Abstract
Consumption of a high-fat, high-fructose diet [Western diet (WD)] promotes vascular stiffness, a critical factor in the development of cardiovascular disease (CVD). Obese and diabetic women exhibit greater arterial stiffness than men, which contributes to the increased incidence of CVD in these women. Furthermore, high-fructose diets result in elevated plasma concentrations of uric acid via xanthine oxidase (XO) activation, and uric acid elevation is also associated with increased vascular stiffness. However, the mechanisms by which increased xanthine oxidase activity and uric acid contribute to vascular stiffness in obese females remain to be fully uncovered. Accordingly, we examined the impact of XO inhibition on endothelial function and vascular stiffness in female C57BL/6J mice fed a WD or regular chow for 16 wk. WD feeding resulted in increased arterial stiffness, measured by atomic force microscopy in aortic explants (16.19 ± 1.72 vs. 5.21 ± 0.54 kPa, P < 0.05), as well as abnormal aortic endothelium-dependent and -independent vasorelaxation. XO inhibition with allopurinol (widely utilized in the clinical setting) substantially improved vascular relaxation and attenuated stiffness (16.9 ± 0.50 vs. 3.44 ± 0.50 kPa, P < 0.05) while simultaneously lowering serum uric acid levels (0.55 ± 0.98 vs. 0.21 ± 0.04 mg/dL, P < 0.05). In addition, allopurinol improved WD-induced markers of fibrosis and oxidative stress in aortic tissue, as analyzed by immunohistochemistry and transmission electronic microscopy. Collectively, these results demonstrate that XO inhibition protects against WD-induced vascular oxidative stress, fibrosis, impaired vasorelaxation, and aortic stiffness in females. Furthermore, excessive oxidative stress resulting from XO activation appears to play a key role in mediating vascular dysfunction induced by chronic exposure to WD consumption in females.
Keywords: Western diet, vascular stiffness, females, oxidative stress, allopurinol
widespread consumption of diets rich in saturated fat and fructose [Western diet (WD)] leads to insulin resistance and obesity, which are critical factors in the pathogenesis of type 2 diabetes mellitus (DM2) and cardiovascular disease (CVD) (31). In turn, CVD accounts for more than 50% of deaths in subjects with DM2 (36, 52). Women affected by obesity, insulin resistance, and DM2 are at an especially high risk of developing CVD, with a 50% increased risk of death from coronary artery disease (36) and 27% increased relative risk of stroke compared with men (57). Therefore, uncovering the mechanisms that link diet-induced obesity to vascular dysfunction is of paramount importance. Notably, vascular stiffness is a parameter that can be used to evaluate vascular dysfunction and can be measured clinically using noninvasive techniques (60). Although it is a physiological phenomenon associated with aging, vascular stiffness is also a biomarker that correlates independently with increased risk of CVD-related morbidity and mortality (4, 12, 14, 34). Conditions such as obesity and DM2 are characterized by accelerated and enhanced vascular stiffness (69, 75). Furthermore, under these conditions, augmented vascular stiffness occurs to a greater extent in women compared with men (56). Therefore, the increased incidence and greater severity of CVD in diabetic women can be partially related to increased vascular stiffness (14, 64).
Fructose in contemporary diets derives mostly from abundantly available sucrose in table sugar and high-fructose corn syrup and appears to be a critical contributor to obesity and associated metabolic abnormalities arising from consumption of WD. Indeed, excess dietary ingestion of fructose correlates strongly with weight gain and impaired glucose tolerance in rodents (38) as well as in humans (68). High-fructose diets lead to increased levels of uric acid via xanthine oxidase (XO) activation in the liver (10), and high uric acid levels are in turn associated with increased vascular stiffness and CVD in women (16, 21, 47). Importantly, uric acid levels in the high-normal range have been associated with increased vascular stiffness in women (21), and beneficial CVD effects of XO inhibition have been documented even in the absence of frank hyperuricemia (39). In addition to uric acid production, XO activation in cardiovascular tissue results in enhanced production of reactive oxygen species (ROS) and increased expression of proinflammatory molecules in rodents and humans (28, 30, 48), which contribute to cardiovascular tissue damage. The association between hyperuricemia, obesity, and CVD is well recognized in the literature and frequently coexists with other features of the metabolic syndrome, such as impaired glucose homeostasis (51). Conversely, pharmacological XO inhibition results in improvements in systemic inflammatory markers in preclinical models of the metabolic syndrome (3, 46) as well as in humans (70).
However, the impact of XO inhibition on vascular stiffness and function remains to be fully elucidated. A recent meta-analysis evaluating the effects of the XO inhibitor allopurinol on vascular stiffness in humans was inconclusive (18), thus underscoring the need for additional research in this area. In the present investigation, we test the hypothesis that XO inhibition results in reduced WD-induced vascular stiffness and impaired aortic vasodilatory responses in part via decreased vascular oxidative stress. Therefore, we utilized C57BL/6J female mice fed a WD for 16 wk in the presence or absence of allopurinol, a widely used XO inhibitor, which reduces uric acid production and oxidative stress. We assessed in vivo and ex vivo vascular stiffness as well as aortic vasomotor responses along with markers of fibrosis and oxidative stress both systemically and in aortic tissue.
METHODS
Animal models.
Three-week-old C57BL/6J female mice (stock no. 00664) were procured from Jackson Laboratories (Bar Harbor, ME). All procedures were approved in advance by the Institutional Animal Care and Use Committee of the University of Missouri, and mice were cared for according to National Institutes of Health guidelines. When mice were 4 wk of age, they were randomly assigned to 1) a control diet feeding (CD; Test Diet 58Y2, Richmond, IN), 2) a CD and allopurinol (CD-ALLO; 125 mg/l in drinking water), 3) a WD containing high fat (46%) and high carbohydrate as sucrose (17.5%) and high fructose corn syrup (17.5%) (Test Diet 58Y1 with high refined carbohydrate and high fat, 5APC) (WD), or 4) a WD and allopurinol (WD-ALLO, 125 mg/l in drinking water) for 16 wk. The dose used was chosen based on previous studies from our laboratory that showed beneficial cardiac effects in males (30). The female mice were housed in pairs under a 12-h light-dark regimen, and water and food were provided ad libitum.
Body composition and biochemical parameters.
Mice were weighed before being euthanized. After 16 wk of feeding, mice underwent body composition analysis for whole body fat mass, lean body mass, and total body water using an EchoMRI-500 for quantitative magnetic resonance analysis (Echo Medical Systems, Houston, TX), as described previously (41). Venous blood samples were collected from a subset of fasting mice in each treatment group, and plasma was stored at −80°C for glucose and uric acid measurements. Measurements of plasma uric acid and glucose were performed by automated clinical chemistry analyzer (AU680; Beckman-Coulter, Brea, CA) as described previously (30).
Atomic force microscopy imaging and force measurement.
Atomic force microscopy (AFM) was used to evaluate stiffness of endothelial cells (ECs) in enface aortic preparations of the thoracic aorta, as described previously (42). Briefly, a 2 × 2 mm segment of the thoracic aorta was obtained after euthanasia and opened longitudinally. The adventitial surface of each explant was fastened to a glass-covered slip using Cell-Tak, which was opened longitudinally. Stiffness (elastic modulus) of the EC surface was measured by AFM using a nano-indentation protocol, as described previously (17). An MFP-3D AFM 89 (Asylum Research, Goleta, CA) mounted on an Olympus IX81 microscope (Olympus) was used for biomechanical measurements and to estimate elastic modulus/stiffness. AFM measurements were conducted at room temperature (~25°C). For stiffness measurements, an AFM cantilever (MLCT; Bruker-nano, Goleta, CA) was used to perform repeated cycles of nano-indentation and retraction cycles on the cell surface. The parameters employed were 0.3 Hz of sampling frequency with an approach/retraction velocity of 960 nm/s, 1,600 nm traveling distance for one sampling cycle (indentation and retraction), and ∼400–600 pN loading force. Force curves were generated over a period of 2 min and analyzed using NForceR software (registration no. TXu1-328-659) and MATLAB. The mean of these elastic modulus (i.e., stiffness) values was computed for each indentation site and then averaged together for each group. Estimations of Young’s modulus (E-modulus) were obtained using a length of 100–300 nm of the AFM indentation curve after the initial point of contact that was fit with a Hertz model as shown in the equation
where E is the E-modulus, F is the force exerted by AFM probe on tissue surface, δ is indentation depth into the sample, α is the half-opening angle of the AFM tip, and ν is the Poisson ratio. The tissues were considered as a gel, and ν was assumed at 0.5. To obtain topographical images of EC or VSMC, the AFM was operated in contact mode. The area of the tissue surface that was scanned in these experiments was 40 × 40 μm, and the digital density of the scanned area was 512 × 512 pixels. Stylus-type AFM probes (model: MLCT-C, k = 15 pN/nm; Bruker, Santa Barbara, CA) were used to perform surface scanning at 0.4-Hz frequency, with ∼300–500 pN of tracking force (17).
Ex vivo vasomotor responses of aortic rings.
Vasomotor responses were evaluated in the aorta via wire myography, as described previously (17, 42). Briefly, a 2-mm segment of thoracic aorta was collected immediately after euthanasia and placed in the bathing physiological salt solution (PSS) containing (in mM) 145 NaCl, 4.7 KCl, 1.2 NaH2PO4, 1.17 MgSO4, 2 CaCl2, 5 glucose, 2 pyruvate, 0.02 EDTA, 3 MOPS, and 1% bovine serum albumin, pH 7.4. Samples were maintained at 37°C and were continuously aerated with 95% O2-5% CO2. Before experimentation, the aortic contractile state was ascertained by KCl (80 mM/l). Aortas were preconstricted with U-46619 (100 nM). Vasorelaxation of arterial rings to acetylcholine (ACh, 10−9 to 10−4 M) and the nitric oxide (NO)-donor sodium nitroprusside (SNP; 10−9 to 10−4 M) were assessed by cumulative addition of agonist to the vessel bath. Aortic relaxation responses are presented as percent maximal relaxation, calculated as [(Fb − Fd)/(Fb − Fmin)] × 100, where Fd is force after a drug intervention, Fb is baseline force, and Fmin is force before the intervention. At the end of each experiment, the PSS bath solution was replaced with Ca2+-free PSS to determine minimal force during passive conditions.
Vascular fibrosis.
A 2-mm segment of thoracic aorta was fixed in 3% paraformaldehyde, dehydrated in ethanol, paraffin embedded, and transversely sectioned in 5-µm slices. Four sections each for four to five mice per group were examined. Slides were stained with picrosirius red stain and Verhoeff-Von Gieson (VVG) stain to measure collagen accumulation. The areas and intensities of red color that were stained with picrosirius red and the intensities of pink color on the VVG-stained sections indicative of collagen deposition were quantified as grayscale intensities, using MetaVue software as described previously (29).
Measurement of vivo aortic stiffness in vivo.
We measured aortic stiffness in vivo by pulse-wave velocity (PWV). Doppler ultrasound (Indus Mouse Doppler System, Webster, TX) was used as described previously in our laboratory (17). Before euthanasia, isoflurane-anesthetized mice (1.75% in 100% oxygen stream) were placed supine on a heating board and legs secured to ECG electrodes. PWV was determined according to the transit time method and calculated as the difference in arrival times of a Doppler pulse wave at two locations along the aorta (aortic arch and descending aorta), which were set at a known distance apart (35 mm). Each of the pulse wave arrival times was measured as the time from the peak of the ECG R-wave to the leading foot of the pulse wave, at which time velocity begins to rise at the start of systole. The distance between the two locations along the aorta was divided by transit time, and data are expressed in millimeters per millisecond. Velocity waveforms were acquired at the aortic arch, followed immediately by measurement at the descending aorta proximal to the iliac bifurcation (17).
Oxidative stress.
Aortic oxidative stress was assessed by immunostaining for 3-nitrotyrosine (3-NT), as described previously (77). 3-NT is a product of tyrosine nitration mediated by ROS such as peroxynitrite, which promotes NO destruction as well as vascular inflammation (17, 29). Briefly, 5-µm paraffin-embedded aorta sections from different treatments were dewaxed and rehydrated, and antigen was retrieved and incubated overnight with 1:150 primary rabbit polyclonal anti-3-NT antibody (Millipore). Sections were washed and incubated for 30 min with secondary antibodies, biotinylated anti-rabbit, and streptavidin-horseradish peroxidase. After several rinses with distilled water, diaminobenzidine was applied for 7 min, and sections were again rinsed and stained with hematoxylin for 90 s, dehydrated, and mounted with permount. For 3-NT quantification, all colors on the sections were deleted except for the brown color, which is indicative of 3-NT formation in the different component of the aorta. The slides were checked under a brightfield (Nikon 50i) microscope (Nikon, Tokyo, Japan), and ×40 images were captured with a Cool SNAP cf camera (Roper Scientific Germany, Trenton, NJ). The images were analyzed by MetaVue (Molecular Devices, Sunnyvale, CA), and the intensity of brown color was quantified as grayscale intensities. To quantify 3-NT in ECs, the endothelial layer was carefully traced and the brown color quantified only in that region. For quantifying 3-NT generation in VSMCs, equal regions of interest were made in the media in all sections, and the intensities of brown color were quantified and analyzed in these specific areas.
XO activity in aortic tissue was determined using a xanthine oxidase activity quantitative colorimetric/fluorimetric assay kit (BioAssay Systems, Hayward, CA). In addition, we measured systemic oxidative stress by quantifying plasma malondialdehyde (MDA) using a colorimetric/flurimetric assay kit (TBARS assay kit; Cayman Chemical, Ann Arbor, MI).
Statistical analysis.
Results are reported as means ± SE. Statistical analysis was done primarily by two-way ANOVA, followed by post hoc tests (Bonferroni) to examine effects of WD and allopurinol (Sigma Plot 13.0, Systat Software). The dose responses to ACh and SNP were analyzed using repeated-measures ANOVA. Aortic dilator responses are presented as percent maximal relaxation, calculated as [(Fb − Fd)/(Fb − Fmin)] × 100.
Transmission electron microscopy.
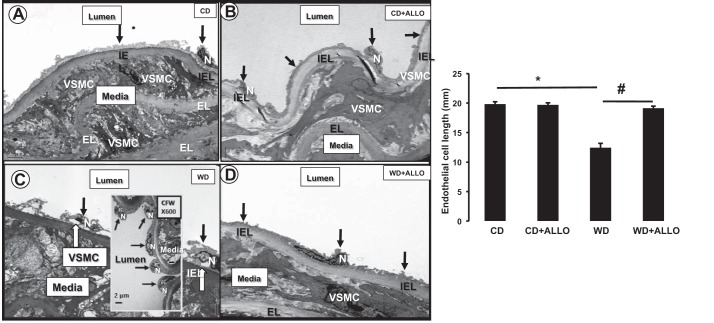
Aorta samples were fixed in 2% paraformaldehyde and 2% glutaraldehyde in 100 mM sodium cacodylate buffer, pH = 7.35. Fixed tissues were rinsed with 100 mM sodium cacodylate buffer containing 10 mM 2 mercaptoethanol and 130 mM sucrose (2-ME buffer). Secondary fixation was performed using 1% osmium tetroxide in 2-ME buffer. Specimens were next incubated at 4°C for 1 h and then rinsed with 2-ME buffer. Block staining was performed using 1% aqueous uranyl acetate and incubated at 4°C overnight and then rinsed with distilled water. Sections were cut to a thickness of 85 nm using an ultramicrotome and stained using Sato’s triple lead solution stain and 5% aqueous uranyl acetate. Multiple images were acquired for study at various magnifications with a JEOL JEM 1400 transmission electron microscope at 80 kV. Specifically, 12 endothelial images per group were acquired at ×800 and/or ×1,000 magnification for measurement of EC length with existing a scale bar on image. To measure EC length, 3 ECs were measured randomly from each sample at their base, where they joined the internal elastic lamina utilizing the scale bar of that image for a total of 12 measurements for each sample.
RESULTS
WD results in increased body weight, adiposity, and fasting glucose.
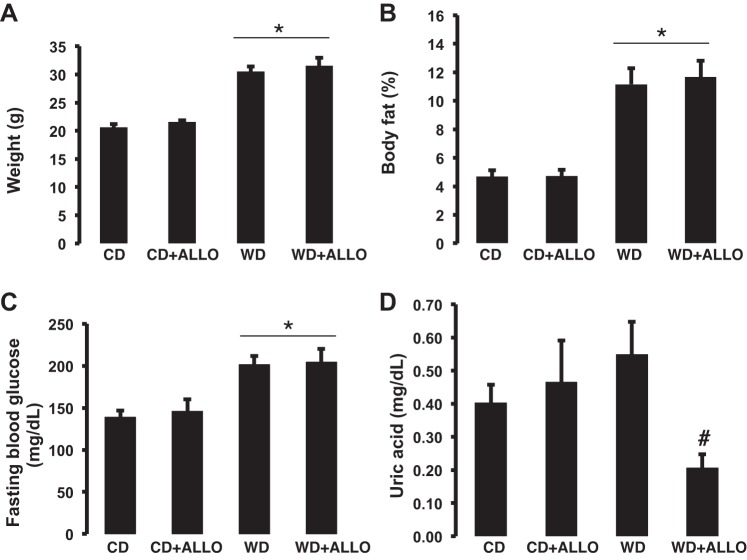
In agreement with previous data from our laboratory (41), 16 wk of WD feeding resulted in a significant increase in body weight as well as fat percentage in all cohorts. Administration of allopurinol did not affect these parameters (Fig. 1, A and B). In parallel, fasting blood glucose was elevated in the WD-fed cohorts relative to animals fed a control diet (281.0 ± 10.30 vs. 217.75 ± 27.30 mg/dl, P < 0.05). Administration of allopurinol did not induce significant changes in body composition or glucose metabolism in either the CD-fed or the WD-fed mice (Fig. 1C). These data support the notion that the effects of XO inhibition are independent of changes on body weight, body composition, or glucose metabolism. In male C57BL/6J mice, we have demonstrated previously and published that 16 wk of WD resulted in a significant increase in uric acid levels (30). In the present investigation, WD resulted in a nonstatistical significant elevation of uric acid in the WD-fed female mice relative to animals fed a CD (0.55 ± 0.09 vs. 0.40 ± 0.05 mg/dl). Treatment with allopurinol did not affect uric acid levels in CD-fed animals, but it substantially lowered uric acid levels in WD-fed female mice compared with untreated WD-fed animals (0.21 ± 0.04 vs. 0.55 ± 0.09 mg/dl, P < 0.05; Fig. 1D). In addition, XO activity was increased ∼1.9-fold in WD female mice relative to animals fed a CD (6.86 ± 2.97 vs. 3.61 ± 0.37 U/l, P = 0.35) and was significantly decreased by allopurinol (1.51 ± 0.27 U/l, P < 0.05). XO activity also trended down in CD-fed mice treated with allopurinol as well (2.07 ± 0.08 U/l, P = 0.17).
Fig. 1.
Xanthine oxidase (XO) inhibition does not impact changes in body weight, adiposity, or fasting glucose induced by Western diet (WD). A: body weight; B: body fat composition (%total body weight) by quantitative magnetic resonance (echoMRI-500); C: fasting plasma glucose concentration; D: plasma uric acid levels (samples obtained under anesthesia). *P < 0.05, control diet (CD) vs. WD; #P < 0.05, WD vs. WD-allopurinol (ALLO); n = 4–8 for all groups.
XO inhibition protects against WD-induced aortic stiffness and impaired vasodilatory responses.
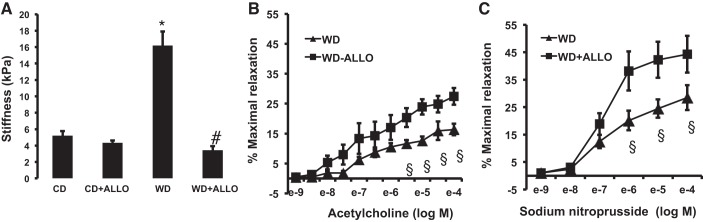
We have demonstrated and published previously that 16 wk of WD feeding results in increased cardiovascular stiffness in female mice (17, 41). In the current investigation, we assessed vascular stiffness via AFM of thoracic aortic explants. We found that WD feeding resulted in a significant increase in aortic stiffness compared with CD (16.9 ± 0.50 vs. 5.21 ± 0.54 kPa; Fig. 2A). XO inhibition with allopurinol significantly reduced aortic stiffness to levels comparable with animals fed a CD (16.9 ± 0.50 vs. 3.44 ± 0.50 kPa, P < 0.05; Fig. 2A). In addition, we measured in vivo aortic stiffness by ultrasound-based PWV. We have previously demonstrated significant increases in PWV in female mice fed a WD for 16 wk relative to mice fed a CD (17, 29, 42). In our experiments, treatment with allopurinol did not significantly affect PWV (3.82 + 0.25 mm/ms for untreated vs. 3.67 + 0.16 mm/ms for allopurinol-treated, all fed a WD, P > 0.05).
Fig. 2.
XO inhibition improves WD-induced aortic stiffness and increases endothelium-dependent and -independent vasodilatory responses in the setting of WD feeding. A: aortic endothelial stiffness was assessed via atomic force microscopy (AFM) of aortic explants from C57BL/6J female mice fed a WD for 16 wk in the presence or absence of ALLO in drinking water. *P < 0.05, control diet (CD) vs. WD; #P < 0.05, WD vs. WD-ALLO; n = 4–5 for all groups. In addition, vasomotor responses were evaluated in C57BL/6J female mice fed a WD for 16 wk in the presence or absence of ALLO in drinking water. B and C: responses of isolated aortic rings to the endothelium-dependent dilator acetylcholine (B) and the endothelium-independent vasodilator sodium nitroprusside (C). §P < 0.05, WD vs. WD + ALLO; n = 4–7.
Structural and functional characteristics of the vasculature are closely linked (72), and vascular endothelial stiffness contributes to impaired vasomotor responses (74). Importantly, we have shown previously that 16 wk of WD feeding in female C57BL/6J results in impaired endothelium-dependent and -independent vasodilatory responses (17). Therefore, we evaluated vasomotor responses only in the WD-fed cohorts. In isolated aortic rings, the cohort treated with XO inhibitor had greater vasodilatory response to ACh when compared with the WD-fed animals (Emax = 16.38 ± 1.94% vs. Emax = 27.46 ± 2.80%, P < 0.05; Fig. 2B). Similarly, the endothelium-independent vasodilatory response to SNP was significantly greater in the WD-ALLO when compared with WD (Emax = 28.50 ± 4.55% vs 44.29%, P < 0.05; Fig. 2C). These responses were restored to a degree comparable with female mice fed a CD (Emax = 35.1 ± 2.20 and 38.9 ± 2.70% for ACh and SNP, respectively), as published previously by our laboratory (17). The present data demonstrate that XO inhibition prevents WD-induced aortic stiffness in parallel with greater endothelium-dependent and -independent vasodilatory responses.
XO inhibition ameliorates vascular stiffness in association with a reduction in vascular oxidative stress and fibrosis.
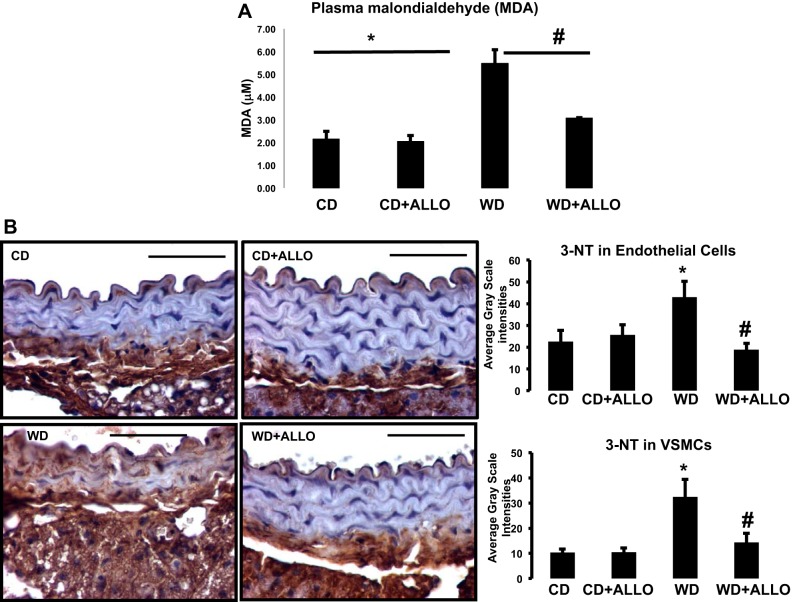
We analyzed systemic markers of oxidative stress by measuring MDA concentrations in plasma. WD-fed mice demonstrated a significant elevation in MDA concentrations (5.48 ± 0.59 vs. 2.15 ± 0.34 μM, P < 0.05), which was prevented by treatment with allopurinol (3.90 ± 0.82 μM) (Fig. 3A). Furthermore, XO inhibition reduced MDA to levels similar to those found in allopurinol-treated control mice (2.05 ± 0.26 μM, P > 0.05).
Fig. 3.
ALLO decreases WD-induced oxidative stress systemically and in aortic tissue. A: levels of malondialdehyde (MDA) were measured in plasma to determine systemic markers of oxidative stress in C57BL/6J female mice fed a WD for 16 wk with and without treatment with ALLO. B: analysis of oxidative stress by 3-nitrotyrosine (3-NT) staining in sections from aortas. *P < 0.05, WD vs. CD; #P < 0.05, WD vs. WD + ALLO; n = 3–6 all groups.
Previously, we have determined that in females WD-induced aortic stiffness is related to increased oxidative stress and vascular remodeling (17). Similarly, in the present investigation, we found increased 3-NT staining in the WD-fed cohort group compared with CD (Fig. 3B). In addition, XO inhibition significantly ameliorated oxidative stress (WD vs. WD-ALLO; Fig. 3B. These data, in addition to our results showing WD-induced increments in XO activity in aortic tissue, which is ameliorated by allopurinol, collectively suggest that XO inhibition is protective against WD-induced vascular oxidative stress in female mice.
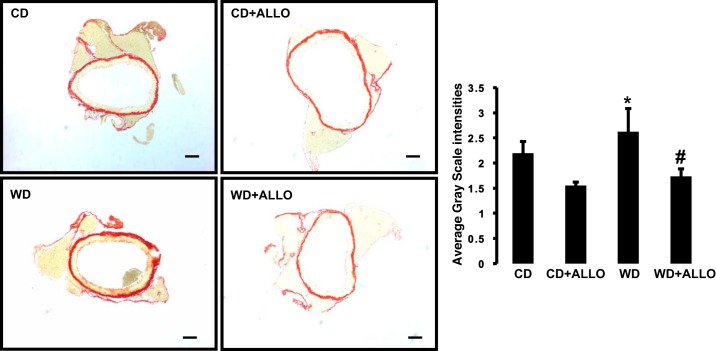
We also studied the impact of WD-induced enhancement of XO activity on fibrosis in aortic tissue by immunohistochemistry. Relative to mice fed a CD, WD feeding for 16 wk resulted in increased collagen deposition, as measured by picrosirius red immunostaining (Fig. 4). Allopurinol administration resulted in significant reductions in this marker of fibrosis in WD-fed animals relative to untreated animals also fed a WD (WD-ALLO vs. WD). These data suggest a beneficial influence of XO inhibition on vascular fibrosis in conditions of WD feeding (Fig. 4).
Fig. 4.
XO inhibition decreases aortic fibrosis. Left: analysis of fibrosis by picrosirius red staining in aortas from C57BL/6J female mice fed WD for 16 wk in the presence or absence of ALLO in drinking water. Right: collagen deposition quantified as average grayscale intensities. *P < 0.05, WD vs. CD; #P < 0.05 WD vs. WD + ALLO; n = 4 all groups.
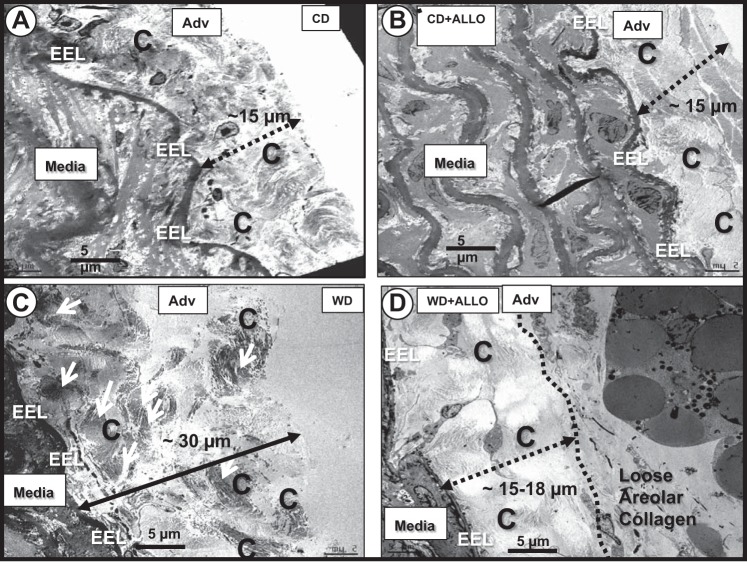
Finally, we analyzed ultrastructural changes via transmission electron microscopy in aortic samples. Our findings demonstrate increased deposition of highly organized fibrillary collagen in WD-fed mice, which correlates with our immunohistochemistry findings reported above (Fig. 5). Furthermore, we also demonstrated an abnormal phenotype characterized by shortening and lifting of ECs, which is suggestive of increased EC contractility (Fig. 6). Similarly to our AFM and vasomotor responses, these abnormalities were corrected by allopurinol.
Fig. 5.
ALLO decreases collagen deposition in WD-fed female mice. Analysis of fibrosis by transmission electron microscopy (TEM) in aortas from C57BL/6J female mice fed WD for 16 wk in the presence or absence of ALLO in drinking water (representative images). CD (A) and CD + ALLO (B) demonstrate the normal appearance and extension of the fibrillar collagen adjacent to the homogeneous electron dense external elastic lamina (EEL) on the abluminal side of the media. C: layering of highly organized, compact, dense, fibrillar collagen (C; demarked by dashed line). D: ALLO treatment protects against additional layering of mature, highly organized, compact, dense, fibrillar collagen relative to WD-fed animals in C. Magnification, ×400. Scale bar, 5 µm; n = 12 for all groups.
Fig. 6.
ALLO improves endothelial cell (EC) phenotype in WD-fed female mice. Analysis of EC morphology by TEM in aortas from C57BL/6J female mice fed WD for 16 wk in the presence or absence of ALLO in drinking water (representative images). C: characteristic abnormal phenotype of descending thoracic aorta EC (luminal side; black arrows) showing lifting and separation (white arrows) from the internal elastic lamina (IEL) compared with the CD (A) and CD treated with ALLO (CD + ALLO; B) models. Importantly, there is notable contraction-retraction of ECs, as depicted in inset within C, where there is loss of EC elongation of cytoplasm and nucleus, which illustrates their contracted phenotype (magnification, ×600; scale bar, 2 µm). D: WD models are protected from lifting; separation from the IEL and contraction of the ECs. Magnification, ×800; scale bar, 2 µm in A–D. EL, medial elastic lamina; N, endothelial nucleus; VSMC, media vascular smooth muscle cell; n = 12 for all groups.
DISCUSSION
The central aim of the current investigation was to evaluate the impact of XO inhibition on vascular stiffness in a female rodent model of chronic overnutrition with a diet high in fat and fructose (WD). Overall, our data demonstrate that XO inhibition improves WD-induced vascular stiffness as well as aortic vasodilatory responses. In addition, we show that these actions of XO inhibition occur in concert with decreased oxidative stress and aortic fibrosis in females.
Fructose is ubiquitously consumed in industrialized countries, derived mostly from sucrose present in table sugar and high-fructose corn syrup, and typically leads to increased levels of uric acid (71). As opposed to glucose, which is readily utilized by most cells to produce energy, fructose must be further metabolized before joining the glycolytic pathway. In the liver, fructokinase phosphorylates fructose to fructose 1-phosphate in an unregulated manner, leading to depletion of adenosine triphosphate, which in turn results in stimulation of key enzymes involved in purine nucleotide synthesis. Ultimately, this pathway leads to increased activity of XO and subsequent production of uric acid (32, 33, 43).
Although there is controversy with regard to the relative contribution of dietary fructose in the development of CVD (62), high-fructose diets are currently under scrutiny due to their potential impact on obesity, DM2, and CVD (6, 9, 40, 50, 63, 67). It is accepted that increased levels of uric acid, as occurs with WD consumption, are linked to increased risk for CVD (61) as well as chronic kidney disease in humans (49). Nonetheless, other mechanisms have been postulated to contribute to the development of vascular dysfunction and CVD, including fructose-induced cardiovascular oxidative stress (30, 62).
We performed our studies in cycling female mice because of the translational relevance related to observations in premenopausal women. To this point, the impact of dietary fructose on vascular stiffness appears to have more severe implications in females than in males. Indeed, in a cohort of more than 88,000 women, the consumption of high-fructose diet in the form of more than two sugar-sweetened beverages resulted in a 35% increased risk of coronary artery disease relative to men (24). In a Japanese cohort of more than 38,000 subjects, sugar-sweetened beverages were associated with ischemic stroke only in women (20). Similarly, it has been shown that high plasma levels of uric acid are linked to increased vascular stiffness in apparently healthy women but not in men (21). Furthermore, women exhibit increased vascular stiffness with aging, and insulin resistance worsens this abnormality to a greater degree compared with men (56). Therefore, the augmented incidence and greater severity of CV disease in obese and diabetic women can be related partially to the presence of increased vascular stiffness (14, 64). Indeed, women with DM2 are at an especially higher risk of developing coronary artery disease (36) and stroke compared with men (57).
Our data support the notion that a key mediator of WD-induced vascular stiffness is increased oxidative stress. The key role played by excess production of ROS in the pathogenesis of CVD is well established and described (13, 45). In addition, data from our laboratory have demonstrated that ROS contributes to vascular remodeling as well as stiffness in rodent models of chronic overnutrition (17, 29, 42). In this study, we evaluated systemically oxidative stress by measuring plasma levels of MDA as well as at the tissue level via measurements of XO and 3-NT in aortic tissue. Our results demonstrate significantly increased plasma levels of MDA and 3-NT in vascular smooth muscle and endothelial cells, which were substantially reduced by administration of allopurinol to levels comparable with mice fed a CD and treated with allopurinol. Moreover, similar results were demonstrated in our measurements of XO activity, which is independently a major source of ROS and is known to participate in the pathogenesis of vascular dysfunction, leading to CVD (23). Therefore, our data do support a critical role for oxidative stress as a mediator of WD-induced vascular stiffness in female mice. Mitochondrial enzymes, activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymatic complex, and endothelial nitric oxide synthase (eNOS) uncoupling are major alternative sources of ROS as well (37). Conversely, there is extensive work about the role of multiple antioxidants in protecting the vasculature against oxidative stress-induced damage. Generic antioxidants, as well as different inhibitors of the NADPH-oxidase enzymatic complex and mitochondrial oxidative stress have been associated with improved markers of vascular stiffness, fibrosis remodeling, and endothelial dysfunction (15, 22, 73). However, our findings that allopurinol decreased WD-induced vascular stiffness and fibrosis strongly support an important role for tissue XO activation as a key mediator of vascular dysfunction as well. Furthermore, our findings are in agreement with previous data in cardiovascular tissue in rodents treated chronically with XO inhibition (19, 44).
High-fructose diets can indeed trigger activation of alternative sources of ROS such as NADPH oxidase (1, 5, 11). However, in our experiments, treatment with allopurinol decreased markers of oxidative stress and fibrosis, thus supporting the role of vascular XO activation as a critical contributor to elevated ROS production in the setting of WD feeding. Indeed, we have reported previously that WD feeding increases cardiovascular XO activity in males and that allopurinol treatment for 16 wk mitigates this WD-mediated increase (30).
Interestingly, administration of allopurinol resulted in improvements in endothelial stiffness in the absence of significant changes in aortic stiffness in vivo (measured by PWV). These findings suggest that improvement in endothelial cell stiffness is an earlier event that precedes further changes in arterial stiffness in whole aorta. In agreement, data from our laboratory obtained in female mice also fed a WD for 16 wk have also reported changes in endothelial stiffness in response to other interventions such as exercise, which do not result in in vivo changes in PWV (54).
We also found improved vasomotor responses associated with XO inhibition ex vivo. Utilizing wire myography, we detected increased endothelium-dependent and -independent responses in aorta in our allopurinol-treated mice in conditions of WD feeding. Interestingly we have previously reported aortic maximal relaxation reaching roughly 35–40% for ACh and ∼70% for SNP in female mice fed a CD (17, 29, 42). Available literature has reported comparable results with our published data (58, 59), whereas others studies report higher vasomotor responses compared with our results in control animals under similar conditions (55, 76). In addition, we have demonstrated under the same conditions as in our experiments that WD feeding for 16 wk results in reduction of maximal vasorelaxation in aorta of ∼30 and 35% for ACh and SNP relative to CD (17, 29, 42). In our current investigation, we found even more substantial reductions in vasomotor responses by WD, ∼54% for ACh and 61% for SNP, thus confirming a very significant impact of WD on vasomotor response, both endothelium dependent and independent. Although reports are variable, reflecting that vascular function is influenced by multiple variables, including genetic background and experimental conditions, our data not only confirm the profound impact of WD on vasomotor responses but demonstrate a critical contribution of enhanced XO activity to vascular abnormalities induced by WD.
We utilized allopurinol for XO inhibition since it is known to be safe and effective in the clinical setting for the treatment of hyperuricemia. Furthermore, available literature has reported potential additional benefits of this medication (18), such as improvements in endothelial function (25), oxidative stress (26), and modulation of inflammation (2), which are contributors to vascular stiffness. Nonetheless, the efficacy of allopurinol to improve vascular stiffness has not been established (18).
Remarkably, consumption of a WD induced only a nonsignificant increase in plasma uric acid levels in our female mice. This modest response has been reported previously in several studies dealing with high-fructose diets used in humans (27) as well as in rodents (65), and several reasons have been argued for this particular finding. Unlike humans in which uricase (the enzyme that catalyzes conversion of uric acid into allantoin and allows its excretion) is absent, this enzyme is present in rodents. Therefore, the impact of high-fructose diets on uric acid levels in mice differs significantly, and elevations in serum levels of uric acid in response to high-fructose diets (66) are expected to be less significant compared with humans. Furthermore, similarly to glucose, fructose can be reabsorbed from the filtrate in the renal proximal tubule via transporters such as glucose transporter (GLUT)5 and GLUT9, and hyperglycemia, which present in our WD-fed mice, is associated with impaired function of proximal tubules, leading to decreased urate reabsorption (7, 8). In addition, also in the setting of hyperglycemia, high concentrations of fructose reaching the renal proximal tubules competitively inhibit urate reabsorption via GLUT9, which can transport both glucose and fructose (35). Collectively, these phenomena result in reduced reabsorption of urate and, therefore, to decreased plasma uric acid levels, as found in our experiments. Furthermore, there is a dimorphic response regarding uric acid handling in mice, and it has been shown that elevations in uric acid are more substantial in males relative to females. Certainly, we have previously published data demonstrating significant increases in plasma uric acid levels in male mice fed a WD for 16 wk (30), which additionally confirms the adequacy of the feeding paradigm chosen for our study. Also, it has been shown that males have greater metabolism of fructose in the proximal tubule, whereas females exhibit more substantial distal tubule abnormalities regarding electrolyte balance (65).
In humans, available clinical studies support the therapeutic role of XO inhibition even in the absence of frank hyperuricemia. In heart failure subjects, a high dose of allopurinol improved forearm endothelial function, as assessed by venous occlusion plethysmography (25). Remarkably, these findings were not dependent on reduction of uric acid levels and might have been related to decreased oxidative stress (25). Another recent analysis of an elderly hypertensive population data set in the UK showed that, in hypertensive individuals, treatment with allopurinol was associated with 50% decreased risk of stroke and 30% risk of cardiac events (39). Importantly, a multicenter, prospective, randomized, open-label, blinded end-point clinical study, the PRIZE study, is currently underway and will assess the effect of a newer XO inhibitor, febuxostat, on carotid intima thickness in a population of asymptomatic hyperuricemia subjects (53). This trial, designed to include 500 participants with uric acid >7.0 mg/dl and carotid intima-media thickness ≥1.1 mm, will likely add valuable information regarding the role of XO inhibition in the treatment of CVD.
Our findings of a reduction in WD-mediated vascular stiffness with allopurinol contribute to a better understanding of the impact of XO activation on vascular stiffness in females. However, additional studies comparing males with females are warranted and will contribute to determining a possible differential CVD benefit from XO inhibition in females compared with males. In addition, additional studies in cardiac tissue in females are needed to better understand the sexual dimorphic impact of a WD, elevated uric acid, and XO activity on cardiovascular stiffness and cardiac-impaired relaxation. In this regard, data from our laboratory in male mice fed a WD have already shown benefits of allopurinol on markers of cardiac stiffness and diastolic dysfunction (30).
Our results do not show a significant impact of XO inhibition on body weight, fat percentage, or fasting glycemia in mice exposed to WD. These data are suggestive that the actions of allopurinol were not mediated by an indirect effect of XO inhibition on these metabolic parameters. Instead, the beneficial actions of XO inhibition are likely resulting from direct vascular effects such as decreased oxidative stress. We did not specifically measure markers of insulin resistance; however, the finding that the above-mentioned parameters remained unchanged also supports the possibility of mechanisms not affecting insulin sensitivity or glucose homeostasis.
In summary, this investigation demonstrates that XO inhibition and subsequent reductions in systemic and vascular tissue oxidative stress play a protective role against WD-induced vascular stiffness in female mice. Because population studies suggest that hyperuricemia has a more severe impact on vascular stiffness in females compared with males, our findings stimulate the possibility that females consuming a WD may benefit specially from pharmacological XO inhibition. Additional studies are required to determine the extent to which reduced vascular stiffness with XO inhibition is mediated by a reduction in uric acid and whether this pharmacological treatment results in a significant reduction in the excessive CVD morbidity and mortality found in insulin-resistant women relative to men.
GRANTS
This work was supported by the National Institutes of Health (1-K08-HL-132012-01A1 to G. Lastra, 1-K08-HL-129074-01 to C. Manrique, K01-HL-125503 and R21-DK-105368 to J. Padilla, and R01-HL-073101 and R01-HL-107910 to J. R. Sowers) and a Department of Veterans Affairs Merit Award (1BX001981 to J. R. Sowers).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.L., C.M., J.P., and J.R.S. conceived and designed research; G.L., A.R.A., and J.P. analyzed data; G.L., C.M., J.P., and J.R.S. interpreted results of experiments; G.L., C.M., G.J., A.R.A., M.R.H., and J.P. prepared figures; G.L. and A.R.A. drafted manuscript; G.L., C.M., G.J., A.R.A., M.R.H., J.P., and J.R.S. edited and revised manuscript; G.L., C.M., G.J., A.R.A., M.R.H., B.J.B., B.N., J.P., and J.R.S. approved final version of manuscript; C.M., G.J., A.R.A., M.R.H., B.J.B., and B.N. performed experiments.
ACKNOWLEDGMENTS
We acknowledge the work by Dr. Javad Habibi, who captured and analyzed immunohistochemistry data. We also acknowledge Brenda Hunter for her valuable editorial assistance and the formatting of this manuscript. We acknowledge the National Institutes of Health for funding this investigation as well as the Department of Veterans Affairs.
REFERENCES
- 1.Almenara CC, Mill JG, Vassallo DV, Baldo MP, Padilha AS. In vitro fructose exposure overactivates NADPH oxidase and causes oxidative stress in the isolated rat aorta. Toxicol In Vitro 29: 2030–2037, 2015. doi: 10.1016/j.tiv.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Bäck M, Hansson GK. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol 12: 199–211, 2015. doi: 10.1038/nrcardio.2015.5. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, Johnson RJ, Sautin YY. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 60: 1258–1269, 2011. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63: 636–646, 2014. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettaieb A, Vazquez Prieto MA, Rodriguez Lanzi C, Miatello RM, Haj FG, Fraga CG, Oteiza PI. (-)-Epicatechin mitigates high-fructose-associated insulin resistance by modulating redox signaling and endoplasmic reticulum stress. Free Radic Biol Med 72: 247–256, 2014. doi: 10.1016/j.freeradbiomed.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ Res 118: 1723–1735, 2016. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjornstad P, Paul Wadwa R, Sirota JC, Snell-Bergeon JK, McFann K, Rewers M, Rivard CJ, Jalal D, Chonchol MB, Johnson RJ, Maahs DM. Serum uric acid and hypertension in adults: a paradoxical relationship in type 1 diabetes. J Clin Hypertens (Greenwich) 16: 283–288, 2014. doi: 10.1111/jch.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjornstad P, Snell-Bergeon JK, McFann K, Wadwa RP, Rewers M, Rivard CJ, Jalal D, Chonchol MB, Johnson RJ, Maahs DM. Serum uric acid and insulin sensitivity in adolescents and adults with and without type 1 diabetes. J Diabetes Complications 28: 298–304, 2014. doi: 10.1016/j.jdiacomp.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, Manolis AJ, Perez-Ruiz F, Mancia G. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens 33: 1729–1741, 2015. doi: 10.1097/HJH.0000000000000701. [DOI] [PubMed] [Google Scholar]
- 10.Bruun JM, Maersk M, Belza A, Astrup A, Richelsen B. Consumption of sucrose-sweetened soft drinks increases plasma levels of uric acid in overweight and obese subjects: a 6-month randomised controlled trial. Eur J Clin Nutr 69: 949–953, 2015. doi: 10.1038/ejcn.2015.95. [DOI] [PubMed] [Google Scholar]
- 11.Cannizzo B, Quesada I, Militello R, Amaya C, Miatello R, Cruzado M, Castro C. Tempol attenuates atherosclerosis associated with metabolic syndrome via decreased vascular inflammation and NADPH-2 oxidase expression. Free Radic Res 48: 526–533, 2014. doi: 10.3109/10715762.2014.889295. [DOI] [PubMed] [Google Scholar]
- 12.Chow B, Rabkin SW. The relationship between arterial stiffness and heart failure with preserved ejection fraction: a systemic meta-analysis. Heart Fail Rev 20: 291–303, 2015. doi: 10.1007/s10741-015-9471-1. [DOI] [PubMed] [Google Scholar]
- 13.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293: H2009–H2023, 2007. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 14.Coutinho T. Arterial stiffness and its clinical implications in women. Can J Cardiol 30: 756–764, 2014. doi: 10.1016/j.cjca.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 15.de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15: 522–530, 2016. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol 10: 364–376, 2014. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in female mice. Hypertension 66: 99–107, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng G, Qiu Z, Li D, Fang Y, Zhang S. Effects of allopurinol on arterial stiffness: a meta-analysis of randomized controlled trials. Med Sci Monit 22: 1389–1397, 2016. doi: 10.12659/MSM.898370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engberding N, Spiekermann S, Schaefer A, Heineke A, Wiencke A, Müller M, Fuchs M, Hilfiker-Kleiner D, Hornig B, Drexler H, Landmesser U. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation 110: 2175–2179, 2004. doi: 10.1161/01.CIR.0000144303.24894.1C. [DOI] [PubMed] [Google Scholar]
- 20.Eshak ES, Iso H, Kokubo Y, Saito I, Yamagishi K, Inoue M, Tsugane S. Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: the Japan Public Health Centre-based study cohort I. Am J Clin Nutr 96: 1390–1397, 2012. doi: 10.3945/ajcn.112.037903. [DOI] [PubMed] [Google Scholar]
- 21.Fang JI, Wu JS, Yang YC, Wang RH, Lu FH, Chang CJ. High uric acid level associated with increased arterial stiffness in apparently healthy women. Atherosclerosis 236: 389–393, 2014. doi: 10.1016/j.atherosclerosis.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell 11: 269–276, 2012. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res 120: 713–735, 2017. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 24.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 89: 1037–1042, 2009. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 114: 2508–2516, 2006. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 26.George J, Struthers AD. The role of urate and xanthine oxidase inhibitors in cardiovascular disease. Cardiovasc Ther 26: 59–64, 2008. doi: 10.1111/j.1527-3466.2007.00029.x. [DOI] [PubMed] [Google Scholar]
- 27.Goicoechea M, Garcia de Vinuesa S, Verdalles U, Verde E, Macias N, Santos A, Pérez de Jose A, Cedeño S, Linares T, Luño J. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis 65: 543–549, 2015. doi: 10.1053/j.ajkd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Hayden MR, Tyagi SC. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutr Metab (Lond) 1: 10, 2004. doi: 10.1186/1743-7075-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 118: 935–943, 2016. doi: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia G, Habibi J, Bostick BP, Ma L, DeMarco VG, Aroor AR, Hayden MR, Whaley-Connell AT, Sowers JR. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension 65: 531–539, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY, Lanaspa MA. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 62: 3307–3315, 2013. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sánchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86: 899–906, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Kanbay M, Sánchez-Lozada LG, Franco M, Madero M, Solak Y, Rodriguez-Iturbe B, Covic A, Johnson RJ. Microvascular disease and its role in the brain and cardiovascular system: a potential role for uric acid as a cardiorenal toxin. Nephrol Dial Transplant 26: 430–437, 2011. doi: 10.1093/ndt/gfq635. [DOI] [PubMed] [Google Scholar]
- 34.Kang S, Fan HM, Li J, Fan LY, Miao AY, Bao Y, Wu LZ, Zhu Y, Zhang DF, Liu ZM. Relationship of arterial stiffness and early mild diastolic heart failure in general middle and aged population. Eur Heart J 31: 2799–2807, 2010. doi: 10.1093/eurheartj/ehq296. [DOI] [PubMed] [Google Scholar]
- 35.Le MT, Shafiu M, Mu W, Johnson RJ. SLC2A9–a fructose transporter identified as a novel uric acid transporter. Nephrol Dial Transplant 23: 2746–2749, 2008. doi: 10.1093/ndt/gfn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care 23: 962–968, 2000. doi: 10.2337/diacare.23.7.962. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Horke S, Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237: 208–219, 2014. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Luo Y, Burrington CM, Graff EC, Zhang J, Judd RL, Suksaranjit P, Kaewpoowat Q, Davenport SK, O’Neill AM, Greene MW. Metabolic phenotype and adipose and liver features in a high-fat Western diet-induced mouse model of obesity-linked NAFLD. Am J Physiol Endocrinol Metab 310: E418–E439, 2016. doi: 10.1152/ajpendo.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacIsaac RL, Salatzki J, Higgins P, Walters MR, Padmanabhan S, Dominiczak AF, Touyz RM, Dawson J. Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension 67: 535–540, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06344. [DOI] [PubMed] [Google Scholar]
- 40.Malik VS, Hu FB. Fructose and cardiometabolic health: what the evidence from sugar-sweetened beverages tells us. J Am Coll Cardiol 66: 1615–1624, 2015. doi: 10.1016/j.jacc.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manrique C, DeMarco VG, Aroor AR, Mugerfeld I, Garro M, Habibi J, Hayden MR, Sowers JR. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology 154: 3632–3642, 2013. doi: 10.1210/en.2013-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manrique C, Lastra G, Ramirez-Perez FI, Haertling D, DeMarco VG, Aroor AR, Jia G, Chen D, Barron BJ, Garro M, Padilla J, Martinez-Lemus LA, Sowers JR. Endothelial estrogen receptor-α does not protect against vascular stiffness induced by Western Diet in female mice. Endocrinology 157: 1590–1600, 2016. doi: 10.1210/en.2015-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr 58, 5 Suppl: 754S–765S, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Minhas KM, Saraiva RM, Schuleri KH, Lehrke S, Zheng M, Saliaris AP, Berry CE, Barouch LA, Vandegaer KM, Li D, Hare JM. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res 98: 271–279, 2006. [Erratum. Circ Res 98: e70, 2006.] doi: 10.1161/01.RES.0000200181.59551.71. [DOI] [PubMed] [Google Scholar]
- 45.Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol 31: 631–641, 2015. doi: 10.1016/j.cjca.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 290: F625–F631, 2006. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 47.Nogi S, Fujita S, Okamoto Y, Kizawa S, Morita H, Ito T, Sakane K, Sohmiya K, Hoshiga M, Ishizaka N. Serum uric acid is associated with cardiac diastolic dysfunction among women with preserved ejection fraction. Am J Physiol Heart Circ Physiol 309: H986–H99, 2015. doi: 10.1152/ajpheart.00402.2015. [DOI] [PubMed] [Google Scholar]
- 48.Nomura J, Busso N, Ives A, Tsujimoto S, Tamura M, So A, Yamanaka Y. Febuxostat, an inhibitor of xanthine oxidase, suppresses lipopolysaccharide-induced MCP-1 production via MAPK phosphatase-1-mediated inactivation of JNK. PLoS One 8: e75527, 2013. doi: 10.1371/journal.pone.0075527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol 19: 2407–2413, 2008. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol 301: F919–F931, 2011. doi: 10.1152/ajprenal.00068.2011. [DOI] [PubMed] [Google Scholar]
- 51.Onat A, Uyarel H, Hergenç G, Karabulut A, Albayrak S, Sari I, Yazici M, Keleș I. Serum uric acid is a determinant of metabolic syndrome in a population-based study. Am J Hypertens 19: 1055–1062, 2006. doi: 10.1016/j.amjhyper.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Orchard TJ. The impact of gender and general risk factors on the occurrence of atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Ann Med 28: 323–333, 1996. doi: 10.3109/07853899608999089. [DOI] [PubMed] [Google Scholar]
- 53.Oyama J, Tanaka A, Sato Y, Tomiyama H, Sata M, Ishizu T, Taguchi I, Kuroyanagi T, Teragawa H, Ishizaka N, Kanzaki Y, Ohishi M, Eguchi K, Higashi Y, Yamada H, Maemura K, Ako J, Bando YK, Ueda S, Inoue T, Murohara T, Node K; PRIZE Study Investigators . Rationale and design of a multicenter randomized study for evaluating vascular function under uric acid control using the xanthine oxidase inhibitor, febuxostat: the PRIZE study. Cardiovasc Diabetol 15: 87, 2016. doi: 10.1186/s12933-016-0409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padilla J, Ramirez-Perez FI, Habibi J, Bostick B, Aroor AR, Hayden MR, Jia G, Garro M, DeMarco VG, Manrique C, Booth FW, Martinez-Lemus LA, Sowers JR. Regular exercise reduces endothelial cortical stiffness in western diet-fed female mice. Hypertension 68: 1236–1244, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park JL, Shu L, Shayman JA. Differential involvement of COX1 and COX2 in the vasculopathy associated with the α-galactosidase A-knockout mouse. Am J Physiol Heart Circ Physiol 296: H1133–H1140, 2009. doi: 10.1152/ajpheart.00929.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park JS, Nam JS, Cho MH, Yoo JS, Ahn CW, Jee SH, Lee HS, Cha BS, Kim KR, Lee HC. Insulin resistance independently influences arterial stiffness in normoglycemic normotensive postmenopausal women. Menopause 17: 779–784, 2010. doi: 10.1097/gme.0b013e3181cd3d60. [DOI] [PubMed] [Google Scholar]
- 57.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 383: 1973–1980, 2014. doi: 10.1016/S0140-6736(14)60040-4. [DOI] [PubMed] [Google Scholar]
- 58.Pojoga LH, Yao TM, Opsasnick LA, Garza AE, Reslan OM, Adler GK, Williams GH, Khalil RA. Dissociation of hyperglycemia from altered vascular contraction and relaxation mechanisms in caveolin-1 null mice. J Pharmacol Exp Ther 348: 260–270, 2014. doi: 10.1124/jpet.113.209189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pojoga LH, Yao TM, Sinha S, Ross RL, Lin JC, Raffetto JD, Adler GK, Williams GH, Khalil RA. Effect of dietary sodium on vasoconstriction and eNOS-mediated vascular relaxation in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol 294: H1258–H1265, 2008. doi: 10.1152/ajpheart.01014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quinn U, Tomlinson LA, Cockcroft JR. Arterial stiffness. JRSM Cardiovasc Dis 1: pii: cvd.2012.012024, 2012. doi: 10.1258/cvd.2012.012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reunanen A, Takkunen H, Knekt P, Aromaa A. Hyperuricemia as a risk factor for cardiovascular mortality. Acta Med Scand Suppl 668: 49–59, 1982. [DOI] [PubMed] [Google Scholar]
- 62.Rosset R, Surowska A, Tappy L. Pathogenesis of cardiovascular and metabolic diseases: are fructose-containing sugars more involved than other dietary calories? Curr Hypertens Rep 18: 44, 2016. doi: 10.1007/s11906-016-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saab KR, Kendrick J, Yracheta JM, Lanaspa MA, Pollard M, Johnson RJ. New insights on the risk for cardiovascular disease in African Americans: the role of added sugars. J Am Soc Nephrol 26: 247–257, 2015. doi: 10.1681/ASN.2014040393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shapiro Y, Mashavi M, Luckish E, Shargorodsky M. Diabetes and menopause aggravate age-dependent deterioration in arterial stiffness. Menopause 21: 1234–1238, 2014. doi: 10.1097/GME.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 65.Sharma N, Li L, Ecelbarger CM. Sex differences in renal and metabolic responses to a high-fructose diet in mice. Am J Physiol Renal Physiol 308: F400–F410, 2015. doi: 10.1152/ajprenal.00403.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.So A, Thorens B. Uric acid transport and disease. J Clin Invest 120: 1791–1799, 2010. doi: 10.1172/JCI42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, Chen GX, Keim NL, Havel PJ. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr 101: 1144–1154, 2015. doi: 10.3945/ajcn.114.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119: 1322–1334, 2009. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia 51: 527–539, 2008. doi: 10.1007/s00125-007-0918-3. [DOI] [PubMed] [Google Scholar]
- 70.Takir M, Kostek O, Ozkok A, Elcioglu OC, Bakan A, Erek A, Mutlu HH, Telci O, Semerci A, Odabas AR, Afsar B, Smits G, ALanaspa M, Sharma S, Johnson RJ, Kanbay M. Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia. J Investig Med 63: 924–929, 2015. doi: 10.1097/JIM.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 71.Tappy L, Lê K-A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90: 23–46, 2010. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 72.Tatchum-Talom R, Martel C, Marette A. Influence of estrogen on aortic stiffness and endothelial function in female rats. Am J Physiol Heart Circ Physiol 282: H491–H498, 2002. doi: 10.1152/ajpheart.00589.2001. [DOI] [PubMed] [Google Scholar]
- 73.Vendrov AE, Vendrov KC, Smith A, Yuan J, Sumida A, Robidoux J, Runge MS, Madamanchi NR. NOX4 NADPH oxidase-dependent mitochondrial oxidative stress in aging-associated cardiovascular disease. Antioxid Redox Signal 23: 1389–1409, 2015. doi: 10.1089/ars.2014.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker AE, Henson GD, Reihl KD, Morgan RG, Dobson PS, Nielson EI, Ling J, Mecham RP, Li DY, Lesniewski LA, Donato AJ. Greater impairments in cerebral artery compared with skeletal muscle feed artery endothelial function in a mouse model of increased large artery stiffness. J Physiol 593: 1931–1943, 2015. doi: 10.1113/jphysiol.2014.285338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Webb DR, Khunti K, Silverman R, Gray LJ, Srinivasan B, Lacy PS, Williams B, Davies MJ. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia 53: 1190–1198, 2010. doi: 10.1007/s00125-010-1689-9. [DOI] [PubMed] [Google Scholar]
- 76.Zemse SM, Hilgers RHP, Webb RC. Interleukin-10 counteracts impaired endothelium-dependent relaxation induced by ANG II in murine aortic rings. Am J Physiol Heart Circ Physiol 292: H3103–H3108, 2007. doi: 10.1152/ajpheart.00456.2006. [DOI] [PubMed] [Google Scholar]
- 77.Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, Demarco VG, Sowers JR. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension 55: 880–888, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145136. [DOI] [PMC free article] [PubMed] [Google Scholar]