Abstract
Intrauterine growth restriction (IUGR) is associated with persistent metabolic complications, but information is limited for IUGR infants. We determined glucose-stimulated insulin secretion (GSIS) and insulin sensitivity in young lambs with placental insufficiency-induced IUGR. Lambs with hyperthermia-induced IUGR (n = 7) were compared with control lambs (n = 8). GSIS was measured at 8 ± 1 days of age, and at 15 ± 1 days, body weight-specific glucose utilization rates were measured with radiolabeled d-glucose during a hyperinsulinemic-euglycemic clamp (HEC). IUGR lambs weighed 23% less (P < 0.05) than controls at birth. Fasting plasma glucose and insulin concentrations were not different between IUGR and controls for either study. First-phase insulin secretion was enhanced 2.3-fold in IUGR lambs compared with controls. However, second-phase insulin concentrations, glucose-potentiated arginine-stimulated insulin secretion, and β-cell mass were not different, indicating that IUGR β-cells have an intrinsic enhancement in acute GSIS. Compared with controls, IUGR lambs had higher body weight-specific glucose utilization rates and greater insulin sensitivity at fasting (1.6-fold) and hyperinsulinemic periods (2.4-fold). Improved insulin sensitivity for glucose utilization was not due to differences in skeletal muscle insulin receptor and glucose transporters 1 and 4 concentrations. Plasma lactate concentrations during HEC were elevated in IUGR lambs compared with controls, but no differences were found for glycogen content or citrate synthase activity in liver and muscle. Greater insulin sensitivity for glucose utilization and enhanced acute GSIS in young lambs are predicted from fetal studies but may promote conditions that exaggerate glucose disposal and lead to episodes of hypoglycemia in IUGR infants.
Keywords: glucose, pancreas, β-cell, insulin sensitivity, sheep
studies in human and animal models demonstrate that low birth weight, a proxy for intrauterine growth restriction (IUGR), predisposes offspring to metabolic diseases, such as glucose intolerance and Type 2 diabetes (21, 26, 45, 55). The causal link between IUGR and glucose intolerance in adulthood provides one example of the developmental origins of health and disease hypothesis, namely that adaptation to detrimental stimuli in development will shape the individual’s health for life (3). In addition to birth weight as a predictor for increased risk of diabetes, neonatal events that impact growth trajectory have also been shown to influence metabolic outcomes (43). There is a paucity of information on the transition of IUGR infants into childhood because insulin action and secretion continue to develop and mature with age (23, 31, 46). To our knowledge, only a few studies in IUGR infants have investigated the early transition into neonatal life (24 to 72 h). Even though these studies associate small for gestational age (SGA) with glucose dysregulation, the results are inconsistent across studies, which might reflect a dynamic interaction between insulin sensitivity and insulin secretion (5, 20, 44, 54). In sheep fetuses with placental insufficiency-induced IUGR, we have shown defects in pancreatic insulin secretion due to a reduced number of β-cells, but in vitro, fetal sheep islets (and, thus, individual β-cells) have increased insulin secretion capacity. IUGR fetal sheep also have increased peripheral insulin sensitivity (35, 36, 47, 52). We also have shown that chronic exposures to high plasma catecholamine concentrations, which are characteristic of IUGR fetuses with placental insufficiency, inhibit insulin secretion in fetal sheep, but decreasing chronic adrenergic stimulation results in a compensatory hypersecretion of insulin that persists for several days (12, 13, 32, 41), likely accounting for at least some of the increased islet and β-cell insulin secretion capacity.
We have not, however, followed these placenta-insufficient IUGR fetuses postnatally, after normalization of plasma catecholamine, glucose, and insulin concentrations. This is important to do, because many human infants with IUGR have persistent evidence of increased glucose requirements to prevent hypoglycemia, indicative of increased insulin secretion and peripheral insulin sensitivity relative to current glucose concentrations. Therefore, we tested the hypothesis that glucose-stimulated insulin secretion (GSIS) and insulin sensitivity for glucose disposal are enhanced in IUGR lambs during their first 2 wk after birth, but the increase in GSIS is relatively greater than the increase in peripheral insulin sensitivity; both of these conditions would predispose IUGR lambs to excessive glucose utilization and hypoglycemia.
MATERIALS AND METHODS
Animal preparation.
Animal experiments were approved by the Institutional Animal Care and Use Committee at The University of Arizona, Tucson, AZ, which is accredited by the American Association for Accreditation of Laboratory Animal Care. Time-mated, Columbia-Rambouillet cross-bred ewes were purchased from Nebeker Ranch (Lancaster, CA). Singleton pregnancies were confirmed by ultrasonography before treatment assignment and animal husbandry was provided, as previously described (10). Briefly, ewes were assigned by simple randomization method to one of two experimental groups—control or IUGR—at receipt. Placental insufficiency-induced IUGR lambs (n = 7) were produced by exposing pregnant ewes to elevated ambient temperatures (40°C for 12 h and 35°C for 12 h; dew point 22°C) from 39 ± 1 day gestational age (dGA) to 85 ± 3 dGA (term 148 ± 1 dGA). Control lambs (n = 8) were from ewes maintained at 25 ± 2°C that were pair fed to the average feed intake of ewes in the IUGR group (term 149 ± 1 dGA). After delivery, lambs were removed from the ewe to eliminate confounding maternal variability and raised in individual pens adjacent to each other. Lambs received colostrum before being placed solely on milk replacer (Milk Specialties, Dundee, IL) with ad libitum access. Weights were measured within 3 h of birth, usually before the lamb suckled, and lambs were also weighed before each study. Crown rump length (poll to tail head), hindlimb length (hip to hoof), and head circumference were measured in lambs at birth.
Surgical preparation.
At 3 ± 1 days of age, indwelling polyvinyl catheters were surgically placed in the femoral artery and vein for blood sampling and intravenous infusions. Prior to surgery, lambs were fasted for 3 or 4 h. Lambs were anesthetized and maintained by inhalation of 1.5–4% isoflurane in oxygen. All catheters were filled with heparinized saline (30 units/ml, 0.9% wt/vol NaCl; Nova-Tech, Grand Island, NE). After placement, catheters were tunneled subcutaneously to the lambʼs flank, exteriorized through a skin incision, and kept in a plastic mesh pouch sutured to the skin. Lambs were allowed to recover for at least 3 days before performing GSIS studies. Catheters were flushed daily with heparinized saline.
Insulin secretion responsiveness.
Glucose-stimulated insulin concentration was determined with a square-wave hyperglycemic clamp at 8 ± 1 days of age. Lambs were fasted for 3 h, placed into a custom-made Panepinto sling, and covered with a cloth drape. After ~20 min of acclimation, basal, fasting blood samples (1.5 ml) were collected in EDTA-lined syringes at −20, −10, and −1 min for plasma glucose and insulin measurements, as described previously (10). All GSIS sample times are presented relative to the dextrose bolus (time 0). The hyperglycemic clamp was initiated with an intravenous dextrose bolus (150 mg/kg) followed by a constant infusion of 33% (wt/vol) dextrose (d-glucose) solution that was adjusted (109 ± 9 µmol·min−1·kg−1) to maintain arterial plasma glucose concentrations at 15.0 ± 0.6 mmol/l, which was approximately twice the baseline arterial plasma glucose concentration. At the onset of the infusion, arterial blood samples (0.5 ml) were collected every 3–5 min for 20 min to establish the steady-state plasma glucose concentrations, after which three blood samples (1.5 ml) were collected between 30 and 60 min (10- to 15-min intervals). Steady-state hyperglycemic conditions were confirmed during the sampling period when arterial plasma glucose concentrations varied less than ± 10% of the mean. Acute, first-phase insulin concentration was calculated as the area under the curve (AUC) for the first 20 min of insulin secretion.
Glucose-potentiated arginine-stimulated insulin concentration (GPAIS) was determined with a follow-on arginine bolus (0.5 mmol/kg) to the GSIS study. Arginine was administered over 4 min after the final GSIS sample was collected at 60 min. Blood samples (0.5 ml) for plasma insulin concentrations were collected at 65, 75, and 90 min.
Insulin sensitivity for glucose utilization.
At 15 ± 1 days of age, insulin sensitivity of glucose utilization was measured in fasted conditions and during a hyperinsulinemic-euglycemic clamp (HEC) (19). Body weight-specific rates of glucose utilization (disposal) were determined by the net disappearance rate of d-[14C(U)]glucose (PerkinElmer Life Sciences, Boston, MA) in fasting and hyperinsulinemic steady-state periods. Lambs were fasted 4 h and placed into the Panepinto sling. A constant infusion (2 ml/h) of radiolabeled glucose (37.2 μCi/ml) in saline was initiated with a 4-ml priming bolus. After 40 min, four arterial blood samples (1.2 ml each) were collected at 8–10-min intervals, and basal (fasted) glucose utilization rates (µmol·min−1·kg−1), plasma concentrations of insulin, glucose, and lactate, and arterial blood gasses were measured. Hyperinsulinemia was initiated with a priming dose of insulin (175 mU/kg; HumulinR; Lilly, Indianapolis IN) followed by a constant infusion (2 mU·min−1·kg−1). Euglycemia was maintained with a 33% (wt/vol) dextrose infusion that was adjusted in response to the plasma glucose concentrations taken every 5–10 min until steady state was achieved, usually within an hour. Steady-state euglycemic conditions were confirmed during the sampling period when arterial plasma glucose concentrations varied less than ± 9% of the mean. Arterial blood samples (1.2 ml each) were collected at 8- to10-min intervals during the HEC.
After an overnight recovery from the HEC study, lambs (16 ± 1 days of age) were euthanized with an intravenous overdose of pentobarbital sodium (86 mg/kg) and phenytoin sodium (11 mg/kg; Euthasol, Virbac Animal Health, Fort Worth, TX). Organs were dissected, weighed, snap frozen in liquid nitrogen, and stored at −80°C. The splenic portion of the pancreas (body and tail from the pancreatic notch) was fixed in 4% paraformaldehyde overnight and then embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek, Torrance, CA), as described previously (14).
Biochemical analysis and calculations.
Concentrations of plasma glucose and lactate were measured immediately with a YSI model 2700 Select Biochemistry Analyzer (Yellow Springs Instruments, Yellow Springs, OH). Blood gasses and oxygen saturations were measured in blood collected in heparin-lined syringes (Elkins-Sinn, Cherry Hill, NJ) using an ABL520 (Radiometer, Copenhagen, Denmark), and values were temperature corrected for the lamb’s core body temperature at the start of the study. Whole blood [14C]glucose (0.5 ml) was determined in supernatants after being deproteinized by mixing whole blood with 0.3 N zinc sulfate heptahydrate and 0.3 M barium hydroxide in a final volume of 5 ml. The deproteinized supernatant was aliquoted in triplicate and dried; radioactivity was measured with a LS 6500 multipurpose scintillation counter (Beckman Coulter, Fullerton, CA) in Hionic Fluor Liquid Scintillation Cocktail (PerkinElmer, Waltham, MA). Blood plasma was stored at −80°C until insulin concentrations were measured with an ovine insulin ELISA (ALPCO Diagnostics, Windham, NH; sensitivity 0.07 ng/ml; intra-assay and interassay coefficients of variation were 2.9% and 5.6%, respectively).
The rate of glucose disappearance (utilization) equals the rate of glucose appearance under steady-state conditions for plasma glucose concentrations. The body weight-specific net glucose utilization (disposal) rate was calculated as the ratio of the rate of [14C]glucose (dpm/min) and the steady-state arterial plasma [14C]glucose specific activity (dpm/μmol glucose). Endogenous (hepatic) glucose production was calculated as the difference between the net [14C]glucose utilization rate and the exogenous net dextrose (d-glucose) infusion rate (µmol/min). Insulin sensitivity for glucose utilization rate (µmol·min−1·kg−1·µg−1·l−1) was calculated as the steady-state net rate of glucose utilization (µmol·min−1·kg−1) divided by the arterial plasma insulin concentration (µg/l). All results were normalized to lamb body weight (kg).
Endocrine pancreas morphology.
Tissue sections (6 μm) for histological evaluation were cut from the tail of the dissected pancreas. Procedures for fluorescent immunostaining on cryosections were performed to detect insulin, glucagon, and the combination of somatostatin and pancreatic polypeptide, as reported previously (14, 32, 33). Fluorescent images were visualized with a Leica microscope, digitally captured, and analyzed with ImagePro software (Media Cybernetics, Silver Spring, MD), as described previously (32, 33). Positive areas were determined for at least 25 fields of view (0.39 mm2) on two pancreas sections per animal, and tissue sections were separated by ≥100-μm interval. Data are expressed as a percentage of total pancreas area, and cell mass was calculated by multiplying the pancreas weight by the percent positive area.
Glycogen content.
Glycogen content in liver and skeletal muscle (semitendinosus muscle) was determined as previously described with modifications (4, 35). Briefly, 200 mg of frozen tissue was pulverized and digested in 2 ml of 30% KOH at 95°C for 30 min. The homogenate (150 μl) was placed on no. 1 Whatman filter paper and washed in 66.6% ethanol with constant stirring for 30 min. The filter paper was removed, dried, and cut into small pieces. Glycogen was converted to glucose with 41.2 mg/ml amyloglucosidase (Sigma-Aldrich, St. Louis, MO) in 0.2 M acetate buffer (pH 4.8, 0.5% glacial acetic acid, 0.12 M sodium acetate) at 37°C for 65 min. Glucose concentration of this solution was determined in triplicate using the YSI 2700 Select Biochemistry Analyzer. Results are expressed as milligrams glucose per gram tissue (wet weight).
Citrate synthase activity.
Citrate synthase content in liver and skeletal muscle was determined using the citrate synthase assay kit (Sigma-Aldrich). Protein was extracted from snap-frozen liver and skeletal muscle (100 mg) into 2 ml of CelLytic MT reagent following the CelLytic MT mammalian tissues lysis procedure (cat. no. C3228; Sigma-Aldrich). After protein extraction, total protein concentrations were determined by Pierce BCA assay (Thermo Fisher, Rockford, IL). Citrate synthase activity of the supernatant was measured in a 96-well plate in triplicates. Each reaction contained 20 μg of protein. Reaction was initiated after adding oxaloacetic acid, and absorbance of the reaction was measured for 1.5 min at a wavelength of 412 nm.
Immunoblotting.
Protein lysates were prepared from semitendinosus muscle (30–40 mg) with CelLytic MT Cell Lysis Reagent (Sigma-Aldrich) and protease inhibitors (0.5 mM PMSF, 2 µg/ml aprotinin, 2.5 µg/ml leupeptin). Lysates were homogenized with the TissueLyser LT (Qiagen, Hilden, Germany) at 50 Hz for 5 min and were centrifuged for 10 min at 16,100 g. Protein concentrations of the supernatant were determined with Pierce BCA Assay (Thermo Fisher). Immunoblots were performed as previously described (35, 52). Primary antibodies against glucose transporter (GLUT)1 (1:250; Millipore, Temecula, CA), GLUT4 (1 µg/ml, Sigma-Aldrich) and insulin receptor β-subunit (1:100, Santa Cruz) were detected with anti-rabbit immunoglobulin G horseradish peroxidase conjugated secondary antibody(1:15,000; Bio-Rad Laboratories, Hercules, CA) and chemiluminescence (West Pico Chemiluminescent Substrate; Thermo Fisher). Protein levels were quantified using photographed images and densitometric analyses (Scion Image Software, Frederick, MD); to accommodate the number of samples, two blots were run simultaneously and contain four overlapping samples for internal controls. Data are presented as a percentage of control means.
Statistical analysis.
The statistical analysis was performed on IUGR (n = 7; 5 males and 2 females) and control (n = 8; 3 males and 5 females) lambs unless otherwise noted. For the HEC analysis, one IUGR lamb did not meet steady-state criteria during the clamp, and another lamb became ill, and both were excluded from the analysis. Skeletal muscle and liver tissues were not collected from one control and two IUGR lambs. After surgery, lambs were assigned a new identification number that did not correspond with treatment or previous identification to facilitate blinding and eliminate bias in subsequent analyses. Lamb morphometric characteristics, pancreas histology, citrate synthase activity, glycogen content, and immunoblots were analyzed by one-way ANOVA using general linear means procedure of SAS software (version 9.4; SAS Institute), and differences were determined with a post hoc least significant difference test. The GSIS and GPAIS studies were analyzed by ANOVA using the MIXED procedure of SAS. The model included experimental groups (control and IUGR), draw time, and their interaction. Acute, first-phase insulin concentration for the first 20 min was calculated as AUC with GraphPad Prism version 6 (GraphPad Software, La Jolla, CA) of insulin secretion and analyzed by one-way ANOVA using general linear means procedure of SAS. Insulin sensitivity study parameters were also analyzed by ANOVA using the MIXED procedure of SAS with lamb as the random effect. Main effects were experimental group (control and IUGR), period (basal and hyperglycemic), and their interaction. By experimental design, insulin sensitivity was analyzed at basal and hyperglycemia independently with one-way ANOVA using general linear means procedure of SAS, and differences were determined with a post hoc least significant difference test. For all data, the sex effect was removed from the model if P > 0.30. Means were separated using the PDIFF option of the LSMEANS statement of SAS and were considered significant when P ≤ 0.05. In the absence of interactions (P > 0.05) for the repeated-measures model, significant main effects are reported. Data are presented as the means ± SE.
RESULTS
Weights.
IUGR lambs were lighter at birth than control lambs (3.6 ± 0.3 kg vs. 4.7 ± 0.3 kg; P < 0.05). In the GSIS study, IUGR lambs weighed less (4.8 ± 0.2 kg; P ≤ 0.01) than control lambs (6.3 ± 0.4 kg). The body weights of the IUGR lambs were less (6.0 ± 0.4 kg; P < 0.01) compared with control lambs (7.4 ± 0.3 kg) at the HEC study. At necropsy the average body weights of IUGR lambs remained 30% lighter (P < 0.05) than control lambs.
Glucose-stimulated insulin secretion.
Basal plasma glucose and insulin concentrations were not different between IUGR and control lambs (Fig. 1). Plasma glucose and insulin concentrations were increased (P < 0.01), with hyperglycemia in both groups. Glucose concentrations (Fig. 1A) during hyperglycemia were similar between IUGR and control lambs, but average insulin concentrations were greater in IUGR (5.79 ± 0.90 µg/l, P = 0.05) than in control lambs (3.14 ± 0.84 µg/l). During the square-wave hyperglycemic clamp, first-phase insulin secretion in response to the clamp-induced hyperglycemia was determined during the first 20 min of hyperglycemia, and the change in insulin concentration by time calculated by AUC was greater (P ≤ 0.05) in IUGR lambs compared with controls (Fig. 1B). However, there were no differences between IUGR and control lambs during steady-state hyperglycemia, which represents the second phase of insulin secretion.
Fig. 1.
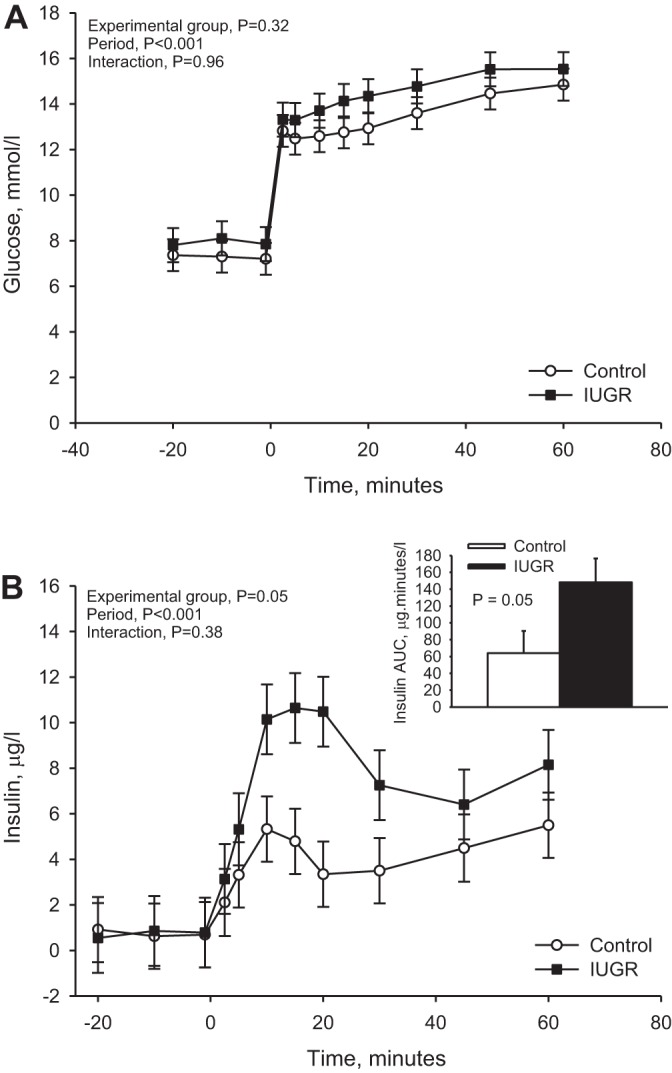
Glucose-stimulated insulin concentration. Square-wave hyperglycemic clamps were performed in control lambs (n = 8) and in lambs with placental insufficiency-induced intrauterine growth restriction (IUGR) (n = 7) at 8 days of age. A: plasma glucose concentrations (mmol/l) are presented for each sample time (y-axis, minutes), which is relative to the start of the glucose bolus (time = 0). B: plasma insulin concentrations (µg/l) are presented for each sampling point. The insulin area under the curve (AUC; µg·min−1·l−1) was calculated for the first 20 min of hyperglycemia, which represents acute, first-phase insulin secretion, and the AUC (means ± SE) is graphed in the inset. P values from the statistical analysis are reported in the top left corner of each graph.
Glucose-potentiated arginine-induced insulin secretion.
Insulin concentrations increased further (P < 0.01) after an arginine bolus (Fig. 2). Maximum insulin concentrations were reached at 5 min and returned to prebolus insulin concentrations by 30 min. No experimental group by time interaction or experimental group effect was found for GPAIS.
Fig. 2.
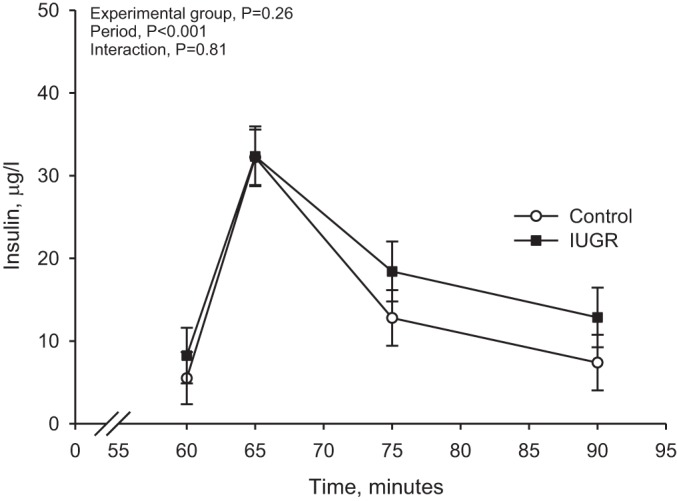
Glucose-potentiated arginine-induced insulin concentration. Insulin concentrations are presented for the glucose-potentiated arginine-stimulated insulin concentration (GPAIS) studies in control (n = 8) and IUGR lambs (n = 7). The arginine bolus is administered at 60 min; therefore, values obtained immediately before the hyperglycemic clamp are baseline samples for this analysis. Results from the statistical analysis are presented in the top left corner.
Insulin sensitivity for net body weight-specific glucose utilization rate.
During the HEC insulin concentrations increased from basal (fasting) period, as expected, but were not different between IUGR and control lambs in either period (Table 1). Glucose concentrations were not different between experimental groups or between study periods. Arterial blood pH, , , O2 content, and hematocrit were not different between IUGR and control lambs (Table 1). Although differences in period means were found, there was no experimental group by period interaction. Hyperinsulinemia decreased pH (7.400 ± 0.010 vs. 7.443 ± 0.001 at basal), O2 content (5.8 ± 0.26 mM vs. 6.12 ± 0.25 mM at basal), and hematocrit (31.6 ± 1.3% vs. 33.3 ± 1.3% at basal) means, but increased (79.7 ± 2.2 mmHg vs. 74.6 ± 2.2 mmHg at basal) from basal levels. Plasma lactate concentrations were 21.8% higher in IUGR lambs compared with control lambs during the HEC.
Table 1.
Arterial blood gases, lactate, glucose, and insulin concentrations during HEC study
| Fasting (Basal) |
Hyperinsulinemia |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Control (n = 8) | IUGR (n = 5) | Control (n = 8) | IUGR (n = 5) | P Value Group | P Value Period | P Value Interaction |
| pH | 7.46 ± 0.01 | 7.43 ± 0.02 | 7.42 ± 0.01 | 7.38 ± 0.02 | 0.12 | <0.01 | 0.85 |
| , mmHg | 76.4 ± 2.8 | 72.8 ± 3.3 | 84.9 ± 2.8 | 74.6 ± 3.5 | 0.10 | 0.02 | 0.11 |
| , mmHg | 39.8 ± 1.5 | 42.4 ± 1.7 | 41.2 ± 1.5 | 44.1 ± 1.8 | 0.23 | 0.06 | 0.76 |
| O2 content, mM | 6.1 ± 0.3 | 6.1 ± 0.4 | 6.0 ± 0.3 | 5.7 ± 0.4 | 0.74 | 0.03 | 0.22 |
| Hematocrit, % | 32.4 ± 1.7 | 34.1 ± 1.8 | 31.1 ± 1.7 | 32.4 ± 1.9 | 0.59 | <0.01 | 0.45 |
| Lactate, mM | 1.01 ± 0.08a,b | 1.09 ± 0.09a,b | 0.84 ± 0.08b | 1.27 ± 0.10a | 0.02 | 0.95 | 0.05 |
| Glucose, mmol/l | 8.54 ± 0.52 | 8.02 ± 0.60 | 8.27 ± 0.52 | 8.51 ± 0.63 | 0.87 | 0.66 | 0.09 |
| Insulin, µg/l | 0.93 ± 0.37 | 0.75 ± 0.42 | 4.33 ± 0.37 | 3.54 ± 0.51 | 0.29 | <0.01 | 0.46 |
Values are expressed as means ± SE; n, number of lambs. HEC, hyperinsulinemic-euglycemic clamp; IUGR, intrauterine growth restriction. Superscript letters denote differences for significant interactions between experimental group and period.
Net body weight-specific glucose utilization rates (µmol·min−1·kg−1) were similar in IUGR and control lambs in the basal (fasting) period (Fig. 3). However, steady-state rates of glucose utilization were 1.7-fold greater (P ≤ 0.05) in IUGR lambs than controls during the HEC. Glucose utilization rates were increased (P ≤ 0.05) during the HEC compared with basal in both IUGR (1.8-fold change) and control (1.4-fold change) lambs.
Fig. 3.
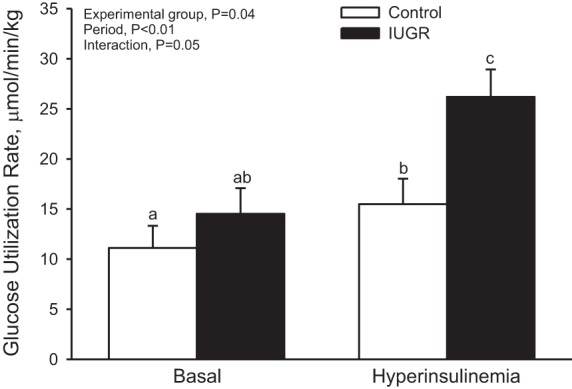
Glucose utilization rate. Rates of glucose utilization were measured with a radiotracer for d-glucose. Mean values are presented for control (n = 8) and IUGR (n = 5) lambs at basal and hyperinsulinemic steady states. There was an experimental group-by-period interaction, and the letters denote significant difference (P < 0.05).
Insulin sensitivity for glucose utilization (µmol·min−1·kg−1·µg−1·l−1; Fig. 4) was 1.6-fold greater (P ≤ 0.05) in IUGR lambs than in control lambs under basal conditions and 2.4-fold greater (P ≤ 0.05) during the HEC. Hepatic glucose production was not different between IUGR (11.8 ± 2.9 mg·min−1·kg−1) lambs and controls (5.4 ± 2.5 mg·min−1·kg−1).
Fig. 4.

Insulin sensitivity for glucose. Values are expressed as means ± SE for insulin sensitivity and are presented for control (n = 8) and IUGR (n = 7) lambs at basal (A) and hyperinsulinemic (B) steady-state periods. *Significant difference (P < 0.05) between IUGR and control groups.
Endocrine pancreas morphology.
Pancreas weights were not different between IUGR (9.87 ± 0.11 g) and control (12.25 ± 0.91 g) lambs. Similarly, the proportion of pancreas weight to body weight was not different between IUGR and control lambs. Insulin-positive area was not different between IUGR (3.2 ± 0.8%) and control (2.8 ± 0.5%) lambs. β-Cell mass also was not different between IUGR (302 ± 50.8 mg) and control (372 ± 113.6 mg) lambs. β-Cell mass relative to lamb body weight was similar between IUGR (50.3 ± 10.7 mg/kg) and control (47.9 ± 12.1 mg/kg) lambs. Glucagon-positive area (IUGR 1.1 ± 0.2% vs. control 1.1 ± 0. 2%) and α-cell mass (IUGR: 117 ± 20 mg vs. control: 132 ± 28 mg) were similar between experimental groups. In addition, α-cell mass relative to lamb body weight was not different between IUGR (19.6 ± 4.3 mg/kg) and control (17.1 ± 2.6 mg/kg) lambs. Total endocrine area, a combination of insulin-, glucagon-, somatostatin-, and pancreatic polypeptide-positive areas, was similar between IUGR (6.2 ± 1.0%) and control (5.0 ± 0.9%) lambs. Total endocrine mass (IUGR: 714 ± 83 mg vs. control: 529 ± 164 mg) and total endocrine mass relative to lamb body weight (IUGR: 88.0 ± 17.9 mg/kg vs. control: 92.1 ± 15.6 mg/kg) were also not different between experimental groups.
Glycogen content and citrate synthase activity.
Glycogen concentrations in muscle were similar between IUGR (17.2 ± 1.9 glucose mg/g) and control lambs (16.0 ± 1.6 glucose mg/g). Liver glycogen concentrations were not different between experimental groups (IUGR: 32.3 ± 9.9 glucose mg/g vs. control: 37.7 ± 7.5 mmol/kg). Similarly, citrate synthase activities were not different between groups in muscle (IUGR: 0.52 ± 0.04 µmol·min−1·µg−1 vs. control: 0.47 ± 0.03 µmol·min−1·µg−1) and liver (IUGR: 0.08 ± 0.01 µmol·min−1·µg−1 vs. control: 0.08 ± 0.01 µmol·min−1·µg−1).
Glucose transporters and insulin receptor levels.
No differences were found in skeletal muscle for GLUT1 (control: 100 ± 12% vs. IUGR: 108 ± 13%), GLUT4 (control 100 ± 5% vs. IUGR: 99 ± 9%), or insulin receptor β concentrations (control: 100 ± 8% vs. IUGR: 104 ± 13%) between groups (Fig. 5).
Fig. 5.
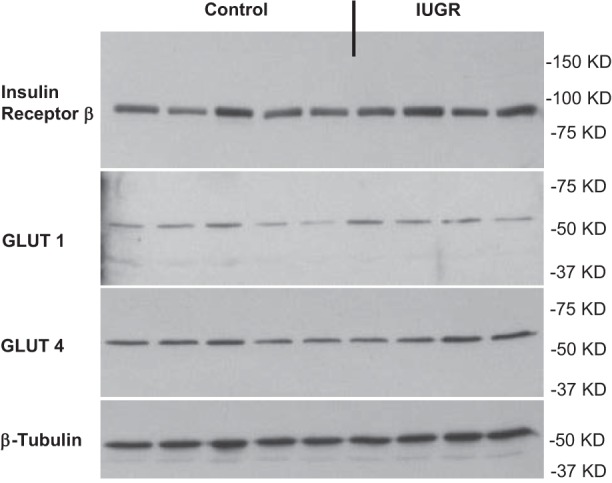
Glucose transporters and insulin receptor levels. The protein expression of insulin receptor β, GLUT1, and GLUT4 was measured in control and IUGR lamb semitendinosus muscle samples by Western blot analysis. Results were quantified and analyzed for control (n = 5) and PI-IUGR (n = 4) semitendinosus muscle. Representative image is shown for β-tubulin, which was run as an internal control for each Western blot.
DISCUSSION
Although the association between IUGR and metabolic complications in adulthood is well known, information is scarce on the metabolic consequences of IUGR in such infants shortly after birth. Here, we show enhanced insulin secretion and insulin action for glucose in young lambs born with IUGR caused by placental insufficiency. Amplification of the acute GSIS response in lambs occurs despite several earlier reports demonstrating impaired GSIS in the IUGR fetus, including studies in this ovine model of placental insufficiency-induced IUGR (25). Recent evidence indicates that inhibition of insulin secretion in the IUGR fetuses with placental insufficiency is due to elevated catecholamines that cause a hypersecretory response of insulin following cessation of the adrenergic stimulation (12, 13, 32). IUGR lambs have improved insulin sensitivity for glucose, which parallels findings in IUGR fetuses, where body weight-specific glucose utilization rates are maintained despite lower insulin concentrations (35, 53). The current results indicate that the increase in insulin sensitivity persists beyond birth without affecting glucose transporter or insulin receptor concentrations in skeletal muscle. These young IUGR lambs have inappropriately increased insulin concentrations for their degree of increased insulin sensitivity, which together could explain the transient relative hyperinsulinemic hypoglycemia observed in some IUGR infants (48, 51).
Analysis of the biphasic insulin secretion response shows greater acute, first-phase GSIS in IUGR lambs that is due to enhanced pancreatic β-cell responsiveness. We based this conclusion on the observations that second-phase insulin secretion, GPAIS, and β-cell mass were not different between IUGR and control lambs. Furthermore, equivalent insulin concentrations during the HEC, when insulin doses are identical, indicate that the metabolic clearance rate of insulin is comparable between IUGR and control lambs. These findings indicate that the enhanced acute insulin concentration response is intrinsic to the β-cells in IUGR lambs.
Our previous work in the IUGR fetus indicates that chronic suppression of insulin secretion from elevated plasma catecholamines promotes the β-cell hyperresponsiveness in IUGR lambs. Placenta insufficiency causes fetal hypoglycemia and hypoxia, which elevate plasma catecholamines (34, 36). We have shown that plasma catecholamine concentrations are chronically elevated in late gestation in such IUGR fetuses and continually inhibit their insulin secretion (40, 41). A pharmacological blockade of adrenergic receptors improves GSIS to a greater extent in IUGR fetuses than controls despite significantly less β-cell mass in the IUGR fetus (32, 40). Isolated islets from IUGR fetal sheep also have a greater fractional insulin secretion relative to their insulin content than control islets, which further supports β-cell hyperresponsiveness in the absence of adrenergic receptor activation (36). We have experimentally isolated the effects of hypercatecholaminemia in otherwise unperturbed sheep fetuses by continuously infusing norepinephrine for 1 wk. After termination of norepinephrine infusion, GSIS studies revealed β-cell hyperresponsiveness to glucose, which persisted for days in the fetus and more specifically their islets (12, 13).
We have previously shown in our ovine model that IUGR fetuses at 0.9 of gestation weighed 58% less than controls and had a similar reduction (59%) in pancreas weight compared with control fetuses (33). In this study, we see a modest, but significant, 23% reduction in birth weight compared with control lambs. However, pancreas weight and β-cell mass were not significantly different between IUGR and control lambs at 16 days of age when the IUGR lambs were 30% lighter in weight than control lambs. We attribute these differences to the shorter length of hyperthermia exposure in the current study, because it was demonstrated previously that the durations of environmental heat stress were associated with the severity of fetal growth restriction (22). To ensure that the lambs were viable without extensive measures to stabilize them after birth, we chose to expose the ewes to a shorter 6-wk duration of environmental heat stress instead of the 80 days previously used for studies on the IUGR fetus (32, 33). Although the severity of fetal growth restriction was less in this cohort of IUGR lambs (23%) compared with fetal cohorts (40–58%), many similarities, such as increased insulin secretion responsiveness (with adrenergic receptor antagonists) and increased insulin sensitivity, were identified, suggesting that previously described impairments in the fetus occur and persist in these IUGR lambs (32, 35, 53). These data are consistent with the uterine carunclectomy model of placental restriction, which also shows a positive association between fetal weight and absolute β-cell mass and that this correlation is lost in 43-day-old lambs (24). These data indicate that the severity of placental insufficiency and IUGR are important for the development of β-cell mass, but additional experiments are required to fully understand the postnatal relationships for molecular mechanisms that regulate cell proliferation, which were identified previously in islets from fetal sheep with placental insufficiency and IUGR (30).
Body-weight specific rates of glucose utilization increase with hyperinsulinemia, and in IUGR lambs, insulin-stimulated glucose utilization rates are increased to a greater extent compared with control lambs. Rates of glucose utilization in relation to insulin concentrations demonstrate increased insulin sensitivity in IUGR lambs at fasting and hyperinsulinemic periods. This increase in insulin sensitivity in IUGR lambs may be a continuation of adaptations seen in utero, where, despite lower insulin concentrations, IUGR fetuses have relatively normal weight-specific glucose utilization rates (35, 53) and increased insulin receptor concentrations (52). In the IUGR fetus, however, the capacity for glucose oxidation is less than in normally grown fetuses, which has been proposed to promote lactate production in skeletal muscle to supply hepatic gluconeogenesis (i.e., via the Cori cycle) (7, 35, 57). Whereas promoting mechanisms to spare glucose from oxidative metabolism is advantageous for the IUGR fetus and most likely dependent on endocrine responses to high catecholamines and low insulin, we also detect disparities between utilization and oxidation in the lambs, because lactate concentrations are greater in IUGR lambs and increased to a greater extent during the HEC. Additional evidence for glucose entry into glycolysis and not glycogenesis is that glycogen concentrations are similar between experimental groups in skeletal muscle and liver. Elevated lactate concentrations may indicate that, despite the maintained rates of glucose nonoxidative disposal, pyruvate entry into the tricarboxylic acid cycle is restricted, or at least not increased in response to increased glucose uptake and glycolysis. Limited oxidation rates of pyruvate do not appear to be dependent on differences in mitochondrial number because activity of citrate synthase, a mitochondrial matrix enzyme, was not different between experimental groups. Therefore, limitations in glucose oxidative capacity are dependent on pyruvate metabolism, which is a proposed site of regulation because pyruvate dehydrogenase kinase 4 expression is increased in IUGR fetuses. Pyruvate dehydrogenase kinase 4 inhibits pyruvate dehydrogenase activity to reduce the flux into the tricarboxylic acid cycle, thereby promoting pyruvate conversion to lactate, which is catalyzed by lactate dehydrogenase (7). Additional studies are needed to fully characterize the metabolic flux, as well as its dysregulation in skeletal muscle and liver, but these studies support the hypothesis that limitations in glucose oxidative rates in IUGR fetuses persist postnatally.
Like the β-cell hyperresponsiveness, the dysregulation of glucose metabolism in skeletal muscle may be produced by chronically elevated catecholamines, because sustained adrenergic stimulation increases insulin-stimulated nonoxidative glucose disposal in adult humans and rats (9, 28, 29, 39, 49). We have shown adrenergic receptor expression is downregulated in insulin-responsive tissues in IUGR fetuses and lambs (56, 58). Studies are ongoing to address the adrenergic-induced developmental programming and its role in glucose metabolism in IUGR fetuses and lambs.
Although the relationship between insulin secretion and sensitivity is enhanced early, this effect is most likely transient, on the basis of studies carried out in adults showing a higher incidence of glucose intolerance. In a sheep model of placental restriction, IUGR lambs displayed increased insulin sensitivity to circulating free fatty acids at 6 wk of age but had impaired insulin secretion to an intravenous glucose tolerance test (17). Normally, insulin sensitivity decreases with age. In sheep with placental restriction IUGR, increased whole body insulin sensitivity for glucose is present at 4 wk after birth, but glucose intolerance is present in adulthood (16, 23, 38, 45). To our knowledge, only a few studies have evaluated the early transition into neonatal life in humans. A human longitudinal study showed that SGA infants have increased insulin sensitivity for glucose at 48 h (5, 44, 50). However, by 1 yr of age SGA infants that showed catch up growth had higher fasting insulin concentrations compared with infants that did not gain weight and those born appropriate for gestational age (AGA) (44, 50), indicating the development of insulin resistance. Interestingly, by 3 yr of age, glucose disposition index in SGA infants was reduced, even though they were the same weight as appropriate weight-for-gestational age infants (44). These studies in human SGA infants and sheep models of IUGR support early enhancement of the insulin disposition index that declines with age to a greater degree than what is observed for normal maturation. This indicates that there is a postnatal component contributing to the developmental origins for glucose intolerance, and although not shown in this study, may be associated with later catch-up growth, particularly of adipose tissue and total body fat (18).
Perspectives and Significance
Placenta insufficiency-induced IUGR causes hypoglycemia and hypoxia, which are present in human IUGR fetuses and in fetuses in our ovine model of IUGR (36). These fetal conditions associated with IUGR are expected to decrease insulin secretion and action, either directly or indirectly through hypercatecholaminemia and other endocrine factors (37). We have shown that catecholamines inhibit insulin secretion in IUGR sheep fetuses and also suppress growth rates independent of circulating insulin concentrations, suggesting direct actions on other metabolic tissues (15, 41). Persistent activation of adrenergic receptors in humans increases insulin-stimulated nonoxidative glucose disposal (49). This adaptation and also potential adaptations to low plasma glucose and insulin concentrations in IUGR fetuses may accentuate glucose extraction in skeletal muscle resulting in the appearance of increased insulin sensitivity for glucose utilization. In addition, chronic adrenergic inhibition of insulin secretion due to hypercatecholaminemia leads to persistent hypersecretion of insulin following the cessation of adrenergic stimulation (13, 32). In this study, we show that both enhanced GSIS and increased insulin sensitivity persist at least transiently (up to 2 wk after birth) in lambs that were IUGR, indicating that the adaptive outcomes developed in utero. A common problem after birth for human IUGR newborns is transient hyperinsulinism and hypoglycemia (1, 5, 27, 51). Although the adaptations observed in IUGR lambs did not result in hypoglycemia, they resemble features that could explain this common problem in human IUGR newborns. Clearly, developmental impairments in insulin secretion, insulin action, and glucose homeostasis are not limited to fetal adaptations in IUGR, but produce a mismatch between secretion and sensitivity of insulin in the neonate that perpetuates such impairments after birth and the consequent metabolic responses, including hypoglycemia.
GRANTS
This work was supported by the National Institutes of Health (NIH) R01DK-084842 (S.W. Limesand, principal investigator). X. Chen was supported by National Natural Science Foundation of China (NSFC; Grant 31602021), Chongqing Science and Technology Commission, Chongqing, China (Grant CSTC2014JCYJA80036) and Southwest University (Grant 20140090). W. W. Hay was supported by NIH T32 HD-007186 (principal investigator and project director) and NIH K12 HD-068372 (project director). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.E.C., W.W.H.J., and S.W.L. conceived and designed research; L.E.C., X.C., and S.W.L. performed experiments; L.E.C., X.C., W.W.H.J., and S.W.L. analyzed data; L.E.C., X.C., W.W.H.J., and S.W.L. interpreted results of experiments; L.E.C. and S.W.L. prepared figures; L.E.C. and S.W.L. drafted manuscript; L.E.C., X.C., W.W.H.J., and S.W.L. edited and revised manuscript; L.E.C., X.C., W.W.H.J., and S.W.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mandie M. Dunham, Miranda J. Anderson, and Rafael A. Leos for technical assistance.
REFERENCES
- 1.Arya VB, Flanagan SE, Kumaran A, Shield JP, Ellard S, Hussain K, Kapoor RR. Clinical and molecular characterisation of hyperinsulinaemic hypoglycaemia in infants born small-for-gestational age. Arch Dis Child Fetal Neonatal Ed 98: F356–F358, 2013. doi: 10.1136/archdischild-2012-302880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJP. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 13: 807–813, 1997. doi: 10.1016/S0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 4.Barry JS, Davidsen ML, Limesand SW, Galan HL, Friedman JE, Regnault TR, Hay WW Jr. Developmental changes in ovine myocardial glucose transporters and insulin signaling following hyperthermia-induced intrauterine fetal growth restriction. Exp Biol Med (Maywood) 231: 566–575, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bazaes RA, Salazar TE, Pittaluga E, Peña V, Alegría A, Iñiguez G, Ong KK, Dunger DB, Mericq MV. Glucose and lipid metabolism in small for gestational age infants at 48 hours of age. Pediatrics 111: 804–809, 2003. doi: 10.1542/peds.111.4.804. [DOI] [PubMed] [Google Scholar]
- 7.Brown LD, Rozance PJ, Bruce JL, Friedman JE, Hay WW Jr, Wesolowski SR. Limited capacity for glucose oxidation in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 309: R920–R928, 2015. doi: 10.1152/ajpregu.00197.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budohoski L, Challiss RA, Dubaniewicz A, Kaciuba-Usciłko H, Leighton B, Lozeman FJ, Nazar K, Newsholme EA, Porta S. Effects of prolonged elevation of plasma adrenaline concentration in vivo on insulin-sensitivity in soleus muscle of the rat. Biochem J 244: 655–660, 1987. doi: 10.1042/bj2440655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Fahy AL, Green AS, Anderson MJ, Rhoads RP, Limesand SW. β2-Adrenergic receptor desensitization in perirenal adipose tissue in fetuses and lambs with placental insufficiency-induced intrauterine growth restriction. J Physiol 588: 3539–3549, 2010. doi: 10.1113/jphysiol.2010.192310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Green AS, Macko AR, Yates DT, Kelly AC, Limesand SW. Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep. Am J Physiol Endocrinol Metab 306: E58–E64, 2014. doi: 10.1152/ajpendo.00517.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Kelly AC, Yates DT, Macko AR, Lynch RM, Limesand SW. Islet adaptations in fetal sheep persist following chronic exposure to high norepinephrine. J Endocrinol 232: 285–295, 2016. doi: 10.1530/JOE-16-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole L, Anderson M, Antin PB, Limesand SW. One process for pancreatic β-cell coalescence into islets involves an epithelial-mesenchymal transition. J Endocrinol 203: 19–31, 2009. doi: 10.1677/JOE-09-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis MA, Macko AR, Steyn LV, Anderson MJ, Limesand SW. Fetal adrenal demedullation lowers circulating norepinephrine and attenuates growth restriction but not reduction of endocrine cell mass in an ovine model of intrauterine growth restriction. Nutrients 7: 500–516, 2015. doi: 10.3390/nu7010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Blasio MJ, Gatford KL, Harland ML, Robinson JS, Owens JA. Placental restriction reduces insulin sensitivity and expression of insulin signaling and glucose transporter genes in skeletal muscle, but not liver, in young sheep. Endocrinology 153: 2142–2151, 2012. doi: 10.1210/en.2011-1955. [DOI] [PubMed] [Google Scholar]
- 17.De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology 148: 1350–1358, 2007. doi: 10.1210/en.2006-0653. [DOI] [PubMed] [Google Scholar]
- 18.De Blasio MJ, Gatford KL, Robinson JS, Owens JA. Placental restriction of fetal growth reduces size at birth and alters postnatal growth, feeding activity, and adiposity in the young lamb. Am J Physiol Regul Integr Comp Physiol 292: R875–R886, 2007. doi: 10.1152/ajpregu.00430.2006. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 20.Falorni A, Fracassini F, Massi-Benedetti F, Maffei S. Glucose metabolism and insulin secretion in the newborn infant. Comparisons between the responses observed the first and seventh day of life to intravenous and oral glucose tolerance tests. Diabetes 23: 172–178, 1974. doi: 10.2337/diab.23.3.172. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Twinn DS, Ozanne SE. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav 88: 234–243, 2006. doi: 10.1016/j.physbeh.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 22.Galan HL, Hussey MJ, Barbera A, Ferrazzi E, Chung M, Hobbins JC, Battaglia FC. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol 180: 1278–1282, 1999. doi: 10.1016/S0002-9378(99)70629-0. [DOI] [PubMed] [Google Scholar]
- 23.Gatford KL, De Blasio MJ, Thavaneswaran P, Robinson JS, McMillen IC, Owens JA. Postnatal ontogeny of glucose homeostasis and insulin action in sheep. Am J Physiol Endocrinol Metab 286: E1050–E1059, 2004. doi: 10.1152/ajpendo.00340.2003. [DOI] [PubMed] [Google Scholar]
- 24.Gatford KL, Mohammad SN, Harland ML, De Blasio MJ, Fowden AL, Robinson JS, Owens JA. Impaired β-cell function and inadequate compensatory increases in β-cell mass after intrauterine growth restriction in sheep. Endocrinology 149: 5118–5127, 2008. doi: 10.1210/en.2008-0233. [DOI] [PubMed] [Google Scholar]
- 25.Green AS, Rozance PJ, Limesand SW. Consequences of a compromised intrauterine environment on islet function. J Endocrinol 205: 211–224, 2010. doi: 10.1677/JOE-09-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303: 1019–1022, 1991. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoe FM, Thornton PS, Wanner LA, Steinkrauss L, Simmons RA, Stanley CA. Clinical features and insulin regulation in infants with a syndrome of prolonged neonatal hyperinsulinism. J Pediatr 148: 207–212, 2006. doi: 10.1016/j.jpeds.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Jacob S, Fogt DL, Dietze GJ, Henriksen EJ. The β2-adrenergic modulator celiprolol reduces insulin resistance in obese Zucker rats. Life Sci 64: 2071–2079, 1999. doi: 10.1016/S0024-3205(99)00154-X. [DOI] [PubMed] [Google Scholar]
- 29.Jensen J, Jebens E, Brennesvik EO, Ruzzin J, Soos MA, Engebretsen EM, O’Rahilly S, Whitehead JP. Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling. Am J Physiol Endocrinol Metab 290: E154–E162, 2006. doi: 10.1152/ajpendo.00330.2005. [DOI] [PubMed] [Google Scholar]
- 30.Kelly AC, Bidwell CA, McCarthy FM, Taska DJ, Anderson MJ, Camacho LE, Limesand SW. RNA sequencing exposes adaptive and immune responses to intrauterine growth restriction in fetal sheep islets. Endocrinology 158: 743–755, 2017. doi: 10.1210/en.2016-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kervran A, Randon J. Development of insulin release by fetal rat pancreas in vitro: effects of glucose, amino acids, and theophylline. Diabetes 29: 673–678, 1980. doi: 10.2337/diab.29.9.673. [DOI] [PubMed] [Google Scholar]
- 32.Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 298: E770–E778, 2010. doi: 10.1152/ajpendo.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limesand SW, Jensen J, Hutton JC, Hay WW Jr. Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 288: R1297–R1305, 2005. doi: 10.1152/ajpregu.00494.2004. [DOI] [PubMed] [Google Scholar]
- 34.Limesand SW, Rozance PJ, Macko AR, Anderson MJ, Kelly AC, Hay WW Jr. Reductions in insulin concentrations and β-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am J Physiol Endocrinol Metab 304: E516–E523, 2013. doi: 10.1152/ajpendo.00435.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limesand SW, Rozance PJ, Smith D, Hay WW Jr. Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 293: E1716–E1725, 2007. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 36.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW Jr. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006. doi: 10.1210/en.2005-0900. [DOI] [PubMed] [Google Scholar]
- 37.Limesand SW, Rozance PJ. Fetal adaptations in insulin secretion result from high catecholamines during placental insufficiency. J Physiol In press. doi: 10.1113/JP273324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Schultz CG, De Blasio MJ, Peura AM, Heinemann GK, Harryanto H, Hunter DS, Wooldridge AL, Kind KL, Giles LC, Simmons RA, Owens JA, Gatford KL. Effect of placental restriction and neonatal exendin-4 treatment on postnatal growth, adult body composition, and in vivo glucose metabolism in the sheep. Am J Physiol Endocrinol Metab 309: E589–E600, 2015. doi: 10.1152/ajpendo.00487.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Pérusse F, Bukowiecki LJ. Mechanisms of the antidiabetic effects of the β3-adrenergic agonist CL-316243 in obese Zucker-ZDF rats. Am J Physiol Regul Integr Comp Physiol 274: R1212–R1219, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Macko AR, Yates DT, Chen X, Green AS, Kelly AC, Brown LD, Limesand SW. Elevated plasma norepinephrine inhibits insulin secretion, but adrenergic blockade reveals enhanced β-cell responsiveness in an ovine model of placental insufficiency at 0.7 of gestation. J Dev Orig Health Dis 4: 402–410, 2013. doi: 10.1017/S2040174413000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macko AR, Yates DT, Chen X, Shelton LA, Kelly AC, Davis MA, Camacho LE, Anderson MJ, Limesand SW. Adrenal demedullation and oxygen supplementation independently increase glucose-stimulated insulin concentrations in fetal sheep with intrauterine growth restriction. Endocrinology 157: 2104–2115, 2016. doi: 10.1210/en.2015-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin A, Connelly A, Bland RM, Reilly JJ. Health impact of catch-up growth in low-birth weight infants: systematic review, evidence appraisal, and meta-analysis. Matern Child Nutr 13: 2017. doi: 10.1111/mcn.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mericq V, Ong KK, Bazaes R, Peña V, Avila A, Salazar T, Soto N, Iñiguez G, Dunger DB. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia 48: 2609–2614, 2005. doi: 10.1007/s00125-005-0036-z. [DOI] [PubMed] [Google Scholar]
- 45.Owens JA, Thavaneswaran P, De Blasio MJ, McMillen IC, Robinson JS, Gatford KL. Sex-specific effects of placental restriction on components of the metabolic syndrome in young adult sheep. Am J Physiol Endocrinol Metab 292: E1879–E1889, 2007. doi: 10.1152/ajpendo.00706.2006. [DOI] [PubMed] [Google Scholar]
- 46.Philipps AF, Dubin JW, Raye JR. Maturation of early-phase insulin release in the neonatal lamb. Biol Neonate 39: 225–231, 1981. doi: 10.1159/000241441. [DOI] [PubMed] [Google Scholar]
- 47.Rozance PJ, Anderson M, Martinez M, Fahy A, Macko AR, Kailey J, Seedorf GJ, Abman SH, Hay WW Jr, Limesand SW. Placental insufficiency decreases pancreatic vascularity and disrupts hepatocyte growth factor signaling in the pancreatic islet endothelial cell in fetal sheep. Diabetes 64: 555–564, 2015. doi: 10.2337/db14-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozance PJ, Hay WW Jr. New approaches to management of neonatal hypoglycemia. Matern Health Neonatol Perinatol 2: 3, 2016. doi: 10.1186/s40748-016-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheidegger K, Robbins DC, Danforth E Jr. Effects of chronic β receptor stimulation on glucose metabolism. Diabetes 33: 1144–1149, 1984. doi: 10.2337/diab.33.12.1144. [DOI] [PubMed] [Google Scholar]
- 50.Soto N, Bazaes RA, Peña V, Salazar T, Avila A, Iñiguez G, Ong KK, Dunger DB, Mericq MV. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. J Clin Endocrinol Metab 88: 3645–3650, 2003. doi: 10.1210/jc.2002-030031. [DOI] [PubMed] [Google Scholar]
- 51.Stanley CA, Rozance PJ, Thornton PS, De Leon DD, Harris D, Haymond MW, Hussain K, Levitsky LL, Murad MH, Simmons RA, Sperling MA, Weinstein DA, White NH, Wolfsdorf JI. Re-evaluating “transitional neonatal hypoglycemia”: mechanism and implications for management. J Pediatr 166: 1520–5.e1, 2015. doi: 10.1016/j.jpeds.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150: 3021–3030, 2009. doi: 10.1210/en.2008-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorn SR, Brown LD, Rozance PJ, Hay WW Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes 62: 65–73, 2013. doi: 10.2337/db11-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Cui Y, Tong X, Ye H, Li S. Glucose and lipid metabolism in small-for-gestational-age infants at 72 hours of age. J Clin Endocrinol Metab 92: 681–684, 2007. doi: 10.1210/jc.2006-1281. [DOI] [PubMed] [Google Scholar]
- 55.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE, Carlsson S, de Rooij SR, Dyck RF, Eriksson JG, Falkner B, Fall C, Forsén T, Grill V, Gudnason V, Hulman S, Hyppönen E, Jeffreys M, Lawlor DA, Leon DA, Minami J, Mishra G, Osmond C, Power C, Rich-Edwards JW, Roseboom TJ, Sachdev HS, Syddall H, Thorsdottir I, Vanhala M, Wadsworth M, Yarbrough DE. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 300: 2886–2897, 2008. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 56.Yates DT, Macko AR, Chen X, Green AS, Kelly AC, Anderson MJ, Fowden AL, Limesand SW. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J Physiol 590: 5439–5447, 2012. doi: 10.1113/jphysiol.2012.237347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yates DT, Green AS, Limesand SW. Catecholamines mediate multiple fetal adaptations during placental insufficiency that contribute to intrauterine growth restriction: lessons from hyperthermic sheep. J Pregnancy 2011: 740408, 2011. doi: 10.1155/2011/740408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yates DT, Macko AR, Nearing M, Chen X, Rhoads RP, Limesand SW. Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. J Pregnancy 2012: 631038, 2012. doi: 10.1155/2012/631038. [DOI] [PMC free article] [PubMed] [Google Scholar]


