Abstract
The δ-protocadherins comprise a small family of homophilic cell adhesion molecules within the larger cadherin superfamily. They are essential for neural development as mutations in these molecules give rise to human neurodevelopmental disorders, such as schizophrenia and epilepsy, and result in behavioral defects in animal models. Despite their importance to neural development, a detailed understanding of their mechanisms and the ways in which their loss leads to changes in neural function is lacking. However, recent results have begun to reveal roles for the δ-protocadherins in both regulation of neurogenesis and lineage-dependent circuit assembly, as well as in contact-dependent motility and selective axon fasciculation. These evolutionarily conserved mechanisms could have a profound impact on the robust assembly of the vertebrate nervous system. Future work should be focused on unraveling the molecular mechanisms of the δ-protocadherins and understanding how this family functions broadly to regulate neural development.
Keywords: protocadherins, adhesion, neurogenesis, development
1. Introduction
The vertebrate brain is remarkable in two apparently contradictory regards. First, the brain exhibits tremendous diversity at the cellular and synaptic levels, and can appear as a chaotic and unintelligible tangle. At the same time, the overall architecture of the brain is remarkably consistent and orderly, both within and across species. A fundamental issue in neurodevelopmental research is to understand the mechanisms that both generate a stereotyped organization, and tolerate and utilize variability. A requirement for recognition mechanisms that determine correct synaptic wiring has been inferred from the tremendous number of connections and their perceived precision. However, as there are a vast number of brain configurations consistent with normal function, it may be the case that development is concerned primarily with establishing the overall architecture and does not need to specify small scale variations. Thus, much of the apparent micro-heterogeneity may provide the substrate for selection during developmental experience-dependent refinement, facilitate changes through evolution, or constitute developmental noise. In this view, the mechanisms responsible for generating the stereotyped features of the nervous system are the most directly relevant for understanding neural network assembly. Two such mechanisms include lineage-based assembly of neuronal modules from sibling neurons and selective axon outgrowth and fasciculation. As outlined below, the δ-protocadherins (pcdhs) play important roles in both of these processes.
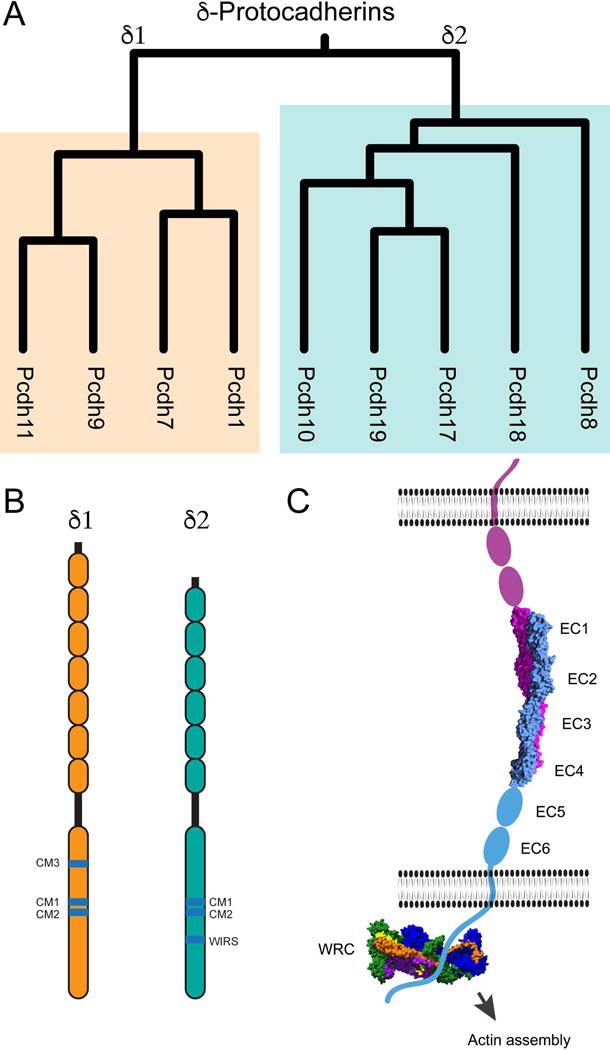
The δ-pcdhs are a small family of non-clustered protocadherins within the larger cadherin superfamily [1, 2]. In mammals, this family consists of 10 genes (Figure 1A). While linked by the presence of common sequence motifs in their intracellular domains, the δ-pcdhs can be further divided into δ1 and δ2 subgroups [3], which have 7 and 6 extracellular cadherin (EC) repeats, respectively (Figure 1B). Expression studies in rodent and zebrafish show that δ-pcdhs are present in all major subdivisions of the developing nervous system [3–6]. Some evidence suggests that different family members are expressed in complementary patterns [7–9], although there can be some overlap [6]. Analogous to their cousins, the classical cadherins, both the δ1- and δ2-pcdhs can mediate homophilic adhesion (discussed below), albeit more weakly. The homophilic preference of the δ-pcdhs (Figure 1C) and their strong expression in the developing nervous system make them intriguing candidates to sculpt patterns of connectivity during neural development. This potential is emphasized by the fact that mutations in δ-pcdhs are increasingly being identified in human neurodevelopmental disorders and experiments are revealing roles for them in directing both local and inter-regional connectivity. Here, we discuss the importance of the δ-pcdhs to neural development, highlighting some new insights into their cellular and molecular functions.
Figure 1. δ-protocadherins.
A. Phylogenetic tree of the human δ-protocadherins, showing the two subfamilies, δ1 (on the left in orange) and δ2 (on the right in green).
B. Domain organization of the δ-pcdhs. The δ1-pcdhs differ from the δ2-pcdhs, as they have 7 extracellular cadherin repeats, rather than 6. In addition, the δ1-pcdhs have conserved motif 3 (CM3) that is absent in the δ2-pcdhs, while the δ2-pcdhs and Pcdh9 have the WIRS motif that is absent in Pcdh1, Pcdh7 and Pcdh11.
C. Structural organization of δ2-pcdhs. Shown is a model based on the crystal structures of the Pcdh19 EC1-4 fragment (Cooper et al., 2016) and the WAVE complex associated with the WIRS peptide (Chen et al., 2014). It appears that the δ2-pcdhs mediate adhesion through the overlap of EC1-4, which differs from the mechanism used by classical cadherins.
2. δ-protocadherins in neurodevelopmental disorders
2.1 Human genetics
A central goal in neural development research is to understand the relationships between genetic mutations, altered development, and neural disorders. In this regard, the δ-pcdhs are emerging as an important family of genes, as accumulating data reveal their involvement in an array of neurodevelopmental disorders, including schizophrenia, autism, intellectual disability, and epilepsy [1, 10]. PCDH19 (δ2 subgroup) provides the clearest case for the involvement of a δ-pcdh in a human neurodevelopmental disorder, as mutations in this gene cause an X-linked, female-limited form of infant-onset epilepsy. First reported by Dibbens et al. (2008), females harboring mutations in PCDH19 suffer seizures as early as 6 months of age, which can persist through early childhood [11]. Subsequently, numerous mutations in PCDH19 have been identified in patients, making PCDH19 Female-limited epilepsy (PCDH19 FE) one of the most prevalent genetic forms of epilepsy [12]. In addition to epilepsy, mis-sense mutations in PCDH19 were found by targeted re-sequencing of X-chromosome synaptic genes in ASD and schizophrenia patients [13]. This suggests a broader clinical relevance for PCDH19 and is consistent with the observations that many developmental genes can act as risk factors for multiple disorders, and that autism, schizophrenia, and epilepsy exhibit high degrees of comorbidity [14].
PCDH19 FE exhibits an unusual pattern of transmission [15]. Although PCDH19 is present on the X-chromosome, females are affected while males are spared. This appears to be due to mosaicism resulting from X-inactivation: cells expressing the “good” copy of PCDH19 are interspersed with those lacking a “good” copy. The resulting mosaicism is more disruptive than the complete inactivation of the gene, a phenomenon which is supported by the identification of affected males that acquired spontaneous somatic mutations in PCDH19 and are mosaic for PCDH19 loss [16, 17]. It remains unknown why mosaicism leads to more severe phenotypes, although the idea of “cellular interference” has been proposed to explain this phenomenon [18]. In this model, cells harboring a mutant copy of PCDH19 have a dominant-interfering effect on development, as they interact with and disrupt normal activity of wild type cells. While cellular mosaicism is clearly more troublesome than a complete loss of PCDH19, both the exact cellular and developmental events being affected and the precise mechanism of cellular interference remain to be determined.
In addition to PCDH19, other members of the δ-pcdh family are increasingly implicated in human neural disorders. Recently, a patient was described who harbors a large chromosomal deletion encompassing PCDH18 (δ2 subgroup) and exhibits intellectual disability, microcephaly, and seizures, among other features [19]. In addition, mutations in PCDH12, which has a protocadherin ectodomain, but lacks the conserved, intracellular sequence motifs of the δ1 or δ2 subgroups, were shown to cause congenital microcephaly and an associated developmental disability [20]. Moreover, a preliminary investigation showed that a single nucleotide polymorphism (SNP) in the sixth EC repeat of PCDH12 had a tendency to associate with cortical asymmetry in schizophrenic patients [21]. PCDH7 (δ1 subgroup) was identified as a downstream target of MeCP2 [22], the causative gene in Rett syndrome, a neurological disorder with a wide range of symptoms including developmental delay, cognitive and behavioral impairment, and seizures. In addition, a micro-deletion of PCDH7 was found in a patient with juvenile myoclonic epilepsy, supporting a role for PCDH7 in generalized, genetic epilepsies [23]. In an investigation of inherited genes related to ASD, Morrow et al. (2008) found that PCDH10 (δ2 subgroup) was the closest gene to the second largest homozygous deletion site (300kbp) identified in one of their patients [24]. Inherited duplication of part of PCDH11X (δ1 subgroup) gene was found in a patient with recurrent seizures [25]. Chang et al. (2017) identified PCDH17 (δ2 subgroup) in a genome wide association study as a novel risk candidate gene for mood disorders, including major depressive disorder and bipolar disorder [26]. SNPs in PCDH17 were associated with vulnerable personality traits, decreased amygdala volume, and increased amygdala activity in response to negative stimuli. Analysis of frontal cortex as well as induced pluripotent stem cell (iPSC) cultures from BPD patients carrying the risk allele showed higher levels of PCDH17 expression. Thus, the δ-pcdhs appear to be essential for human brain development.
2.2 Behavioral defects in animal models
In addition to the mounting human data, animal models are also identifying functional and behavioral defects in δ-pcdh mutants. Analysis of Pcdh9 (δ1 subgroup) mutant mice reveals defects in object and social recognition, as well as sensorimotor development [27]. These behavioral phenotypes are accompanied by a reduction of Pcdh9+ neurons in layers 5/6 of somatosensory cortex, in addition to reduced complexity of dendritic arbors and increased spine density of pyramidal neurons. Reminiscent of these results with Pcdh9, male mice heterozygous for Pcdh10 exhibit a deficit in social approach behavior, which is accompanied by reduced transmission of gamma oscillations in the amygdala [28]. Additionally, amygdala neurons exhibit an increase in dendritic filopodia, as well as a reduction in NMDA receptor levels, suggesting alterations in amygdala circuitry. Hoshina et al. (2013) investigated a knockout mouse model of Pcdh17 [8]. While the neuronal organization of basal ganglia circuits was not obviously disrupted, knockouts showed increased activity in these pathways in conjunction with an anti-depressant-like behavioral phenotype. This was accompanied by changes in short-term synaptic plasticity. Finally, zebrafish lacking pcdh19 exhibit defects in visually-guided behaviors [7]. Mutant larvae display a less robust visually-evoked escape response, as well as impaired phototaxis. These results suggest that processing of visual information is compromised in pcdh19 mutants. Collectively, the evidence supports the idea that δ-pcdhs are important contributors to the assembly of neural networks, as mutations in these genes result in both subtle structural alterations as well as behavioral deficits.
3. Cellular roles of δ-protocadherins
3.1 Roles in neuronal differentiation
One of the initial steps in assembling brain circuits is the production of neurons; neural stem cells divide asymmetrically to produce either intermediate progenitors (that will give rise to neurons) or differentiated neurons. In the mammalian cortex, successive divisions of neural progenitors give rise to clones of neurons, and accumulating data indicate that lineage plays an important role in circuit assembly [29]. Several lines of evidence suggest that δ-pcdhs play essential roles in neuronal differentiation. For example, in both mouse brain tissue and stem cell culture, immunohistochemistry reveals that Pcdh11x is expressed alongside Sox2 and Nestin, markers of neuronal progenitor cells [30]. Inhibition of Pcdh11x promoted neuronal differentiation and reduced the number of layer V/VI neurons in the cortex. Conversely, ectopic expression decreased differentiation and cell migration away from the ventricular zone.
Micro-duplication of the human chromosomal locus 16p13.11 is associated with multiple neurodevelopmental disorders, including developmental delay, intellectual disability, attention deficit disorder, and autism spectrum disorder [31, 32]. Investigating the micro-duplication of 16p13.11 in a mouse model, Fujitani et al. (2017) found that the microRNA miR484 was responsible for a hyperactivity phenotype in mice, as well as increased proliferation and neuronal differentiation [33]. The authors showed that Pcdh19 is a target of miR484 and that miR484 negatively regulates Pcdh19 expression. Forced expression of Pcdh19 rescued the effects of miR484 duplication on neuronal differentiation, suggesting that Pcdh19 acts as an important regulator of neurogenesis. Further reinforcing the notion that δ-pcdhs function in neuronal progenitors, two ChIP-Seq studies looking for targets of the B1 Sox transcription factors, Sox2 [34] or Sox3 [35], identified all of the δ-pcdhs as direct targets. Similarly, the B1 Sox genes regulate the expression of pcdh18a and pcdh18b in zebrafish [36]. The B1 Sox genes are important for maintenance of neuronal progenitor cell identity [37], which highlights the conclusion that a major aspect of δ-pcdh function involves the regulation of neuronal differentiation.
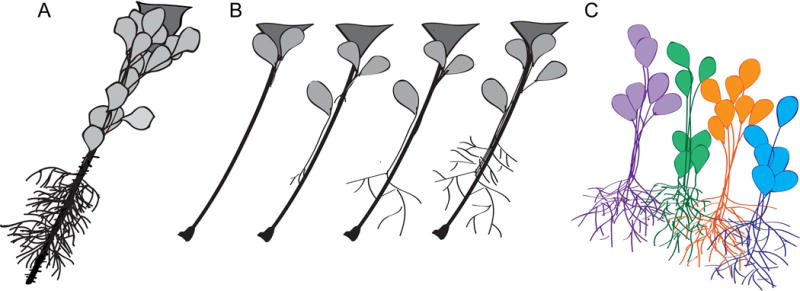
Recent work in zebrafish also corroborates a role for δ-pcdhs in neuronal progenitors and provides additional evidence that these molecules guide the modular assembly of local circuitry. Cooper et al. (2015) showed that pcdh19 is expressed discontinuously as radial columns of neurons within the developing optic tectum (Figure 2A) [7], which is homologous to the mammalian superior colliculus. Each column consists of a radial array of tightly associated neurons and multiple lines of evidence suggest that the cells within each column are siblings derived from a common progenitor. Additional δ-pcdhs reveal a similar columnar organization, and 2-color in situ hybridization suggests that expression of individual δ-pcdhs may be mostly non-overlapping. Advancing the idea that δ-pcdhs play an essential role in neuronal progenitors, elimination of pcdh19 leads to increased proliferation in the optic tectum. Combined with an apparent loss of cohesion among siblings, this leads to degradation of the columnar organization.
Figure 2.
A. In the zebrafish optic tectum, neurons expressing a common δ-pcdh are arranged into radial clusters, or columns. These columns are tightly associated with a radial glia cell. The primary processes fasciculate as they project to the synaptic neuropil where their dendrites and local axon collaterals arborize within a restricted volume. Neurons within a column are siblings, derived from a common progenitor that expresses the same δ-pcdh.
B. Based on data from pcdh19, the δ-pcdhs influence the production of neurons, as there is an increase in proliferation and the production of pcdh19+ neurons in pcdh19 mutants. Mutants exhibit defects in fasciculation during their projection to the synaptic neuropil, and in their arborization. The restricted volume over which neurons within a column arborize, and δ-pcdh-mediated adhesion between axons and dendrites could bias synaptogenesis toward sibling neurons.
C. Shown here is a model in which the distinct colors represent clones that express a distinct δ-pcdh. If each protocadherin acts in a similar manner and in parallel, differential expression of the δ-pcdhs provides a mechanism for partitioning the optic tectum into discreet functional modules.
These observations suggest a model for the assembly of neuronal modules by δ-pcdhs: collections of sibling neurons co-expressing a δ-pcdh remain tightly associated and arborize within a restricted volume (Figure 2B). As axonal and dendritic arbors grow, homophilic adhesion could bias their growth, promoting contacts of dendrites and axons that express a common δ-pcdh and facilitating preferential synapse formation among sibling neurons. This could provide an efficient way for wiring local neuronal modules (Figure 2C) as well as a mechanism for the observation that sibling neurons in the mammalian cortex are preferentially connected and have similar receptive fields [29, 38]. Some evidence suggests that the clustered protocadherins may also play a similar role [39]. It has yet to be shown whether the radial columns observed in the optic tectum constitute functional units, if cells within these clones are synaptically connected, and if δ-pcdhs influence arborization and synaptogenesis. It will be important to investigate these questions in the future.
3.2 Roles in axon outgrowth
Perhaps the most conspicuous step in assembling neural networks is axon guidance, which lays down the long tracts and pathways that connect distinct brain regions. While neuronal lineage helps provide an initial scaffold for local network assembly, axon guidance establishes the scaffolding for broader networks. Accumulating evidence suggests that the δ-pcdhs are heavily involved in this process as well. The expression of dominant-interfering forms of Pcdh7 (also known as NF-pcdh) in Xenopus retinal ganglion cells (RGCs) results in decreased axon outgrowth and extension [40], similar to what had previously been shown for N-cadherin [41]. In those neurons that do extend axons, their growth through the optic tract is impaired and average axon length is reduced. Further work showed that proper axon outgrowth and guidance from the retina to the optic tectum requires Pcdh7 in both the RGCs and in the neuroepithelial substrate along which their growth cones migrate [42]. Moreover, some evidence suggests that Pcdh7 signaling may intersect with the Semaphorin and Netrin guidance pathways [42, 43].
Further implicating δ-pcdhs in axonal development, analysis of motor axons in zebrafish suggests that Pcdh18b and the WAVE complex may also play a role in promoting axon arborization [44]. Knockdown of either pcdh18b or nap1, a core component of the WAVE complex, reduces the complexity of motor axons. This reduction can be at least partly attributed to a decreased rate of filopodia formation. Arborization defects are also observed in the tectum of zebrafish lacking pcdh19 [7].
Mice lacking Pcdh17 show phenotypes that also support a role for δ-pcdhs in axon outgrowth [45]. Pcdh17 is strongly expressed in neurons of the amygdala, which project to the hypothalamus and ventral striatum. Focusing on this axon tract, electron microscopy reveals a disorganization of axons in the mutants, and DiI tracing of these axons further reveals defects in their extension. An in vitro analysis suggests that Pcdh17 is required for growth cones to migrate along adjacent Pcdh17+ axons, consistent with data from non-neuronal cells showing that Pcdh10 promotes contact-dependent motility [46]. Co-immunoprecipitation demonstrates that the intracellular domain of Pcdh17 interacts with the WAVE complex, as well as Lamellipodin, which binds Ena/VASP and regulates actin dynamics. Pcdh17 recruits the WAVE complex, Lamellipodin, and Ena/VASP to cell-cell contacts and promotes cell motility (Figure 3A). All δ2-pcdhs have the intercellular region that mediates WAVE complex interactions, called WIRS (WAVE Interaction Receptor Site) [47]. Taken together, these observations lead to a model in which cell-cell interactions mediated by δ2-pcdhs lead to axon sorting and selective axon extension (Figure 3B). As a growth cone expressing a δ-pcdh enters a tract, contact-dependent motility will promote its growth along those axons expressing the same δ-pcdh, resulting in a bundle of axons that segregate from similar axonal bundles expressing various other δ-pcdhs. The expression of δ-pcdhs could be analogous to the different colored insulation on electrical wires, and would help organize axons within tracts. Loss of δ-pcdhs might then result in subtle defects in axon targeting. Further work will be required to extrapolate the observations of Pcdh17 to other δ-pcdhs and to investigate the relationships of the family members to patterns of axonal projections.
Figure 3. Contact-dependent axon outgrowth mediated by δ2-protocadherins.
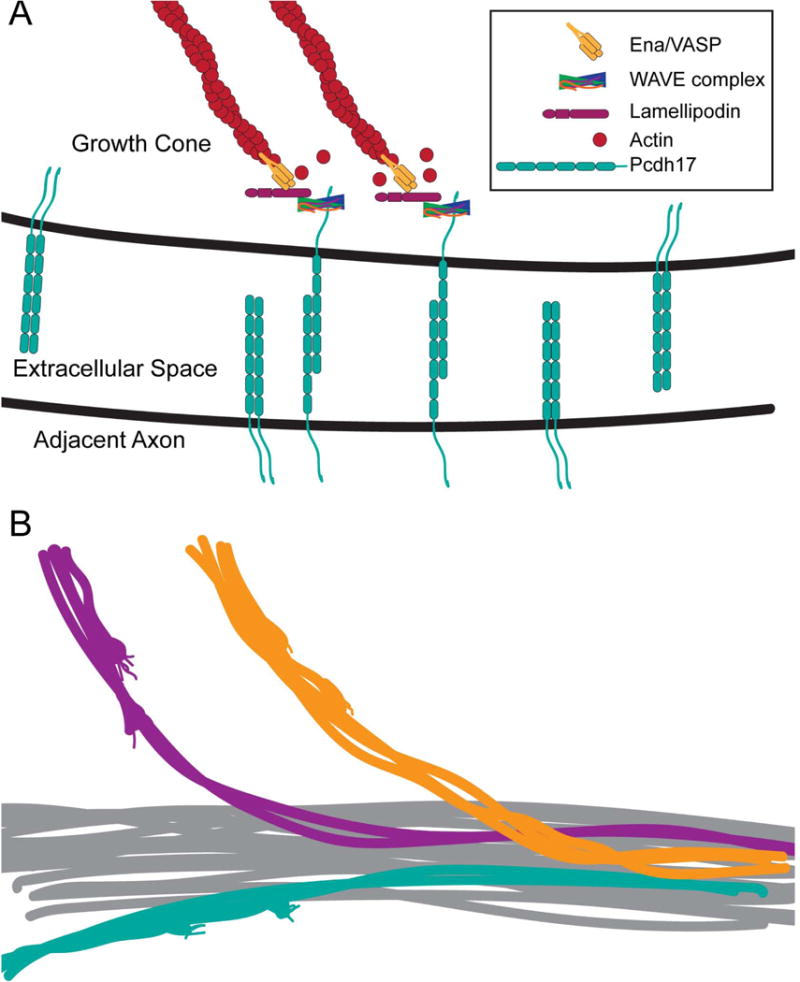
A. Model based on Hayashi et al. (2014). Shown here are homophilic interactions between Pcdh17 molecules on the apposing plasma membranes of a migrating growth cone and an axon. Pcdh17 recruits the WAVE complex, which interacts directly with a conserved motif. Pcdh17-WAVE complex recruits the scaffolding protein, Lamellipodin, and Ena/VASP to the site of contact. Ena/VASP promotes the addition of actin monomers to the plus-ends of actin filaments. This contact-dependent growth both enhances and directs motility, so that follower axons that express Pcdh17 move efficiently along Pcdh17+ axons.
B. If each of the δ2-pcdhs, which also have the conserved WAVE binding motif, acts similarly to Pcdh17, this would promote the selective formation of axon bundles on the basis of differential protocadherin expression, as well as segregation of axons withina larger axon tract. Similarly, this could also promote the selective de-fasciculation at exit points. Promoting selective axon bundling would increase the robustness of axon targeting. If active at early stages of axon guidance, this could also increase the reliability of path finding, as clusters of growth cones would collaborate to respond to cues, rather than individual pioneers.
3.2 Synaptic defects
As yet, there are no data that show a direct involvement of the δ-pcdhs in synaptogenesis. However, some studies reveal roles in mature synapses. Originally identified in a search for genes regulated by electrical activity [48], Pcdh8/Arcadlin (δ2 subgroup) may play a role in synapse disassembly. Initially absent in cultured hippocampal neurons, Pcdh8 expression is up-regulated in response to electroconvulsive stimulation [49]. Once expressed, Pcdh8 localizes to dendritic spines, where it associates with and promotes internalization of N-cadherin by receptor-mediated endocytosis. Loss of Pcdh8 leads to increased spine density, suggesting a model in which Pcdh8 negatively regulates spine stability by antagonizing N-cadherin adhesion. Similar increases in spine and filopodia density are observed in both Pcdh9 and Pcdh10 mutant mice [27, 28]. A possible role in synapse stability has also been described for Pcdh10, as some data suggest that Pcdh10 could facilitate the delivery of ubiquitinated PSD-95 to the proteasome for degradation [50], though the mechanism is unclear. As further evidence that δ-pcdhs can antagonize synapse stability, synapses in the anterior striatum and the lateral globus pallidus of mice lacking Pcdh17 exhibit an increased number of docked vesicles [8]. Thus, the presence of Pcdh17 may regulate and limit the activity potential of these terminals. Overall, published evidence supports the idea that δ-pcdhs antagonize the maintenance of mature synapses, either destabilizing them and promoting their disassembly, or limiting their size. Further work will be required to determine whether these observations relate to the broader collection of δ1- and δ2-subfamily members. Moreover, it is not clear how these data in mature synapses are relevant to the developmental functions of the δ-pcdhs.
4. Molecular mechanisms of δ-protocadherin function
4.1 Adhesive interactions
As they are members of the cadherin superfamily, it is widely presumed that the δ-pcdhs function as homophilic adhesion molecules. Classical cadherins, such as N-cadherin or E-cadherin, exhibit robust adhesion in both cell-based assays and in bead-aggregation assays that employ purified ectodomains [51, 52]. Adhesion by classical cadherins is promiscuous, as they can interact heterophilically, although they exhibit homophilic preferences [53–56]. As revealed by x-ray crystallography, classical cadherins mediate adhesion through a reciprocal swap of amino-terminal β-strands, anchored by the burying of a conserved Tryptophan side chain into a non-polar pocket on the partner cadherin [57, 58]. In contrast, δ-pcdhs do not have this conserved tryptophan residue, suggesting that they must mediate adhesive interactions through an alternative mechanism [59]. Moreover, adhesion by δ-pcdhs is generally weaker than that found for classical cadherins. In the case of Paraxial Protocadherin (PAPC, which is Pcdh8-like), several assays failed to find any evidence for adhesion [60].
Recently, x-ray crystallography was used to determine an atomic model of cadherin repeats 1–4 (EC1-4) of zebrafish Pcdh19 [61]. The structure revealed an antiparallel dimer involving the full overlap of EC1-4 with extensive contacts between EC2-EC3 and EC1-EC4. Intriguingly, 5 pathogenic mutations identified in patients with PCDH19 FE mapped to these contact surfaces. Site-directed mutagenesis was used to make two of these mutations, which abolished adhesion in bead aggregation assays. Similar antiparallel dimers have also been observed for γ-pcdhs, suggesting that the observed mechanism of adhesion is applicable to the broader protocadherin family [62, 63]. Identification of the mechanism of δ2-pcdh adhesion provides an important new set of analytical tools, as point mutations with known biochemical effects can be introduced into animal models and their functional consequences determined. The adhesion mechanism of δ1-pcdhs, with their additional EC repeat, has yet to be determined. Identifying the adhesive interface for the δ1-pcdhs will be important, as it will allow the design of similar tools to those now available for the δ2-pcdhs.
In addition to homophilic trans interactions, δ-pcdhs may fulfill additional functions by interacting in cis with other cadherin family members. For example, PAPC forms cis-oligomers [64], as do other δ-pcdhs (unpublished observations). As clustered protocadherins can form hetero-oligomers [65, 66], in vitro, the same may be true for the δ-pcdhs. This could drastically complicate the interpretation of in vivo function studies. In addition, δ-pcdhs appear to have an intimate functional relationship with classical cadherins. Expression of Pcdh8 is induced in hippocampal neurons by electroconvulsive shock [48, 49]. When expressed, Pcdh8 associates in cis with synaptic N-cadherin and promotes its internalization by endocytosis. Thus, Pcdh8 negatively regulates homophilic adhesion by N-cadherin, similar to the self-avoidance role proposed for γ-Pcdhs [67]. Paraxial protocadherin, PAPC, mediates cell sorting [68], yet exhibits no adhesive activity [60]. The effects of PAPC on adhesion are indirect, as expression of PAPC suppresses adhesion by C-cadherin [60]. Kraft et al. (2012) suggest that Frizzled7 and Wnt11 can form complexes with either PAPC or C-cadherin [69]. When PAPC is present, C-cadherin is displaced from Frizzled7-Wnt11, destabilizing its presence on the plasma membrane and weakening adhesion. In addition, zebrafish N-cadherin can form a cis-complex with Pcdh19 during early brain morphogenesis [70], and N-cadherin can enhance homophilic adhesion by Pcdh19 in bead aggregation assays [71]. These examples suggest that in addition to mediating homophilic adhesion directly, δ-pcdhs can act through cis complexes with other members of the cadherin superfamily.
4.2 Intracellular pathways
While the developmental roles of the δ-pcdhs are beginning to be revealed, the intracellular pathways and molecular mechanisms of δ-pcdh function remain poorly understood. The intracellular domains of the δ-pcdhs are intrinsically disordered proteins, and family members exhibit poor sequence conservation apart from a few short motifs. The presence of intracellular sequence motifs CM1 and CM2 are partly responsible for defining the δ-pcdh family [72]. In addition to these motifs, an additional motif (CM3) was identified in the δ1-pcdh subfamily [73]. As first shown by Yoshida et al. (1999), the CM3 motif of PCDH7 interacts with the protein phosphatase, PP1α [73], and inhibits phosphatase activity. Subsequently, this interaction was verified for the remaining δ1-pcdh subfamily members [3]. Recently, the δ2-pcdh subfamily was shown to interact with the WAVE complex through a conserved WIRS (WAVE interaction receptor site) motif [47], as described above. The interaction of the intracellular domains of Pcdh19 and Pcdh10 with the WAVE complex promotes actin assembly in in vitro assays, which is consistent with the observation (outlined above) that Pcdh17 recruits the WAVE complex, Lamellipodin, and Ena/VASP to cell-cell contacts and promotes motility.
In addition to the interactions described above, three “orphan” binding partners have been identified. Pcdh18 contains a binding site for the scaffolding protein, Dab1 [74], which is involved in regulating neuronal migration as part of the Reelin pathway. This motif does not appear to be present in other δ-pcdhs, and its functional significance remains undetermined. Additionally, Pcdh7 (NF-Pcdh) has been shown to interact with the transcription factor, TAF1/SET [75]. TAF1 appears to be important for Pcdh7 function, both in the neural tube and during axon outgrowth [40, 75]. It is not known whether other δ1-pcdhs similarly interact with TAF1. Finally, Pcdh8 interacts with the kinase TAO2β to initiate the internalization of N-cadherin, as discussed above [49].
Perhaps the biggest impediment to understanding the δ-pcdhs is the relatively small number of known downstream intracellular pathways. Determining intracellular binding partners for the δ-pcdhs will be the most direct way of identifying their cellular roles and unraveling their molecular mechanisms. For example, the interaction of the δ2-pcdhs with the WAVE complex directly reveals a role for them in regulation of actin dynamics and cell motility. A more systematic proteomics approach to the δ-pcdhs will be required to elucidate the range of cellular pathways linked to their cell surface interactions.
5. Conclusions
Increasingly, members of the δ-pcdh family are being revealed as essential to the robust assembly of the vertebrate nervous system. Mutations in human δ-pcdhs can lead to neural disorders including schizophrenia, autism spectrum disorders, and epilepsy, and animal models reveal a variety of behavioral defects. The fundamental questions are: what roles do the δ-pcdhs play during neural development and how does loss of δ-pcdhs affect the assembly of neural architecture and lead to changes in neural function? As outlined above, recent observations reveal roles for the δ-pcdhs in establishing both long-range and short-range neural connections. The δ2-pcdhs promote contact-dependent motility and, at least in the case of Pcdh17, this facilitates collective axon extension. Extrapolated across the δ2-pcdh subfamily, this suggests an important role in selective fasciculation and pathway selection, which would help organize axon tracts and connectivity between brain regions.
Evidence also supports the idea that both δ1- and δ2-pcdhs regulate neuronal differentiation and that their expression defines clones of sibling neurons. This suggests the possibility that expression of δ-pcdhs could partition the developing nervous system into discrete neuronal modules. Homophilic adhesion and/or heterophilic repulsion could then facilitate the assembly of local synaptic sub-networks. However, both of the above scenarios presume that each δ-pcdh is equivalent and performs comparable, parallel functions. It remains to be determined whether this is the case, or whether each individual family member constitutes a new problem to be solved. Future work will require extending observations from individual molecules to other family members and other brain regions. Additionally, the functional relationship of the δ-pcdhs to other pathways needs to be clarified. The physical links to classical cadherins and other known intracellular pathways, such as Wnt signaling, are important clues to understanding the molecular mechanisms of the δ-pcdhs and the full range of their activities.
Acknowledgments
We would like to thank Dr. Michelle Emond for critical reading of the manuscript.
Funding Source
Our work is supported by grants from the National Institutes of Health (R01EY0273009) and the National Science Foundation (IOS1457126).
Abbreviations
- CM
conserved motif
- EC
extracellular cadherin repeat
- pcdh
protocadherin
- SNP
single nucleotide polymorphism
- WIRS
WAVE interacting receptor site
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Hirano S, Takeichi M. Cadherins in brain morphogenesis and wiring. Physiol Rev. 2012;92(2):597–634. doi: 10.1152/physrev.00014.2011. [DOI] [PubMed] [Google Scholar]
- 2.Hulpiau P, van Roy F. New insights into the evolution of metazoan cadherins. Mol Biol Evol. 2011;28(1):647–57. doi: 10.1093/molbev/msq233. [DOI] [PubMed] [Google Scholar]
- 3.Vanhalst K, Kools P, Staes K, van Roy F, Redies C. delta-Protocadherins: a gene family expressed differentially in the mouse brain. Cell Mol Life Sci. 2005;62(11):1247–59. doi: 10.1007/s00018-005-5021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blevins CJ, Emond MR, Biswas S, Jontes JD. Differential expression, alternative splicing, and adhesive properties of the zebrafish delta1-protocadherins. Neuroscience. 2011;199:523–34. doi: 10.1016/j.neuroscience.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 5.Kim SY, Chung HS, Sun W, Kim H. Spatiotemporal expression pattern of non-clustered protocadherin family members in the developing rat brain. Neuroscience. 2007;147(4):996–1021. doi: 10.1016/j.neuroscience.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 6.Krishna KK, Hertel N, Redies C. Cadherin expression in the somatosensory cortex: evidence for a combinatorial molecular code at the single-cell level. Neuroscience. 2011;175:37–48. doi: 10.1016/j.neuroscience.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 7.Cooper SR, Emond MR, Duy PQ, Liebau BG, Wolman MA, Jontes JD. Protocadherins control the modular assembly of neuronal columns in the zebrafish optic tectum. J Cell Biol. 2015;211(4):807–14. doi: 10.1083/jcb.201507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshina N, Tanimura A, Yamasaki M, Inoue T, Fukabori R, Kuroda T, Yokoyama K, Tezuka T, Sagara H, Hirano S, Kiyonari H, Takada M, Kobayashi K, Watanabe M, Kano M, Nakazawa T, Yamamoto T. Protocadherin 17 regulates presynaptic assembly in topographic corticobasal Ganglia circuits. Neuron. 2013;78(5):839–54. doi: 10.1016/j.neuron.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Neudert F, Nuernberger KK, Redies C. Comparative analysis of cadherin expression and connectivity patterns in the cerebellar system of ferret and mouse. J Comp Neurol. 2008;511(6):736–52. doi: 10.1002/cne.21865. [DOI] [PubMed] [Google Scholar]
- 10.Redies C, Hertel N, Hubner CA. Cadherins and neuropsychiatric disorders. Brain Res. 2012;1470:130–44. doi: 10.1016/j.brainres.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Dibbens LM, Tarpey PS, Hynes K, Bayly MA, Scheffer IE, Smith R, Bomar J, Sutton E, Vandeleur L, Shoubridge C, Edkins S, Turner SJ, Stevens C, O’Meara S, Tofts C, Barthorpe S, Buck G, Cole J, Halliday K, Jones D, Lee R, Madison M, Mironenko T, Varian J, West S, Widaa S, Wray P, Teague J, Dicks E, Butler A, Menzies A, Jenkinson A, Shepherd R, Gusella JF, Afawi Z, Mazarib A, Neufeld MY, Kivity S, Lev D, Lerman-Sagie T, Korczyn AD, Derry CP, Sutherland GR, Friend K, Shaw M, Corbett M, Kim HG, Geschwind DH, Thomas P, Haan E, Ryan S, McKee S, Berkovic SF, Futreal PA, Stratton MR, Mulley JC, Gecz J. X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nat Genet. 2008;40(6):776–81. doi: 10.1038/ng.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Depienne C, LeGuern E. PCDH19-related infantile epileptic encephalopathy: an unusual X-linked inheritance disorder. Hum Mutat. 2012;33(4):627–34. doi: 10.1002/humu.22029. [DOI] [PubMed] [Google Scholar]
- 13.Piton A, Gauthier J, Hamdan FF, Lafreniere RG, Yang Y, Henrion E, Laurent S, Noreau A, Thibodeau P, Karemera L, Spiegelman D, Kuku F, Duguay J, Destroismaisons L, Jolivet P, Cote M, Lachapelle K, Diallo O, Raymond A, Marineau C, Champagne N, Xiong L, Gaspar C, Riviere JB, Tarabeux J, Cossette P, Krebs MO, Rapoport JL, Addington A, Delisi LE, Mottron L, Joober R, Fombonne E, Drapeau P, Rouleau GA. Systematic resequencing of X-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol Psychiatry. 2011;16(8):867–80. doi: 10.1038/mp.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu X, Need AC, Petrovski S, Goldstein DB. One gene, many neuropsychiatric disorders: lessons from Mendelian diseases. Nat Neurosci. 2014;17(6):773–81. doi: 10.1038/nn.3713. [DOI] [PubMed] [Google Scholar]
- 15.Ryan SG, Chance PF, Zou CH, Spinner NB, Golden JA, Smietana S. Epilepsy and mental retardation limited to females: an X-linked dominant disorder with male sparing. Nat Genet. 1997;17(1):92–5. doi: 10.1038/ng0997-92. [DOI] [PubMed] [Google Scholar]
- 16.de Lange IM, Rump P, Neuteboom RF, Augustijn PB, Hodges K, Kistemaker AI, Brouwer OF, Mancini GMS, Newman HA, Vos YJ, Helbig KL, Peeters-Scholte C, Kriek M, Knoers NV, Lindhout D, Koeleman BPC, van Kempen MJA, Brilstra EH. Male patients affected by mosaic PCDH19 mutations: five new cases. Neurogenetics. 2017 doi: 10.1007/s10048-017-0517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Depienne C, Bouteiller D, Keren B, Cheuret E, Poirier K, Trouillard O, Benyahia B, Quelin C, Carpentier W, Julia S, Afenjar A, Gautier A, Rivier F, Meyer S, Berquin P, Helias M, Py I, Rivera S, Bahi-Buisson N, Gourfinkel-An I, Cazeneuve C, Ruberg M, Brice A, Nabbout R, Leguern E. Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females. PLoS Genet. 2009;5(2):e1000381. doi: 10.1371/journal.pgen.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wieland I, Jakubiczka S, Muschke P, Cohen M, Thiele H, Gerlach KL, Adams RH, Wieacker P. Mutations of the ephrin-B1 gene cause craniofrontonasal syndrome. Am J Hum Genet. 2004;74(6):1209–15. doi: 10.1086/421532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasnauskiene J, Ciuladaite Z, Preiksaitiene E, Matuleviciene A, Alexandrou A, Koumbaris G, Sismani C, Pepalyte I, Patsalis PC, Kucinskas V. A single gene deletion on 4q28.3: PCDH18–a new candidate gene for intellectual disability? Eur J Med Genet. 2012;55(4):274–7. doi: 10.1016/j.ejmg.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Aran A, Rosenfeld N, Jaron R, Renbaum P, Zuckerman S, Fridman H, Zeligson S, Segel R, Kohn Y, Kamal L, Kanaan M, Segev Y, Mazaki E, Rabinowitz R, Shen O, Lee M, Walsh T, King MC, Gulsuner S, Levy-Lahad E. Loss of function of PCDH12 underlies recessive microcephaly mimicking intrauterine infection. Neurology. 2016;86(21):2016–24. doi: 10.1212/WNL.0000000000002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregorio SP, Sallet PC, Do KA, Lin E, Gattaz WF, Dias-Neto E. Polymorphisms in genes involved in neurodevelopment may be associated with altered brain morphology in schizophrenia: preliminary evidence. Psychiatry Res. 2009;165(1–2):1–9. doi: 10.1016/j.psychres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Miyake K, Hirasawa T, Soutome M, Itoh M, Goto Y, Endoh K, Takahashi K, Kudo S, Nakagawa T, Yokoi S, Taira T, Inazawa J, Kubota T. The protocadherins, PCDHB1 and PCDH7, are regulated by MeCP2 in neuronal cells and brain tissues: implication for pathogenesis of Rett syndrome. BMC Neurosci. 2011;12:81. doi: 10.1186/1471-2202-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lal D, Ruppert AK, Trucks H, Schulz H, de Kovel CG, Kasteleijn-Nolst Trenite D, Sonsma AC, Koeleman BP, Lindhout D, Weber YG, Lerche H, Kapser C, Schankin CJ, Kunz WS, Surges R, Elger CE, Gaus V, Schmitz B, Helbig I, Muhle H, Stephani U, Klein KM, Rosenow F, Neubauer BA, Reinthaler EM, Zimprich F, Feucht M, Moller RS, Hjalgrim H, De Jonghe P, Suls A, Lieb W, Franke A, Strauch K, Gieger C, Schurmann C, Schminke U, Nurnberg P, Consortium E. Sander T. Burden analysis of rare microdeletions suggests a strong impact of neurodevelopmental genes in genetic generalised epilepsies. PLoS Genet. 2015;11(5):e1005226. doi: 10.1371/journal.pgen.1005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321(5886):218–23. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linhares ND, Valadares ER, da Costa SS, Arantes RR, de Oliveira LR, Rosenberg C, Vianna-Morgante AM, Svartman M. Inherited Xq13.2-q21.31 duplication in a boy with recurrent seizures and pubertal gynecomastia: Clinical, chromosomal and aCGH characterization. Meta Gene. 2016;9:185–90. doi: 10.1016/j.mgene.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang H, Hoshina N, Zhang C, Ma Y, Cao H, Wang Y, Wu DD, Bergen SE, Landen M, Hultman CM, Preisig M, Kutalik Z, Castelao E, Grigoroiu-Serbanescu M, Forstner AJ, Strohmaier J, Hecker J, Schulze TG, Muller-Myhsok B, Reif A, Mitchell PB, Martin NG, Schofield PR, Cichon S, Nothen MM, G. Swedish Bipolar Study. Moo DSBC, Walter H, Erk S, Heinz A, Amin N, van Duijn CM, Meyer-Lindenberg A, Tost H, Xiao X, Yamamoto T, Rietschel M, Li M. The protocadherin 17 gene affects cognition, personality, amygdala structure and function, synapse development and risk of major mood disorders. Mol Psychiatry. 2017 doi: 10.1038/mp.2016.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruining H, Matsui A, Oguro-Ando A, Kahn RS, Van’t Spijker HM, Akkermans G, Stiedl O, Engeland Hvan, Koopmans B, van Lith HA, Oppelaar H, Tieland L, Nonkes LJ, Yagi T, Kaneko R, Burbach JP, Yamamoto N, Kas MJ. Genetic Mapping in Mice Reveals the Involvement of Pcdh9 in Long-Term Social and Object Recognition and Sensorimotor Development. Biol Psychiatry. 2015;78(7):485–95. doi: 10.1016/j.biopsych.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Schoch H, Kreibich AS, Ferri SL, White RS, Bohorquez D, Banerjee A, Port RG, Dow HC, Cordero L, Pallathra AA, Kim H, Li H, Bilker WB, Hirano S, Schultz RT, Borgmann-Winter K, Hahn CG, Feldmeyer D, Carlson GC, Abel T, Brodkin ES. Sociability Deficits and Altered Amygdala Circuits in Mice Lacking Pcdh10, an Autism Associated Gene. Biol Psychiatry. 2017;81(3):193–202. doi: 10.1016/j.biopsych.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458(7237):501–4. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang P, Wu C, Liu N, Niu L, Yan Z, Feng Y, Xu R. Protocadherin 11 × regulates differentiation and proliferation of neural stem cell in vitro and in vivo. J Mol Neurosci. 2014;54(2):199–210. doi: 10.1007/s12031-014-0275-x. [DOI] [PubMed] [Google Scholar]
- 31.Ullmann R, Turner G, Kirchhoff M, Chen W, Tonge B, Rosenberg C, Field M, Vianna-Morgante AM, Christie L, Krepischi-Santos AC, Banna L, Brereton AV, Hill A, Bisgaard AM, Muller I, Hultschig C, Erdogan F, Wieczorek G, Ropers HH. Array CGH identifies reciprocal 16p13.1 duplications and deletions that predispose to autism and/or mental retardation. Hum Mutat. 2007;28(7):674–82. doi: 10.1002/humu.20546. [DOI] [PubMed] [Google Scholar]
- 32.Williams NM, Zaharieva I, Martin A, Langley K, Mantripragada K, Fossdal R, Stefansson H, Stefansson K, Magnusson P, Gudmundsson OO, Gustafsson O, Holmans P, Owen MJ, O’Donovan M, Thapar A. Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. Lancet. 2010;376(9750):1401–8. doi: 10.1016/S0140-6736(10)61109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujitani M, Zhang S, Fujiki R, Fujihara Y, Yamashita T. A chromosome 16p13.11 microduplication causes hyperactivity through dysregulation of miR-484/protocadherin-19 signaling. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergsland M, Ramskold D, Zaouter C, Klum S, Sandberg R, Muhr J. Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 2011;25(23):2453–64. doi: 10.1101/gad.176008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAninch D, Thomas P. Identification of highly conserved putative developmental enhancers bound by SOX3 in neural progenitors using ChIP-Seq. PLoS One. 2014;9(11):e113361. doi: 10.1371/journal.pone.0113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okuda Y, Ogura E, Kondoh H, Kamachi Y. B1 SOX coordinate cell specification with patterning and morphogenesis in the early zebrafish embryo. PLoS Genet. 2010;6(5):e1000936. doi: 10.1371/journal.pgen.1000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39(5):749–65. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Lu H, Cheng PL, Ge S, Xu H, Shi SH, Dan Y. Clonally related visual cortical neurons show similar stimulus feature selectivity. Nature. 2012;486(7401):118–21. doi: 10.1038/nature11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tarusawa E, Sanbo M, Okayama A, Miyashita T, Kitsukawa T, Hirayama T, Hirabayashi T, Hasegawa S, Kaneko R, Toyoda S, Kobayashi T, Kato-Itoh M, Nakauchi H, Hirabayashi M, Yagi T, Yoshimura Y. Establishment of high reciprocal connectivity between clonal cortical neurons is regulated by the Dnmt3b DNA methyltransferase and clustered protocadherins. BMC Biol. 2016;14(1):103. doi: 10.1186/s12915-016-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piper M, Dwivedy A, Leung L, Bradley RS, Holt CE. NF-protocadherin and TAF1 regulate retinal axon initiation and elongation in vivo. J Neurosci. 2008;28(1):100–5. doi: 10.1523/JNEUROSCI.4490-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17(5):837–48. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 42.Leung LC, Harris WA, Holt CE, Piper M. NF-Protocadherin Regulates Retinal Ganglion Cell Axon Behaviour in the Developing Visual System. PLoS One. 2015;10(10):e0141290. doi: 10.1371/journal.pone.0141290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung LC, Urbancic V, Baudet ML, Dwivedy A, Bayley TG, Lee AC, Harris WA, Holt CE. Coupling of NF-protocadherin signaling to axon guidance by cue-induced translation. Nat Neurosci. 2013;16(2):166–73. doi: 10.1038/nn.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biswas S, Emond MR, le Duy PQ, Hao T, Beattie CE, Jontes JD. Protocadherin-18b interacts with Nap1 to control motor axon growth and arborization in zebrafish. Mol Biol Cell. 2014;25(5):633–42. doi: 10.1091/mbc.E13-08-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi S, Inoue Y, Kiyonari H, Abe T, Misaki K, Moriguchi H, Tanaka Y, Takeichi M. Protocadherin-17 mediates collective axon extension by recruiting actin regulator complexes to interaxonal contacts. Dev Cell. 2014;30(6):673–87. doi: 10.1016/j.devcel.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Nakao S, Uemura M, Aoki E, Suzuki ST, Takeichi M, Hirano S. Distribution of OL-protocadherin in axon fibers in the developing chick nervous system. Brain Res Mol Brain Res. 2005;134(2):294–308. doi: 10.1016/j.molbrainres.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 47.Chen B, Brinkmann K, Chen Z, Pak CW, Liao Y, Shi S, Henry L, Grishin NV, Bogdan S, Rosen MK. The WAVE regulatory complex links diverse receptors to the actin cytoskeleton. Cell. 2014;156(1–2):195–207. doi: 10.1016/j.cell.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamagata K, Andreasson KI, Sugiura H, Maru E, Dominique M, Irie Y, Miki N, Hayashi Y, Yoshioka M, Kaneko K, Kato H, Worley PF. Arcadlin is a neural activity-regulated cadherin involved in long term potentiation. J Biol Chem. 1999;274(27):19473–1979. doi: 10.1074/jbc.274.27.19473. [DOI] [PubMed] [Google Scholar]
- 49.Yasuda S, Tanaka H, Sugiura H, Okamura K, Sakaguchi T, Tran U, Takemiya T, Mizoguchi A, Yagita Y, Sakurai T, De Robertis EM, Yamagata K. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron. 2007;56(3):456–71. doi: 10.1016/j.neuron.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai NP, Wilkerson JR, Guo W, Maksimova MA, DeMartino GN, Cowan CW, Huber KM. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell. 2012;151(7):1581–94. doi: 10.1016/j.cell.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chappuis-Flament S, Wong E, Hicks LD, Kay CM, Gumbiner BM. Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J Cell Biol. 2001;154(1):231–43. doi: 10.1083/jcb.200103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977;75(2 Pt 1):464–74. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR, Shapiro L. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell. 2006;124(6):1255–68. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 54.Katsamba P, Carroll K, Ahlsen G, Bahna F, Vendome J, Posy S, Rajebhosale M, Price S, Jessell TM, Ben-Shaul A, Shapiro L, Honig BH. Linking molecular affinity and cellular specificity in cadherin-mediated adhesion. Proc Natl Acad Sci U S A. 2009;106(28):11594–9. doi: 10.1073/pnas.0905349106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niessen CM, Gumbiner BM. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol. 2002;156(2):389–399. doi: 10.1083/jcb.200108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prakasam AK, Maruthamuthu V, Leckband DE. Similarities between heterophilic and homophilic cadherin adhesion. Proc Natl Acad Sci U S A. 2006;103(42):15434–9. doi: 10.1073/pnas.0606701103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296(5571):1308–13. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 58.Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374(6520):327–37. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 59.Morishita H, Umitsu M, Murata Y, Shibata N, Udaka K, Higuchi Y, Akutsu H, Yamaguchi T, Yagi T, Ikegami T. Structure of the cadherin-related neuronal receptor/protocadherin-alpha first extracellular cadherin domain reveals diversity across cadherin families. J Biol Chem. 2006;281(44):33650–63. doi: 10.1074/jbc.M603298200. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol. 2006;174(2):301–13. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooper SR, Jontes JD, Sotomayor M. Structural determinants of adhesion by Protocadherin-19 and implications for its role in epilepsy. Elife. 2016;5 doi: 10.7554/eLife.18529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goodman KM, Rubinstein R, Thu CA, Bahna F, Mannepalli S, Ahlsen G, Rittenhouse C, Maniatis T, Honig B, Shapiro L. Structural Basis of Diverse Homophilic Recognition by Clustered alpha- and beta-Protocadherins. Neuron. 2016;90(4):709–23. doi: 10.1016/j.neuron.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nicoludis JM, Vogt BE, Green AG, Scharfe CP, Marks DS, Gaudet R. Antiparallel protocadherin homodimers use distinct affinity- and specificity-mediating regions in cadherin repeats 1–4. Elife. 2016;5 doi: 10.7554/eLife.18449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Molino C, Liu L, Gumbiner BM. Structural elements necessary for oligomerization, trafficking, and cell sorting function of paraxial protocadherin. J Biol Chem. 2007;282(44):32128–37. doi: 10.1074/jbc.M705337200. [DOI] [PubMed] [Google Scholar]
- 65.Rubinstein R, Thu CA, Goodman KM, Wolcott HN, Bahna F, Mannepalli S, Ahlsen G, Chevee M, Halim A, Clausen H, Maniatis T, Shapiro L, Honig B. Molecular logic of neuronal self-recognition through protocadherin domain interactions. Cell. 2015;163(3):629–42. doi: 10.1016/j.cell.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schreiner D, Weiner JA. Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc Natl Acad Sci U S A. 2010;107(33):14893–8. doi: 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature. 2012;488(7412):517–21. doi: 10.1038/nature11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SH, Yamamoto A, Bouwmeester T, Agius E, Robertis EM. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 1998;125(23):4681–90. doi: 10.1242/dev.125.23.4681. [DOI] [PubMed] [Google Scholar]
- 69.Kraft B, Berger CD, Wallkamm V, Steinbeisser H, Wedlich D. Wnt-11 and Fz7 reduce cell adhesion in convergent extension by sequestration of PAPC and C-cadherin. J Cell Biol. 2012;198(4):695–709. doi: 10.1083/jcb.201110076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biswas S, Emond MR, Jontes JD. Protocadherin-19 and N-cadherin interact to control cell movements during anterior neurulation. J Cell Biol. 2010;191(5):1029–41. doi: 10.1083/jcb.201007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Emond MR, Biswas S, Blevins CJ, Jontes JD. A complex of Protocadherin-19 and N-cadherin mediates a novel mechanism of cell adhesion. J Cell Biol. 2011;195(7):1115–21. doi: 10.1083/jcb.201108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolverton T, Lalande M. Identification and characterization of three members of a novel subclass of protocadherins. Genomics. 2001;76(1–3):66–72. doi: 10.1006/geno.2001.6592. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida K, Watanabe M, Kato H, Dutta A, Sugano S. BH-protocadherin-c, a member of the cadherin superfamily, interacts with protein phosphatase 1 alpha through its intracellular domain. FEBS Lett. 1999;460(1):93–8. doi: 10.1016/s0014-5793(99)01309-5. [DOI] [PubMed] [Google Scholar]
- 74.Homayouni R, Rice DS, Curran T. Disabled-1 interacts with a novel developmentally regulated protocadherin. Biochem Biophys Res Commun. 2001;289(2):539–47. doi: 10.1006/bbrc.2001.5998. [DOI] [PubMed] [Google Scholar]
- 75.Heggem MA, Bradley RS. The cytoplasmic domain of Xenopus NF-protocadherin interacts with TAF1/set. Dev Cell. 2003;4(3):419–29. doi: 10.1016/s1534-5807(03)00036-4. [DOI] [PubMed] [Google Scholar]


