Abstract
Primary cilia (PC) are solitary cellular organelles that play critical roles in development, homeostasis, and disease pathogenesis by modulating key signaling pathways such as Sonic Hedgehog and calcium flux. The antenna-like shape of PC enables them also to facilitate sensing of extracellular and mechanical stimuli into the cell, and a critical role for PC has been described for mesenchymal cells such as chondrocytes. However, nothing is known about the role of PC in airway smooth muscle cells (ASMCs) in the context of airway remodeling. We hypothesized that PC on ASMCs mediate cell contraction and are thus integral in the remodeling process. We found that PC are expressed on ASMCs in asthmatic lungs. Using pharmacological and genetic methods, we demonstrated that PC are necessary for ASMC contraction in a collagen gel three-dimensional model both in the absence of external stimulus and in response to the extracellular component hyaluronan. Mechanistically, we demonstrate that the effect of PC on ASMC contraction is, to a small extent, due to their effect on Sonic Hedgehog signaling and, to a larger extent, due to their effect on calcium influx and membrane depolarization. In conclusion, PC are necessary for the development of airway remodeling by mediating calcium flux and Sonic Hedgehog signaling.
Keywords: smooth muscle cells, contraction, extracellular matrix, lung, hyaluronan
wound healing after injury is characterized by an intricately coordinated process of tissue regeneration, mesenchymal cell expansion, and scar formation (29). Chronic lung injury promotes an imbalanced wound-healing response leading to airway remodeling (5, 35) that is characteristic of chronic airway diseases such as asthma and chronic obstructive pulmonary disease (COPD; 2, 4). The extracellular matrix mediates the wound-healing response to injury by providing signals to mesenchymal cells. For example, during tissue injury, hyaluronan (HA) is fragmented into short fragments (short HA, or sHA), which promote inflammation, cell migration, contraction, and tissue remodeling (16, 27, 41). Therefore, understanding the communication between mesenchymal cells and matrix components such as sHA may provide novel treatments for airway remodeling in asthma and COPD.
Communication between mesenchymal cells and the extracellular matrix requires a means of sensing changes within the matrix that occur as result of injury or disease development. Primary cilia (PC) have emerged as major contributors to cellular sensing of the microenvironment (13). PC are structurally related to motile cilia, but unlike motile cilia, PC are solitary (1 per cell), are ubiquitous (almost every cell can develop a PC at some point in its lifetime; 59, 60) and play crucial roles in cell division, differentiation, and migration (14, 28, 36). Mutations in PC-related genes are a common genetic cause of polycystic kidney disease and syndromes such as Bardet-Biedl, Joubert, Meckel disease, and others (8, 28).
Recent literature supports a connection of PC to lung biology, because several of the genetic syndromes of PC-related mutations have associated lung phenotypes, such as bronchiectasis (23). In addition, in vertebrates, PC play a crucial role in Sonic Hedgehog (Shh) signaling (28, 37), which is a an important regulator of lung morphogenesis (42, 47), is activated in lung fibrosis (9), and has recently been shown to mediate cellular quiescence and repair after lung injury (53). PC also have important roles in mechanotransduction and regulation of calcium flux (19, 45, 69). It has long been known that mechanical loading is closely linked to remodeling (56). Persistent airway smooth muscle cell (ASMC) contraction contributes to airway remodeling through mechanotransduction pathways (56), and ASMC contraction is mediated in part by changes in intracellular calcium flux (7, 34). Because PC modulate calcium influx in response to mechanical stimuli (45, 55, 69), connections between PC and ASMC contraction, calcium flux, and airway remodeling are possible.
PC have been described in many mesenchymal cells, e.g., chondrocytes, osteocytes, and fibroblasts (33, 40, 72), but only a single report has described the presence of PC in ASMCs (71). In that report, mechanotransduction sensory molecules such as α2-, α5-, and β1-integrins and EGF receptor were localized on PC, and PC loss resulted in deficient migration and wound repair in vitro, suggesting an important role of PC in airway repair and remodeling. ASMCs are central players of airway remodeling [reviewed by Noble et al. (51)]. We thus hypothesized that PC in ASMCs are necessary for cell contractility either at baseline or in response to stimuli in their microenvironment. We found that PC are expressed in asthmatic remodeled airways. Furthermore, inhibition of PC by pharmacological or genetic means significantly curtails the ability of human ASMCs to contract in a three-dimensional gel model. PC mediation of cell contractility occurs to a lesser degree through the Shh pathway, but mainly because PC are necessary for calcium flux and membrane depolarization of ASMCs. Thus we provide for the first time evidence linking PC with ASMC function in remodeling.
MATERIALS AND METHODS
Detection of PC in ASMCs and asthmatic lung tissue.
Normal human ASMCs (Lonza, Walkerville, MD) were grown to confluence in smooth muscle cell growth media (SmGM-2; Lonza) and then serum starved overnight (18–24 h) in basal media. Briefly, cells were fixed with acetone-methanol (1:1 vol/vol) and then blocked for 1 h at room temperature with 1% BSA, 1% milk, and 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) in Tris-buffered saline with Tween 20 (TBST). Following incubation with primary antibodies [mouse anti-acetylated tubulin (1:1,000; Sigma-Aldrich, St. Louis, MO), rabbit anti-γ-tubulin (1:800; Sigma-Aldrich), rabbit anti-ADP-ribosylation factor-like protein 13b (anti-Arl13b; 1:500; Proteintech, Chicago, IL), mouse anti-rootletin (1:500; Santa Cruz Biotechnology; Santa Cruz, CA), or rabbit anti-intraflagellar transport protein 88 homolog (anti-IFT88; 1:250; Proteintech)] or with matching host isotype control 1 to 2 h at room temperature, primary cilia were detected with secondary antibodies, including Alexa Fluor 594 donkey anti-rabbit and donkey anti-mouse and Alexa Fluor 488 donkey anti-rabbit and donkey anti-mouse (Molecular Probes, Thermo Fisher Scientific, Hudson, NH). Four subjects with severe asthma were recruited from the Durham, North Carolina, community. These patients fulfilled criteria for asthma (50) exhibiting a provocative concentration of methacholine resulting in a 20% fall in the forced expiratory volume in 1 s (PC20) of <8 mg/ml and reversibility, as demonstrated by at least a 12% and 200-ml increase in the forced expiratory volume during the first second (FEV1) or the forced vital capacity (FVC) with inhaled albuterol. All subjects provided written informed consent in this Duke University Institutional Review Board-approved protocol (Pro00010753). Subjects underwent bronchoscopy with endobronchial biopsy. Formalin-fixed sections of normal human lung were procured from Abcam (Cambridge, MA; n = 1), OriGene (Rockville, MD; n = 3), and Amsbio (Cambridge, MA; n = 1). Sections of endobronchial biopsies from asthmatic patients and normal lung were deparaffinized in xylenes and rehydrated to TBST. Following antigen retrieval in a pressure cooker (Biocare Medical, Concord, CA) in 1× EDTA, tissues were blocked as described above and stained with rabbit anti-α-smooth muscle actin (1:800; secondary Alexa Fluor 594 donkey anti-rabbit; Abcam) or rabbit anti-Arl13b (1:500; secondary Alexa Fluor 488 donkey anti-rabbit) combined with anti-rootletin (1:500, secondary Alexa Fluor 594 donkey anti-mouse) in adjacent sections to identify primary cilia localization in airway smooth muscle. Coverslips were affixed with ProLong Gold plus 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies, Thermo Fisher Scientific, Hudson, NH), and PC expression was imaged using an Olympus BX-51 microscope (Olympus Scientific Solutions Americas, Waltham, MA) fitted with an Olympus DP-10 digital camera, or captured on a Zeiss LSM-780 (Carl Zeiss, Oberkochen, Germany) using a Plan-Apochromat ×40/1.4 Oil DIC objective. To determine the percentage of cells expressing primary cilia in culture, nuclei (stained with DAPI) and primary cilia (stained with Arl13b and rootletin) were counted from each of four ×400 images of stained cells.
Mechanical stretch of cultured ASMCs.
Six-well amino-treated BioFlex culture plates (Flexcell International, Hillsborough, NC) were coated with type I collagen solution (250 μg/ml; Sigma-Aldrich) 2 h before plating cells. Human ASMCs (Lonza) were plated in SmGM-2 media (Lonza), incubated overnight, and then switched to basal media before cell stretching. Wells were cyclically stretched at 5 or 15% elongation at 0.86 Hz for 24 h using a Flexcell Tension Plus System (Flexcell International), with statically cultured wells as controls. To visualize PC, samples were fixed in 4% paraformaldehyde (PFA) and then incubated with rabbit anti-Arl13b. Alexa Fluor 488 goat anti-rabbit IgG (Proteintech) was used as secondary antibody. Samples were mounted in ProLong Gold with DAPI and imaged on a Zeiss LSM-700 confocal microscope.
Lentiviral knockdown of IFT88 expression in ASMCs.
All lentiviruses were packaged in HEK293T/17 cells (ATCC no. CRL-11268) according to Barde et al. (3). Briefly, 293T cells were transiently transfected with pMD2G, psPAX2, and transfer vector containing the short hairpin RNA (shRNA)-hIFT88 clone (MISSION shRNA, pLKO.1 plasmid backbone; Sigma-Aldrich) or scrambled control using Lipofectamine 2000. ASMCs were infected at a multiplicity of infection (MOI) of 5 for experimental assays. IFT88 expression knockdown was assessed by quantitative real-time PCR using human IFT88-specific KiCqStart SYBR Green predesigned primer pairs (Sigma-Aldrich) in combination with Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) as well as staining for IFT88 localization in the ciliary axoneme as described above.
Collagen gel contraction assay.
Type 1 collagen solution from bovine skin (~3 mg/ml stock; Sigma-Aldrich) was neutralized with 10× PBS and 1 N NaOH to a pH of ~6.5 and then mixed 1:1 with ASMCs in media supplemented with 1 M HEPES buffer to achieve a cell-collagen mixture of 300,000 cells/ml and 1.2 mg collagen/ml. The collagen-cell mixtures were polymerized 2 h in a 37°C incubator, 300 μl of media were added to the gels, and images were collected using an Epson Perfection V-30 scanner and Image Capture software (Epson America, Long Beach, CA). Images were collected at intervals of 0–40 h, and the extent of gel contraction was determined with National Institutes of Health ImageJ software. In some experiments, the gels were supplemented with high-molecular weight hyaluronan (HMW HA; Healon ophthalmic viscoelastic device; Abbott, North Chicago, IL) or Healon sonicated to form short-fragment hyaluronan (sHA; 250–500 kDA), at 0.5 mg/ml, as well as Sonic Hedgehog pathway inhibitors HPI-4 (ciliobrevin A; R&D Systems, Minneapolis, MN) at 5, 10, and 15 µM, or vismodegib (LC Laboratories, Woburn, MO) at 15, 30, or 45 µM. Contracted gels were fixed in 4% PFA, and paraffin sections were prepared for immunofluorescent detection of PC as described above.
Measurement of intracellular calcium levels.
Studies were performed as previously described (44). Briefly, ASMCs, either uninfected or infected with lentiviral IFT88 shRNA or scrambled controls were seeded onto 25-mm glass coverslips in six-well cell culture plates with normal medium and used at confluency 3–4 days postseeding. To measure intracellular calcium, the cells underwent serum starvation for 24 h and then were incubated for 20 min with fura-2 AM (3 μg/ml) in HBSS containing 25 mM MOPS at pH 7.4. Cells were rinsed for an additional 20 min using HBSS + MOPS buffer to remove excess fura-2 AM. Fresh HBSS + MOPS was added to the cells upon transfer of the coverslip to the Attofluor chamber. Data were acquired using a Nikon Eclipse Ti inverted microscope fitted with a ×40 oil immersion objective and Nikon Elements software. Changes in intracellular calcium are reported as the change in the emission ratio of calcium-bound fura (340 nm) to calcium-unbound fura (380 nm). The presence of primary cilia was confirmed under these growth conditions by immunofluorescence detection as described above.
Measurement of ASMC membrane potential.
Serum-starved ASMCs were seeded on glass coverslips and transferred to a recording chamber on the stage of an inverted microscope (Olympus IMT-2) and perfused at a rate of 1 ml/min with a solution containing (in mM) 135 NaCl, 5.4 KCl, 1 MgCl2, 1.8 CaCl2, 5.5 glucose, and 10 HEPES, pH 7.4 (following addition of 1 N NaOH) at room temperature. Membrane potential (Vm) was measured with a Molecular Devices amplifier (Axopatch 200B; Sunnyvale, CA) in fast current-clamp conditions. The procedure consists of establishing a stable gigaseal (10–20 GΩ) between a glass pipette and the cell membrane, followed by rupture of the membrane patch under the pipette. Vm values were read on the digital display of the patch amplifier and simultaneously recorded and stored on a hard drive of a computer equipped with pClamp software (Molecular Devices). The pipette resistance varied from 2 to 3 MΩ when filled with a solution containing (in mM) 135 KCl, 10 NaCl, 2 MgCl2, 10 glucose, 0.1 EGTA, 0.2 Na2ATP, and 10 HEPES, pH 7.2 (1 N KOH). The data were digitized with a Digidata 1440A low-noise digitizer (Molecular Devices) connected to a computer equipped with pClamp software (Molecular Devices).
Statistics.
Data were analyzed with Student’s t-test or one-way ANOVA with Tukey’s post hoc testing using GraphPad Prism software, version 7b.
RESULTS
Human ASMCs express PC.
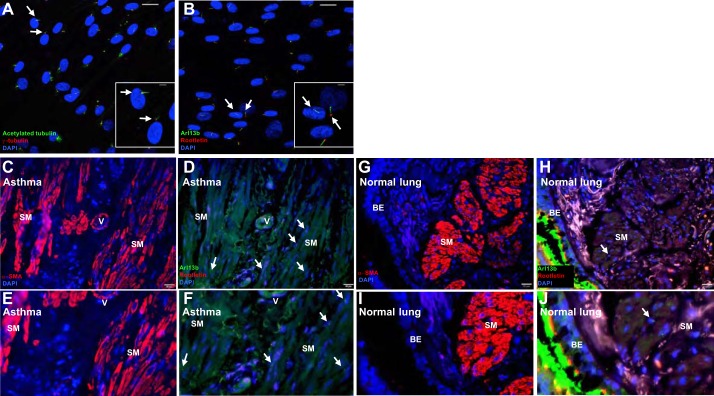
To develop experimental protocols to assess the functional role of PC in airway remodeling, we first determined whether PC were expressed in primary human ASMCs. When stained for proteins expressed in either the axoneme (acetylated tubulin and Arl13b) or basal body (γ-tubulin and rootletin) of the PC, most primary human ASMCs were found to express PC in culture (Fig. 1, A and B). The percentage of ciliated nuclei was determined from four ×400 images of Arl13b plus rootletin-stained cells. About 80% of ASMC nuclei are ciliated when grown under these conditions.
Fig. 1.
Primary cilia are expressed in human airway smooth muscle cells. Primary cilia (PC) were assessed in ASMCs in culture and in airway smooth muscle in human normal and asthmatic lung. A and B: normal ASMCs (Lonza) were grown to confluence on eight-well glass chamber slides and then serum starved overnight. PC were detected with antibodies to acetylated tubulin (green) and γ-tubulin (red; A) or with antibodies to Arl13b (green) and rootletin (red; B). Arrows point to PC. Scale bars = 20 μm (5 μm for insets). C–J: adjacent sections of formalin-fixed human asthmatic (C–F) or healthy (G–J) lung biopsies were stained with antibodies to either α-smooth muscle actin (red; C, E, G, and I) or Arl13b and rootletin (green and red, respectively; D, F, H, and J). Arrows point to PC, to show localization of PC to regions positive for α-smooth muscle actin staining. Nuclei for all images are marked by DAPI (blue). SM, smooth muscle cells; V, vessel; BE, bronchoepithelial cells.
Primary cilia, as marked by Arl13b and rootletin, are present in smooth muscle cells in airways from patients with severe asthma (Fig. 1, C–F), and, as was previously published (71), in normal human lung (Fig. 1, G–F). PC were found in smooth muscle cells in the airways in four out of four asthmatic lung sections and in three out of five normal lung sections, although PC were comparatively less abundant in normal lung. This provides the first evidence of PC expression in human pulmonary disease, localizing them to a region characterized by extensive smooth muscle expansion typical of airway remodeling.
PC elongate in response to cyclic tensile stress.
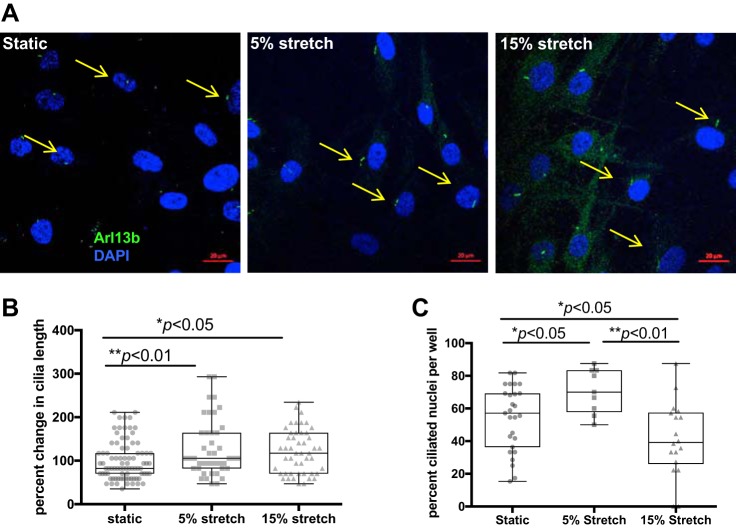
PC are mechanosensory organelles (11, 67, 69). Because mechanical strain and smooth muscle shear stress are central hallmarks of airway remodeling in pulmonary disease, the effect of mechanical stretch on PC was tested in cultured ASMCs. Cells seeded onto collagen-coated BioFlex plates were serum starved overnight and then subjected to cyclical stretch at a 5 and 15% elongation over a 24-h period. PC were identified in fixed ASMCs on the basis of Arl13b expression (Fig. 2A). Compared with static controls, PC length increased significantly at both 5% (P < 0.01) and 15% (P < 0.05) stretch (Fig. 2B). The percentage of ciliated nuclei significantly increased at 5% stretch (P < 0.05) but decreased at 15% (P < 0.05) compared with static controls (Fig. 2C), suggesting that the increasing cyclic tension may result in a trend toward cilia disassembly. These data show that PC respond in a dynamic fashion to mechanical strain exerted on ASMCs, with possible consequences on cellular responses to airway remodeling.
Fig. 2.
Primary cilia length increases in response to mechanical stretch of ASMCs. Cells were cultured on collagen-coated BioFlex culture plates, serum starved overnight, and then subjected to cyclic tensile stretch at a 5 or 15% change in surface area. A: PC were visualized with an antibody to Arl13b (green; nuclei were stained with DAPI in blue). B: percent change in cilia length was determined using ImageJ software to measure individual cilia from confocal images, to compare 5% stretch (n = 41 cilia) and 15% stretch (n = 47 cilia) to static controls (n = 82 cilia). C: percentage of ciliated nuclei was determined, counting the number of nuclei that expressed primary cilia per confocal image (51.6, 69.1, and 41.7% for static, 5% stretch, and 15% stretch, respectively). Data are presented as means ± SE. Scale bar = 20 μm.
Pharmacological inhibition of PC reduces contractile activity in collagen.
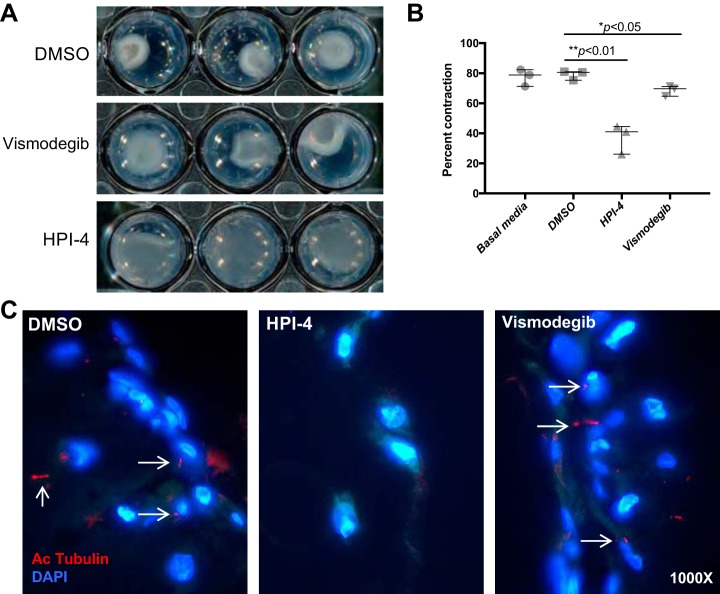
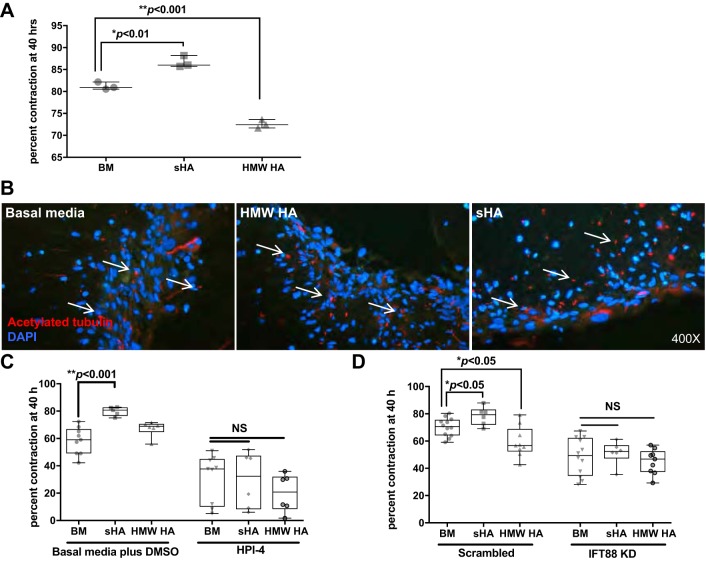
To test the role of PC in airway remodeling, the collagen gel contraction assay was used as an in vitro method of determining how contractile cells such as smooth muscle cells (SMCs) functionally interact with the extracellular matrix. Because PC are critical for the activation of the Shh pathway, which is associated with lung injury response, the effect of inhibition of Shh on ASMC function and PC expression was tested. ASMCs were prepared with media containing the Shh pathway inhibitors vismodegib [which affects localization of Smoothened (Smo) to the ciliary membrane (1, 62)] or HPI-4 [which not only blocks Shh signaling downstream of Smo but also directly impacts ciliogenesis (24, 38)]. On the basis of results of a dose-response test of both inhibitors for ASMC contractility in collagen gels (Table 1), we used a dose of 15 μM for both HPI-4 and vismodegib for experimental assays. Both inhibitors significantly reduced the contraction of collagen gels (P < 0.01 and 0.05 for HPI-4 and vismodegib, respectively), but gels prepared with HPI-4 exhibited significantly decreased contraction compared with those prepared with vismodegib (Fig. 3, A and B, and Table 1). PC were found in ASMCs in gels prepared with DMSO vehicle and vismodegib but were not detectable in the presence of HPI-4 (Fig. 3C). These data suggest that although Shh signaling may mediate SMC contractility to some extent, total PC ablation has a more profound impact on ASMC-mediated collagen gel contraction. These data support potential PC effects on airway remodeling that are independent of Shh signaling.
Table 1.
Dose response of HPI-4 and vismodegib on ASMCs in the collagen gel contraction assay
| Drug | Contraction at 24 h, % | Contraction at 40 h, % | Percent Contraction 0–24 h* | Percent Contraction 0–40 h* |
|---|---|---|---|---|
| HPI-4 | ||||
| DMSO control | 60.896 ± 2.322 | 65.595 ± 2.464 | ||
| HPI-4 (5 μM) | 58.033 ± 2.550 | 56.229 ± 2.971 | NS | P < 0.05 |
| HPI-4 (10 μM) | 56.501 ± 1.521 | 61.011 ± 1.825 | NS | NS |
| HPI-4 (15 μM) | 41.134 ± 3.115 | 48.655 ± 1.769 | P < 0.001 | P < 0.001 |
| Vismodegib | ||||
| DMSO control | 60.832 ± 1.525 | 73.952 ± 1.620 | ||
| Vismodegib (15 μM) | 55.219 ± 0.4298 | 66.982 ± 1.323 | P < 0.05 | P < 0.01 |
| Vismodegib (30 μM) | 49.281 ± 3.609 | 62.673 ± 4.078 | P < 0.05 | P < 0.05 |
| Vismodegib (45 μM) | 60.465 ± 2.295 | 68.383 ± 3.207 | NS | NS |
Values are means ± SE; n = 3 gels analyzed per group.
Statistical comparisons were made using one-way ANOVA, Holm-Sidak post hoc testing, using Prism GraphPad Prism software, version 7.0b. Comparisons were made between DMSO control and dose groups. NS, not significant.
Fig. 3.
Pharmacological inhibition of PC reduces ASMC contractility. A: ASMCs were prepared in basal media supplemented with 15 μM Sonic Hedgehog pathway inhibitors vismodegib (Smoothened inhibitor) or HPI-4 (inhibits downstream of Smoothened) and then combined with neutralized collagen and allowed to contract for up to 20 h. B: gel areas were measured with ImageJ software (n = 3 gels per group); data presented as means ± SE. C: contracted collagen gels were fixed in 4% PFA, and paraffin sections were stained with acetylated tubulin (Ac tubulin; red); nuclei were stained with DAPI (blue). Images are at ×1,000 magnification.
In ASMCs, shRNA-mediated knockdown of IFT88 results in reduced collagen gel contractility.
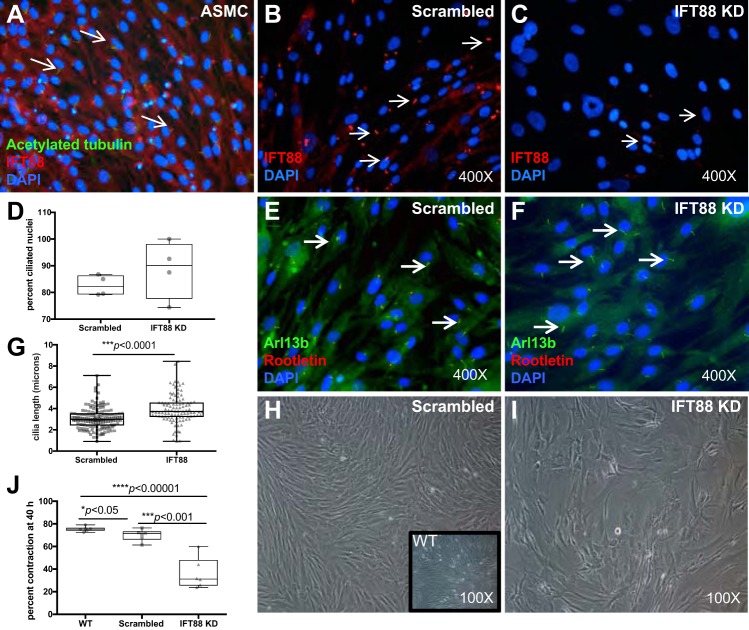
Because pharmacological inhibitors of cilia formation may have off-target effects, we confirmed our observations by genetically disrupting ciliogenesis. This was performed by shRNA-mediated knockdown of the essential ciliary protein, IFT88 (46, 52). IFT88 was expressed in ASMCs (Fig. 4A), and the level of IFT88 mRNA expression was reduced by ~50% in shRNA-transfected cells compared with controls (data not shown). However, staining for IFT88 showed a marked decrease in the IFT88 knockdown (KD) population compared with scrambled control cells (Fig. 4, B and C), demonstrating that IFT88 expression knockdown was affected at the ciliary axoneme level. In addition, expression of the gene Gli1, which is downstream of PC (Shh) signaling, was also decreased by 50%, demonstrating that the IFT88 KD had led to a decrease in PC function. Interestingly, the percentage of ciliated nuclei was similar between scrambled and IFT88 KD cells (Fig. 4D), and PC in the KD cells were slightly longer (P < 0.0001) compared with controls (Fig. 4, E–G). Elongated PC in response to genetic perturbations of ciliary genes have been described before (66). There was also a significant effect of IFT88 knockdown on ASMC morphology (Fig. 4, H and I) and a lower cell yield from flasks containing IFT88 KD cells compared with similarly seeded and cultured control cells (data not shown).
Fig. 4.
Knockdown of IFT88 expression in ASMCs reduces contractile activity in collagen gels. A: confluent and serum-starved ASMCs were stained with antibodies to acetylated tubulin (green) and IFT88 (red) to assess IFT88 expression and localization in PC. IFT88 colocalizes in the ciliary axoneme with acetylated tubulin (arrows). Image is at ×400 magnification. B and C: ASMCs were infected with 5 MOI of lentiviral vector containing shRNA to IFT88 or with scrambled control shRNA. Cells were stained with an antibody to IFT88 (red) to determine levels of expression and localization to PC. Nuclei are shown in blue (DAPI), and images are at ×400 magnification. D: percentage of ciliated nuclei was determined using four ×400 images of Arl13b and rootletin staining to indicate cilia and using DAPI to localize nuclei, comparing scrambled control (82.4%) with IFT88 KD (87.6%). Data are presented as box-and-whiskers plots, whereby boxes represent 25-75 percentile and whickers minimum to maximum values. E and F: to assess PC morphology in ASMCs following knockdown of IFT88 expression, cells were stained with antibodies to Arl13b (green) and rootletin (red). Arrows indicate cilia; images are at ×400 magnification. G: cilia length was measured with ImageJ software, using four ×400 images of Arl13b and rootletin staining to determine relative cilia length comparing scrambled control (n = 139 cilia measured) with IFT88 KD (n = 103 cilia measured). Data are presented as box-and-whiskers plots, whereby boxes represent 25-75 percentile and whickers minimum to maximum values. H and I: scrambled control and IFT88 KD cells were grown for 3 wk in culture, and light microscope images were taken (×100 magnification) to assess cellular morphology. Inset shows noninfected wild-type (WT) cells, at ×100 magnification. J: IFT88 KD and scrambled control ASMCs were combined with neutralized collagen and allowed to contract for 40 h. Gel areas were measured (n = 6 IFT88 KD and n = 6 scrambled control), and percent contraction from time 0 was determined. Data are presented as box-and-whiskers plots, whereby boxes represent 25-75 percentile and whickers minimum to maximum values.
Next, the effect of IFT88 KD on ASMC contractility in collagen gels was determined (Fig. 4J). Over a 40-h period, gels containing IFT88 KD cells were significantly less contracted than those with scrambled control cells (P < 0.0002), indicating that the presence of PC is necessary for constrictive remodeling within the collagen gel matrix. Taken together, both pharmacological and genetic disruption of PC function results in decreased contractility in collagen gels, demonstrating that PC are an important functional component in this process.
PC mediate hyaluronan-induced gel contraction.
In asthma, airway smooth muscle layer expansion is accompanied by deposition of extracellular matrix (ECM) components in the subepithelial space, including the glycosaminoglycan hyaluronan (HA; 25). HA is fragmented into smaller-molecular weight species and can be detected in the bronchial alveolar lavage fluid of patients with virtually every chronic lung disease (10, 43, 58). HA in remodeled lung tissue is associated with airway inflammation and hyperresponsiveness (26, 43). Although PC can interact directly with the ECM through integrins (48, 49, 71), no association has been made between PC and HA. The hypothesis was tested that HA effects on ASMC contractility are mediated by PC, using both high-molecular weight HA (HMW HA; >5,000 kDa) and short-fragment HA (sHA; <500 kDa) to determine whether differences exist between normal and pathological HA.
Both pharmacological inhibition, with HPI-4, and genetic knockdown of IFT88 were used to test the effect of disrupted PC on HA-mediated ASMC contractility in collagen matrices (Fig. 5). Contractility was consistently increased by sHA in ASMCs (Fig. 5A). The effect of HMW HA was more variable, but overall, there was decreased contraction over time in gels supplemented with HMW HA (Fig. 5A), confirming prior observations that sH acts as an ASMC constrictor, whereas HMW HA inhibits ASMC contractility (25, 44). PC formation was not affected by HMW HA or sHA (Fig. 5B). When PC were disrupted with either HPI-4 or IFT88 expression knockdown, gel contraction was reduced, but the addition of either HMW HA or sHA in the presence of disrupted PC failed to affect contraction (Fig. 5, C and D). These data indicate that PC are at least partly necessary for the ASMC contractility response to HA.
Fig. 5.
Hyaluronan-induced ASMC contraction is mediated by PC. A: to determine the effect of high-molecular weight hyaluronan (HMW HA) and short-fragment HA (sHA) on ASMC contraction in collagen gels, cells were prepared in basal media alone (BM) or supplemented with 0.5 mg/ml HMW HA (n = 3) or sHA (n = 3) and combined with neutralized collagen. Gels contracted over 40 h, and gel areas were measured at time 0 and at 40 h to calculate percent contraction over time. Data are presented as means ± SE. B: contracted gels were fixed in 4% PFA and paraffin embedded, and sections were stained with acetylated tubulin (red; nuclei were stained with DAPI in blue) to visualize PC (arrows); images are at ×400 magnification. C: ASMCs were prepared in basal media supplemented with DMSO vehicle control (n = 3) or supplemented with either 0.5 mg/ml sHA plus DMSO (n = 3) or 0.5 mg/ml HMW HA plus DMSO (n = 3; left side of graph). Another set of gels was prepared with ASMCs in basal media plus DMSO as vehicle control (n = 9), with 15 μM HPI-4 plus 0.5 mg/ml sHA (n = 6) or with 15 μM HPI-4 plus 0.5 mg/ml HMW HA (n = 6; right side of graph). Percent contraction over 40 h was determined; data are presented as means ± SE. D: collagen gels were prepared with scrambled control of IFT88 KD ASMCs in basal media alone (n = 12) or supplemented with either sHA (n = 6) or HMW HA (n = 9; left side of graph) and allowed to contract for 40 h. Percent contraction was determined at 40 h; data are presented as means ± SE. NS, not significant.
PC mediate HA effects on calcium flux.
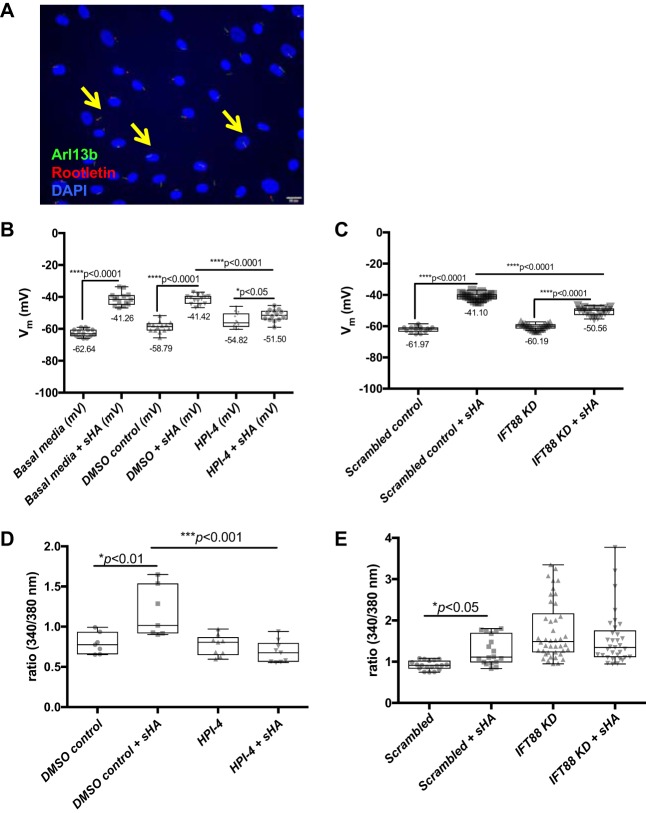
Recent work has demonstrated that sHA induces membrane depolarization and increases intracellular calcium concentration in human ASMCs (44), which is associated with increased ASMC contractility (44). Therefore the effect of disrupted PC on sHA-induced membrane depolarization and calcium flux was investigated to determine whether this pathway provides a potential mechanism through which PC mediate sHA effects on ASMC contractility. PC were detected in ASMCs grown under conditions necessary for depolarization and calcium flux experiments (Fig. 6A). ASMCs in basal media have a resting membrane potential of −62.64 mV, which, upon exposure to sHA, was depolarized to −41.26 mV (Fig. 6B). The same effect was observed in ASMCs grown in basal media supplemented with DMSO as the vehicle control for HPI-4 (−58.79 mV, resting, to −41.42 mV upon sHA exposure; P < 0.0001). HPI-4 significantly attenuated the effect of sHA exposure on cell membrane depolarization, indicating that functional PC are necessary for sHA-mediated membrane depolarization (Fig. 6B). IFT88 KD cells also had a significantly attenuated depolarization response to sHA compared with scrambled shRNA-treated cells, (Fig. 6C), strengthening the conclusion that functional PC facilitate sHA-induced membrane depolarization.
Fig. 6.
Hyaluronan effects on calcium flux are mediated by PC. A: ASMCs were grown on 25-mm coverslips in six-well dishes to confluence and serum starved, and then fixed cells were stained with antibodies to Arl13b (green) and rootletin (red). Scale bar = 20 μm. B and C: membrane depolarization was measured in the following: ASMCs in basal media or basal media plus sHA, basal media plus DMSO vehicle control or DMSO + sHA, or basal media plus HPI-4 or HPI-4 plus sHA (B); scrambled control or IFT88 KD ASMCs with and without sHA (C). D and E: changes in the ratio of calcium-bound to calcium-unbound fura-2 AM were used to determine intracellular calcium flux in the following: ASMCs prepared in basal media plus DMSO or DMSO + sHA compared with HPI-4 or HPI-4 plus sHA (D); scrambled control or IFT88 ASMCs with and without sHA (E). Data are presented as means ± SE.
To investigate further the role of PC in HA-mediated effects on ASMC contractility, the effect of disrupted PC function on HA-mediated influx of calcium (Ca2+) into the intracellular space was assessed. Previous work demonstrated that sHA, but not HMW HA, induced an increase in cytosolic Ca2+ concentration (44). In our hands, sHA also induced a significant increase in cytosolic Ca2+ compared with control (Fig. 6, D and E). The sHA effect on Ca2+ flux was attenuated both in HPI-4-treated ASMCs and in IFT88 KD cells (Fig. 6, D and E), indicating that as with sHA-increased ASMC contraction in collagen gels and sHA-mediated membrane depolarization, functional PC are necessary for sHA-induced Ca2+ current flux in ASMCs. Taken together, these data support the hypothesis that PC play an important role in airway SMC remodeling: PC show a dynamic response to mechanical strain, are necessary for cell contraction in unstimulated cells and in response to the pathological ECM component sHA, and are necessary for sHA-induced Ca2+ flux and membrane depolarization, which are the cellular events leading up to ASMC contraction.
DISCUSSION
In aggregate, these data suggest a mechanistic connection between PC, cell-matrix interactions, and ASMC contraction, which provides important insights into the pathogenesis of airway remodeling. Our results provide evidence suggesting that PC mediate the response of ASMCs to their microenvironment.
Even though PC are, in principle, expressed in every cell type, there is remarkably little knowledge about their expression in the lung. Jain et al. (39) showed that PC are expressed in airway epithelia after cell injury and indicated that PC expression was a marker of undifferentiated, proliferating epithelial cells (39). Wu et al., however, demonstrated PC on ASMCs in situ in human airways and showed that PC on ASMCs mediate cell migration (71). Our findings expand on these results and suggest further mechanisms for PC involvement in airway remodeling.
A primary function of PC is to sense extracellular strain or shear stress, and this function has been described in diverse cells such as renal tubular epithelia (61, 63), chondrocytes (32, 67), and osteoblasts (18). Increased mechanical strain is a central component of airway remodeling (20, 21). Our results suggest that mechanical strain may activate ASMC PC assembly, because PC on ASMCs subjected to cyclic tensile strain responded with increased PC length compared with static cultured cells. In addition, the number of ciliated nuclei increased at low-grade stretch, again supporting a dynamic effect on cilia assembly. IFT88 mRNA expression was not changed with cyclic stretch (data not shown), indicating that stress effects on cilia length are posttranslational in nature. This finding supports the notion that PC become activated in response to strain, which is common in airway constriction or remodeling, and are thus primed to mediate the cellular response to their environment. Our results suggest a positive feedback mechanism between airway remodeling and PC. Remodeling activates PC through mechanical strain, and activated PC are necessary for remodeling. Thus inhibiting PC activity in ASMCs may be a way to disrupt the vicious cycle of remodeling and airway strain.
PC mediate cell responses to both chemical and mechanical triggers (6). HA is an abundant component of the cellular microenvironment, and HA metabolism is altered in airway remodeling. Significantly, sHA expression is upregulated in many diseases in which airway remodeling is a central feature, such as asthma, COPD, idiopathic pulmonary fibrosis, and lung transplant rejection (25, 43). Because sHA in the pathological matrix promotes cell proliferation and contraction, we studied whether PC mediate sHA effects. Both pharmacological and genetic inhibition of PC function significantly abrogated the effect of HA on ASMC contraction. Importantly, PC seem to mediate both the contractile response to sHA and the relaxation response to HMW HA.
Interestingly, in our hands, IFT88 KD cells did not have a decrease in PC abundance and had a slight increase in PC size. Although perhaps counterintuitive, this result is not without precedent. There are several examples of mutations in primary cilia genes that lead to increased primary cilia length, e.g., mutations or deficiency in Kif7 (31), Mks1 and Mks3 (65), Bbs1 (17), and DYNC2LI1 (66). There also may be a cell-specific response to the effect of a genetic perturbation of primary cilia genes on cilia formation. For example, Mks1krc, Mks1del64–323, and B9d1-null mutant mice have disrupted cilia on the embryonic node and limb bud mesenchyme, whereas tracheal and bile duct cilia numbers are not affected (15, 22, 70). Also, cilia size and function may not always be correlated. For example, embryonic fibroblasts isolated from B9d1-null mice possess structurally normal cilia but still demonstrate reduced Shh signaling capacity (22). Our results suggest that IFT88 knockdown can lead to an inhibition of PC function, even though size is not reduced.
Because PC mediate the ASMC response to its pericellular HA matix, the possible mechanism was investigated. PC are essential hubs for Sonic Hedgehog signaling (28, 37). Shh genes (HHIP, PTCH, and CROCC) have recently been implicated in large gene-wide association studies as determinants of decreased lung function (30, 47, 57) and susceptibility to obstructive airway disease (12, 68, 74), and Shh genes have also been implicated in lung fibrosis (73). Recent evidence provides a direct role for Shh in injury, repair, and cellular quiescence in the adult mouse lung (53). Taken together, these studies support a role for PC in lung function and response to injury, because PC are an integral part of Shh signaling. Our results, however, suggest that the effect of PC in ASMC contraction is only partly mediated through Shh signaling. Inhibition of Shh signaling through vismodegib had a modest effect on collagen gel contraction, whereas pharmacological (through HPI-4) or genetic (through IFT88 shRNA) disruption of PC caused a strong inhibition of contraction. Thus PC may mediate other signaling pathways that are important for smooth muscle contractility, such as calcium flux.
Calcium flux is important for many mesenchymal cell functions such as cell contractility and migration and is necessary for the development of airway remodeling and hyperresponsiveness. For example, we recently showed that oxidative lung injury through inhalation of chlorine gas leads to the release of sHA, which promotes ASMC contraction through the induction of calcium influx into the cell and subsequent membrane depolarization (44). Recent research has suggested that PC are necessary for orderly calcium signaling (19, 54, 64), which prompted investigation into how PC modulate calcium flux into the smooth muscle cell as induced by sHA. Our data suggest that PC are necessary for sHA-mediated membrane depolarization and changes in intracellular calcium that ultimately result in SMC contraction. Both pharmacological and genetic disruption of PC function significantly decreased membrane depolarization and completely abolished sHA-induced calcium flux into ASMCs, suggesting that PC must be included in the calcium signaling pathway that mediates airway remodeling and contractility. This does not mean that sHA is directly activating PC or that PC are directly interacting with sHA. However, our findings do suggest that PC are an integral part of the signaling pathway downstream of sHA engagement with its receptors, which leads to calcium flux, membrane depolarization, and, ultimately, cell contraction.
In conclusion, our results show that PC are expressed by ASMCs both in vivo and in vitro, are necessary for ASMC contraction in the three-dimensional matrix, mediate the contractile response of ASMCs to microenvironmental stimuli such as extracellular sHA, and are necessary for calcium flux into the ASMCs in response to sHA. In aggregate, our data suggest a possible mechanistic role for PC in the cellular response to extracellular matrix perturbations (i.e., hyaluronan degradation) and implicate PC in the pathogenesis of airway disease.
GRANTS
This work was conducted in part within the Intramural Research Department of the National Institute of Environmental Health Sciences (C. S. Trempus and S. Garantziotis). This study was supported by National Institutes of Health (NIH) Grants R01-AG-041823 (R. L. Heise) and IU01-ES-026458-01A1 (S. Matalon), a Foundation of Anesthesia Education and Research Research Fellowship (W. Song), and NIH Grants 1R01-HL-086887-01 (J. L. Ingram) and HL-105537 (R. M. Tighe).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.G. conceived and designed research; C.S.T., W.S., A.L., Z.Y., J.R.C., B.M.Y., R.L.H., and S.G. performed experiments; C.S.T., W.S., A.L., Z.Y., J.R.C., R.L.H., Y.R.Y., J.L.I., R.M.T., S.M., and S.G. analyzed data; C.S.T., W.S., A.L., J.R.C., R.L.H., Y.R.Y., J.L.I., R.M.T., S.M., and S.G. interpreted results of experiments; C.S.T., W.S., A.L., Z.Y., J.R.C., B.M.Y., R.L.H., and S.G. prepared figures; C.S.T. and S.G. drafted manuscript; C.S.T., W.S., A.L., J.R.C., R.L.H., Y.R.Y., J.L.I., R.M.T., S.M., and S.G. edited and revised manuscript; C.S.T., W.S., A.L., Z.Y., J.R.C., B.M.Y., R.L.H., Y.R.Y., J.L.I., R.M.T., S.M., and S.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Chip Romeo and Negin Martin [National Institute of Environmental Health Sciences (NIEHS) Viral Vector Core], Jeff Tucker (NIEHS Fluorescence Microscopy and Imaging Center), and Julie Foley and Teena Jones (Special Techniques, National Toxicology Program, Cellular and Molecular Pathology Branch) for excellent technical assistance. We thank Dr. Steve Akiyama and Dr. John Roberts (NIEHS) for critical review of the manuscript.
REFERENCES
- 1.Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol 46: 3–12, 2014. doi: 10.4103/0253-7613.124884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bara I, Ozier A, Tunon de Lara JM, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J 36: 1174–1184, 2010. doi: 10.1183/09031936.00019810. [DOI] [PubMed] [Google Scholar]
- 3.Barde I, Salmon P, Trono D. Production and titration of lentiviral vectors. Curr Protoc Neurosci 53: 4.21, 2010. doi: 10.1002/0471142301.ns0421s53. [DOI] [PubMed] [Google Scholar]
- 4.Berair R, Hollins F, Brightling C. Airway smooth muscle hypercontractility in asthma. J Allergy (Cairo) 2013: 185971, 2013. doi: 10.1155/2013/185971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berair R, Saunders R, Brightling CE. Origins of increased airway smooth muscle mass in asthma. BMC Med 11: 145, 2013. doi: 10.1186/1741-7015-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol 19: R526–R535, 2009. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol 586: 5047–5061, 2008. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development 133: 4131–4143, 2006. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- 9.Bolaños AL, Milla CM, Lira JC, Ramírez R, Checa M, Barrera L, García-Alvarez J, Carbajal V, Becerril C, Gaxiola M, Pardo A, Selman M. Role of Sonic Hedgehog in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 303: L978–L990, 2012. doi: 10.1152/ajplung.00184.2012. [DOI] [PubMed] [Google Scholar]
- 10.Bousquet J, Chanez P, Lacoste JY, Enander I, Venge P, Peterson C, Ahlstedt S, Michel FB, Godard P. Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. J Allergy Clin Immunol 88: 649–660, 1991. doi: 10.1016/0091-6749(91)90159-L. [DOI] [PubMed] [Google Scholar]
- 11.Chen JC, Hoey DA, Chua M, Bellon R, Jacobs CR. Mechanical signals promote osteogenic fate through a primary cilia-mediated mechanism. FASEB J 30: 1504–1511, 2016. doi: 10.1096/fj.15-276402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho MH, Castaldi PJ, Hersh CP, Hobbs BD, Barr RG, Tal-Singer R, Bakke P, Gulsvik A, San José Estépar R, Van Beek EJ, Coxson HO, Lynch DA, Washko GR, Laird NM, Crapo JD, Beaty TH, Silverman EK; NETT Genetics, ECLIPSE, and COPDGene Investigators . A genome-wide association study of emphysema and airway quantitative imaging phenotypes. Am J Respir Crit Care Med 192: 559–569, 2015. doi: 10.1164/rccm.201501-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic 8: 97–109, 2007. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 14.Christensen ST, Pedersen SF, Satir P, Veland IR, Schneider L. The primary cilium coordinates signaling pathways in cell cycle control and migration during development and tissue repair. Curr Top Dev Biol 85: 261–301, 2008. doi: 10.1016/S0070-2153(08)00810-7. [DOI] [PubMed] [Google Scholar]
- 15.Cui C, Chatterjee B, Francis D, Yu Q, SanAgustin JT, Francis R, Tansey T, Henry C, Wang B, Lemley B, Pazour GJ, Lo CW. Disruption of Mks1 localization to the mother centriole causes cilia defects and developmental malformations in Meckel-Gruber syndrome. Dis Model Mech 4: 43–56, 2011. doi: 10.1242/dmm.006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cyphert JM, Trempus CS, Garantziotis S. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol 2015: 563818, 2015. doi: 10.1155/2015/563818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, Berry CJ, Thedens DR, Yang B, Weiss RM, Cassell MD, Stone EM, Sheffield VC. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci USA 104: 19422–19427, 2007. doi: 10.1073/pnas.0708571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaine-Smith RM, Sittichokechaiwut A, Reilly GC. Primary cilia respond to fluid shear stress and mediate flow-induced calcium deposition in osteoblasts. FASEB J 28: 430–439, 2014. doi: 10.1096/fj.13-231894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE. Primary cilia are specialized calcium signalling organelles. Nature 504: 311–314, 2013. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol (1985) 62: 1324–1330, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Dixon AE, Kaminsky DA. Mechanical strain and airway responsiveness: how long does it take, how long will it last? J Appl Physiol (1985) 114: 1504–1505, 2013. doi: 10.1152/japplphysiol.00421.2013. [DOI] [PubMed] [Google Scholar]
- 22.Dowdle WE, Robinson JF, Kneist A, Sirerol-Piquer MS, Frints SG, Corbit KC, Zaghloul NA, van Lijnschoten G, Mulders L, Verver DE, Zerres K, Reed RR, Attié-Bitach T, Johnson CA, García-Verdugo JM, Katsanis N, Bergmann C, Reiter JF. Disruption of a ciliary B9 protein complex causes Meckel syndrome. Am J Hum Genet 89: 94–110, 2011. doi: 10.1016/j.ajhg.2011.06.003. [Erratum. Am J Hum Genet 89(Oct.): 589, 2011. doi:.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driscoll JA, Bhalla S, Liapis H, Ibricevic A, Brody SL. Autosomal dominant polycystic kidney disease is associated with an increased prevalence of radiographic bronchiectasis. Chest 133: 1181–1188, 2008. doi: 10.1378/chest.07-2147. [DOI] [PubMed] [Google Scholar]
- 24.Firestone AJ, Weinger JS, Maldonado M, Barlan K, Langston LD, O’Donnell M, Gelfand VI, Kapoor TM, Chen JK. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature 484: 125–129, 2012. doi: 10.1038/nature10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009. doi: 10.1074/jbc.M802400200. [Erratum. J Biol Chem 291(Sept.): 19257–19258, 2016. doi:. ] [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 26.Garantziotis S, Zudaire E, Trempus CS, Hollingsworth JW, Jiang D, Lancaster LH, Richardson E, Zhuo L, Cuttitta F, Brown KK, Noble PW, Kimata K, Schwartz DA. Serum inter-alpha-trypsin inhibitor and matrix hyaluronan promote angiogenesis in fibrotic lung injury. Am J Respir Crit Care Med 178: 939–947, 2008. doi: 10.1164/rccm.200803-386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghatak S, Maytin EV, Mack JA, Hascall VC, Atanelishvili I, Moreno Rodriguez R, Markwald RR, Misra S. Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int J Cell Biol 2015: 834893, 2015. doi: 10.1155/2015/834893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11: 331–344, 2010. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 89: 219–229, 2010. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, Marciante KD, Franceschini N, van Durme YM, Chen TH, Barr RG, Schabath MB, Couper DJ, Brusselle GG, Psaty BM, van Duijn CM, Rotter JI, Uitterlinden AG, Hofman A, Punjabi NM, Rivadeneira F, Morrison AC, Enright PL, North KE, Heckbert SR, Lumley T, Stricker BH, O’Connor GT, London SJ. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 42: 45–52, 2010. doi: 10.1038/ng.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He M, Subramanian R, Bangs F, Omelchenko T, Liem KF Jr, Kapoor TM, Anderson KV. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol 16: 663–672, 2014. doi: 10.1038/ncb2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Z, Leong DJ, Zhuo Z, Majeska RJ, Cardoso L, Spray DC, Goldring MB, Cobelli NJ, Sun HB. Strain-induced mechanotransduction through primary cilia, extracellular ATP, purinergic calcium signaling, and ERK1/2 transactivates CITED2 and downregulates MMP-1 and MMP-13 gene expression in chondrocytes. Osteoarthritis Cartilage 24: 892–901, 2016. doi: 10.1016/j.joca.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Hellio Le Graverand MP, Ou Y, Schield-Yee T, Barclay L, Hart D, Natsume T, Rattner JB. The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. J Anat 198: 525–535, 2001. doi: 10.1046/j.1469-7580.2000.19850525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol 3: a004549, 2011. doi: 10.1101/cshperspect.a004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirota N, Martin JG. Mechanisms of airway remodeling. Chest 144: 1026–1032, 2013. doi: 10.1378/chest.12-3073. [DOI] [PubMed] [Google Scholar]
- 36.Hsiao YC, Tuz K, Ferland RJ. Trafficking in and to the primary cilium. Cilia 1: 4, 2012. doi: 10.1186/2046-2530-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426: 83–87, 2003. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 38.Hyman JM, Firestone AJ, Heine VM, Zhao Y, Ocasio CA, Han K, Sun M, Rack PG, Sinha S, Wu JJ, Solow-Cordero DE, Jiang J, Rowitch DH, Chen JK. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci USA 106: 14132–14137, 2009. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain R, Pan J, Driscoll JA, Wisner JW, Huang T, Gunsten SP, You Y, Brody SL. Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am J Respir Cell Mol Biol 43: 731–739, 2010. doi: 10.1165/rcmb.2009-0328OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, Bowser SS. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int 28: 101–110, 2004. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 23: 435–461, 2007. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 42.Kugler MC, Joyner AL, Loomis CA, Munger JS. Sonic Hedgehog signaling in the lung. From development to disease. Am J Respir Cell Mol Biol 52: 1–13, 2015. doi: 10.1165/rcmb.2014-0132TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lauer ME, Dweik RA, Garantziotis S, Aronica MA. The rise and fall of hyaluronan in respiratory diseases. Int J Cell Biol 2015: 712507, 2015. doi: 10.1155/2015/712507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazrak A, Creighton J, Yu Z, Komarova S, Doran SF, Aggarwal S, Emala CW Sr, Stober VP, Trempus CS, Garantziotis S, Matalon S. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 308: L891–L903, 2015. doi: 10.1152/ajplung.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee KL, Guevarra MD, Nguyen AM, Chua MC, Wang Y, Jacobs CR. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia 4: 7, 2015. doi: 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehman JM, Michaud EJ, Schoeb TR, Aydin-Son Y, Miller M, Yoder BK. The Oak Ridge Polycystic Kidney mouse: modeling ciliopathies of mice and men. Dev Dyn 237: 1960–1971, 2008. doi: 10.1002/dvdy.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Howard TD, Moore WC, Ampleford EJ, Li H, Busse WW, Calhoun WJ, Castro M, Chung KF, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, Teague WG, Wenzel SE, Peters SP, Hawkins GA, Bleecker ER, Meyers DA. Importance of hedgehog interacting protein and other lung function genes in asthma. J Allergy Clin Immunol 127: 1457–1465, 2011. doi: 10.1016/j.jaci.2011.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu CJ, Du H, Wu J, Jansen DA, Jordan KL, Xu N, Sieck GC, Qian Q. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res 31: 171–184, 2008. doi: 10.1159/000132462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem 54: 1005–1014, 2006. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 50.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma–summary report 2007. J Allergy Clin Immunol 120, Suppl: S94–S138, 2007. doi: 10.1016/j.jaci.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 51.Noble PB, Pascoe CD, Lan B, Ito S, Kistemaker LE, Tatler AL, Pera T, Brook BS, Gosens R, West AR. Airway smooth muscle in asthma: linking contraction and mechanotransduction to disease pathogenesis and remodelling. Pulm Pharmacol Ther 29: 96–107, 2014. doi: 10.1016/j.pupt.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151: 709–718, 2000. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng T, Frank DB, Kadzik RS, Morley MP, Rathi KS, Wang T, Zhou S, Cheng L, Lu MM, Morrisey EE. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature 526: 578–582, 2015. doi: 10.1038/nature14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184: 71–79, 2001. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 55.Raghavan V, Rbaibi Y, Pastor-Soler NM, Carattino MD, Weisz OA. Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. Proc Natl Acad Sci USA 111: 8506–8511, 2014. doi: 10.1073/pnas.1402195111. [Erratum. Proc Natl Acad Sci USA 113(March): E1587, 2016. doi:. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redd SC. Asthma in the United States: burden and current theories. Environ Health Perspect 110, Suppl 4: 557–560, 2002. doi: 10.1289/ehp.02110s4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Repapi E, Sayers I, Wain LV, Burton PR, Johnson T, Obeidat M, Zhao JH, Ramasamy A, Zhai G, Vitart V, Huffman JE, Igl W, Albrecht E, Deloukas P, Henderson J, Granell R, McArdle WL, Rudnicka AR, Barroso I, Loos RJ, Wareham NJ, Mustelin L, Rantanen T, Surakka I, Imboden M, Wichmann HE, Grkovic I, Jankovic S, Zgaga L, Hartikainen AL, Peltonen L, Gyllensten U, Johansson A, Zaboli G, Campbell H, Wild SH, Wilson JF, Gläser S, Homuth G, Völzke H, Mangino M, Soranzo N, Spector TD, Polasek O, Rudan I, Wright AF, Heliövaara M, Ripatti S, Pouta A, Naluai AT, Olin AC, Torén K, Cooper MN, James AL, Palmer LJ, Hingorani AD, Wannamethee SG, Whincup PH, Smith GD, Ebrahim S, McKeever TM, Pavord ID, MacLeod AK, Morris AD, Porteous DJ, Cooper C, Dennison E, Shaheen S, Karrasch S, Schnabel E, Schulz H, Grallert H, Bouatia-Naji N, Delplanque J, Froguel P, Blakey JD, Britton JR, Morris RW, Holloway JW, Lawlor DA, Hui J, Nyberg F, Jarvelin MR, Jackson C, Kähönen M, Kaprio J, Probst-Hensch NM, Koch B, Hayward C, Evans DM, Elliott P, Strachan DP, Hall IP, Tobin MD; Wellcome Trust Case Control Consortium; NSHD Respiratory Study Team . Genome-wide association study identifies five loci associated with lung function. Nat Genet 42: 36–44, 2010. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sahu S, Lynn WS. Hyaluronic acid in the pulmonary secretions of patients with asthma. Biochem J 173: 565–568, 1978. doi: 10.1042/bj1730565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol 69: 377–400, 2007. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 60.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci 123: 499–503, 2010. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol Renal Physiol 272: F132–F138, 1997. [DOI] [PubMed] [Google Scholar]
- 62.Singh BN, Fu J, Srivastava RK, Shankar S. Hedgehog signaling antagonist GDC-0449 (vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PLoS One 6: e27306, 2011. doi: 10.1371/journal.pone.0027306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313: 629–633, 2006. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 64.Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods 10: 1105–1107, 2013. doi: 10.1038/nmeth.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tammachote R, Hommerding CJ, Sinders RM, Miller CA, Czarnecki PG, Leightner AC, Salisbury JL, Ward CJ, Torres VE, Gattone VH II, Harris PC. Ciliary and centrosomal defects associated with mutation and depletion of the Meckel syndrome genes MKS1 and MKS3. Hum Mol Genet 18: 3311–3323, 2009. doi: 10.1093/hmg/ddp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taylor SP, Dantas TJ, Duran I, Wu S, Lachman RS, Nelson SF, Cohn DH, Vallee RB, Krakow D; University of Washington Center for Mendelian Genomics Consortium . Mutations in DYNC2LI1 disrupt cilia function and cause short rib polydactyly syndrome. Nat Commun 6: 7092, 2015. doi: 10.1038/ncomms8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson CL, Chapple JP, Knight MM. Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: a feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthritis Cartilage 22: 490–498, 2014. doi: 10.1016/j.joca.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Durme YM, Eijgelsheim M, Joos GF, Hofman A, Uitterlinden AG, Brusselle GG, Stricker BH. Hedgehog-interacting protein is a COPD susceptibility gene: the Rotterdam Study. Eur Respir J 36: 89–95, 2010. doi: 10.1183/09031936.00129509. [DOI] [PubMed] [Google Scholar]
- 69.Wann AK, Zuo N, Haycraft CJ, Jensen CG, Poole CA, McGlashan SR, Knight MM. Primary cilia mediate mechanotransduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J 26: 1663–1671, 2012. doi: 10.1096/fj.11-193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weatherbee SD, Niswander LA, Anderson KV. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum Mol Genet 18: 4565–4575, 2009. doi: 10.1093/hmg/ddp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu J, Du H, Wang X, Mei C, Sieck GC, Qian Q. Characterization of primary cilia in human airway smooth muscle cells. Chest 136: 561–570, 2009. doi: 10.1378/chest.08-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, Quarles LD. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem 281: 30884–30895, 2006. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang IV, Coldren CD, Leach SM, Seibold MA, Murphy E, Lin J, Rosen R, Neidermyer AJ, McKean DF, Groshong SD, Cool C, Cosgrove GP, Lynch DA, Brown KK, Schwarz MI, Fingerlin TE, Schwartz DA. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax 68: 1114–1121, 2013. doi: 10.1136/thoraxjnl-2012-202943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou X, Baron RM, Hardin M, Cho MH, Zielinski J, Hawrylkiewicz I, Sliwinski P, Hersh CP, Mancini JD, Lu K, Thibault D, Donahue AL, Klanderman BJ, Rosner B, Raby BA, Lu Q, Geldart AM, Layne MD, Perrella MA, Weiss ST, Choi AM, Silverman EK. Identification of a chronic obstructive pulmonary disease genetic determinant that regulates HHIP. Hum Mol Genet 21: 1325–1335, 2012. doi: 10.1093/hmg/ddr569. [DOI] [PMC free article] [PubMed] [Google Scholar]