Abstract
Pulmonary hypertension (PH) is a progressive disorder whose cellular pathogenesis involves enhanced smooth muscle cell (SMC) proliferation and resistance to apoptosis signals. Existing evidence demonstrates that the tumor suppressor programmed cell death 4 (PDCD4) affects patterns of cell growth and repair responses in the systemic vasculature following experimental injury. In the current study, the regulation PDCD4 and its functional effects on growth and apoptosis susceptibility in pulmonary artery smooth muscle cells were explored. We previously demonstrated that pharmacological activation of the nuclear transcription factor peroxisome proliferator-activated receptor-γ (PPARγ) attenuated hypoxia-induced proliferation of human pulmonary artery smooth muscle cells (HPASMCs) by inhibiting the expression and mitogenic functions of microRNA-21 (miR-21). In the current study, we hypothesize that PPARγ stimulates PDCD4 expression and HPASMC apoptosis by inhibiting miR-21. Our findings demonstrate that PDCD4 is reduced in the mouse lung upon exposure to chronic hypoxia (10% O2 for 3 wk) and in hypoxia-exposed HPASMCs (1% O2). HPASMC apoptosis was reduced by hypoxia, by miR-21 overexpression, or by siRNA-mediated PPARγ and PDCD4 depletion. Activation of PPARγ inhibited miR-21 expression and resultant proliferation, while restoring PDCD4 levels and apoptosis to baseline. Additionally, pharmacological activation of PPARγ with rosiglitazone enhanced PDCD4 protein expression and apoptosis in a dose-dependent manner as demonstrated by increased annexin V detection by flow cytometry. Collectively, these findings demonstrate that PPARγ confers growth-inhibitory signals in hypoxia-exposed HPASMCs through suppression of miR-21 and the accompanying derepression of PDCD4 that augments HPASMC susceptibility to undergo apoptosis.
Keywords: pulmonary hypertension, smooth muscle cell proliferation
pulmonary hypertension (PH) is defined by increased mean pulmonary artery pressure ≥25 mmHg (38). PH develops in response to stimuli that progressively increase pulmonary vascular resistance (PVR) and lead to maladaptive right ventricular (RV) structural, functional, and metabolic changes that culminate in RV failure (31, 42). Pulmonary vascular remodeling, a central pathophysiological phenomenon that increases PVR, develops in association with enhanced proliferation of pulmonary artery smooth muscle cells (PASMCs) in the medial vascular compartment (35, 42). Cellular mechanisms that contribute to PASMC proliferation and vascular remodeling in PH are complex and involve imbalances in molecular signals that regulate cell survival and death (1, 32, 42). Among the cell death pathways, apoptosis is characterized by highly regulated cellular events involving proteolytic digestion of organelles, cytoskeletal destruction, and morphological changes that include cell shrinkage, membrane blebbing, DNA fragmentation, and phagocytic engulfment of membrane-enclosed vesicles (5). Apoptosis plays a critical role in eliminating unwanted cells and maintaining tissue homeostasis under normal physiological conditions. In disease states, cellular resistance to apoptosis is an increasingly recognized contributor to the pathogenesis of disorders defined by dysregulated proliferation (5, 16).
Programmed cell death 4 (PDCD4) is a central mediator of apoptosis. Genetic mutations or stimuli that reduce PDCD4 expression are associated with cancer progression (24, 25, 45) and vascular smooth muscle cell (VSMC) proliferation following carotid injury (27). PDCD4 also regulates endothelial cell (EC) apoptosis, a sentinel event associated with the subsequent selection for apoptosis-resistant ECs, development of intimal hyperplasia, and vascular remodeling (36, 43). However, studies examining PDCD4 in the context of PASMC apoptosis resistance and proliferation in PH pathogenesis are limited. To gain insight into stimuli and therapeutics that influence PASMC apoptosis, we examined the expression and regulation of the prototype apoptosis mediator PDCD4 in HPASMCs exposed to hypoxia. PDCD4 is expressed in nearly all tissues, but relative expression levels in selected tissues may be cell type and stimulus specific. The protein is found in both nuclear and cytoplasmic compartments, and its function and location may be regulated by kinase-mediated phosphorylation of multiple sites (24, 25). Consistent with its known role as a tumor suppressor, PDCD4 confers inhibitory effects on cell growth by blocking growth-promoting transcription factors such as activated protein-1 (AP-1) (41, 45) or inhibiting the translation of mRNA involved in cell proliferation. Although the exact mechanisms by which PDCD4 induces apoptosis are being elucidated, it is known to activate the effector caspase-3 (3), which cleaves central protein substrates necessary for the maintenance of cell structure, function, and survival. PDCD4 also initiates programmed cell death in HEK293 cells in response to adenoviral transfer of the important apoptosis mediator Fas ligand (8).
Stimuli that regulate the expression and activity of PDCD4 influence the balance of cellular proliferation or apoptosis in numerous cell types. For example, PDCD4 exerts inhibitory effects on rat VSMCs in response to experimentally induced carotid artery injury (27). Furthermore, the enhanced migration and proliferation of HPASMCs are associated with reduced cellular expression of PDCD4 (37). These studies confirm the central role of PDCD4 in the regulation of patterns of VSMC growth. However, little is known about the regulation of PDCD4 by available pharmacological therapies. Because the thiazolidinedione class of peroxisome proliferator-activated receptor-γ (PPARγ) ligands attenuate experimental PH (11, 13, 34, 39), the current report further examined the relationship between PPARγ activation and PDCD4 expression and function in HPASMCs.
Our group previously demonstrated that the pharmacological PPARγ activator rosiglitazone (RSG) attenuated hypoxia-induced PH and vascular remodeling in vivo (11, 34) and HPASMC proliferation in vitro (10, 29). These studies demonstrated that PPARγ inhibited HPASMC growth by regulating the expression of target genes that enhance HPASMC proliferation, including platelet-derived growth factor receptor-β (PDGFRβ) (34), transforming growth factor-β1 (TGF-β1) (12), endothelin-1 (ET-1) (20), nuclear factor-κB (NF-κB), NADPH oxidase 4 (Nox4) (29), and thrombospondin-1 (10). Recently, we determined that RSG also inhibited HPASMC proliferation by attenuating hypoxia-induced microRNA (miR)-21 levels (12).
miR-21, a prototype oncomiR that is overexpressed in most solid organ tumors and known for enhancing cell survival and metastasis in cancer models (15, 17, 23, 33), has emerged as an important small endogenous noncoding RNA species that regulates vascular wall cell proliferation in PH (12, 37, 44, 46). miR-21 binds to a complimentary sequence in the 3′-UTR of PDCD4 resulting in reduced PDCD4 luciferase activity and PDCD4 protein translation (19, 30). Because RSG negatively regulated miR-21 expression (12) and PDCD4 is a miR-21 target gene, the current study extends these observations by examining the hypothesis that PDCD4 is a central target through which PPARγ acts to restore apoptosis signals and inhibit proliferation of HPASMCs. Our findings identify a signaling axis involving miR-21 that contributes to the apoptosis resistance found in hypoxia-exposed HPASMCs and defines PDCD4 as a central target through which PPARγ confers its antiproliferative effects. Ultimately, this study adds to a growing body of literature that identifies an important role of miR-21 in the pathogenesis of diseases like PH that are characterized by excessive cellular proliferation and further highlights the potential benefit of developing therapies that target miRs in PH.
MATERIALS AND METHODS
Mouse model of chronic hypoxia.
All animals used in the current study were male C57/BL6J mice obtained from Jackson Laboratories. Mice were housed in cages in room air (21% O2) or in a hypoxia chamber (10% O2) for 3 wk. This hypoxia-exposure regimen induced PH, RV hypertrophy, and vascular remodeling (11, 34). At the conclusion of the study, mice were euthanized by insufflating CO2 from a compressed gas tank into a clear plastic chamber in which they were housed for 3–4 min. Protein and mRNA were extracted from homogenized lung tissue. All animals had access to standard mouse chow and water ad libitum. All scientific investigations and procedures that involved animals were reviewed and formally approved by the Atlanta Veterans Affairs Medical Center Institutional Animal Care and Use Committee.
Cell culture and in vitro hypoxia studies.
HPASMCs obtained from ScienCell (Carlsbad, CA) originated from three different male donors. Contamination by other human cell lines was not detected as confirmed by the BioSYNTHESIS DNA Identity Testing Center (Case No. AS1996; Biosynthesis, Lewisville, TX). Additionally, the MycoAlert Mycoplasma Detection Kit (Lonza) failed to detect mycoplasma cell culture contaminants. Each cell line derived from a separate male donor represented an individual experimental n. In each experiment, each of the three human cell lines were plated separately into designated experimental groups. In the experimental groups within each cell line, two to three experimental replicates were plated to ensure the consistency and reproducibility of the individual experimental result. Significant effort was made to conduct experiments using three human cell lines (n = 3) more than once to increase the number of observations and enhance the reproducibility of the study results. To increase the experimental ns, HPASMCs derived from later passages were subjected to the identical experimental conditions utilized in studies using HPASMCs derived from earlier passages. The ns from these identical studies were added together to increase the total number of observations. Before the initiation of experiments, HPASMCs were incubated at 37°C in complete smooth muscle growth medium until they reached 60–80% confluence. Selected HPASMC monolayers were then placed in a normobaric hypoxia chamber (1% O2-5% CO2; Biospherix, Lacuna, NY) or propagated in a cell culture incubator under normoxic conditions (21% O2-5% CO2) for 48–72 h. In selected studies, to activate PPARγ, the pharmacological PPARγ agonist RSG (10–25 μM) or an equal volume of dimethyl sulfoxide (DMSO; 1%; Fisher Scientific, Fair Lawn, NJ) vehicle was added to the HPASMC culture media.
HPASMC transfection.
The Lipofectamine RNAiMAX Reagent (Life Technologies, Grand Island, NY) was used in experiments that examined the cellular consequences of miR-21 overexpression or miR-21 depletion. In these studies, HPASMCs were transiently transfected with small interfering RNA sequences that targeted miR-21 (hsa-antimiR-21 miRCURY LNA; 5′-CAACATCAGTCTGATAAGCT-3′) or scrambled RNA sequences (Control A miRCURY LNA; 5′-TAACACGTCTATACGCCCA-3′) (Exiqon, Woburn, MA). Transfection of hsa-antimiR-21 (1 nM) achieved robust miR-21 knockdown as we recently reported (12); therefore, this concentration was used in the current study to deplete miR-21. To overexpress miR-21, mature miR-21 mimic (30 nM; 5′-UAGCUUAUCAGACUGAUGUUGA-3′; Ambion; Life Technologies) was applied to HPASMCs. In our previous report, transfection of HPASMCs with miR-21 mimic (30 nM) robustly increased the detection of miR-21 and significantly increased proliferation (12). Dicer-substrate RNAi methods using TriFecta (IDT, Coralville, IA) were employed in experiments that examined cellular effects of depleting PPARγ (5′-GGAAUUAGUGACAGCGACUUGGCA-3′) or PDCD4 (5′-CCAUUAACGAAGCUAGAAUUAAUGC-3′). Control HPASMCs were transfected with nontargeting scrambled oligonucleotides (All Stars Negative Control nontargeting siRNA; Qiagen, Valencia, CA). All transient transfections were conducted for 6 h.
PPARγ adenovirus vector construction and HPASMC transfection.
To examine the effects of PPARγ overexpression on HPASMC proliferation and cellular signaling, selected HPASMCs were transfected with nonreplicating adenovirus encoding PPARγ (AdPPARγ) or green fluorescent protein (AdGFP; Vector Biolabs, Philadelphia, PA) at a multiplicity of infection (MOI) of 5–25. HPASMCs grown to 60–80% confluence were transfected with the respective adenovirus constructs in 1% fetal bovine serum and smooth muscle growth media. After a 6-h transfection period, the media were replaced with complete growth media, and the HPASMC monolayers were placed in the cell culture incubator. Twenty-four hours after adenovirus transfection, PPARγ was activated by treatment with RSG (10 μM), and HPASMCs were subjected to various treatment conditions for the remaining study period.
Assessment of HPASMC proliferation and apoptosis.
The influence of hypoxia, PPARγ, PDCD4, and miR-21 on HPASMC proliferation was assessed by cell counting using the Invitrogen Countess II Automated Cell Counter (ThermoFisher Scientific). At the onset of experiments, equal numbers of HPASMCs were seeded onto 24-well plates. The Countess was employed following the application of specified treatment conditions to determine the final HPASMC number. In selected studies, the effect of hypoxia, PPARγ, PDCD4, and miR-21 on HPASMC apoptosis was assessed using the ApoAlert Caspase Colorimetric Assay Kit (Clontech, Mountain View, CA). In brief, The ApoAlert Caspase Colorimetric Assay measures caspase-3 activity using spectrophotometric detection of the chromophore p-nitroaniline (pNA) after its cleavage by caspases from the labeled caspase-specific substrates (405 nM). For each sample analyzed, 50 μg of total protein were measured and diluted in cell lysis buffer to generate 50 μl total volume of cell lysis buffer at the onset of the assay, which proceeded as recommended by the manufacturer. In separate studies, the water soluble mitochondrial inner membrane protein cytochrome-c was detected by enzyme-linked-immunosorbent assay (ELISA; Invitrogen, Camarillo, CA) to mark an early critical component required for apoptosis initiation, caspase-3 activation, and DNA fragmentation. Following exposure to experimental conditions, HPASMCs were lysed, and 5 μg of total protein were measured and added to a diluent buffer to generate a total volume of 100 μl at the onset of the assay. This volume was added to wells of a 96-well plate prefixed with immobilized capture antibodies capable of binding to released cytochrome-c antigen. Biotinylated secondary anti-cytochrome-c antibodies were then added. Following wash steps, streptavidin peroxidase enzyme and a substrate solution were added to produce a color detected at 450 nm that correlated with the quantity of cytochrome-c detected in the sample.
Annexin V binding assay.
HPASMC monolayers grown to 60–80% confluence were treated with RSG (10–25 μM) or an equal volume of DMSO and placed in normoxic or hypoxic conditions for 72 h. Following the treatment period, apoptosis was determined using the Annexin V Apoptosis Detection Kit (BD Biosciences, Mountain View, CA). HPASMCs were detached using Accutase (BD Biosciences), centrifuged, and washed in PBS. Annexin V staining was performed in accordance with the manufacturer’s instructions (BD Biosciences). HPASMCs were passed through a 35-μm cell strainer (Corning, New York, NY) before undergoing fluorescence-activated cell sorting (FACS) analysis. FACS was conducted using BD FACSAria II, and data were analyzed using FACSDiva software. Apoptosis was measured by quantifying the percentage of cells upon which annexin V was detected and graphed in absolute units using the median fluorescence intensity.
Western blot analysis.
Equivalent amounts of protein from HPASMC lysates were resolved by SDS-PAGE and transferred to nitrocellulose membranes. After blocking, membranes were probed with the following antibodies: anti-GAPDH (1:10,000; Sigma, St. Louis, MO), anti-PDCD4 (1:500, Cell Signaling Technologies, Danvers, MA), and anti-PPARγ (1:1,000; Santa Cruz Biotechnology, Dallas TX). Primary antibodies were targeted by infrared dye-based secondary antibodies (Li-Cor Biotechnology, Lincoln, NE). Immunodetection was performed using infrared dye imaging (Li-Cor). Relative protein levels were quantified using Li-Cor proprietary software.
RNA and microRNA isolation, reverse transcription, and quantitative PCR.
The mirVana miRNA Isolation Kit was used to extract and purify large RNA and small (micro) RNA that were enriched from HPASMC monolayer lysates according to the manufacturer’s protocol (Life Technologies). Following organic extraction and purification procedures, both large and small RNA fractions were eluted in separate vials, and RNA concentration and purity were determined by ultraviolet absorption using a spectrophotometer (BioTek, Winooski, VT). Complementary DNA (cDNA) was generated from 125 to 250 ng RNA per sample using the miScript II RT kit (Qiagen, Valencia, CA), and quantitative RT-PCR was conducted with the 7500 Fast Real-Time PCR (Applied Biosystems Foster City, CA) using the primer sequence 5′-UAGCUUAUCAGACUGAUGUUGA-3′ to detect hsa-miR-21-5p. The amplified miR gene product was quantified with QuantiTect SYBR Green PCR Kit (Qiagen). In parallel extractions, large RNA species were isolated and purified from HPASMC monolayer lysates using the RNeasy Mini Kit (Qiagen) as recently described (12). In each sample, the expression of large RNA species was normalized to the respective endogenous content of the 9S reference gene. The relative abundance of target mRNA in each sample was calculated using the ΔΔCt method (28). Primer sequences were as follows: 9S: forward, 5′-CTGACGCTTGATGAGAAGGAC-3′ and reverse, 5′-CAGCTTCATCTTGCCCTCAT-3′; PDCD4: forward, 5′-GAGGTGGATGTGAAAGATCCTAA-3′ and reverse, 5′-CCAAAGGCAAAACTACAGTTTCAT-3′; and PPARγ: forward, 5′-GGGTGATGTGTTTGAACTTGATT-3′ and reverse, 5′-TGACAGGAAAGACAACAGACAAAT-3′ (Eurofins MWG Operon, Huntsville, AL).
Statistical analysis.
All statistical analyses were conducted using the statistical software Graphpad Prism Version 6.04 (1992–2014 GraphPad Software). Differences between two groups were detected using two-tailed unpaired t-tests. Experimental groups in mouse studies were graphically depicted as fold change with respect to the averaged control values. In cell culture studies, fold changes in experimental groups made with respect to the control condition each cell line were averaged. Means ± SE were used to calculate error bars for each experimental condition. A one-way ANOVA was employed to detect treatment effects in studies with three or more experimental groups. A two-way ANOVA was used in studies that examined the influence of two independent variables on a continuous dependent variable. The main effect of independent variables on the dependent variable and interactions within other independent variables was assessed for overall statistical significance. The α to determine significance was 0.05. In studies where overall significance was detected, pairwise comparisons among groups were determined using Tukey’s method for multiple comparisons.
RESULTS
Hypoxia reduces PDCD4 expression and apoptosis in vitro and in vivo.
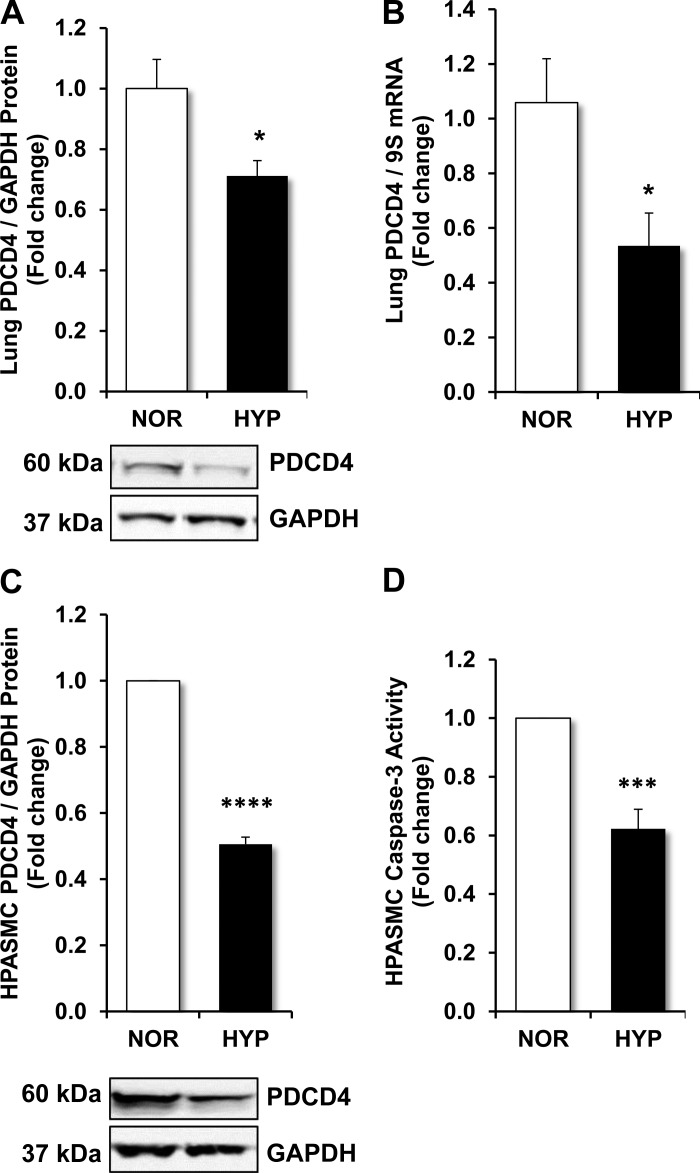
Our laboratory previously demonstrated that proliferation induced by hypoxia was mediated by the enhanced expression of major redox responsive targets, including thrombospondin-1, TGF-β1 (10), PDGF (34), and Nox4 (11, 29). To determine the effects of hypoxia on the expression of a major mediator of apoptosis, PDCD4 levels were examined in the mouse lung in vivo and in HPASMCs in vitro. As shown in Fig. 1, A and B, hypoxia reduced PDCD4 protein and mRNA levels, respectively, in the mouse lung. Hypoxia also significantly reduced PDCD4 protein in HPASMCs (Fig. 1C). PDCD4 mRNA levels were similarly reduced in hypoxia-exposed HPASMCs in comparison with normoxia-exposed cells (data not shown). Since PDCD4 activates the effector caspase-3 (3), a key step in apoptosis, we examined the effect of hypoxia-induced alterations in PDCD4 expression on apoptosis using an colorimetric assay that analyzes caspase-3 activity by spectrophotometric detection of the chromophore pNA after its cleavage by caspases. As shown in Fig. 1D, in comparison with normoxia-exposed controls, apoptosis, as measured by caspase-3 activity, was significantly reduced in hypoxic conditions.
Fig. 1.
Hypoxia reduces programmed cell death 4 (PDCD4) expression in vivo and in vitro. A and B: C57BL/6J mice were exposed to normoxia (21% O2) or hypoxia (10% O2) for 3 wk. Lungs were collected, and samples were subjected to determination of PDCD4 protein and mRNA levels. C and D: human pulmonary artery smooth muscle cells (HPASMCs) were exposed to normoxic (NOR; 21% O2) or hypoxic (HYP; 1% O2) environments for 72 h. C: total protein was isolated from HPASMC lysates and PDCD4 levels were detected by Western blot analysis. D: caspase-3 activity was determined by spectrophotometric detection of the cleavage of labeled caspase-specific substrates. A and C: representative immunoblots. Bars represent means ± SE for PDCD4 levels or caspase-3 activity. A and B: n = 5. *P < 0.05 vs. normoxia (NOR). C and D: n = 6. ***P < 0.001 vs. NOR; ****P < 0.0001 vs. NOR.
Depletion of PPARγ reduces HPASMC PDCD4 levels and apoptosis.
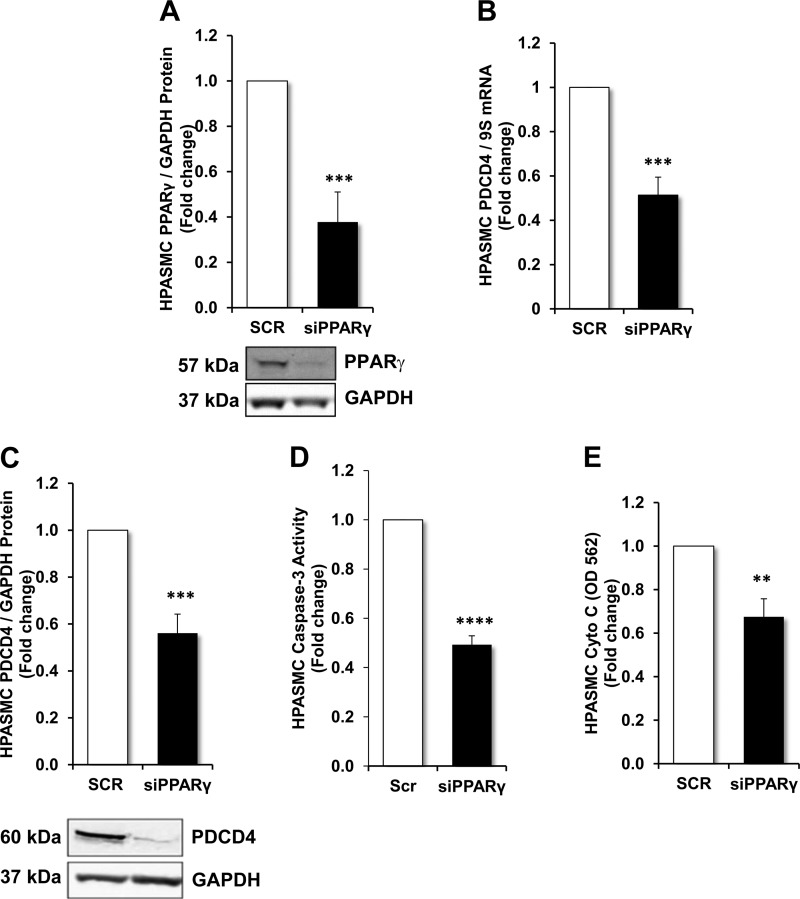
Previous reports demonstrated that hypoxia reduces PPARγ levels in the lung in vivo (9, 34) and in pulmonary vascular wall cells in vitro (9). Furthermore, knockdown of PPARγ was sufficient to stimulate PH in vivo (14, 21) and pulmonary vascular wall cell proliferation in vitro (2, 21). Therefore to further explore the role of reductions in PPARγ on PDCD4 expression and HPASMC apoptosis, PPARγ siRNA was employed and compared with studies in which HPASMCs were transfected with nontargeting scrambled oligonucleotides. As shown in Fig. 2A, transfection of HPASMCs with PPARγ siRNA significantly depleted PPARγ protein expression. PDCD4 levels were then measured in HPASMCs transfected with nontargeting scrambled nucleotides or PPARγ siRNA. As shown in Fig. 2, B and C, PDCD4 mRNA and protein levels, respectively, were significantly reduced in HPASMCs following PPARγ depletion. The functional effects of PPARγ depletion on HPASMC apoptosis were determined by measuring caspase-3 activity. As demonstrated in Fig. 2D, apoptosis was significantly reduced following knockdown of PPARγ when compared with HPASMCs transfected with nontargeting scrambled oligonucleotides. To further confirm alterations in HPASMC apoptosis, the release of the inner mitochondrial membrane protein cytochrome-c into the cytosol was determined. As demonstrated in Fig. 2E, cytochrome-c antigen abundance was decreased in HPASMCs in which PPARγ was depleted, in comparison with scrambled-transfected controls. These findings provide evidence that depletion of PPARγ is sufficient to reduce PDCD4 expression and apoptosis in HPASMCs and are consistent with our previous reports that PPARγ depletion stimulates PASMC proliferation (2).
Fig. 2.
PDCD4 levels and apoptosis in peroxisome proliferator-activated receptor-γ (PPARγ)-depleted HPASMCs. HPASMCs were transfected with nontargeting scrambled oligonucleotides or PPARγ (20 nM) dicer-substrate short interfering (si)RNA constructs for the first 6 h of 48- or 72-h incubation periods. A: PPARγ protein expression in HPASMCs transfected with PPARγ siRNA. B: PDCD4 mRNA levels in HPASMCs transfected with PPARγ siRNA. C: PDCD4 protein levels in HPASMCs transfected with PPARγ siRNA. D: caspase-3 activity in HPASMCs transfected with nontargeting scrambled oligonucleotides or siPPARγ. E: cytochrome-c levels were analyzed using an ELISA on HPASMCs transfected with nontargeting scrambled (SCR) oligonucleotides or siPPARγ. A and C: representative immunoblots. Bars represent means ± SE for PPARγ, PDCD4, caspase-3 activity, or cytochrome-c abundance; n = 6–7. **P < 0.01 vs. SCR; ***P < 0.001 vs. scrambled (SCR; ****P < 0.0001 vs. SCR.
Loss of PDCD4 reduces apoptosis in HPASMCs.
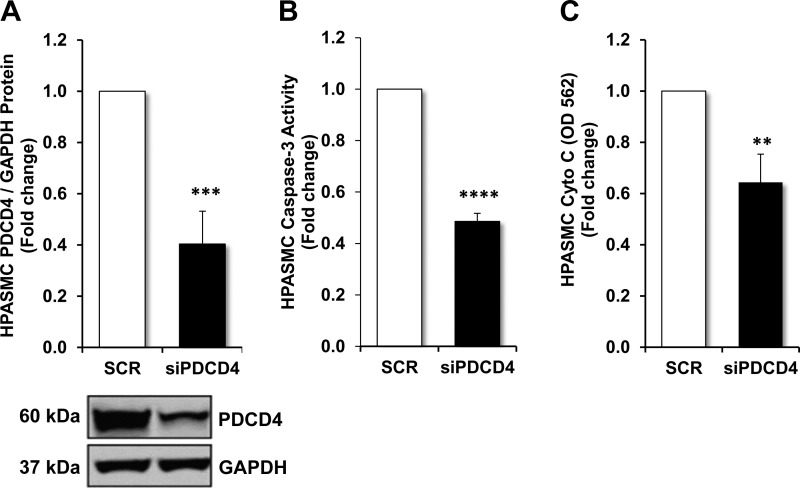
To mimic the hypoxic environment in which PDCD4 expression is reduced, HPASMCs were transfected with PDCD4 siRNA or nontargeting scrambled oligonucleotides. Following transfection, PDCD4 protein was decreased as demonstrated in Fig. 3A. Similarly, PDCD4 mRNA levels were significantly reduced in HPASMCs in which PDCD4 was depleted (data not shown). To demonstrate the effects of PDCD4 depletion on apoptosis, assays were employed to detect caspase-3 activity and cytochrome-c abundance. As shown in Fig. 3, B and C, detection of caspase-3 activity and cytochrome-c antigen presence was significantly reduced in PDCD4-depleted HPASMCs in comparison with nontargeting scrambled-transfected control HPASMCs.
Fig. 3.
Effects of PDCD4-depletion on apoptosis in HPASMCs. HPASMC monolayers were transfected with nontargeting scrambled oligonucleotides or PDCD4 (20 nM) dicer-substrate siRNA construct for the first 6 h of a 72-h incubation period. A: PDCD4 protein expression in HPASMCs transfected with nontargeting scrambled oligonucleotides or siPDCD4 with representative immunoblot. B and C: upon the conclusion of the 72-h incubation period, caspase-3 activity or cytochrome-c levels were determined in HPASMCs transfected with nontargeting scrambled oligonucleotides or siPDCD4, respectively. Bars represent means ± SE for PDCD4 expression, caspase-3, or cytochrome-c enzymatic activity; n = 6–7. **P < 0.01 vs. SCR; ***P < 0.001 vs. SCR; ****P < 0.0001 vs. SCR.
PPARγ adenovirus causes concentration- and PPARγ ligand-dependent increases in PDCD4 and PPARγ expression.
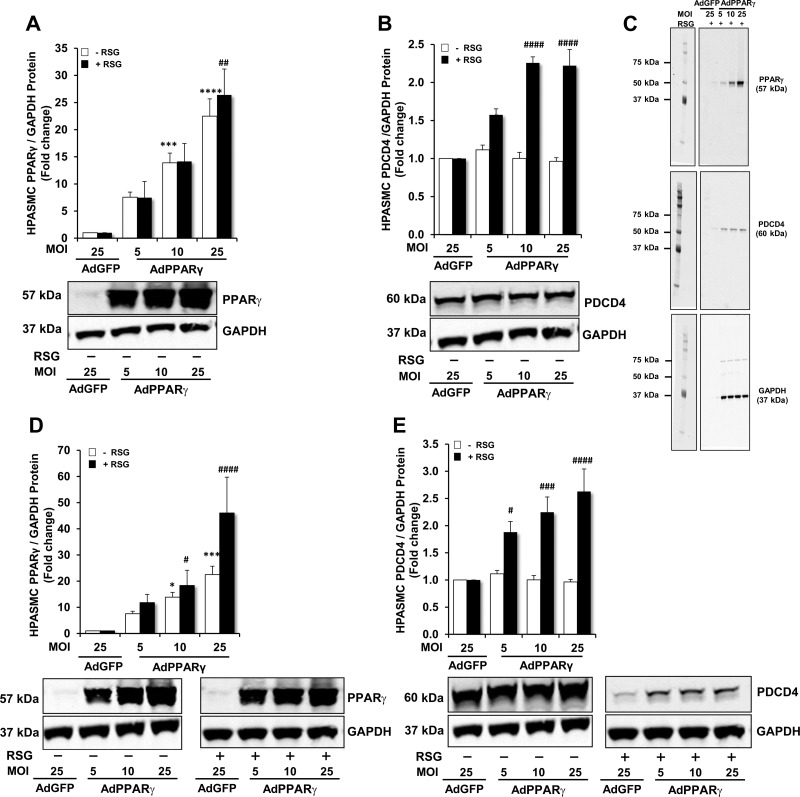
In Fig. 2, we demonstrated that loss of PPARγ reduced PDCD4 expression and apoptosis. To further examine the relationship between PPARγ and PDCD4, we performed studies to increase PPARγ expression and/or activity by transfecting HPASMCs with a control GFP-encoded adenovirus (AdGFP; 25 MOI) or with escalating concentrations of a PPARγ-encoded adenovirus (AdPPARγ) to attain MOIs of 5, 10, or 25 for 48 or 72 h. To stimulate PPARγ activity, selected HPASMCs were also treated with the synthetic PPARγ ligand RSG (10 μM) or with an equal volume of DMSO vehicle. As demonstrated in Fig. 4A, transfection with AdPPARγ caused concentration-dependent increases in PPARγ protein expression at 48 h. As shown in Fig. 4B, in comparison with AdGFP-transfected HPASMCs, PDCD4 protein expression was unchanged in AdPPARγ-transfected HPASMCs in the absence of RSG at 48 h. However, when the PPARγ ligand RSG was added to HPASMC monolayers transfected with AdPPARγ, PDCD4 protein expression significantly increased in comparison with AdGFP-transfected HPASMCs in a concentration-dependent manner. Representative immunoblots that demonstrate the concentration-dependent effects of AdPPARγ + RSG on PPARγ and PDCD4 protein levels at 48 h are shown in the immunoblot displayed in Fig. 4C. Similarly, as demonstrated in Fig. 4, D and E, transfection with AdPPARγ for 72 h caused concentration- and PPARγ ligand-dependent effects on PDCD4 and PPARγ protein levels. Collectively, these findings demonstrate that AdPPARγ significantly increases the PPARγ ligand-dependent expression of PDCD4 in a concentration-dependent fashion. In subsequent studies that utilized the AdPPARγ constructs to overexpress PPARγ, a MOI of 10 was used because it produced a consistent increase in PDCD4 protein at both 48 and 72 h in RSG-treated HPASMCs.
Fig. 4.
Time- and dose-dependent effects of PPARγ ± rosiglitazone (RSG) on PDCD4 and PPARγ protein expression. HPASMC monolayers were propagated in a cell culture incubator at 37◦ C until reaching 60–80% confluence. Selected HPASMCs were transfected with AdGFP [25 multiplicity of infection (MOI)] or escalating doses of AdPPARγ (5–25 MOI) for 6 h. After 24 h, RSG (10 μM) or an equal volume of DMSO was added to the cell culture media. Following the conclusion of 48- or 72-h incubation periods, total protein was isolated from monolayer lysates. A and B: PPARγ or PDCD4 protein levels were detected in HPASMCs transfected with AdGFP or AdPPARγ ± RSG for 48 h. C: full-length representative immunoblots are shown to demonstrate PPARγ, PDCD4 and GAPDH proteins in HPASMCs transfected with AdGFP or AdPPARγ + RSG for 48 h. D and E: PPARγ or PDCD4 protein levels were detected in HPASMCs transfected with AdGFP or AdPPARγ ± RSG for 72 h. Representative immunoblots are displayed in A, B, D, and E; n = 6. *P < 0.05 vs. AdGFP (−RSG); ***P < 0.001 vs. AdGFP (−RSG); ****P < 0.0001 vs. AdGFP (−RSG); #P < 0.05 vs. AdGFP (+RSG); ##P < 0.01 vs. AdGFP (+RSG); ###P < 0.001 vs. AdGFP (+RSG); ####P < 0.0001 vs. AdGFP (+RSG).
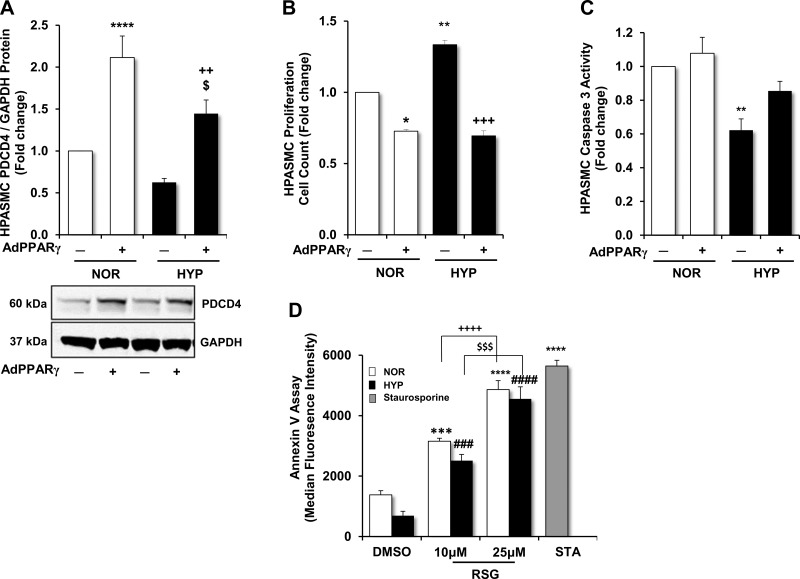
PPARγ activation reduces apoptosis resistance and restores PDCD4 levels in hypoxia-exposed HPASMCs.
PPARγ activation with RSG treatment inhibited proliferation in HPASMCs exposed to hypoxia (10, 29). To further examine the role of PDCD4 as a potential mediator through which PPARγ reduces proliferation, PDCD4 expression, proliferation, and apoptosis were analyzed in HPASMCs transfected with AdPPARγ or AdGFP + RSG and then exposed to normoxic or hypoxic conditions. As shown in Fig. 5A, PDCD4 protein levels were reduced in hypoxia-exposed HPASMCs; however, AdPPARγ enhanced PDCD4 protein levels in both normoxic and hypoxic conditions. As displayed in Fig. 5B, HPASMC proliferation detected by automated cell counting was significantly increased in hypoxia-exposed HPASMCs. In normoxia- and hypoxia-exposed HPASMCs in which PPARγ was overexpressed, proliferation was attenuated in comparison with AdGFP-transfected controls. As demonstrated in Fig. 5C, hypoxia significantly reduced caspase-3 activity in HPASMCs. Conversely, in hypoxia-exposed HPASMCs in which PPARγ was overexpressed, caspase-3 activity was augmented to a level that was comparable to normoxia-exposed AdGFP-transfected control HPASMCs.
Fig. 5.
PPARγ modulates PDCD4 protein, HPASMC proliferation, and apoptosis in response to hypoxia. HPASMC monolayers were exposed to normoxic (21% O2) or hypoxic (1% O2) conditions for 72 h. At the onset of the study, selected HPASMCs were transfected with AdGFP (10 MOI) or AdPPARγ (10 MOI) for 6 h. RSG (10 μM) was added to the culture medium after 24 h of incubation. Upon the conclusion of the study period, total protein was extracted from HPASMC monolayer lysates, and proliferation and apoptosis assays were conducted. A: demonstration of PDCD4 protein expression in HPASMCs with representative immunoblot. B: proliferation was detected in HPASMCs using automated cell counting. C: demonstration of caspase-3 activation as an indicator of apoptosis in normoxia- or hypoxia-exposed HPASMCs treated with AdPPARγ or AdGFP + RSG. D: HPASMCs exposed to normoxia or hypoxia for 72 h were treated with RSG (10–25 μM) or an equal volume of DMSO at the onset of the study period. With the use of FACS analysis, apoptosis was examined by detecting annexin V staining in HPASMCs. Staurosporine (0.1 μM) was applied to selected normoxia-exposed HPASMCs as a positive control to enhance apoptosis. Bars represent means ± SE for PDCD4 protein (A), proliferation (B), caspase-3 activation (C), or annexin V (D) detection by median fluorescence. A: n = 12; ****P < 0.0001 vs. NOR(−); $P < 0.05 vs. NOR (+); ++P < 0.01 vs. HYP(−). B: n = 3; and C: n = 6. *P < 0.05 vs. NOR(−); **P < 0.01 vs. NOR(−); +++P < 0.001 vs. HYP(−). D: n = 3; ***P < 0.001 vs. NOR (DMSO); ****P < 0.0001 vs. NOR (DMSO); ++++P < 0.0001 vs. NOR (10 μM); ###P < 0.001 vs. HYP (DMSO); ####P < 0.0001 vs. HYP (DMSO); $$$P < 0.001 vs. HYP (10 μM).
To further confirm that PPARγ modulates apoptosis in hypoxia-exposed HPASMCs, an annexin V detection assay was performed. As shown in Fig. 5D, upon exposure to hypoxia, HPASMC apoptosis was reduced as reflected by lower annexin V median fluorescence intensity. Upon the addition of RSG (10–25 μM) to cell monolayers, concentration-dependent increases in apoptosis were observed in both normoxia- and hypoxia-exposed HPASMCs. In these studies, a low concentration of the apoptosis inducer staurosporine (0.1 μM) was added to the cells as a positive control. As shown in Fig. 5D, when added to the culture medium, staurosporine yielded the greatest increase in apoptosis. Collectively, these findings identify PDCD4 as an important mediator of HPASMC apoptosis susceptibility. Hypoxia reduces PDCD4 expression and apoptosis; however, activation of PPARγ increases PDCD4 expression and restores apoptosis susceptibility in hypoxia-exposed HPASMCs.
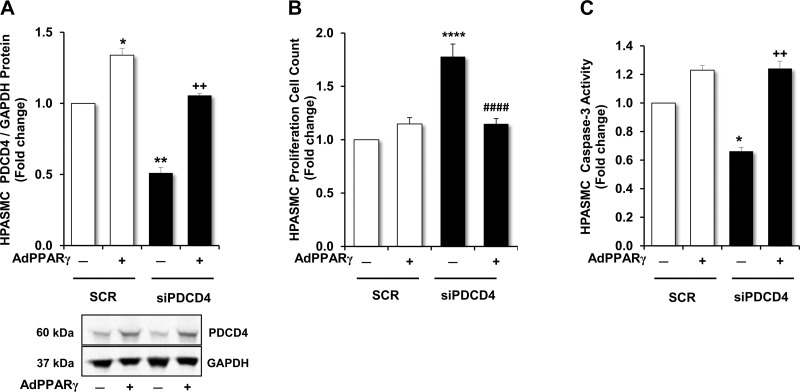
In PDCD4-depleted HPASMCs, activation of PPARγ reduced cell proliferation and restored apoptosis.
To mimic hypoxic conditions in which PDCD4 is reduced, HPASMCs were transfected with PDCD4 siRNA. The functional effects of PPARγ activation on proliferation and apoptosis were then examined. As demonstrated in Fig. 6A, overexpression of PPARγ increased PDCD4 levels in scrambled-transfected groups and blunted the expected reductions in PDCD4 expression in groups transfected with PDCD4 siRNA. As shown in Fig. 6B, siRNA-induced depletion of PDCD4 caused a significant increase in HPASMC proliferation, whereas activation of PPARγ suppressed the growth-stimulating effects of PDCD4 depletion. Examination of caspase-3 activity in PDCD4-depleted groups revealed opposing trends. Whereas caspase-3 activity was reduced in HPASMCs in which PDCD4 was depleted, caspase-3 activity was restored in groups in which PPARγ was overexpressed (Fig. 6C).
Fig. 6.
Effects of PPARγ overexpression on PDCD4 expression, proliferation, and apoptosis in PDCD4-depleted HPASMCs. PDCD4 levels, proliferation, and apoptosis were measured in PDCD4-depleted HPASMCs in which PPARγ was overexpressed. HPASMCs were transfected with AdGFP or AdPPARγ for 6 h at the onset of the experiment. After 24 h of incubation, RSG (10 μM) was added to all HPASMC monolayers, and selected HPASMCs were transfected with nontargeting scrambled oligonucleotides or siPDCD4 (20 nM). Upon the conclusion of the 72-h study period, total protein was extracted from HPASMC monolayer lysates, and proliferation and apoptosis assays were conducted. A: demonstration of PDCD4 protein expression in HPASMCs with representative immunoblot. B: proliferation was measured in HPASMCs using automated cell counting. C: demonstration of caspase-3 activation as an indicator of apoptosis in HPASMCs. Bars represent means ± SE for PDCD4 protein (A), HPASMC proliferation (B), or caspase-3 cleavage (C). A and C: n = 3; *P < 0.05 vs. SCR(−); **P < 0.01 vs. SCR(−); ++P < 0.01 vs. siPDCD4(−). B: n = 7; ****P < 0.0001 vs. SCR(−); ####P < 0.0001 vs. siPDCD4 (−).
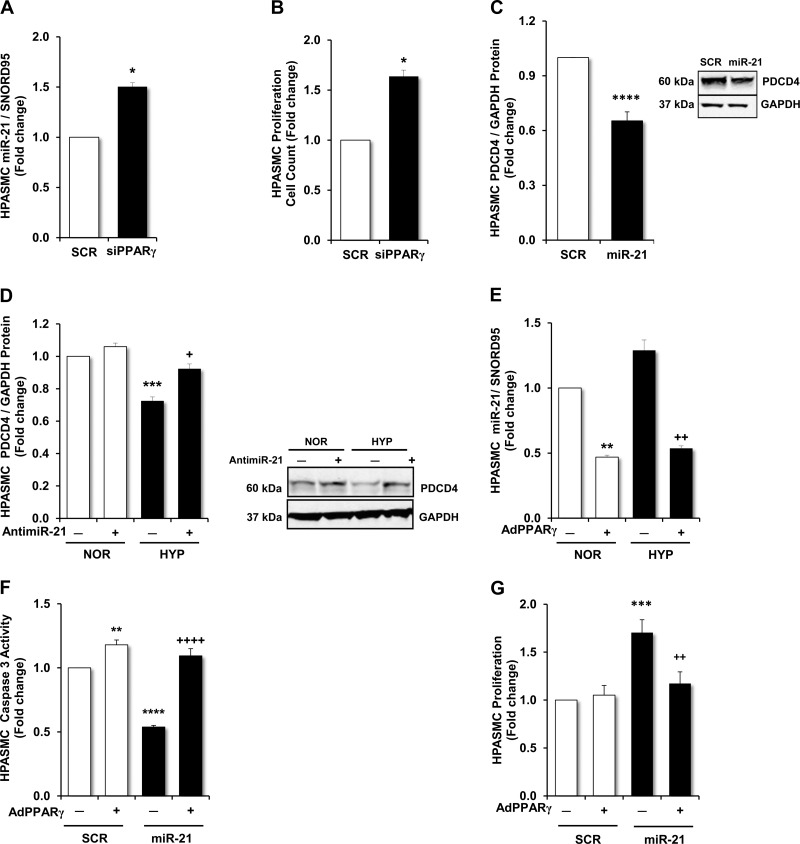
PPARγ suppresses hypoxia-induced HPASMC miR-21 expression, proliferation, and apoptosis resistance.
In light of our findings in Figs. 2 and 4 that demonstrate that PPARγ stimulates PDCD4 expression, we further examined the signaling mechanisms that underlie this observation. We conducted an in silico analysis of the PDCD4 promoter and did not detect any PPAR response elements that would be predicted to stimulate PDCD4 transcription. This observation suggested that alternative, potentially indirect, mechanisms of regulation were present. Based on previous reports that hypoxia stimulates miR-21 expression in HPASMCs (12, 37) and on evidence that PDCD4 is regulated by miR-21 in vascular wall cells (37, 43), we explored the regulation of miR-21 and its target, PDCD4, in response to PPARγ. As shown in Fig. 7, A and B, siRNA-mediated PPARγ depletion increased HPASMC miR-21 expression and proliferation, respectively. To further explore the involvement of miR-21 in the regulation of PDCD4, we conducted miR-21 gain and loss of function studies by transfecting HPASMCs with mature miR-21 mimics (30 nM) or locked nucleic acid antisense miR-21 oligonucleotides (1 nM), respectively. As shown in Fig. 7C, overexpression of mature miR-21 mimics reduced PDCD4 protein levels. In contrast, while antimiR-21 had no significant effect on PDCD4 levels in normoxia-exposed HPASMCs, it significantly blunted hypoxia-induced reductions in PDCD4 protein expression (Fig. 7D).
Fig. 7.
Effects of PPARγ on miR-21 expression and HPASMC proliferation and apoptosis. In selected studies, PPARγ was depleted by transfecting HPASMCs with siPPARγ (20 nM). A: demonstration of the expression of miR-21 in PPARγ-depleted HPASMCs compared with HPASMCs transfected with nontargeting scrambled oligonucleotides (20 nM). B: demonstration of the functional effects of PPARγ on HPASMC proliferation in PPARγ-depleted HPASMCs compared with HPASMCs transfected with nontargeting scrambled oligonucleotides. The effects of gain or loss of miR-21 expression on PDCD4 protein levels were examined. C: PDCD4 protein levels were measured in HPASMCs transfected with mature miR-21 mimics (30 nM) or nontargeting scrambled oligonucleotides (30 nM) with a representative immunoblot. D: PDCD4 protein levels were measured in HPASMCs exposed to normoxic (21% O2) or hypoxic (1% O2) conditions for 72 h. Six hours before the cells were placed in normoxic or hypoxic conditions, HPASMC monolayers were transfected with locked nucleic acid siRNA sequences targeting miR-21 (anti-miR-21; 1 nM) or nontargeting control locked nucleic acid scrambled RNA sequences (1 nM). Representative immunoblot is shown. E: miR-21 expression was measured in HPASMC monolayers exposed to normoxic (21% O2) or hypoxic (1% O2) conditions. Selected HPASMCs were transfected with AdGFP (10 MOI) or AdPPARγ (10 MOI) for 6 h at the onset of the study. RSG (10 μM) was added to the culture medium after 24 h of incubation and miR-21 levels were detected using quantitative RT-PCR upon the conclusion of the 72-h study. F and G: effects of miR-21 overexpression on apoptosis and proliferation in HPASMCs were examined. HPASMCs were transfected with AdGFP (10 MOI) or AdPPARγ (10 MOI) for 6 h at the onset of the study. After 24 h of incubation, RSG (10 μM) was added to all HPASMC monolayers and selected HPASMCs were transfected with mature miR-21 mimics (30 nM) or nontargeting scrambled oligonucleotides (30 nM). F: demonstration of the spectrophotometric detection of absorbance at 405 nm reflecting caspase-3 activity as an indicator of apoptosis. G: proliferation was measured in HPASMCs using automated cell counting. A and B: n = 3; *P < 0.05 vs. SCR. C: n = 9; ****P < 0.0001 vs. SCR. D: n = 9 and E: n = 3; **P < 0.01 vs. NOR(−); ***P < 0.001 vs. NOR(−); +P < 0.05 vs. HYP(−); ++P < 0.01 vs. HYP(−). F and G: n = 5–6; **P < 0.01 vs. SCR(−); ***P < 0.001 vs. SCR(−); ****P < 0.0001 vs. SCR(−); ++P < 0.01 vs. miR-21(−); ++++P < 0.0001 vs. miR-21(−).
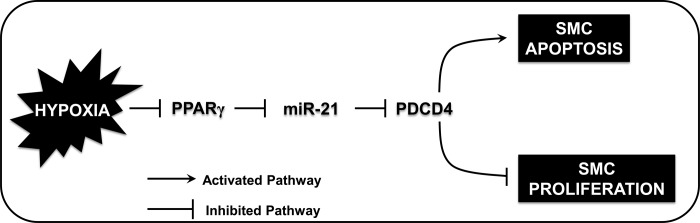
To confirm the proposed inhibitory relationship between PPARγ and miR-21 in this model, HPASMCs were transfected with AdPPARγ or AdGFP + RSG, exposed to normoxic or hypoxic conditions, and miR-21 mRNA was assayed. As shown in Fig. 7E, hypoxia increased HPASMC miR-21 levels, whereas miR-21 expression was significantly reduced in both hypoxia- and normoxia-exposed groups transfected with AdPPARγ in comparison with AdGFP-transfected groups. Finally, we examined the impact of miR-21 overexpression on HPASMC apoptosis signals. As shown in Fig. 7F, in HPASMCs transfected with mature miR-21 mimics, caspase-3 activity was significantly reduced. However, transfection of AdPPARγ enhanced caspase-3 activity in groups in which both scrambled oligonucleotides or miR-21 mimics were transfected. The functional effects of PPARγ overexpression in miR-21- or scrambled-transfected HPASMCs on proliferation were diametrically opposed to their effects on apoptosis signaling displayed in Fig. 7F. Whereas proliferation in HPASMCs transfected with mature miR-21 mimics significantly increased, proliferation was significantly reduced in miR-21-transfected cells in which PPARγ was overexpressed (Fig. 7G). These results demonstrate that PPARγ plays an important upstream role in the suppression of miR-21 levels in HPASMCs, which concomitantly inhibits HPASMC proliferation and enhances apoptosis signals. Collectively, as illustrated in the proposed schema in Fig. 8, our findings indicate that stimuli such as hypoxia that reduce PPARγ levels in HPASMCs increase miR-21 levels, which enhance the translational suppression of PDCD4 and reduce apoptosis in HPASMCs.
Fig. 8.
PPARγ modulates hypoxia-induced alterations in miR-21 and PDCD4 expression to enhance apoptosis and attenuate HPASMC proliferation. The schema depicts the proposed signaling interactions involved in hypoxia-induced HPASMC proliferation. Hypoxia-induced HPASMC proliferation is associated with enhanced expression of miR-21, which causes posttranscriptional reduction in PDCD4 gene translation and contributes to apoptosis resistance. Activation of PPARγ inhibits HPASMC proliferation by blunting the excitatory effects of hypoxia on miR-21 expression, which derepresses PDCD4 and restores the susceptibility of HPASMCs to undergo apoptosis. Collectively, a finely tuned balance of mediators of proliferation and apoptosis is required to maintain vascular wall cell homeostasis.
DISCUSSION
The current study examines the postulate that the miR-21-targeted gene PDCD4 is a central apoptosis mediator whose expression and function is enhanced by PPARγ to increase apoptosis susceptibility and inhibit proliferation of HPASMCs in response to hypoxia. To examine this hypothesis, we transfected HPASMCs with a PPARγ-encoded adenovirus to determine its effects on PDCD4 expression and apoptosis. In addition, miR-21, a PPARγ-regulated target and a key mediator of proliferation in hypoxia-exposed HPASMCs (12), also reduces PDCD4 levels through binding to sites in the PDCD4 3′-UTR (4, 33). PDCD4 translational suppression by miR-21 has been demonstrated in the pathobiology of cancer cell proliferation (26), in proliferative responses to systemic vascular injury (18, 27), and more recently in endothelial apoptosis (43) and HPASMC proliferation (37). The current study therefore extended this existing knowledge by elucidating signaling interactions between PPARγ and miR-21 in the context of the functional effects on PDCD4 regulation, HPASMC proliferation, and apoptosis susceptibility. Our primary findings demonstrated that activation of PPARγ enhanced the expression of PDCD4, restored apoptosis, and inhibited proliferation in hypoxia-exposed HPASMCs. Additional studies designed to characterize the role of the PPARγ ligand activation on the increased expression of PDCD4 were conducted. As shown in Fig. 4, B, C, and E, PDCD4 protein expression only increased in AdPPARγ-transfected HPASMCs when the PPARγ ligand RSG was administered. PPARγ protein levels increased as expected upon transfection of AdPPARγ with or without RSG. These results suggest that levels of endogenous PPARγ ligands may be inadequate to optimally stimulate the overexpression of PDCD4 protein. Ultimately, these findings demonstrate that AdPPARγ significantly increases the PPARγ ligand-dependent expression of PDCD4 in a time-and concentration-dependent fashion.
The absence of putative PPAR-response elements in the PDCD4 promoter suggested that the observed effects of PPARγ on PDCD4 expression occurred through indirect mechanisms. This consideration led us to examine the PPARγ-miR-21-PDCD4 signaling axis in hypoxia-exposed HPASMCs. In a recent study, we demonstrated that treatment with RSG, a pharmacological activator of PPARγ, reduced miR-21 levels in hypoxia-exposed HPASMCs (12). Therefore, we first examined the effects of gain or loss of PPARγ function on miR-21 expression. Consistent with the expected findings, we demonstrated that directly overexpressing PPARγ with the PPARγ adenovirus construct similarly inhibited miR-21 levels, whereas siRNA-mediated PPARγ depletion augmented miR-21 levels in HPASMCs. To mimic hypoxic alterations in these targets, we then examined the effects of PPARγ and PDCD4 depletion and miR-21 overexpression on proliferation in HPASMCs. We demonstrated that basal proliferation increased in all conditions and the magnitude of the proliferative response was similar to that detected in hypoxia-exposed HPASMCs. Furthermore, overexpression of mature miR-21 mimics resulted in an increase in basal proliferation in association with reduced caspase-3 activity and PDCD4 expression. Therapeutically, adenovirus-mediated PPARγ overexpression reversed the aforementioned signaling derangements by 1) attenuating hypoxia-induced miR-21 expression and 2) restoring PDCD4 levels and apoptosis through activation of caspase-3. To our knowledge, this study is to first to describe miR-21 as a therapeutic target of PPARγ, capable of regulating PDCD4 expression and apoptosis in HPASMCs. Collectively, the current study identifies a unique PPARγ-miR-21-PDCD4 signaling axis that regulates the proliferation of hypoxia-exposed HPASMCs by enhancing the susceptibility of HPASMCs to undergo apoptosis.
Although PDCD4 is best known as a tumor suppressor, our study adds to a growing body of literature that identifies PDCD4 as an essential regulator of VSMC apoptosis and proliferation (18, 27, 37). The importance of PDCD4 in the maintenance of VSMC apoptosis and proliferation was demonstrated in a rat carotid artery balloon injury model, wherein PDCD4 levels and apoptosis were significantly reduced in injured vessels in vivo and in PDGF-stimulated aortic VSMCs in vitro (27). The relationship between miR-21 and PDCD4 was further suggested by a related study where balloon-induced carotid vessel injury increased miR-21 expression and proliferation of intimal and medial vascular compartments (18). These findings suggest that vascular injury-induced expression of miR-21 reduces levels of its target gene, PDCD4, and stimulates the proliferation of vascular wall cells in the systemic circulation. Few studies examined miR-21 and PDCD4 in the context of its effects on pulmonary vascular wall cell proliferation. Previous reports demonstrated that hypoxia was associated with increased miR-21 expression in remodeled pulmonary arteries and in the lungs of mice with PH (44). In this study, the delivery of antimiR-21 in vivo attenuated hypoxia-induced vascular remodeling and RV hypertrophy, thus providing additional evidence that confirms the pathogenic role of miR-21 in the development of PH.
In vitro studies further confirmed that miR-21 was associated with HPASMC proliferation and migration, as well as the downregulation of several antiproliferative targets, including PDCD4, Sprouty, PPARα (37), and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) (12). Contrastingly, another study suggested that inactivation of miR-21 stimulated PDCD4 and caspase-3 to promote EC apoptosis, vascular remodeling, and PH (43). The observed heterogeneous effects of miR-21 on EC apoptosis and SMC proliferation may relate to the timing of vascular insults and highlight compartment-specific effects. For example, early reductions in miR-21 enhance PDCD4 expression and stimulate pathogenic EC apoptosis, an increasingly described initial event in development of vascular dysfunction associated with PH (36). In PASMCs, however, early increases in miR-21 enhance proliferation, which contributes to vascular remodeling, increased PVR, and PH. Collectively these considerations highlight the complexity of PH pathobiology and the heterogeneity of molecular events that occur simultaneously in the vascular wall. Although our study sheds light on the role of PPARγ as a potential therapy that inhibits apoptosis resistance in hypoxia-exposed HPASMCs, the complexity and interconnectedness of signaling events required to maintain homeostasis in the vasculature are not adequately captured by our data and are beyond the scope of the current study.
The precise mechanisms by which PPARγ regulates miR-21 expression remain to be defined and constitute an active area of investigation in our laboratory. Hypoxia is a well-known stimulus that activates the expression of the transcription factor AP-1. AP-1 is capable of regulating miR-21 transcription by binding to conserved sites in the miR-21 promoter (6, 33). In ECs, activation of PPARγ decreased AP-1 and NF-κB transcriptional activity (7). Given the totality of these findings, we postulated that the activation of PPARγ attenuates the hypoxia-induced activation of AP-1, thereby inhibiting the transcription of miR-21. Furthermore, because miR-21 suppresses the translation of PDCD4, activation of PPARγ in essence abrogates the repression of PDCD4 and enhances its expression by inhibiting the stimulatory effects of hypoxia on miR-21 transcription.
Furthermore, we observed that increased PDCD4 expression in PPARγ-treated groups occurred in association with reduced HPASMC proliferation in both normoxic and hypoxic conditions. On the other hand, PPARγ had no effect on caspase-3 activity in normoxia-exposed HPASMCs, despite the observed stimulatory effects on PDCD4 expression and inhibitory effects on HPASMC proliferation in this group. In hypoxic conditions, activation of PPARγ jointly stimulated PDCD4 and caspase-3 activity while reducing proliferation. These findings may reflect the occurrence of several possible scenarios. First, PPARγ appears to stimulate apoptosis most substantially in the hypoxic environment in which resistance to apoptosis is enhanced and apoptosis itself is reduced. This observation is underscored by the lack of effect of PPARγ on apoptosis as detected by caspase-3 activity in normoxia. This effect may be explained by the presence of other redox-sensitive apoptosis mediators such as PTEN and bone morphogenetic protein receptor 2, whose expression levels in normoxic environments are sufficient to maintain apoptosis and counterbalance proliferative signals in the absence of additional PPARγ stimulation.
Second, the absence of a normoxia-associated increase in caspase-3 activation upon the stimulation of PPARγ may signify that although PDCD4 is an important mediator of apoptosis, its functional effects on apoptosis may not completely depend upon caspase-3 activation. In fact, caspase-independent cell death has been observed as demonstrated by clear evidence of apoptosis and DNA fragmentation in a caspase-9−/− thymocyte model (22, 40). Third, our findings may reflect the limited capability of the caspase-3 activity assay to capture the complexity of apoptosis signaling events in the stressed cell. We therefore further analyzed our findings by employing an ELISA-based cytochrome-c detection assay and conducting an annexin V detection assay using FACS cell sorting analysis. Our data confirmed that in PPARγ and PDCD4-depleted HPASMCs, the detection of the critical apoptosis protein cytochrome-c was significantly reduced. Furthermore, we demonstrated that pharmacological activation of PPARγ through administration of RSG to HPASMC monolayers enhanced apoptosis in normoxia-and hypoxia-exposed cells (Fig. 5D). Collectively, our findings demonstrate that activation of PPARγ attenuates proliferation through increases in PDCD4 signaling and apoptosis.
The current study has several important limitations. By focusing on the miR-21-PDCD4 axis as a prototypical pathway that causes apoptosis, our study does not examine the effects of PPARγ on other well-known mediators of apoptosis, such as Fas ligand or B cell lymphoma-2 (BCL-2) family members, Bax/Bak or Bad, which may be involved in the growth inhibitory responses to PPARγ. Unlike PDCD4 and PTEN, the BCL-2 family apoptosis mediators are not directly regulated by miR-21. Although conceptually important to identify other apoptosis mediators involved in pathogenic HPASMC proliferation, our study focus explored the role of miR-21 in the development of the apoptosis-resistant phenotype that characterizes hypoxia-exposed HPASMCs. In the current study, we primarily explore the balance of proliferative and apoptosis signaling mechanisms in vitro in HPASMCs. Although our use of human-derived PASMC enhances the translational relevance of our findings, the preferred use of ex vivo pulmonary artery SMCs derived from mice exposed to hypoxia or SMCs derived from patients with idiopathic pulmonary arterial hypertension would further improve the translational relevance of our findings. Future studies designed to evaluate the effects of PPARγ in mice with targeted knockdown or overexpression of PDCD4 in vascular SMCs will enhance our understanding of the compartment-specific role of PDCD4 on the development of apoptosis resistance and PH in the hypoxic mouse lung in vivo.
In conclusion, we determined that in proliferating HPASMCs exposed to hypoxia, growth-inhibitory signals conferred by PPARγ involved the enhanced expression of PDCD4 and increased caspase-3 activity and apoptosis. We additionally determined that activation of PPARγ restored PDCD4 levels indirectly by inhibiting the expression of miR-21. Collectively, our findings highlight additional mechanisms through which PPARγ achieves desired the antiproliferative end points in PH and underscore the potential utility of future clinical investigations that explore the effects of pharmacological therapies that activate PPARγ.
GRANTS
This study is supported by VA Career Development Award 1IK2 BX001707-01A1 [to D. E. Green, principal investigator (PI)], VA Merit Review Award BX-001786 (to R. T. Sadikot, PI), National Heart, Lung, and Blood Institute Grant R01-HL-102167 (to C. M. Hart, PI), and VA Merit Review Award 1l01BX001910 (to C. M. Hart, PI).
DISCLAIMERS
The contents of the manuscript do not represent the views of the United States Department of VA or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
D.E.G., T.C.M., and C.M.H. conceived and designed research; D.E.G., T.C.M., B.B., and Z.Y. performed experiments; D.E.G., T.C.M., B.-Y.K., B.B., Z.Y., and C.M.H. analyzed data; D.E.G., T.C.M., B.-Y.K., B.B., Z.Y., R.T.S., and C.M.H. interpreted results of experiments; D.E.G., T.C.M., B.-Y.K., B.B., and Z.Y. prepared figures; D.E.G. drafted manuscript; D.E.G., T.C.M., B.-Y.K., R.T.S., and C.M.H. edited and revised manuscript; D.E.G., R.T.S., and C.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
D. E. Green, R. T. Sadikot, and C. M. Hart are staff physicians at the Veterans Affairs (VA) Medical Center, Atlanta, GA, and hold faculty appointments in the Division of Pulmonary Allergy, Critical Care and Sleep Medicine, at Emory University.
REFERENCES
- 1.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation 121: 2045–2066, 2010. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bijli KM, Kleinhenz JM, Murphy TC, Kang BY, Adesina SE, Sutliff RL, Hart CM. Peroxisome proliferator-activated receptor gamma depletion stimulates Nox4 expression and human pulmonary artery smooth muscle cell proliferation. Free Radic Biol Med 80: 111–120, 2015. doi: 10.1016/j.freeradbiomed.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitomsky N, Wethkamp N, Marikkannu R, Klempnauer KH. siRNA-mediated knockdown of Pdcd4 expression causes upregulation of p21(Waf1/Cip1) expression. Oncogene 27: 4820–4829, 2008. doi: 10.1038/onc.2008.115. [DOI] [PubMed] [Google Scholar]
- 4.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454: 56–61, 2008. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 35: 495–516, 2007. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujita S, Ito T, Mizutani T, Minoguchi S, Yamamichi N, Sakurai K, Iba H. miR-21 gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J Mol Biol 378: 492–504, 2008. doi: 10.1016/j.jmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Gao M, Hu Z, Zheng Y, Zeng Y, Shen X, Zhong D, He F. Peroxisome proliferator-activated receptor γ agonist troglitazone inhibits high mobility group box 1 expression in endothelial cells via suppressing transcriptional activity of nuclear factor κB and activator protein 1. Shock 36: 228–234, 2011. doi: 10.1097/SHK.0b013e318225b29a. [DOI] [PubMed] [Google Scholar]
- 8.Göke A, Göke R, Knolle A, Trusheim H, Schmidt H, Wilmen A, Carmody R, Göke B, Chen YH. DUG is a novel homologue of translation initiation factor 4G that binds eIF4A. Biochem Biophys Res Commun 297: 78–82, 2002. doi: 10.1016/S0006-291X(02)02129-0. [DOI] [PubMed] [Google Scholar]
- 9.Gong K, Xing D, Li P, Aksut B, Ambalavanan N, Yang Q, Nozell SE, Oparil S, Chen YF. Hypoxia induces downregulation of PPAR-γ in isolated pulmonary arterial smooth muscle cells and in rat lung via transforming growth factor-β signaling. Am J Physiol Lung Cell Mol Physiol 301: L899–L907, 2011. doi: 10.1152/ajplung.00062.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DE, Kang BY, Murphy TC, Hart CM. Peroxisome proliferator-activated receptor gamma (PPARγ) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation. Pulm Circ 2: 483–491, 2012. doi: 10.4103/2045-8932.105037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DE, Murphy TC, Kang BY, Kleinhenz JM, Szyndralewiez C, Page P, Sutliff RL, Hart CM. The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am J Respir Cell Mol Biol 47: 718–726, 2012. doi: 10.1165/rcmb.2011-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green DE, Murphy TC, Kang BY, Searles CD, Hart CM. PPARγ ligands attenuate hypoxia-induced proliferation in human pulmonary artery smooth muscle cells through modulation of microRNA-21. PLoS One 10: e0133391, 2015. doi: 10.1371/journal.pone.0133391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green DE, Sutliff RL, Hart CM. Is peroxisome proliferator-activated receptor gamma (PPARγ) a therapeutic target for the treatment of pulmonary hypertension? Pulm Circ 1: 33–47, 2011. doi: 10.4103/2045-8932.78101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-γ in mice causes PDGF receptor-β-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol 297: L1082–L1090, 2009. doi: 10.1152/ajplung.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha TY. MicroRNAs in human diseases: from cancer to cardiovascular disease. Immune Netw 11: 135–154, 2011. doi: 10.4110/in.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter AL, Choy JC, Granville DJ. Detection of apoptosis in cardiovascular diseases. Methods Mol Med 112: 277–289, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Jazbutyte V, Thum T. MicroRNA-21: from cancer to cardiovascular disease. Curr Drug Targets 11: 926–935, 2010. doi: 10.2174/138945010791591403. [DOI] [PubMed] [Google Scholar]
- 18.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 100: 1579–1588, 2007. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 19.Jiang LH, Ge MH, Hou XX, Cao J, Hu SS, Lu XX, Han J, Wu YC, Liu X, Zhu X, Hong LL, Li P, Ling ZQ. miR-21 regulates tumor progression through the miR-21-PDCD4-Stat3 pathway in human salivary adenoid cystic carcinoma. Lab Invest 95: 1398–1408, 2015. doi: 10.1038/labinvest.2015.105. [DOI] [PubMed] [Google Scholar]
- 20.Kang BY, Kleinhenz JM, Murphy TC, Hart CM. The PPARγ ligand rosiglitazone attenuates hypoxia-induced endothelin signaling in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 301: L881–L891, 2011. doi: 10.1152/ajplung.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang BY, Park KK, Kleinhenz JM, Murphy TC, Green DE, Bijli KM, Yeligar SM, Carthan KA, Searles CD, Sutliff RL, Hart CM. Peroxisome proliferator-activated receptor γ and microRNA 98 in hypoxia-induced endothelin-1 signaling. Am J Respir Cell Mol Biol 54: 136–146, 2016. doi: 10.1165/rcmb.2014-0337OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94: 325–337, 1998. doi: 10.1016/S0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 23.Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol 8: 706–713, 2011. doi: 10.4161/rna.8.5.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lankat-Buttgereit B, Göke R. Programmed cell death protein 4 (pdcd4): a novel target for antineoplastic therapy? Biol Cell 95: 515–519, 2003. doi: 10.1016/j.biolcel.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Lankat-Buttgereit B, Göke R. The tumour suppressor Pdcd4: recent advances in the elucidation of function and regulation. Biol Cell 101: 309–317, 2009. doi: 10.1042/BC20080191. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Huang K, Yu J. Inhibition of microRNA-21 upregulates the expression of programmed cell death 4 and phosphatase tensin homologue in the A431 squamous cell carcinoma cell line. Oncol Lett 8: 203–207, 2014. doi: 10.3892/ol.2014.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Cheng Y, Yang J, Krall TJ, Huo Y, Zhang C. An essential role of PDCD4 in vascular smooth muscle cell apoptosis and proliferation: implications for vascular disease. Am J Physiol Cell Physiol 298: C1481–C1488, 2010. doi: 10.1152/ajpcell.00413.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Murphy TC, Nanes MS, Hart CM. PPARgamma regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. Am J Physiol Lung Cell Mol Physiol 299: L559–L566, 2010. doi: 10.1152/ajplung.00090.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 27: 4373–4379, 2008. doi: 10.1038/onc.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundgrin EL, Park MM, Sharp J, Tang WH, Thomas JD, Asosingh K, Comhair SA, DiFilippo FP, Neumann DR, Davis L, Graham BB, Tuder RM, Dostanic I, Erzurum SC. Fasting 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year. Ann Am Thorac Soc 10: 1–9, 2013. doi: 10.1513/AnnalsATS.201206-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J; American College of Cardiology Foundation Task Force on Expert Consensus Documents; American Heart Association; American College of Chest Physicians; American Thoracic Society, Inc.; Pulmonary Hypertension Association . ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 53: 1573–1619, 2009. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Mudduluru G, George-William JN, Muppala S, Asangani IA, Kumarswamy R, Nelson LD, Allgayer H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci Rep 31: 185–197, 2011. doi: 10.1042/BSR20100065. [DOI] [PubMed] [Google Scholar]
- 34.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010. doi: 10.1165/rcmb.2008-0132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 122: 4306–4313, 2012. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19: 1178–1180, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol 299: L861–L871, 2010. doi: 10.1152/ajplung.00201.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D34–D41, 2013. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 39.Sutliff RL, Kang BY, Hart CM. PPARgamma as a potential therapeutic target in pulmonary hypertension. Ther Adv Respir Dis 4: 143–160, 2010. doi: 10.1177/1753465809369619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tait SW, Green DR. Caspase-independent cell death: leaving the set without the final cut. Oncogene 27: 6452–6461, 2008. doi: 10.1038/onc.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talotta F, Cimmino A, Matarazzo MR, Casalino L, De Vita G, D’Esposito M, Di Lauro R, Verde P. An autoregulatory loop mediated by miR-21 and PDCD4 controls the AP-1 activity in RAS transformation. Oncogene 28: 73–84, 2009. doi: 10.1038/onc.2008.370. [DOI] [PubMed] [Google Scholar]
- 42.Toshner M, Tajsic T, Morrell NW. Pulmonary hypertension: advances in pathogenesis and treatment. Br Med Bull 94: 21–32, 2010. doi: 10.1093/bmb/ldq012. [DOI] [PubMed] [Google Scholar]
- 43.White K, Dempsie Y, Caruso P, Wallace E, McDonald RA, Stevens H, Hatley ME, Van Rooij E, Morrell NW, MacLean MR, Baker AH. Endothelial apoptosis in pulmonary hypertension is controlled by a microRNA/programmed cell death 4/caspase-3 axis. Hypertension 64: 185–194, 2014. doi: 10.1161/HYPERTENSIONAHA.113.03037. [DOI] [PubMed] [Google Scholar]
- 44.Yang S, Banerjee S, Freitas A, Cui H, Xie N, Abraham E, Liu G. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 302: L521–L529, 2012. doi: 10.1152/ajplung.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young MR, Yang HS, Colburn NH. Promising molecular targets for cancer prevention: AP-1, NF-kappa B and Pdcd4. Trends Mol Med 9: 36–41, 2003. doi: 10.1016/S1471-4914(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 46.Zhou G, Chen T, Raj JU. MicroRNAs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 52: 139–151, 2015. doi: 10.1165/rcmb.2014-0166TR. [DOI] [PMC free article] [PubMed] [Google Scholar]