Abstract
We studied acute effects of tumor necrosis factor-α (TNFα) on the sensitivity of isolated rat vagal pulmonary sensory neurons. Our results showed the following. First, a brief pretreatment with a low dose of TNFα (1.44 nM, 9 min) enhanced the sensitivity of transient receptor potential vanilloid type 1 (TRPV1) receptors in these neurons in two distinct phases: the inward current evoked by capsaicin was amplified (Δ = 247%) immediately following the TNFα pretreatment, which gradually declined toward control and then increased again reaching another peak (Δ = 384%) after 60–90 min. Second, the immediate phase of this potentiating effect of TNFα was completely abolished by a pretreatment with a selective cyclooxygenase-2 (COX-2) inhibitor, NS-398, whereas the delayed potentiation was only partially attenuated. Third, in sharp contrast, TNFα did not generate any potentiating effect on the responses to non-TRPV1 chemical activators of these neurons. Fourth, the selectivity of the TNFα action on TRPV1 was further illustrated by the responses to acid (pH 6.0); TNFα did not affect the rapid transient current mediated by acid-sensing ion channels but significantly augmented the slow sustained current mediated by TRPV1 in the same neurons. Fifth, in anesthetized rats, a similar pattern of acute sensitizing effects of TNFα on pulmonary C-fiber afferents and the involvement of COX-2 were also clearly shown. In conclusion, a brief pretreatment with TNFα induced both immediate and delayed potentiating effects on the TRPV1 sensitivity in pulmonary sensory neurons, and the production of COX-2 arachidonic acid metabolites plays a major role in the immediate sensitizing effect of TNFα.
Keywords: TNFα, TRPV1, COX, airway inflammation
tumor necrosis factor-alpha (TNFα) is a proinflammatory cytokine and plays an important role in the pathogenesis of allergic asthma. TNFα is released from a variety of cell types in the airways, particularly macrophages, monocytes, and mast cells. TNFα is detected in bronchoalveolar lavage fluid, sputum, and serum of asthmatic patients during acute asthmatic exacerbation or following antigen inhalation challenge (8, 27, 41). Indeed, inhalation of TNFα can induce airway hyperresponsiveness accompanied by neutrophil infiltration and airway inflammation in healthy humans (56). TNFα is also known to exert multiple potent effects on several types of cells in the airways: for example, it is a chemoattractant for neutrophils and eosinophils, upregulates the leukocyte-endothelial cell adhesion molecule E-selectin, enhances the production of Th2 cytokines, and increases microvascular permeability (5, 28, 56).
Previous investigators have shown that a prolonged (3–24 h) pretreatment with TNFα induced a sensitizing effect on the nociceptive neurons in dorsal root ganglia (DRG) and contributed to the development of lingering inflammatory pain in somatic tissues (10, 45). It has been suggested that this hyperalgesic effect was mediated through an increase in the sensitivity and/or expression of transient receptor potential vanilloid type 1 (TRPV1) receptors in DRG neurons (19). TRPV1 is also abundantly expressed in vagal bronchopulmonary C-fiber sensory nerves that are known to play an important role in regulating cardiopulmonary functions in healthy and pathophysiological conditions (9, 36), especially in airway inflammatory diseases such as asthma (16, 34). Indeed, a recent study performed in our laboratory has demonstrated that incubation with TNFα for 24–48 h induced a significant increase in the intracellular Ca2+ transient evoked by TRPV1 agonists and other chemical activators in isolated pulmonary sensory neurons (22). However, whether the increase in Ca2+ influx was mediated through TRPV1 or voltage-gated Ca2+ channels is not known.
In addition to these chronic effects described above, our pilot study has recently shown that instillation of TNFα into the lung of anesthetized rats almost immediately (within 10 min) increased the pulmonary C-fiber response to capsaicin (Cap), which sustained for more than 1 h (37). However, we could not draw a more definitive conclusion from that study because data interpretation was complicated by the acute systemic effects, such as an increase in tracheal pressure, generated by the procedure of tracheal instillation. Furthermore, the responses may have also involved actions of TNFα on other target cells in the airways, such as neutrophils, eosinophils, epithelial cells, etc. TNFα is also known to trigger an increase in the production of cyclooxygenase-2 (COX-2) metabolites of arachidonic acid, particularly prostaglandin E2 (PGE2) (13) and thromboxane A2 (TxA2) (38). These COX-2 metabolites are known to enhance the sensitivity of pulmonary sensory neurons (17, 26, 32, 35, 39). However, whether TNFα induces an acute sensitizing effect on vagal pulmonary sensory nerves remains to be determined.
In view of these unanswered questions and the important role of TNFα in the inflammation-induced airway hypersensitivity, this study was performed to investigate the possibility of an acute effect of a brief pretreatment with TNFα on the sensitivity of isolated vagal pulmonary sensory neurons and the potential role of COX-2-dependent inflammatory mediators in generating these effects.
METHODS
The study protocol was approved by the University of Kentucky Institutional Animal Care and Use Committee and performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health.
In Vivo Study
Animal preparation.
Male adult Sprague-Dawley rats were initially anesthetized by intraperitoneal injection of α-chloralose (0.1 mg/g) and urethane (0.5 mg/g), and supplemental doses were injected intravenously (iv) to maintain abolition of pain reflexes. The trachea was cannulated and the lungs were artificially ventilated by a respirator (Ugo Basile model 7025, Comerio, Italy) after a tracheotomy. The expiratory outlet of the respirator was placed under 3 cmH2O pressure to maintain a near-normal functional residual capacity. The left jugular vein and left femoral artery were cannulated for administering chemicals and measuring arterial blood pressure (ABP), respectively. Body temperature was maintained at ∼36°C by a heating pad. At the end of the experiment, the animals were euthanized by intravenous injections of KCl during deep anesthesia.
Afferent activities of single-unit vagal pulmonary C-fibers were recorded, as described in detail previously (20). Briefly, pulmonary C-fiber activity was searched initially by their mild responses to lung inflation (3 to 4 times of tidal volume) and by an immediate (delay < 1 s) response to bolus injection of a higher concentration of Cap (1.0 μg/kg), a TRPV1 agonist, into the right atrium. The general locations of C-fiber terminals were identified by their responses to gentle pressing of the lungs with a blunt-end glass rod at the end of the experiment. Action potential, tracheal pressure, and ABP were recorded on a thermal writer (Gould TW11, Cleveland, OH); data were digitized and analyzed by a data-acquisition system (Biocybernetics TS-100, Taipei, Taiwan).
Experimental protocol.
To evaluate the acute effects of TNFα on the pulmonary C-fiber sensitivity and to avoid the acute respiratory changes caused by intratracheal instillation, TNFα (10 µg/ml, 0.6 ml volume) or vehicle (Veh, phosphate-buffered isotonic saline, 0.6 ml) was aerosolized by a nebulizer (Aeroneb AG-AP6000-US, Galway, Ireland) that was connected to the breathing circuit between the inspiratory outlet of the respirator and the tracheal cannula; the 0.6 ml solution (either TNFα or Veh) was nebulized and delivered over 1.5 ± 0.3 min under the experimental conditions. Bolus injection of Cap (0.25–1.0 µg/kg iv) was tested before (as control) and at different time points after the inhalation of TNFα or Veh.
To investigate the involvement of COX-2, the acute effects of TNFα on the pulmonary C-fiber response to Cap (0.25–1.0 µg/kg iv) was determined after the rat was pretreated with either NS-398 (0.32 ± 0.13 mg·kg−1·min−1), a selective COX-2 inhibitor, or its vehicle (DMSO) infused slowly (0.1 ml/min for 5 min, iv) in two separate groups of rats ~60 min before the administration of TNFα. The same protocol was also used to determine a possible involvement of COX-2 in the effect of TNFα on the C-fiber response to injection of lactic acid (12 mg/kg iv) in two other groups of rats; pH of the lactic acid solution was 2.26. The volume of both Cap and lactic acid injections was 0.15 ml.
In Vitro Study
Identification of vagal pulmonary sensory neurons.
Vagal sensory neurons innervating the lungs and airways were identified by retrograde labeling with the fluorescent tracer, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), following a protocol described previously (32). Briefly, young male Sprague-Dawley rats (4–8 wk old) were anesthetized with isoflurane inhalation. DiI (0.2 mg/ml, 1% ethanol vol/vol) was initially dissolved in ethanol, diluted in saline, and then instilled into the lungs (0.05 ml).
Isolation of nodose and jugular ganglion neurons.
After 7–21 days, the DiI-labeled rats were anesthetized with isoflurane inhalation and decapitated. The head was immediately immersed in ice-cold DMEM/F-12 solution. Nodose and jugular ganglia were extracted and cultured separately. Each ganglion was desheathed, cut into 8 pieces, placed in a mixture of 0.04% type IV collagenase and 0.02% dispase, and incubated in a humidified chamber for 1 h in 5% CO2 in air at 37°C. The ganglion suspension was centrifuged (150 g, 5 min), and the supernatant was aspirated. The cell pellet was then resuspended in a modified-DMEM/F-12 solution [DMEM/F-12 supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin, and 100 µM minimal essential medium nonessential amino acids] and gently titrated with a small-bore fire-polished Pasteur pipette. The dispersed cell suspension was centrifuged (500 g, 8 min) through a layer of 15% bovine serum albumin to separate the cells from the myelin debris. The cell pellets were then resuspended in a modified-DMEM solution and plated onto dry poly-l-lysine-coated glass coverslips. After 3 h, the coverslips were immersed in the modified-DMEM media and incubated overnight (5% CO2 in air at 37°C).
Electrophysiological recording.
Cells were selected for electrophysiological recording based upon the following criteria: 1) labeled with DiI fluorescence, 2) spherical shape with no neurite outgrowths, and 3) activated by Cap (0.1 or 0.3 µM). Whole cell perforated patch-clamp recordings were performed as described previously (21). Briefly, recording was performed at room temperature in a small-volume (0.2 ml) perfusion chamber that was continuously perfused by gravity-feed (VC-6, Warner Instruments, Hamden, CT) with extracellular solution (ECS). The tip resistance of borosilicate glass electrodes was 1.5–3.0 MΩ. The series resistance was 6–10 MΩ and was not compensated. The regular ECS contained (in mM) 136 NaCl, 10 HEPES, 10 glucose, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, and 0.33 NaH2PO4; the pH was adjusted to 7.4 with NaOH. For ECS with pH ≤ 6.0, 2-(4-morpholino)-ethanesulfonic acid (MES) was used instead of HEPES for more stable pH buffering. The intracellular solution (ICS) contained (in mM) 92 potassium gluconate, 40 KCl, 8 NaCl, 1 CaCl2, 0.5 MgCl2, 10 EGTA, and 10 HEPES; the pH was adjusted to 7.2 with KOH.
Chemical solutions were applied by using a three-channel fast-stepping perfusion system (SF-77B, Warner Instruments), with its tip positioned to ensure that the cell was fully within the stream of the perfusate. All experiments were performed in the voltage-clamp mode, and the resting membrane potential was held at −70 mV. Data were digitized at 5 kHz and filtered at 5 kHz.
Experimental protocol.
We performed the following four study series.
series 1.
To determine the effect of a pretreatment with TNFα (1.44 nM, 9 min) on the inward current evoked by Cap challenge in isolated vagal pulmonary sensory neurons, the current responses to the same concentration of Cap (0.1 or 0.3 µM, 3 s) were tested before (as control), and at 0, 10, 20, 30, 45, 60, 75, 90, 105, 120, 150, and 180 min after termination of TNFα pretreatment in the same neurons. Nodose and jugular neurons were tested separately for comparison of their responses because sensory neurons derived from these two different ganglionic origins are known to express different physiological and pharmacological properties (49).
series 2.
To evaluate if the potentiating effect of TNFα was mediated selectively through an activation of the TRPV1 receptors, Cap was replaced by one of these known chemical activators of vagal pulmonary sensory neurons: 1) H+ (pH 6.0, 6 s), which is known to activate both acid-sensing ion channels (ASICs) and TRPV1 receptors (7, 18); 2) ATP (0.3 or 1 µM, 3 s), an agonist of P2X2 and P2X3 receptors (31); 3) icilin (100 µM, 4 s), an agonist of transient receptor potential melastatin 8 and transient receptor potential ankyrin 1 receptors (42, 55); and 4) phenylbiguanide (PBG; 10 µM, 3 s), an agonist of 5-HT3 receptors (40). These chemical activator-evoked responses were tested before and at different time points after termination of the TNFα pretreatment. To compare the effects of TNFα on ASICs and TRPV1 receptors in the H+-induced inward currents in the same vagal pulmonary sensory neurons, amiloride (a known blocker of ASICs; 500 µM, 2 min) and AMG9810 (a specific antagonist of TRPV1; 1 µM, 5 min) were applied separately to determine their individual contributions. The concentrations and durations of applications of amiloride and AMG9810 were selected based upon our previous studies (18, 44).
series 3.
To investigate if an increase in the synthesis and release of COX-2 metabolites of arachidonic acid was involved, the effects of TNFα pretreatment were compared between the pulmonary sensory neurons that were pretreated with vehicle (0.1% DMSO) and with NS-398 (10 μM, 10 min) immediately before the TNFα pretreatment.
series 4.
This series was performed to investigate if the TNFα pretreatment induced an increased production of prostanoids (first study); and if so, whether the effect could be blocked by the COX-2 inhibitor (second study). The rat nodose and jugular ganglion cells were prepared in the same manner as described for the patch-clamp recording experiment, except that the cell pellet (after separation from the myelin debris) was resuspended and plated onto a cell culture dish (Nest scientific, Rahway, NJ) instead of the coverslip, immersed in the modified-DMEM/F-12, and incubated overnight (5% CO2 in air at 37°C). To reach a sufficient quantity of cell mass, neurons collected from 3 rats were pooled into one sample, and a total of 3 samples (from 9 rats) was prepared for each of these two studies. In the first study, each sample was divided in two halves, and each half was selected randomly to receive either TNFα (1.44 nM) or vehicle treatment for 9 min (matching the protocol of the patch-clamp recording experiment). In the second study, each sample was divided into three equal parts: receiving the treatment with TNFα, TNFα + indomethacin (a nonselective COX inhibitor), or TNFα + NS-398; indomethacin (30 µM) and NS-398 (10 µM) were administered 20 min and 10 min, respectively, before the TNFα treatment (1.44 µM, 9 min). Supernatants (~0.05 ml each) were then collected from each dish for enzyme-linked immunosorbent assay (ELISA; Cayman chemical, Ann Arbor, MI) of PGE2 and thromboxane B2 (TxB2); optical density was read at a wavelength 410 nm, and the sample in each dish was assayed in duplicate and the data averaged.
Chemicals.
TNFα was purchased from Prospec Bio (Rehovot, Israel); NS-398 from Cayman (Ann Arbor, MI). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). A stock solution of TNFα (1.2 µM) was prepared in distilled water. Stock solutions of amiloride (500 mM), AMG9810 (1 mM), NS-398 (10 mM), indomethacin (30 mM), and icilin (0.1 M) were prepared in DMSO. A stock solution of Cap (1 mM) was prepared in 1% ethanol, 1% Tween 80, and 98% saline and that of ATP (10 mM) and PBG (2 mM) in saline. Solutions of these chemicals at desired concentrations were then prepared daily by dilution with ECS (final concentration of DMSO: 0.1%).
Statistical Analysis
Data were analyzed with one-way or two-way repeated-measures ANOVA, followed by a post hoc Fisher’s test. A P value < 0.05 was considered significant. All data are means ± SE.
RESULTS
In Vivo Study
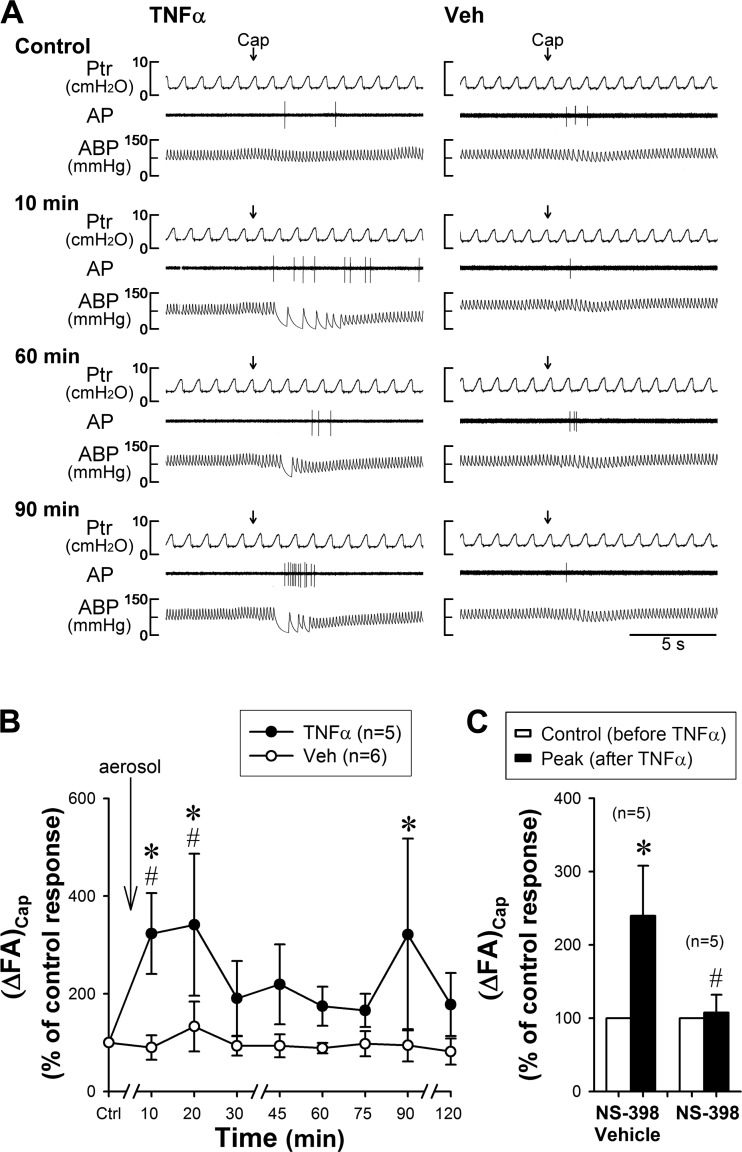
The in vivo study was performed in 16 rats (wt: 367 ± 8 g). In both TNFα and Veh groups, a bolus injection of Cap (0.75 µg/kg) triggered a short and mild burst of discharge of pulmonary C-fibers immediately followed the right atrial injection (e.g., Fig. 1A, top). After TNFα aerosol (10 µg/ml, 0.6 ml volume) was delivered into the lung by the respirator over 1.5 ± 0.3 min (n = 5), the same dose of Cap evoked a significantly higher fiber activity (FA) in the same pulmonary C-fiber (Fig. 1, A and B); ΔFA triggered by the same dose of Cap was elevated to 323 ± 83% of the control (pre-TNFα) response at 10 min (P < 0.01, n = 5; Fig. 1B). The increased response to Cap (ΔFA) sustained at 20 min (341 ± 145%; P < 0.01, n = 5), and then declined toward the control level after 30 to 75 min, but ΔFA began to increase again and reached another peak (321 ± 196%; P < 0.01, n = 5) at ~90 min (Fig. 1, A and B). To allow the baseline condition to reach a steady state after the aerosol delivery, Cap challenge was not performed between 0 and 10 min. In sharp contrast, the Veh aerosol delivered in the same manner did not alter the responses of pulmonary C-fibers to Cap at any of the time points following the same protocol (all P > 0.05, n = 6; Fig. 1, A and B). TNFα aerosol induced a small (~9%) but significant fall in ABP at 20 min after TNFα and that sustained for ~1 h; in contrast, Veh aerosol did not induce any change in ABP. Heart rate and peak tracheal pressure did not change after the TNFα challenge.
Fig. 1.
Effect of inhalation of aerosolized TNFα on the responses of pulmonary C-fibers to capsaicin (Cap) in anesthetized, open-chest, artificially ventilated rats. The solution of TNFα (10 µg/ml, 0.6 ml) or vehicle (Veh, phosphate-buffered isotonic saline, 0.6 ml) was aerosolized and delivered into the lung by the respirator. A: representative experimental records. Cap solution at a low concentration (0.25 µg/kg, 0.15 ml volume) was delivered first into the venous catheter (dead space volume 0.2 ml) and then flushed (at arrow) into the circulation by a bolus of 0.3 ml saline. Control, before TNFα or Veh pretreatment. Ptr, tracheal pressure; AP, action potential; ABP, arterial blood pressure. Receptor locations: middle lobe in the TNFα rat (left panel) and accessory lobe in the Veh rat (right panel). Body weights: 353 g (TNFα) and 365 g (Veh). B: group data before (Ctrl) and after the TNFα (n = 5 from 5 rats) or Veh (n = 6 from 4 rats) pretreatment. (ΔFA)Cap, increase in fiber activity expressed as percentage of the control response to Cap. *Significantly different from the corresponding control. #Significant difference between TNFα and Veh groups at the same time points. C: group data illustrating the immediate effect of TNFα on pulmonary C-fiber responses to Cap after pretreatments with NS-398 (right; n = 5 from 4 rats) and its vehicle (left; n = 5 from 3 rats). *Significantly different from the control response (before TNFα). #Significant difference between corresponding data points from groups pretreated with NS-398 and its vehicle. Data are means ± SE.
The immediate potentiating effect of TNFα on the C-fiber response to Cap was completely abolished by the pretreatment with NS-398, but unaffected by its vehicle (Fig. 1C): (ΔFA)Cap, the TNFα-induced increase in FA response to Cap expressed as percentage of the control response, was 107 ± 24% (n = 5) and 240 ± 68% (n = 5, P < 0.05) after NS-398 and its vehicle, respectively. A similar effect of NS-398 was also found in blocking the TNFα-induced potentiation of C-fiber response to intravenous injection of lactic acid: (ΔFA)lactic acid was 95 ± 8% (n = 10) and 172 ± 25% (n = 6, P < 0.05) after NS-398 and vehicle, respectively.
In Vitro Study
The patch-clamp recording experiments were performed in 104 vagal pulmonary sensory neurons isolated from nodose and jugular ganglia of 29 rats (135 ± 9 g) and identified by the fluorescent intensity of DiI. The whole cell capacitance of these neurons was 19.5 ± 1.0 pF (n = 104); the majority of them (92 out of 104) were small in size (capacitance ≤ 30 pF); 51% (n = 53) of these cells were nodose neurons, and 49% (n = 51) were jugular neurons. To normalize the responses between cells of different sizes, the current density (current/capacitance; pA/pF) was calculated for comparison.
Series 1.
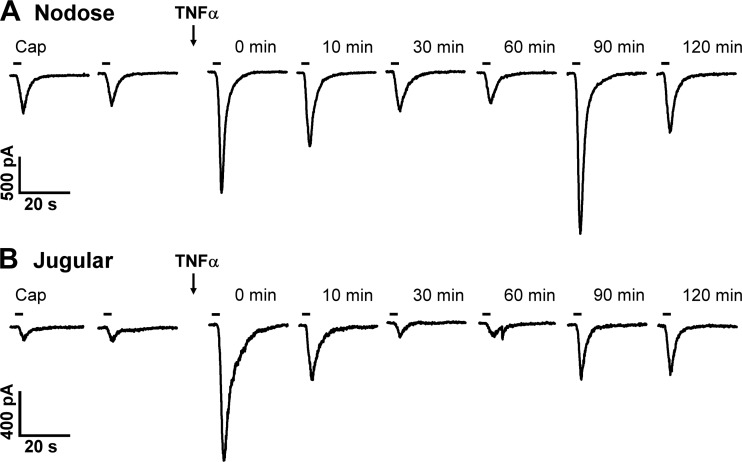
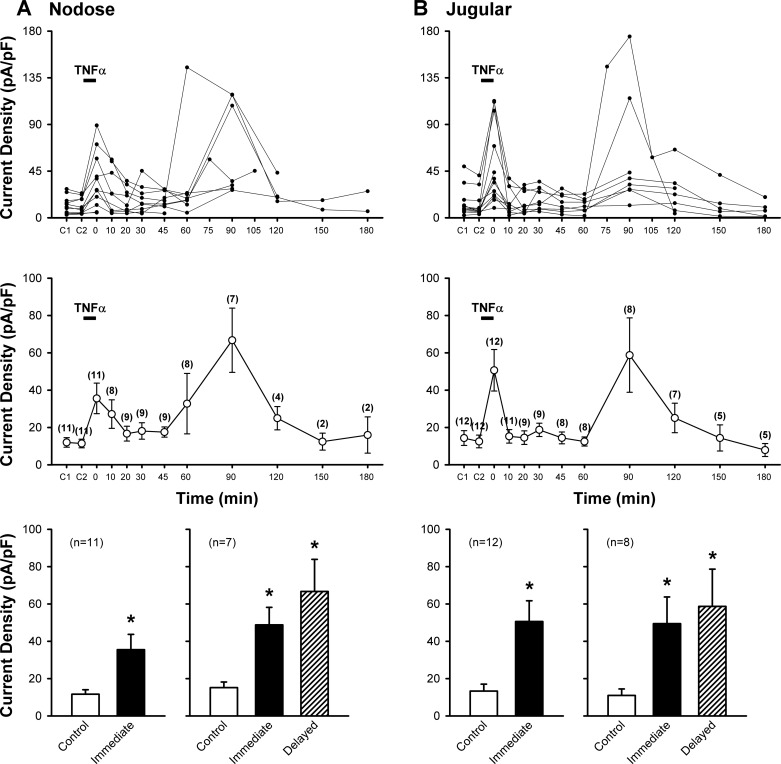
Application of a low dose of Cap (0.1 or 0.3 µM, 3 s) evoked an abrupt and short-duration inward current in both pulmonary nodose (11.6 ± 2.4 pA/pF; n = 11) and jugular (13.3 ± 3.7 pA/pF; n = 12) sensory neurons (Figs. 2 and 3); the responses were reproducible in the same neurons when the Cap challenge was repeated 10 min later (Figs. 2 and 3). Immediately after a pretreatment with TNFα (1.44 nM, 9 min), the Cap-evoked current density was significantly augmented (the immediate phase; Figs. 2 and 3). These elevated responses gradually declined and returned to control after ~20 min; then the response began to increase again and reached another peak at 60–90 min after the termination of TNFα pretreatment in the same neurons (the delayed phase; Figs. 2 and 3). During the immediate phase after TNFα, the Cap-induced current densities increased 3.1-fold in nodose (35.5 ± 8.2 pA/pF; P < 0.01, n = 11) and 3.8-fold in jugular (50.6 ± 11.1 pA/pF; P < 0.01, n = 12) neurons (Fig. 3). In the delayed phase, the Cap-induced current densities increased 4.4-fold in nodose (from 15.2 ± 3.0 pA/pF to 66.7 ± 17.2 pA/pF; P < 0.01, n = 7) and 5.3-fold in jugular (from 11.0 ± 3.5 pA/pF to 58.7 ± 19.9 pA/pF; P < 0.01, n = 8) neurons (Fig. 3). Because a similar pattern and magnitude of the potentiated responses were found between nodose and jugular neurons (Fig. 3), the data from these two groups were pooled together for the later study series.
Fig. 2.
Representative experimental records illustrating the inward currents evoked by Cap before and at 0, 10, 30, 60, 90, and 120 min after pretreatment with TNFα in a nodose (A: 18.6 pF) and a jugular (B: 15.1 pF) sensory neuron. Arrows indicate the TNFα (1.44 nM, 9 min) pretreatment. 0 min: the Cap response immediately after the termination of TNFα pretreatment. Cap (0.1 µM, 3 s) application was marked by the horizontal bar; two consecutive Cap challenges before TNFα pretreatment were separated by 10 min.
Fig. 3.
Effect of TNFα pretreatment on Cap-evoked current densities in rat vagal pulmonary nodose (A) and jugular (B) sensory neurons. Top: individual responses of each neuron recorded at different time points. TNFα (1.44 nM, 9 min) pretreatment was depicted by the horizontal bar. C1 and C2: control responses to two consecutive Cap (0.1 or 0.3 µM, 3 s) challenges separated by 10 min before TNFα; 0 min: responses immediately after the termination of TNFα pretreatment. The number of neurons decreased progressively toward later stage of the experiment due to the difficulty of maintaining a complete seal of the patch recording in some of the neurons. Middle: group data calculated from the same neurons in top. The number of neurons tested at each time point is shown in parenthesis. Bottom: left panels in both A and B show group data illustrating the immediate effects of TNFα (0 min) on the Cap-evoked current density in all the neurons studied; right panels in both A and B show group data obtained from the neurons in which both the immediate and the delayed phases (the peak response occurring between 60 and 90 min after TNFα) of the TNFα effect were recorded. *Significantly different from the corresponding control. Data in middle and bottom are means ± SE.
Series 2.
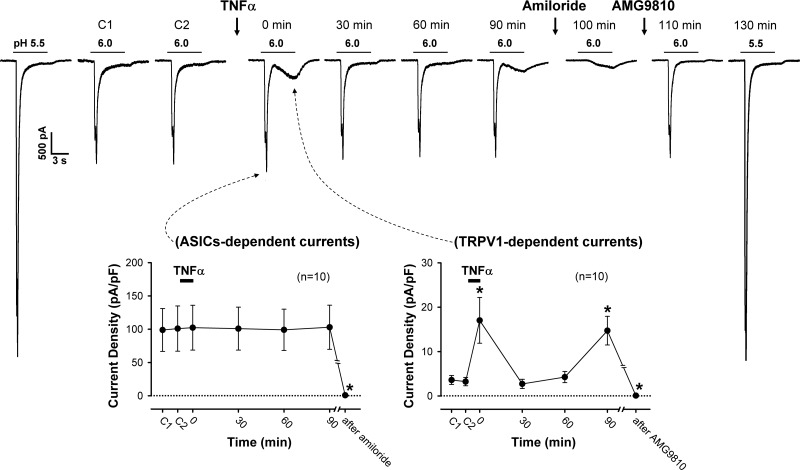
To determine whether the potentiating effect of TNFα pretreatment occurred only selectively on the TRPV1 receptor, the study protocol was repeated in different groups of neurons when Cap was replaced by one of four other known chemical activators of vagal pulmonary sensory neurons. Acidic ECS (pH 6.0) evoked a rapid but transient inward current that was rapidly inactivated before termination of the 6-s acid application in these neurons (Fig. 4, top). Immediately (0 min) after pretreatment with TNFα (1.44 nM, 9 min), the same acid challenge (pH 6.0) did not change the amplitude of this rapid transient current, but it evoked distinctly a slow but more sustained inward current (17.0 ± 5.2 pA/pF, n = 10; Fig. 4). More interestingly, this acid-evoked slow sustained current declined after the TNFα pretreatment and then reemerged at ~90 min (14.7 ± 3.2 pA/pF, n = 10), following a pattern similar to the immediate and delayed phases of TNFα potentiation of the Cap responses (e.g., Fig. 2). In contrast, TNFα had no effect on the acid-evoked rapid transient current at any of the time points (Fig. 4). The lack of potentiating effect of TNFα was not due to this rapid transient current has already reached its maximum because a larger current was evoked by a lower pH (5.5) in each of these neurons both before and after the study of the TNFα effect (e.g., Fig. 4, top).
Fig. 4.
Effect of TNFα pretreatment on H+-evoked responses in rat vagal pulmonary sensory neurons. Top: representative experimental records of H+ (pH 6.0, 6 s; horizontal bar)-evoked inward currents before and at 0, 30, 60, and 90 min after TNFα (1.44 nM, 9 min) pretreatment in a jugular sensory neuron (24.7 pF). Pretreatment of amiloride (500 µM, 2 min) blocked the transient (ASICs-dependent) current evoked by H+ (pH 6.0); after washout of amiloride, pretreatment of AMG9810 (1 µM, 5 min) blocked the sustained (TRPV1-dependent) current evoked by the same acid application. The response to a lower pH (pH 5.5) was tested at both the beginning and the end of experiment to determine if the lack of a potentiating effect on ASICs-dependent currents was due to that the maximal currents were already reached at pH 6.0. Bottom: group data indicating that the H+ (pH 6.0)-evoked ASICs-dependent currents were not altered by the TNFα pretreatment (left), whereas TRPV1-dependent currents were clearly potentiated at 0 and 90 min after the TNFα pretreatment (right). C1 and C2: control responses to two consecutive H+ challenges separated by 10 min before TNFα. *Significantly different from control. Data are means ± SE (n = 10, except for the data points after amiloride and AMG9810: n = 5 in each).
The acid-evoked rapid transient current was completely abolished by pretreatment with amiloride (500 µM, 2 min), a known blocker of ASICs (from 99.9 ± 22.8 pA/pF to 0.8 ± 0.4 pA/pF; P < 0.05, n = 5), whereas the slow sustained current resulting from the TNFα pretreatment was blocked by pretreatment with AMG9810 (1 µM, 5 min), a specific antagonist of TRPV1 (from 3.4 ± 0.7 pA/pF to 0.1 ± 0.1 pA/pF; P < 0.05, n = 5) (e.g., Fig. 4, top). Thus these two currents were the ASICs-dependent currents and the TRPV1-dependent currents that were characterized in pulmonary sensory neurons in our previous study (18).
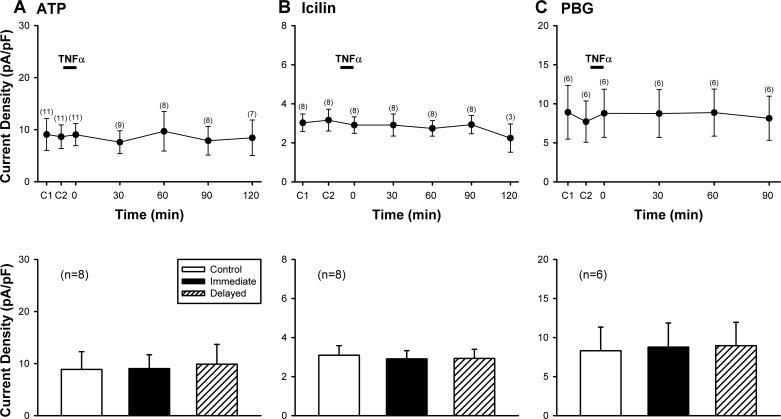
The same TNFα pretreatment (1.44 nM, 9 min) did not generate any potentiation of the current densities evoked by ATP (0.3 or 1 µM, 3 s), icilin (100 µM, 4 s), and PBG (10 µM, 3 s), the non-TRPV1 activators of pulmonary sensory neurons, at any of the time points (P > 0.05; n = 8 for Fig. 5, A and B, bottom; n = 6 for Fig. 5C, bottom). Only nodose sensory neurons were tested in Fig. 5A because jugular sensory neurons did not respond to ATP challenge in our pilot study (data not shown), which confirmed a previous report by Kwong et al. (31).
Fig. 5.
A lack of potentiating effect of TNFα pretreatment when Cap was replaced by non-TRPV1 activators of rat vagal pulmonary sensory neurons. Top: histograms of group data illustrating the current densities evoked by ATP (0.3 or 1 µM, 3 s) in A, icilin (100 µM, 4 s) in B, and phenylbiguanide (PBG; 10 µM, 3 s) in C, before and at different time points after TNFα (1.44 nM, 9 min) pretreatment in nodose and jugular sensory neurons, except that ATP responses were only studied in nodose neurons. C1 and C2: control (before TNFα) responses to two consecutive challenges separated by 10 min in A and C; and by 20 min in B. The number of neurons tested at each time point is shown in parenthesis. Bottom: comparisons between responses to the same challenges (ATP, icilin or PBG) between control, immediate (0 min) and delayed (60–90 min) phases after the TNFα pretreatment; these are the same time points when the responses to Cap were potentiated by TNFα as shown in Fig. 3. Data are means ± SE.
Series 3.
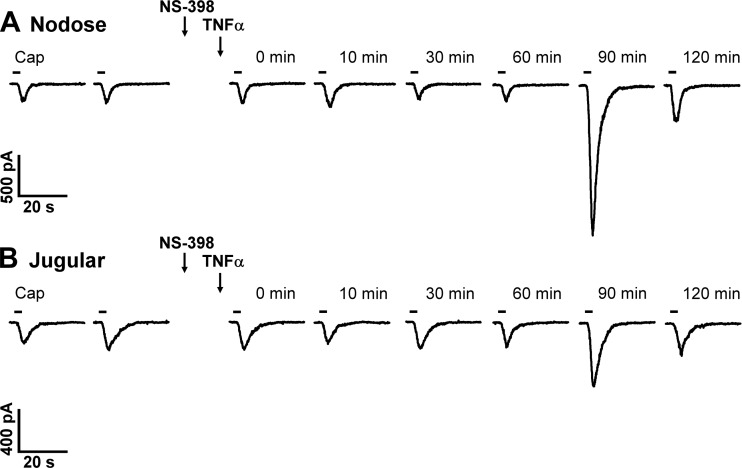
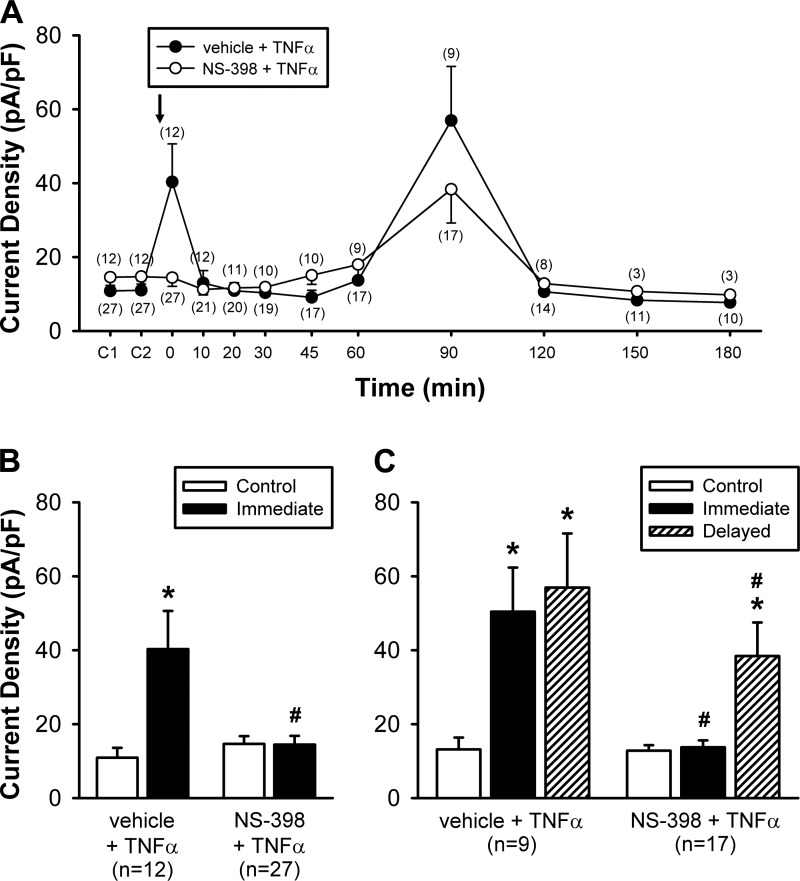
The immediate phase of the potentiating effect of TNFα was totally abolished by the NS-398 pretreatment (10 µM, 10 min), a selective COX-2 inhibitor (e.g., Fig. 6); after the NS-398 pretreatment, the current density induced by Cap at 0 min after TNFα was 14.5 ± 2.4 pA/pF (n = 27), which was not significantly different from the control (pre-TNFα) response (14.6 ± 2.1 pA/pF; P > 0.05, n = 27) in the same neurons (Fig. 7B), and distinctly smaller than the 0 min (post-TNFα) response to Cap in the group of neurons with the pretreatment of the vehicle of NS-398 (0.1% DMSO) (40.3 ± 10.3 pA/pF; P < 0.01, n = 12; Fig. 7B). In comparison, the delayed phase of the potentiating effect of TNFα was only partially blocked by NS-398 pretreatment; the current densities induced by Cap were 57.0 ± 14.6 pA/pF (n = 9) and 38.4 ± 9.1 pA/pF (n = 17) in vehicle + TNFα group and in NS-398 + TNFα group, respectively (P < 0.05; Fig. 7C). To determine whether the blocking effect of NS-398 on the TNFα-induced potentiation was due to that the Cap-evoked current was attenuated by the NS-398 pretreatment, we compared the Cap-evoked currents before and after NS-398 pretreatment; the current densities evoked by Cap before (14.2 ± 4.5 pA/pF) and after NS-398 (13.8 ± 6.8 pA/pF) were not significantly different (P > 0.05, n = 7).
Fig. 6.
Representative experimental records illustrating the effect of NS-398 pretreatment on the increased response to Cap induced by TNFα in a nodose (A: 14.4 pF) and a jugular (B: 8.2 pF) sensory neuron. TNFα (1.44 nM, 9 min) was administered separately and immediately following the termination of NS-398 (10 µM, 10 min) pretreatment, as depicted by the two arrows. 0 min: responses immediately after the termination of TNFα pretreatment. Cap (0.1 µM, 3 s) challenge was marked by the horizontal bar; two consecutive Cap challenges were separated by 10 min before NS-398 and TNFα pretreatments.
Fig. 7.
Group data illustrating the effect of NS-398 pretreatment on the TNFα-induced hypersensitivity of vagal pulmonary sensory neurons. A: histogram of the responses of current densities to the same concentration of Cap (0.1 or 0.3 µM, 3 s) challenges in the vehicle (of NS-398) + TNFα group (closed circles) and the NS-398 + TNFα group (opened circles); TNFα (1.44 nM, 9 min) was applied immediately following the NS-398 vehicle (0.1% DMSO) or NS-398 pretreatment (10 µM, 10 min), as depicted by the arrow. C1 and C2: responses to two consecutive Cap challenges separated by 10 min before TNFα; 0 min: responses immediately after the termination of TNFα. The number of neurons tested at each time point is shown in parenthesis. The number of neurons decreased progressively toward later stage of the experiment due to the difficulty of maintaining a complete seal of the patch recording in some of the neurons. B: comparison of the Cap-evoked peak current density responses between before (Control) and immediately after TNFα (0 min) in two groups of neurons: vehicle + TNFα group [n = 12: 6 nodose (N) and 6 jugular (J)] and NS-398 + TNFα group (n = 27: 12 N and 15 J). C: in only some of these neurons in B, both the immediate (0 min after TNFα) and delayed (60–90 min after TNFα) phases of the TNFα effect were recorded, and a comparison of the Cap-evoked peak responses between control (before), immediate and delayed phases in two groups of neurons: vehicle + TNFα group (n = 9: 3 N and 6 J) and NS-398 + TNFα group (n = 17: 8 N and 9 J). *Significantly different from the corresponding control; #significant difference between vehicle + TNFα and NS-398 + TNFα groups at the same time points. Data are means ± SE.
Series 4.
Results obtained from ELISA in the first study showed that both PGE2 (0.42 ± 0.01 ng/ml) and TxB2 (0.52 ± 0.05 ng/ml) levels in the samples collected from the TNFα-treated nodose and jugular cells were significantly higher than PGE2 (0.37 ± 0.01 ng/ml; n = 3, P < 0.01) and TxB2 (0.42 ± 0.05 ng/ml; n = 3, P < 0.01) from the Veh-treated cells, respectively.
Results in the second study showed that the PGE2 level (0.55 ± 0.07 ng/ml) in the samples collected from the TNFα-treated nodose and jugular cells were significantly reduced by pretreatments with indomethacin (0.37 ± 0.04 ng/ml; n = 3, P < 0.05) and NS-398 (0.33 ± 0.01 ng/ml; n = 3, P < 0.05). Similarly, the TxB2 level (0.63 ± 0.03 ng/ml) in the samples collected from the TNFα-treated nodose and jugular cells were also significantly reduced by pretreatments with indomethacin (0.40 ± 0.08 ng/ml; n = 3, P < 0.05) and NS-398 (0.38 ± 0.05 ng/ml; n = 3, P < 0.05). No significant difference was found between the data obtained after indomethacin and NS-398 in either PGE2 or TxB2.
DISCUSSION
Results of the present study showed that a brief pretreatment with TNFα potentiated the response to TRPV1 activators in isolated rat vagal pulmonary sensory neurons. The peak inward current evoked by the same Cap challenge was consistently augmented immediately following the TNFα pretreatment, which gradually declined toward control and then increased again reaching another peak at 60–90 min (Figs. 2 and 3). In contrast, the same pretreatment with TNFα did not generate any potentiating effect on the response to non-TRPV1 chemical activators of vagal pulmonary sensory neurons (Fig. 5). The immediate phase of TNFα-induced increase in Cap response was completely abolished by pretreatment with a selective COX-2 inhibitor, NS-398, whereas the delayed phase was only partially attenuated (Figs. 6 and 7). A similar pattern of acute sensitizing effects of TNFα on pulmonary C-fiber afferents and the involvement of COX-2 in this action was also clearly demonstrated in anesthetized rats (Fig. 1C). All these results suggest that COX-2 arachidonic acid metabolites are primarily responsible for generating the immediate phase of the potentiating effect of TNFα on the TRPV1 sensitivity in these neurons.
COX-2 can be activated by several proinflammatory cytokines in inflamed tissues (54). It has been shown that COX-2 can be activated by TNFα in airways (47), which is associated with various airway inflammatory disorders including asthma (33). Previous investigators have reported that a sensitizing effect on the response of isolated DRG neurons to Cap was induced by a substantially longer duration of TNFα pretreatment (4–24 h), and the effect was attenuated by nonselective COX inhibitors or selective COX-2 inhibitors (11, 45, 50). Furthermore, Fehrenbacher and coworkers reported that the expression of COX-2 and production of COX-2 metabolites, such as PGE2, were elevated in rat DRG cultured cells after incubation with TNFα for >4 h (13). In anesthetized rats, the production of TxB2, another COX-2 metabolite, in the airways was also significantly increased by a pretreatment with TNFα 24 h earlier (38). Our results obtained in this study further showed that the releases of both PGE2 and TxB2 were increased in the cultured rat nodose and jugular cells after treatment with TNFα for a relatively short duration (9 min). Furthermore, the TNFα-induced increases in both PGE2 and TxB2 released from these neurons were significantly reduced after the pretreatment with NS-398; but no further reductions were found after the pretreatment with indomethacin, a non-selective COX inhibitor, lending strong support of the involvement of COX-2 mechanism.
It is well documented that both PGE2 and TxA2, the precursor of TxB2, can activate and/or enhance the sensitivity of vagal pulmonary sensory neurons (17, 26, 32, 35, 39). Taken together, these findings suggest that a brief treatment with TNFα can activate COX-2 and release chemical mediators which, in turn, enhance the TRPV1 sensitivity in pulmonary sensory neurons. A possible involvement of activations of different subtypes of EP and TP receptors remains to be explored in future studies.
The COX-2-like immunoreactivity is present in both neurons and nonneuronal cells (microglia, endothelial cells, epithelial cells, etc.) (2, 13, 23, 52, 59). Although the present study was performed in isolated pulmonary sensory neurons, we cannot completely rule out the possibility that TNFα may activate the microglial cells remaining with the neurons in the culture and induce release of inflammatory mediators and other cytokines, which may in turn exert a sensitizing effect on these neurons.
Acid solution can directly stimulate isolated vagal pulmonary sensory neurons in a dose-dependent manner, primarily through the activation of two ion channels: ASICs and TRPV1 (14, 18, 29); ASICs are proton-gated ion channels that are voltage-insensitive, and belong to the superfamily of degenerins/epithelial Na+ channels (1, 30). Gu and Lee have shown that the inward current evoked by lowering the pH of ECS to 7.0 consisted of only a small, transient and rapidly inactivating component, which increased progressively in amplitude when the H+ concentration was further elevated (18). In addition, a slow and sustained current began to emerge when pH was lowered to below 6.5. Of this biphasic response to acid, the transient component was inhibited by amiloride, a common blocker of ASIC channels (58), whereas the sustained component was completely abolished by capsazepine, a selective TRPV1 antagonist (18). Results of the present study demonstrated a distinct potentiating effect of TNFα on the acid (pH 6.0)-evoked TRPV1-dependent currents in vagal pulmonary sensory neurons. This potentiating effect is also mediated through the COX-2, as shown in the pretreatment effect of NS-398 in the TNFα-induced hypersensitivity of pulmonary C-fiber response to lactic acid in anesthetized rats (Fig. 1C). In a sharp contrast, TNFα had no effect on the ASICs-dependent currents in the same neurons (Fig. 4); the absence of potentiating effect of TNFα was not due to the limit of the ASICs-dependent current because a lower pH (5.5) was able to evoke a much greater ASIC current in the same neurons (Fig. 4). These results clearly illustrated the selectivity of the acute potentiating effect of TNFα on TRPV1, and this observation was further substantiated by the absence of any potentiating effect of TNFα on the responses to ATP, icilin or PBG; all of them are known non-TRPV1 activators of these sensory neurons (Fig. 5). Once again, the lack of potentiating effect of TNFα was not due to that maximal currents were already reached by these activators at the concentrations applied in this study (1 µM ATP and 10 µM PBG) based upon the concentration-dependent responses to ATP in pulmonary nodose neurons and PBG in pulmonary nodose and jugular neurons documented in our recent study (21). In addition, in our pilot study the current density evoked by a higher concentration of icilin (300 µM) was more than twofold higher than that by icilin (100 µM) applied in this study (unpublished data).
Although the signaling pathway(s) mediating the potentiating effect of TNFα on TRPV1 cannot be identified in this study, an involvement of enhanced sensitivity and/or an increase in expression of TRPV1 induced by the TNFα pretreatment should be considered (22). Another possible contributing factor is the critical role of the A-kinase anchoring protein AKAP150 in the activation of the PGE2/cAMP/PKA signaling pathway and phosphorylation of TRPV1; AKAP150 is known to be coexpressed and coimmunoprecipitated with TRPV1 in DRG neurons (51, 61). There are several known PKA phosphorylation sites on TRPV1, including S116, S502, T144, and T370 (43, 48). In particular, the AKAP150 aligns PKA to control phosphorylation of the S502 site on TRPV1. Furthermore, S502 is the major phosphorylation site in the AKAP150-dependent translocation of TRPV1 to the surface membrane (61). Notwithstanding this suggestive evidence, a possible involvement of the AKAP150 and these PKA phosphorylation sites in TRPV1 sensitization by TNFα remains to be determined.
The results of this study indicate that COX-2 arachidonic acid metabolites only play a partial role in the delayed potentiating effect of TNFα because ~60% of the delayed TNFα potentiation still persisted even after the NS-398 pretreatment (Fig. 7C). This was not due to an inadequate antagonistic dose of NS-398 because previous investigators have shown that the same dose of NS-398 completely blocked TNFα-induced PGE2 production in DRG neurons (13) and the lipopolysaccharide-induced PGE2 production in neuronal-glial cultures (3). In addition, we have tested the blocking effect of a higher dose of NS-398 (100 µM) in our pilot experiments, but it did not produce any additional attenuation in both immediate and delayed phases of the potentiating effects of TNFα (unpublished data). The type(s) of COX-2 metabolites that are partially responsible for this delayed sensitizing effect of TNFα in this study is not known.
The primary mechanism underlying the delayed potentiating effect of TNFα on the response to TRPV1 agonists in this study is not yet fully understood. Several cell signaling pathways that have been previously reported under similar experimental conditions could be potentially involved; for example, TNFα exerts its biological effects via a direct action on TNFα receptors (TNFRs), which upon activation can activate specific signaling pathways (60). TNFα is initially produced as a membrane-anchored precursor protein, which is subsequently cleaved into free proteins. These proteins form biologically active homotrimers and interact with two distinct TNFRs, TNFR1 and TNFR2, on the cell surface (6, 53). We have previously demonstrated the expression of both TNFR1 and TNFR2 in vagal pulmonary sensory neurons (22). TNFR1 activation by TNFα is known to activate nuclear factor-kappa B (15) and mitogen activated protein kinases (MAPK) (4). A previous study has demonstrated that exposure of DRG neurons to TNFα for 24–48 h significantly increased the proportion of DRG neurons expressing TRPV1 receptor-like immunoreactivity via TNFR1 and extracellular signal-regulated kinase (ERK) activation (19). ERK is a member of MAPK pathway and known to be activated by TNFα (57). Furthermore, it has been shown that the p38 MAPK pathway is involved in the heat hyperalgesia evoked by increasing TRPV1 expression (25). However, whether these signaling pathways mediated through the activation of these TNFRs are involved in the immediate and delayed phases of potentiating effects of TNFα on vagal pulmonary sensory neurons in this study remains to be determined. One characteristic feature of the delayed effect of TNFα observed in this study is its consistent occurrence in a relatively narrow window of 60–90 min after the pretreatment. Whether this intriguing feature of timing was related to an involvement of certain specific signaling pathways is yet not known.
TRPV1 is a polymodal transducer and a nonselective cation channel with high permeability to Ca2+ (46). TRPV1 is extensively and selectively expressed in C-fibers that represent a majority (>75%) of vagal afferents innervating the lungs and airways (20, 24). Bronchopulmonary C-fiber afferents can be activated by a number of endogenous chemical mediators and under certain pathophysiological conditions, including acid, bradykinin, certain lipoxygenase metabolites, and hyperthermia (9, 36). Upon activation, they can elicit centrally mediated reflex responses including bronchoconstriction and mucus hypersecretion via the cholinergic pathway (9, 36). Activation of TRPV1 can also trigger Ca2+ influx and release of tachykinins and calcitonin gene-related peptide from the sensory terminals (12). These sensory neuropeptides can act on a number of effector cells in the respiratory tract (e.g., smooth muscles, cholinergic ganglia, mucous glands, immune cells), and elicit the local “axon reflexes” such as bronchoconstriction, protein extravasation, and inflammatory cell chemotaxis (12). Indeed, increasing evidence suggests that the TRPV1 channel plays an important role in the manifestation of various symptoms of airway hypersensitivity, a common pathophysiological feature in patients with airway inflammatory diseases (16, 34). Taken together, results obtained from this study suggest that the acute sensitizing effects of TNFα on TRPV1 expressed in pulmonary sensory nerves may contribute, at least in part, to the pathogenesis of airway hypersensitivity associated with the production and release of this cytokine during airway inflammation.
GRANTS
This study was supported in part by National Institutes of Health Grants HL-96914, AI-123832, and UL1-TR-001998 (to L.-Y. Lee) and Taipei Medical University Grant TMU105-AE1-B17 (to C.-C. Hsu).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
C.-C.H., and L.-Y.L. conceived and designed research; C.-C.H., Y.S.L., and R.-L.L. performed experiments; C.-C.H., Y.S.L., R.-L.L., and L.-Y.L. analyzed data; C.-C.H., Y.S.L., R.-L.L., and L.-Y.L. interpreted results of experiments; C.-C.H., R.-L.L., and L.-Y.L. prepared figures; C.-C.H., R.-L.L., Y.S.L., and L.-Y.L. drafted manuscript; C.-C.H., Y.S.L., R.-L.L., and L.-Y.L. edited and revised manuscript; C.-C.H., Y.S.L., R.-L.L., and L.-Y.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. J. Geer for technical assistance.
REFERENCES
- 1.Alvarez de la Rosa D, Canessa CM, Fyfe GK, Zhang P. Structure and regulation of amiloride-sensitive sodium channels. Annu Rev Physiol 62: 573–594, 2000. doi: 10.1146/annurev.physiol.62.1.573. [DOI] [PubMed] [Google Scholar]
- 2.An Y, Belevych N, Wang Y, Zhang H, Herschman H, Chen Q, Quan N. Neuronal and nonneuronal COX-2 expression confers neurotoxic and neuroprotective phenotypes in response to excitotoxin challenge. J Neurosci Res 92: 486–495, 2014. doi: 10.1002/jnr.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araki E, Forster C, Dubinsky JM, Ross ME, Iadecola C. Cyclooxygenase-2 inhibitor ns-398 protects neuronal cultures from lipopolysaccharide-induced neurotoxicity. Stroke 32: 2370–2375, 2001. doi: 10.1161/hs1001.096057. [DOI] [PubMed] [Google Scholar]
- 4.Barbin G, Roisin MP, Zalc B. Tumor necrosis factor alpha activates the phosphorylation of ERK, SAPK/JNK, and P38 kinase in primary cultures of neurons. Neurochem Res 26: 107–112, 2001. doi: 10.1023/A:1011086426652. [DOI] [PubMed] [Google Scholar]
- 5.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol 10: 471–480, 1994. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 6.Brockhaus M, Schoenfeld HJ, Schlaeger EJ, Hunziker W, Lesslauer W, Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci USA 87: 3127–3131, 1990. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 8.Cembrzynska-Nowak M, Szklarz E, Inglot AD, Teodorczyk-Injeyan JA. Elevated release of tumor necrosis factor-alpha and interferon-gamma by bronchoalveolar leukocytes from patients with bronchial asthma. Am Rev Respir Dis 147: 291–295, 1993. doi: 10.1164/ajrccm/147.2.291. [DOI] [PubMed] [Google Scholar]
- 9.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- 10.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol 107: 660–664, 1992. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunha TM, Verri WA Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA 102: 1755–1760, 2005. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Swert KO, Joos GF. Extending the understanding of sensory neuropeptides. Eur J Pharmacol 533: 171–181, 2006. doi: 10.1016/j.ejphar.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 13.Fehrenbacher JC, Burkey TH, Nicol GD, Vasko MR. Tumor necrosis factor alpha and interleukin-1beta stimulate the expression of cyclooxygenase II but do not alter prostaglandin E2 receptor mRNA levels in cultured dorsal root ganglia cells. Pain 113: 113–122, 2005. doi: 10.1016/j.pain.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Fox AJ, Urban L, Barnes PJ, Dray A. Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience 67: 741–752, 1995. doi: 10.1016/0306-4522(95)00115-Y. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem 70: 1876–1886, 1998. doi: 10.1046/j.1471-4159.1998.70051876.x. [DOI] [PubMed] [Google Scholar]
- 16.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol 533: 207–214, 2006. doi: 10.1016/j.ejphar.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 17.Gu Q, Kwong K, Lee LY. Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: role of cAMP/PKA signaling pathway. J Neurophysiol 89: 1985–1993, 2003. doi: 10.1152/jn.00748.2002. [DOI] [PubMed] [Google Scholar]
- 18.Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 291: L58–L65, 2006. doi: 10.1152/ajplung.00517.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hensellek S, Brell P, Schaible HG, Bräuer R, Segond von Banchet G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci 36: 381–391, 2007. doi: 10.1016/j.mcn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127: 113–124, 2001. doi: 10.1016/S0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CC, Lee LY. Role of calcium ions in the positive interaction between TRPA1 and TRPV1 channels in bronchopulmonary sensory neurons. J Appl Physiol (1985) 118: 1533–1543, 2015. doi: 10.1152/japplphysiol.00043.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y, Gu Q, Lin RL, Kryscio R, Lee LY. Calcium transient evoked by TRPV1 activators is enhanced by tumor necrosis factor-alpha in rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 299: L483–L492, 2010. doi: 10.1152/ajplung.00111.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iadecola C, Forster C, Nogawa S, Clark HB, Ross ME. Cyclooxygenase-2 immunoreactivity in the human brain following cerebral ischemia. Acta Neuropathol 98: 9–14, 1999. doi: 10.1007/s004010051045. [DOI] [PubMed] [Google Scholar]
- 24.Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst 5: 165–176, 1982. doi: 10.1016/0165-1838(82)90037-6. [DOI] [PubMed] [Google Scholar]
- 25.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36: 57–68, 2002. doi: 10.1016/S0896-6273(02)00908-X. [DOI] [PubMed] [Google Scholar]
- 26.Karla W, Shams H, Orr JA, Scheid P. Effects of the thromboxane A2 mimetic, U46,619, on pulmonary vagal afferents in the cat. Respir Physiol 87: 383–396, 1992. doi: 10.1016/0034-5687(92)90019-S. [DOI] [PubMed] [Google Scholar]
- 27.Keatings VM, O’Connor BJ, Wright LG, Huston DP, Corrigan CJ, Barnes PJ. Late response to allergen is associated with increased concentrations of tumor necrosis factor-alpha and IL-5 in induced sputum. J Allergy Clin Immunol 99: 693–698, 1997. doi: 10.1016/S0091-6749(97)70032-0. [DOI] [PubMed] [Google Scholar]
- 28.Kips JC, Tavernier J, Pauwels RA. Tumor necrosis factor causes bronchial hyperresponsiveness in rats. Am Rev Respir Dis 145: 332–336, 1992. doi: 10.1164/ajrccm/145.2_Pt_1.332. [DOI] [PubMed] [Google Scholar]
- 29.Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 543: 591–600, 2002. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci 26: 477–483, 2003. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 31.Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol 295: L858–L865, 2008. doi: 10.1152/ajplung.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwong K, Lee LY. PGE(2) sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol (1985) 93: 1419–1428, 2002. doi: 10.1152/japplphysiol.00382.2002. [DOI] [PubMed] [Google Scholar]
- 33.Lee IT, Yang CM. Inflammatory signalings involved in airway and pulmonary diseases. Mediators Inflamm 2013: 791231, 2013. doi: 10.1155/2013/791231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol 9: 243–249, 2009. doi: 10.1016/j.coph.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee LY, Morton RF. Pulmonary chemoreflex sensitivity is enhanced by prostaglandin E2 in anesthetized rats. J Appl Physiol (1985) 79: 1679–1686, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol 4: 287–324, 2014. doi: 10.1002/cphy.c130020. [DOI] [PubMed] [Google Scholar]
- 37.Lin RL, Lee LY. Tumor necrosis factor alpha (TNFα) potentiates pulmonary chemoreflex responses in anesthetized rats (Abstract). FASEB J 23: 1010.14, 2009. [Google Scholar]
- 38.Lin RL, Lin YJ, Geer MJ, Kryscio R, Lee LY. Pulmonary chemoreflex responses are potentiated by tumor necrosis factor-alpha in mice. J Appl Physiol (1985) 114: 1536–1543, 2013. doi: 10.1152/japplphysiol.01301.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin S, Li H, Xu L, Moldoveanu B, Guardiola J, Yu J. Arachidonic acid products in airway nociceptor activation during acute lung injury. Exp Physiol 96: 966–976, 2011. doi: 10.1113/expphysiol.2011.058263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mair ID, Lambert JJ, Yang J, Dempster J, Peters JA. Pharmacological characterization of a rat 5-hydroxytryptamine type3 receptor subunit (r5-HT3A(b)) expressed in Xenopus laevis oocytes. Br J Pharmacol 124: 1667–1674, 1998. doi: 10.1038/sj.bjp.0702037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsunaga K, Yanagisawa S, Ichikawa T, Ueshima K, Akamatsu K, Hirano T, Nakanishi M, Yamagata T, Minakata Y, Ichinose M. Airway cytokine expression measured by means of protein array in exhaled breath condensate: correlation with physiologic properties in asthmatic patients. J Allergy Clin Immunol 118: 84–90, 2006. doi: 10.1016/j.jaci.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 42.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 43.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain 1: 3, 2005. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni D, Gu Q, Hu HZ, Gao N, Zhu MX, Lee LY. Thermal sensitivity of isolated vagal pulmonary sensory neurons: role of transient receptor potential vanilloid receptors. Am J Physiol Regul Integr Comp Physiol 291: R541–R550, 2006. doi: 10.1152/ajpregu.00016.2006. [DOI] [PubMed] [Google Scholar]
- 45.Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci 17: 975–982, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 47.Pang L, Knox AJ. Effect of interleukin-1 beta, tumour necrosis factor-alpha and interferon-gamma on the induction of cyclo-oxygenase-2 in cultured human airway smooth muscle cells. Br J Pharmacol 121: 579–587, 1997. doi: 10.1038/sj.bjp.0701152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci 22: 4740–4745, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riccio MM, Myers AC, Undem BJ. Immunomodulation of afferent neurons in guinea-pig isolated airway. J Physiol 491: 499–509, 1996. doi: 10.1113/jphysiol.1996.sp021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schäfers M, Marziniak M, Sorkin LS, Yaksh TL, Sommer C. Cyclooxygenase inhibition in nerve-injury- and TNF-induced hyperalgesia in the rat. Exp Neurol 185: 160–168, 2004. doi: 10.1016/j.expneurol.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci 28: 4904–4917, 2008. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, Wang D, FitzGerald GA, Dingledine R. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci 31: 14850–14860, 2011. doi: 10.1523/JNEUROSCI.3922-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith RA, Baglioni C. The active form of tumor necrosis factor is a trimer. J Biol Chem 262: 6951–6954, 1987. [PubMed] [Google Scholar]
- 54.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem 271: 33157–33160, 1996. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 55.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003. doi: 10.1016/S0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 56.Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med 152: 76–80, 1995. doi: 10.1164/ajrccm.152.1.7599866. [DOI] [PubMed] [Google Scholar]
- 57.Utreras E, Futatsugi A, Rudrabhatla P, Keller J, Iadarola MJ, Pant HC, Kulkarni AB. Tumor necrosis factor-alpha regulates cyclin-dependent kinase 5 activity during pain signaling through transcriptional activation of p35. J Biol Chem 284: 2275–2284, 2009. doi: 10.1074/jbc.M805052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 59.Watkins DN, Peroni DJ, Lenzo JC, Knight DA, Garlepp MJ, Thompson PJ. Expression and localization of COX-2 in human airways and cultured airway epithelial cells. Eur Respir J 13: 999–1007, 1999. doi: 10.1034/j.1399-3003.1999.13e12.x. [DOI] [PubMed] [Google Scholar]
- 60.Wu H. Assembly of post-receptor signaling complexes for the tumor necrosis factor receptor superfamily. Adv Protein Chem 68: 225–279, 2004. doi: 10.1016/S0065-3233(04)68007-7. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Li L, McNaughton PA. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron 59: 450–461, 2008. doi: 10.1016/j.neuron.2008.05.015. [DOI] [PubMed] [Google Scholar]