Abstract
Fish routinely experience environmental hypoxia and have evolved various strategies to tolerate this challenge. Given the key role of the CRF system in coordinating the response to stressors and its cardioprotective actions against ischemia in mammals, we sought to characterize the cardiac CRF system in zebrafish and its role in hypoxia tolerance. We established that all genes of the CRF system, the ligands CRFa, CRFb, urotensin 1 (UTS1), and urocortin 3 (UCN3); the two receptor subtypes (CRFR1 and CRFR2); and the binding protein (CRFBP) are expressed in the heart of zebrafish: crfr1 > crfr2 = crfbp > crfa > ucn3 > crfb > uts1. In vivo, exposure to 5% O2 saturation for 15 min and 90 min of recovery resulted in four- to five-fold increases in whole heart crfb and ucn3 mRNA levels but did not affect the gene expression of other CRF system components. In vitro, as assessed by monitoring caspase 3 activity and the number of terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive cells, pretreatment of excised whole hearts with CRF or UCN3 for 30 min prevented the increase in apoptosis associated with exposure to 1% O2 saturation for 30 min with a 24-h recovery. Lastly, the addition of the nonselective CRF receptor antagonist αh-CRF(9–41) prevented the cytoprotective effects of CRF. We show that the CRF system is expressed in fish heart, is upregulated by hypoxia, and is cytoprotective. These findings identify a novel role for the CRF system in fish and a new strategy to tolerate hypoxia.
Keywords: CRH, UCN3, heart, hypoxia, cytoprotection
corticotropin-releasing factor (CRF) is the primary neuropeptide that controls the vertebrate hypothalamic-pituitary-adrenal/interrenal (HPA/HPI) axis (28, 74). In mammals, CRF, together with three paralogous peptides, urocortin 1 (UCN1), UCN2, and UCN3; two receptor subtypes, CRFR1 and CRFR2; and a binding protein, CRFBP, coordinate the endocrine, autonomic, and behavioral responses to stressors (9). In the periphery, the mammalian CRF system has also been attributed to numerous actions in the regulation of the cardiovascular (83), digestive (76), reproductive (43), and immune (42) systems. In contrast, in nonmammalian species, while several studies have shown that components of the CRF system are broadly expressed outside the central nervous system (CNS) (16, 24, 38), to date, the functional roles of CRF and CRF-related peptides in peripheral tissues and the evolutionary origin of these actions remain largely unknown.
At the cellular level, a growing number of reports have attributed cytoprotective functions to CRF-related peptides in various tissues and species. For example, CRF treatment reduced spontaneous apoptosis in Xenopus laevis tadpole tail explant cultures (16). Similarly, CRF reduced caspase cleavage in human retinoblastoma cells (69) and is neuroprotective in primary cultures of rat neuronal cell lines (12, 32, 62). Urocortins have also been shown to have similar cytoprotective and neuroprotective properties in various cell types (11, 15, 40, 78). In the mammalian heart, powerful cytoprotective effects have been attributed to UCN1 that is released from cardiomyocytes in response to sublethal ischemia and reperfusion injury (26). In fact, there is now considerable interest in the therapeutic use of urocortins for the treatment of heart failure (77). Therefore, beyond their key role in regulating the neuroendocrine stress response, emerging evidence suggests that CRF-related peptides are involved in the cellular balance between survival and apoptosis. Although CRF, UCN3, and their receptors have been detected by RT-PCR in the heart of several fish species (6, 20, 24, 38, 67), whether CRF-related peptides are also cytoprotective in the heart of nonmammalian species is not known.
Unlike mammals, fish routinely experience hypoxia in their natural habitat (5, 29). The reduced solubility and content of oxygen in water compared with air contribute to making hypoxia an important environmental challenge faced by aquatic species (54). Indeed, the evolutionary adaptations of teleost fish to low oxygen may have contributed to their extensive radiation among aquatic vertebrate species (71). However, delivery of oxygen to the heart remains a critical challenge (4, 31). In most fish species, the coronary circulation, which provides oxygenated blood to the outer compact layer of the heart, is either absent or limited, and the heart mainly comprises spongy myocardium, which relies on deoxygenated venous blood for its oxygen supply (31). Moreover, the percent oxygen saturation of the venous return to the heart can decrease from 25 to 40% at rest to less than 3% during environmental hypoxia (27, 36, 80). Although many behavioral, physiological, and biochemical adaptations to maintain oxygen delivery to the heart and match cardiac ATP demand with supply have been described in fish (4, 34, 55, 81), little is known of the cellular mechanisms that may mitigate the potential damage of hypoxia.
Therefore, given the expression of CRF-related peptides and receptors in the heart of several fish species, the significance of hypoxia as an evolutionary force shaping adaptive strategies in fish and the known cardioprotective effects of the CRF system in mammals, we hypothesized that the cardiac CRF system is involved in the protective response to acute hypoxia in zebrafish (Danio rerio) by contributing to the maintenance of cardiac integrity. To test this hypothesis, we first determined which members of the CRF system are expressed in zebrafish hearts. The CRF system in zebrafish has four ligands, CRFa (an ortholog of medaka teleocortin; 37) and CRFb, paralogs that evolved via duplication of crh1 early in the teleost lineage (35), urotensin 1 (UTS1), the teleost UCN1 ortholog (49), and UCN3 (20), as well as CRFBP, CRFR1, and CRFR2 (3). We then assessed the effects of an acute hypoxic challenge on the gene expression of the cardiac CRF system members. Finally, we used excised whole hearts (65) to test the cytoprotective effects of CRF and UCN3. In support of our hypothesis, we found that all components of the CRF system are expressed in the heart; crfb and ucn3 expression increased in response to hypoxia/reoxygenation; and both CRF and UCN3 inhibited hypoxia-induced cell death and caspase 3 activity in cultured hearts.
MATERIALS AND METHODS
All of the procedures used were approved by the University of Guelph’s Animal Care and Use Committee in accordance with the Canadian Council on Animal Care and conformed to the “Guide for the Care and Use of Laboratory Animals.”
Experimental Animals.
Wild-type adult zebrafish (Danio rerio) were obtained from AQuality (Mississauga, ON, Canada) and were reared in the Aqualab at the University of Guelph following standard practices described by Westerfield (85). Fish were maintained at 28°C with a 14:10-h light-dark cycle and fed Artemia nauplii and fish flakes ad libitum.
Experimental Protocol
Assessment of basal cardiac CRF system gene expression.
Male fish (n = 11) were terminally anesthetized by rapid cooling in ice water (86), as locally approved. Hearts were immediately isolated, snap frozen on dry ice, stored at −80°C until total RNA extraction and quantification of gene expression by quantitative real-time RT-PCR (qRT-PCR).
Effects of hypoxia exposure and reoxygenation on cardiac CRF system gene expression and whole body cortisol levels.
Four groups of male adult fish were placed in 3-liter tanks with a ceramic air stone and a perforated barrier 3 cm below the surface of the water to prevent aquatic surface respiration (n = 31–32 per group). Air was bubbled in the tanks for 24 h before hypoxia exposure to acclimate the fish and maintain dissolved O2 (DO) above 90%. Fish in the normoxic control treatment were mock-treated with air for 130 min to match the time taken for the longest treatment group. In the three hypoxic treatments, nitrogen was bubbled through the water to achieve 5% DO in 24 ± 1 min. The hypoxic fish were exposed to 5% DO for 15 min and either euthanized immediately, following 15 min recovery, or following 90 min of recovery. Reoxygenation was accomplished by bubbling air through the water to achieve 90–100% DO in 7 ± 1 min. Water O2 was monitored continuously using an O2 electrode (liquid dissolved O2 field probe, Hach, Loveland, CO). All fish were euthanized by rapid cooling in ice water. Hearts, including the sinus venosus, atrium, ventricle, and bulbus arteriosus, were immediately isolated, snap frozen on dry ice, and stored at −80°C until total RNA extraction and quantification of gene expression by qRT-PCR. The remainder of each fish was frozen for measurement of whole body cortisol levels.
Effects of CRF or UCN3 on hypoxia/reoxygenation-induced cell death in cultured zebrafish hearts.
On the basis of the heart culture protocol described by Pieperhoff et al. (65), hearts from adults males were cultured in 1 ml of supplemented L-15 medium (see Heart culture for all compositions) with 0, 0.1, or 1.0 nM of rat/human CRF (American Peptide Company, Sunnyvale, CA; n = 6–8 per dosage) or mouse UCN3 (GenScript, Piscataway, NJ; n = 5–7 per dosage) for 30 min and then rinsed twice in supplemented 1× PBS. Hearts were then kept in normoxic conditions or exposed to hypoxia in 1 ml of supplemented 1× PBS by bubbling nitrogen through the medium using 21 gauge needles. Oxygen was decreased from >90% DO to 1% DO (over 5.9 ± 0.3 min), reflective of the venous blood supply observed in fish experiencing hypoxia (80), and maintained for 30 min. Hearts were then returned to supplemented L-15 medium and recovered for 24 h. After recovery, all hearts were fixed in fresh 2% paraformaldehyde (PFA) overnight at 4°C and processed for terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL).
Effects of CRF or UCN3 on hypoxia/reoxygenation-induced caspase 3 activation in cultured zebrafish hearts.
To examine whether the effects of CRF and UCN3 on cell death are at least partially due to changes in apoptosis, four treatment groups were used per peptide. As above, hearts from male adults were pretreated with 0 or 1 nM of CRF (n = 10–14 per dosage) or UCN3 (n = 10–12 per dosage) and then kept in normoxic conditions (>90% DO) or exposed to 1% DO for 30 min. After the exposures, hearts were recovered in supplemented L-15 medium for 24 h, and caspase 3 activity was immediately assessed. It should be noted that in a pilot study to confirm the applicability of this method, caspase activity did not increase over 48 h under control conditions, and neither fish body mass nor heart rate following excision correlated with caspase activity (data not shown).
Effects of cotreatment with αh-CRF(9–41) and CRF on hypoxia/reoxygenation-induced caspase 3 activation in cultured zebrafish hearts.
To determine whether the antiapoptotic effects of CRF are CRF receptor-dependent, hearts from adult males (n = 9–12 per treatment) were pretreated with the nonselective CRF receptor antagonist α-helical CRF(9–41) [αh-CRF(9–41)] alone (10 nM) or with CRF (1 nM), and either kept in normoxic conditions or exposed to 1% DO for 30 min. After the exposures, hearts were recovered in supplemented L-15 medium for 24 h and snap frozen on dry ice, and caspase 3 activity was immediately assessed.
Analytical Techniques
Quantification of gene expression.
Total RNA extraction was performed on pools of two or three hearts. The hearts were homogenized using an RNA extraction reagent (Solution A; Bio Basic, Markham, ON, Canada) by micropestle followed by sonication (2 × 8 s at 50 W) on wet ice. The manufacturer’s recommendations were followed and to increase recovery, 10 μg of RNA-grade glycogen (Roche Diagnostics, Laval, QC, Canada) was added to each sample before precipitation of nucleic acid by isopropanol and incubation at −80°C overnight. RNA was quantified by ultraviolet spectrophotometry at 260 nm (Nanodrop 8000; Nanodrop Products, Wilmington, DE). From each total RNA pool, 200 ng of RNA was treated with Ambion DNase (Thermo Fisher, Waltham, MA) and used to synthesize cDNA using Quanta qScript (Quanta Biosciences, Gaithersburg, MD), both as per the manufacturer’s instructions.
Quantitative PCR was performed on a CFX96 system (Bio-Rad, Hercules, CA) with Quanta Perfecta Supermix (Quanta Biosciences); the primers are listed in Table 1. The 20-µl reactions contained 10 µl 2× master mix, 5 µl of 10-fold diluted first-strand cDNA template or no-RT controls, and 2.5 µl of both forward and reverse primers (0.4 µM). Default cycling conditions were used and followed by a melting curve analysis to verify the specificity of each PCR product. Only samples with a unimodal dissociation curve and the predicted melting point temperature were analyzed. To account for differences in amplification efficiency, standard curves were constructed for each gene using known dilutions of cDNA from heart tissue. Input values for each gene were obtained by fitting the average threshold cycle (Ct) value to the antilog of the gene-specific standard curve, thereby correcting for differences in primer amplification efficiency. To correct for minor variations in template input and transcriptional efficiency, the input values were normalized to the housekeeping gene elongation factor 1α (ef1α). Note that the expression of ef1α did not differ between any of the treatments (P > 0.05). Gene expression data are reported as a fold change relative to the normoxic control treatment mean value.
Table 1.
Nucleotide sequences of zebrafish primers used for quantitative RT-PCR
| Gene | Accession no. | Sequence (5′-3′) | Efficiency, % |
|---|---|---|---|
| crfa | XM_009298729 | F: ACAGCAGACTCTCACCGACAA | 98% |
| R: CGGTAATCTCAGTCGGTGGTCC | |||
| crfb | BC085458.1 | F: GCCGCGCAAAGTTCAAAA | 105% |
| R: GCGAGGAGAATCTGTGCGTAA | |||
| crfbp | NM_001003459 | F: CGAGAGTTACCAGAGGAGTTTGTGTA | 98% |
| R: ACCCTCTACGGCCACCATATC | |||
| crfr1 | XM_691254 | F: AGGCAGGCTGTTCACCTCAT | 100% |
| R: CTGTTGCCCTGGAGATTAGTGTAA | |||
| crfr2 | XM_681362 | F: TTACCAAGGGCCTGTGATTCTAG | 104% |
| R: GCGCACAATGTTGAAAAGAAC | |||
| ef1α | NM_131263 | F: GGGCAAGGGCTCCTTCAA | 105% |
| R: CGCTCGGCCTTCACTTTG | |||
| ucn3 | BC163845 | F: GAGTGCAGGGCAGAACAATGT | 98% |
| R: GAAACTGGTTGCGCAAAGGA | |||
| uts1 | NM_001030180 | F: CACGCTTCCTCAACCGCTACT | 104% |
| R: CAGTCGCGCACTTCTCGAT |
crf, corticotropin-releasing factor; crfbp, CRF binding protein; crfr, CRF receptor; ef1α, elongation factor 1α; F, forward; R, reverse; ucn3, urocortin 3; uts1, urotensin 1.
Whole body cortisol.
Steroids were extracted following Lister and Van Der Kraak (48). Briefly, zebrafish were removed from −80°C storage, thawed on ice, blotted dry, weighed, and homogenized in 3 ml of PBS with 1 mM EDTA using a Euro Turrax T 20b mechanical homogenizer (IKA Labortechnik, Staufen, Germany). A portion of the homogenate was methanol extracted twice (4 volume, vol/vol). The soluble fractions were combined, dried, and reconstituted in 50 mM sodium acetate (pH 4.0) and then purified using C-18 solid-phase extraction columns (Agela Technologies, Wilmington, DE). Whole body cortisol concentrations were measured in triplicate by RIA, as previously described (33). The lower detection of the assay was 16 pg/ml. The intra-assay and interassay coefficients of variation were 3.2% (n = 6) and 5.3% (n = 6), respectively. Cortisol values are given as nanograms per gram body weight and are corrected for extraction efficiency.
Heart culture.
The in vitro heart culture method of Pieperhoff (65) was used to assess whether CRF or UCN3 has cytoprotective effects during hypoxia exposure in zebrafish hearts. Briefly, adult zebrafish were anesthetized in 28°C tricaine methanesulfonate (MS-222; 0.02%; Syndel International, Qualicum Beach, BC, Canada) and were dissected immediately. Whole hearts, including the sinus venosus, atrium, ventricle, and bulbus arteriosus, were excised and transferred to supplemented 1× PBS (with 100 IU/ml penicillin-streptomycin, 150 IU/ml heparin, 1.25 mM CaCl2, 0.8 g/l glucose), rinsed, and then transferred to supplemented L-15 medium (L15 medium with 10% FBS, 100 IU/ml penicillin-streptomycin, 1.25 mM CaCl2, 0.8 g/l glucose), and incubated with gentle shaking (50 rpm) at 28°C. All experiments were performed at 28°C, in 12-well tissue culture plates (Sarstedt, Nümbrecht, Germany) with 1 ml of supplemented medium. All hypoxia exposures and controls were performed in supplemented 1× PBS for O2 electrode compatibility. O2 saturation of the wells was monitored with a MI-730 micro-O2 electrode (Microelectrodes, Bedford, NH).
TUNEL staining.
Fixed hearts were incubated in 1× PBS with 100 mM glycine and 10 mM EDTA for 30 min and then sequentially transferred to 30% sucrose in 1× PBS, 15% sucrose in 1× PBS, 1:1 PBS:embedding medium (Tissue-Plus OCT Compound; Thermo Fisher, Waltham, MA), and 100% embedding medium for 1 day each. All solutions used for cryopreservation were made with 10 mM EDTA. Hearts were then cryosectioned at 10 µm and postfixed for 2 min in 2% PFA and again quenched in 1× PBS with 100 µM glycine and 10 mM EDTA. Sections were then cleared following Kuwajima et al. (46) using ClearT2. For permeabilization, samples were treated with proteinase K (20 µg/ml) for 30 min at 37°C, 0.5% Triton-X PBS for 30 min, and citrate buffer for 30 min. TUNEL staining was done using an in situ cell death detection kit (TMR Red; Roche, Mississauga, ON, Canada), with samples being stained for 2 h in a 37°C humidified chamber and then washed twice with 1× PBS. Finally, sections were incubated with the nuclear stain Hoechst 33342 (1 µg/ml; Sigma-Aldrich, Oakville, ON, Canada) for 30 min and then rinsed twice with 1× PBS for 15 min. TUNEL-stained hearts were imaged on a Nikon Ti at 580 nm for TUNEL (TMR Red) and 461 nm for all nuclei (Hoechst 33342). Images were processed in Fiji (73). TUNEL- and Hoechst-positive cells were manually counted to establish automatic cell-counting parameters that gave equal results. Results are the percentage of TUNEL-positive cells taken from entire heart sections and averaged by treatment group; each heart was considered an n of one.
Caspase 3 enzymatic activity.
Whole heart caspase 3 activity was quantified, according to Stankiewicz et al. (75). Briefly, the maximum enzymatic breakdown of the caspase-3 fluorogenic substrate Ac-DEVD-AMC was quantified as a measure of caspase-3 activation. Hearts were homogenized in lysis buffer (50 mM HEPES, 0.1% wt/vol CHAPS, 0.1 mM EDTA, 1 mM DTT, pH 7.4, 10 mg/ml aprotinin, leupeptin, and pepstatin A; Sigma-Aldrich) by micropestle and sonication (2 × 8 s at 50 W) on ice then centrifuged at 12,000 g for 10 min. Lysate was mixed with assay buffer (50 mM HEPES, 100 mM NaCl, 0.1% wt/vol CHAPS, 10 mM DTT, 1 mM EDTA, 10% glycerol) containing 40 mM Ac-DEVD-AMC (Amresco, Solon, OH), and fluorescence (380-nm excitation and 460-nm emission) was measured for 2 h at 28°C using a fluorescence plate reader (BioTek FLx800; BioTek, Winooski, VT). The maximum reaction rate was normalized to total protein determined by Bradford assay (Bio-Rad Protein Assay Dye Reagent; Bio-Rad, Hercules, CA) on the remaining lysate.
Statistical Analysis
Results are presented as means ± SE. All data were analyzed in SigmaPlot 12.5 (Systat Software, Chicago, IL). The changes in cardiac CRF system gene expression following an acute hypoxic challenge were analyzed by one-way ANOVA and Holm-Sidak post hoc multiple-comparisons tests. The effects of CRF or UCN3 on hypoxia/reoxygenation-induced cell death and caspase 3 activation in cultured zebrafish hearts were analyzed by two-way ANOVA and Holm Sidak post hoc multiple-comparisons tests. Where assumptions of normality and equal variance were not met, the data were log10-transformed before the above analysis; when the data still did not meet assumptions, the general linear model with Dunnett’s post hoc test was used on untransformed data using IBM SPSS Statistics 23.0. For TUNEL staining differences, a logistic binomial regression was used since binary outcomes were measured. The significance level for all statistical tests was P < 0.05.
RESULTS
Assessment of Basal Cardiac CRF System Gene Expression
All known members of the CRF family in zebrafish were expressed in the heart (Fig. 1). Relative to the expression of uts1, the mRNA levels of crfb, ucn3, and crfa were 3.6-, 8.7-, and 21.9-fold higher, respectively. Overall, the mRNA levels of crfbp and both receptor types, crfr1 and crfr2, were greater than the expression of the four ligands. More specifically, crfbp, crfr2, and crfr1 gene expression was 249-, 362-, and 3,122-fold higher than the mRNA levels of uts1, respectively.
Fig. 1.
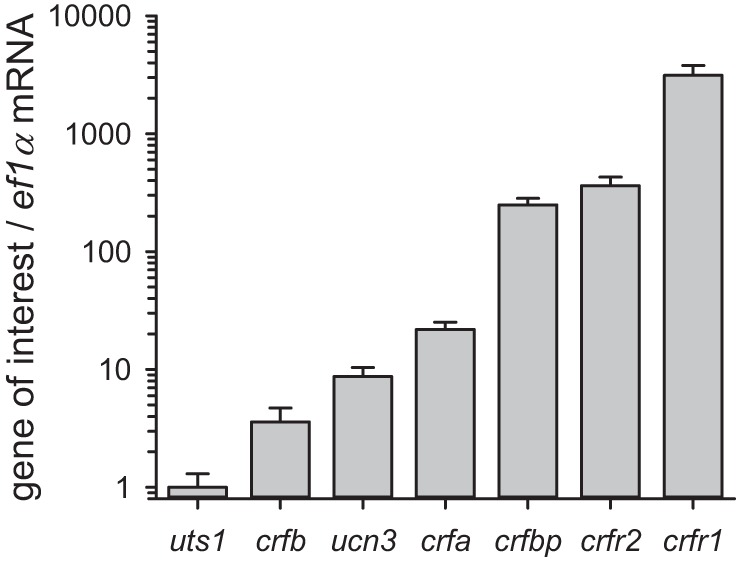
Assessment of basal cardiac CRF system gene expression in zebrafish. mRNA expression values are normalized with ef1α, and the expression ratios are presented relative to uts1. Values are expressed as means ± SE (n = 11).
Effects of Hypoxia Exposure and Reoxygenation on Cardiac CRF System Gene Expression and Whole Body Cortisol Levels
The changes in cardiac CRF system gene expression following an acute hypoxic challenge were investigated. Most notably, cardiac crfb mRNA levels showed a strong increase following hypoxia (F3,44 = 5.339, P = 0.003). Although the hypoxia treatments with 0 and 15 min of reoxygenation elicited nonsignificant 1.3- and 2.0-fold increases in crfb gene expression, the hypoxia treatment followed by 90 min of reoxygenation caused a significant 4.9-fold increase over control fish (P = 0.003) (Fig. 2A). Hypoxia exposure also significantly increased the mRNA levels of cardiac ucn3 (F3,43 = 3.32, P = 0.028). Overall, the expression of ucn3 changed by 0.9-, 2.6-, and 4.1-fold relative to the control treatment after 0, 15, and 90 min of reoxygenation, but the post hoc pairwise test failed to identify individual significant treatment effects (P > 0.05) (Fig. 2B). In contrast, the expression of cardiac utsI, crfa, crfbp, crfr1, and crfr2 did not change in response to hypoxia or reoxygenation (data not shown). The hypoxia exposure also elicited an increase in whole body cortisol levels during reoxygenation (F3,36 = 3.681, P = 0.021). From a resting value of 4.7 ng/g body wt in the normoxic treatment, whole body cortisol levels did not change immediately after the hypoxia treatment, increased four-fold after 15 min of reoxygenation (P = 0.029), and returned to basal levels in the 90 min of reoxygenation treatment (Fig. 2C).
Fig. 2.
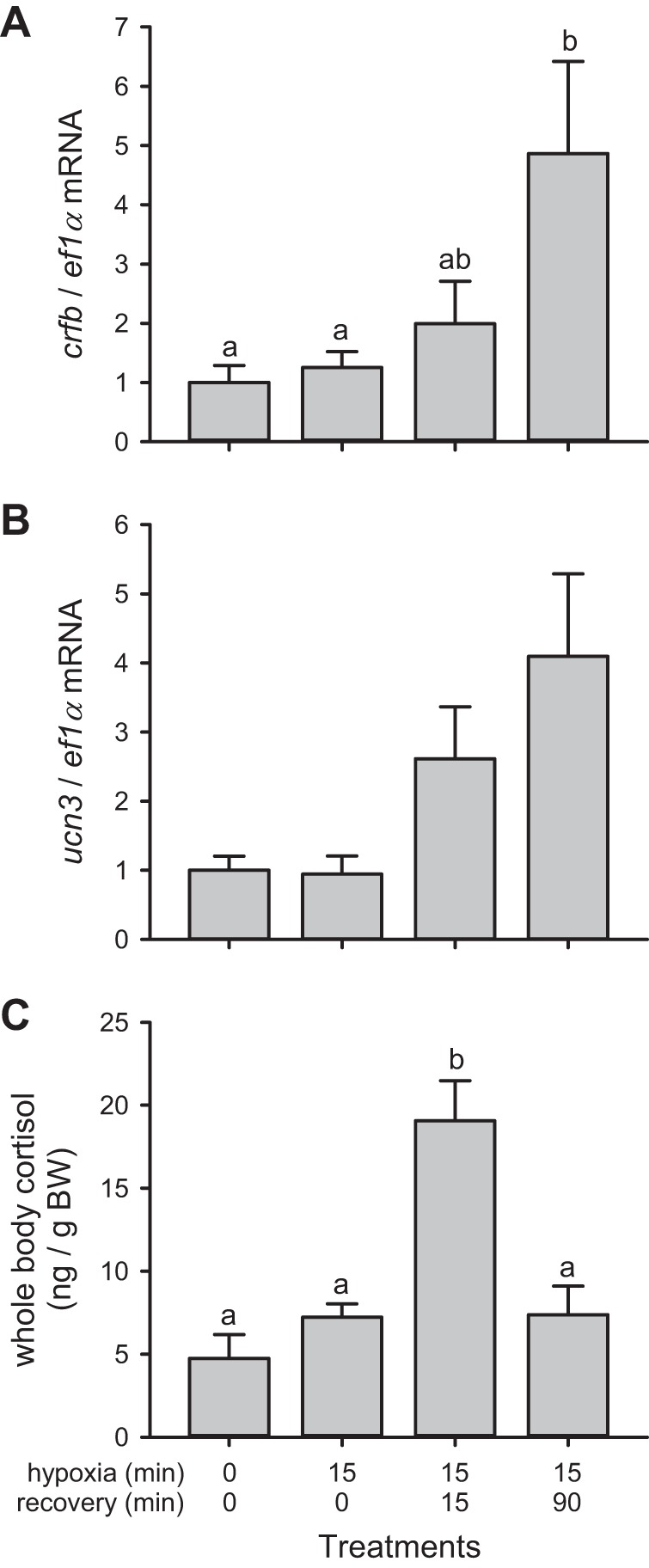
Effects of hypoxia exposure and reoxygenation on cardiac CRF system gene expression and whole body cortisol levels in zebrafish. Whole heart crfb (A) and ucn3 gene expression (B), and whole body cortisol (C) of adult zebrafish either kept under normoxic conditions (0/0 treatment) or exposed to 5% dissolved oxygen for 15 min and euthanized immediately (15/0 treatment) after 15 min reoxygenation (15/15 treatment) or 90 min reoxygenation (15/90 treatment). mRNA values are normalized with ef1α, and the expression ratio for each gene is presented relative to the normoxic treatment. For a given parameter, treatments that do not share a common letter are significantly different from each other. Overall, the expression of ucn3 differed between treatments (P = 0.018), although the post hoc pairwise test failed to identify individual significant treatment effects (P > 0.05). Values are expressed as means ± SE (n = 11–14).
Effects of CRF or UCN3 on Hypoxia/Reoxygenation-Induced Cell Death in Cultured Zebrafish Hearts
Overall CRF had a significant effect on the percentage of TUNEL-positive cells (CRF: F2,36 = 7.02, P = 0.003) and the effects of hypoxia (hypoxia: P > 0.05) were dependent on CRF dosage (hypoxia × CRF: F2,36 = 8.73, P < 0.001) (Fig. 3A). In the normoxic treatment, 0.84% of Hoechst-positive cells were TUNEL-positive, and hypoxia exposure increased the percentage of TUNEL-positive cells five-fold (P < 0.001). Under normoxic conditions, incubating hearts in either 0.1 or 1.0 nM CRF for 30 min did not affect the percentage of TUNEL-positive cells. In contrast, pretreatment with either CRF concentration abolished hypoxia-induced cell death. The percentage of TUNEL-positive cells did not differ between the normoxia- and hypoxia-exposed hearts pretreated with 0.1 nM CRF. However, the percentage of apoptotic cells was lower in the hypoxia-exposed hearts than in the normoxic ones pretreated with 1.0 nM CRF (P = 0.049).
Fig. 3.
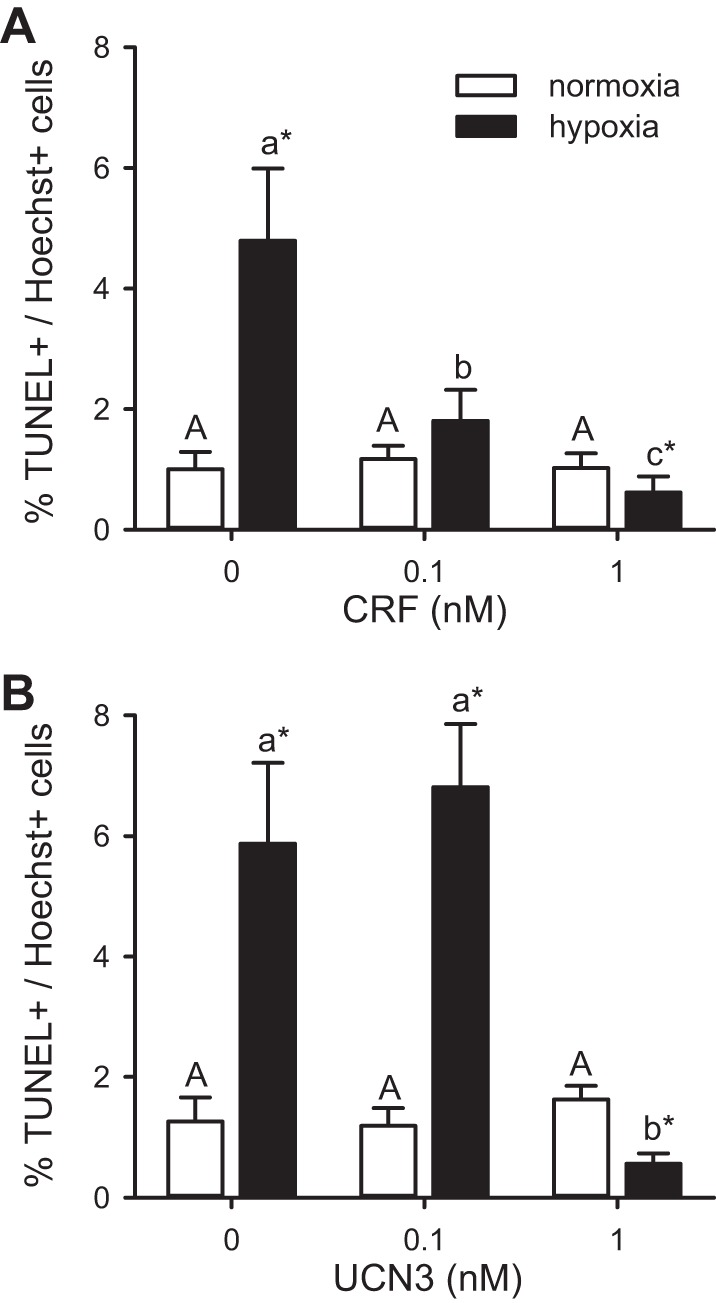
Effects of CRF or UCN3 on hypoxia/reoxygenation-induced cell death in cultured zebrafish hearts. Effects of CRF (A) or UCN3 (B) on the percentage of TUNEL-positive heart cells either kept in normoxic culture media for 24 h (normoxia) or exposed to ≤1% dissolved oxygen for 30 min and cultured for 24 h (hypoxia). Bars for a given peptide and treatment that do not share a common letter are significantly different from one another (P < 0.05). For a given peptide dosage, a difference between treatments is indicated by an asterisk (*P < 0.05). Values are expressed as means ± SE. CRF (n = 6–8) and UCN3 (n = 5–7).
Similar to CRF, UCN3 inhibited the hypoxia-induced cell death (hypoxia: F1,30 = 10.30, P = 0.003; UCN3: F2,30 = 8.06, P = 0.002), and the effects were dose-dependent (hypoxia × UCN3: F2,30 = 18.29, P < 0.001) (Fig. 3B). Normoxia-treated hearts had an average of 1.06% TUNEL-positive cells, and this value increased fivefold when exposed to hypoxia (P < 0.001). Under normoxic conditions, incubating hearts in either 0.1 or 1.0 nM UCN3 for 30 min did not affect the percentage of TUNEL-positive cells. Whereas pretreatment with 0.1 nM UCN3 did not inhibit the cell death caused by hypoxia exposure, the percentage of cell death was lower in the hypoxia-exposed hearts than in the normoxic ones pretreated with 1.0 nM UCN3 (P = 0.004).
Effects of CRF or UCN3 on Hypoxia/Reoxygenation-Induced Caspase 3 Activation in Cultured Zebrafish Hearts
To provide further support for the cytoprotective effects of CRF-related peptides in the zebrafish heart, we tested whether CRF or UCN3 can inhibit hypoxia-induced caspase-3 activity. CRF had a significant effect on caspase 3 activity (CRF: F1,48 = 13.97, P < 0.001), and the effects of hypoxia (hypoxia: P > 0.05) were dependent on CRF dosage (hypoxia × CRF: F1,48 = 25.06, P < 0.001) (Fig. 4A). In the absence of CRF, hypoxia exposure increased heart caspase 3 activity 2.3-fold relative to the normoxic treatment (P < 0.001). In contrast, while pretreatment with CRF had no effect on caspase 3 activity in normoxic hearts, it lowered caspase activity in the hypoxia-exposed hearts below the levels observed in the normoxic treatment (P < 0.001).
Fig. 4.
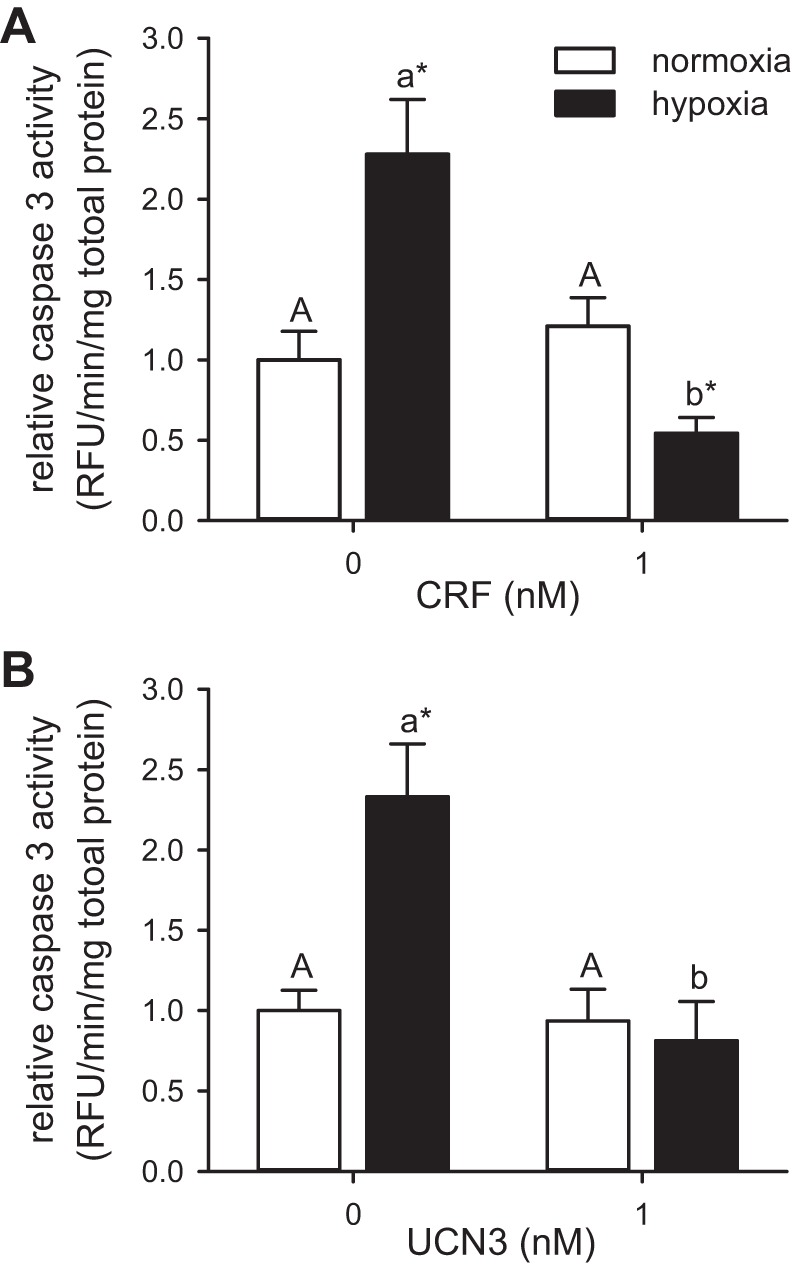
Effects of CRF or UCN3 on hypoxia/reoxygenation-induced caspase 3 activation in cultured zebrafish hearts. Relative caspase activity in extracts prepared from cultured zebrafish hearts treated for 30 min with CRF (A) or UCN3 (B) and either kept in normoxic culture media for 24 h (normoxia) or exposed to ≤1% dissolved oxygen for 30 min and cultured for 24 h (hypoxia). Within a given experiment, caspase activities are presented relative to the nontreated normoxic hearts. Bars for a given peptide and treatment that do not share a common letter are different from one another (P < 0.05). For a given peptide dosage, a difference between treatments is indicated by an asterisk (*P < 0.05). Values are expressed as means ± SE. CRF (n = 10–14) and UCN3 (n = 10–12).
Similar results were obtained with UCN3 preconditioning, where both hypoxia and UCN3 were significant factors affecting caspase 3 activity (hypoxia: F1,39 = 5.38, P = 0.026; UCN3: F1,39 = 13.38, P < 0.001), and the effects of hypoxia were dependent on UCN3 dosage (hypoxia × UCN3: F1,39 = 8.279, P = 0.006) (Fig. 4B). Without UCN3 pretreatment, hypoxia increased caspase 3 activity by 2.3-fold relative to the normoxic treatment (P = 0.026). Although UCN3 pretreatment had no effect in normoxic hearts, it abolished hypoxia-induced caspase activation.
Effects of Cotreatment with αh-CRF(9–41) and CRF on Hypoxia/Reoxygenation-Induced Caspase 3 Activation in Cultured Zebrafish Hearts
To determine whether CRF-mediated reduction in hypoxia-induced caspase 3 activity results from the specific activation of CRF receptors, we tested whether CRF can inhibit hypoxia-induced caspase 3 activity following coincubation with αh-CRF(9–41). Overall, although hypoxia was still a significant factor (F1,34 = 18.19, P < 0.001), CRF had no significant effect in the presence of αh-CRF(9–41) (Fig. 5). In response to pretreatment with αh-CRF(9–41) alone, hypoxia exposure increased heart caspase 3 activity 2.0-fold (P = 0.001). In response to pretreatment with both CRF and αh-CRF(9–41), hypoxia exposure increased heart caspase 3 activity 1.6-fold (P = 0.024). Finally, pretreatment with αh-CRF(9–41) had no effect on caspase 3 activity in normoxic hearts.
Fig. 5.
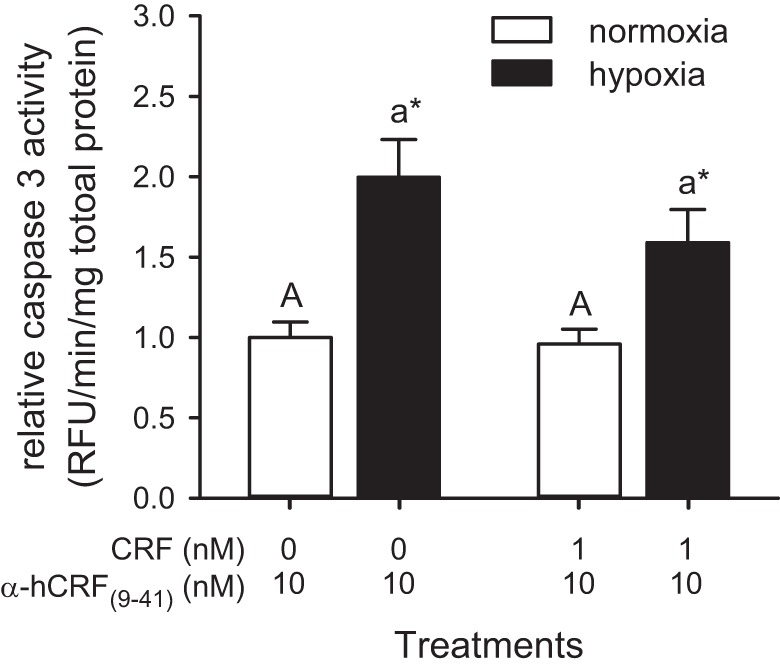
Effects of cotreatment with αh-CRF(9–41) and CRF on hypoxia/reoxygenation-induced caspase 3 activation in cultured zebrafish hearts. Relative caspase activity in extracts prepared from cultured zebrafish hearts treated for 30 min with the CRF receptor antagonist αh-CRF(9–41) alone or with CRF, and either kept in normoxic culture media for 24 h (normoxia) or exposed to ≤1% dissolved oxygen for 30 min and cultured for 24 h (hypoxia). Caspase activities are presented relative to the normoxic and αh-CRF(9–41)-alone treatment. Bars for a given peptide and treatment that do not share a common letter are different from one another (P < 0.05). For a given peptide dosage, a difference between treatments is indicated by an asterisk (*P < 0.05). Values are expressed as means ± SE (n = 9–12).
DISCUSSION
This is the first report on the expression profile and the functional role of the cardiac CRF system in a nonmammalian vertebrate. Our analysis shows that all four teleosts paralogous CRF-related peptides, both CRF receptor subtypes and the CRF binding protein are expressed in the zebrafish heart. In vivo, acute exposure to a severe hypoxic challenge elicits a robust increase in the expression of two of the ligands, namely CRFb and UCN3. Furthermore, we show that pretreatment with CRF or UCN3 can prevent apoptosis and cell death associated with acute exposure to severe hypoxia in intact ex vivo zebrafish hearts. Finally, the addition of the nonselective CRF receptor antagonist, αh-CRF(9–41), prevented the antiapoptotic effects of CRF, demonstrating that the cardioprotective effects of CRF-related peptides in the zebrafish heart are mediated by CRF receptor-dependent mechanisms.
Our findings demonstrate that all known members of the CRF family of ligands in teleosts are expressed under basal conditions in the zebrafish heart. Previous studies have reported cardiac expression of ucn3 (20) and uts1 (60) in zebrafish, crfa in medaka (Oryzias latipes; Ref. 37), and crfb in Burton’s mouthbrooder (Astatotilapia burtoni; Ref. 24). Under basal conditions, our results show that crfa is the most abundant CRF ligand expressed in the zebrafish heart, followed by ucn3, crfb, and uts1. In contrast, although the dominant ligand varies across species, the urocortins (ucn1, ucn2, and ucn3) are consistently the most abundantly expressed CRF-related ligands in mammalian cardiomyocytes and crf is either undetectable or expressed at low levels (8, 17, 23, 44, 66, 88). Although crfa was only recently identified, the results of Grone and Maruska (35) suggest that the expression patterns of crfa in teleosts are species-specific. Therefore, although the dominant cardiac mRNA levels of crfa in zebrafish is consistent with the high expression levels of teleocortin (an ortholog of zebrafish crfa) in the heart of medaka (37), further studies may be needed to confirm whether CRFa is the dominant cardiac CRF ligand in teleost fish.
Our study also provides an original quantitative assessment of the expression of CRF receptors and CRFBP in the heart of a teleost species. Whereas previous qualitative studies have reported cardiac expression of crfr1 in pufferfish (Fugu rubripes; Ref. 22) and common carp (Cyprinus carpio; Ref. 38), crfr1 and crfr2 in chum salmon (Oncorhynchus keta; Ref. 67), and crfr2 in catfish (Ameiurus nebulosus; Ref. 6) and in Burton’s mouthbrooder (24), here, we demonstrate that the cardiac expression levels of crfr1 in zebrafish are significantly higher than those of crfr2. Moreover, the expression levels of crfr1 and crfr2 in the zebrafish heart are one to two orders of magnitude higher than those of the CRF-related ligands, as previously observed in the skin and gills of common carp (52). Consistent with previous observations of crfbp expression in the heart of common carp (38) and Burton’s mouthbrooder (24), we also determined that under basal conditions, zebrafish hearts have a high level of crfbp expression, which is greater than that of its ligands. These results are in sharp contrast with those previously observed in the mammalian heart, where CRFR2 is the dominant CRF receptor (8, 17, 44), the expression of the principle ligands is much higher than that of the receptors (66), and crfbp expression is absent (8). In common carp, a member of the Cyprinidae family as is zebrafish, CRFBP inhibits CRF- and UTS1-mediated activation of CRF receptors in a ligand- and receptor subtype-specific fashion (51). As such, CRFBP likely reduces the biological activity of CRF-related ligands in the fish cardiac CRF system, and the robust expression of crfbp in zebrafish hearts may provide an explanation for the higher receptor mRNA levels than ligands under basal conditions. It should be noted that while these whole heart differences in gene expression have biologically relevant implications and the expression pattern of the CRF system components is generally uniform in mammalian hearts (44, 84), our results could underestimate differences in the expression levels of the various cardiac CRF system components if the changes are restricted to any particular heart compartment, as is the case for the CRF-R2β splice variant in humans (44). Future examination of the localization and existence of splice variants would be pertinent to fully understand the roles of these genes in the heart.
Consistent with our hypothesis that components of the cardiac CRF system in fish are responsive to hypoxia/reoxygenation, we demonstrate that severe acute hypoxia increases endogenous expression of crfb and ucn3 in the zebrafish heart. Similarly, ischemia has been shown to affect the expression and tissue levels of several cardiac CRF system components in various mammalian models of heart disease. For example, ischemia increases rat UCN1 perfusate levels from isolated hearts (45), supernatant UCN from cultured cardiomyocytes (19), and the cardiomyocyte gene expression of ucn1, ucn2, and ucn3 (19, 23). In contrast, human heart failure patients are characterized by elevated cardiac crf, ucn3, and crfr1, lower crf2α, and unchanged ucn1 and unc2 mRNA levels (66). In this study, severe acute hypoxia did not affect the expression of crfa, uts1, crfr1, crfr2 and crfbp mRNA levels, suggesting that the components of the cardiac CRF system that are hypoxia-responsive are species-specific. However, previous studies have shown that hypoxia can induce human ucn2 expression in cardiac cells (21), and patients with dilated cardiomyopathy are characterized by elevated immunoreactive UCN1 levels (56). Additionally, hypoxia-responsive elements have been identified in human ucn2 and ucn3 (21, 39). Therefore, beyond species differences, the results from human studies suggest that the components of the cardiac CRF system, which contribute to the expression profile of a hypoxic insult, varies across experimental conditions. As such, although our results suggest that cardiac crfb and ucn3 are responsive to hypoxia/reoxygenation, more research is needed to determine whether the remaining components of the zebrafish cardiac CRF system are also regulated by hypoxic conditions.
Beyond its effects on the cardiac CRF system, our results show for the first time that severe acute hypoxia exposure can stimulate the HPI axis in adult zebrafish, resulting in a transient increase in whole body cortisol levels. Similarly, asphyxia due to transient air exposure in zebrafish and other species is associated with a sharp increase in whole body cortisol and a return to baseline levels within 1–2 h of the acute stressor (7, 33, 61, 70). Common carp, rainbow trout (Oncorhynchus mykiss; Ref. 82), and turbot (Scophthalmus maximus; Ref. 64) exposed transiently to severe hypoxia are also characterized by rapid and short-lived increases in plasma cortisol levels. In rainbow trout, activation of the HPI axis by hypoxia is associated with increases in forebrain preoptic area CRF and UTS1 gene expression and chronic intracranial infusion of αh-CRF(9–41) blocked the hypoxia-induced increase in plasma cortisol (14). Whether the CRF-related peptides produced in the nucleus preopticus in zebrafish (2) are similarly implicated in the physiological regulation of the HPI axis under hypoxic conditions remains to be determined.
Unique attributes of the central CRF system in fish may also have consequences for the cardiac CRF system in this group of vertebrates. In addition to the forebrain, fish possess a second major source of CRF-related peptides in the central nervous system, the caudal neurosecretory system (CNSS; Refs. 3 and 25). Located in the terminal segments of the spinal cord, the CNSS is a neuroendocrine organ from which peptides can be released into the caudal vein (50, 87). In rainbow trout, chronic hypoxia is associated with an increase in CNSS CRF mRNA levels (13). Although studies are needed to determine whether hypoxia-induced increases in CNSS CRF gene expression translate into higher circulating levels of the peptide, our previous results suggest that in addition to the paracrine actions of locally produced CRF-related peptides, cardiac CRF receptors in fish may mediate the endocrine actions of CRF ligands produced by the CNSS.
The functional roles of CRF-related peptides in the fish heart have not previously been investigated. Here, we provide original evidence of cytoprotective effects by CRF and UCN3 in a teleost species using isolated zebrafish hearts in culture (65). More specifically, we show that in vitro CRF and UCN3 offer cardioprotection when added before a severe hypoxia/reoxygenation treatment by reducing cardiac cell death, specifically, apoptosis via caspase 3. Similarly, in mammals, administration of UCN1, UCN2, UCN3, and CRF before a lethal ischemic insult and/or during reperfusion has been shown to improve cell survival in cultured cardiomyocytes, in isolated hearts ex vivo, and in vivo (18, 41, 72). Although the doses used in our study (10−9 and 10−10 M) are toward the lower end of those most commonly applied exogenously in mammalian studies (10−8 to 10−10 M), the chosen doses are comparable to the endogenous, basal levels measured in human hearts (10−9 M; 44) and in the plasma of stressed tilapia (Oreochromis mossambicus; 10−10 M; Ref. 63). In general, while a similar range of CRF-related peptide concentrations appears to be needed for cardiac cytoprotection in fish and mammals, our results also suggest that the cardioprotective potency of individual CRF-related ligands differs between these two groups of vertebrates. For example, when tested at a concentration of 10−10 M in myocytes from neonatal rat hearts, UCN2 and UCN3 exhibited higher levels of protection from apoptosis than UNC1 (23), and UCN1 (10−10 M) was significantly more protective than CRF (10−10 M; Ref. 19). In contrast, while 10−10 M CRF reversed the hypoxia-induced increase in the percentage of TUNEL-positive cells in zebrafish hearts, 10−10 M UCN3 had no effect. Moreover, at a concentration of 10−9 M, CRF in this study exerted a greater potency than UCN3 at reducing cardiac caspase 3 activity following exposure to hypoxia. In rodents, CRFR2 mediates the cytoprotective effects of CRF-related peptides in the heart and differences in the cardioprotective potency of the individual CRF-related ligands are due, at least in part, to differences in their affinity for this receptor (19, 23, 41). As such, our results suggest that the contribution of CRF receptors in mediating the cardioprotective actions of CRF-related peptides in zebrafish differs from that observed in mammals.
Our observation that αh-CRF(9–41) prevents the antiapoptotic effects of CRF suggest that the cardioprotective effects of CRF-related peptides in the zebrafish heart are mediated by CRF receptor-dependent mechanisms. Since UCN3 signals only through CRFR2 in fish (51), the cytoprotective effects of UCN3 in zebrafish are likely mediated through this receptor. In contrast, the fact that CRFR1 is more sensitive for CRF than CRF-R2 in fish (51), that cardiac expression levels of crfr1 in zebrafish are significantly higher than those of crfr2, and that CRF has a higher level of cardioprotective potency than UCN3, together, suggest that the cytoprotective effects of CRF in the zebrafish heart are at least partially mediated through CRFR1. Interestingly, since CRFBP is more potent in inhibiting the activation of CRFR2 than CRFR1 in fish (51), the expression of CRFBP in the zebrafish heart may also play a role in determining the relative contribution of the CRF receptor subtypes to the cardioprotective effects of CRF-related peptides. In heart failure patients, although CRFR2 is the dominant CRF receptor, upregulation of CRFR1 mRNA levels and the expression of a CRFR1 splice variant that can modulate cardiac CRFR1 signaling suggest that both CRF receptor subtypes contribute to the coordinated compensatory response of the cardiac CRF system to this condition (66). Similarly, although more studies are needed to delineate the relative contribution of the CRF receptor subtypes and their splice variants to the cardioprotective effects of CRF-related peptides, our results suggest that both CRFR1 and CRFR2 contribute to cytoprotection in the zebrafish heart.
Perspectives and Significance
We have demonstrated the expression of genes encoding all members of the CRF system in the zebrafish heart and provide novel functional evidence, suggesting that cardiac CRF-related peptides may protect the heart against the cellular stress associated with severe hypoxia exposure in fish. Interestingly, however, the relationships among the different CRF ligands and their receptors, and the robust expression of CRFBP in the zebrafish heart, suggest that the relative contribution of the various cardiac CRF system components differs significantly between fish and mammals. Beyond its cytoprotective functions, the cardiac CRF system also mediates hemodynamic and bioenergetic effects that enhance cardiovascular function and promotes recovery from ischemia/reoxygenation exposure (1, 57). For example, administration of urocortins in mammals potently elevates cardiac contractility and output, improves coronary blood flow, and preserves the high-energy phosphate stores of the heart (10, 59, 68, 72, 79). While central and peripheral administration of CRF and UTS1 has diverse and differential cardiovascular actions in rainbow trout (47, 53), the direct actions of CRF-related peptides on the contractile and bioenergetics properties of the heart in fish are not known. Because fish species vary broadly in their hypoxia tolerance, the response of fish hearts to hypoxia, unlike mammalian hearts, involves bradycardia associated with increased stroke volume (30, 34), and cell proliferation in some species can lead to complete myocardial regeneration after a hypoxic/reperfusion event (58), we suggest that future research into the physiological actions of the cardiac CRF system across teleost species will advance our basic understanding of the cellular mechanisms that protect the heart during hypoxia and ischemic conditions.
GRANTS
This research was supported by a Natural Sciences and Engineering Research Council of Canada Discovery grant to N. J. Bernier.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.A.W. and N.J.B. conceived and designed research; T.A.W., J.C.B., and J.S. performed experiments; T.A.W. and N.J.B. analyzed data; T.A.W. and N.J.B. interpreted results of experiments; T.A.W. and N.J.B. prepared figures; T.A.W. drafted manuscript; T.A.W. and N.J.B. edited and revised manuscript; T.A.W., J.C.B., J.S., and N.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the laboratory Dr. Richard D. Mosser for assistance with the caspase assay and the staff of the Aqualab for their ongoing technical support.
REFERENCES
- 1.Adão R, Santos-Ribeiro D, Rademaker MT, Leite-Moreira AF, Brás-Silva C. Urocortin 2 in cardiovascular health and disease. Drug Discov Today 20: 906–914, 2015. doi: 10.1016/j.drudis.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 2.Alderman SL, Bernier NJ. Localization of corticotropin-releasing factor, urotensin I, and CRF-binding protein gene expression in the brain of the zebrafish, Danio rerio. J Comp Neurol 502: 783–793, 2007. doi: 10.1002/cne.21332. [DOI] [PubMed] [Google Scholar]
- 3.Alderman SL, Bernier NJ. Ontogeny of the corticotropin-releasing factor system in zebrafish. Gen Comp Endocrinol 164: 61–69, 2009. doi: 10.1016/j.ygcen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Alderman SL, Harter TS, Wilson JM, Supuran CT, Farrell AP, Brauner CJ. Evidence for a plasma-accessible carbonic anhydrase in the lumen of salmon heart that may enhance oxygen delivery to the myocardium. J Exp Biol 219: 719–724, 2016. doi: 10.1242/jeb.130443. [DOI] [PubMed] [Google Scholar]
- 5.Altieri AH, Gedan KB. Climate change and dead zones. Glob Change Biol 21: 1395–1406, 2015. doi: 10.1111/gcb.12754. [DOI] [PubMed] [Google Scholar]
- 6.Arai M, Assil IQ, Abou-Samra AB. Characterization of three corticotropin-releasing factor receptors in catfish: a novel third receptor is predominantly expressed in pituitary and urophysis. Endocrinology 142: 446–454, 2001. doi: 10.1210/endo.142.1.7879. [DOI] [PubMed] [Google Scholar]
- 7.Arends RJ, Mancera JM, Muñoz JL, Wendelaar Bonga SE, Flik G. The stress response of the gilthead sea bream (Sparus aurata L.) to air exposure and confinement. J Endocrinol 163: 149–157, 1999. doi: 10.1677/joe.0.1630149. [DOI] [PubMed] [Google Scholar]
- 8.Baigent SM, Lowry PJ. mRNA expression profiles for corticotrophin-releasing factor (CRF), urocortin, CRF receptors and CRF-binding protein in peripheral rat tissues. J Mol Endocrinol 25: 43–52, 2000. doi: 10.1677/jme.0.0250043. [DOI] [PubMed] [Google Scholar]
- 9.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44: 525–557, 2004. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 10.Bale TL, Hoshijima M, Gu Y, Dalton N, Anderson KR, Lee K-F, Rivier J, Chien KR, Vale WW, Peterson KL. The cardiovascular physiologic actions of urocortin II: acute effects in murine heart failure. Proc Natl Acad Sci USA 101: 3697–3702, 2004. doi: 10.1073/pnas.0307324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basappa J, Turcan S, Vetter DE. Corticotropin-releasing factor-2 activation prevents gentamicin-induced oxidative stress in cells derived from the inner ear. J Neurosci Res 88: 2976–2990, 2010. doi: 10.1002/jnr.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayatti N, Zschocke J, Behl C. Brain region-specific neuroprotective action and signaling of corticotropin-releasing hormone in primary neurons. Endocrinology 144: 4051–4060, 2003. doi: 10.1210/en.2003-0168. [DOI] [PubMed] [Google Scholar]
- 13.Bernier NJ, Alderman SL, Bristow EN. Heads or tails? Stressor-specific expression of corticotropin-releasing factor and urotensin I in the preoptic area and caudal neurosecretory system of rainbow trout. J Endocrinol 196: 637–648, 2008. doi: 10.1677/JOE-07-0568. [DOI] [PubMed] [Google Scholar]
- 14.Bernier NJ, Craig PM. CRF-related peptides contribute to stress response and regulation of appetite in hypoxic rainbow trout. Am J Physiol Regul Integr Comp Physiol 289: R982–R990, 2005. doi: 10.1152/ajpregu.00668.2004. [DOI] [PubMed] [Google Scholar]
- 15.Blaabjerg L, Christensen GL, Matsumoto M, van der Meulen T, Huising MO, Billestrup N, Vale WW. CRFR1 activation protects against cytokine-induced β-cell death. J Mol Endocrinol 53: 417–427, 2014. doi: 10.1530/JME-14-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boorse GC, Denver RJ. Widespread tissue distribution and diverse functions of corticotropin-releasing factor and related peptides. Gen Comp Endocrinol 146: 9–18, 2006. doi: 10.1016/j.ygcen.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, Mjøs OD, Latchman DS, Lee K-F, Vale W. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology 145: 24–35, 2004. doi: 10.1210/en.2003-0689. [DOI] [PubMed] [Google Scholar]
- 18.Brar BK, Stephanou A, Knight R, Latchman DS. Activation of protein kinase B/Akt by urocortin is essential for its ability to protect cardiac cells against hypoxia/reoxygenation-induced cell death. J Mol Cell Cardiol 34: 483–492, 2002. doi: 10.1006/jmcc.2002.1529. [DOI] [PubMed] [Google Scholar]
- 19.Brar BK, Stephanou A, Okosi A, Lawrence KM, Knight RA, Marber MS, Latchman DS. CRH-like peptides protect cardiac myocytes from lethal ischaemic injury. Mol Cell Endocrinol 158: 55–63, 1999. doi: 10.1016/S0303-7207(99)00183-5. [DOI] [PubMed] [Google Scholar]
- 20.Bräutigam L, Hillmer JM, Söll I, Hauptmann G. Localized expression of urocortin genes in the developing zebrafish brain. J Comp Neurol 518: 2978–2995, 2010. doi: 10.1002/cne.22375. [DOI] [PubMed] [Google Scholar]
- 21.Bühler K, Plaisance I, Dieterle T, Brink M. The human urocortin 2 gene is regulated by hypoxia: identification of a hypoxia-responsive element in the 3′-flanking region. Biochem J 424: 119–127, 2009. doi: 10.1042/BJ20090311. [DOI] [PubMed] [Google Scholar]
- 22.Cardoso JC, Power DM, Elgar G, Clark MS. Isolation and characterisation of the corticotropin releasing factor receptor 1 (CRFR1) gene in a teleost fish, Fugu rubripes. DNA Seq 14: 215–218, 2003. doi: 10.1080/1042517031000112624. [DOI] [PubMed] [Google Scholar]
- 23.Chanalaris A, Lawrence KM, Stephanou A, Knight RD, Hsu SY, Hsueh AJ, Latchman DS. Protective effects of the urocortin homologues stresscopin (SCP) and stresscopin-related peptide (SRP) against hypoxia/reoxygenation injury in rat neonatal cardiomyocytes. J Mol Cell Cardiol 35: 1295–1305, 2003. doi: 10.1016/S0022-2828(03)00244-X. [DOI] [PubMed] [Google Scholar]
- 24.Chen CC, Fernald RD. Sequences, expression patterns and regulation of the corticotropin-releasing factor system in a teleost. Gen Comp Endocrinol 157: 148–155, 2008. doi: 10.1016/j.ygcen.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craig PM, Al-Timimi H, Bernier NJ. Differential increase in forebrain and caudal neurosecretory system corticotropin-releasing factor and urotensin I gene expression associated with seawater transfer in rainbow trout. Endocrinology 146: 3851–3860, 2005. doi: 10.1210/en.2005-0004. [DOI] [PubMed] [Google Scholar]
- 26.Davidson SM, Yellon DM. Urocortin: a protective peptide that targets both the myocardium and vasculature. Pharmacol Rep 61: 172–182, 2009. doi: 10.1016/S1734-1140(09)70019-2. [DOI] [PubMed] [Google Scholar]
- 27.Davie PS, Farrell AP. The coronary and luminal circulation s of the myocardium of fishes. Can J Zool 69: 1993–2001, 1991. doi: 10.1139/z91-278. [DOI] [Google Scholar]
- 28.Denver RJ. Structural and functional evolution of vertebrate neuroendocrine stress systems. Ann NY Acad Sci 1163: 1–16, 2009. doi: 10.1111/j.1749-6632.2009.04433.x. [DOI] [PubMed] [Google Scholar]
- 29.Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD. Climate change impacts on marine ecosystems. Annu Rev Mar Sci 4: 11–37, 2012. doi: 10.1146/annurev-marine-041911-111611. [DOI] [PubMed] [Google Scholar]
- 30.Farrell AP. Cardiac output in fish: regulation and limitations. In: The Vertebrate Gas Transport Cascade: Adaptions to Environment and Mode of Life, edited by Bicudo E. Boca Raton, FL: CRC, 1993. [Google Scholar]
- 31.Farrell AP, Farrell ND, Jourdan H, Cox GK. A perspective on the evolution of the coronary circulation in fishes and the transition to terrestrial life. In: Ontogeny and Phylogeny of the Vertebrate Heart, edited by Sedmera D, Wang. New York: Springer, 2012. doi: 10.1007/978-1-4614-3387-3_4. [DOI] [Google Scholar]
- 32.Fox MW, Anderson RE, Meyer FB. Neuroprotection by corticotropin releasing factor during hypoxia in rat brain. Stroke 24: 1072–1075, 1993. doi: 10.1161/01.STR.24.7.1072. [DOI] [PubMed] [Google Scholar]
- 33.Fuzzen ML, Van Der Kraak G, Bernier NJ. Stirring up new ideas about the regulation of the hypothalamic-pituitary-interrenal axis in zebrafish (Danio rerio). Zebrafish 7: 349–358, 2010. doi: 10.1089/zeb.2010.0662. [DOI] [PubMed] [Google Scholar]
- 34.Gamperl AK, Driedzic WR. Cardiovascular responses to hypoxia. In: Fish Physiology, edited by Richards JG, Farrell AP, Brauner CJ. London: Academic, 2009. [Google Scholar]
- 35.Grone BP, Maruska KP. Divergent evolution of two corticotropin-releasing hormone (CRH) genes in teleost fishes. Front Neurosci 9: 365, 2015. doi: 10.3389/fnins.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holeton GF, Randall DJ. The effect of hypoxia upon the partial pressure of gases in the blood and water afferent and efferent to the gills of rainbow trout. J Exp Biol 46: 317–327, 1967. [DOI] [PubMed] [Google Scholar]
- 37.Hosono K, Kikuchi Y, Miyanishi H, Hiraki-Kajiyama T, Takeuchi A, Nakasone K, Maehiro S, Okubo K. Teleocortin: a novel member of the CRH family in teleost fish. Endocrinology 156: 2949–2957, 2015. doi: 10.1210/en.2015-1042. [DOI] [PubMed] [Google Scholar]
- 38.Huising MO, van der Aa LM, Metz JR, de Fátima Mazon A, Kemenade BM, Flik G. Corticotropin-releasing factor (CRF) and CRF-binding protein expression in and release from the head kidney of common carp: evolutionary conservation of the adrenal CRF system. J Endocrinol 193: 349–357, 2007. doi: 10.1677/JOE-07-0070. [DOI] [PubMed] [Google Scholar]
- 39.Imperatore A, Rolfo A, Petraglia F, Challis JR, Caniggia I. Hypoxia and preeclampsia: increased expression of urocortin 2 and urocortin 3. Reprod Sci 17: 833–843, 2010. doi: 10.1177/1933719110373147. [DOI] [PubMed] [Google Scholar]
- 40.Intekhab-Alam NY, White OB, Getting SJ, Petsa A, Knight RA, Chowdrey HS, Townsend PA, Lawrence KM, Locke IC. Urocortin protects chondrocytes from NO-induced apoptosis: a future therapy for osteoarthritis? Cell Death Dis 4: e717, 2013. doi: 10.1038/cddis.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonassen AK, Wergeland A, Helgeland E, Mjøs OD, Brar BK. Activation of corticotropin releasing factor receptor type 2 in the heart by corticotropin releasing factor offers cytoprotection against ischemic injury via PKA and PKC dependent signaling. Regul Pept 174: 90–97, 2012. doi: 10.1016/j.regpep.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Kalantaridou S, Makrigiannakis A, Zoumakis E, Chrousos GP. Peripheral corticotropin-releasing hormone is produced in the immune and reproductive systems: actions, potential roles and clinical implications. Front Biosci 12: 572–580, 2007. doi: 10.2741/2083. [DOI] [PubMed] [Google Scholar]
- 43.Kiapekou E, Zapanti E, Mastorakos G, Loutradis D. Update on the role of ovarian corticotropin-releasing hormone. Ann NY Acad Sci 1205: 225–229, 2010. doi: 10.1111/j.1749-6632.2010.05685.x. [DOI] [PubMed] [Google Scholar]
- 44.Kimura Y, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Darnel AD, Suzuki T, Ebina M, Nukiwa T, Sasano H. Expression of urocortin and corticotropin-releasing factor receptor subtypes in the human heart. J Clin Endocrinol Metab 87: 340–346, 2002. doi: 10.1210/jcem.87.1.8160. [DOI] [PubMed] [Google Scholar]
- 45.Knight RA, Chen-Scarabelli C, Yuan Z, McCauley RB, Di Rezze J, Scarabelli GM, Townsend PA, Latchman D, Saravolatz L, Faggian G, Mazzucco A, Chowdrey HS, Stephanou A, Scarabelli TM. Cardiac release of urocortin precedes the occurrence of irreversible myocardial damage in the rat heart exposed to ischemia/reperfusion injury. FEBS Lett 582: 984–990, 2008. doi: 10.1016/j.febslet.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 46.Kuwajima T, Sitko AA, Bhansali P, Jurgens C, Guido W, Mason C. ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development 140: 1364–1368, 2013. doi: 10.1242/dev.091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Mével JC, Mimassi N, Lancien F, Mabin D, Conlon JM. Cardiovascular actions of the stress-related neurohormonal peptides, corticotropin-releasing factor and urotensin-I in the trout Oncorhynchus mykiss. Gen Comp Endocrinol 146: 56–61, 2006. doi: 10.1016/j.ygcen.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Lister AL, Van Der Kraak G. An investigation into the role of prostaglandins in zebrafish oocyte maturation and ovulation. Gen Comp Endocrinol 159: 46–57, 2008. doi: 10.1016/j.ygcen.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Lovejoy DA. Structural evolution of urotensin-I: reflections of life before corticotropin releasing factor. Gen Comp Endocrinol 164: 15–19, 2009. doi: 10.1016/j.ygcen.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Lovejoy DA, Balment RJ. Evolution and physiology of the corticotropin-releasing factor (CRF) family of neuropeptides in vertebrates. Gen Comp Endocrinol 115: 1–22, 1999. doi: 10.1006/gcen.1999.7298. [DOI] [PubMed] [Google Scholar]
- 51.Manuel R, Metz JR, Flik G, Vale WW, Huising MO. Corticotropin-releasing factor-binding protein (CRF-BP) inhibits CRF- and urotensin-I-mediated activation of CRF receptor-1 and -2 in common carp. Gen Comp Endocrinol 202: 69–75, 2014. doi: 10.1016/j.ygcen.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Mazon AF, Verburg-van Kemenade BML, Flik G, Huising MO. Corticotropin-releasing hormone-receptor 1 (CRH-R1) and CRH-binding protein (CRH-BP) are expressed in the gills and skin of common carp Cyprinus carpio L. and respond to acute stress and infection. J Exp Biol 209: 510–517, 2006. doi: 10.1242/jeb.01973. [DOI] [PubMed] [Google Scholar]
- 53.Mimassi N, Shahbazi F, Jensen J, Mabin D, Conlon JM, Le Mével JC. Cardiovascular actions of centrally and peripherally administered trout urotensin-I in the trout. Am J Physiol Regul Integr Comp Physiol 279: R484–R491, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Nilsson GE, Randall DJ. Adaptations to hypoxia in fishes. In: Respiratory Physiology of Vertebrates: Life With and Without Oxygen, edited by Nilsson GE. Cambridge, UK: Cambridge University Press, 2010. doi: 10.1017/CBO9780511845178. [DOI] [Google Scholar]
- 55.Nilsson GE, Vaage J, Stensløkken KO. Oxygen- and temperature-dependent expression of survival protein kinases in crucian carp (Carassius carassius) heart and brain. Am J Physiol Regul Integr Comp Physiol 308: R50–R61, 2015. doi: 10.1152/ajpregu.00094.2014. [DOI] [PubMed] [Google Scholar]
- 56.Nishikimi T, Miyata A, Horio T, Yoshihara F, Nagaya N, Takishita S, Yutani C, Matsuo H, Matsuoka H, Kangawa K. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. Am J Physiol Heart Circ Physiol 279: H3031–H3039, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Onorati F, Chen-Scarabelli C, Knight R, Stephanou A, Mohanti B, Santini F, Tessari M, Kini A, Narula J, Saravolatz L, Mazzucco A, Scarabelli T, Faggian G. Targeting urocortin signaling pathways to enhance cardioprotection: is it time to move from bench to bedside? Cardiovasc Drugs Ther 27: 451–463, 2013. doi: 10.1007/s10557-013-6468-7. [DOI] [PubMed] [Google Scholar]
- 58.Parente V, Balasso S, Pompilio G, Verduci L, Colombo GI, Milano G, Guerrini U, Squadroni L, Cotelli F, Pozzoli O, Capogrossi MC. Hypoxia/reoxygenation cardiac injury and regeneration in zebrafish adult heart. PLoS One 8: e53748, 2013. doi: 10.1371/journal.pone.0053748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parkes DG, Vaughan J, Rivier J, Vale W, May CN. Cardiac inotropic actions of urocortin in conscious sheep. Am J Physiol Heart Circ Physiol 272: H2115–H2122, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Parmentier C, Hameury E, Lihrmann I, Taxi J, Hardin-Pouzet H, Vaudry H, Calas A, Tostivint H. Comparative distribution of the mRNAs encoding urotensin I and urotensin II in zebrafish. Peptides 29: 820–829, 2008. doi: 10.1016/j.peptides.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 61.Pavlidis M, Theodoridi A, Tsalafouta A. Neuroendocrine regulation of the stress response in adult zebrafish, Danio rerio. Prog Neuropsychopharmacol Biol Psychiatry 60: 121–131, 2015. doi: 10.1016/j.pnpbp.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen WA, McCullers D, Culmsee C, Haughey NJ, Herman JP, Mattson MP. Corticotropin-releasing hormone protects neurons against insults relevant to the pathogenesis of Alzheimer’s disease. Neurobiol Dis 8: 492–503, 2001. doi: 10.1006/nbdi.2001.0395. [DOI] [PubMed] [Google Scholar]
- 63.Pepels PPLM, Van Helvoort H, Wendelaar Bonga SE, Balm PHM. Corticotropin-releasing hormone in the teleost stress response: rapid appearance of the peptide in plasma of tilapia (Oreochromis mossambicus). J Endocrinol 180: 425–438, 2004. doi: 10.1677/joe.0.1800425. [DOI] [PubMed] [Google Scholar]
- 64.Pichavant K, Maxime V, Thébault MT, Ollivier H, Garnier JP, Bousquet B, Diouris M, Boeuf G, Nonnotte G. Effects of hypoxia and subsequent recovery on turbot Scophthalmus maximus: hormonal changes and anaerobic metabolism. Mar Ecol Prog Ser 225: 275–285, 2002. doi: 10.3354/meps225275. [DOI] [Google Scholar]
- 65.Pieperhoff S, Wilson KS, Baily J, de Mora K, Maqsood S, Vass S, Taylor J, Del-Pozo J, MacRae CA, Mullins JJ, Denvir MA. Heart on a plate: histological and functional assessment of isolated adult zebrafish hearts maintained in culture. PLoS One 9: e96771, 2014. doi: 10.1371/journal.pone.0096771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pilbrow AP, Lewis KA, Perrin MH, Sweet WE, Moravec CS, Tang WH, Huising MO, Troughton RW, Cameron VA. Cardiac CRFR1 expression is elevated in human heart failure and modulated by genetic variation and alternative splicing. Endocrinology 157: 4865–4874, 2016. doi: 10.1210/en.2016-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pohl S, Darlison MG, Clarke WC, Lederis K, Richter D. Cloning and functional pharmacology of two corticotropin-releasing factor receptors from a teleost fish. Eur J Pharmacol 430: 193–202, 2001. doi: 10.1016/S0014-2999(01)01391-7. [DOI] [PubMed] [Google Scholar]
- 68.Rademaker MT, Cameron VA, Charles CJ, Richards AM. Urocortin 3: haemodynamic, hormonal, and renal effects in experimental heart failure. Eur Heart J 27: 2088–2098, 2006. doi: 10.1093/eurheartj/ehl138. [DOI] [PubMed] [Google Scholar]
- 69.Radulovic M, Hippel C, Spiess J. Corticotropin-releasing factor (CRF) rapidly suppresses apoptosis by acting upstream of the activation of caspases. J Neurochem 84: 1074–1085, 2003. doi: 10.1046/j.1471-4159.2003.01594.x. [DOI] [PubMed] [Google Scholar]
- 70.Ramsay JM, Feist GW, Varga ZM, Westerfield M, Kent ML, Schreck CB. Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture 297: 157–162, 2009. doi: 10.1016/j.aquaculture.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Randall DJ, Rummer JL, Wilson JM, Wang S, Brauner CJ. A unique mode of tissue oxygenation and the adaptive radiation of teleost fishes. J Exp Biol 217: 1205–1214, 2014. doi: 10.1242/jeb.093526. [DOI] [PubMed] [Google Scholar]
- 72.Scarabelli TM, Pasini E, Stephanou A, Comini L, Curello S, Raddino R, Ferrari R, Knight R, Latchman DS. Urocortin promotes hemodynamic and bioenergetic recovery and improves cell survival in the isolated rat heart exposed to ischemia/reperfusion. J Am Coll Cardiol 40: 155–161, 2002. doi: 10.1016/S0735-1097(02)01930-7. [DOI] [PubMed] [Google Scholar]
- 73.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682, 2012. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci USA 78: 6517–6521, 1981. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stankiewicz AR, Lachapelle G, Foo CPZ, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem 280: 38729–38739, 2005. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 76.Stengel A, Taché Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol 71: 219–239, 2009. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stirrat CG, Venkatasubramanian S, Pawade T, Mitchell AJ, Shah AS, Lang NN, Newby DE. Cardiovascular effects of urocortin 2 and urocortin 3 in patients with chronic heart failure. Br J Clin Pharmacol 82: 974–982, 2016. doi: 10.1111/bcp.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szabadfi K, Kiss P, Reglodi D, Fekete EM, Tamas A, Danyadi B, Atlasz T, Gabriel R. Urocortin 2 treatment is protective in excitotoxic retinal degeneration. Acta Physiol Hung 101: 67–76, 2014. doi: 10.1556/APhysiol.100.2013.020. [DOI] [PubMed] [Google Scholar]
- 79.Terui K, Higashiyama A, Horiba N, Furukawa KI, Motomura S, Suda T. Coronary vasodilation and positive inotropism by urocortin in the isolated rat heart. J Endocrinol 169: 177–183, 2001. doi: 10.1677/joe.0.1690177. [DOI] [PubMed] [Google Scholar]
- 80.Thomas S, Fritsche R, Perry SF. Pre-and post-branchial blood respiratory status during acute hypercapnia or hypoxia in rainbow trout, Oncorhynchus mykiss. J Comp Physiol B 164: 451–458, 1994. doi: 10.1007/BF00714582. [DOI] [PubMed] [Google Scholar]
- 81.Tota B, Angelone T, Mancardi D, Cerra MC. Hypoxia and anoxia tolerance of vertebrate hearts: an evolutionary perspective. Antioxid Redox Signal 14: 851–862, 2011. doi: 10.1089/ars.2010.3310. [DOI] [PubMed] [Google Scholar]
- 82.Van Raaij MTM, Van den Thillart GEEJM, Vianen GJ, Pit DSS, Balm PHM, Steffens AB. Substrate mobilization and hormonal changes in rainbow trout (Oncorhynchus mykiss, L.) and common carp (Cyprinus carpio, L.) during deep hypoxia and subsequent recovery. J Comp Physiol B 166: 443–452, 1996. doi: 10.1007/BF02337889. [DOI] [Google Scholar]
- 83.Walczewska J, Dzieza-Grudnik A, Siga O, Grodzicki T. The role of urocortins in the cardiovascular system. J Physiol Pharmacol 65: 753–766, 2014. [PubMed] [Google Scholar]
- 84.Waser B, Rehmann R, Rivier J, Vale W, Reubi JC. CRF receptors in the rodent and human cardiovascular systems: species differences. Peptides 27: 3029–3038, 2006. doi: 10.1016/j.peptides.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 85.Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: Univ. of Oregon Press, 2000. [Google Scholar]
- 86.Wilson JM, Bunte RM, Carty AJ. Evaluation of rapid cooling and tricaine methanesulfonate (MS222) as methods of euthanasia in zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 48: 785–789, 2009. [PMC free article] [PubMed] [Google Scholar]
- 87.Winter MJ, Ashworth A, Bond H, Brierley MJ, McCrohan CR, Balment RJ. The caudal neurosecretory system: control and function of a novel neuroendocrine system in fish. Biochem Cell Biol 78: 193–203, 2000. doi: 10.1139/o00-059. [DOI] [PubMed] [Google Scholar]
- 88.Yamauchi N, Otagiri A, Nemoto T, Sekino A, Oono H, Kato I, Yanaihara C, Shibasaki T. Distribution of urocortin 2 in various tissues of the rat. J Neuroendocrinol 17: 656–663, 2005. doi: 10.1111/j.1365-2826.2005.01354.x. [DOI] [PubMed] [Google Scholar]


