INTRODUCTION
Global Hepatitis B Virus Burden
Hepatitis B virus (HBV) infection is a global public health problem.1,2 Worldwide estimates suggest that more than 2 billion people have been infected with HBV, and that 248 million of these people are chronically infected (defined as hepatitis B surface antigen [HBsAg] positivity).3 About 15% to 25% of persons with chronic HBV infection die from cirrhosis or liver cancer.3 The Global Burden of Disease study estimated that there were 686,000 deaths caused by hepatitis B in 2013 and a 5.9 per 100,000 age-standardized death rate globally,4 of which 300,000 deaths were attributed to liver cancer and 317,400 deaths to cirrhosis of the liver secondary to hepatitis B.4 This rate represents a substantial global burden, with wide global geographic variation. Hepatitis B prevalence (HBsAg) is highest in the sub-Saharan African and western Pacific regions, considered high-intermediate to high endemicity countries (5% to ≥ 8% prevalence), and prevalence estimates exceed 15% in several countries. Low-intermediate regions (2%–4.99%) include the eastern Mediterranean and European regions. The Americas and Western Europe regions are considered low endemicity, with HBsAg prevalence generally less than 2%.3,5 There has been an overall decrease in HBsAg prevalence over time in most countries, but with notable increases in African and eastern European countries.3
United States Hepatitis B Virus Burden
In the United States, estimates of chronic hepatitis B infection range from 700,000 to more than 2 million people.6–9 Estimating the number of chronically infected persons globally and in the United States is challenging because the disease is asymptomatic in most infected persons, leading to underdiagnosis, and passive surveillance often results in under reporting.7,9 Despite declines in chronic hepatitis B cases among children and adolescents, due to increasing immunity following universal vaccine recommendations, the number of chronically infected adults in the United States has been increasing as a result of immigration of infected persons from highly endemic countries.10,11 As many as 70% of HBV infections in the United States are estimated to be among foreign-born persons.12 Yearly, 40,000 to 45,000 people from HBV-endemic countries where the prevalence of chronic HBV infection is > 2%, enter the United States legally11; an estimated 3.9 million foreign-born persons from eastern Asia and sub-Saharan Africa currently reside in the United States.13
The prevalence of HBV infection is analyzed each year by the National Health and Examination Survey (NHANES), a survey representative of the US noninstitutionalized household population.6,14 Recent NHANES data from 2011 to 2012 indicate that there are approximately 850,000 Americans living with chronic HBV infection in the United States.9 Oversampling of non-Hispanic Asians, who represent about 5% of the US population, indicate that non-Hispanic Asians account for almost 50% (400,000) of all chronic HBV infections in the United States.12,15
In the United States, rates of acute infection have remained about 1.0 per 100,000 population since 2009, and have been reported from more nonurban than urban regions.16 African American adults have the highest rate of acute HBV infection in the United States.15 Recently (2006–2013) an increase in incident cases of acute HBV infection in Kentucky, Tennessee, and West Virginia has occurred among non-Hispanic white people aged 30 to 39 years, who reported injection-drug use as a common risk factor.17
TRANSMISSION OF HEPATITIS B VIRUS
There are 2 major modes of transmission of HBV that occur in the world. Perinatal transmission, occurring at birth from infected mothers to their newborns, accounts for the majority of HBV transmission worldwide. Horizontal transmission can occur through open cuts and scratches; transfusion of blood products; breaks in good practices to prevent blood-borne infections in the health care setting; sexual transmission and risky behavior, including injecting-drug use or tattooing, body piercing, and scarification procedures without the use of sterilized equipment and needles.
The risk of developing chronic HBV infection among susceptible persons decreases with age at infection and thus depends on the mode of transmission. Up to 90% of perinatal infections become chronically infected; approximately 20% to 60% of children aged 1 to 5 years become chronically infected, and 5% to 10% of older children and adults.18–21
Perinatal Transmission
Hepatitis B “e” antigen (HBeAg) is a serologic marker for high viral levels of HBV DNA. Perinatal transmission occurs almost universally in mothers who are positive for but also can occur in mothers who have very high levels of HBV DNA, > 200,000 IU/ml in their blood. The risk of an unvaccinated infant acquiring HBV at birth is up to 100% in an infant born to an HBeAg-positive mother. The classic study by Palmer Beasley in Taiwan in the 1970’s, before vaccine was available, demonstrated that among women who were HBeAg-positive, 85% of their infants became chronically infected as compared to 32% among HBeAg negative women.22 An estimated 90% risk of developing chronic HBV exists among infants infected perinatally.
Prevention of Perinatal Transmission
The most impactful strategy for reducing mother to newborn transmission of HBV is incorporating the birth dose into the hepatitis B vaccine schedule. A birth dose followed by 2 more doses of hepatitis B vaccine can reduce the prevalence of chronic HBV in the infant by approximately 90% in infants of HBeAg-positive mothers and almost all HBeAg-negative mothers. This birth dose is especially important in areas of the world where a significant proportion of HBsAg-positive mothers are also positive for HBeAg, such as in China, south east Asia, and the Pacific Islands. In these areas, if the birth dose is not given, the effectiveness of hepatitis B vaccine could be reduced to as low as only 50% to 75%.23 In regions such as sub-Saharan Africa and Russia where less than 25% of HBsAg-positive pregnant women are also HBeAg positive,24 the impact of missing the birth dose is not as severe but is still significant. Including a dose of HBIG at birth to infants born to HBsAg-positive mothers can further reduce the risk of transmission to less than 5%. Beasley and his colleagues showed in a randomized-controlled trial, that with administration of the birth dose plus HBIG to infants born to HBsAg/HBeAg-positive mothers only 6% of those infants became HBsAg-positive verses 88% of infants who received placebo.25
Horizontal Transmission
Horizontal transmission of HBV, if it occurs in young children, has a high risk of leading to chronic HBV. Three prospective studies conducted before the availability of hepatitis B vaccine have shown this.26–28 A study of 1280 persons who were seronegative for HBV markers conducted in Alaskan villages in the 1970s found that, of 189 persons who acquired HBV during a 4-year period, 29% of those less than the age of 5 years developed chronic HBV versus 16% of those between 5 and 10 years and 8% of those more than 30 years of age.26 In a study from Taiwan following children born without HBV infection who acquired HBV before 5 years of age, 23% developed chronic HBV.27 A third study from Senegal found that 50% of children infected horizontally before the age of 2 years became chronically infected.28 In the Senegal study the rate of chronic HBV decreased from 68% at 1 year to 6.3% after 4 years of age. Furthermore, for those infected at less than 6 months of age, the rate of chronic HBV was 82%, and for those infected between 6 months and 1 year it was 54%. Inclusion of the birth dose and subsequent doses not only prevents perinatal transmission but also reduces acquisition of infection in the first few months of life when there is the greatest risk of developing chronic infection via horizontal transmission.
In young children and some adults, horizontal transmission likely occurs because of the presence of infectious HBV on environmental surfaces. In a study from Alaska 40 years ago, before HBV DNA testing was available, HBsAg was found by environmental sampling on school lunch room table tops; on walls, toys, and baby bottles in homes where HBsAg-positive persons were living; and filtered from impetigo sores.29 Furthermore, when HBV was left at room temperature, after at least 7 days viral replication was found to occur.30 Virus may be shed via open cuts, scratches, and sores from persons with chronic HBV onto environmental surfaces and then can infect other persons with open lesions through their contact with the contaminated surfaces. Horizontal transmission also occurs via unsterile injections from health care encounters or injection-drug use and tattooing as well as scarification practices, sexual transmission, and via high-risk health care environments, including renal dialysis units and emergency rooms.31
Among young adults in the United States, injection-drug use is an increasing cause of horizontal hepatitis B transmission in some areas. A 114% increase in acute HBV infection from 2006 to 2013 was found in Kentucky, Tennessee, and West Virginia.17 The increase occurred primarily among white people aged 30 to 39 years who reported the use of injection-drugs. Outbreaks in health care settings is a further cause of horizontal transmission. From 2008 to 2014, 23 acute hepatitis B outbreaks occurred related to health care.32 These outbreaks were associated with 175 cases and more than 10,700 persons were notified for screening. Of these outbreaks, 17 outbreaks occurred in long-term care facilities, and most were associated with infection-control lapses during assisted monitoring of blood glucose levels.32
Prevention of horizontal transmission requires education, appropriate infection-control practices, and vaccination of hepatitis B household contacts and other persons at high risk of hepatitis B.
STRATEGIES FOR CONTROL AND PREVENTION OF HEPATITIS B INFECTION
This article discusses the good practice principals that can effectively halt transmission of HBV. It uses as examples global and US programs in HBV vaccination (eg, Alaska) to demonstrate how effective infant vaccination strategies can accomplish this goal, starting with the first dose of hepatitis B vaccine administered immediately after birth followed by full vaccination during infancy and the use of catch-up vaccination programs for children. In addition, it highlights how programs targeting adults at the highest risk of HBV infection can prevent acute icteric HBV infection and transmission in this age group.
Global Vaccine Policy
In 1991, the Global Advisory Group of the Expanded Programme on Immunization (EPI) recommended integration of hepatitis B vaccination into national immunization programs by 1995 in countries with an HBV carrier prevalence of 8% or higher, and by 1997 in countries with a lower prevalence.2 By the end of 2014, hepatitis B vaccine had been introduced nationwide in 184 countries.1,2
World Health Organization Recommendations and Strategy on Vaccination for Control of Hepatitis B
There are 5 key World Health Organization (WHO) strategic areas for hepatitis B prevention through vaccination summarised in a WHO policy document from the Western Pacific region.33
Vaccination of infants
The WHO recommends the use of monovalent HBV vaccination within 24 hours of birth, followed by completion of the HBV vaccine series within 6 to 12 months as the most cost-effective strategy for the prevention and control of hepatitis B.33,34 This strategy provides the earliest possible protection to future birth cohorts and reduces the pool of chronic carriers in the population. Timely vaccination of newborn infants can prevent perinatal HBV transmission. Table 1 shows a typical schedule of hepatitis B and pentavalent vaccination in the western Pacific region. Strengthening of routine immunization services to achieve and sustain high coverage with 3 doses of hepatitis B vaccine by 1 year of age is the most important strategy for hepatitis B control. Mathematical modeling suggests that very high vaccine coverage rates (≥ 90%) are needed to interrupt transmission and prevent deaths, with the goal to protect the entire birth cohort and achieve health equity.35
Table 1.
Typical schedule of hepatitis B (HepB) and pentavalent (diphtheria-tetanus-pertussis [DTP], Haemophilus influenzae B [Hib], HepB) vaccination in the Western Pacific Region
| Agea | Vaccine |
|---|---|
| At birth (within 24 h) | HepB monovalent |
| 6 wk | DTP-HepB-Hib1 |
| 10 wk | DTP-HepB-Hib2 |
| 14 wk | DTP-HepB-Hib3 |
Although 3 doses are sufficient to induce immunity, for programmatic reasons most countries in the region use a combination vaccine, resulting in a 4-dose schedule.
Ages given are recommended as the earliest possible, but they are flexible. Immunization programs should emphasize vaccination at birth and completing the hepatitis B series by 6 months of age.
Data from Hepatitis B control through immunization: a reference guide. Available at: http://www.who.int/immunization/sage/meetings/2015/october/8_WPRO_Hepatitis_B_Prevention_Through_Immunization_Regional_Reference_Guide.pdf.
Delivery of a timely birth dose also provides an opportunity to link immunization delivery systems with maternal health programs, and to ensure that HBV vaccine is included in the essential care package for newborn infants, and to harmonize training and programmatic issues, including where, when, and by whom the birth dose is given.
Hepatitis B immune globulin (HBIG)
Where resources allow, HBIG may be given in addition to the vaccine to children born to HBsAg-positive mothers. However, the option for HBIG is conditional on the existence of a comprehensive antenatal screening program for hepatitis B infection, and is of limited value in settings with poor antenatal coverage.33
Catch-up vaccination
The WHO also recommends catch-up vaccination for older children who missed immunization as infants as a secondary strategy after routine vaccination reaches target levels. This strategy depends on whether a country has additional financial and human resources for enhanced hepatitis B control, and should be based on careful epidemiologic and economic analysis.
Vaccination of priority adult population groups may be prioritized after infant immunization
Priority or high-risk population groups include health care workers, contacts of HBsAg-positive persons, men who have sex with men, sex workers, people who inject drugs, frequent recipients of blood/plasma transfusions, and any other population groups coming in regular contact with blood and blood products. Incidence of acute HBV is highest among adolescents and adults, although the risk of developing chronic HBV is low compared with infants and children. Vaccination programs targeting high-risk adults can be difficult to implement because of challenges in identifying and vaccinating persons engaged in high-risk activity before they become infected. Universal vaccination of health care workers is an effective strategy to protect high-risk adult groups from HBV infection.36
Vaccine supply and quality
Key goals are elimination of vaccine stock-outs at the national and district levels through improved training in vaccine management, prevention of vaccine freezing through improved training in temperature monitoring, and promotion of the use of controlled temperature chain for hepatitis B birth dose delivery.33
Advocacy and social mobilization
The primary goal is to increase awareness among decision makers, health care workers, and caretakers of the risks and consequences of HBV infection and the need for hepatitis B vaccination through community and civil society engagement, use of media outlets, education materials, and mass awareness campaigns such as World Hepatitis Day and World immunization week.33
Measurement of program performance and impact
Because frequent serologic surveys are not feasible, vaccine coverage rates serve as an interim proxy for program performance, and can help identify areas of poor performance where increased resources and efforts should be focused. Although routinely collected administrative data may be used for regular monitoring, it is useful to supplement this with periodic vaccination coverage surveys to identify any major problems. Such surveys usually target children aged 12 to 33 months to allow estimation of vaccine coverage for the most recent birth cohort. In order to assess the status of the impact of the vaccination program, seroprevalence surveys are essential. There is no set schedule for conducting these surveys and they should be undertaken when it is programmatically important to do so.33
VACCINATION OF INFANTS AND ADMINISTRATION OF HEPATITIS B IMMUNE GLOBULIN
United States Vaccine Policy
In the United States in 1991, the Advisory Committee on Immunization Practices (ACIP) published a comprehensive strategy for eliminating transmission in the United States through universal childhood. The strategy included recommendations for the prevention of perinatal HBV infection, universal vaccination of infants born to HBsAg-negative mothers, vaccination of adolescents, and vaccination of selected high-risk groups.37 The recommendations have been revised and expanded since 1991.
United States Universal Infant and Childhood and Adult Vaccination Policy
The 2005 ACIP recommendations38 state that infants born to mothers who are HBsAg-positive should receive hepatitis B vaccine and HBIG within the first 12 hours of birth. Infants born to mothers whose HBsAg status is unknown should receive hepatitis B vaccine in the first 12 hours after birth. The mother should have blood drawn as soon as possible to determine her HBsAg status; if she is HBsAg-positive, the infant should receive HBIG as soon as possible (no later than age 1 week). Full-term infants who are medically stable and weigh more than 2000 g born to HBsAg-negative mothers should receive single-antigen hepatitis B vaccine before hospital discharge. Preterm infants weighing less than 2000 g born to HBsAg-negative mothers should receive the first dose of vaccine 1 month after birth or at hospital discharge.38
After the birth dose, all infants should complete the hepatitis B vaccine series with either single-antigen vaccine or combination vaccine, according to a recommended vaccination schedule38 (Box 1). Postvaccination serologic testing consisting of HBsAg and anti-HBs should be ordered at age 9 to 12 months (or 1–2 months after the final dose of the vaccine series, if delayed) for infants born to HBsAg-positive mothers only,39 to enable prompt identification and revaccination of nonresponders who were exposed at birth.
Box 1. Hepatitis B (HepB) vaccine in the United States (minimum age: birth).
Routine vaccination
At birth:
Administer monovalent HepB vaccine to all newborns before hospital discharge.
For infants born to HBsAg-positive mothers, administer HepB vaccine and 0.5 mL of HBIG within 12 hours of birth. These infants should be tested for HBsAg and anti-HBs at age 9 to 12 months (preferably at the next well-child visit) or 1 to 2 months after completion of the HepB series if the series was delayed; see http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6439a6.htm.
If mother’s HBsAg status is unknown, within 12 hours of birth administer HepB vaccine regardless of birth weight.
For infants weighing less than 2000 g, administer HBIG in addition to HepB vaccine within 12 hours of birth. Determine the mother’s HBsAg status as soon as possible and, if the mother is HBsAg-positive, also administer HBIG for infants weighing 2000 g or more as soon as possible, but no later than age 7 days.
Doses following the birth dose
The second dose should be administered at age 1 or 2 months. Monovalent HepB vaccine should be used for doses administered before age 6 weeks.
Infants who did not receive a birth dose should receive 3 doses of a HepB-containing vaccine on a schedule of 0, 1 to 2 months, and 6 months starting as soon as feasible.
Administer the second dose 1 to 2 months after the first dose (minimum interval of 4 weeks), administer the third dose at least 8 weeks after the second dose and at least 16 weeks after the first dose. The final (third or fourth) dose in the HepB vaccine series should be administered no earlier than age 24 weeks.
Administration of a total of 4 doses of HepB vaccine is permitted when a combination vaccine containing HepB is administered after the birth dose.
Catch-up vaccination
Unvaccinated persons should complete a 3-dose series.
A 2-dose series (doses separated by at least 4 months) of adult formulation Recombivax HB® is licensed for use in children aged 11 through 15 years.
Data from http://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed August 15, 2016.
Catch-up Vaccination
All unvaccinated children and adolescents aged less than 19 years old should receive the hepatitis B vaccine series according to the hepatitis B catch-up schedule.40
Adult Vaccination
High-risk adults should also receive the 3 doses of hepatitis B vaccine41 (Box 2). High-risk adults include persons at risk for infection by sexual exposure, and persons at risk for infection by percutaneous or mucosal exposure to blood and other infectious fluids. Persons at risk of sexual exposure include sex partners of HBsAg-positive persons; sexually active persons who are not in a long-term, mutually monogamous relationship; persons seeking evaluation or treatment of a sexually transmitted disease; and men who have sex with men. Persons at risk for percutaneous exposure include current or recent injection-drug users; household contacts of HBsAg-positive persons; residents and staff of facilities for developmentally disabled persons; health care and public safety workers with reasonably anticipated risk for exposure to blood or blood-contaminated body fluids; persons with end-stage renal disease, including predialysis, hemodialysis, peritoneal dialysis, and home dialysis patients. Others who should receive this vaccine include international travelers to regions with high or intermediate levels (HBsAg prevalence of ≥ 2%) of endemic HBV infection, persons with chronic liver disease, persons with human immunodeficiency virus (HIV) infection, and all other persons seeking protection from HBV infection (Box 3).41
Box 2. HepB vaccination for adults aged 19 years or older.
Vaccinate any person seeking protection from HBV infection and persons with risk factors for HBV infection with the 3-dose series (http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf).
- Catch-up
-
○Administer missing doses to complete a 3-dose series of HepB vaccine to those persons not vaccinated or not completely vaccinated. The second dose should be administered at least 1 month after the first dose; the third dose should be administered at least 2 months after the second dose (and at least 4 months after the first dose). If the combined hepatitis A and HepB vaccine (Twinrix®) is used, give 3 doses at 0, 1, and 6 months; alternatively, a 4-dose Twinrix® schedule may be used, administered on days 0, 7, and 21 to 30, followed by a booster dose at 12 months.
-
○
Adult patients receiving hemodialysis or with other immunocompromising conditions should receive 1 dose of 40 μg/mL (Recombivax HB®) administered on a 3-dose schedule at 0, 1, and 6 months or 2 doses of 20 μg/mL (Engerix-B®) administered simultaneously on a 4-dose schedule at 0, 1, 2, and 6 months.
Box 3. Persons recommended to receive HepB vaccination by risk factor.
All infants, children and adolescents
Persons at risk for infection by sexual exposure
Sex partners of HBsAg-positive persons
Sexually active persons who are not in a long-term, mutually monogamous relationship (eg, persons with more than 1 sex partner during the previous 6 months)
Persons seeking evaluation or treatment of a sexually transmitted disease
Men who have sex with men
Persons at risk for infection by percutaneous or mucosal exposure to blood
Current or recent injection-drug users
Household contacts of HBsAg-positive persons
Residents and staff of facilities for developmentally disabled persons
Health care and public safety workers with reasonably anticipated risk for exposure to blood or blood-contaminated body fluids
Persons with end-stage renal disease, including predialysis, hemodialysis, peritoneal dialysis, and home dialysis patients
Adults with diabetes mellitus who are aged 19 through 59 years
Others
International travelers to regions with high or intermediate levels (HBsAg prevalence of > 2%) of endemic HBV infection
Persons with chronic liver disease
Persons with HIV infection
All other persons seeking protection from HBV infection
Over the next 4 years, 53,000 AN persons were tested for HBV serologic markers, which represented two-thirds of the statewide AN population and 90% of children and adults living in western Alaska; 40,000 serologically negative persons were vaccinated.46,47 In addition, in all hospitals, screening of pregnant women with HBsAg was initiated and infants of HBsAg-positive mothers received both HBIG and 3 doses of hepatitis B vaccination starting at birth, plus universal vaccination was initiated for all infants, starting with a birth dose. Since the mid-1990s, universal hepatitis B vaccination has been given to all newborns in Alaska, regardless of race or ethnicity, starting at birth, and there has been a catch-up program for all children through age 19 years consistent with the ACIP recommendations.
IMPACT OF VACCINATION GLOBALLY AND IN THE UNITED STATES
Impact and Current Status of Implementation of World Health Organization Recommendations
The 1991 EPI recommendations for universal hepatitis B vaccine at birth and other hepatitis B vaccination strategies have resulted in substantial reductions of HBV transmission in previously high endemic countries.2 In 2014, global coverage with 3 doses of hepatitis B vaccine is estimated at 82% (compared to 1% in 1990) and is as high as 92% in the Western Pacific.1 For example, the national Taiwan vaccine program, which started in 1984, has been 78% to 87% effective in reducing HBsAg seroprevalence in children.48 China has also made remarkable progress in increasing immunization coverage of hepatitis B vaccine. From 2005 to 2009, demonstration projects focused on promoting hospital delivery, strengthening collaboration between the EPI and the maternal and child health program, and developing strategies for both hospital and home births.49
By the end of 2014, hepatitis B vaccine had been introduced nationwide in 184 countries. Fig. 1 shows the geographic variation in coverage of the third dose of HBV vaccine in 2014. Fig. 2 shows the temporal trends in vaccination coverage for both the third dose and birth dose in the western Pacific region, and the marked increases in coverage that occurred in 2000 and 2004 respectively. A birth dose for hepatitis B vaccine was introduced in 96 countries by 2014, and global coverage was estimated at 38%, reaching 80% in the western Pacific, but only 10% in the African region.50 Data from 2012 also show that only 52% of countries recommend giving the first HBV vaccine dose within 24 hours of birth.51 Even fewer were administering HBIG to infants born to mothers infected with HBV. Such data represent a challenge for the goal of HBV elimination because nearly half of all births worldwide are estimated to occur in high HBV-endemic countries.
Fig. 1.

Immunization coverage with third dose of HepB vaccines in infants, 2014. (From WHO/UNICEF coverage estimates 2015 revision. Map production: Immunization Vaccines and Biologicals, (IVB). Geneva: World Health Organization; 2015.)
Fig. 2.
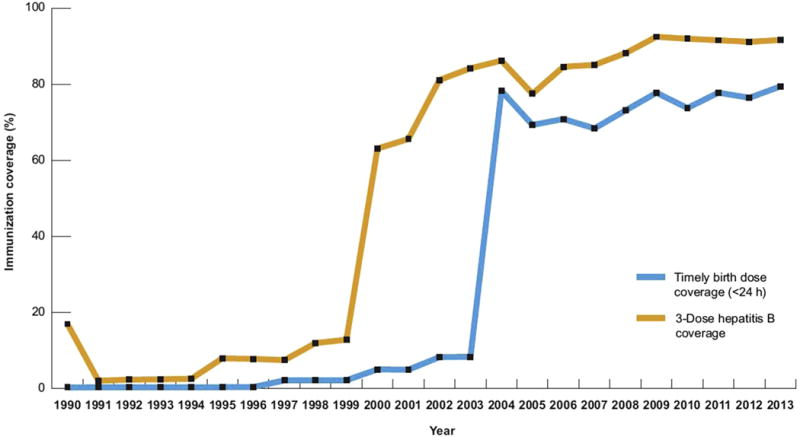
Immunization coverage with hepatitis B birth dose and third dose, western Pacific region, 1990 to 2013. (From World Health Organization. Hepatitis B control through immunization: a reference guide. Geneva: WHO Press, 2014. Available at: http://www.who.int/immunization/sage/meetings/2015/october/8_WPRO_Hepatitis_B_Prevention_Through_Immunization_Regional_Reference_Guide.pdf.)
At present, there is limited vaccination in low-income and middle-income countries of high-risk adults, particularly susceptible household and sexual contacts of HBsAg-positive persons identified in screening programs.
Current Vaccine Coverage and Impact in the United States
Children
The number of cases of HBV infection has decreased dramatically in the United States because of routine vaccination of infants. A 68% decrease in HBV infection prevalence among US children, regardless of country of origin, was observed within 10 years of initiation of universal hepatitis B vaccination in 1991.6 Forty-eight perinatal hepatitis B cases were reported.52 In 2014, vaccination coverage among children aged 19 to 35 months was 91.6% for greater than or equal to 3 doses and 72.4% for the birth dose (1 dose administered by 3 days of life).53 Vaccine coverage was 91.4% among adolescents age 13–17 years.54
High-risk adults
Adult vaccination coverage for hepatitis B in the United States is low. The 2014 National Health Interview Survey reported hepatitis B vaccination coverage (≥ 3 doses) among adults was 24.5% for adults aged greater than or equal to 19 years, 32.2% among adults aged 19 to 49 years, and 15.7% among adults aged greater than or equal to 50 years.55 Among adults aged 19 to 49 years, vaccination coverage was lower for black (29.9%) and Hispanic (20.2%) people compared with white people (36.3%). Among high-risk groups, vaccination coverage was 30.5% among adults aged greater than or equal to 19 years who had traveled outside the United States since 1995 to a country in which hepatitis B is of high or intermediate endemicity (regions other than the countries of Europe, Japan, Australia, New Zealand, or Canada); was 29.8% among adults aged greater than or equal to 19 years with chronic liver conditions; and 23.5% for those aged 19 to 59 years with diabetes mellitus (DM). The group with the highest hepatitis B vaccination coverage was health care providers (HCPs); 60.7% overall among HCP aged greater than or equal to 19 years with direct patient care responsibilities (70.9% among white HCPs and 56.6% among black HCPs).
The number of HBV infections among HCPs decreased substantially after hepatitis B vaccine was first recommended for HCPs in 1982 because of the implementation of routine preexposure vaccination and improved infection-control precautions.56 At present, all unvaccinated persons whose work-related and training-related activities involve reasonably anticipated risk for exposure to blood or other infectious body fluids should be vaccinated with the complete, ≥ 3-dose hepatitis B vaccine series and undergo postvaccination serologic testing to show protective antibody levels.57,58
Hepatitis B vaccination coverage among persons with DM has remained low since the recommendation in 2011 to vaccinate all unvaccinated adults with DM age 19–59 years and adults with DM age ≥ 60 years at the provider’s discretion. Infection-control lapses during assisted monitoring of blood glucose levels32 continue to occur, emphasizing the need to increase hepatitis B vaccination in this population.
Incidence of acute hepatitis B virus infection
Age specific prevalence of HBV vaccine immunity (anti-HBs) was estimated at about 25% of the US non-institutionalized population, based on the last NHANES survey from 2007–2012,6,9 largely due to routine vaccination policies in newborns, children and adults. Routine vaccination programs in newborns, children, and adults has also had a dramatic effect on reducing the transmission of HBV infection. Routine vaccination has had a dramatic effect on lowering transmission of HBV infection as well. One measure of vaccine impact is the incidence of acute symptomatic HBV in populations. The number of reported cases of acute hepatitis B decreased by 62%, from 8036 in 2000 to 2,953 (0.9/100,000) in 2014 (Fig. 3).16,52
Fig. 3.
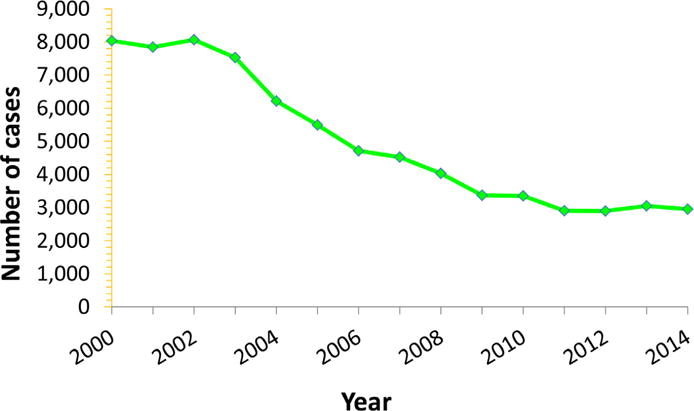
Reported number of acute hepatitis B cases in the United States, 2000 to 2014. (From Centers for Disease Control and Prevention. Viral hepatitis statistics and surveillance. Surveillance for viral hepatitis—United States, 2013. Atlanta (GA): US Department of Health and Human Services; 2015. Available at: http://www.cdc.gov/hepatitis/statistics/2013surveillance/index.htm; with permission.)
Impact of Alaskan vaccination program
In western Alaska, where one of the highest incidences of acute symptomatic HBV infection was found, mass vaccination of susceptible persons coupled with universal vaccination of newborns starting at birth resulted in a dramatic decrease in the annual incidence of acute icteric HBV from more than 200 in 100,000 in 1981 to 0 in 1995, where it has remained.46,57 The State of Alaska implemented universal infant vaccination for all newborns in 1993, followed by a catch-up vaccination program in children up to 20 years of age, coupled with required proof of HBV vaccination for school entry. The overall incidence of acute HBV in the State of Alaska has decreased to less than 1 in 100,000. In AN children in Alaska, the incidence of acute symptomatic HBV has decreased from 35 in 100,000 in 1983 to 0 in 1993 and no cases have been reported since then.46 Two countries in which the rates have fallen substantially due to vaccine implementation include Taiwan and Italy. In Taiwan, the incidence of acute symptomatic HBV in children has decreased significantly since the implementation of universal newborn vaccination in the 1980s.59 In Italy, hepatitis B vaccine was strongly recommended for high risk groups in 1984 and for 3 month olds and 12 years olds in 1991.60
Hepatitis B–related Mortality and Liver Cancer
An estimated 39,230 new cases of liver cancer are expected to occur in the United States during 2016; most will be HCC.61 Chronic infection with HBV or hepatitis C virus is the strongest risk factor for hepatocellular carcinoma, which is the most common type of liver cancer. In the United States, 10% to 15% of patients with HCC are infected with HBV.62 Prospective cohort studies have shown a 5-fold to 100-fold increase in the risk of developing HCC among persons chronically infected with HBV. However, the age distribution of patients with HCC has shifted to younger ages, with increases among persons 45 to 60 years old. HCC in children is rare.56 The number and rate of hepatitis B–related deaths overall has been stable for all age group in recent years (2010–2014), at ≤ 0.03 per 100,000 population for persons aged 0 to 34 years and increasing up to 1.8/100,000 in older age groups. In 2014, the overall hepatitis B-related mortality rate was 0.5 deaths/100,000 population (n=51,843).16
Incidence of Hepatocellular Carcinoma in Children
Although HCC is not common in children, vaccination programs from Alaska, Taiwan, and Thailand that target newborns and children studies have shown a significant decrease in the incidence of HCC. Before the vaccination campaign in Alaska, the incidence of HCC in children was the highest reported in the world, at 3 per 100,000. Since 1993, no cases of HCC in children caused by HBV have occurred, which is a result of the universal infant and child vaccination program.63 Furthermore, currently there are no AN children less than 20 years of age in Alaska known to be HBsAg-positive, showing that, with a concerted vaccination program, acute and chronic HBV can be eliminated from a generation. In Taiwan and Thailand, a reduction in the incidence of HCC in children was also shown 10 years after introduction of universal infant vaccination.64,65
Hepatocellular Carcinoma in Adults
After the successful implementation of a universal newborn vaccination program followed by a child catch-up program, there will be a several-decade gap before the largest impact on the reduction of the serious sequelae of HBV takes place. The incidence of HCC and decompensated cirrhosis increases slowly in persons infected at birth or early childhood then accelerates exponentially when infected persons reach their late 30s and early 40s. Goldstein and colleagues36 developed a model to display this graphically (Fig. 4). In order to affect the incidence of these serious complications sooner, programs are needed to identify persons infected with HBV, link them to regular care, and intervene with antiviral therapy in those eligible based on current guidelines.66,67 With currently licensed and recommended antiviral medications (tenofovir and entecavir), HBV cannot be cured, but many new drugs are in clinical development that target different sites in the HBV life cycle and offer potential for a curative strategy.68
Fig. 4.
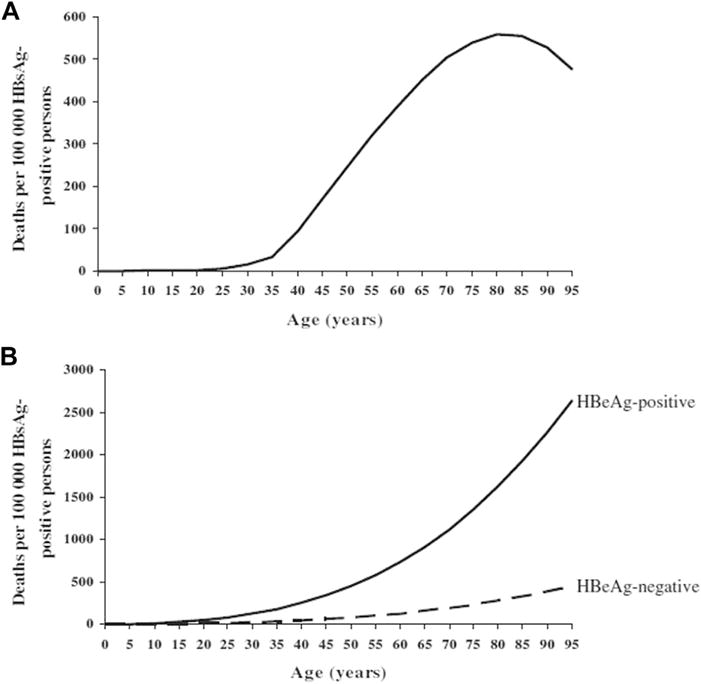
Mathematical model: age-specific hepatitis B–related cirrhosis (A) and HCC (B) mortality. (From Goldstein ST, Zhou FJ, Hadler SC, et al. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 2005;34:1329–39; with permission.)
DISCUSSION
Challenges in Implementation of Birth Dose Vaccination in Low-Income and Middle-Income Countries
There are several reasons why many low-resource countries are having difficulty implementing birth dose vaccination within 24 hours of birth. These include:
Out-of-hospital births are common in countries with high HBV endemicity, and home deliveries are frequently handled by traditional birth attendants, most of whom have no formal training.
In many resource-limited settings, particularly in Africa and Asia, HBV vaccine is only available in a pentavalent preparation (with diphtheria, pertussis, tetanus, and Haemophilus influenzae B) and not as a monovalent vaccine for use in newborn babies. The GAVI Alliance (formerly Global Alliance for Vaccines and Immunisation) no longer supports the monovalent vaccine, but only the pentavalent one, which cannot be given in the first 6 weeks of life and is therefore not useful as the birth dose vaccine.
Despite extensive evidence that hepatitis B vaccine is extremely safe for neonates and infants, many health care workers still hold misconceptions regarding contraindications for hepatitis B birth dose vaccination.
Of note, HBIG is also not available or feasible in many low-income countries because its storage requires a cold chain and complex production.2
Challenges in Vaccinating Adults at High Risk of Acquiring Hepatitis B Virus as Adults
In the United States, adult vaccination coverage rates are low overall,62 and this has been attributed to multiple factors, such as limited awareness among the public of the benefit of adult vaccination, inadequate needs assessment for vaccination in adult patient care, payment challenges for patients and providers, and limited stock in health provider offices. Strategies to improve adult immunization in persons at risk for HBV infection and for whom hepatitis B vaccine is recommended include clinical decision support tools, standing orders or protocols for vaccination, and provider and patient reminders. Provider recommendation and convenient access to vaccination are also important.69
In the United States, a recent National Academy of Sciences report concluded that control of hepatitis B is feasible, but that eliminating hepatitis B disease will require resources, public support, and addressing barriers.8 Barriers to elimination identified include surveillance, vaccine tracking, stigma, difficulty reaching foreign-born adults, overworked primary care providers, and need to better understand HBV and management of chronic infection.
Future Goals in Prevention of Mother-to-child Transmission and Vaccination
Some of the key strategies to address current barriers to implementation of the birth dose include the following (summarized in Box 4)49:
Ensuring the provision of monovalent HBV vaccine at an affordable price in low-income countries with high HBV endemicity, which may require further support from GAVI.
Storing the HBV vaccine out of the cold chain, which is possible for up to a month (2006 WHO operational field guidelines). The availability of monovalent vaccine in prefilled, single-dose injection devices can also facilitate the administration of the vaccine by birth attendants to infants delivered at home.70
In addition, collaboration with maternal and child health programs to promote access to skilled birth attendants and postnatal care is critical, together with engagement of community leaders to increase access to available outreach services. Ensuring population confidence in the safety of the vaccine is also important to achieve high vaccine coverage. Education and training of health workers along with communications targeted at the community are needed to address concerns about the timing and safety of administering vaccine immediately after birth and about false contraindications.
Box 4. Strategies to improve implementation of birth dose and infant vaccination.
Service delivery arrangements
Encourage health-facility deliveries through subsidized deliveries, provide education to mothers during antenatal care, and enhance links with communities.
Issue standing orders for birth dose vaccination in all health facilities.
Orientate health and administrative staff on the HBV policy.
- Integrate birth dose with maternal and newborn care in health facilities by:
-
○Ensuring vaccine is available in the delivery room or postnatal ward
-
○Establishing clear health-facility policies on where/when/who is to vaccinate
-
○Positioning birth dose vaccination as part of essential newborn care package
-
○Providing supportive supervision
-
○
Ensure private facilities provide birth dose vaccination.
- Where infants are born outside health facilities:
-
○Conduct home visits to provide timely vaccination
-
○Integrate timely birth dose with home visits for other early postnatal care
-
○Store vaccine outside the standard cold chain in controlled temperature chain
-
○Engage village health volunteers to inform health facility of all home births
-
○
Health workforce considerations
Conduct well-structured training for health workers, including education on perinatal transmission, backed up by frequent follow-up and supportive supervision.
Health information system strengthening
Maintain birth registries and community birth notification, including tracking home births.
Incorporate birth dose and its timing within vaccination records.
Use accurate definition of timely birth dose in coverage reporting.
Education
Address community concerns or lack of knowledge regarding birth dose.
Address fear of adverse events, including planning for the risk of coincidental newborn death or disease.
Respond to parental refusal of vaccination.
Data from Practices to improve coverage of the hepatitis B birth dose vaccine. Geneva (Switzerland): World Health Organization; 2012. WHO/IVB/12.11. Available at: http://www.who.int/immunization/documents/control/who_ivb_12.11/en/. Accessed May 13, 2016.
The key strategies for control of the HBV epidemic are a combination of universal infant vaccination with three doses of hepatitis B vaccine in the first year of life, together with diagnosis of mothers at high risk of transmitting HBV, those HBeAg-positive or having a level of HBV DNA > 200,000 IU/ml in their sera, and use of antiviral agents in the late first or early second trimester to decrease maternal DNA concentrations to undetectable concentrations.
Use of Antiviral Therapy in Women with High Viral Loads
Despite timely administration of HBIG and hepatitis B vaccine starting immediately after birth, 5% to 10% of newborns whose mothers have very high levels of HBV DNA can still acquire HBV infection and develop chronic HBV.71 Recently, the American Association for the Study of Liver Diseases (AASLD) conducted a systemic review of the evidence that administering antiviral therapy in the third trimester reduces the incidence of HBV transmission to women with very high levels of HBV DNA.72 From this review, the AASLD, in their updated guideline that used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, recommended that antiviral therapy be administered to HBsAg-positive pregnant mothers in the third trimester whose levels of HBV DNA exceed 200,000 IU/mL73 but WHO HBV guidelines have not yet included such a recommendation on use of antiviral therapy.67 Although lamivudine and telbivudine are both efficacious and considered safe in pregnancy (though long-term safety data are lacking), tenofovir is the drug of choice based on both its potency and high barrier to resistance.
SUMMARY
The hepatitis B vaccine which has been proven to be safe and effective with protection after the vaccine series estimated to persist for at least 30 years among greater than 90% of persons vaccinated,74 is a key component of chronic hepatitis B prevention and eventual elimination. Routine infant vaccination in over 180 countries has resulted in a reduction in global HBV transmission and declines in chronic HBV prevalence.1,2 Particular success in reduction of HBV burden from high to low endemicity was achieved in Taiwan and Alaska, US and serve as examples for other areas.2,75
Substantial obstacles to global hepatitis B vaccination have been overcome since it was introduced. For example, the cost of vaccine has decreased, the GAVI Alliance has provided targeted assistance to specific countries and implementation of infant immunization programs has broadened.76 However, many barriers still need to be overcome to achieve elimination of hepatitis B, such as sustained support in developing countries, improved identification of hepatitis B positive mothers, improved implementation of hepatitis B infant vaccination programs including the timely administration of the birth dose, increasing facility-based births to improve access to vaccination, improved cold-chain management for vaccine storage and transport, further exploration of outside the cold chain storage of vaccine,77 and reducing horizontal transmission among high risk adults. Integrating hepatitis B vaccination with global health initiatives may help sustain existing hepatitis B vaccination programs.78 Integrating hepatitis B vaccination with screening, care and treatment might also promote general hepatitis B prevention, particularly among pregnant women and is essential for hepatitis Belimination.60,71
KEY POINTS.
The Global Advisory Group of the Expanded Programme on Immunization recommendations to integrate hepatitis B vaccination into national immunization programs have resulted in substantial reductions of hepatitis B virus (HBV) transmission in previously high endemic countries.
A 68% decrease in HBV infection prevalence among US children, regardless of country of origin, was observed within 10 years of initiation of universal hepatitis B vaccination.
The key strategy for control of the HBV epidemic is universal infant vaccination with administration of birth dose and 3 doses of hepatitis B vaccine in the first year of life. Additional measures include use of hepatitis B immunoglobulin (HBIG) and together with diagnosis of mothers at high risk of transmitting HBV with use of antiviral agents in the late first or early second trimester to decrease maternal DNA concentrations to undetectable concentrations.
Despite the substantial decrease in HBV cases since vaccination introduction, implementation of birth dose vaccination in low-income and middle-income countries and vaccination of high-risk adults remain challenging.
Alaska Vaccination Program.
In the 1970s epidemiologic studies found that HBV infection was endemic in western Alaska. Rates of acute icteric HBV infection were more than 200 per 100,000 and the prevalence of chronic HBV was 6% to 8%.42 Furthermore, the incidence of hepatocellular carcinoma (HCC) was the highest in the United States at the time and was especially high in children and young adults.43 Five different HBV genotypes were found in Alaska and, because of that, transmission was predominantly perinatal in northwest Alaska, where genotype C predominated, similar to what is observed in China and southeast Asia, but there was horizontal child to child transmission in southwest Alaska, similar to the predominant mode of transmission in Africa.26,44 Studies from Alaska found that persons infected with genotype C on average cleared HBeAg after reaching their mid-40’s and thus most were positive throughout most of their childbearing years. In contrast most of those infected with genotypes A, B6, D and F cleared HBeAg in the teens and early 20’s early in their childbearing years.44 The approach to interrupting transmission therefore had to address both the perinatal and horizontal routes. To accomplish this, a combination of vaccination starting at birth and 2 further doses, coupled with a rapid catch-up program was aimed at children as well as adults. The rationale for including adults was based on studies that showed that although children were passing the infection through open cuts and scratches, a large component of transmission in adults was sexual.26,31
The program began in 1980 with screening of pregnant women for HBsAg in the 2 largest hospitals serving Alaska Native (AN) persons in the state and administering HBIG at birth, 1 month, and 3 months to infants born of HBsAg-positive mothers. In 1981 and 1982, a trial with plasma-derived vaccine (Heptavax) was conducted in 16 villages in western Alaska in adults and children more than 6 months of ages and showed that this vaccine was highly efficacious in preventing transmission.45 In 1984, funding was obtained to start a statewide comprehensive program designed to completely eradicate transmission of HBV in the AN population.
Footnotes
Disclosure: The authors have nothing to disclose.
References
- 1.World Health Organization. Hepatitis B. Available at: www.who.int/topics/hepatitis/factsheets/en. Accessed June 22, 2016.
- 2.WHO position paper on hepatitis B vaccines - October 2009. Wkly Epidemiol Rec. 2009;84:405–20. [Google Scholar]
- 3.Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco E, Bagnato B, Marino MG, et al. Hepatitis B: epidemiology and prevention in developing countries. World J Hepatol. 2012;4:74–80. doi: 10.4254/wjh.v4.i3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasley A, Kruszon-Moran D, Kuhnert W, et al. The prevalence of hepatitis B infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 7.Cohen C, Evans AA, London WT, et al. Underestimation of chronic hepatitis B infection in the United States of America. J Viral Hepat. 2008;15:12–3. doi: 10.1111/j.1365-2893.2007.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Academies of Sciences, Engineering, and Medicine. Eliminating the public health problem of hepatitis B and C in the United States: Phase one report. Washington, DC: The National Academies Press; 2016. [PubMed] [Google Scholar]
- 9.Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology. 2016;63(2):388–97. doi: 10.1002/hep.28109. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell T, Armstrong GL, Hu DJ, et al. The increasing burden of imported chronic hepatitis B—United States, 1974–2008. PLoS One. 2011;6:e27717. doi: 10.1371/journal.pone.0027717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine (US) Committee on the Prevention and Control of Viral Hepatitis Infections. In: Grossblatt N, editor. Hepatitis and liver cancer: national strategy for prevention and control of hepatitis B and C. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 12.Weinbaum CM, Williams I, Mast EE, et al. Centers for Disease Control and Prevention (CDC) Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 13.Kowdley KV, Wang C, Welch S, et al. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–33. doi: 10.1002/hep.24804. [DOI] [PubMed] [Google Scholar]
- 14.Available at: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Accessed August 15, 2016
- 15.Centers for Disease Control and Prevention. Asian Americans and hepatitis B. 2013 Available at: http://www.cdc.gov/features/aapihepatitisb/. Accessed May 1, 2015.
- 16.CDC. Surveillance for viral hepatitis—United States, 2014. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. Available at: http://www.cdc.gov/hepatitis/statistics/2014surveillance/index.htm. [Google Scholar]
- 17.Harris AM, Iqbal K, Schillie S, et al. Increases in acute hepatitis B virus infections - Kentucky, Tennessee, and West Virginia, 2006–2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):47–50. doi: 10.15585/mmwr.mm6503a2. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Update: recommendations to prevent hepatitis B virus transmission-United States. MMWR Morb Mortal Wkly Rep. 1995;44:574–5. [PubMed] [Google Scholar]
- 19.Edmunds WJ, Medley GF, Nokes DJ, et al. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253:197–201. doi: 10.1098/rspb.1993.0102. [DOI] [PubMed] [Google Scholar]
- 20.Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992–1000. doi: 10.1093/clinids/20.4.992. [DOI] [PubMed] [Google Scholar]
- 21.Available at: http://www.cdc.gov/hepatitis/Resources/Professionals/PDFs/ABCTable.pdf. Accessed August 15, 2016.
- 22.Beasley RP, Trepo C, Stevens CE, et al. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol. 1977;105:94–8. doi: 10.1093/oxfordjournals.aje.a112370. [DOI] [PubMed] [Google Scholar]
- 23.McMahon BJ. Two key components to address chronic hepatitis B in children: detection and prevention. J Pediatr. 2015;167:1186–7. doi: 10.1016/j.jpeds.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Marinier E, Barrois V, Larouze B, et al. Lack of perinatal transmission of hepatitis B virus infection in Senegal, West Africa. J Pediatr. 1985;106:843–9. doi: 10.1016/s0022-3476(85)80371-1. [DOI] [PubMed] [Google Scholar]
- 25.Beasley RP, Hwang LY, Lee GC, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099–102. doi: 10.1016/s0140-6736(83)90624-4. [DOI] [PubMed] [Google Scholar]
- 26.McMahon BJ, Alward WL, Hall DB, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 27.Beasley RP, Hwang LY, Lin CC, et al. Incidence of hepatitis B virus infections in preschool children in Taiwan. J Infect Dis. 1982;146:198–204. doi: 10.1093/infdis/146.2.198. [DOI] [PubMed] [Google Scholar]
- 28.Coursaget P, Yvonnet B, Chotard J, et al. Age- and sex-related study of hepatitis B virus chronic carrier state in infants from an endemic area (Senegal) J Med Virol. 1987;22:1–5. doi: 10.1002/jmv.1890220102. [DOI] [PubMed] [Google Scholar]
- 29.Petersen NJ, Barrett DH, Bond WW, et al. Hepatitis B surface antigen in saliva, impetiginous lesions, and the environment in two remote Alaskan villages. Appl Environ Microbiol. 1976;32:572–4. doi: 10.1128/aem.32.4.572-574.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bond WW, Favero MS, Petersen NJ, et al. Survival of hepatitis B virus after drying and storage for one week. Lancet. 1981;1:550–1. doi: 10.1016/s0140-6736(81)92877-4. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Recommendations for preventing transmission of human immunodeficiency virus and hepatitis B virus to patients during exposure-prone invasive procedures. MMWR Recomm Rep. 1991;40(RR-8):1–9. [PubMed] [Google Scholar]
- 32.Available at: http://www.cdc.gov/hepatitis/outbreaks/pdfs/healthcareinvestigationtable.pdf. Accessed August 15, 2016.
- 33.Hepatitis B control through immunization: a reference guide. Available at: http://www.who.int/immunization/sage/meetings/2015/october/8_WPRO_Hepatitis_B_Prevention_Through_Immunization_Regional_Reference_Guide.pdf. Accessed August 15, 2016.
- 34.Introduction of hepatitis B vaccine into childhood immunization services. Management guidelines, including information for health workers and parents. Available at: http://www.wpro.who.int/hepatitis/whovb0131.pdf. Accessed May 13, 2016.
- 35.Goldstein ST, Zhou FJ, Hadler SC, et al. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 36.WHO global plan of action on workers’ health (2008–2017) baseline for implementation. Available at: http://www.who.int/occupational_health/who_workers_health_web.pdf. Accessed August 15, 2016.
- 37.Forde KA, Tanapanpanit O, Reddy KR. Hepatitis B and C in African Americans: current status and continued challenges. Clin Gastroenterol Hepatol. 2014;12(5):738–48. doi: 10.1016/j.cgh.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1–31. [PubMed] [Google Scholar]
- 39.Schillie S, Murphy TV, Fenlon N, et al. Update: shortened interval for postvaccination serologic testing of infants born to hepatitis B-infected mothers. MMWR Morb Mortal Wkly Rep. 2015;64(39):1118–20. doi: 10.15585/mmwr.mm6439a6. [DOI] [PubMed] [Google Scholar]
- 40.Available at: http://www.cdc.gov/vaccines/schedules/index.html. Accessed August 15, 2016.
- 41.Mast EE, Weinbaum CM, Fiore AE, et al. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC) A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55(RR-16):1–33. [quiz: CE1–4; Erratum appears in MMWR Morb Mortal Wkly Rep 2007;56(42):1114] [PubMed] [Google Scholar]
- 42.Schreeder MT, Bender TR, McMahon BJ, et al. Prevalence of hepatitis B in selected Alaskan Eskimo villages. Am J Epidemiol. 1983;118:543–9. doi: 10.1093/oxfordjournals.aje.a113659. [DOI] [PubMed] [Google Scholar]
- 43.Lanier AP, McMahon BJ, Alberts SR, et al. Primary liver cancer in Alaskan natives. 1980–1985. Cancer. 1987;60:1915–20. doi: 10.1002/1097-0142(19871015)60:8<1915::aid-cncr2820600841>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Livingston SE, Simonetti JP, Bulkow LR, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology. 2007;133:1452–7. doi: 10.1053/j.gastro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Heyward WL, Bender TR, McMahon BJ, et al. The control of hepatitis B virus infection with vaccine in Yupik Eskimos. Demonstration of safety, immunogenicity, and efficacy under field conditions. Am J Epidemiol. 1985;121:914–23. doi: 10.1093/oxfordjournals.aje.a114061. [DOI] [PubMed] [Google Scholar]
- 46.McMahon BJ, Rhoades ER, Heyward WL, et al. A comprehensive programme to reduce the incidence of hepatitis B virus infection and its sequelae in Alaskan natives. Lancet. 1987;2:1134–6. doi: 10.1016/s0140-6736(87)91557-1. [DOI] [PubMed] [Google Scholar]
- 47.McMahon BJ, Schoenberg S, Bulkow L, et al. Seroprevalence of hepatitis B viral markers in 52,000 Alaska natives. Am J Epidemiol. 1993;138:544–9. doi: 10.1093/oxfordjournals.aje.a116888. [DOI] [PubMed] [Google Scholar]
- 48.Peng CY, Chien RN, Liaw YF. Hepatitis B virus-related decompensated liver cirrhosis: benefits of antiviral therapy. J Hepatol. 2012 Aug;57(2):442–50. doi: 10.1016/j.jhep.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 49.Expanded Programme on Immunization (EPI) of the Department of Immunization, Vaccines and Biologicals. Practices to improve coverage of the hepatitis B birth dose vaccine. Geneva (Switzerland): World Health Organization; 2012. WHO/IVB/12.11. Available at: http://www.who.int/immunization/documents/control/who_ivb_12.11/en/. Accessed May 13, 2016. [Google Scholar]
- 50.WHO/UNICEF coverage estimates 2014 revision. 2015 Available at: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragebcg.html. Accessed August 15, 2016.
- 51.Centers for Disease Control and Prevention (CDC) Global routine vaccination coverage–2012. MMWR Morb Mortal Wkly Rep. 2013;62(43):858–61. [PMC free article] [PubMed] [Google Scholar]
- 52.Adams D, Fullerton K, Jajosky R, et al. Summary of notifiable infectious diseases and conditions - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;62(53):1–122. doi: 10.15585/mmwr.mm6253a1. [DOI] [PubMed] [Google Scholar]
- 53.Hill HA, Elam-Evans LD, Yankey D, et al. National, state, and selected local area vaccination coverage among children aged 19–35 months - United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(33):889–96. doi: 10.15585/mmwr.mm6433a1. [DOI] [PubMed] [Google Scholar]
- 54.Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years–United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784–92. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations - United States, 2014. MMWR Surveill Summ. 2016;65(1):1–36. doi: 10.15585/mmwr.ss6501a1. [DOI] [PubMed] [Google Scholar]
- 56.Alter MJ, Hadler SC, Margolis HS, et al. The changing epidemiology of hepatitis B in the United States. Need for alternative vaccination strategies. JAMA. 1990;263:1218–22. [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60(RR-7):1–45. [PubMed] [Google Scholar]
- 58.Schillie S, Murphy TV, Sawyer M, et al. CDC guidance for evaluating healthcare personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep. 2013;62(RR-10):1–19. [PubMed] [Google Scholar]
- 59.Chien Y-C, Jan C-F, Kuo H-S, et al. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev. 2006;28:126–35. doi: 10.1093/epirev/mxj010. [DOI] [PubMed] [Google Scholar]
- 60.Stroffolini T, Mele A, Tosti ME, et al. The impact of the hepatitis B mass immunisation campaign on the incidence and risk factors of acute hepatitis B in Italy. J Hepatol. 2000;33:980–5. doi: 10.1016/s0168-8278(00)80132-4. [DOI] [PubMed] [Google Scholar]
- 61.Cancer facts and figures. 2016 Available at: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed August 15, 2016.
- 62.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McMahon BJ, Bulkow LR, Singleton RJ, et al. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54:801–7. doi: 10.1002/hep.24442. [DOI] [PubMed] [Google Scholar]
- 64.Wichajarn K, Kosalaraksa P, Wiangnon S. Incidence of hepatocellular carcinoma in children in Khon Kaen before and after National Hepatitis B Vaccine Program. Asian Pac J Cancer Prev. 2008;9:507–9. [PubMed] [Google Scholar]
- 65.Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N Engl J Med. 1997;336:1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 66.Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. 2015 Available at: http://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/. Accessed August 15, 2016. [PubMed]
- 68.Liang TJ, Block TM, McMahon BJ, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology. 2015;62:1893–908. doi: 10.1002/hep.28025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bridges CB, Hurley LP, Williams WW, et al. Meeting the challenges of immunizing adults. Am J Prev Med. 2015;49:S455–64. doi: 10.1016/j.amepre.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 70.Sutanto A, Suarnawa IM, Nelson CM, et al. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Organ. 1999;77(2):119–26. [PMC free article] [PubMed] [Google Scholar]
- 71.Chen HL, Lin LH, Hu FC, et al. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology. 2012;142:773–81.e2. doi: 10.1053/j.gastro.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 72.Brown RS, Jr, McMahon BJ, Lok AS, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatology. 2016;63:319–33. doi: 10.1002/hep.28302. [DOI] [PubMed] [Google Scholar]
- 73.Terrault NA, Bzowej NH, Chang K, et al. AASLD Guidelines for Treatment of chronic Hepatitis B. Hepatology. 2015 doi: 10.1002/hep.28156. Availabe at: https://www.aasld.org/sites/default/files/guideline_documents/hep28156.pdf. Accessed September 7, 2016. [DOI] [PMC free article] [PubMed]
- 74.Bruce MG, Bruden D, Hurlburt D, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016;214(1):16–22. doi: 10.1093/infdis/jiv748. [DOI] [PubMed] [Google Scholar]
- 75.Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27(47):6550–7. doi: 10.1016/j.vaccine.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 76.Hadler SC, Fuqiang C, Averhoff F, et al. The impact of hepatitis B vaccine in China and in the China GAVI Project. Vaccine. 2013;31(Suppl 9):J66–72. doi: 10.1016/j.vaccine.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 77.Kolwaite AR, Xeuatvongsa A, Ramirez-Gonzalez A, et al. Hepatitis B vaccine stored outside the cold chain setting: a pilot study in rural Lao PDR. Vaccine. 2016;34(28):3324–30. doi: 10.1016/j.vaccine.2016.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van Damme P, Ward J, Shouval D, et al. Hepatitis B vaccines. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. China: Saunders; 2012. pp. 183–204. [Google Scholar]


