Abstract
Objectives The pedicled nasoseptal flap (NSF) has dramatically reduced postoperative cerebrospinal fluid leakage following endoscopic endonasal approach (EEA) surgery. Although rare, its arterial supply may be damaged during harvest or may be preoperatively damaged for numerous reasons. Early recognition permits harvesting a contralateral flap before sacrificing its pedicle as part of the surgical exposure or use of an alternative flap.
Design Technical feasibility study and case series.
Setting Tertiary care university-associated medical center.
Participants Five patients requiring an EEA with NSF reconstruction.
Main Outcome Measures During NSF harvest, intravenous indocyanine green (IVICG) was administered, and a customized endoscopic system was used to visualize the emerging fluorescence. At the end of each case, just before final positioning of the NSF, additional IVICG was administered, and the custom endoscope was again introduced to evaluate fluorescence.
Results In four patients, the entire NSF fluoresced brightly with IVICG on initial harvest and before final positioning. One patient showed heterogeneous fluorescence of the pedicle and distal parts of the NSF at both stages. All NSFs healed well without complication.
Conclusion IVICG facilitates real-time evaluation NSF's arterial supply. This may provide early recognition of arterial compromise, allowing the harvest of alternate flaps or modification of surgery.
Keywords: indocyanine green, ICG, fluorescence, indocyanine green fluorescence, indocyanine green endoscope, endoscopic endonasal, nasoseptal flap, nasoseptal artery, skull base
Introduction
Endoscopic endonasal approaches (EEA) to the cranial base are increasingly being utilized to address a variety of cranial base pathological processes. 1 2 3 4 5 6 7 Historically, one limitation of this approach was that large cranial base defects would not rapidly heal after reconstruction with synthetic materials or free tissue grafts; therefore, patients suffered unacceptably high rates of postoperative cerebrospinal fluid (CSF) leakage. This limitation was dramatically reduced (from an incidence of over 30% to approximately 3–5%) with the introduction of the pedicled nasoseptal flap. This technique provided viable, vascularized tissue capable of rapidly healing and adhering to the bone surrounding the defect; thus, effectively sealing it. 8 9
When harvesting the flap, it is crucial to avoid injuring the nasoseptal artery on which it is pedicled. Intraoperative Doppler acoustic ultrasonography may be utilized to identify its presence and location; however it provides poor spatial resolution and sensitivity. 10 Real-time evaluation of the location and patency of the vessel would be helpful not only during the process of harvesting the nasoseptal flap but also in assessing the patency of its blood supply should cause iatrogenic arterial injury (such as with electrocautery near its origin). In this case, an alternate flap for reconstruction could be harvested during the early stages of surgery before sacrificing the arterial supply to potential alternate flaps.
Indocyanine green (ICG) fluorescence has long been utilized for real-time angiography in vascular neurosurgery. 11 12 13 14 15 With the advent of the ICG-responsive endoscope, surgeons began exploring its use in endonasal skull base surgery. 16 17 However, its utility in delineating the arterial supply of the nasal mucosa has not yet been investigated. We report our prospective case series utilizing ICG to investigate the integrity of blood supply during the harvest and use of nasoseptal flaps for cranial defect repair during endonasal skull base surgery.
Materials and Methods
Patient Population
This investigation was performed at the Ohio State University Wexner Medical Center with the approval of its Institutional Review Board. We prospectively identified adult patients with skull base lesions who were candidates for an EEA for resection and a nasoseptal flap for reconstruction. Exclusion criteria included patient age less than 18 years, history of iodide allergy, previous anaphylactic reaction to ICG, pregnancy, history of septal surgery for any reason, and preoperative lack of indication for nasoseptal flap harvest. All surgeries were performed by a team comprising of a neurosurgeon and an otolaryngologist-head and neck surgeon.
Indocyanine Green Fluorescence Endoscopy
All cases were performed with standard, commercially available rigid 0 and 30-degree endoscopes (4 mm diameter, 18 cm length; Karl Storz Endoscopy-America, Inc., El Segundo, California, United States) for all aspects of the surgery except at two distinct times: immediately prior to the nasoseptal flap harvest and just before its final positioning over the defect. Specialized endoscopic equipment was used during these time intervals to evaluate the fluorescence of the tissues. To evaluate the nasoseptal flap fluorescence, 25 mg of ICG were dissolved in 10 mL of normal saline solution and injected intravenously as a bolus followed by a flush of 10 mL of normal saline solution. The emerging and resulting fluorescence was observed with a 0-degree rigid endoscope (5.8 mm diameter, 19 cm length) with a selectable 790 nm filter, an Image 1 Camera Head (H3-Z F1 3-CCD ICG Full HD), an Image 1 UB HD camera control unit, a Cold Light Fountain D-LIGHT P, and a separate high-definition display connected to this custom apparatus (all Karl Storz Endoscopy-America, Inc.). As described by Litvack et al, 17 the light source can switch between white light and near-infrared light output with a foot switch, thus, allowing the user to visualize the anatomy both in the standard visible light spectrum and in the near-infrared spectrum. Video and photographic data were captured and stored directly by an AIDA recording system (Karl Storz Endoscopy-America, Inc.).
Results
During this pilot study, we enrolled two men and three women, ranging in age from 24 to 78 years (mean: 48.8 years). Indications for surgery included one type-2 craniopharyngioma, 18 two planum sphenoidale meningiomas, one esthesioneuroblastoma, and one encephalocele. Table 1 summarizes the relevant information.
Table 1. Patient Information.
| Patient number | Age (y) | Sex | Pathology |
|---|---|---|---|
| 1 | 45 | Female | Craniopharyngioma (type 2) |
| 2 | 78 | Male | Planum meningioma |
| 3 | 24 | Female | Esthesioneuroblastoma |
| 4 | 52 | Female | Planum meningioma |
| 5 | 45 | Male | Encephalocele |
ICG was successfully administered intravenously to all patients without adverse event. Approximately 20 seconds after injection, the ICG fluorescence, indicating the vascularity of the nasal mucosa, was clearly visible. In four patients, the entire flap fluoresced brightly after administration of ICG upon initial harvest ( Fig. 1 ) and before final positioning ( Fig. 2 ). One patient showed heterogeneous fluorescence of the pedicle and distal parts of the flap. The surgeons decided to still use this flap for skull base reconstruction since there was perfusion. This patient was followed closely postoperatively, and the flap proved to survive with no CSF leak thus far after 8 months. The fluorescent nasoseptal artery could be clearly seen through the mucosa in two patients, and the position of the artery was obscured by the intense fluorescence of the mucosal pedicle in the other three patients. All nasoseptal flaps fluoresced after final positioning. No patient experienced an adverse reaction to the ICG. Postoperative magnetic resonance images of the brain with and without contrast were obtained from each patient, and all demonstrated avid nasoseptal flap enhancement, indicating a patent blood supply. Fig. 3 provides a characteristic example of this enhancement.
Fig. 1.
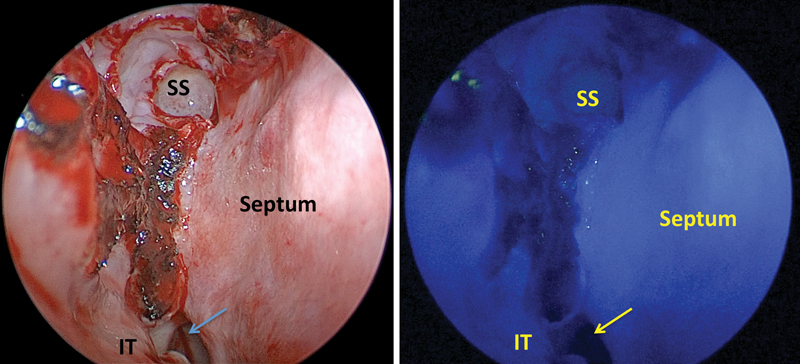
True-color and ICG-fluorescence images of the nasoseptal flap just before harvest in the patient with a type-2 craniopharyngioma (patient 1). In this case, the flap enhanced so avidly and uniformly that the enhancement of the underlying arterial supply was not discernable. ICG, indocyanine green; IT, inferior turbinate; SS, sphenoid sinus.
Fig. 2.
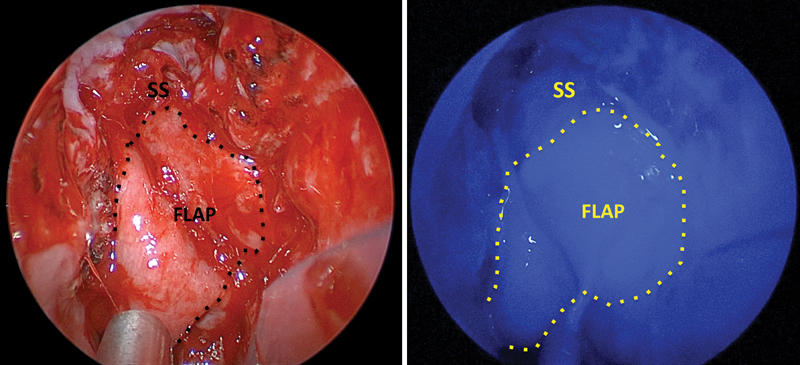
True-color and ICG-fluorescence images of the nasoseptal flap in its final position covering the skull base defect in patient 1. ICG, indocyanine green; SS, sphenoid sinus.
Fig. 3.
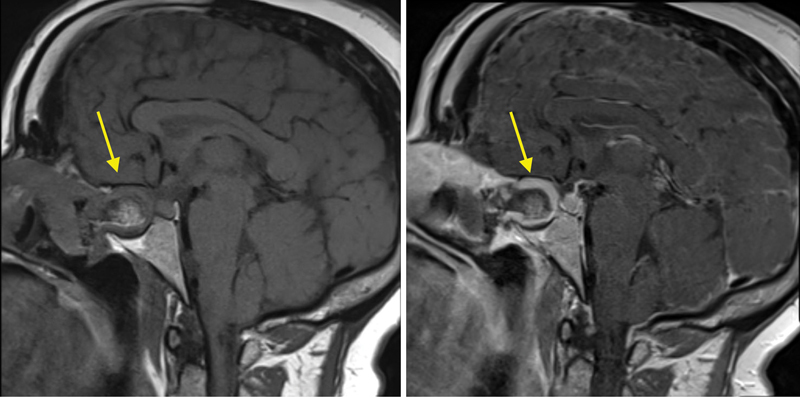
Postoperative precontrast (left) and postcontrast (right) magnetic resonance images demonstrating nasoseptal flap contrast enhancement (patient 1), indicating a patent blood supply to the flap.
Discussion
Originally Food and Drug Administration approved for human use in 1959, ICG is a tricarbocyanine dye with near-infrared fluorescence. Since 1972, ICG has been used routinely in a variety of cerebrovascular procedures for real-time noninvasive intraoperative angiography; thus, it has long safety history. 11 12 13 14 15 It is administered as an intravenous bolus, typically in a dose of 25 mg (dissolved in 10 mL of sterile water), binds to large plasma proteins within 2 seconds, remains intravascular, is metabolized exclusively by the liver with a plasma half-life of 150 seconds, and is excreted in bile. 17 Angiography is typically most useful after about 45 seconds of ICG administration in distinct arterial, capillary, and venous phases. 17
ICG administration resulted in avid fluorescence in the nasoseptal mucosa in each of our patients approximately 20 seconds post administration, and the fluorescence persisted for minutes. We did not measure the time for the fluorescence to dissipate, as doing so would needlessly have prolonged surgery. However, in each case, no residual fluorescence was observed after final positioning of the nasoseptal flap following the administration of the second bolus of ICG. In our series, the ICG administration was well tolerated by the patients. This was expected, as ICG is routinely used in open vascular neurosurgery and for the evaluation of other reconstructive flaps (i.e., traditional open surgeries) at our institution without adverse event.
With the advent of the nasoseptal flap, postoperative CSF leak rates following expanded (extrasellar) EEA cases have declined from around 30 to about 5%. Any flap, however, can fail due to poor blood supply caused by injury to its supplying vessels or thrombosis of its vasculature. Unfortunately, after harvesting the flap, arterial insufficiency is not immediately evident with a visual inspection or even with Doppler ultrasonography. It should be considered that sinonasal dissection beyond this point in the surgery involves sacrificing the contralateral nasoseptal artery as the surgeon either prepares a reverse flap, 19 or simply removes the mucosal pedicle that carries the nasoseptal artery to the septal mucosa along the anterior face of the sphenoid sinus. Consequently, if ICG is administered just after harvesting the flap and the flap does not fluoresce (and the surrounding mucosa does, providing a positive control), then the surgeon could harvest a contralateral nasoseptal flap while the option still exists. Similarly, if a nasoseptal flap does not fluoresce after final positioning of the repair, then the surgeon can surmise that its arterial supply has either become occluded by thrombus or was injured during the course of the case, and an alternate technique such as multilayered grafting with or without a substitute flap such as a lateral nasal wall, 20 pericranial or temporoparietal fascia 21 flap may be considered before terminating the case. Finally, it is important to consider cost when evaluating any novel technology. ICG has been commercially available for decades as a generic medication, generally under US$100 as of the time of this study for a 25 mg vial, and the additional operating room time required to administer and observe the fluorescence twice was approximately 10 minutes total in our patients. As such, the cost of ICG administration is minimal, especially when compared with the savings in both direct costs and morbidity if doing so helps avoid a postoperative CSF leak requiring further management.
Conclusion
This novel technique for performing real-time endoscopic intranasal angiography provides immediate feedback regarding the success of arterial preservation during nasoseptal flap harvest and will similarly provide the surgeon with vital information regarding the viability of the flap at the conclusion of the reconstructive effort. This information, which is unavailable without real-time angiography, allows for further surgical action/intervention to avoid the complications associated with a nonviable flap and the potential CSF leak that would otherwise result.
Acknowledgments
Karl Storz Endoscopy (El Segundo, California, United States) provided commercially unavailable endoscopy equipment for use in this study at no cost and without any linked funding. All additional materials and methods used in this study were self-funded.
Note
No portion of this article has been previously presented or published.
References
- 1.Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19(01):E3. [PubMed] [Google Scholar]
- 2.Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19(01):E4. [PubMed] [Google Scholar]
- 3.Attia M, Kandasamy J, Jakimovski Det al. The importance and timing of optic canal exploration and decompression during endoscopic endonasal resection of tuberculum sella and planum sphenoidale meningiomas Neurosurgery 201271(1, Suppl Operative):58–67. [DOI] [PubMed] [Google Scholar]
- 4.Zada G, Du R, Laws E R., Jr Defining the “edge of the envelope”: patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy. J Neurosurg. 2011;114(02):286–300. doi: 10.3171/2010.8.JNS10520. [DOI] [PubMed] [Google Scholar]
- 5.Cavallo L M, Solari D, Esposito F, Cappabianca P.The endoscopic endonasal approach for the management of craniopharyngiomas involving the third ventricle Neurosurg Rev 2013360127–37., discussion 38 [DOI] [PubMed] [Google Scholar]
- 6.Schmidt R F, Choudhry O J, Raviv J et al. Surgical nuances for the endoscopic endonasal transpterygoid approach to lateral sphenoid sinus encephaloceles. Neurosurg Focus. 2012;32(06):E5. doi: 10.3171/2012.3.FOCUS1267. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin N, Bresson D, Ditzel Filho L Fet al. Vidian nerve neurofibroma removed via a transpterygoid approach Minim Invasive Neurosurg 201154(5-6):250–252. [DOI] [PubMed] [Google Scholar]
- 8.Kassam A B, Thomas A, Carrau R Let al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap Neurosurgery 2008630101ONS44–ONS52., discussion ONS52–ONS53 [DOI] [PubMed] [Google Scholar]
- 9.Liu J K, Schmidt R F, Choudhry O J, Shukla P A, Eloy J A. Surgical nuances for nasoseptal flap reconstruction of cranial base defects with high-flow cerebrospinal fluid leaks after endoscopic skull base surgery. Neurosurg Focus. 2012;32(06):E7. doi: 10.3171/2012.5.FOCUS1255. [DOI] [PubMed] [Google Scholar]
- 10.Pinheiro-Neto C D, Carrau R L, Prevedello D M et al. Use of acoustic Doppler sonography to ascertain the feasibility of the pedicled nasoseptal flap after prior bilateral sphenoidotomy. Laryngoscope. 2010;120(09):1798–1801. doi: 10.1002/lary.20996. [DOI] [PubMed] [Google Scholar]
- 11.Ambekar S, Babu A, Pandey P, Devi I B. Intraoperative assessment of STA-MCA bypass patency using near-infrared indocyanine green video-angiography: a preliminary study. Neurol India. 2012;60(06):604–607. doi: 10.4103/0028-3886.105194. [DOI] [PubMed] [Google Scholar]
- 12.Imizu S, Kato Y, Sangli A, Oguri D, Sano H. Assessment of incomplete clipping of aneurysms intraoperatively by a near-infrared indocyanine green-video angiography (Niicg-Va) integrated microscope. Minim Invasive Neurosurg. 2008;51(04):199–203. doi: 10.1055/s-2008-1080916. [DOI] [PubMed] [Google Scholar]
- 13.Kim D L, Cohen-Gadol A A. Indocyanine-green videoangiogram to assess collateral circulation before arterial sacrifice for management of complex vascular and neoplastic lesions: technical note. World Neurosurg. 2013;79(02):4040–4.04E8. doi: 10.1016/j.wneu.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Son Y J, Kim J E, Park S B, Lee S H, Chung Y S, Yang H J. Quantitative analysis of intraoperative indocyanine green video angiography in aneurysm surgery. J Cerebrovasc Endovasc Neurosurg. 2013;15(02):76–84. doi: 10.7461/jcen.2013.15.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson E M, Halsey J H, Jr, Vitek J J. Validation of jugular venous flow as an index of total cerebral blood flow. Stroke. 1972;3(03):300–321. doi: 10.1161/01.str.3.3.300. [DOI] [PubMed] [Google Scholar]
- 16.Hide T, Yano S, Shinojima N, Kuratsu J. Usefulness of the indocyanine green fluorescence endoscope in endonasal transsphenoidal surgery. J Neurosurg. 2015;122(05):1185–1192. doi: 10.3171/2014.9.JNS14599. [DOI] [PubMed] [Google Scholar]
- 17.Litvack Z N, Zada G, Laws E R., Jr Indocyanine green fluorescence endoscopy for visual differentiation of pituitary tumor from surrounding structures. J Neurosurg. 2012;116(05):935–941. doi: 10.3171/2012.1.JNS11601. [DOI] [PubMed] [Google Scholar]
- 18.Kassam A B, Gardner P A, Snyderman C H, Carrau R L, Mintz A H, Prevedello D M. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg. 2008;108(04):715–728. doi: 10.3171/JNS/2008/108/4/0715. [DOI] [PubMed] [Google Scholar]
- 19.Kasemsiri P, Carrau R L, Otto B A et al. Reconstruction of the pedicled nasoseptal flap donor site with a contralateral reverse rotation flap: technical modifications and outcomes. Laryngoscope. 2013;123(11):2601–2604. doi: 10.1002/lary.24088. [DOI] [PubMed] [Google Scholar]
- 20.Rivera-Serrano C M, Bassagaisteguy L H, Hadad G et al. Posterior pedicle lateral nasal wall flap: new reconstructive technique for large defects of the skull base. Am J Rhinol Allergy. 2011;25(06):e212–e216. doi: 10.2500/ajra.2011.25.3693. [DOI] [PubMed] [Google Scholar]
- 21.Fortes F S, Carrau R L, Snyderman C H et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope. 2007;117(06):970–976. doi: 10.1097/MLG.0b013e3180471482. [DOI] [PubMed] [Google Scholar]


