Abstract
Background
From December 2012-March 2014, three randomized trials, each implementing a unique intervention in primary care settings (mail recruitment [repeated-mailing], an electronic health record best practice alert [BPA], and patient-solicitation [patient-solicitation]), evaluated HCV antibody testing, diagnosis, and costs for each of the interventions compared to standard-of-care testing. Multilevel multivariable models were used to estimate the adjusted risk ratio (aRR) for receiving an HCV antibody test, and costs were estimated using activity-based costing.
Rationale
To estimate the effects of interventions conducted as part of the Birth-cohort Evaluation to Advance Screening and Testing for Hepatitis C study on hepatitis C virus (HCV) testing and costs among persons of the 1945–1965 birth-cohort (BC).
Main Results
Intervention resulted in substantially higher HCV testing rates compared to standard-of-care (26.9% vs. 1.4% for repeated-mailing, 30.9% vs. 3.6% for BPA, and 63.5% vs. 2.0% for patient-solicitation), and significantly higher aRR for testing after controlling for sex, birth year, race, insurance type, and median household income (19.2 [95% Confidence Interval (CI) 9.7–38.2] for repeated-mailing, 13.2 [95% CI 3.6–48.6] for BPA, and 32.9 [95% CI 19.3–56.1] for patient-solicitation). The BPA intervention had the lowest incremental cost per completed test ($24 with fixed startup costs, $3 without) and also the lowest incremental cost per new case identified after omitting fixed startup costs ($1,691).
Conclusion
HCV testing interventions resulted in an increase in BC testing compared to standard-of-care but also increased costs. The effect size and incremental costs of BPA intervention (excluding startup costs) support more widespread adoption compared to the other interventions.
Keywords: testing, cost-effectiveness, experimental design, evidenced based practice, implementation
Hepatitis C virus (HCV) infection, a blood-borne infectious disease of the liver, currently affects as many as 185 million people worldwide. In the United States, approximately 4.7 million individuals are HCV-antibody positive, and approximately 3.5 million of those are chronically infected.[1–3] Chronic HCV infection is largely asymptomatic for years prior to the development of serious complications. However, if left undetected and untreated, HCV infection is estimated to result in decompensated cirrhosis, hepatocellular carcinoma and premature death in as many as 30–40% of those chronically infected.[4] HCV infection has been historically underdiagnosed in clinical settings, and the increasing number of HCV-attributable deaths suggests that increased case identification is needed.[2, 5, 6] Furthermore, since November, 2013, a series of new generation, highly effective, direct acting antiviral (DAA) medications, have been approved for the treatment of HCV, including several different all-oral, interferon-free, DAA combination therapies that each cured greater than 90% of the patients treated in clinical trials[1], making case identification even more critical.
In the United States, individuals born as part of the 1945–1965 birth cohort (BC) have a prevalence of HCV infection as high as five times greater than other BCs.[7] To increase hepatitis C case identification within the BC, the Centers for Disease Control and Prevention (CDC) and the U.S Preventive Services Task Force expanded prior risk-based testing recommendations to include one-time HCV testing for U.S. residents born during 1945–1965.[8–10] HCV testing and DAA treatment is highly cost-effective and insensitive to 25% increases in testing costs at the list price of the first two DAA combinations, and treatment costs have fallen dramatically due to price discounts negotiated by insurers and the competitive pricing of the newest DAA combinations.[11–13] However, the effect and costs of expanded testing recommendations has not been empirically assessed in primary care settings.[14–18]
In response to the HCV BC testing recommendation, the CDC Foundation sponsored the Birth-cohort Evaluation to Advance Screening and Testing for Hepatitis C (BEST-C) study to understand the effect and costs of BC testing in primary care settings. As part of the BEST-C project, an initial retrospective assessment examined the prevalence and predictors of undiagnosed HCV among primary care patients, as well as the proportion of HCV antibody positive (anti-HCV+) patients overlooked with risk-based testing, and found that risk-based testing may have missed 4 of 5 anti-HCV+ patients.[19]
Between December 2012-March 2014, using the BEST-C infrastructure, a prospective study was implemented in three large healthcare systems (centers); each center implemented a unique randomized HCV testing trial, each evaluating one of three different interventions to increase HCV testing among the BC.[20] The interventions were: letters sent via the postal service (center 1), an electronic health record (EHR) best practice alert (BPA) (center 2), or physician office based direct patient-solicitation (center 3). Each intervention aimed to increase HCV testing and compare the number of HCV infections identified using the intervention versus standard-of-care (risk-based testing was the standard at the time of these trials).[20] Each center developed and evaluated their center’s intervention. Each center collected data on the cost of BC testing (cost of the intervention and cost of the HCV antibody test), the percentage of patients who were offered and accepted testing, and the percentage of patients who tested positive; each center also assessed HCV treatment and care, and the impact of BC screening on staff and health systems.[21] The purpose of the current study was to evaluate the effect of testing interventions on the probability of HCV antibody testing among BC patients as compared to the standard-of-care, to assess patient characteristics associated with testing, and to estimate incremental costs per-person-tested and per-HCV-positive patient identified for each intervention.
Experimental Procedures and Human Subjects
Three centers implemented independent interventions and each used a unique research design. Center 1 (repeated-mailings) used the EHR system to identify eligible patients, defined as those born during 1945–1965, with at least one primary care visit to a system-affiliated physician in the past year, and no prior evidence of HCV testing in their medical records. From February 2013-October 2013, eligible patients were sent letters containing HCV screening information and preregistered laboratory order forms mailed at 0, 4, and 12 weeks, and reminder letters sent at 1 and 8 weeks.[22, 23] A sample of 9,000 patients was selected for inclusion. Center 1 implemented its intervention using a stratified multi-clinic individually randomized design, (i.e. within each of the nine primary care clinics randomized to “intervention”, patients were randomized to the intervention or standard-of-care (control group)). Clinics were stratified by major characteristics (size, urban location) before selection at the clinic level to intervention vs. control, and then patients were randomized within intervention clinics at a ratio of 1:2 to receive BC testing letters or to be tracked as controls.[23] The center delivered a list of eligible patients to the coordinating center that performed the randomization and delivered the list back to the center.
From April 2013–March 2014, center 2 utilized an EHR BPA notifying a medical assistant that a scheduled patient was eligible for HCV testing. Eligible patients were defined as patients born during 1945–1965 who had no record of an HCV antibody or viral load test or HCV diagnosis in the EHR system. To help reduce potential physician BPA fatigue, the assistant placed an electronic HCV test order that would prompt the physician to review, discuss, and accept or reject the order for each eligible patient. (The assistant did not discuss HCV testing with the patient). If the medical assistant did not respond to the alert, a BPA automatically appeared when the physician opened the patient medical record recommending HCV testing alongside the HCV test order form.[23, 24] Center 2 used a cluster randomized design among 10 primary care practices. Each of the ten primary care practices were defined as a cluster and each cluster was randomized in a 1:1 ratio to implement BC testing with a BPA or to provide standard-of-care.[23] NORC at the University of Chicago, the coordinating center, randomized the primary care practices and participants were eligible patients visiting the primary care practices.
Center 3 implemented direct patient-solicitation to recruit patients for HCV testing. Study coordinators approached patients following their visit with the physician at four internal medicine clinics from December 2012-January 2014. Eligible patients were defined as those born during 1945–1965 who had no prior evidence of HCV testing in their medical record and who had previously visited one of the four internal medicine clinics. Center 3 conducted a cluster randomized crossover study with two intervention clinics and two control clinics; randomized assignment crossed over midway through the study.[23, 25] Thus, the randomization of clinics to perform BC testing or standard-of-care was a 1:1 ratio.
All centers collected information on test occurrence and results, patient demographic characteristics (sex, birth year [1945–50, 1950–54, 1955–59, or 1960–65], ethnicity [Hispanic, non-Hispanic, unknown], and race [black, white, Asian, other, or unknown]), insurance type (private, Medicare/Medicaid, or uninsured/unknown), and median household income based on patient’s ZIP code of residence (<$30,000, $30,000–49,999, $50,000–69,999, $70,000–99,999, ≥$100,000 or Unknown) as reported by the American Community Survey.[26]
Intervention assignments for all trials were performed at CDC using Proc SurveySelect in SAS (SAS Institute, Cary, NC) and were implemented by institution staff and providers with technical support from CDC and at the coordinating center, NORC at the University of Chicago.[23] This study underwent institutional review board review and received approval at each center, University of Alabama, Henry Ford Health System and Mount Sinai Hospital and NORC at the University of Chicago. Neither research staff nor providers were blinded to intervention assignments. This study was registered with clinicaltrials.gov (identifier: NCT02123212).
Hepatitis C Intervention and Testing Costs
For each center, we obtained intervention and testing costs using an activity-based costing approach. We asked center staff to divide their intervention into mutually exclusive activities and assign labor and material costs to each activity.[27, 28] For center 1, labor costs included staff hourly wages for personnel to conduct project coordination, database management and screening, while material costs included paper and postage costs for each mailing and the institution charge for HCV antibody test. For center 2, labor costs included technical development of the BPA, and training staff to use the BPA. No material costs were documented. Fixed startup costs included technical design and development of the BPA system. For center 3, labor costs included staff hourly wages for program set-up, program and database management, while material costs included cellphones, printers, printer cartridges, and HCV antibody test. The cost of antibody testing itself was set equal to the system-wide average reimbursement amount for antibody tests, using unique estimates as reported by each center, and using the same cost for both the standard-of-care and intervention groups.
Statistical Analysis
For each center, we (1) compared differences in patient characteristics between the intervention and standard-of-care groups using Chi-square tests; and (2) estimated the adjusted risk ratio (aRR) and 95% confidence interval (CI) for receiving an HCV antibody test using multilevel, multivariable models with a Poisson distribution, log link and empirical estimator (“classic sandwich estimator”).[29–33] To adjust for correlations between patients within clinics, a random effect for clinic was included in each model. We refit each model restricting the analysis to the intervention group only to estimate the effect of patient characteristics on HCV antibody testing. Data analysis was conducted in SAS (SAS Institute, Inc., Cary North Carolina) version 9.4.
Calculation of Testing Costs
We assumed that the aggregated cost of the standard-of-care group was equal to the average reimbursement costs for HCV antibody testing multiplied by the number of tests conducted. Standard-of-care (risk-based testing) may also incur costs due to providers taking time for risk ascertainment, but these costs were not measured in the study. We estimated the aggregate cost of the intervention group as the sum of: 1) fixed startup costs, 2) cost of unsuccessful testing recruitment multiplied by the number of individuals who were unsuccessfully recruited, 3) cost of successful testing recruitment multiplied by the number of people successfully recruited, and, 4) the average reimbursement costs for HCV antibody testing multiplied by the number of tests conducted. For both the standard-of-care and the intervention costs per person tested were estimated as aggregated costs divided by the number of individuals tested. The incremental cost per additional person tested was calculated by subtracting cost per-person-tested in the standard-of-care group from the cost per-person-tested in the intervention group (test costs and intervention costs). The incremental cost per-HCV positive case identified was calculated as the aggregated program and testing costs in the intervention group minus the aggregated testing costs in the standard-of-care group divided by the number of positive patients identified in the intervention group minus the number of positive patients identified in the standard-of-care group.
Results
Study Sample
At centers 1, 2, and 3, 8,992, 14,475 and 8,873 patients were eligible for participation, respectively (Appendix Figures 1–3). Patients randomized to the intervention and standard-of-care groups at the repeated-mailings center were similar with respect to sex, ethnicity, race, median household income, birth year and insurance type (Table 1). At centers 2 and 3, statistically significant differences (p ≤ 0.05) in the intervention and standard-of-care groups were observed with regard to sex, ethnicity, race, MHI, and insurance type. Further, at center 3, there were statistically significant differences between the intervention and standard-of-care groups by birth year.
Table 1.
Patient demographic characteristics for intervention and standard-of-care groups by intervention type to increase hepatitis C virus antibody testing among persons born during 1945–1965
| Center (Intervention Type) | ||||||
|---|---|---|---|---|---|---|
| Center 1 (Repeated-mailings) | Center 2 (Best Practice Alert) | Center 3 (Patient-solicitation) | ||||
| Patient Characteristics | Birth Cohort (%) |
Standard-of-care (%) |
Birth Cohort %) |
Standard-of-care (%) |
Birth Cohort (%) |
Standard-of-care (%) |
| Sexa | ||||||
| Female | 1686 (56.3) | 3372 (56.2) | 5616 (62.9) | 3260 (58.8) | 2560 (59.4) | 2614 (57.3) |
| Male | 1307 (43.7) | 2627 (43.8) | 3309 (37.1) | 2285 (41.2) | 1747 (40.6) | 1952 (42.7) |
|
| ||||||
| Ethnicitya | ||||||
| Non-Hispanic | 1825 (61.0) | 3715 (61.9) | 5135 (57.5) | 2944 (53.1) | 4290 (99.6) | 4289 (93.9) |
| Hispanic | 36 (1.2) | 89 (1.5) | 899 (10.1) | 467 (8.4) | 15 (0.4) | 18 (0.4) |
| Unknown | 1132 (37.8) | 2195 (36.6) | 2894 (32.4) | 2136 (38.5) | 2 (0.1) | 259 (5.7) |
|
| ||||||
| Racea | ||||||
| Black | 924 (30.9) | 1878 (31.3) | 1002 (11.2) | 366 (6.6) | 2195 (51.0) | 1854 (40.6) |
| White | 1540 (51.5) | 3054 (50.9) | 5587 (62.6) | 3931 (70.9) | 1993 (46.3) | 2544 (55.7) |
| Asian | 86 (2.9) | 228 (3.8) | 154 (1.7) | 104 (1.9) | 73 (1.7) | 94 (2.1) |
| Other | 22 (0.7) | 41 (0.7) | 471 (5.3) | 226 (4.1) | 24 (0.6) | 20 (0.4) |
| Unknown | 421 (14.1) | 798 (13.3) | 1714 (19.2) | 920 (16.6) | 22 (0.5) | 54 (1.2) |
|
| ||||||
| Median Household Income (in dollars) of ZIP Code of Residencea | ||||||
| <30,000 | 357 (11.9) | 728 (12.1) | 394 (4.4) | 195 (3.5) | 849 (19.7) | 745 (16.3) |
| 30,000–49,999 | 665 (22.2) | 1335 (22.3) | 1472 (16.5) | 634 (11.4) | 1817 (42.2) | 1794 (39.3) |
| 50,000–69,999 | 679 (22.7) | 1364 (22.7) | 1041 (11.7) | 371 (6.7) | 745 (17.3) | 872 (19.1) |
| 70,000–99,999 | 552 (18.4) | 1167 (19.5) | 2287 (25.6) | 1259 (22.7) | 752 (17.5) | 966 (21.2) |
| >100,000 | 282 (9.4) | 537 (9.0) | 3604 (40.4) | 2978 (53.7) | 62 (1.4) | 119 (2.6) |
| Unknown | 458 (15.3) | 868 (14.5) | 130 (1.5) | 110 (2.0) | 82 (1.9) | 70 (1.5) |
|
| ||||||
| Birth Yearb | ||||||
| 1945–50 | 722 (24.1) | 1425 (23.8) | 2030 (22.7) | 1240 (22.4) | 1077 (25.0) | 993 (21.8) |
| 1950–54 | 752 (25.1) | 1443 (24.1) | 2043 (22.9) | 1258 (22.7) | 1182 (27.4) | 1231 (27.0) |
| 1955–59 | 732 (24.5) | 1571 (26.2) | 2236 (25.0) | 1375 (24.8) | 1103 (25.6) | 1106 (24.2) |
| 1960–1965 | 787 (26.3) | 1560 (26.0) | 2619 (29.3) | 1674 (30.2) | 945 (21.9) | 1236 (27.1) |
|
| ||||||
| Insurance Typea | ||||||
| Private | 2522 (84.3) | 5043 (84.1) | 5807 (65.0) | 3610 (65.1) | 2788 (64.7) | 3377 (74.0) |
| Public | 455 (15.2) | 934 (15.6) | 1858 (20.8) | 1026 (18.5) | 1444 (33.5) | 1141 (25.0) |
| Uninsured or unknown | 16 (0.5) | 22 (0.4) | 1263 (14.2) | 911 (16.4) | 75 (1.7) | 48 (1.1) |
Differences in the intervention and control group are statistically significant at the best practice alert and the patient-solicitation centers;
Differences in the intervention and control group are statistically significant at the patient-solicitation center
Characteristics of Patients Tested for HCV
Overall 9.9%, 20.4% and 31.9% of patients received an HCV antibody test at centers 1, 2 and 3, respectively. Across centers HCV antibody testing occurred more frequently in the intervention than the standard-of-care group: 26.9% vs. 1.4% for center 1, 30.9% vs. 3.6% for center 2, and 63.5% vs. 2.0% for center 3. Multivariable modelling revealed that HCV antibody testing was significantly more common in the intervention than the standard-of-care group, aRR 19.2 (95% CI 9.70–38.15) at center 1; aRR 13.2 (95% CI 3.58–48.62) at center 2; and aRR 32.93 (95% CI 19.34–56.05) at t center 3 (Table 2). Other predictors of HCV antibody testing varied by center.
Table 2.
Estimated Adjusted Risk Ratios (aRR) and 95% Confidence Intervals (CI) of birth cohort hepatitis C antibody testing compared with standard-of-care testing by center and patient characteristics
| Center (Intervention type) | |||
|---|---|---|---|
| Center 1 (Repeated, mailings) aRR (95% CI) |
Center 2 (Best Practice Alert) aRR (95% CI) |
Center 3 (Patient, solicitation RR (95% CI) |
|
| Testing Group | |||
| Standard-of-care (Risk based) | ref. | ref. | ref. |
| Birth Cohort (Intervention) |
19.24 (9.70, 38.15) | 13.19 (3.58, 48.62) | 32.93 (19.34, 56.05) |
|
| |||
| Sex | |||
| Female | ref. | ref. | ref. |
| Male | 0.94 (0.82, 1.06) | 1.08 (0.97, 1.20) | 1.03 (ref., 1.07) |
|
| |||
| Ethnicity | |||
| Non, Hispanic | ref. | ref. | ref. |
| Hispanic | 0.70 (0.48, 1.01) | 0.98 (0.88, 1.09) | 2.94 (1.24, 6.98) |
| Unknown | 0.66 (0.57, 0.77) | 0.95 (0.92, 0.98) | 1.60 (1.05, 2.45) |
|
| |||
| Race | |||
| Black | 0.79 (0.67, 0.92) | 1.00 (0.91, 1.10) | 0.94 (0.83, 1.07) |
| White | ref. | ref. | ref. |
| Asian | 0.89 (0.68, 1.16) | 1.09 (0.82, 1.44) | 0.95 (0.79, 1.13) |
| Other | 0.77 (0.42, 1.41) | 0.93 (0.79, 1.09) | 0.81 (0.36, 1.85) |
| Unknown | 1.07 (0.92, 1.24) | 0.98 (0.91, 1.06) | 0.35 (0.18, 0.67) |
|
| |||
| Median Household Income (in dollars) of ZIP Code of Residence | |||
| <30,000 | 0.87 (0.75, 1.01) | 0.96 (0.84, 1.10) | 1.03 (0.96, 1.10) |
| 30,000, 49,999 | 0.87 (0.77, 0.98) | 0.87 (0.78, 0.96) | 1.04 (0.97, 1.10) |
| 50,000, 69,999 | ref. | ref. | ref. |
| 70,000, 99,999 | 0.98 (0.83, 1.16) | 1.00 (0.93, 1.08) | 0.92 (0.81, 1.05) |
| >100,000 | 1.13 (0.95, 1.35) | 1.01 (0.96, 1.06) | 0.71 (0.54, 0.95) |
| Unknown | 0.60 (0.52, 0.70) | 0.88 (0.58, 1.36) | 1.02 (0.92, 1.12) |
|
| |||
| Birth Year | |||
| 1945, 50 | 1.50 (1.31, 1.71) | 1.11 (0.98, 1.26) | 1.11 (1.04, 1.19) |
| 1950, 54 | 1.41 (1.26, 1.57) | 1.05 (0.99, 1.11) | 1.01 (0.96, 1.07) |
| 1955, 59 | 1.16 (ref., 1.34) | 1.01 (0.94, 1.09) | 1.02 (0.98, 1.05) |
| 1960, 65 | ref. | ref. | ref. |
|
| |||
| Insurance Type | |||
| Private | ref. | ref. | ref. |
| Public | 1.27 (1.10, 1.48) | 0.82 (0.72, 0.94) | 0.81 (0.75, 0.89) |
| Uninsured or Unknown | 0.70 (0.28, 1.71) | 0.89 (0.74, 1.07) | 0.84 (0.59, 1.20) |
When restricting the analysis to those in the intervention groups, multivariable modelling revealed that at center 1, the strongest statistically significant predictors of testing were median household income ≥ $100,000 aRR 1.17, (95% CI 1.03–1.32) compared with $50,000–69,000; being born before 1950 aRR 1.59 (95% CI 1.38–1.84) or 1950–54 aRR 1.46 (95% CI 1.2–1.66) compared with those born in or after 1960; and having Medicare or Medicaid as the primary insurance aRR 1.26 (95% CI 1.12–1.41) compared with having with private insurance. In the contrast, at centers 2 and 3, there were no strong demographic predictors of testing within the intervention groups (Table 3).
Table 3.
Estimated adjusted risk ratios (aRR) and 95% confidence intervals (CI) of hepatitis C antibody testing for patients assigned to the intervention group
| Center (Intervention Type) | |||
|---|---|---|---|
| Patient Characteristics | Center 1 (Repeated-mailings) aRR 95% CI |
Center 2 (Best Practice Alert) aRR 95% CI |
Center 3 (Patient-solicitation) aRR 95% CI |
|
| |||
| Sex | |||
| Female | ref. | ref. | ref. |
| Male | 0.88 (0.80, 0.98) | 1.06 (0.95, 1.19) | 1.01 (0.96, 1.07) |
|
| |||
| Ethnicitya | |||
| Non-Hispanic | ref. | ref. | |
| Hispanic | 0.72 (0.43, 1.21) | 1.03 (0.94, 1.12) | |
| Unknown | 0.66 (0.56, 0.77) | 0.94 (0.91, 0.98) | |
|
| |||
| Race | |||
| Black | 0.76 (0.66, 0.89) | 1.04 (0.98, 1.10) | 0.97 (0.85, 1.11) |
| White | ref. | ref. | ref. |
| Asian | 0.75 (0.56, 1.00) | 1.13 (0.87, 1.48) | 0.90 (0.76, 1.05) |
| Other | 0.82 (0.46, 1.45) | 0.95 (0.82, 1.10) | 0.95 (0.49, 1.86) |
| Unknown | 1.02 (0.85, 1.22) | 1.00 (0.93, 1.08) | 0.72 (0.48,1.07) |
|
| |||
| Median Household Income (in dollars) of Zip Code of Residence | |||
| <30,000 | 0.81 (0.69, 0.97) | 1.00 (0.85, 1.16) | 1.02 (0.97, 1.07) |
| 30.000–49,999 | 0.86 (0.77, 0.97) | 0.91 (0.84, 0.97) | 1.03 (0.99, 1.08) |
| 50.000–69,999 | ref. | ref. | ref. |
| 70.000–99,999 | 1.01 (0.88, 1.16) | 1.01 (0.95, 1.08) | 0.95 (0.86, 1.05) |
| ≥100,000 | 1.17 (1.03, 1.32) | 1.01 (0.96, 1.06) | 0.77(0.58, 1.03) |
| Unknown | 0.61 (0.49, 0.76) | 0.91 (0.59, 1.39) | 0.96 (0.85, 1.08) |
|
| |||
| Birth Year | |||
| 1945–50 | 1.59 (1.38, 1.84) | 1.12 (0.98, 1.27) | 1.14 (1.09, 1.19) |
| 1950–54 | 1.46 (1.29, 1.66) | 1.07 (1.02, 1.13) | 1.01 (0.96, 1.06) |
| 1955–59 | 1.19 (0.99, 1.42) ref. | 1.00 (0.94, 1.07) ref. | 1.00 (0.94, 1.06) ref. |
| 1960–65 | ref. | ref. | ref. |
|
| |||
| Insurance Type | |||
| Private | ref. | ref. | ref. |
| Public | 1.26 (1.12, 1.41) | 0.84 (0.74, 0.96) | 0.80 (0.75, 0.84) |
| Uninsured or unknown | 0.75 (0.29, 1.97) | 0.92 (0.76, 1.10) | 0.91 (0.69, 1.20) |
Hispanic ethnicity was excluded from the model of patient-solicitation intervention group because the model would not converge when it was included
Costs of HCV Antibody Testing
In the standard-of-care groups, cost per HCV antibody test (including supplies and processing) was $19, $20, and $25 (Table 4) for centers 1, 2 and 3, respectively. The cost per HCV-positive patient identified for the standard-of-care group ranged $798, $644, and $459 for centers 1, 2 and 3, respectively. Intervention costs per patient tested and per HCV-positive patient varied substantially by intervention. The BPA intervention at center 2 had the lowest cost per completed test ($44), and when omitting fixed startup costs the BPA intervention had an even lower cost per test completed ($23). The cost per test completed was higher for the interventions at centers 1 and 3 (repeated-mailings $63 and patient-solicitation $53). Over the study period, the patient-solicitation intervention at center 3 had lowest cost per new HCV positive patient identified ($4,230), but cost per HCV-positive patient identified for the BPA intervention at center 2 was similar ($4,527). The costs per HCV-positive patient identified for the repeated-mailing intervention at center 1 was $7,005. At center 1, testing resulted in the detection of 8 cases in the intervention group vs. 2 cases in the standard-of-care group; at center 2, there were 27 cases in intervention group and 6 in the standard-of-care group; and, at center 3, there were 34 cases in the intervention group and 2 in the standard-of-care group. Incremental cost per additional person tested was lowest for center 2 (the BPA intervention $24 including fixed startup costs, and $3 without fixed startup costs); the incremental cost per additional HCV positive test identified was also lowest for the BPA intervention when excluding fixed startup costs ($1,691), however including startup costs the incremental cost was $3,883, very similar to the patient-solicitation intervention at center 3 ($3,771).
Table 4.
The number of patients for hepatitis C antibody, the number positive, total cost of testing over the study time period, costs per-person-tested, and costs per-positive-identified by centers intervention group
| Center (Intervention Type) | Testing Group | No. Tested | No. Positive (%) | Total Costs in $ | Cost Per-Person-Tested in $ | Cost Per-Positive-Identified in $ | Incremental Cost Per Additional Person Tested in $ | Incremental Cost Per Additional Positive Identified in $ |
|---|---|---|---|---|---|---|---|---|
| Center 1 (Repeated-mailings) | Standard-of-care (Risk-Based) Birth Cohort (Intervention) |
84 805 |
2 (2.4) 8 (1.0) |
1,596 56,039 |
19 63 |
798 7,005 |
44 | 6,207 |
| Center 2 (Best Practice Alert All costs) | Standard-of-care (Risk-Based) Birth Cohort (Intervention) |
197 2757 |
6 (3.0) 27 (1.0) |
1,648 122,223 |
20 44 |
644 4,527 |
24 | 3,883 |
| Center 2 (Best Practice Alert Omitting fixed startup costs) | Standard-of-care (Risk-Based) Birth Cohort (Intervention) |
197 2757 |
6 (3.0) 27 (1.0) |
1,648 63,059 |
20 23 |
644 2,336 |
3 | 1,691 |
| Center 3 (Patient-Solicitation) | Standard-of-care (Risk-Based) Birth Cohort (Intervention) |
92 2736 |
5 (5.4) 34 (1.2) |
2,295 143,815 |
25 53 |
459 4,230 |
28 | 3,771 |
Discussion
The results of this study show that the HCV testing interventions were successful at increasing HCV antibody testing in the 1945–1965 BC compared with standard-of-care, but also increased the aggregate and per person costs of testing. In multivariable models, patients born during 1945–1950, and those who had Medicare or Medicaid insurance were more likely to be tested for HCV. Other factors associated with HCV testing varied by center. Different interventions may be effective at eliciting participation from different patient populations; for example, individuals receiving a mailed reminder requires active participation and interpretation of mailed content, whereas with other two interventions are incorporated into a routine patient visit and involve direct communication between a provider and patient, likely decreasing barriers to testing.
The overall findings of increased HCV antibody testing rates due to testing interventions are consistent with other studies.[34–37] An intervention that aimed to provide HCV BC testing in conjunction with colonoscopy screening reported an increase in HCV testing.[38] A systematic review of observational and randomized controlled studies that examined the effectiveness of interventions aiming to raise awareness about, or engagement in HCV testing found that several interventions increased HCV testing among high-risk groups.[34] While the effect size for each intervention varied across studies of high-risk populations, the highest were for interventions that provided testing in community settings.[34] A separate systematic review of observational and randomized controlled studies examined the effectiveness of targeted testing interventions on HCV test uptake and found that practitioner-based interventions were effective in increasing test uptake, but media/information-based interventions were less effective.[39] A serial cross-sectional evaluation of two community-based primary care interventions in New York, found that instituting clinical reminders was associated with significantly increased HCV testing rates.[35] A related study that implemented a paper-based clinical reminder sticker to prompt practitioners to order an HCV test if a patient had any HCV risk factor at three urban primary care clinics in New York, also resulted in increased HCV testing.[36]
The optimal strategy for engaging patients to increase HCV antibody testing is not known and likely involves a center-specific context-dependent multiple-strategy approach. In the current study, each intervention resulted in varied testing rates and costs per-person tested. All centers reported that the interventions were a considerable resource burden, which was reflected in both aggregate and per [40–44] person-tested intervention costs.[24] While HCV testing rates increased most with the repeated-mailings and patient-solicitation interventions, these interventions were also more costly, in terms of cost per person-tested. The BPA intervention had the lowest cost per HCV test, and the lowest cost per HCV-positive patient identified when omitting fixed startup costs. The fixed costs of the BPA intervention would be regardless of the patients tested, thus total costs per person tested for the intervention at Center 2 would decrease over time. The BPA intervention costs per HCV-positive patient identified were comparable to costs demonstrated in a cost simulation study of HCV testing; however the simulation study was based on sexually active males, age ≥ 40 years, reporting < 100 lifetime sexual partners and no history of injection drug use.[45]
Multiple studies have demonstrated that the overall strategy of HCV case identification followed by treatment is cost-effective relative to commonly accepted thresholds.[11, 14, 40–44] Our study differs from these in that it estimates only the cost per case and positive case identified without subsequent simulation steps to capture the benefits of such testing. Cost-per-outcome studies such as this one are used to assess the relative costs of different interventions designed to achieve an essential process indicator. Additional HCV testing interventions will always increase costs when their benefits are not taken into account, and the fact that more testing resulted in higher costs says nothing about the cost-effectiveness strategies. However, a previous cost-effectiveness study found that HCV testing followed by DAA treatment was cost-effective ($25,000 per quality adjusted life year gained) at a cost per person tested that was the same as what we estimated for the BPA intervention after its fixed startup costs were excluded ($25 per person tested). This result was almost entirely unchanged in sensitivity analyses which used higher testing costs ($32 per person tested) and was estimated using the list price of sofusbuvir and ledipasvir or a combination of ombitasvir/paritaprevir/ritonavir tablet taken with a dasabuvir tablet.[11, 14] When compared to standard-of-care, HCV test and treat strategies would likely be cost-effective using any of the three intervention cost estimates found by this study.[12] While every effort should be made to reduce the costs of testing interventions by implementing BPAs, interventions to increase testing can also be considered in settings that lack electronic health records or the technical capacity to modify them.
This study is subject to several notable limitations. First, while this investigation examined data from three randomized HCV antibody testing trials, each study examined a different intervention and had a distinct methodology and research team. While each center utilized randomization, imbalances between baseline patient characteristics was observed between the intervention and the standard-of-care groups in two of the trials because of differences in randomization designs-simple randomization, cluster randomization, and cluster crossover randomization; we controlled for the imbalances in patient characteristics using multivariable analysis. Second, the expanded CDC and U.S Preventive Services Task Force HCV BC screening recommendations were released during the study period, and these recommendations could have influenced a patient’s decision to receive an HCV test. However, given the temporal difference of the testing period, both patients in the intervention and the standard-of-care groups would have been exposed to these recommendations and thus we would expect the reported findings to be an underestimate of the effects of testing interventions. Third, this paper describes the experience of three interventions implemented in primary care settings; it is not an exhaustive examination of HCV testing interventions in primary care or other settings. Fourth, this study did not account for the cost of doing risk elicitation among patients in the control group, which would result in the control testing costs to be lower than they really are. Fifth, because of the nature of the intervention at center 1, patients were sent repeated mailings, center 1 reached the desired sample size sooner and thus, center 1 had a shorter implementation period than centers 2 and 3.
Conclusions
Compared with the standard-of-care (risk-based testing), interventions designed to increase HCV testing among the 1945–1965 BC in primary care settings resulted in increased HCV testing, but also increased costs.19 Careful consideration of the increases in HCV testing and HCV diagnoses, as well as the resources needed and the costs associated with implementing an intervention are needed to ascertain which interventions are feasible to implement. The cost per additional person tested and the cost per HCV infected person identified excluding startup costs were lowest for the BPA intervention, suggesting that integrating BC testing into standard-of-care is likely to be more cost-effective and practical than instituting an intervention in addition to standard-of-care, such as repeated-mailings and patient-solicitation.
Acknowledgments
Source of Support: This study was funded by through the National Viral Hepatitis Action Coalition a charitable mechanism set up by the National Foundation for the Centers for Disease Control and Prevention, Inc. MOU # 527-11 SC. In 2015, there were ten corporate members, of the CDC Foundation including Abbott Laboratories, AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme Corporation, OraSure Technologies, Quest Diagnostics and Siemens Healthcare, Inc.
Two non-profit organizations, the Association of State and Territorial Health Officials, and the National Viral Hepatitis Roundtable (NVHR), and a representative from the US Department of Health and Human Services (HHS), Office of HIV/AIDS and Infectious Disease Policy also participated in Coalition activities.
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
List of Abbreviations
- Anti-HCV+
hepatitis C virus antibody positive
- aRR
adjusted risk ratio
- BC
birth-cohort
- BPA
best practice alert
- BEST-C
Birth-cohort Evaluation to Advance Screening and Testing for Hepatitis C
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- DAA
direct acting antiviral
- HER
electronic health record
- HCV
hepatitis C virus
Appendix Figure 1a. Repeated-Mailing Outreach: Participant Flow.
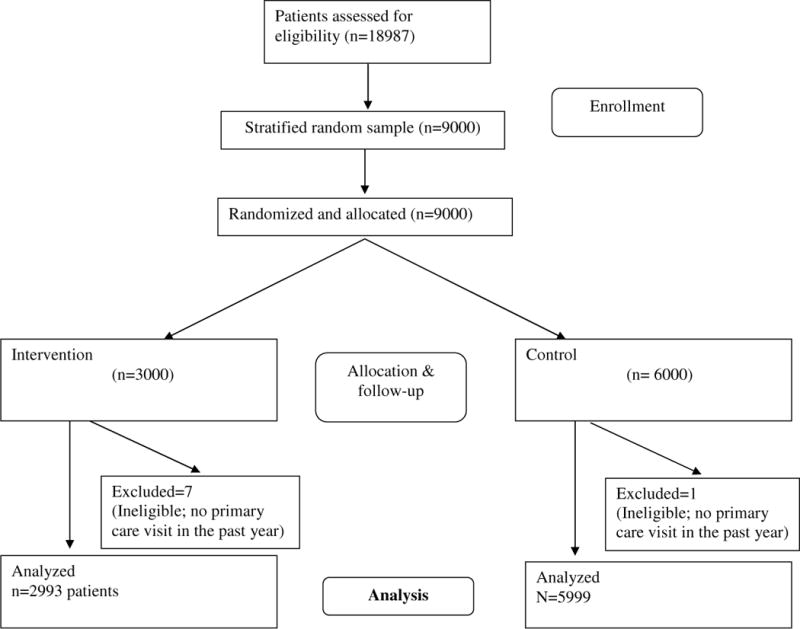
This flowchart was developed as part of another manuscript that is currently undergoing peer review: Yartel AK; Rein DB; Brown KA; Krauskopf K; Massoud OI; Jordan C; Kil N; Federman AD; Nerenz DR; Brady JE; Liffman DK; Smith, BD. “Effectiveness of Hepatitis C Virus Testing for Case Identification in Persons Born during 1945–1965: Results from Three Randomized Controlled Trials.” Annals of Internal Medicine.
Appendix Figure 1b. Electronic Medical Record Best Practice Alert: Participant Flow.
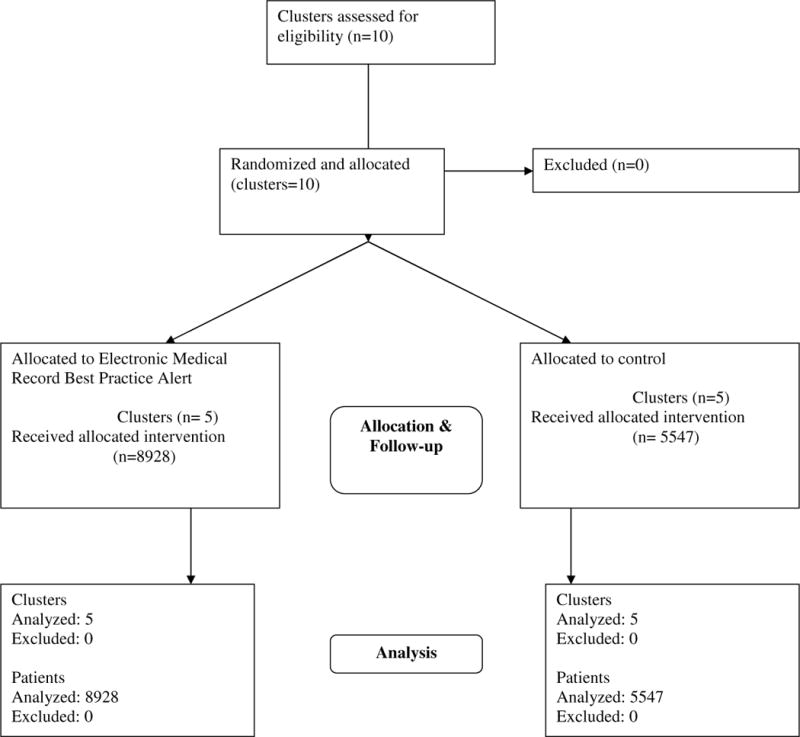
This flowchart was developed as part of another manuscript that is currently undergoing peer review: Yartel AK; Rein DB; Brown KA; Krauskopf K; Massoud OI; Jordan C; Kil N; Federman AD; Nerenz DR; Brady JE; Liffman DK; Smith, BD. “Effectiveness of Hepatitis C Virus Testing for Case Identification in Persons Born during 1945–1965: Results from Three Randomized Controlled Trials.” Annals of Internal Medicine.
Figure 1c. Patient Solicitation Outpatient Visit: Cluster and Participant Flow.
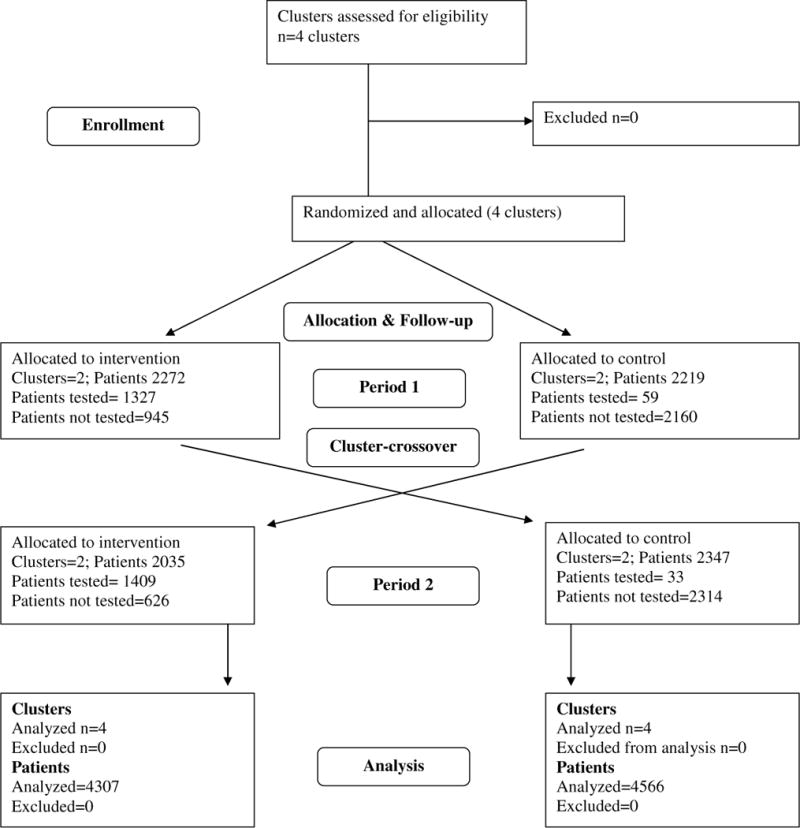
This flowchart was developed as part of another manuscript that is currently undergoing peer review: Yartel AK; Rein DB; Brown KA; Krauskopf K; Massoud OI; Jordan C; Kil N; Federman AD; Nerenz DR; Brady JE; Liffman DK; Smith, BD. “Effectiveness of Hepatitis C Virus Testing for Case Identification in Persons Born during 1945–1965: Results from Three Randomized Controlled Trials.” Annals of Internal Medicine.
Appendix 2
Sample size calculations were based on projected hepatitis C antibody positive rates, not hepatitis C antibody testing rates.
Sample size estimation for repeated-mailing trial
For the repeated-mailing trial, based on projections from pilot data, we estimated that 8,400 patients would be needed to achieve a minimum power of 80% assuming a two-tailed type I error of 5%, and hepatitis C antibody positive identification rates, respectively, of 3.8 and 0.5 cases per 1000 eligible patients in the BC and control groups.
Sample size estimation for BPA trial
The initial sample size calculation was based on eight clusters with two extras to preserve statistical power in the event of cluster loss. We estimated that 440 patients per cluster (4400 patients total) would be required to achieve a power of 80% assuming equal number of participants per cluster, a 5% two-tailed type I error, an intracluster correlation coefficient (ICC) of 0.005, and HCV antibody positive identification rates of 3.5% and 1%, respectively, in the BC and controls groups. This trial was terminated at the beginning of March 2014, immediately after a law in New York State requiring BC testing as standard went into effect.
Sample size estimation for patient-solicitation trial
We determined that 1,240 patients per cluster (4960 patients total) would be sufficient to produce a minimum power of 80% assuming equal participants per cluster, a two-tailed type I error of 5%, ICC of 0.005, interperiod correlation coefficient of 0.0025 (assumed to be half of ICC), and hepatitis C antibody positive identification rates of 1.5% and 0.03%, respectively, in the BC and control groups. In each cluster, after accounting for about 10% participant inflation, it was estimated that 682 patients would receive the intervention in period one and another 682 patients would receive usual care in period two.
This write up was developed as part of another manuscript that is currently undergoing peer review: Yartel AK; Rein DB; Brown KA; Krauskopf K; Massoud OI; Jordan C; Kil N; Federman AD; Nerenz DR; Brady JE; Liffman DK; Smith, BD. “Effectiveness of Hepatitis C Virus Testing for Case Identification in Persons Born during 1945–1965: Results from Three Randomized Controlled Trials.” Annals of Internal Medicine.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1002/hep.28880
References
- 1.Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. Jama. 2014;312:631–640. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
- 2.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353–1363. doi: 10.1002/hep.27978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66–72. doi: 10.1016/j.dld.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Spradling PR, Rupp L, Moorman AC, Lu M, Teshale EH, Gordon SC, Nakasato C, et al. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55:1047–1055. doi: 10.1093/cid/cis616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis – United States 2013. Atlanta: 2015. Division of Viral Hepatitis and National Center for HIV/AIDS VH, STD, and TB Prevention. [Google Scholar]
- 7.Asrani SK, Davis GL. Impact of birth cohort screening for hepatitis C. Curr Gastroenterol Rep. 2014;16:381. doi: 10.1007/s11894-014-0381-5. [DOI] [PubMed] [Google Scholar]
- 8.Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 9.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 10.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 11.Rein DB, Wittenborn JS, Smith BD, Liffmann DK, Ward JW. The cost-effectiveness, health benefits, and financial costs of new antiviral treatments for hepatitis C virus. Clin Infect Dis. 2015;61:157–168. doi: 10.1093/cid/civ220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rein DB. Reply to Messori. Clin Infect Dis. 2015;61:1892–1893. doi: 10.1093/cid/civ668. [DOI] [PubMed] [Google Scholar]
- 13.Nisen M. Merck Throws a Wrench in Drug Pricing. BloombergGadfly. 2016 [Google Scholar]
- 14.Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, Patel N, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–270. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis. 2012;54:1259–1271. doi: 10.1093/cid/cis011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGarry LJ, Pawar VS, Panchmatia HR, Rubin JL, Davis GL, Younossi ZM, Capretta JC, et al. Economic model of a birth cohort screening program for hepatitis C virus. Hepatology. 2012;55:1344–1355. doi: 10.1002/hep.25510. [DOI] [PubMed] [Google Scholar]
- 17.McEwan P, Kim R, Yuan Y. Assessing the cost utility of response-guided therapy in patients with chronic hepatitis C genotype 1 in the UK using the MONARCH model. Appl Health Econ Health Policy. 2013;11:53–63. doi: 10.1007/s40258-012-0002-0. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Cipriano LE, Holodniy M, Goldhaber-Fiebert JD. Cost-effectiveness analysis of risk-factor guided and birth-cohort screening for chronic hepatitis C infection in the United States. PLoS One. 2013;8:e58975. doi: 10.1371/journal.pone.0058975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith BD, Yartel AK, Krauskopf K, Massoud OI, Brown KA, Fallon MB, Rein DB. Hepatitis C Virus Antibody Positivity and Predictors Among Previously Undiagnosed Adult Primary Care Outpatients: Cross-Sectional Analysis of a Multisite Retrospective Cohort Study. Clin Infect Dis. 2015 doi: 10.1093/cid/civ002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith BDYA, Rein DB, Brown K, Krauskopf K, Massoud O, Federman AD, Jordan CE, Kil N, Nerenz DR, Liffmann D. Effectiveness of Hepatitis C Virus (HCV) Testing for Persons Born during 1945–1965 – Summary Results from Three Randomized Controlled Trials. Annual Meeting of the American Association for the Study of Liver Disease (AASLD); Boston, MA. 2014. [Google Scholar]
- 21.CDC Foundation. Birth-cohort Evaluation to Advance Screening and Testing for Hepatitis C. 2014 [Google Scholar]
- 22.Hoddinott SN, Bass MJ. The dillman total design survey method. Can Fam Physician. 1986;32:2366–2368. [PMC free article] [PubMed] [Google Scholar]
- 23.Yartel AKRD, Brown KA, Krauskopf K, Massoud OI, Jordan C, Kil N, Federman AD, Nerenz DR, Brady JE, Liffmann DK, Smith BD. Effectiveness of Hepatitis C Virus Testing for Persons Born during 1945–1965 – Summary Results from Three Randomized Controlled Trials. Annals of Internal Medicine In preparation [Google Scholar]
- 24.Liffmann DKRD, Kil N, Jordan C, Brown KA, Yartel A, Smith BD. Implementation of Birth Cohort Testing for Hepatitis C Virus: Lessons Learned from 3 Primary Care Sites. Health Promotion Practice. 2016 doi: 10.1177/1524839916661495. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rietbergen Charlotte, Mirjam M. The Design of Cluster Randomized Crossover Trials. Journal of Educational and Behavioral Statistics. 2011;36:72–490. [Google Scholar]
- 26.United States Census Bureau. American Community Survey. 2014 [Google Scholar]
- 27.Canby JBt. Applying activity-based costing to healthcare settings. Healthc Financ Manage. 1995;49:50–52. 54–56. [PubMed] [Google Scholar]
- 28.Zarkin GA, Bray JW, Mitra D, Cisler RA, Kivlahan DR. Cost methodology of COMBINE. J Stud Alcohol Suppl. 2005:50–55. doi: 10.15288/jsas.2005.s15.50. discussion 33. [DOI] [PubMed] [Google Scholar]
- 29.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Lu N, Feng C, Thurston SW, Xia Y, Zhu L, Tu XM. On fitting generalized linear mixed-effects models for binary responses using different statistical packages. Stat Med. 2011;30:2562–2572. doi: 10.1002/sim.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu M. Analyzing multilevel models with the glimmix procedure. 2014 [Google Scholar]
- 32.Li L. Fitting multivariable random-effects models using SAS Proc Glimmix. 2010 [Google Scholar]
- 33.Ying GaL C. Statistical analysis of clustered data using SAS system. NorthEast SAS Users Group; Portland ME: 2006. pp. 1–13. [Google Scholar]
- 34.Jones L, Bates G, McCoy E, Beynon C, McVeigh J, Bellis MA. Effectiveness of interventions to increase hepatitis C testing uptake among high-risk groups: a systematic review. Eur J Public Health. 2014;24:781–788. doi: 10.1093/eurpub/ckt156. [DOI] [PubMed] [Google Scholar]
- 35.Litwin AH, Smith BD, Drainoni ML, McKee D, Gifford AL, Koppelman E, Christiansen CL, et al. Primary care-based interventions are associated with increases in hepatitis C virus testing for patients at risk. Dig Liver Dis. 2012;44:497–503. doi: 10.1016/j.dld.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Drainoni ML, Litwin AH, Smith BD, Koppelman EA, McKee MD, Christiansen CL, Gifford AL, et al. Effectiveness of a risk screener in identifying hepatitis C virus in a primary care setting. Am J Public Health. 2012;102:e115–121. doi: 10.2105/AJPH.2012.300659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson EM, Mandeville RP, Hutchinson SJ, Cameron SO, Mills PR, Fox R, Ahmed S, et al. Evaluation of a general practice based hepatitis C virus screening intervention. Scott Med J. 2009;54:3–7. doi: 10.1258/RSMSMJ.54.3.3. [DOI] [PubMed] [Google Scholar]
- 38.Sears DM, Cohen DC, Ackerman K, Ma JE, Song J. Birth cohort screening for chronic hepatitis during colonoscopy appointments. Am J Gastroenterol. 2013;108:981–989. doi: 10.1038/ajg.2013.50. [DOI] [PubMed] [Google Scholar]
- 39.Aspinall EJ, Doyle JS, Corson S, Hellard ME, Hunt D, Goldberg D, Nguyen T, et al. Targeted hepatitis C antibody testing interventions: a systematic review and meta-analysis. Eur J Epidemiol. 2015;30:115–129. doi: 10.1007/s10654-014-9958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chahal HS, Marseille EA, Tice JA, Pearson SD, Ollendorf DA, Fox RK, Kahn JG. Cost-effectiveness of Early Treatment of Hepatitis C Virus Genotype 1 by Stage of Liver Fibrosis in a US Treatment-Naive Population. JAMA Intern Med. 2015:1–9. doi: 10.1001/jamainternmed.2015.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162:397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leidner AJ, Chesson HW, Xu F, Ward JW, Spradling PR, Holmberg SD. Cost-effectiveness of hepatitis C treatment for patients in early stages of liver disease. Hepatology. 2015;61:1860–1869. doi: 10.1002/hep.27736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linas BP, Barter DM, Morgan JR, Pho MT, Leff JA, Schackman BR, Horsburgh CR, et al. The cost-effectiveness of sofosbuvir-based regimens for treatment of hepatitis C virus genotype 2 or 3 infection. Ann Intern Med. 2015;162:619–629. doi: 10.7326/M14-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Najafzadeh M, Andersson K, Shrank WH, Krumme AA, Matlin OS, Brennan T, Avorn J, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162:407–419. doi: 10.7326/M14-1152. [DOI] [PubMed] [Google Scholar]
- 45.Honeycutt AAHJ, Khavjou O, Buffington J, Jones JT, Rein DB. The Costs and Impacts of testing for Hepatitis C Virus Antibodies In Public STD Clinics. Public Health Reports. 2007;122:55–62. doi: 10.1177/00333549071220S211. [DOI] [PMC free article] [PubMed] [Google Scholar]


