Abstract
Objective Spontaneous cerebrospinal fluid rhinorrhea (SCSFR) might be the only clinical manifestation of idiopathic intracranial hypertension (IIH), which has been historically related to overweight. Our goal was to search for an association between SCSFR and increased body weight on the one hand and SCSFR and imaging findings suggestive of IIH on the other hand.
Materials and Methods We retrospectively collected clinical and radiological data of patients operated on endoscopically for SCSFR in our institution from 1993 to 2013. Analyzed factors were body mass index (BMI), extended sphenoid sinus pneumatization on computed tomography, and empty sella and distention of the optic nerve sheath on magnetic resonance imaging.
Results There were 15 patients: 8 females/7 males; mean age 50 years. Primary surgical success rate was 86.7%. Regarding body weight, 80% were overweight (BMI ≥ 25) versus 32% in the French general population ( p < 0.001). Among patients with SCSFR, 20% were obese (BMI ≥ 30) versus 15% in French individuals without SCSFR ( p = 0.483). Increased pneumatization of sphenoid sinuses was observed in 92.9 versus 27.5% in the general population ( p < 0.0001). Empty sella was found in 46.2 versus 3% in the general population ( p < 0.00001). Dilation of the optic nerve sheath was observed in 46.2 versus 15% in the general population ( p < 0.01).
Conclusion We found statistically significant associations between SCSFR and overweight, increased pneumatization of sphenoid sinuses, empty sella, and dilation of optic nerve sheath, but not with obesity, which did not have any additional impact of CSF leak than did overweight.
Keywords: spontaneous cerebrospinal fluid rhinorrhea, intracranial pressure, sphenoid sinus, pneumatization, empty sella, optic nerve sheath
Introduction
Cerebrospinal fluid (CSF) rhinorrhea is due to an abnormal communication between subarachnoid spaces and sinonasal cavities, or tympanic cavity through the eustachian tube. CSF rhinorrhea of sinonasal origin can be traumatic, iatrogenic, tumor induced, or spontaneous. Spontaneous forms are not exceptional and follow traumatic and iatrogenic forms in frequency. 1 2 3 Spontaneous leaks can be congenital, idiopathic, or related to a meningoencephalocele. 1 4 5 According to Wise and Schlosser, the incidence of spontaneous idiopathic CSF leaks was of 3 to 5% in the 1990s only to become 14 to 46% in the 2000s. 4 This underlines an earlier and more frequent diagnosis of the spontaneous form, which was probably underestimated in the past.
Classically, spontaneous CSF rhinorrhea (SCSFR) has been related to elevated intracranial pressure (ICP), more commonly seen in females who are middle aged and overweight. 2 3 6 7 Elevated CSF pressure without any intracranial mass or ventricular dilation is called idiopathic intracranial hypertension (IIH). 8 It may manifest clinically by headache, pulsatile tinnitus, visual disturbances, papilledema, and/or spontaneous CSF leak. However, CSF leak may be isolated in IIH. This disease entity may be highly suspected on magnetic resonance imaging (MRI) by an empty sella appearance, dilation of the optic nerve sheath, tortuosity of the optic nerve, posterior flattening of the globe, and/or meningoencephalocele. 3 8 Signs of IIH on computed tomography (CT) scan may include enlargement of the foramen ovale and increased sphenoid sinus pneumatization. 3 6 9 These radiological signs are inconstant, however. The goal of our work was to seek an association between SCSFR and body weight on the one hand and SCSFR and imaging findings on the other hand. To achieve this goal, we reviewed the clinical and radiological data of our series of SCSFR.
Materials and Methods
This is a retrospective study conducted on patients with CSF rhinorrhea of sinonasal origin, between 1993 and 2013, in our institution. Patients included were those with SCSFR, that is, without any past history of trauma, surgery, or tumor in the sinonasal cavity and skull base. All patients have been operated on by endonasal endoscopic surgery (EES).
Collected data were age, gender, diagnostic symptoms, body mass index (BMI), CT scan findings (bone defect, sinus opacity, pneumocephalus, and extended pneumatization of sphenoid sinuses), MRI scan findings (dural defect, meningocele or encephalocele, pneumocephalus, empty sella turcica, and dilation of optic nerve sheath), localization of the leaking site, material of closure, postoperative complications, hospital stay, and follow-up duration. One senior radiologist with certificate of added qualification qualitatively analyzed CT and MRI scans.
Extended pneumatization of sphenoid sinuses on CT scan was defined on axial sections as pneumatization of the lateral recess of the sphenoid sinus extending into the greater wing of the sphenoid bone, and/or pneumatization of the space between medial and lateral pterygoid processes ( Fig. 1 ).
Fig. 1.
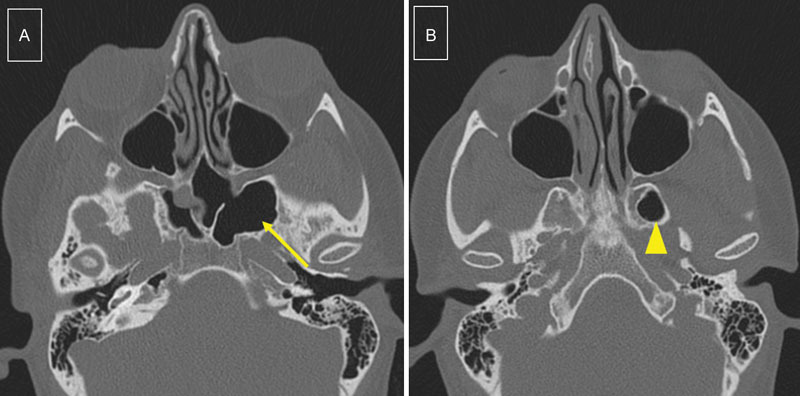
Extended pneumatization of the sphenoid sinuses: CT scan, axial sections, reveals extended pneumatization on the left side. ( A ) Laterally into the greater wing of sphenoid bone (arrow) and ( B ) into the space between medial and lateral pterygoid processes (arrowhead). This is the scan of a 31-year-old woman, obese, who presented CSF leak from the cribriform plate. CSF, cerebrospinal fluid; CT, computed tomography.
Empty sella on MRI scan was defined on axial and sagittal sections as a pituitary fossa filled with CSF, thus appearing as a high-signal intensity on T2-weighted sequence or heavily-weighted T2 sequence ( Fig. 2 ).
Fig. 2.
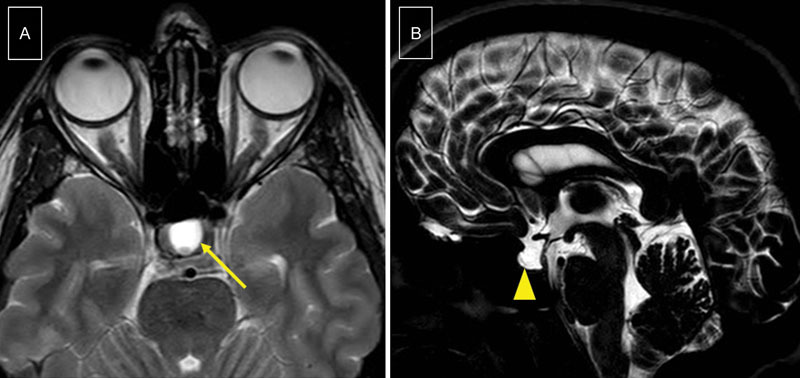
Empty sella appearance: MRI scan reveals CSF filling the pituitary space. ( A ) High-signal intensity on axial T2-weighted sequence (arrow). ( B ) High-signal intensity on sagittal heavily-weighted T2 sequence (arrowhead). This is the scan of a 44-year-old woman, overweight, who presented CSF leak from the cribriform plate. CSF, cerebrospinal fluid; MRI, magnetic resonance imaging.
Distention of the optic nerve sheath on MRI scan was defined on axial sections as obvious filling of the sheath with CSF along the whole nerve length, thus appearing as a high-signal intensity image bordering the optic nerve on T2-weighted sequence or heavily-weighted T2 sequence ( Fig. 3 ).
Fig. 3.
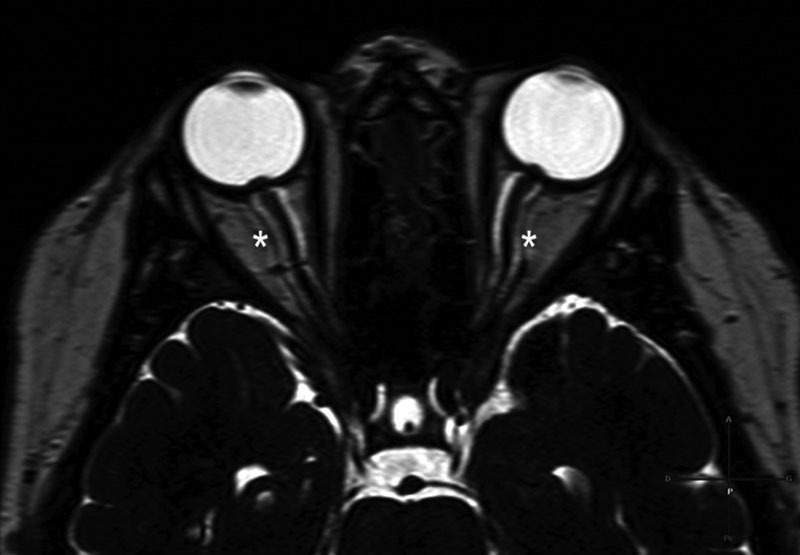
Dilation of optic nerve sheaths: MRI scan, axial section, heavily-weighted T2 sequence, shows enlarged CSF spaces appearing as a high-signal intensity around the optic nerves (asterisks). This is the scan of a 76-year-old woman, overweight, who presented CSF leak from the posterior wall of the right sphenoid sinus. CSF, cerebrospinal fluid; MRI, magnetic resonance imaging.
Exact binomial 95% confidence intervals (95CI) and two-sided tests were used to describe and compare the proportions of overweight/imaging criteria in our patients with CSF rhinorrhea and in the general population. We considered p -values of < 0.05 as being significant. Analyzes were performed using Stata software release 13 (StataCorp. 2013; StataCorp LP).
Study ethics approval was obtained on July 29, 2015 (CECIC Rhône-Alpes-Auvergne, Clermont-Ferrand, Institutional Review Board 5891).
Results
In total, 65 patients with sinonasal CSF rhinorrhea have been operated on by EES within the 20-year period defined earlier. CSF leak was iatrogenic in 26 patients (40%), traumatic in 22 (33.8%), and spontaneous in 17 (26.2%). There was no case of CSF leak of tumoral origin in our series. Among the 17 patients with SCSFR, there were 2 children (ages 3 and 3.5 years) who were excluded from the study because the etiology was most probably congenital and sphenoid sinuses are not developed at this age. Besides, MRI was not performed in those children to avoid general anesthesia. There were eight females and seven males. The age range at surgery was 15 to 84 years with a mean of 50 years. As for BMI, 12 out of 15 patients (80%) were overweight (BMI ≥ 25) at diagnosis. Among the 15 patients with SCSFR, 3 (20%) were obese (BMI ≥ 30). All patients (100%) had presented CSF rhinorrhea at some point of the disease. Three (20%) have had meningitis. Apart from CSF rhinorrhea, no patient had any other sign of intracranial hypertension. Diagnosis was based on finding overt CSF rhinorrhea or CSF leak on flexible or rigid nasal endoscopy. Dosage of β2-transferrin was performed in seven cases (46.7%) in case of intermittent rhinorrhea, doubtful diagnosis, and/or absence of CSF rhinorrhea during office examination.
CT was performed in 14 out of 15 (93.3%) patients. In one patient who did not have CT, CSF leak was an incidental finding intraoperatively. This patient had presented preoperatively with profuse clear bloody rhinorrhea. MRI was performed in 13 patients (86.7%). There was the case of incidental finding, and in one case, we have no explanation for not scheduling an MRI.
Findings on CT scans were bone defect in 11 out of 14 cases (78.6%), sinus opacity in 9 out of 14 (64.3%), pneumocephalus in 1 out of 14 (7.1%), and extended sphenoid sinus pneumatization in 13 out of 14 (92.9%). Findings on MRI scans were dural defect in 8 out of 13 cases (61.5%), meningocele or encephalocele in 6 out of 13 (46.2%), pneumocephalus in 1 out of 13 (7.7%), empty sella in 6 out of 13 (46.2%), and dilation of optic nerve sheath in 6 out of 13 (46.2%).
All patients had been operated on by endonasal endoscopic repair of the osteodural defect, using a fat graft in all but one patient, in whom a middle turbinate graft was placed. A silicone sheath was used in nine cases to hold the fat graft in place and a nasoseptal flap was performed in two cases. Intraoperatively, the site of leakage was found to be situated in the sphenoid sinus in seven patients (lateral recess three, posterior wall three, and planum sphenoidale one), cribriform plate in three, roof of anterior ethmoidal cells in two, roof of posterior ethmoidal cells in one, posterior wall of frontal sinus in one, and roof of nasal fossa in one. Intraoperative image guidance and intrathecal fluorescein injection were used in no case.
CSF rhinorrhea ceased postoperatively in 13 out of 15 patients, yielding a primary success rate of 86.7%. Two patients necessitated a second EES (at 1 month from first surgery) which was successful. The mean hospital stay was 4 days (2–8 days); patients who necessitated a long hospital stay had other comorbidities. There were no postoperative complications apart from recurrent CSF rhinorrhea in two patients. The mean follow-up period was of 6 months (2–18 months).
Regarding body weight, 12 out of 15 patients (80%, exact 95CI [51.9–95.7%]) were overweight versus 32% in the French general population ( p < 0.001). 10 Among patients with SCSFR, 3 out of 15 (20% [4.3–48.1%]) were obese versus 15% in French individuals without SCSFR ( p = 0.483). 10 Regarding CT findings, extended pneumatization of the sphenoid sinuses was observed in 13 out of 14 patients (92.9% [66.1–99.8%]) versus 27.5% in the general population ( p < 0.0001), according to three non-French studies. 3 9 11 As for MRI findings, an empty sella was found in 6 out of 13 patients (46.2% [19.2–74.9%]) versus 3% in the general population without any symptoms of increased ICP ( p < 0.00001), according to two non-French studies. 3 6 Distention of the optic nerve sheath was observed in 6 out of 13 patients (46.2% [19.2–74.9%]) versus 15% in the general population ( p < 0.01), according to two non-French studies. 6 12 Hence, overweight and the three imaging criteria cited earlier were statistically associated with SCSFR.
Discussion
Relationship between Spontaneous CSF Rhinorrhea and Idiopathic Intracranial Hypertension
From a pathophysiological point of view, it is believed that spontaneous CSF leaks through the skull base are secondary to an inadequate resorption of CSF in arachnoid villi, leading to chronic intracranial hypertension. These arachnoid villi are enlarged and ectopic: they are usually found outside the dural venous sinuses, and thus are incapable of resorbing CSF into the venous system; they are defined as aberrant arachnoid granulations and might be present on aerated portions of the skull base. 3 5 7 13 14 Elevated pressure and chronic CSF pulsations result in weakening of the thin parts of the skull base and lead to CSF leak when the arachnoid membrane ruptures and/or to meningoencephaloceles. 3 4 9 13 15 16 Among the weakest parts of the skull base, prone to leaks and cerebral hernias are the cribriform plate, the ethmoid roof, and the lateral recess of the sphenoid sinus. 1 2 3 4 17 18 Another reason is that at these sites, the dura firmly adheres to the bone of the skull base, making it easier to develop an abnormal communication between the subarachnoid space and sinonasal cavities. 1 These sites were most frequently involved in our series as well, in addition to the posterior wall of the sphenoid sinus. Besides CSF leak, almost half of the patients had a meningocele or an encephalocele in our study. This rate is variable in the literature: 50 to 100%. 3 16
The underlying mechanism through which overweight causes spontaneous CSF leakage is that central obesity increases abdominal pressure, which decreases cerebral venous return. This in turn raises ICP by preventing normal CSF absorption due to decreased venous return. This mechanism, together with aberrant arachnoid granulations (which is the predisposing factor), leads to spontaneous CSF leak. 3 5 13 18 In our study, the rate of overweight in patients with SCSFR (80%) was statistically higher than that in the normal population (32%). However, the rate of obesity in patients with SCSFR (20%) was not statistically higher than that in individuals without SCSFR (15%). Although we cannot draw conclusions because of the small number of the sample and thus a weak statistical power for obesity, we can note that obesity among patients with SCSFR did not significantly add any further risk on SCSFR than did overweight alone. One can hypothesize that there could be a threshold of weight, the pressure above which causes an increased risk of intracranial hypertension.
CT and MRI Findings in Patients with Spontaneous CSF Rhinorrhea
Regarding the sphenoid sinus, extended posterolateral pneumatization might be a contributive cause to IIH. Indeed, the extension of the pneumatization process leads to thinning of the sinus walls, thus facilitating bone erosion by aberrant arachnoid granulations, which ultimately results in CSF leak and cerebral herniation. 3 It also seems that extended sinus pneumatization is sufficient to cause CSF leak in the presence of aberrant arachnoid granulations, through thinning of sinus bony walls, without even the presence of overweight. 18 This might explain the presence of SCSFR in 20% of patients in our series not being overweight. Furthermore, pneumatization of the lateral recess of the sphenoid bone is a mandatory precondition for spontaneous CSF leak through the lateral recess.
We found a significant association between SCSFR and MRI findings: empty sella appearance and dilation of the optic nerve sheath. These two findings have been classically related to IIH. 3 4 6 7 8 9 19 We did not analyze other findings, such as posterior flattening of the globe (on MRI), tortuosity of the optic nerve (on MRI), or enlargement of the foramen ovale (on CT) because our study is retrospective and some specific image reconstructions were lacking.
The underlying mechanism of empty sella syndrome in IIH and SCSFR is the following: the pulsatile forces of increased ICP follow the paths of least resistance. One of these paths is the fascia of the sellar diaphragm. Herniation of arachnoid and CSF through the sellar diaphragm results in compression of the pituitary gland and possibly erosion of the sellar floor, leading to the appearance of an empty sella on MRI and eventually CSF rhinorrhea. 3 4 6 13
The rationale behind distention of the optic nerve sheath in IIH is that pulsatile cerebral pressure is transmitted to the subarachnoid space of the nerve sheath, filled with CSF, which is in communication with cerebral CSF spaces. This results in distention of this subarachnoid space and increased diameter of optic nerve sheath, as seen on MRI, and sometimes papilledema. 3 19 With regard to the latter sign, increased ICP in spontaneous CSF leak patients was recently found not to be associated with papilledema, due to CSF pressure diversion by the leak itself. 20
Spontaneous CSF rhinorrhea is a rare pathology and has long been underdiagnosed. It is classically described in middle-aged women, who are overweight. These patients might present signs of IIH, other than spontaneous CSF leak: headache, vomiting, pulsatile tinnitus, and visual disturbances. However, all these signs can be very subtle and even absent. Indeed, no patient in our series presented with signs typical of IIH, probably because of some degree of “pressure relief” through the CSF leakage. Furthermore, there was no female predominance in our series (eight females/seven males). This stresses the importance of MRI data seeking for eventual indirect signs of elevated ICP, namely, empty sella and distended optic nerve sheath, these being often associated with meningoencephaloceles. These three criteria were found in less than half of patients on MRI scans in our series; this might once again be explained by the pressure relief through spontaneous CSF leakage found in these patients. A CT should also be performed seeking extended pneumatization of the sphenoid sinuses. Patients with SCSFR should be diagnosed accurately for CSF leak and eventual IIH, and then managed surgically, primarily because of the high risk of meningitis secondary to contamination of CSF by bacteria of sinonasal cavities. Three patients out of 15 (20%) in our series had meningitis preoperatively. The endoscopic endonasal approach for closure of CSF leaks provides better results than old external approaches while being less morbid. 1 2 18 21 22 However, the recurrence rate might be higher in spontaneous CSF leaks as compared with nonspontaneous forms (traumatic, iatrogenic). 4 One of the reasons is the presence of elevated ICP in the spontaneous forms, which transmits forces against the closure material. As for our series, we obtained similar primary success rates in spontaneous and nonspontaneous forms.
The retrospective nature is the main drawback of this study, thereby limiting the number of factors analyzed in SCSFR. The reason is that SCSFR is a relatively rare disease thus rendering prospective data collection difficult in our institution. However, these results are promising for further longitudinal diagnostic trials notably focusing on visual impairment and additional radiological signs in patients with presumed IIH. Spontaneous CSF rhinorrhea should be considered a potential precursor sign of IIH that may be suspected on imaging, more often in the presence of overweight, leading to appropriate management of these patients with fundoscopic examination and eventually CSF pressure measurement through a lumbar puncture.
Conclusion
Spontaneous CSF rhinorrhea is one of the etiologies of CSF rhinorrheas and has been increasingly diagnosed. We found statistically significant associations between SCSFR and overweight, increased pneumatization of sinuses, empty sella, and dilation of optic nerve sheath, but not with obesity, which did not have any additional impact of CSF leak than did overweight. Although we used a statistical test appropriate for small samples, a large number of patients are needed in the future to confirm our preliminary results.
Conflict of Interest The authors have no conflict of interest to disclose.
Notes
This is a retrospective study analyzing charts of operated patients, thus involving human participants. This was in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. No informed consent was obtained from the patients because of the retrospective nature of the study. In other terms, at the time of surgery and follow-up, no study was ongoing. Years later, at the time of the study, only a chart analysis was performed and was kept anonymous. Besides, study ethics approval was obtained on July 29, 2015 (CECIC Rhône-Alpes-Auvergne, Clermont-Ferrand, IRB 5891).
References
- 1.Locatelli D, Rampa F, Acchiardi I, Bignami M, De Bernardi F, Castelnuovo P.Endoscopic endonasal approaches for repair of cerebrospinal fluid leaks: nine-year experience Neurosurgery 2006580402ONS-246–ONS-256., ONS-256–ONS-257 [DOI] [PubMed] [Google Scholar]
- 2.Banks C A, Palmer J N, Chiu A G, O'Malley B W, Jr, Woodworth B A, Kennedy D W. Endoscopic closure of CSF rhinorrhea: 193 cases over 21 years. Otolaryngol Head Neck Surg. 2009;140(06):826–833. doi: 10.1016/j.otohns.2008.12.060. [DOI] [PubMed] [Google Scholar]
- 3.Alonso R C, de la Peña M J, Caicoya A G, Rodriguez M R, Moreno E A, de Vega Fernandez V M. Spontaneous skull base meningoencephaloceles and cerebrospinal fluid fistulas. Radiographics. 2013;33(02):553–570. doi: 10.1148/rg.332125028. [DOI] [PubMed] [Google Scholar]
- 4.Wise S K, Schlosser R J. Evaluation of spontaneous nasal cerebrospinal fluid leaks. Curr Opin Otolaryngol Head Neck Surg. 2007;15(01):28–34. doi: 10.1097/MOO.0b013e328011bc76. [DOI] [PubMed] [Google Scholar]
- 5.Karkas A, Atallah I, Son H J, Schmerber S. Berlin, Heidelberg: Springer; 2013. Temporal bone meningocele/encephalocele; pp. 2724–2736. [Google Scholar]
- 6.Butros S R, Goncalves L F, Thompson D, Agarwal A, Lee H K. Imaging features of idiopathic intracranial hypertension, including a new finding: widening of the foramen ovale. Acta Radiol. 2012;53(06):682–688. doi: 10.1258/ar.2012.110705. [DOI] [PubMed] [Google Scholar]
- 7.Son H J, Karkas A, Buchanan P et al. Spontaneous cerebrospinal fluid effusion of the temporal bone: repair, audiological outcomes, and obesity. Laryngoscope. 2014;124(05):1204–1208. doi: 10.1002/lary.24484. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Takanashi J, Kobayashi K, Nagasawa K, Tashima K, Kohno Y. MR imaging of idiopathic intracranial hypertension. AJNR Am J Neuroradiol. 2001;22(01):196–199. [PMC free article] [PubMed] [Google Scholar]
- 9.Shetty P G, Shroff M M, Fatterpekar G M, Sahani D V, Kirtane M V. A retrospective analysis of spontaneous sphenoid sinus fistula: MR and CT findings. AJNR Am J Neuroradiol. 2000;21(02):337–342. [PMC free article] [PubMed] [Google Scholar]
- 10.Eschwege E, Charles M A, Basdevant Aet al. Epidemiological study on French overweight and obesityAvailable at:http://www.roche.fr/content/dam/corporate/roche_fr/doc/obepi_2012.pdf. Accessed October 16, 2012
- 11.Scuderi A J, Harnsberger H R, Boyer R S. Pneumatization of the paranasal sinuses: normal features of importance to the accurate interpretation of CT scans and MR images. AJR Am J Roentgenol. 1993;160(05):1101–1104. doi: 10.2214/ajr.160.5.8470585. [DOI] [PubMed] [Google Scholar]
- 12.Sotoudeh H, Bowerson M, Parsons M et al. Effect of spatial resolution of T2-weighted imaging on diagnostic efficacy of MRI in detection of papilledema. AJR Am J Roentgenol. 2015;204(03):602–607. doi: 10.2214/AJR.14.12662. [DOI] [PubMed] [Google Scholar]
- 13.Schlosser R J, Woodworth B A, Wilensky E M, Grady M S, Bolger W E. Spontaneous cerebrospinal fluid leaks: a variant of benign intracranial hypertension. Ann Otol Rhinol Laryngol. 2006;115(07):495–500. doi: 10.1177/000348940611500703. [DOI] [PubMed] [Google Scholar]
- 14.Gacek R R, Gacek M R, Tart R. Adult spontaneous cerebrospinal fluid otorrhea: diagnosis and management. Am J Otol. 1999;20(06):770–776. [PubMed] [Google Scholar]
- 15.Brainard L, Chen D A, Aziz K M, Hillman T A. Association of benign intracranial hypertension and spontaneous encephalocele with cerebrospinal fluid leak. Otol Neurotol. 2012;33(09):1621–1624. doi: 10.1097/MAO.0b013e318271c312. [DOI] [PubMed] [Google Scholar]
- 16.Woodworth B A, Bolger W E, Schlosser R J.Nasal cerebrospinal fluid leaks and encephaloceles Oper Tech Otolaryngol--Head Neck Surg 200617111–116.. DOI: http://dx.doi.org/10.1016/j.otot.2006.03.001 [Google Scholar]
- 17.Giannetti A V, Guimarães R E, Santiago A P, Perpétuo F O, Machado M A. A tomographic study of the skull base in primary spontaneous cerebrospinal fluid leaks. Neuroradiology. 2012;54(05):459–466. doi: 10.1007/s00234-011-0901-z. [DOI] [PubMed] [Google Scholar]
- 18.Lopatin A S, Kapitanov D N, Potapov A A. Endonasal endoscopic repair of spontaneous cerebrospinal fluid leaks. Arch Otolaryngol Head Neck Surg. 2003;129(08):859–863. doi: 10.1001/archotol.129.8.859. [DOI] [PubMed] [Google Scholar]
- 19.Shofty B, Ben-Sira L, Constantini S, Freedman S, Kesler A. Optic nerve sheath diameter on MR imaging: establishment of norms and comparison of pediatric patients with idiopathic intracranial hypertension with healthy controls. AJNR Am J Neuroradiol. 2012;33(02):366–369. doi: 10.3174/ajnr.A2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aaron G, Doyle J, Vaphiades M S, Riley K O, Woodworth B A. Increased intracranial pressure in spontaneous CSF leak patients is not associated with papilledema. Otolaryngol Head Neck Surg. 2014;151(06):1061–1066. doi: 10.1177/0194599814551122. [DOI] [PubMed] [Google Scholar]
- 21.Carrau R L, Snyderman C H, Kassam A B. The management of cerebrospinal fluid leaks in patients at risk for high-pressure hydrocephalus. Laryngoscope. 2005;115(02):205–212. doi: 10.1097/01.mlg.0000154719.62668.70. [DOI] [PubMed] [Google Scholar]
- 22.Psaltis A J, Schlosser R J, Banks C A, Yawn J, Soler Z M. A systematic review of the endoscopic repair of cerebrospinal fluid leaks. Otolaryngol Head Neck Surg. 2012;147(02):196–203. doi: 10.1177/0194599812451090. [DOI] [PubMed] [Google Scholar]


