Abstract
Exposure to environmental particles during pregnancy increases asthma susceptibility of the offspring. We tested the hypothesis that this transmission continues to F2 and F3 generations and occurs via epigenetic mechanisms. We compared allergic susceptibility of three generations of BALB/c offspring after a single maternal exposure during pregnancy to diesel exhaust particles or concentrated urban air particles. After pregnant dams received intranasal instillations of particle suspensions or control, their F1, F2, and F3 offspring were tested in a low-dose ovalbumin protocol for sensitivity to allergic asthma. We found that the elevated susceptibility after maternal exposure to particles during pregnancy persists into F2 and, with lesser magnitude, into F3 generations. This was evident from elevated eosinophil counts in bronchoalveolar lavage (BAL) fluid, histopathological changes of allergic airway disease, and increased BAL levels of IL-5 and IL-13. We have previously shown that dendritic cells (DCs) can mediate transmission of risk upon adoptive transfer. Therefore, we used an enhanced reduced representation bisulfite sequencing protocol to quantify DNA methylation in DCs from each generation. Distinct methylation changes were identified in F1, F2, and F3 DCs. The subset of altered loci shared across the three generations were not linked to known allergy genes or pathways but included a number of genes linked to chromatin modification, suggesting potential interaction with other epigenetic mechanisms (e.g., histone modifications). The data indicate that pregnancy airway exposure to diesel exhaust particles (DEP) triggers a transgenerationally transmitted asthma susceptibility and suggests a mechanistic role for epigenetic alterations in DCs in this process.
Keywords: transgenerational, epigenetics, DNA methylation, asthma
asthma results from a complex interaction of genome, epigenome, and environment (27). Recent advances suggest an important role for epigenetic alterations that may predispose to allergen responsiveness. Epigenetic changes may either occur during an individual’s lifespan, or may be inherited from ancestors (18). DNA methylation changes, e.g., aberrant methylation in certain gene promoter areas have been linked to exposure to environmental (air) particulates and to increased asthma risk (34, 40).
Evidence for inheritance of epigenetic changes in humans is limited, in part, because of the practical difficulties of relating human phenotypes to events decades prior. Smoking during pregnancy is associated with increased asthma susceptibility in grandchildren (24), but this does not meet a strict definition of transgenerational inheritance. To qualify as transgenerational, as opposed to parental or “intragenerational”, an effect must be observed in generations that were not exposed to the triggering stimulus, even as germ cells that are present in an embryo exposed in utero (16). This condition has been satisfied in a rat model, where exposure of pregnant rats to nicotine leads to increased airway resistance and reduced compliance during methacholine challenge in the F3 generation (32). We have shown that F1 offspring of female mice sensitized using the ovalbumin (OVA) challenge asthma model are predisposed to asthma development and that this is associated with altered DNA methylation in dendritic cells (DCs) (8). Furthermore, adoptive transfer of DCs from pups of asthmatic mothers to normal pups also transfers asthma predisposition, indicating a central role for DCs in inheritance of asthma risk. Exposure of pregnant mice to diesel exhaust particles (DEP) or concentrated urban air particles (CAP) results in a similar increase in asthma susceptibility in F1 pups. However, it is not clear whether the effect of these triggers on increased asthma susceptibility can be transmitted transgenerationally by an epigenetic mechanism. Therefore, in this study, we adapted our sensitization model to investigate the role of DC promoter methylation in transgenerational inheritance of asthma predisposition following exposure to particulate air pollution. Here, we report that increased asthma susceptibility after a single maternal exposure to DEP or CAP during pregnancy transmits risk to F1, F2, and, to a lesser extent, F3 generations. This was associated with altered methylation of promoters of genes associated with lung development, IL-4 signaling, and chromatin dynamics. This report, therefore, demonstrates transgenerational inheritance of asthma susceptibility following exposure to environmental particles and provides a candidate mechanism by which epigenetic changes in DCs may mediate this phenomenon. Better understanding of epigenetic origins of asthma may open new avenues to prevention or therapies.
METHODS
Animal model.
BALB/C mice were obtained from Charles River Laboratories (Cambridge, MA). Animal care complied with the Guide for the Care and Use of Laboratory Animals, and all experiments were approved by the Institutional Review Board/Institutional Animal Care and Use Committee: Harvard Center for Comparative Medicine.
Pregnant BALB/c female mice were exposed at gestational days 14 and 15 to intranasal instillations of environmental particles or vehicle control, as described previously (6) and in Fig. 1. Additional F0 mothers included females sensitized to and challenged with OVA, as in our prior work, to serve as positive controls and comparisons; see detailed protocols in Ref. 14. In each generation (F1, F2, and F3), part of the litter (both females and males) was tested at postnatal day 14 (P14) in our intentionally suboptimal low-dose protocol (14) to determine the extent of the response to allergen. Most of the litter remained intact, and the females were allowed to grow and breed into the next generation. A subset of female mice served as donors of DCs; the harvest was performed at P14. In a separate set of experiments, we injected a subset of F1 females with a DNA methyltransferase (DNMT) inhibitor (DNMTi) decitabine immediately before mating into F2 generation (0.1 mg/kg ip) and then tested F2 and F3 generations separately. In each cohort (trial) of the study, we started with seven F0 females per exposure group, assuming ~6 pups per litter and that ~42 pups would be sufficient for analysis and further breeding. The results of four such trials (cohorts) are reported; one additional trial was discontinued due to an unexplained episode of lower breeding rates, producing an insufficient number of pups at F2.
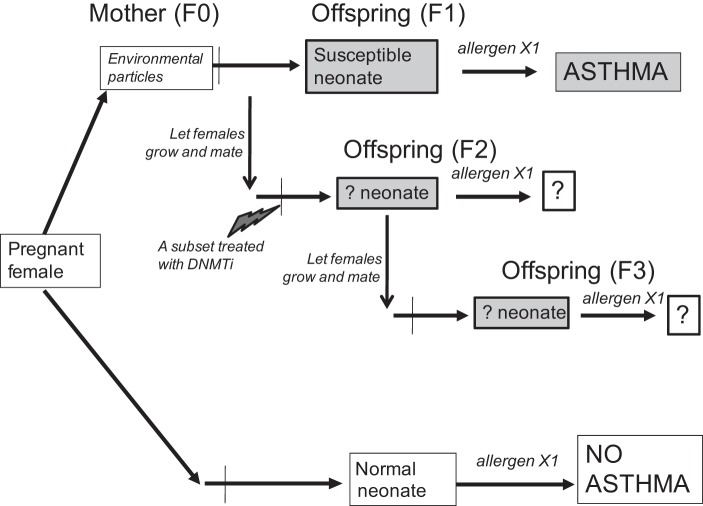
Fig. 1.
Schematic of the transgenerational model. F0 mice were exposed at embryonic day (E) E14–E15 to intranasal instillation of environmental particles; part of their F1 offspring was tested in the X1 low-dose allergen protocol to assess the transmission of asthma risk, while others remained naïve. These naïve females were then mated to normal males, and the study continued to F2, and then similarly to F3.
Exposure protocol.
We used the same well-characterized DEP sample, as reported previously (9), generously provided by Dr. Ian Gilmour at the U.S. Environmental Protection Agency.
In a subset of experiments, we used CAP collected in Boston, as previously described (17). The particles were sonicated on ice before administration. We instilled (50 µg/mouse) under light isoflurane anesthesia. The offspring were divided into three groups: 1) allergen-naïve females (unexposed) for breeding to develop the next generation, 2) allergen-naïve females for DC harvesting, and 3) females and males for testing in our low-dose suboptimal allergen exposure protocol to assay asthma susceptibility as in Ref. 6. Briefly, sensitization was achieved by initial intraperitoneal injections of 5 μg chicken OVA (Sigma) with 1 mg alum in 0.1 ml PBS at P3 followed by aerosols of allergen [3% (wt/vol) OVA in PBS, pH 7.4] for 10 min on three consecutive days at p12–14. The aerosol exposure was performed within individual compartments of a mouse pie chamber (Braintree Scientific, Braintree, MA) using a Pari IS2 nebulizer (Sun Medical Supply, Kansas City, KS) connected to air compressor (PulmoAID; DeVilbiss, Somerset, PA). Analysis was performed the following day after the last aerosol challenge and involved bronchoalveolar lavage and collection of lavage fluid, blood serum, and lung tissue. In a subset of experiments we included a fourth group: allergen-naïve females injected with decitabine.
As a result, the designations of groups in data graphs include first-generation (F1), second-generation (F2), and third-generation (F3) offspring that have arisen in the lineages of either DEP-exposed (DEP), CAP-exposed (CAP), or control vehicle-exposed (Neg Ctrl) mothers. A positive control group (POS Ctrl) comprised OVA-sensitized age-matched asthmatic mice that received two OVA sensitization injections to induce a robust allergic phenotype, which serves to control for the batch of antigen, level of eosinophilia detectable in this group of mice, and other technical causes. In the DNMTi trial, groups labeled “decitabine” designate F2 and F3 offspring from the DEP-exposed F0 grandmother and whose F1 mother was treated with decitabine.
Pathology analysis.
We followed standard protocols for bronchoalveolar lavage and lung tissue analyses. Briefly, animals were euthanized with pentobarbital sodium (Veterinary Laboratories, Lenexa, KS). The chest wall was opened, and the animals were exsanguinated by cardiac puncture. The trachea was cannulated after blood collection. Bronchoalveolar lavage (BAL) was performed five times with 0.3 ml of sterile PBS instilled and harvested gently. Lavage fluid (recovery volume was ~90% of instilled) was collected and centrifuged at 1,200 rpm (300 g) for 10 min, and the cell pellet was resuspended in 0.1 ml PBS. Total cell yield was quantified by hemocytometer. BAL differential cell counts were performed on cytocentrifuge slides prepared by centrifugation of samples at 800 rpm for 5 min (Cytospin 2; Shandon, Pittsburgh, PA). These slides were fixed in 95% methanol and stained with Diff-Quick (VWR, Boston, MA), a modified Wright-Giemsa stain, and a total of 200 cells were counted for each sample by microscopy. Macrophages, lymphocytes, neutrophils, and eosinophils were enumerated. After lavage, the lungs were instilled with 10% buffered formalin, removed, and fixed in the same solution. After paraffin embedding, sections for microscopy were stained with hematoxylin and eosin. For allergy responses, an index of pathological changes in coded hematoxylin-and-eosin slides was derived by scoring the inflammatory cell infiltrates around airways and vessels for greatest severity (0, normal; 1, <3 cell diameter thick; 2, 4–10 cells thick; 3, >10 cells thick) and overall prevalence (0, normal; 1, <25% of sample; 2, 25–50%; 3, >50%). The index was calculated by multiplying severity by prevalence, with a maximum possible score of 9. Levels of IL-5 and IL-13 cytokines in BAL fluid were measured via an ELISA (eBioscience). All samples were tested in duplicates.
Dendritic cell purification for DNA isolation.
Splenic DCs were prepared from collagenase D-treated (Roche) sterile cell suspensions using positive selection (retaining of CD11c+ cells) via the MACS magnetic bead system (Miltenyi Biotec, Auburn, CA). Purity was routinely monitored via flow cytometry (FACSCanto II Becton-Dickinson, Franklin Lakes, NJ) by labeling for CD11c and MHC-II. More than 95% of the purified cells were double-positive for these antigens, and viability was >93% by propidium iodide or trypan blue staining. After purification, the cells were washed two times in LPS-free sterile PBS with 5% BSA and once in pure ice-cold LPS-free sterile PBS.
DNA was isolated using Qiagen DNeasy kit, in complete adherence with the instructions, and after spectrophotometrical assessment of quality, submitted for epigenome-wide methylation profiling.
Epigenomic profiling.
DNA methylation analysis was performed via enhanced reduced representation bisulfite sequencing (eRRBS) at the Weill Cornell Medical Center epigenomic facility (http://epicore.med.cornell.edu/). eRRBS is a method used to prepare DNA for base-pair resolution methylation sequencing analysis, based on the use of a restriction enzyme to enrich for CpG fragments (1). This method is a modification of the original RRBS protocol described by Gu et al. (13), resulting in a 2× increased CpG detection and coverage. ERRBS starts with MspI digestion, followed by NGS library preparation and bisulfite conversion of cytosines. ERRBS yields ~10% of genomic CpG sites (roughly 3M CpGs in the human genome) and provides enrichment in CpG islands and CpG shores, promoters, exons, introns, and intergenic regions.
Pyrosequencing.
Validation of the eRRBS results was performed by targeted evaluation of select targets via pyrosequencing. We designed custom primers for amplification and sequencing using the Pyromark Assay Design SW 2.0 (Qiagen), and the primers were obtained from IDT. DNA samples were bisulfite converted using the EpiTect kit (Qiagen), amplified via the Pyromark PCR Master Mix with HotStartTaq polymerase (Qiagen) for 40 cycles (melt temperature, Tm 54°C), and tested on the Pyromark Q96MD pyrosequencer (Qiagen) using the manufacturer’s recommended procedure.
Data analysis.
With the exception of the eRRBS analysis discussed below, the data are presented as box-and-whisker plots using Prism 5.0 (GraphPad Software, San Diego, CA), where line is the median, plus sign is the mean, box shows the 25th and 75th percentile, and whiskers indicate the 5th and 95th percentiles. Statistical significance was determined using nonparametric criteria: Mann-Whitney U-test for pairwise comparisons and Kruskal-Wallis ANOVA with post hoc tests for multiple comparisons. Significance was accepted when P < 0.05, as designated in the charts via asterisk (usually vs. the negative control group).
eRRBS data were processed as follows. Raw sequencing data were processed into C/T calls, as described previously (11) and CpG sites with more than 10× coverage carried forward for analysis. Data were imported into Methylkit (1) for quality control, and outlier samples (as determined from PCA analysis) and samples without clear sex (as determined by coverage, β value distributions, and β value PCA analysis of CpG sites on the X and Y chromosomes) were discarded. The data were then reimported and converted into bsseq format (15), summing C/T calls from both negative and positive strands into single C/T values for each CpG dyad. Statistical tests were performed at each CpG dyad site to identify differential methylation between control and experimental samples using dispersion shrinkage for sequencing (DSS) (10). DSS models the bisulfite sequencing results using a Bayesian hierarchical model based on the β-binomial distribution to share information across different CpG sites and to account for both biological and sampling variation. All DSS statistical tests were performed by Wald test without smoothing.
Using this strategy, we have identified differentially methylated regions (DMRs) for F1 vs. control, F2 vs. control, and F3 vs. control samples. Venn analysis provided lists of overlapping “hits”, which became sources for network analysis, performed with Metacore software (GeneGo). We used two network algorithms: either “direct interactions” or “Dijkstra’s shortest paths over two intermediaries” to determine known biological links between the factors (genes) associated with the DMRs.
RESULTS
A single DEP exposure induces transgenerational transmission of asthma risk to F1, F2, and to a lesser extent, F3 generation.
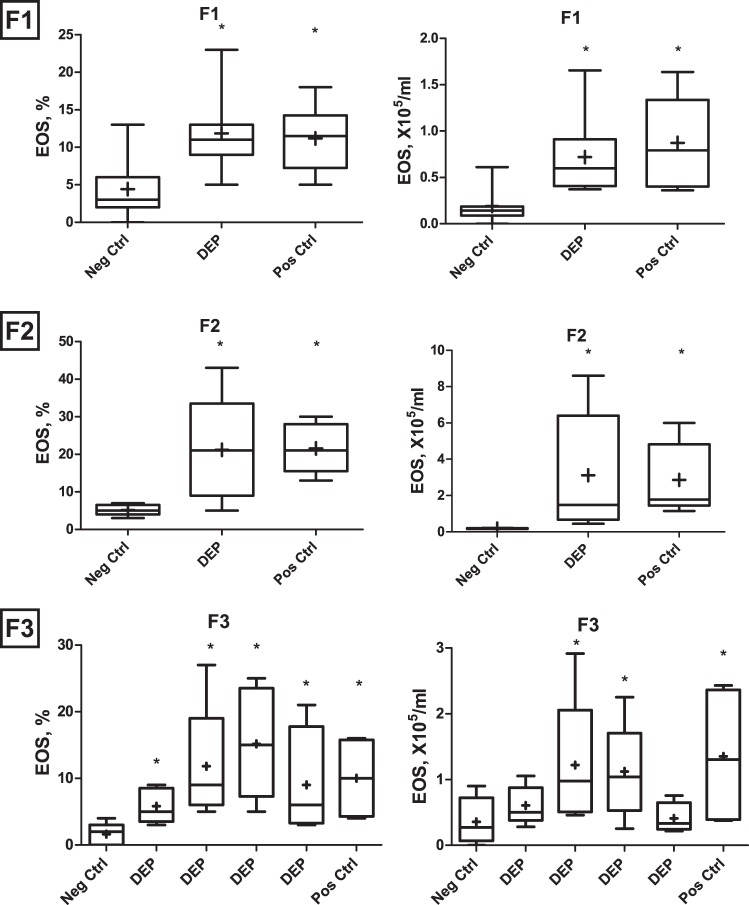
Figure 2 shows BAL eosinophilia in the p14 neonates of F1, F2, and F3 generations descended from F0 mothers exposed during pregnancy to DEP. These neonates received a low-dose OVA sensitization and challenge protocol, which induces little or no response in neonates born from the PBS-exposed ancestor (Neg Ctrl). BAL eosinophilia was assessed as a measure of allergic asthma phenotype. Pups in F1 and F2 showed invariably positive responses, indistinguishable from that in the positive control (higher-dose dual-injection OVA age-matched controls, designated as PosCtrl). Greater variability was apparent in F3 offspring (litters shown separately), especially in total cell numbers, although all litters showed significantly increased percentage of eosinophilia relative to offspring of naïve mothers.
Fig. 2.
Bronchoalveolar lavage (BAL) eosinophil percentages (left) and counts (right) in the F1, F2, and F3 generation offspring of DEP-exposed (DEP) and PBS-exposed control (Neg Ctrl) mothers, as well as positive control asthmatic mice of the same age that received a dual intraperitoneal OVA sensitization protocol (Pos Ctrl). In F3, the four litters of the DEP group are shown separately to illustrate the variability in transmission of the phenotype. We tested multiple litters using from each litter n = 4–6 pups, for a total N behind each bar in the chart to be 6–10 (varies with birth rate and technical issues). *P < 0.05.
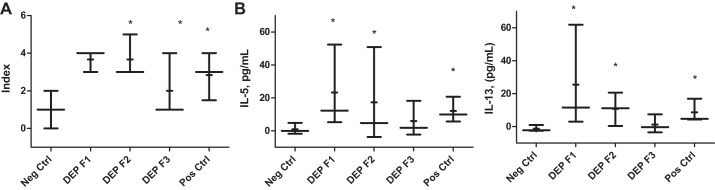
Figure 3 summarizes lung histopathology scores (Fig. 3A) and BAL fluid cytokine levels (Fig. 3, B and C). The histopathological changes and elevated levels of IL-5 and IL-13 are consistent with the BAL counts shown in Fig. 2. Values in the F1 and F2 groups are significantly elevated compared with the negative control; values in F3 show a trend for elevation, but less so than that seen in F1 and F2, suggesting waning of the transmission of susceptibility.
Fig. 3.
A: inflammatory lung histopathology scores in the F1, F2, and F3 generation offspring of DEP-exposed and PBS-exposed control (Neg Ctrl) mothers, as well as X2 OVA-sensitized age-matched positive control asthmatic mice (Pos Ctrl). B: BAL fluid cytokine levels via ELISA. n = 3 per litter. *P < 0.05, Mann-Whitney U-test one-tailed.
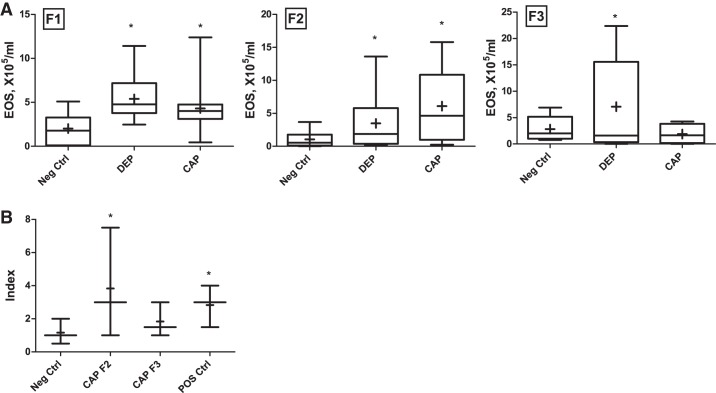
The effects of a different air particulate sample, from CAP, were also evaluated. A comparative analysis of the effect of CAP vs. DEP on transgenerational asthma susceptibility is shown in Fig. 4 (BAL eosinophilia counts are shown in Fig. 4A, while lung histopathology inflammatory index is shown in Fig. 4B). Overall, similar results were obtained. The elevated eosinophil counts and histologic changes indicate that in CAP cohorts, the effect persisted to F2 generation but waned by F3.
Fig. 4.
A: BAL eosinophil counts in the F1, F2, and F3 generation offspring of DEP-exposed in comparison to concentrated urban air particles (CAP)-exposed group, vs. PBS-exposed control (Neg Ctrl). B: lung histopathology scores in the CAP cohorts (F1, data not shown). Number of animals tested was 3–6 per litter, n = 5–10 per group. *P < 0.05.
Epigenome-wide acting DNMT inhibitor treatment abrogates transmission.
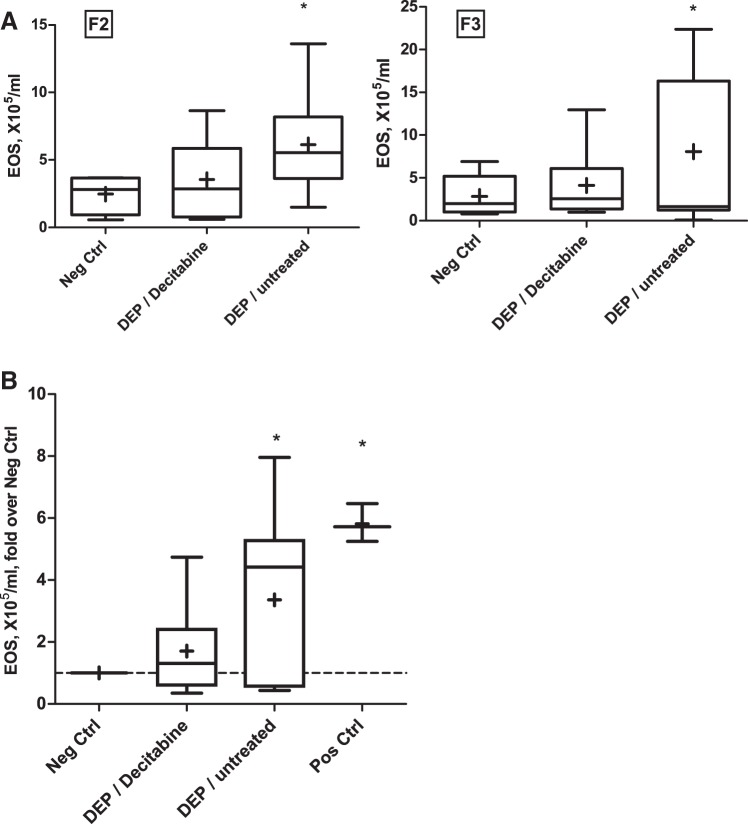
We postulated that an intervention that modifies DNA methylation in the maternal epigenome could abrogate the transmission of the phenotype. Hence, we treated a subset of F1 females from the DEP group (where the transmission of the asthma risk has been confirmed) with a DNMT inhibitor decitabine via intraperitoneal injections for 3 days immediately before mating them to produce the F2 generation. The results indicate that DNMT inhibition led to an attenuation of the asthma risk phenotype in F2 and F3 generations born to the treated F1 females (Fig. 5).
Fig. 5.
Effect of decitabine treatment in F1 on transmission of the phenotype to F2 and F3 generations. A: BAL eosinophil counts from a representative experiment. n = 4–8 per group B: eosinophil counts from three experiments over two separate cohorts were normalized to negative control and pooled. n = 6–12 per group. *P < 0.05.
Dendritic cell methylome.
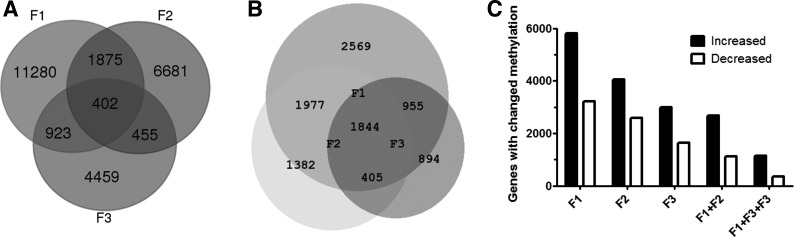
We performed eRRBS methylation profiling on DCs isolated from F1, F2, and F3 mice after the single maternal DEP exposure, and controls (PBS exposed). The percent methylation at ~300,000 individual CpG loci was determined, and F1, F2, and F3 values were compared with control. There were 26,075 loci significantly changed in at least one generation. DEP exposure altered methylation at 14,480 individual CpG loci in the F1 generation, which decreased to 9,413 in F2 and 6,239 in F3. The large majority of these changes were found in only one of the generations (Fig. 6), making it unlikely that they mediate inheritance of DEP-induced asthma susceptibility. The Venn diagrams (Fig. 6) show the extent of overlapping loci. In Fig. 6A, the overlap is based on the exact chromosomal coordinate of the DMRs and shows that a significant methylation change in all three generations vs. control has occurred in 402 specific CpGs. The specific cytosines were then mapped to the nearest annotated gene (see details in methods), so there can be multiple differentially methylated CpGs per gene name. In Fig. 6B, the overlap was dictated by the nearest RefSeq gene; hence, significant methylation changes have been seen in areas associated with 1,844 genes. Interestingly, nearly twice as many sites showed increased methylation as decreased, relative to control (Fig. 6C). This effect is larger when loci that show the same change in two or three generations are considered, implying that genuine, resistant changes in methylation are more likely to be increases than decreases, and, therefore, likely repressive of transcription. This is consistent with the ability of DNMT inhibition to block transmission.
Fig. 6.
Significant differentially methylated regions (DMRs) in F1, F2, and F3 asthma-at-risk dendritic cells (DCs) vs. Control. In A, the overlap is based on the genomic coordinate of the DMR; this demonstrates how many CpGs were altered. B: the specific cytosines were mapped to a nearest annotated gene; we then tested how many genes were involved. There were multiple differentially methylated CpGs per gene name; hence, the numbers differ from A. C: direction of the methylation changes: number of loci with increased or decreased methylation relative in each generation relative to control is plotted. Columns F1+F2, F1+F2+F3 indicate loci that are significantly changed in the same direction in both or all of the indicated generations.
Network analysis.
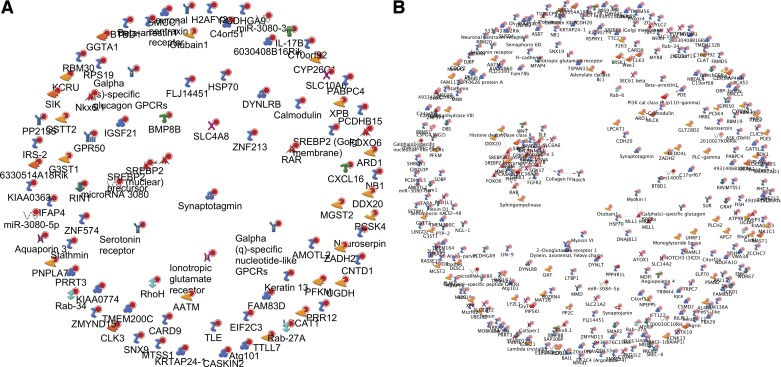
We used GeneGo Metacore to identify networks of interest among the genes with methylation change in all three generations. The results indicate that the interactions among the genes are minimal: most refer to separate networks. This may reflect the fact that most of the change occurs outside of gene bodies or key regulatory areas and relates to noncoding DNA.
Indeed, many of the DMRs were located a great distance away from a nearest known transcript. Specifically, out of the 402 “hits,” only 89 (~22%) were within 10,000 bp of the nearest transcript. Analysis of these factors using the “direct links” pathway analysis algorithm did not reveal any interactions (Fig. 7A). Analysis of the entire 402 DMR list (324 were analyzable by Metacore), i.e., including those mappings that were quite far from the nearest transcript, identified only 30 to be linked in an interacting cluster (~9%, Fig. 7B). A partial list of these DMRs is summarized in Table 1 and shows the substantial magnitude of the methylation changes we observed.
Fig. 7.
Network analysis of a list of genes with associated CpG methylation change within 10-kb distance of the transcript (n = 89) (A) and without the 10 kb filtration (n = 324) (B) seen in F1+F2+F3 generations; “direct links” algorithm. Notice minimal or no involvement in interacting pathways. Various symbols for the genes reflect whether the respective protein is membranous, nuclear, or cytoplasmic, and sometimes a functional designation; these are automatically generated by the software for illustrative purposes only.
Table 1.
Representative loci aberrantly methylated in F1, F2, and F3 DCs vs. control DCs
| Name | RefSeq | Location | Controls | F1 | P Value | F2 | P Value | F3 | P Value |
|---|---|---|---|---|---|---|---|---|---|
| 4931408D14Rik | NR_040298 | chr19:37264008 | 0.057 | 0.820 | 6.09E-06 | 0.944 | 1.48E-11 | 0.899 | 4.27E-07 |
| Prune2 | NM_181348 | chr19:16956275 | 0.891 | 0.035 | 1.66E-07 | 0.070 | 2.11E-05 | 0.052 | 1.72E-06 |
| Rab34 | NM_001159482 | chr11:78188182 | 0.908 | 0.069 | 1.51E-06 | 0.070 | 2.13E-06 | 0.079 | 1.60E-05 |
| Bmp8b | NM_007559 | chr4:123105430 | 0.867 | 0.036 | 2.16E-05 | 0.059 | 0.000134 | 0.039 | 2.32E-05 |
| Snx9 | NM_025664 | chr17:5841662 | 0.934 | 0.026 | 0 | 0.087 | 1.79E-06 | 0.105 | 0.000118 |
| 4930556N09Rik | NR_045358 | chr10:97035459 | 0.039 | 0.737 | 1.39E-07 | 0.730 | 3.99E-05 | 0.871 | 1.10E-05 |
| Grik5 | NM_008168 | chr7:25072054 | 0.911 | 0.040 | 4.15E-10 | 0.089 | 3.16E-05 | 0.076 | 3.70E-06 |
| Rin1 | NM_145495 | chr19:5051147 | 0.919 | 0.102 | 1.69E-08 | 0.036 | 1.57E-11 | 0.052 | 3.04E-09 |
| Cntd1 | NM_026562 | chr11:101279419 | 0.043 | 0.829 | 1.83E-07 | 0.873 | 1.54E-05 | 0.875 | 5.17E-06 |
| Krtap24–1 | NM_001163141 | chr16:88611856 | 0.067 | 0.898 | 8.95E-06 | 0.873 | 0.000168 | 0.955 | 8.15E-12 |
| Pcsk4 | NM_008793 | chr10:80329013 | 0.057 | 0.814 | 1.09E-06 | 0.675 | 0.000118 | 0.926 | 6.12E-10 |
| Krt13 | NM_001313949 | chr11:100121032 | 0.039 | 0.880 | 1.55E-15 | 0.873 | 1.08E-05 | 0.944 | 0 |
| Ago3 | NM_153402 | chr4:126430048 | 0.908 | 0.024 | 1.07E-10 | 0.029 | 2.99E-10 | 0.038 | 1.39E-09 |
| Gstt2 | NM_010361 | chr10:75834324 | 0.760 | 0.015 | 2.12E-05 | 0.047 | 6.91E-05 | 0.045 | 0.000152 |
| Ercc3 | NM_133658 | chr18:32239599 | 0.939 | 0.020 | 0 | 0.089 | 2.28E-06 | 0.103 | 4.71E-05 |
| Gm38404 | NR_073373 | chr6:127308792 | 0.152 | 0.957 | 1.46E-09 | 0.952 | 5.16E-09 | 0.901 | 2.10E-05 |
| Glp1r | NM_021332 | chr17:30902695 | 0.034 | 0.737 | 2.33E-08 | 0.798 | 1.21E-08 | 0.871 | 6.75E-06 |
| 4933440J02Rik | NR_045344 | chr10:111595104 | 0.067 | 0.859 | 1.15E-06 | 0.830 | 1.68E-05 | 0.871 | 0.00013 |
| Serpini1 | NM_009250 | chr3:75558383 | 0.943 | 0.049 | 2.09E-14 | 0.087 | 5.70E-07 | 0.105 | 6.86E-05 |
| Sbk1 | NM_145587 | chr7:126271768 | 0.923 | 0.036 | 7.04E-13 | 0.040 | 4.66E-12 | 0.047 | 1.38E-10 |
| Bloodlinc | NR_131196 | chr11:102376086 | 0.919 | 0.069 | 2.00E-07 | 0.037 | 2.16E-11 | 0.076 | 1.85E-06 |
| Sik1 | NM_010831 | chr17:31856803 | 0.055 | 0.694 | 2.64E-06 | 0.621 | 3.33E-05 | 0.748 | 9.57E-07 |
| Ckmt1 | NM_009897 | chr2:121358868 | 0.940 | 0.029 | 0 | 0.161 | 3.86E-07 | 0.047 | 5.04E-14 |
| Btbd1 | NM_146193 | chr7:81828290 | 0.891 | 0.079 | 1.15E-06 | 0.156 | 0.00018 | 0.051 | 1.36E-06 |
| Naa10 | NM_001177965 | chrX:73920632 | 0.080 | 0.907 | 2.39E-07 | 0.935 | 4.06E-07 | 0.936 | 4.83E-08 |
| Mgst2 | NM_001310482 | chr3:51659781 | 0.458 | 0.919 | 0.00014 | 0.933 | 0.000111 | 0.974 | 7.76E-06 |
| Pcdhb22 | NM_053147 | chr18:37515197 | 0.050 | 0.916 | 4.29E-09 | 0.914 | 1.12E-08 | 0.896 | 4.64E-07 |
| Ccdc177 | NM_001008423 | chr12:80759053 | 0.925 | 0.085 | 2.18E-06 | 0.087 | 3.37E-06 | 0.103 | 9.47E-05 |
| Ugdh | NM_009466 | chr5:65437562 | 0.065 | 0.784 | 0.000231 | 0.895 | 1.01E-05 | 0.912 | 1.91E-07 |
| Rara | NM_001176528 | chr11:98939509 | 0.891 | 0.058 | 4.09E-06 | 0.059 | 4.95E-06 | 0.079 | 8.51E-05 |
| 6030408B16Rik | NR_033803 | chr15:101295042 | 0.133 | 0.716 | 5.59E-05 | 0.818 | 6.72E-06 | 0.772 | 7.97E-05 |
| Nacad | NM_001081652 | chr11:6604158 | 0.867 | 0.096 | 0.00014 | 0.031 | 1.59E-05 | 0.044 | 3.32E-05 |
| Htr6 | NM_021358 | chr4:139076730 | 0.048 | 0.868 | 7.72E-05 | 0.873 | 2.46E-05 | 0.923 | 9.11E-11 |
| H2afy2 | NM_207000 | chr10:61786232 | 0.079 | 0.749 | 3.24E-05 | 0.952 | 1.75E-08 | 0.937 | 1.76E-08 |
| Socs5 | NM_019654 | chr17:87109926 | 0.067 | 0.872 | 1.90E-04 | — | — | 0.896 | 6.19E-06 |
| Caskin2 | NM_080643 | chr11:115810775 | 0.080 | 0.859 | 2.11E-06 | 0.791 | 4.59E-05 | 0.949 | 4.64E-09 |
| Col13a1 | NM_001304757 | chr10:61975935 | 0.927 | 0.246 | 1.06E-04 | — | — | 0.105 | 0.000173 |
| Ikbkb | NM_001159774 | chr8:22703014 | 0.080 | 0.915 | 5.91E-06 | — | — | 0.875 | 1.85E-04 |
| Irs2 | NM_001081212 | chr8:11004807 | 0.041 | 0.965 | 0.00E + 00 | 0.951 | 0.00E + 00 | 0.928 | 1.20E-13 |
| Mfap4 | NM_029568 | chr11:61481270 | 0.067 | 0.823 | 2.16E-05 | 0.732 | 1.94E-04 | 0.747 | 1.12E-04 |
| Slc10a6 | NM_029415 | chr5:103624498 | 0.041 | 0.670 | 3.74E-05 | 0.935 | 2.49E-14 | 0.945 | 0.00E + 00 |
A subset of aberrantly methylated differentially methylated regions (DMRs). DMRs that lie within 2.5 kb of the nearest transcriptional start site and that are significantly changed in F1, F2, and F3 dendritic cells (DCs) vs. control DCs, are shown, as well as DMRs discussed in the text.
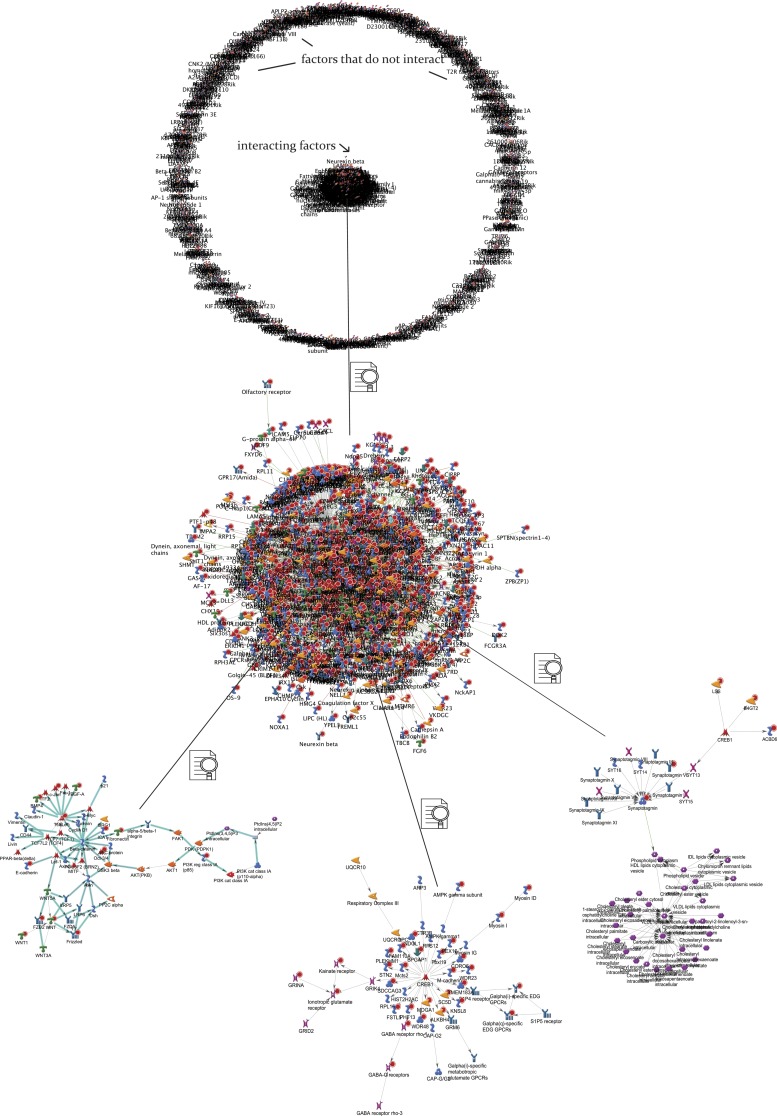
Seeing that the DMRs extending through all three generations did not include familiar asthma-related “suspects,” we have analyzed the F1 DC profiles separately (compared with control DCs) using the same parameters and stringency. In the list of significant DMRs overlapping F1 vs. control only, there were 11,281 “hits,” out of which 2,330 (1,888 annotated in Metacore) were within the 10,000-bp cutoff for proximity to an annotated gene (~21%, similar to the F1F2F3 list). In contrast to the previous results, out of this list, 941 hits (~50%) were interacting in a cluster (Fig. 8); this cluster is expanded in Fig. 8 to reveal in detail three of the more relevant pathways. This suggests that although known biological pathways are associated with the origin of asthma and maternal transmission of asthma risk [as we reported earlier in the maternal allergen model (6, 7, 9, 25) and the DC methylome analysis at F1 level (26)], the transgenerational transmission involves previously unrecognized interactions.
Fig. 8.
A: network analysis of a list of genes with associated CpG methylation change within 10-kb distance of the transcript seen in F1 vs. control within the 10 kb cutoff (n = 1888). Notice a central cluster of genes with known direct interactions in biological pathways. B: central cluster (n = 941) expanded. C: three representative examples dissecting the network interactions within the cluster. The interaction lines illustrate whether the factors are richly or poorly interconnected into nodes.
Many of the F1–F3 altered loci lie at substantial distances from known genes. The nearest—and, therefore, most likely regulated—genes to these sites will likely depend more on three-dimensional arrangement of the chromosome than linear DNA sequence. In the absence of satisfactory methods to identify such targets, we focused on genes that contain differentially methylated CpGs within 5 kb of the transcriptional start site for functional analysis. 51 genes showed significantly increased methylation, while 31 showed decreased methylation that persisted across the three generations. We used Enrichr (23) to obtain functional insight into the roles that these genes may play in predisposing offspring to asthma. Interestingly, the Mouse Gene Atlas score indicates that four of these genes (Mfap4, Slc10a6, Caskin2, and Col13a1) are expressed particularly strongly in lungs.
Because the incomplete genome coverage of CpG sites and the need for multiple-hypothesis P value correction may make requiring differential methylation in each generation overly stringent, we also considered genes that were differentially methylated in F3 and either F1 or F2. Of these, 188 showed an increase in methylation, and 90 showed a decrease. Among the genes with increased methylation was a statistically significant enrichment (three genes, Ikbkb, Irs2, and Socs5, with an enrichment of P = 0.01217) of genes associated with the WikiPathways IL-4 signaling term. Comparing the whole group of differentially methylated genes against the ChIP Enrichment Analysis (ChEA) database, there was a strong overrepresentation of genes that formed a physical association with Suz12 (Enrichr adjusted P = 1.5 × 10−28) and other chromatin remodeling factors, including Sin3B (adjusted P = 2.4 × 10−28), Rcor3 (adjusted P = 2.7 × 10−14), and Tet1 (adjusted P = 1.3 × 10−10). We speculate this may provide insight as to the mechanism by which the methylome of dendritic cells is maintained across generations and/or read to affect gene expression, as discussed next.
DISCUSSION
Here, we show that a single maternal intra-airway exposure to environmental particulate DEP during pregnancy leads to increased asthma risk in F1–F3 progeny. This study extends our prior observations that maternal particle or allergen exposure can be linked to increased asthma onset risk in the immediate (F1) progeny (6, 8, 9). Because we used a gestational exposure here, we traced the phenotype transmission to F3 to follow the stringent definition of “transgenerational” inheritance, as the gametes of the F2 progeny are within the F1 fetus, they might be directly exposed to a toxicant during the F0 exposure (16).
In F3, we observed partial waning of the phenotype in DEP cohorts and complete waning in CAP cohorts. This scenario is frequently seen in models of transgenerational epigenetic transmission (16).
Our methylome analysis suggests that the transmission is associated with aberrant DNA methylation. This is consistent with studies by Rehan et al. (31, 32), showing alterations in global lung methylation levels in a rat model of asthma transmission after a nicotine exposure. One advantage of our model is that we can focus on epigenetic analyses of a key immune cell that we have previously shown is critical in early-life onset of asthma and maternal transmission of asthma risk—the dendritic cell (DC) (8). We also found that using a DNMT inhibitor to reshuffle the methylome-affected transmission. While decitabine and other drugs of this group may have nonmethylome effects, in addition to their effects on an epigenome wide scale, the observation is consistent with the hypothesis that altered CpG methylation in DCs plays a causative role in transmission. Decitabine is known to be teratogenic (2, 35, 38)—this precluded us from using the drug in F0 dams. However, this finding with experimental DNMTi treatment is an encouraging proof-of-principle of the feasibility of new experimental (and potentially therapeutic) approaches that can mitigate aberrant methylation. As reviewed by Krauss-Etschmann et al. (22), studies of transgenerational and truly epigenetic asthma mechanisms are quite limited (27, 31, 36). However, the emerging paradigm that asthma is a disease with a causative epigenetic component (27) could inform curative or preventative approaches that otherwise would not seem to be attainable. We are encouraged that, however indirectly, we can provide a clue to support these future directions. Hence, we focused on the details of aberrant methylation changes.
The methylome analysis indicates that 1) aberrant methylation can be tracked though all three generations of at-risk offspring (we emphasize that the cells were harvested from allergen-naïve neonates at risk for asthma); 2) the majority of these traceable alterations occurs in noncoding areas; 3) the genes involved do not interact in any known pathways, or minimally interact (an area for future exploration); and 4) known asthma-associated genes and pathways are present in F1 offspring but not beyond, which may suggest that while early-life asthma development, in general, involves many known genes, its transgenerational transmission is mediated either by unrecognized ones; however, only a few of these genes are affected throughout.
To expand on this last point, our methylome analysis provided three interesting details. In the first, four genes typically expressed in lung show altered methylation in at least the F1 and F3 generations following DEP exposure. Of these, Mfap4, which encodes an extracellular matrix glycoprotein, microfibrillar associated protein-4, has been associated with both asthma and chronic obstructive pulmonary disease (COPD). Increased MFAP4 expression has been reported in lungs of COPD patients (3, 20) and in bronchial smooth muscle cells of some asthma patients (30). Furthermore, Mfap4 knockout mice show attenuated experimental asthma responses (30), raising the possibility that altered expression of Mfap in F2 and F3 progeny contributes to the enhanced asthma risk. However, although the use of the zebrafish ortholog, mfap4, as a macrophage marker (39) indicates expression on cells of the hematopoietic lineage, the predominant source of MFAP4 in lungs appears to be bronchial smooth muscle cells (30). Therefore, it is unclear to what extent altered promoter methylation in dendritic cells will affect pulmonary MFAP4 and asthma susceptibility. Slc10a6 is another differentially regulated gene strongly expressed in lungs and encodes a transporter for sulfated steroids (12). It is induced by LPS in both mouse livers and Raw267.4 macrophages (21), indicating a role for SLC10A6 and the steroids it transports in inflammation. However, in the lungs, SLC10A6 is predominantly expressed on bronchial epithelial cells (12), so the contribution of dendritic cell Slc10a6 expression to asthma susceptibility again remains to be established.
Second, the contribution of IL-4 signaling to allergic asthma is well established (4), so our observation of enhanced promoter methylation at three regulators of IL-4 signaling is particularly interesting. Of these, expression of both Ikbkb, which encodes IκB inhibitor kinase β, and Socs5, which encodes a member of the suppressors of cytokine signaling family, has been linked to OVA-induced asthma development and severity (28, 37). Thus, the altered methylation of these promoters in dendritic cells of progeny mice may mediate transmission of asthma by altering the Th1/Th2 balance.
The third observation of interest is the strongly significant association of chromatin remodeling factors with differentially methylated genes. This may provide insight into the mechanism by which the differential methylation is achieved, or how the DNA methylation is translated into larger-scale changes in chromatin structure. Suz12 is a component of the polychrome repressor complex-2 (PRC2). It not only recruits DNA methyltransferases (19, 33), but is also essential for histone H3-K27 methylation (5, 29). Thus, its presence at genes whose methylation is altered following DEP exposure raises two mechanistic possibilities, which are not mutually exclusive. 1) Maintenance of baseline methylation at these loci depends on PRC2, and PRC2 activity is affected by DEP exposure. 2) PRC2 transmits the changes in methylation that follow DEP exposure into changes in H3-K27 methylation, which presumably impacts chromatin structure and gene expression. Interestingly, ChIP studies also show that the DNA demethylase Tet1 is significantly associated with genes that show differential methylation following DEP exposure. This hints that methylation of these genes may be dynamically regulated by a balance of PRC2 and Tet1 activities. Studies addressing the role of Suz12 and related factors in effecting DNA methylation changes and interpreting these changes to affect gene expression may, therefore, be informative.
The methylome changes that we detect in the DCs are likely to be reflected in the germline; however, directly studying oocytes to reveal the mechanism of transgenerational transmission is highly challenging: 1) the yield of oocytes from mice is very small; 2) pooling of DNA from different animals (even of the same group) is undesirable in epigenomic studies, and 3) primordial germ cells that could be obtained in larger quantities comprise an epigenetically heterogeneous population and are unlikely to accurately reflect oocytes. Another consideration is that germ cells generally undergo vast epigenomic reprogramming as they mature, and the changes in the DCs that we observe may not be directly derived from something detectable in the gametes as a DMR. Finally, Rehan et al. (31, 32) in the nicotine model have performed a low-resolution, all-genome analysis (and found differences) in the gonads in F1, but it is unclear how to derive mechanistic value from this.
Overall, we conclude that a single gestational exposure to DEP and CAP leads to transgenerational maternal transmission of increased asthma risk to F1, F2, and to a lesser extent F3 generations. The transmission is associated with epigenome-wide DNA methylation changes in dendritic cells and can be abrogated by a DNMT inhibitor treatment.
GRANTS
The study was supported by National Institute of Environmental Health Sciences Grants ES 023281, 025379, 023936, and 00002.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
D.J.G., L.K., and A.V.F. analyzed data; D.J.G., L.K., and A.V.F. edited and revised manuscript; L.K. and A.V.F. conceived and designed research; Z.Y., C.C.M., and A.V.F. performed experiments; A.V.F. interpreted results of experiments; A.V.F. prepared figures; A.V.F. drafted manuscript; A.V.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank John Hutchinson and the Harvard T. H. Chan School of Public Health Bioinformatics team for help with analysis of eRRBS data. We thank Dr. Baldu Pinati for stimulating insights.
REFERENCES
- 1.Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, Mason CE. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol 13: R87, 2012. doi: 10.1186/gb-2012-13-10-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branch S, Francis BM, Brownie CF, Chernoff N. Teratogenic effects of the demethylating agent 5-aza-2′-deoxycytidine in the Swiss Webster mouse. Toxicology 112: 37–43, 1996. doi: 10.1016/0300-483X(96)88183-2. [DOI] [PubMed] [Google Scholar]
- 3.Brandsma C-A, van den Berge M, Postma DS, Jonker MR, Brouwer S, Paré PD, Sin DD, Bossé Y, Laviolette M, Karjalainen J, Fehrmann RSN, Nickle DC, Hao K, Spanjer AIR, Timens W, Franke L. A large lung gene expression study identifying fibulin-5 as a novel player in tissue repair in COPD. Thorax 70: 21–32, 2015. doi: 10.1136/thoraxjnl-2014-205091. [DOI] [PubMed] [Google Scholar]
- 4.Brusselle G, Kips J, Joos G, Bluethmann H, Pauwels R. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am J Respir Cell Mol Biol 12: 254–259, 1995. doi: 10.1165/ajrcmb.12.3.7873190. [DOI] [PubMed] [Google Scholar]
- 5.Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell 15: 57–67, 2004. doi: 10.1016/j.molcel.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Fedulov A, Silverman E, Xiang Y, Leme A, Kobzik L. Immunostimulatory CpG oligonucleotides abrogate allergic susceptibility in a murine model of maternal asthma transmission. J Immunol 175: 4292–4300, 2005. doi: 10.4049/jimmunol.175.7.4292. [DOI] [PubMed] [Google Scholar]
- 7.Fedulov AV, Kobzik L. Immunotoxicologic analysis of maternal transmission of asthma risk. J Immunotoxicol 5: 445–452, 2008. doi: 10.1080/15476910802481765. [DOI] [PubMed] [Google Scholar]
- 8.Fedulov AV, Kobzik L. Allergy risk is mediated by dendritic cells with congenital epigenetic changes. Am J Respir Cell Mol Biol 44: 285–292, 2011. doi: 10.1165/rcmb.2009-0400OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, Kobzik L. Pulmonary exposure to particles during pregnancy causes increased neonatal asthma susceptibility. Am J Respir Cell Mol Biol 38: 57–67, 2008. doi: 10.1165/rcmb.2007-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng H, Conneely KN, Wu H. A Bayesian hierarchical model to detect differentially methylated loci from single nucleotide resolution sequencing data. Nucleic Acids Res 42: e69, 2014. doi: 10.1093/nar/gku154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett-Bakelman FE, Sheridan CK, Kacmarczyk TJ, Ishii J, Betel D, Alonso A, Mason CE, Figueroa ME, Melnick AM. Enhanced reduced representation bisulfite sequencing for assessment of DNA methylation at base pair resolution. J Vis Exp (96): e52246, 2015. doi: 10.3791/52246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosser G, Fietz D, Günther S, Bakhaus K, Schweigmann H, Ugele B, Brehm R, Petzinger E, Bergmann M, Geyer J. Cloning and functional characterization of the mouse sodium-dependent organic anion transporter Soat (Slc10a6). J Steroid Biochem Mol Biol 138: 90–99, 2013. doi: 10.1016/j.jsbmb.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Gu H, Smith ZD, Bock C, Boyle P, Gnirke A, Meissner A. Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat Protoc 6: 468–481, 2011. doi: 10.1038/nprot.2010.190. [DOI] [PubMed] [Google Scholar]
- 14.Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, Coull B, Hubeau C, Kobzik L. Allergen-independent maternal transmission of asthma susceptibility. J Immunol 170: 1683–1689, 2003. doi: 10.4049/jimmunol.170.4.1683. [DOI] [PubMed] [Google Scholar]
- 15.Hansen KD, Langmead B, Irizarry RA. BSmooth: from whole genome bisulfite sequencing reads to differentially methylated regions. Genome Biol 13: R83, 2012. doi: 10.1186/gb-2012-13-10-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157: 95–109, 2014. doi: 10.1016/j.cell.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imrich A, Ning Y, Lawrence J, Coull B, Gitin E, Knutson M, Kobzik L. Alveolar macrophage cytokine response to air pollution particles: oxidant mechanisms. Toxicol Appl Pharmacol 218: 256–264, 2007. doi: 10.1016/j.taap.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33, Suppl: 245–254, 2003. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 19.Jin B, Tao Q, Peng J, Soo HM, Wu W, Ying J, Fields CR, Delmas AL, Liu X, Qiu J, Robertson KD. DNA methyltransferase 3B (DNMT3B) mutations in ICF syndrome lead to altered epigenetic modifications and aberrant expression of genes regulating development, neurogenesis and immune function. Hum Mol Genet 17: 690–709, 2008. doi: 10.1093/hmg/ddm341. [DOI] [PubMed] [Google Scholar]
- 20.Johansson SL, Roberts NB, Schlosser A, Andersen CB, Carlsen J, Wulf-Johansson H, Sækmose SG, Titlestad IL, Tornoe I, Miller B, Tal-Singer R, Holmskov U, Vestbo J, Sorensen GL. Microfibrillar-associated protein 4: a potential biomarker of chronic obstructive pulmonary disease. Respir Med 108: 1336–1344, 2014. doi: 10.1016/j.rmed.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Kosters A, Abebe DF, Felix JC, Dawson PA, Karpen SJ. Inflammation-associated upregulation of the sulfated steroid transporter Slc10a6 in mouse liver and macrophage cell lines. Hepatol Res 46: 794–803, 2016. doi: 10.1111/hepr.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krauss-Etschmann S, Meyer KF, Dehmel S, Hylkema MN. Inter- and transgenerational epigenetic inheritance: evidence in asthma and COPD? Clin Epigenetics 7: 53, 2015. doi: 10.1186/s13148-015-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44: W90––W97., 2016. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y-F, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest 127: 1232–1241, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Lim R, Fedulov AV, Kobzik L. Maternal stress during pregnancy increases neonatal allergy susceptibility: role of glucocorticoids. Am J Physiol Lung Cell Mol Physiol 307: L141–L148, 2014. doi: 10.1152/ajplung.00250.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikhaylova L, Zhang Y, Kobzik L, Fedulov AV. Link between epigenomic alterations and genome-wide aberrant transcriptional response to allergen in dendritic cells conveying maternal asthma risk. PLoS One 8: e70387, 2013. doi: 10.1371/journal.pone.0070387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RL, Ho SM. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med 177: 567–573, 2008. doi: 10.1164/rccm.200710-1511PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohshima M, Yokoyama A, Ohnishi H, Hamada H, Kohno N, Higaki J, Naka T. Overexpression of suppressor of cytokine signalling-5 augments eosinophilic airway inflammation in mice. Clin Exp Allergy 37: 735–742, 2007. doi: 10.1111/j.1365-2222.2007.02707.x. [DOI] [PubMed] [Google Scholar]
- 29.Pasini D, Bracken AP, Jensen MR, Lazzerini Denchi E, Helin K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J 23: 4061–4071, 2004. doi: 10.1038/sj.emboj.7600402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilecki B, Schlosser A, Wulf-Johansson H, Trian T, Moeller JB, Marcussen N, Aguilar-Pimentel JA, de Angelis MH, Vestbo J, Berger P, Holmskov U, Sorensen GL. Microfibrillar-associated protein 4 modulates airway smooth muscle cell phenotype in experimental asthma. Thorax 70: 862–872, 2015. doi: 10.1136/thoraxjnl-2014-206609. [DOI] [PubMed] [Google Scholar]
- 31.Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, Akbari O, Torday JS. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med 10: 129, 2012. doi: 10.1186/1741-7015-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rehan VK, Liu J, Sakurai R, Torday JS. Perinatal nicotine-induced transgenerational asthma. Am J Physiol Lung Cell Mol Physiol 305: L501–L507, 2013. doi: 10.1152/ajplung.00078.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds PA, Sigaroudinia M, Zardo G, Wilson MB, Benton GM, Miller CJ, Hong C, Fridlyand J, Costello JF, Tlsty TD. Tumor suppressor p16INK4A regulates polycomb-mediated DNA hypermethylation in human mammary epithelial cells. J Biol Chem 281: 24,790–24,802, 2006. doi: 10.1074/jbc.M604175200. [DOI] [PubMed] [Google Scholar]
- 34.Salam MT, Byun HM, Lurmann F, Breton CV, Wang X, Eckel SP, Gilliland FD. Genetic and epigenetic variations in inducible nitric oxide synthase promoter, particulate pollution, and exhaled nitric oxide levels in children. J Allergy Clin Immunol 129: 232–239, 2012. doi: 10.1016/j.jaci.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmahl W, Török P, Kriegel H. Embryotoxicity of 5-azacytidine in mice. Phase- and dose-specificity studies. Arch Toxicol 55: 143–147, 1984. doi: 10.1007/BF00346054. [DOI] [PubMed] [Google Scholar]
- 36.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab 21: 214–222, 2010. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugita A, Ogawa H, Azuma M, Muto S, Honjo A, Yanagawa H, Nishioka Y, Tani K, Itai A, Sone S. Antiallergic and anti-inflammatory effects of a novel I κB kinase β inhibitor, IMD-0354, in a mouse model of allergic inflammation. Int Arch Allergy Immunol 148: 186–198, 2009. doi: 10.1159/000161579. [DOI] [PubMed] [Google Scholar]
- 38.Svatá M, Raska K Jr, Sorm F. Interruption of pregnancy by 5-azacytidine. Experientia 22: 53, 1966. doi: 10.1007/BF01897769. [DOI] [PubMed] [Google Scholar]
- 39.Walton EM, Cronan MR, Beerman RW, Tobin DM. The macrophage-specific promoter mfap4 allows live, long-term analysis of macrophage behavior during mycobacterial infection in zebrafish. PLoS One 10: e0138949, 2015. doi: 10.1371/journal.pone.0138949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang IV, Pedersen BS, Liu A, O’Connor GT, Teach SJ, Kattan M, Misiak RT, Gruchalla R, Steinbach SF, Szefler SJ, Gill MA, Calatroni A, David G, Hennessy CE, Davidson EJ, Zhang W, Gergen P, Togias A, Busse WW, Schwartz DA. DNA methylation and childhood asthma in the inner city. J Allergy Clin Immunol 136: 69–80, 2015. doi: 10.1016/j.jaci.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]