Abstract
Scope
The uptake of dietary plant small RNAs (sRNAs) in consumers remains controversial, which is mainly due to low dietary content in combination with poor fractional absorption. MIR2911, among all the plant sRNAs including microRNAs, has been shown to be one of the most robustly absorbed sRNAs. Here we analyze the unusual abundance and unique genesis of MIR2911 during vegetable processing.
Methods and Results
Using qRT-PCR, the abundance of MIR2911 increased dramatically in macerated tissues while other microRNAs degraded. The accumulation of MIR2911 correlated with the degradation of the rRNAs, consistent with MIR2911 being derived from the 26S rRNA. Bioinformatic analysis predicts a microRNA-like precursor structure for MIR2911; however, no reciprocal increase in the putative star-strand was noted, and using an Arabidopsis mutation deficient in miRNA processing the accumulation of MIR2911 appeared Dicer independent. MIR2911 was incorporated into the mammalian RNA-induced silencing complex as demonstrated in HEK293T cells, where transfected synthetic MIR2911 modestly suppressed the activity of a cognate luciferase reporter.
Conclusion
The genesis and increase of MIR2911 post-harvest is atypical, as traditional plant bioactives are less plentiful as vegetables lose freshness. These findings explain the disparity in sera detection between MIR2911 and canonical plant-based miRNAs.
Keywords: absorption, bioavailability, microRNA, food consumption marker, vegetables
Introduction
Contentious reports suggest that small RNAs (sRNAs) ingested from plant-based foods act as bioactives [1–6]. Contemporaneously, several groups have failed to detect dietary RNAs in animals [7–9]. Bioavailability is defined as the fraction of a dietary substance that reaches the systemic circulation and the bioavailability of plant-based dietary sRNAs appears to be, at best, low [5, 7, 10]
The activity of plant based microRNAs (miRNAs) in animals consuming plant-based foods was first reported with a rice-based miRNA MIR168a, and subsequent studies focused on the 26S ribosomal RNA (rRNA)-derived sRNA MIR2911 [1, 11]. Recent work has shown that MIR2911 is found in high levels in various herbs and vegetables, and uptake appears to be more efficient than other dietary miRNAs [11–13].
The ability to detect this sRNA in sera has been attributed to both the digestive stability and dietary abundance of the RNA[13]. However, it remains unknown how MIR2911 is derived in plants and whether it is processed as a bona fide miRNA. In plants, miRNAs recognize their targets with essentially perfect complementarity while in animals, the endogenous miRNAs inhibit translation and alter transcript stability by binding to target transcripts with largely imperfect complementarity [14]. In fact, in mice MIR2911 binds influenza A virus with imperfect complementarity and inhibits viral replication[14, 15]. The mammalian RNA-induced silencing complex (RISC) is compatible with a broad spectrum of miRNA modifications [14, 15]. In contrast to mammalian miRNAs, plant miRNAs have a 2′ O-methylation on the ribose of the 3′ nucleotide [16, 17]. Given the potential of plant-based sRNA delivery, it is important to readdress whether this 2′ O-methylation has any effect on incorporation of plant miRNAs into the host RISC and if MIR2911 can act as a miRNA or small interfering RNA (siRNA) to regulate consumer gene expression [1].
We report here an examination of the quantity of MIR2911 during vegetable maceration and compare these levels to several different plant miRNAs. During maceration, we assessed if this sRNA is derived via typical miRNA biogenesis and measured levels of MIR2911 in relationship to the integrity of plant rRNA. Meanwhile the potential of this sRNA to alter expression of a reporter gene in human cell culture was examined. Theses results provide insights into the atypical bioavailability of MIR2911 and offer engineering strategies for plant-based sRNA therapeutics.
Materials and Methods
Plant growth
The growth conditions of Arabidopsis Dicer-like 1 mutant and its Landsberg erecta ecotype background (Ler22) were as described [18]. dcl1-9/dcl1-9 homozygous mutant plants were distinguished from DCL1/dcl1-9 heterozygous plants or wildtypes by PCR amplification of the octopine synthase terminator sequence of the T-DNA (portion of the tumor-inducing plasmid that is transferred to plant cells) by using genomic DNA as a template and the primers 5′-CTC CGT TCA ATT TAC TGA TTG TAC-3′ and 5′-TTG AAT GGT GCC CGT AAC TTT CG-3. The Arabidopsis plants were grown on soil for 4 weeks before they were processed in small RNA degradation experiment.
Preparation of cabbage extract and cabbage/chow diet
Fresh cabbage was purchased from a local market. Plant material was first mixed with ice-cold water at 1 g/mL and blended in a mixer. The slurry was sequentially centrifuged at 1000 × g for 10 min, 3000 × g for 20 min, and 10,000 × g for 20 min to remove large particles. The supernatant was then centrifuged at 150,000 × g for 90 min. Supernatant from the ultracentrifuged sample was noted as the cabbage extract. Cabbage/chow diets were prepared as previously described [19]. Briefly, fresh cabbage leaves were cut into shreds and were freeze-dried to 30% of the fresh weight before they were finely ground and used to prepare diets. The cabbage-chow diets were prepared by mixing finely ground chow, plant material, and water at 2:1:2 weight ratios.
Small RNA degradation in plant samples
Fifty mg of fresh Arabidopsis or cabbage leaves were finely ground in liquid nitrogen and were kept frozen. Five mg of frozen tissue was immediately mixed with Qiazol lysis solution (Qiagen, Valencia, CA). This represented pre-degradation samples. The remaining frozen tissue was then mixed with water and allowed to degrade on ice. 100 μL aliquots were taken at various time points and mixed with Qiazol lysis solution. To isolate total RNA from the plant samples, miRNEASY RNA isolation kit (Qiagen, Valencia, CA) was used according to manufacturer’s instructions. 1 pmole of an artificial miRNA termed C7 was used as an exogenous spike-in control. The sequence of C7 is 5′- GGA UCA UCU CAA GUC UUA CGU -3′.
Analysis of miRNA levels by qRT-PCR
Taqman microRNA Assays for MIR2911, MIR161, MIR166a, MIR167a, MIR159a, MIR156a, MIR168a, MIR172a, were obtained from Life Technologies (Grand Island, NY). Quantitative Reverse Transcriptase-Polymerase Chain reaction (qRT-PCR) was performed using a Biorad CFX96 Real-Time PCR Detection System, and data were analyzed using Biorad CFX software [13]. Delta-Delta-Ct method was used to calculate relative levels of miRNAs. Absolute concentrations of miRNAs were calculated based on standard curves obtained from serial dilutions of synthetic miRNAs.
Construction of luciferase reporter sensors for miRNAs
Renilla luciferase expression vector was constructed based on the pRL-TK backbone [20] . The cytomegalovirus (CMV) promoter and luciferase gene from pRL-CMV (Promega, Madison, MI) were excised using the BglII and XbaI sites and then inserted into the pRL-TK backbone digested with the same restriction enzymes. Subsequently, a miRNA targeting sequence containing tandem complementary sequence of MIR2911 or MIR168a was inserted into the 3′-untranslated regions (3′-UTRs) downstream of the luciferase (LUC) reading frame, thereby creating plasmids LUC-mir-168 and LUC-mir-2911 (supplementary information, Fig. S2).
miRNA luciferase reporter assay
The effects of plant-based small RNAs on the expression of human genes were assessed in cell cultures using a luciferase reporter assay. Our rationale for choosing HEK-293T cells was that these cells can be transfected with near 100% efficiency [21] and have been used with other dietary miRNA studies [22]. For studies in cell cultures, HEK-293T cells were cultured in minimum essential media (MEM) (Life technologies, Grand Island, NY) containing 10% exosome-depleted fetal bovine serum (FBS), 100,000 U/L penicillin, and 100 mg/L streptomycin for 3 days. Bovine serum was depleted of exosomes by ultracentrifugation at 130,000 × g for 2 h. Cells were transfected using lipofectamine 2000 (Invitrogen, Carlsbad, CA) with miRNA duplexes, renilla luciferase reporter construct and a firefly luciferase control construct pGL3-Firefly (Promega, Madison, MI) as described previously [2]. 24 hours after transfection, luciferase activities were measured using dual luciferase assay (Promega, Madison, MI).
Statistical analysis
Statistical analyses were performed with the student T-Test formula in Microsoft Excel. Significance was set at P < 0.05. Data are presented as means ± SEMs.
Results
Levels of plant miRNAs in cabbage
Previously, we demonstrated that various vegetables including cabbage contain high levels of MIR2911 and mouse diets rich in these vegetable provide bioavailable MIR2911[19]. However, we have failed to detect other plant based miRNAs in mouse sera during these feeding regimes. To assess if initial dietary abundance of the RNAs plays a role in the ability to detect measurable uptake, we sought to quantify the levels of various plant miRNAs relative to that of MIR2911. Our results show that the level of MIR2911 in the fresh tissue was comparable to dietary miRNAs such as MIR166a, MIR167a and MIR172a (Fig. S1).
Impact of food processing on dietary miRNA content
Given the potential for degradation of RNAs during diet preparation, we sought to compare and contrast MIR2911 levels with other dietary miRNAs during processing. First, we analyzed cabbage extract obtained by mechanical maceration and subsequent ultracentrifugation-based clarification. While the levels of various miRNAs are similar in fresh cabbage and in the liquid extract, MIR2911 was over 100-fold more abundant in the liquid extract than in the fresh tissue (Fig. 1A).
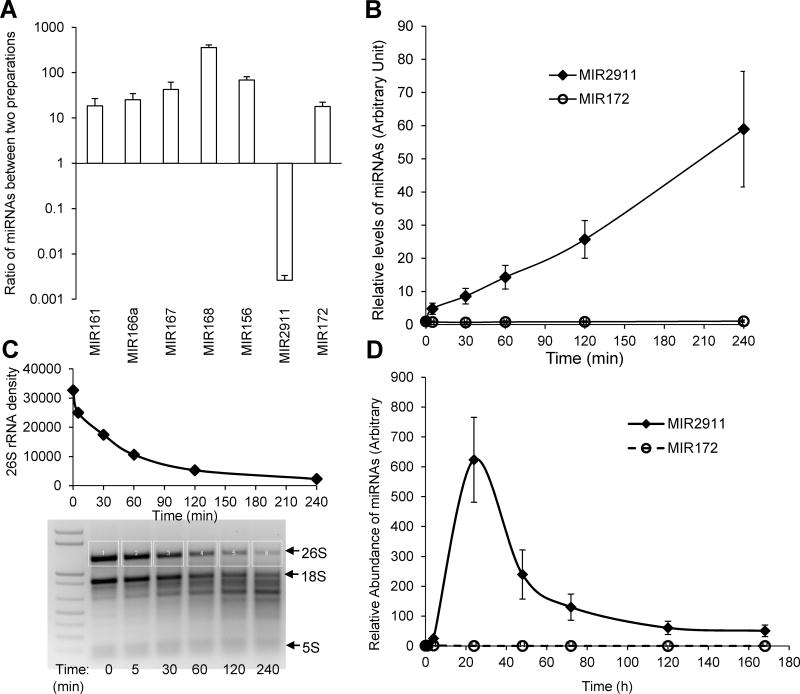
FIGURE 1.
Propagation of MIR2911 is correlated with degradation of plant 26S rRNA. A) The levels of various microRNAs and MIR2911 were assayed in two preparations: fresh cabbage ground in liquid nitrogen (fresh); liquid cabbage extract (extract). The ratio of abundance in fresh tissue over that in the extract is presented. B) Time course analysis of abundance of MIR2911 and MIR172 in cabbage extract incubated on ice. C) RNA gel electrophoresis analysis of total RNA isolated from cabbage extracts incubated on ice from various time points. D) Time course analysis of abundance of MIR2911 and MIR172 in cabbage/chow diet incubated at room temperature over 7 days. N=3; error bars = S.E.M.. Experiments were replicated three times. For panel C, the data is representative of three replicates.
A time course analysis of MIR2911 levels in cabbage extract stored at 4 °C demonstrated a 60-fold increase during a 4-hour interval while MIR172a slowly degraded (Fig. 1B). Given that MIR2911 is derived from the 26S rRNA, we sought to monitor the integrity of the 26S rRNA during this same interval. The results showed that increased levels of MIR2911 correlated with the degradation of the 26S rRNA (Fig. 1C). To evaluate if this MIR2911 propagation also occurred during preparation of the solid cabbage diets, a similar analysis was performed on cabbage diets stored at room temperature. This analysis showed that MIR2911 abundance increased by almost 600 fold (to 57 pmoles/g) after 24 hours, and then gradually decreased over a span of 7 days (Fig. 1D).
Biogenesis of MIR2911
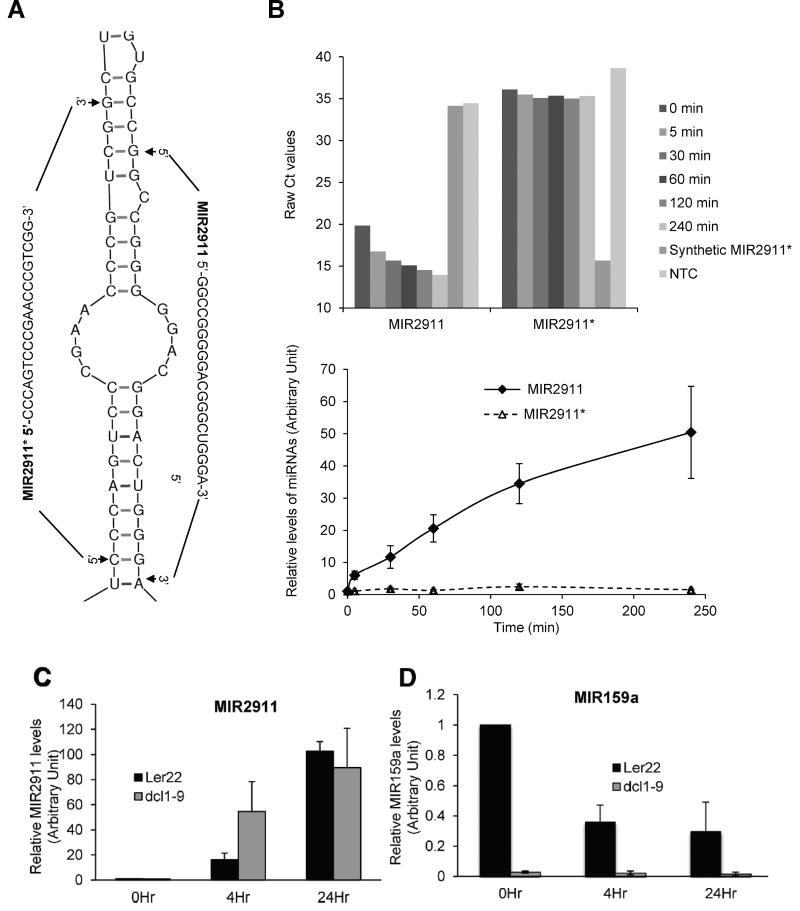
The miRNA biogenesis pathway has been viewed as conserved and universal [23]; however, MIR2911 is derived from the 26S rRNA and may not undergo canonical processing. RNA structure prediction analysis [24]of the 26S rRNA predicted a miRNA precursor for MIR2911 that contains a hairpin structure (Fig. 2A). We thus sought to detect and measure the levels of the predicted star strand of MIR2911 (MIR2911*) during cabbage degradation. However, no MIR2911* was detected at the various time points tested (Fig. 2B). A core component of the miRNA processing apparatus in Arabidopsis is DICER-LIKE1 (DCL1), and the mutation of DCL1 causes reduction in miRNA abundance [25]. Comparing plants homozygous for the nonlethal weak dcl1-9 allele to wild-type, we demonstrated that the generation of MIR2911 occurred with comparable fidelity in the two lines (Fig. 2C). Meanwhile the biogenesis of MIR159a was impaired in the dcl1-9 plants (Fig. 2D). This observation suggests that the generation of MIR2911 does not depend on DCL1.
FIGURE 2.
MIR2911 is unlikely to be a canonical microRNA. A) The folding structure of precursor MIR2911 showing MIR2911 and its predicted star strand (MIR2911*) (RNA folding structure is predicted by mfold program). B) Raw Ct values and relative quantification of MIR2911 and MIR2911* from qRT-PCR quantification of their abundance in cabbage extract incubated on ice at various time points. C) MIR2911 and D) MIR159a levels in Arabidopsis thaliana dcl1-9 and Landsberg erecta (Ler22) wild type plant extracts incubated on ice at 0, 4 and 24 hours. For panel B), the data is representative of three replicates. For panel C) and D), N=3; error bars = S.E.M..
Effects of plant small RNAs on human gene expression
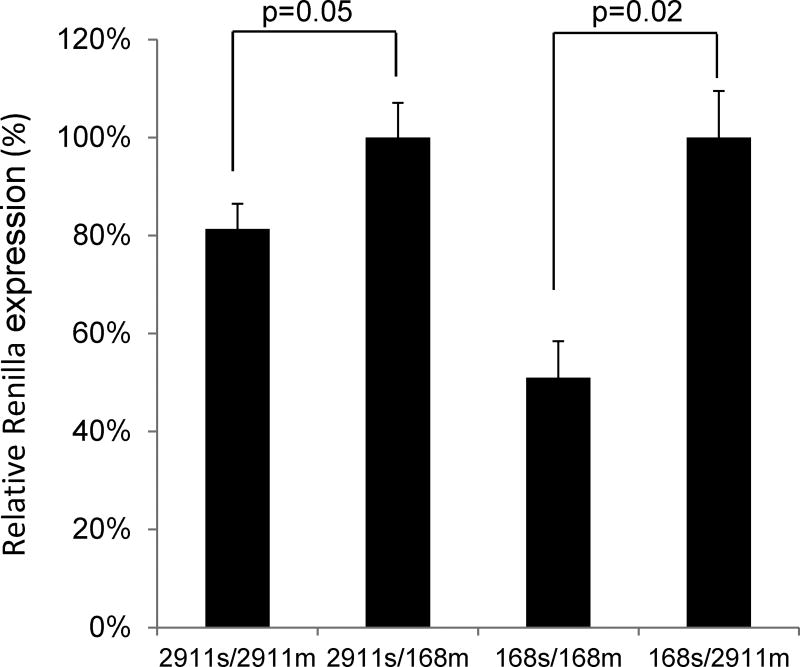
MIR2911 is a fragment of ribosomal RNA, and appears to reduce viral symptoms in mice when delivered orally [1]. In a cell culture system using putative MIR2911-binding sites from the virus in a plasmid containing luciferase, MIR2911 reduced activity by more than 50% [1]. Here MIR2911 was further tested for short interfering RNA (siRNA) activity in comparison to MIR168a, another miRNA formerly indicated to be bioavailable to the mice from plant-based diets [2]. In order to assess whether 2′ O-methylation that is typically present in plant derived miRNAs or sRNAs, hinders function in animal system, siRNA duplexes corresponding to the predicted post-Dicer processing products of MIR2911 and MIR168a were synthesized with methylation at 2-OH of the ribose at the 3′ nucleotide. Cotransfection of HEK-293T cells with MIR2911 RNA and a luciferase reporter containing tandem binding sites for MIR2911 decreased the activity of the reporter gene by approximately 20% (P = 0.05) compared to cotransfection of the reporter with MIR168a. By comparison, MIR168a was able to reduce its cognate reporter gene activity by approximately 50% (Fig. 3).
FIGURE 3.
Synthetic MIR2911 was able to suppress luciferase reporters in HEK293T cells. MIR2911 (2911m) or MIR168a (168m) duplexes were co-transfected with either MIR2911-(2911s) or MIR168a-(168s) luciferase sensors to test their efficacy as siRNAs. N=3; error bars = S.E.M..
Discussion
Dietary MIR2911 can come from a variety of plant foods and the levels of this sRNA appear to be able to increase by more than a hundred fold in macerated samples (Fig. 1). Our previous studies demonstrate this sRNA has a robustness that makes it bioavailable [19]. We further hypothesize that a plant-specific chaperone enhances MIR2911 stability [19]. Our results here implicate post ingestion degradation of 26S rRNA as a means of MIR2911 synthesis (Fig. 1) and provide another credible explanation for our previous work that demonstrated MIR2911’s enhanced bioavailability.
The bioavailability of this plant-derived sRNA during diet maceration deviates from the characteristics of most plant-derived bioactive compounds. In general, the stability and potency of plant-derived bioactive compounds decreases post-harvest [26–28]. For example, about 25% of vitamin C and a greater percentage of folate are lost during the blanching process that occurs before foods are frozen [29]. Meanwhile, anthocyanin flavonoids may be well preserved during freezing but they certainly do not increase in concentration at any point during storage, nor do they increase in abundance during maceration of the food. As a result, MIR2911 represent a novel class of bioactive compounds whose abundance amplifies while the tissue deteriorates.
The processing of precursor miRNAs give rise to two strands, the primary mature strand and the minor *-strand (star-strand) [30]. These represent the two arms of the stem in the precursor hairpin. By assaying for the presence of the star strand, we can indirectly assess whether MIR2911 is processed as a bona fide miRNA. We demonstrate here that we could not detect MIR2911* in plants (Fig. 2), suggesting MIR2911 is processed differently from normal miRNAs. Alternatively, the star-strand might be processed by Dicer and the business strand is more stable, possibly by being incorporated into an Argonaute. To analyze MIR2911 biogenesis in more detail we used a plant line defective in miRNA accumulation. Arabidopsis encodes 4 partially redundant DCL proteins [31] but miRNAs appear to be produced by only DCL1 [25]. Accumulation of miRNA is substantially reduced in the partial-loss-of-function dcl1-9 mutants; however, this mutation did not impair MIR2911 biogenesis (Fig. 2C). While reduced, the minimal accumulation of MIR159a (Fig. 2D) using dcl1-9 may be the result from partial activity of the mutant proteins because dcl1 null alleles are embryo lethal [32]. It is tempting to suggest from our inability to detect MIR2911* in plants, and the DCL1 independent formation of MIR2911, that MIR2911 is not a canonical miRNA.
Using NCBI-blast, no perfect match was found between the sequence of MIR2911 and the Arabidopsis mRNA transcriptome. Using human and mouse mRNA as presumptive targets, and the assumed seed region (2–8 bp) of MIR2911, 10 candidate target genes were identified (data not shown). However, due to the low stringency of the TargetScan predictions the possibility of false positives is high and experimental verification of the targets is important.
MIR2911 is able to reduce H1N1 infection-associated symptoms and death in mice when delivered orally [1]. MIR2911 reduces luciferase activity by 52– 71% through binding to various target sequences using assays similar to those reported here. We demonstrated that MIR2911 has the potential to inefficiently act as a siRNA in animal tissue culture. We consistently saw that MIR168a was twice as efficient suppressing the luciferase reporter as the MIR2911 to its own reporter. Nevertheless, our results showed that MIR2911 was ineffectively assembled into the RISC complex in human cells and modestly regulated gene expression. siRNAs, the active agent mediating RNA interference (RNAi), have been extensively studied in terms of tolerance to mutations [33]. Previous work has shown that no single 2’-hydroxyl group in a siRNA duplex is indispensable in RNAi [34]. Similarly, we demonstrate here that the 2' O-methylation did not impact the activity of the plant miRNA in the animal tissue culture conditions tested.
Given both the bioinformatics analysis and our tissue culture results, we favor a model where MIR2911 is part of an ensemble of sequence-independent plant-based dietary sRNAs that have anti-inflammatory potency [35]. A recent study shows a wide range of small RNAs from diverse plant species appear to modify dendritic cells ability to respond to inflammatory agents by limiting T cell proliferation and consequently dampening inflammation [35].
MIR2911 has three characteristics that aid its bioavailability: first, a high GC content appears to confer digestive stability [1, 19]; secondly, a protein-complex enhances MIR2911 stability and dietary uptake [19]; third, synthesis via rRNA degradation increases MIR2911 abundance (Fig 1). To harness this triumvirate of characteristics to express novel therapeutic sRNA in plants would require innovative approaches in agbiotechnology, but may prove to be a viable strategy due to the ability to produce miRNAs at a level hundreds of fold higher than traditional expression systems.
Sequencing small RNA fractions always identifies RNAs derived from abundant RNA species like rRNAs, or tRNAs[36]. A significant number of the sequences are derived from precise processing at the 5′ or 3′ end of mature or precursor RNAs. These sequences constitute a class of short RNAs that are magnitudes more abundant than miRNAs [37]. The plant RNA degradome is a crucial component of the total cellular RNA pool [38]. Furthermore, emerging evidence suggests that plant sRNAs may have therapeutic effects in consumers that are sequence independent [35]. Since the rRNAs in plants are the most abundant RNAs in nature, one could envision strategies designed to use rRNAs as dietary importers of bioavailable therapeutic RNAs.
Concluding Remarks
The field of dietary RNA has suffered from conflicting reports regarding the measurement of plant based small RNAs in circulation. We document here that the amount of a specific plant small RNA increased when the plant food has been degraded. We demonstrate that as the plant-based food ages, the levels of this dietary compound increase. To our knowledge, this represents the first time a bioactive compound increases while the food loses freshness. These dietary RNAs may be like wine and cheese-- getter better over time!! We offer a plausible, and intuitive, explanation for the inability of numerous groups to detect dietary miRNAs in animal sera. We believe this work is an important step in unifying the field of plant-based dietary small RNA delivery.
Supplementary Material
Acknowledgments
We thank Mark Yarmarkovich, Ismail Elbaz-Younes and LaCassidy Broadnax for technical assistance.
Abbreviations
- 3′-UTRs
3′-untranslated regions
- DCL1
Dicer-like 1
- FBS
fetal bovine serum
- LUC
luciferase
- miRNAs
microRNAs
- MEM
minimum essential media
- qRT-PCR
Quantitative Reverse Transcriptase-Polymerase Chain reaction
- rRNA
ribosomal RNA
- RISC
RNA-induced silencing complex
- RNAi
RNA interference
- sRNAs
small RNAs
- siRNA
small interfering RNA
Footnotes
Author contributions
J.Y. and K.D.H. designed the majority of the studies. N.K. and J.R.N designed the luciferase reporter study. J.Y. performed the majority of the experiments. C.P.P performed the Arabidopsis work. J.Y. and N.K. carried out the luciferase reporter study. J.Y., J.R.N. and K.D.H wrote the paper.
Conflict of interest
The authors declare no conflict of interests or competing financial interests.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Zhou Z, Li X, Liu J, Dong L, et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Research. 2015;25:39–49. doi: 10.1038/cr.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L, Hou D, Chen X, Li D, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: Evidence of cross-kingdom regulation by microRNA. Cell Research. 2012;22:107–126. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mlotshwa S, Pruss GJ, MacArthur JL, Endres MW, et al. A novel chemopreventive strategy based on therapeutic microRNAs produced in plants. Cell Research. 2015;25:521–524. doi: 10.1038/cr.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin AR, Fong MY, Somlo G, Wu J, et al. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res. 2016 doi: 10.1038/cr.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witwer KW, Hirschi KD. Transfer and functional consequences of dietary microRNAs in vertebrates: Concepts in search of corroboration: Negative results challenge the hypothesis that dietary xenomiRs cross the gut and regulate genes in ingesting vertebrates, but important questions persist. Bioessays. 2014;36:394–406. doi: 10.1002/bies.201300150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zempleni J, Baier SR, Hirschi KD. Diet-responsive MicroRNAs are likely exogenous. Journal of Biological Chemistry. 2015;290:25197. doi: 10.1074/jbc.L115.687830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snow JW, Hale AE, Isaacs SK, Baggish AL, Chan SY. Ineffective delivery of diet-derived microRNAs to recipient animal organisms. RNA biology. 2013;10:1107–1116. doi: 10.4161/rna.24909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witwer KW, McAlexander MA, Queen SE, Adams RJ. Real-time quantitative PCR and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs: limited evidence for general uptake of dietary plant xenomiRs. RNA biology. 2013;10:1080–1086. doi: 10.4161/rna.25246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson B, Zhang Y, Petrick JS, Heck G, et al. Lack of detectable oral bioavailability of plant microRNAs after feeding in mice. Nature Biotechnology. 2013;31:965–967. doi: 10.1038/nbt.2737. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Hirschi KD, Farmer LM. Dietary RNAs: New stories regarding oral delivery. Nutrients. 2015;7:3184–3199. doi: 10.3390/nu7053184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Farmer LM, Agyekum AAA, Hirschi KD. Detection of dietary plant-based small RNAs in animals. Cell Research. 2015;25:517–520. doi: 10.1038/cr.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirschi KD, Pruss GJ, Vance V. Dietary delivery: A new avenue for microRNA therapeutics? Trends Biotechnology. 2015;33:431–432. doi: 10.1016/j.tibtech.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Farmer LM, Agyekum AAA, Elbaz-Younes I, Hirschi KD. Detection of an abundant plant-based small RNA in healthy consumers. PLoS One. 2015;10:e0137516, 0137511–0137514. doi: 10.1371/journal.pone.0137516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amarzguioui M, Holen T, Babaie E, Prydz H. Tolerance for mutations and chemical modifications in a siRNA. Nucleic Acids Research. 2003;31:589–595. doi: 10.1093/nar/gkg147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu B, Yang Z, Li J, Minakhina S, et al. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braasch DA, Jensen S, Liu Y, Kaur K, et al. RNA interference in mammalian cells by chemically-modified RNA. Biochemistry. 2003;42:7967–7975. doi: 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- 18.Tsuzuki M, Takeda A, Watanabe Y. Recovery of dicer-like 1-late flowering phenotype by miR172 expressed by the noncanonical DCL4-dependent biogenesis pathway. RNA. 2014;20:1320–1327. doi: 10.1261/rna.044966.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Hotz T, Broadnax L, Yarmarkovich M, et al. Anomalous uptake and circulatory characteristics of the plant-based small RNA MIR2911. Scientific Reports. 2016;6:1–9. doi: 10.1038/srep26834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamaci N, Emnacar T, Karakas N, Arikan G, et al. Selective silencing of DNA topoisomerase IIbeta in human mesenchymal stem cells by siRNAs (small interfering RNAs) Cell biology international reports. 2011;18:e00010. doi: 10.1042/CBR20110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. Journal of Nutrition. 2014;144:1495–1500. doi: 10.3945/jn.114.196436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nature cell biology. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz R, Bernhart SH, Honer Zu Siederdissen C, Tafer H, et al. ViennaRNA Package 2.0. Algorithms for molecular biology : AMB. 2011;6:26. doi: 10.1186/1748-7188-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurihara Y, Watanabe Y. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc Natl Acad Sci U S A. 2004;101:12753–12758. doi: 10.1073/pnas.0403115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalska A, Lysiak G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. International journal of molecular sciences. 2015;16:18642–18663. doi: 10.3390/ijms160818642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shashirekha MN, Mallikarjuna SE, Rajarathnam S. Status of bioactive compounds in foods, with focus on fruits and vegetables. Crit Rev Food Sci Nutr. 2015;55:1324–1339. doi: 10.1080/10408398.2012.692736. [DOI] [PubMed] [Google Scholar]
- 28.Neves LC, Tosin JM, Benedette RM, Cisneros-Zevallos L. Post-harvest nutraceutical behaviour during ripening and senescence of 8 highly perishable fruit species from the Northern Brazilian Amazon region. Food Chem. 2015;174:188–196. doi: 10.1016/j.foodchem.2014.10.111. [DOI] [PubMed] [Google Scholar]
- 29.Severi S, Bedogni G, Manzieri AM, Poli M, Battistini N. Effects of cooking and storage methods on the micronutrient content of foods. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 1997;6(Suppl 1):S21–24. doi: 10.1097/00008469-199703001-00005. [DOI] [PubMed] [Google Scholar]
- 30.Shin C. Cleavage of the star strand facilitates assembly of some microRNAs into Ago2-containing silencing complexes in mammals. Molecules and cells. 2008;26:308–313. [PubMed] [Google Scholar]
- 31.Gasciolli V, Mallory AC, Bartel DP, Vaucheret H. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Curr Biol. 2005;15:1494–1500. doi: 10.1016/j.cub.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Schauer SE, Jacobsen SE, Meinke DW, Ray A. DICER-LIKE1: blind men and elephants in Arabidopsis development. Trends Plant Sci. 2002;7:487–491. doi: 10.1016/s1360-1385(02)02355-5. [DOI] [PubMed] [Google Scholar]
- 33.Amarzguioui M, Holen T, Babaie E, Prydz H. Tolerance for mutations and chemical modifications in a siRNA. Nucleic Acids Research. 2003;31:589–595. doi: 10.1093/nar/gkg147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson AL, Burchard J, Leake D, Reynolds A, et al. Position-specific chemical modification of siRNAs reduces "off-target" transcript silencing. RNA. 2006;12:1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavalieri D, Rizzetto L, Tocci N, Rivero D, et al. Plant microRNAs as novel immunomodulatory agents. Sci Rep. 2016;6:25761. doi: 10.1038/srep25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole C, Sobala A, Lu C, Thatcher SR, et al. Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA. 2009;15:2147–2160. doi: 10.1261/rna.1738409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YS, Shibata Y, Malhotra A, Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs) Genes Dev. 2009;23:2639–2649. doi: 10.1101/gad.1837609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowacka M, Strozycki PM, Jackowiak P, Hojka-Osinska A, et al. Identification of stable, high copy number, medium-sized RNA degradation intermediates that accumulate in plants under non-stress conditions. Plant Mol Biol. 2013;83:191–204. doi: 10.1007/s11103-013-0079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.