood samples to perform future WES studies in this important control population. Another important limitation is the lack of METH-PAH PMVECs available for in vitro studies. Unfortunately, we were only able to establish a cell culture from one of the four patients, as the others failed to grow in vitro. Furthermore, PMVEC cultures from this single METH-PAH could endure only four passages, as their growth progressively slowed down. It is worth speculating why these cells may have less growth potential compared with other PAH isolates, as it could provide critical insight into unique phenotypical and molecular attributes of METH-PAH cells and how they could be affecting cell survival. To overcome this obstacle, our group has started using inducible pluripotent stem cells derived from skin biopsies and PBMCs to generate endothelial cells (iPSC-ECs), a promising approach that will hopefully allow us to expand our mechanistic studies of METH-PAH.
In conclusion, we propose a model by which reduced expression and/or reduced CES1 activity could predispose to PAH by increasing susceptibility of PMVECs to METH-induced apoptosis (Fig. 7). CES1 holds potential as a biomarker that could be used to identify METH users at risk of developing PAH and as a potential candidate target for agents capable of restoring expression and/or activity, which could then help increase cell viability and reduce disease progression in this population. It is our hope that characterizing CES1 and other genes identified by WES will allow us to increase our chances of identifying high-risk individuals and improve our capacity to better care for those who suffer from this devastating disease. Although the findings presented here are pertinent to METH-PAH, we anticipate that our findings will serve to open the field for investigations that will seek to uncover deeper links between gene interactions and other relevant exposures associated with D + T PAH.
GRANTS
This work was supported by a seed grant from the Vera Moulton Wall Center, a career development award from the Robert Wood Johnson Foundation, a National Heart, Lung, and Blood Institute (NHLBI) Award (R01-HL-134776, K08-HL-105884-01), and a Pulmonary Hypertension Association, American Lung Association Biomedical Research Grants, and American Heart Association Beginning Grant in Aid to V. de Jesus Perez. R. T. Zamanian is supported by funds from the NHLBI, the National Institute of Allergy and Infectious Diseases, and the Vera Moulton Wall Center. This work was partially funded by the National Institute of Environmental Health Sciences (NIEHS; P42-ES-04699). S. D. Kodani was supported by a National Institute of General Medical Sciences-funded Pharmacology Training Program Grant (T32-GM-099608). B. Hammock was supported by an NIEHS Grant (R01-ES-002710).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
M.E.O., A.K., E.A.S., K.Y., I.Y.C., S.D.K., C.M., L.A., G.B., and V.A.d.J.P. performed experiments; M.E.O., A.K., E.A.S., K.Y., I.Y.C., S.D.K., C.M., B.D.H., L.A., T.-P.A., R.T.Z., and V.A.d.J.P. analyzed data; M.E.O., A.K., E.A.S., K.Y., I.Y.C., S.D.K., C.M., B.D.H., L.A., T.-P.A., G.B., R.T.Z., and V.A.d.J.P. interpreted results of experiments; M.E.O., E.A.S., R.T.Z., and V.A.d.J.P. prepared figures; M.E.O., I.Y.C., R.T.Z., and V.A.d.J.P. drafted manuscript; M.E.O., K.Y., I.Y.C., S.D.K., C.M., B.D.H., L.A., T.-P.A., R.T.Z., and V.A.d.J.P. edited and revised manuscript; M.E.O., A.K., K.Y., I.Y.C., S.D.K., C.M., B.D.H., L.A., T.-P.A., R.T.Z., and V.A.d.J.P. approved final version of manuscript; E.A.S., E.M.H., and R.T.Z. conceived and designed research.
ACKNOWLEDGMENTS
Lung tissues from IPAH and control patients were provided by the Pulmonary Hypertension Breakthrough Initiative, which is funded by the Cardiovascular Medical Research and Education Fund (CMREF) and managed at Stanford by Drs. Marlene Rabinovitch and Roham T. Zamanian. The tissues were procured at the Transplant Procurement Centers at Stanford University, Cleveland Clinic, and Allegheny General Hospital, and de-identified patient data were obtained via the Data Coordinating Center at the University of Michigan. We thank all patients and their proxies who participated in this study. We are also grateful to Patricia Angeles del Rosario for helping with the collection and processing of blood samples and Andrew Hsi for helping organize the patient database.
REFERENCES
- 1.Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G, Bégaud B; International Primary Pulmonary Hypertension Study Group . Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med 335: 609–616, 1996. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 2.Amouzadeh HR, Bourdi M, Martin JL, Martin BM, Pohl LR. UDP-glucose:glycoprotein glucosyltransferase associates with endoplasmic reticulum chaperones and its activity is decreased in vivo by the inhalation anesthetic halothane. Chem Res Toxicol 10: 59–63, 1997. doi: 10.1021/tx9601364. [DOI] [PubMed] [Google Scholar]
- 3.Beauvais G, Atwell K, Jayanthi S, Ladenheim B, Cadet JL. Involvement of dopamine receptors in binge methamphetamine-induced activation of endoplasmic reticulum and mitochondrial stress pathways. PLoS One 6: e28946, 2011. doi: 10.1371/journal.pone.0028946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borazjani A, Edelmann MJ, Hardin KL, Herring KL, Allen Crow J, Ross MK. Catabolism of 4-hydroxy-2-trans-nonenal by THP1 monocytes/macrophages and inactivation of carboxylesterases by this lipid electrophile. Chem Biol Interact 194: 1–12, 2011. doi: 10.1016/j.cbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenot F, Herve P, Petitpretz P, Parent F, Duroux P, Simonneau G. Primary pulmonary hypertension and fenfluramine use. Br Heart J 70: 537–541, 1993. doi: 10.1136/hrt.70.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruxel EM, Salatino-Oliveira A, Genro JP, Zeni CP, Polanczyk GV, Chazan R, Rohde LA, Hutz MH. Association of a carboxylesterase 1 polymorphism with appetite reduction in children and adolescents with attention-deficit/hyperactivity disorder treated with methylphenidate. Pharmacogenomics J 13: 476–480, 2013. doi: 10.1038/tpj.2012.25. [DOI] [PubMed] [Google Scholar]
- 7.Calcaterra S, Binswanger IA. National trends in psychostimulant-related deaths: 1999-2009. Subst Abus 34: 129–136, 2013. doi: 10.1080/08897077.2012.726959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandramani Shivalingappa P, Jin H, Anantharam V, Kanthasamy A, Kanthasamy A. N-Acetyl Cysteine Protects against Methamphetamine-Induced Dopaminergic Neurodegeneration via Modulation of Redox Status and Autophagy in Dopaminergic Cells. Parkinsons Dis 2012: 424285, 2012. doi: 10.1155/2012/42428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherner M, Bousman C, Everall I, Barron D, Letendre S, Vaida F, Atkinson JH, Heaton R, Grant I; HNRC Group . Cytochrome P450-2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: preliminary findings. J Int Neuropsychol Soc 16: 890–901, 2010. doi: 10.1017/S1355617710000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest 130: 1657–1663, 2006. doi: 10.1378/chest.130.6.1657. [DOI] [PubMed] [Google Scholar]
- 11.da Silva DD, Silva E, Carmo H. Combination effects of amphetamines under hyperthermia - the role played by oxidative stress. J Appl Toxicol 34: 637–650, 2014. doi: 10.1002/jat.2889. [DOI] [PubMed] [Google Scholar]
- 12.de Jesus Perez VA, Yuan K, Lyuksyutova MA, Dewey F, Orcholski ME, Shuffle EM, Mathur M, Yancy L Jr, Rojas V, Li CG, Cao A, Alastalo TP, Khazeni N, Cimprich KA, Butte AJ, Ashley E, Zamanian RT. Whole-exome sequencing reveals TopBP1 as a novel gene in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 189: 1260–1272, 2014. doi: 10.1164/rccm.201310-1749OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Torre R, Yubero-Lahoz S, Pardo-Lozano R, Farré M. MDMA, methamphetamine, and CYP2D6 pharmacogenetics: what is clinically relevant? Front Genet 3: 235, 2012. doi: 10.3389/fgene.2012.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunham-Snary KJ, Hong ZG, Xiong PY, Del Paggio JC, Herr JE, Johri AM, Archer SL. A mitochondrial redox oxygen sensor in the pulmonary vasculature and ductus arteriosus. Pflugers Arch 468: 43–58, 2016. doi: 10.1007/s00424-015-1736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duysen EG, Lockridge O. Prolonged toxic effects after cocaine challenge in butyrylcholinesterase/plasma carboxylesterase double knockout mice: a model for butyrylcholinesterase-deficient humans. Drug Metab Dispos 39: 1321–1323, 2011. doi: 10.1124/dmd.111.039917. [DOI] [PubMed] [Google Scholar]
- 16.Elliott CG, Glissmeyer EW, Havlena GT, Carlquist J, McKinney JT, Rich S, McGoon MD, Scholand MB, Kim M, Jensen RL, Schmidt JW, Ward K. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation 113: 2509–2515, 2006. doi: 10.1161/CIRCULATIONAHA.105.601930. [DOI] [PubMed] [Google Scholar]
- 17.Funakoshi-Hirose I, Aki T, Unuma K, Funakoshi T, Noritake K, Uemura K. Distinct effects of methamphetamine on autophagy-lysosome and ubiquitin-proteasome systems in HL-1 cultured mouse atrial cardiomyocytes. Toxicology 312: 74–82, 2013. doi: 10.1016/j.tox.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barberà J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol Ç, Falk V, Funck-Brentano C, Gorenflo M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Völler H, Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37: 67–119, 2016. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 20.Galiè N, Simonneau G. The Fifth World Symposium on Pulmonary Hypertension. J Am Coll Cardiol 62, Suppl: D1–D3, 2013. doi: 10.1016/j.jacc.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Gotway MB, Marder SR, Hanks DK, Leung JW, Dawn SK, Gean AD, Reddy GP, Araoz PA, Webb WR. Thoracic complications of illicit drug use: an organ system approach. Radiographics 22: S119–S135, 2002. doi: 10.1148/radiographics.22.suppl_1.g02oc01s119. [DOI] [PubMed] [Google Scholar]
- 22.Greberman SB, Wada K. Social and legal factors related to drug abuse in the United States and Japan. Public Health Rep 109: 731–737, 1994. [PMC free article] [PubMed] [Google Scholar]
- 23.Gross AS, Phillips AC, Rieutord A, Shenfield GM. The influence of the sparteine/debrisoquine genetic polymorphism on the disposition of dexfenfluramine. Br J Clin Pharmacol 41: 311–317, 1996. doi: 10.1046/j.1365-2125.1996.03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruenewald PJ, Ponicki WR, Remer LG, Waller LA, Zhu L, Gorman DM. Mapping the spread of methamphetamine abuse in California from 1995 to 2008. Am J Public Health 103: 1262–1270, 2013. doi: 10.2105/AJPH.2012.300779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guidice JM, Marez D, Sabbagh N, Legrand-Andreoletti M, Spire C, Alcaïde E, Lafitte JJ, Broly F. Evidence for CYP2D6 expression in human lung. Biochem Biophys Res Commun 241: 79–85, 1997. doi: 10.1006/bbrc.1997.7775. [DOI] [PubMed] [Google Scholar]
- 26.Gurney MA, Huang C, Ramil JM, Ravindran N, Andres AM, Sin J, Linton PJ, Gottlieb RA. Measuring cardiac autophagic flux in vitro and in vivo. Methods Mol Biol 1219: 187–197, 2015. doi: 10.1007/978-1-4939-1661-0_14. [DOI] [PubMed] [Google Scholar]
- 27.Haritos VS, Ching MS, Ghabrial H, Gross AS, Taavitsainen P, Pelkonen O, Battaglia SE, Smallwood RA, Ahokas JT. Metabolism of dexfenfluramine in human liver microsomes and by recombinant enzymes: role of CYP2D6 and 1A2. Pharmacogenetics 8: 423–432, 1998. doi: 10.1097/00008571-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Hatfield MJ, Umans RA, Hyatt JL, Edwards CC, Wierdl M, Tsurkan L, Taylor MR, Potter PM. Carboxylesterases: General detoxifying enzymes. Chem Biol Interact 259: 327–331, 2016. doi: 10.1016/j.cbi.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humbert M, Deng Z, Simonneau G, Barst RJ, Sitbon O, Wolf M, Cuervo N, Moore KJ, Hodge SE, Knowles JA, Morse JH. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J 20: 518–523, 2002. doi: 10.1183/09031936.02.01762002. [DOI] [PubMed] [Google Scholar]
- 30.Itzhak Y, Ergui I, Young JI. Long-term parental methamphetamine exposure of mice influences behavior and hippocampal DNA methylation of the offspring. Mol Psychiatry 20: 232–239, 2015. doi: 10.1038/mp.2014.7. [DOI] [PubMed] [Google Scholar]
- 31.Krupp M, Marquardt JU, Sahin U, Galle PR, Castle J, Teufel A. RNA-Seq Atlas--a reference database for gene expression profiling in normal tissue by next-generation sequencing. Bioinformatics 28: 1184–1185, 2012. doi: 10.1093/bioinformatics/bts084. [DOI] [PubMed] [Google Scholar]
- 32.Laizure SC, Herring V, Hu Z, Witbrodt K, Parker RB. The role of human carboxylesterases in drug metabolism: have we overlooked their importance? Pharmacotherapy 33: 210–222, 2013. doi: 10.1002/phar.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin LY, Di Stefano EW, Schmitz DA, Hsu L, Ellis SW, Lennard MS, Tucker GT, Cho AK. Oxidation of methamphetamine and methylenedioxymethamphetamine by CYP2D6. Drug Metab Dispos 25: 1059–1064, 1997. [PubMed] [Google Scholar]
- 34.Ma J, Wan J, Meng J, Banerjee S, Ramakrishnan S, Roy S. Methamphetamine induces autophagy as a pro-survival response against apoptotic endothelial cell death through the Kappa opioid receptor. Cell Death Dis 5: e1099, 2014. doi: 10.1038/cddis.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauvezin C, Neufeld TP. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 11: 1437–1438, 2015. doi: 10.1080/15548627.2015.1066957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClay JL, Adkins DE, Vunck SA, Batman AM, Vann RE, Clark SL, Beardsley PM, van den Oord EJ. Large-scale neurochemical metabolomics analysis identifies multiple compounds associated with methamphetamine exposure. Metabolomics 9: 392–402, 2013. doi: 10.1007/s11306-012-0456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 115: 1479–1491, 2005. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merali Z, Ross S, Paré G. The pharmacogenetics of carboxylesterases: CES1 and CES2 genetic variants and their clinical effect. Drug Metabol Drug Interact 29: 143–151, 2014. doi: 10.1515/dmdi-2014-0009. [DOI] [PubMed] [Google Scholar]
- 39.Nemoda Z, Angyal N, Tarnok Z, Gadoros J, Sasvari-Szekely M. Carboxylesterase 1 gene polymorphism and methylphenidate response in ADHD. Neuropharmacology 57: 731–733, 2009. doi: 10.1016/j.neuropharm.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Nousiainen U, Törrönen R. Differentiation of microsomal and cytosolic carboxylesterases in the rat liver by in vivo and in vitro inhibition. Gen Pharmacol 15: 223–227, 1984. doi: 10.1016/0306-3623(84)90163-0. [DOI] [PubMed] [Google Scholar]
- 41.Numachi Y, Shen H, Yoshida S, Fujiyama K, Toda S, Matsuoka H, Sora I, Sato M. Methamphetamine alters expression of DNA methyltransferase 1 mRNA in rat brain. Neurosci Lett 414: 213–217, 2007. doi: 10.1016/j.neulet.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 42.Park M, Hennig B, Toborek M. Methamphetamine alters occludin expression via NADPH oxidase-induced oxidative insult and intact caveolae. J Cell Mol Med 16: 362–375, 2012. doi: 10.1111/j.1582-4934.2011.01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122: 4306–4313, 2012. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramirez SH, Potula R, Fan S, Eidem T, Papugani A, Reichenbach N, Dykstra H, Weksler BB, Romero IA, Couraud PO, Persidsky Y. Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab 29: 1933–1945, 2009. doi: 10.1038/jcbfm.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rayo J, Muñoz L, Rosell G, Hammock BD, Guerrero A, Luque FJ, Pouplana R. Reactivity versus steric effects in fluorinated ketones as esterase inhibitors: a quantum mechanical and molecular dynamics study. J Mol Model 16: 1753–1764, 2010. doi: 10.1007/s00894-010-0807-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rehman J, Archer SL. A proposed mitochondrial-metabolic mechanism for initiation and maintenance of pulmonary arterial hypertension in fawn-hooded rats: the Warburg model of pulmonary arterial hypertension. Adv Exp Med Biol 661: 171–185, 2010. doi: 10.1007/978-1-60761-500-2_11. [DOI] [PubMed] [Google Scholar]
- 47.Rich S, Rubin L, Walker AM, Schneeweiss S, Abenhaim L. Anorexigens and pulmonary hypertension in the United States: results from the surveillance of North American pulmonary hypertension. Chest 117: 870–874, 2000. doi: 10.1378/chest.117.3.870. [DOI] [PubMed] [Google Scholar]
- 48.Richards JR, Bretz SW, Johnson EB, Turnipseed SD, Brofeldt BT, Derlet RW. Methamphetamine abuse and emergency department utilization. West J Med 170: 198–202, 1999. [PMC free article] [PubMed] [Google Scholar]
- 49.Sa S, Gu M, Chappell J, Shao NY, Ameen M, Elliott KA, Li D, Grubert F, Li CG, Taylor S, Cao A, Ma Y, Fong R, Nguyen L, Wu JC, Snyder MP, Rabinovitch M. Induced Pluripotent Stem Cell Model of Pulmonary Arterial Hypertension Reveals Novel Gene Expression and Patient Specificity. Am J Respir Crit Care Med 195: 930–941, 2017. doi: 10.1164/rccm.201606-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sajja RK, Rahman S, Cucullo L. Drugs of abuse and blood-brain barrier endothelial dysfunction: A focus on the role of oxidative stress. J Cereb Blood Flow Metab 36: 539–554, 2016. doi: 10.1177/0271678X15616978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaiberger PH, Kennedy TC, Miller FC, Gal J, Petty TL. Pulmonary hypertension associated with long-term inhalation of “crank” methamphetamine. Chest 104: 614–616, 1993. doi: 10.1378/chest.104.2.614. [DOI] [PubMed] [Google Scholar]
- 52.Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci 40: 141–148, 2015. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shima N, Tsutsumi H, Kamata T, Nishikawa M, Katagi M, Miki A, Tsuchihashi H. Direct determination of glucuronide and sulfate of p-hydroxymethamphetamine in methamphetamine users’ urine. J Chromatogr B Analyt Technol Biomed Life Sci 830: 64–70, 2006. doi: 10.1016/j.jchromb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 54.Solus JF, Arietta BJ, Harris JR, Sexton DP, Steward JQ, McMunn C, Ihrie P, Mehall JM, Edwards TL, Dawson EP. Genetic variation in eleven phase I drug metabolism genes in an ethnically diverse population. Pharmacogenomics 5: 895–931, 2004. doi: 10.1517/14622416.5.7.895. [DOI] [PubMed] [Google Scholar]
- 55.Sun Z, Murry DJ, Sanghani SP, Davis WI, Kedishvili NY, Zou Q, Hurley TD, Bosron WF. Methylphenidate is stereoselectively hydrolyzed by human carboxylesterase CES1A1. J Pharmacol Exp Ther 310: 469–476, 2004. doi: 10.1124/jpet.104.067116. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka M, Iio T, Tabata T. Purification and characterization of a carboxylesterase from rabbit liver lysosomes. J Biochem 101: 619–624, 1987. doi: 10.1093/jb/101.3.619. [DOI] [PubMed] [Google Scholar]
- 56a.Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC); European Respiratory Society (ERS); International Society of Heart and Lung Transplantation (ISHLT), Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 34: 1219–1263, 2009. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 57.Thomsen R, Rasmussen HB, Linnet K; INDICES Consortium . In vitro drug metabolism by human carboxylesterase 1: focus on angiotensin-converting enzyme inhibitors. Drug Metab Dispos 42: 126–133, 2014. doi: 10.1124/dmd.113.053512. [DOI] [PubMed] [Google Scholar]
- 58.Volkow ND, Fowler JS, Wang GJ, Shumay E, Telang F, Thanos PK, Alexoff D. Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PLoS One 5: e15269, 2010. doi: 10.1371/journal.pone.0015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walter L, Hajnóczky G. Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J Bioenerg Biomembr 37: 191–206, 2005. doi: 10.1007/s10863-005-6600-x. [DOI] [PubMed] [Google Scholar]
- 60.Williams ET, Bacon JA, Bender DM, Lowinger JJ, Guo WK, Ehsani ME, Wang X, Wang H, Qian YW, Ruterbories KJ, Wrighton SA, Perkins EJ. Characterization of the expression and activity of carboxylesterases 1 and 2 from the beagle dog, cynomolgus monkey, and human. Drug Metab Dispos 39: 2305–2313, 2011. doi: 10.1124/dmd.111.041335. [DOI] [PubMed] [Google Scholar]
- 61.Zaitsu K, Hayashi Y, Kusano M, Tsuchihashi H, Ishii A. Application of metabolomics to toxicology of drugs of abuse: A mini review of metabolomics approach to acute and chronic toxicity studies. Drug Metab Pharmacokinet 31: 21–26, 2016. doi: 10.1016/j.dmpk.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol 369: 23–37, 2004. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]
- 63.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138: 103–141, 2013. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Banerjee A, Banks WA, Ercal N. N-Acetylcysteine amide protects against methamphetamine-induced oxidative stress and neurotoxicity in immortalized human brain endothelial cells. Brain Res 1275: 87–95, 2009. doi: 10.1016/j.brainres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu HJ, Patrick KS, Yuan HJ, Wang JS, Donovan JL, DeVane CL, Malcolm R, Johnson JA, Youngblood GL, Sweet DH, Langaee TY, Markowitz JS. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet 82: 1241–1248, 2008. doi: 10.1016/j.ajhg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu JP, Xu W, Angulo JA. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience 140: 607–622, 2006. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu W, Song L, Zhang H, Matoney L, LeCluyse E, Yan B. Dexamethasone differentially regulates expression of carboxylesterase genes in humans and rats. Drug Metab Dispos 28: 186–191, 2000. [PubMed] [Google Scholar]