Abstract
While the psychoactive inhalant toluene causes behavioral effects similar to those produced by other drugs of abuse, the persistent behavioral and anatomical abnormalities induced by toluene exposure are not well known. To mimic human “binge-like” inhalant intoxication, adolescent, male Sprague-Dawley rats were exposed to toluene vapor (5700 ppm) twice daily for five consecutive days. These rats remained in their home cages until adulthood (P60), when they were trained in operant boxes to respond to a palatable food reward and then challenged with several different cognitive tasks. Rats that experienced chronic exposure to toluene plus abstinence (“CTA”) showed enhanced performance in a strategy set-shifting task using a between-session, but not a within-session test design. CTA also blunted operant and classical conditioning without affecting responding during a progressive ratio task. While CTA rats displayed normal latent inhibition, previous exposure to a non-reinforced cue enhanced extinction of classically conditioned approach behavior of these animals compared to air controls. To determine whether CTA alters the structural plasticity of brain areas involved in set-shifting and appetitive behaviors, we quantified basal dendritic spine morphology in DiI-labeled pyramidal neurons in layer 5 of the medial prefrontal cortex (mPFC) and medium spiny neurons in the nucleus accumbens (NAc). There were no changes in dendritic spine density or subtype in the mPFC of CTA rats while NAc spine density was significantly increased due to an enhanced prevalence of long-thin spines. Together, these findings suggest that the persistent effects of CTA on cognition are related to learning and memory consolidation/recall, but not mPFC-dependent behavioral flexibility.
Keywords: Inhalant abuse, Abstinence, Behavioral flexibility, Latent inhibition, Dendritic spines, Nucleus accumbens core
1. Introduction
Toluene abuse typically consists of inhaling a concentrated vapor over a short period of time in order to achieve a hedonic, intoxicated state similar to other addictive substances (Howard et al., 2011). Like other drugs of abuse, this “high” is likely due to modulation of dopamine release in the striatum, potentially via enhancing excitatory signaling on mesolimbic dopamine neurons (Beckley et al., 2013; Lubman, Yücel, & Lawrence, 2008). In preclinical rodent studies, acute administration of abuse-level concentrations of toluene is an anxiolytic, has anti-depressant-like properties, produces a conditioned place preference, and impairs learning and motor coordination(Batis, Hannigan, & Bowen, 2010; Bowen, Wiley, & Balster, 1996; Gerasimov et al., 2003; Lopez-Rubalcava, Hen, & Cruz, 2000). These data parallel the behavioral profile of acute exposure to other addictive substances.
Since drug addiction may develop following chronic use, it is important to understand the effects of repeated, long-term toluene exposure on behavior and cognition. Results from preclinical studies reveal that chronic exposure to toluene can cause a wide range of cognitive and behavioral impairments including sensitization to drug-induced hyperlocomotion, impaired novel object recognition, spatial learning, and inhibitory avoidance (Batis et al., 2010; Baydas et al., 2005; Huerta-Rivas et al., 2012). In addition, clinical studies in humans also report cognitive deficits such as decreases in IQ and impairments in executive functions such response inhibition, behavioral flexibility, working memory, and attention (Howard, Balster, Cottler, Wu, & Vaughn, 2008; Lubman et al., 2008; Yuncu et al., 2015). Although these studies of chronic toluene exposure are essential to understanding the drugs’ effects on cognition, most of them assessed behavioral performance shortly following the last toluene exposure. While important, it is also critical to examine whether there are changes in cognitive function following a more protracted period of abstinence.
Drug abstinence results in a negative emotional state, increased anxiety, and social withdrawal – all of which increase an individual’s risk of relapse (Goodwin et al., 2002; McGregor, Callaghan, & Hunt, 2008; Wise & Koob, 2014). Understanding the behavioral profile during drug abstinence is essential for effective treatment of substance use disorders. The few studies concerning toluene’s effects following protracted abstinence are somewhat inconsistent and results vary based on the cognitive measure tested. For example, while deficits in object recognition, operant conditioning, delay discounting, progressive ratio responding, and contingency monitoring have been observed, protracted abstinence from chronic toluene exposure does not affect Pavlovian-to-instrumental transfer, outcome devaluation, anxiety or spatial memory (Dick et al., 2014; Lin et al., 2010; Furlong et al., 2016). Further, while inhalant-induced deficits in behavioral flexibility have been detected in humans after a short abstinence period (5–9 days), their effects in a protracted abstinence rodent model are subtle (Dick et al., 2014; Furlong et al., 2016; Yuncu et al., 2015).
One of the more commonly studied forms of behavioral flexibility involves training a subject to respond to a certain set of rules for a reward, and measuring the ability to adjust behavior when a new rule is introduced unexpectedly. Efficient completion of these tasks is critically-dependent on the integrity of the prefrontal cortex (Hamilton & Brigman, 2015). Moreover, disrupting communication between the medial prefrontal cortex (mPFC) and nucleus accumbens core (NAc) impairs shifting between strategies by increasing perseverative responding (Block et al., 2007). This circuitry is part of a larger network that controls the transition to habitual drug use, where prelimbic mPFC-NAc connectivity is essential for the initiation of drug-seeking behaviors (Everitt & Robbins, 2005; Stefanik et al., 2013). Both behavioral flexibility and drug addiction require structural modifications in the mPFC and NAc to permit the formation and maintenance of new synapses. The postsynaptic dendritic spine is a key component of this neuroplasticity, with long-thin immature spines giving way to mushroom-headed spines over the course of excitatory synaptic growth (Holtmaat et al., 2006). While nearly every drug of abuse examined to date alters dendritic spine morphology in the mPFC and NAc (Mulholland, Chandler, & Kalivas, 2016; Spiga et al., 2014), it is not known whether similar changes occur following toluene exposure.
There is a particularly high incidence of inhalant abuse in adolescents due to the low cost and high availability of toluene-containing products (e.g. paint thinners, nail polish, permanent markers) (Johnston et al., 2015). In the present study, adolescent rats were chronically exposed to abuse levels of toluene vapor and then allowed to recover in their home cage for a protracted abstinence (CTA). When rats reached adulthood we assessed two types of behavioral flexibility – strategy set-shifting and reversal learning – and examined the density and sub-types of dendritic spines in mPFC and NAc. The results from these studies show that toluene exposure during adolescence produces selective impairments in cognitive function during adulthood that are accompanied by alterations in dendritic spine morphology that are region- and spine-subtype specific.
2. Materials and Methods
2.1 Animals
Sixty-seven male Sprague-Dawley Rats (post-natal day (P) 32 on arrival; Harlan Laboratories, Indianapolis, IN) were housed in pairs in polypropylene cages on a reverse light cycle (lights off at 0900 h) in a climate controlled room with ad libitum access to food and water unless otherwise noted. Rat identification numbers were written on the base of each tail using a permanent marker. Each rat was acclimated to handling for 5 min per day for at least 2 days prior to toluene exposure. All procedures were performed in compliance with the Medical University of South Carolina IACUC protocols.
2.2 Toluene Exposure
On the day before the first toluene exposure, adolescent rats (P38) were habituated to the exposure chamber (30×30×30cm) for 15 min. On each of the following 5 days (P39–43), a binge-like regimen was used to mimic adolescent human toluene abuse. Sessions consisted of two, 15 min exposures to 5700 ppm toluene generated using a sevoflurane vaporizer (Penlon Limited; flow rate 4L/min, 8% volume). Each exposure was separated by 2 h of recovery in the home cage. We have previously used gas chromatography to validate this protocol for generating abuse-level toluene concentrations (Beckley et al., 2013). Importantly, these exposures fall within human consumption patterns: 15 min to several hours at 5000 to 15000 ppm (Brouette & Anton, 2001; Bukowski, 2001). Interestingly, Gmaz et al. (2012) exposed Long-Evans rats to 5000 ppm toluene for 30 min and determined that the resulting brain toluene concentrations (500–1000 µmol/l) would be similar to those experienced by humans inhaling toluene-containing products. Similar exposure protocols have been used to study the effect of chronic exposure to abuse levels of toluene vapor (Moser & Balster, 1981; Bowen, Hannigan, & Cooper, 2009; Dick et al., 2014; Furlong et al., 2016). Control rats were exposed to chambers filled with air on the same schedule as above. Housing pairs were placed in the same drug treatment group to avoid potential exposure of air-treated controls to toluene. Animal weights were recorded every day following toluene exposure, and once every 3–5 days thereafter.
2.3 Behavioral Flexibility
2.3.1. Lever Press Training
Rats (eighteen toluene-, seventeen air-treated) were first habituated to 20% sweetened condensed milk (SCM), the reward used throughout these studies. During reward exposure, each rat pair was given free access to 10 ml SCM for two days before exposure to the operant chambers. Subjects were monitored to ensure both rats sampled the SCM. Lever press training was subsequently conducted in operant chambers (Med Associates, St. Albans, VT) that began during adulthood (P60) and proceeded as described previously (Brady & Floresco, 2015). Briefly, rats were first trained to lever press on a fixed-ratio (FR) 1 schedule for 45 µl SCM dispensed from a central feeding well over the course of three phases (1–3). Phase 1 (30 min session) began with both levers extending, each of which were reinforced on an FR1 schedule. Rodents moved on to phase 2 if they made 50 responses for two consecutive days. Phase 2 was identical to phase 1, except that levers retracted 20 s when pressed and then were presented again. Rodents progressed to phase 3 if they made 50 responses for two consecutive days. Phase 3 lasted 30 to 45 min and consisted of 100 trials. During each trial, one of the two levers were extended for 10 s in a pseudorandom order. If the rat responded on the lever, it was retracted for 20 s and a reward was delivered to the feeding well. If the extended lever was not pressed during a trial, it was retracted and the house light was illuminated for a 30 s time out period which was recorded as an “omission”. Once a subject reached criteria (10 or fewer omissions per session for two consecutive days) a side preference test was performed as previously described (Brady & Floresco, 2015; Floresco, Block, & Tse, 2008). For each of 60 trials, both levers extended simultaneously and were reinforced on an FR1 schedule. A trial concluded when two presses occurred, which resulted in lever retraction for 20 s. The preferred side was defined as the side that a rat pressed first most often across trials.
2.3.2. Visual Cue Discrimination
Rats were trained to respond to only the lever under an illuminated light (visual cue) in order to receive reinforcement. Rats received daily session of 100 trials. Each trial started with a visual cue light turning on above one of two lever slots. 3 s later the houselight turned on and both levers were inserted into the chamber. The visual cue was presented in a pseudorandom order across trials to indicate which lever would elicit a reward when pressed. Responses on this “active” lever delivered reward on a FR1 schedule. Responding on either lever caused both levers to retract for 20 s. If neither lever was pressed within 10 s, both were retracted and the house light was illuminated for a 30 s time out period, recorded as an “omission”. Rats were trained to a criterion of two consecutive sessions with less than 10 omissions. They were then subjected to the strategy shift to response discrimination.
2.3.3. Strategy Set-Shift to a Response Discrimination
During this phase, rats were required to shift their strategy and use an egocentric spatial response strategy, wherein responding on one lever (i.e. left vs right lever) now delivered the reward irrespective of the position of the visual cue (Brady & Floresco, 2015). We chose to use this visual cue-response shift because our primary interest was in ascertaining how toluene exposure may affect PFC functioning, and previous studies have shown that performance on this type of shift is more sensitive to disruption following PFC inactivation (Floresco et al., 2008). Reinforced levers were counter-balanced against the rats preferred location as determined by the side preference task. The manner in which the set-shift was administered was varied across two experiments. Group “A” consisted of eight toluene- and nine air-treated rats that received a “within-session” shift, where the session started with 20 “reminder trials” of the visual cue rule. On the 21st trial, rats were required to use a response rule to obtain reward. A separate group (“B”) consisted of eight toluene- and eight air-treated rats that completed this task “between-sessions”, where the strategy shift occurred without any reminder trials (i.e. the last training trial and first training trial were separated by 24 h). Sessions ended once at least 30 trials were completed and a rat achieved criterion performance (8 consecutive correct responses). Primary dependent variables for this task were trials to criterion and errors to criterion. Incorrect responses on illuminated levers were further categorized as either perseverative errors (if >4 in a block of 16 trials) or regressive errors (if ≤4 in a block of 16). During the strategy shift, an incorrect response on an unlit lever was classified as a never reinforced error. Lever press latency, and accuracy during reminder trials were also recoded.
2.3.4. Reversal Learning
Once rats achieved criterion performance (8 consecutive correct lever presses under 30 trials for two days in a row) on the strategy shift task, the response discrimination was reversed so that responses on the previously incorrect lever were now reinforced, as described previously (Brady & Floresco, 2015). Again, the visual cue lights were illuminated above one of the levers on each trial, but here, they served as distractors. Rats from group A were tested for reversal learning using a within-session reversal shift while rats from group B were tested using a between-session task design. Other than the reinforced and unreinforced levers being switched, the same program as in 2.3.3 was used for this task. Primary dependent variables for this task were trials to completion and errors to criterion. Sessions ended once 10 consecutive correct responses were made and at least 30 trials had occurred. Errors were further categorized as either congruent or noncongruent with visual cue. Lever press latency, and accuracy during reminder trials were also recorded.
2.4. Progressive Ratio Test
After achieving criterion performance on the reversal phase of the task, animals from both group A and group B performed a progressive ratio task using the following schedule of reinforcement: responses per reward (rounded) = 5ereward number*0.2 − 5 (Richardson & Roberts, 1996). Each reward delivery preceded a 4 s timeout period. All testing occurred over a single 16 h period that terminated if no rewards were delivered within a 1 h period.
2.5 Classical Conditioning
2.5.1. Training
A separate cohort of 32 rats were divided into 4 groups (n=8/group) to test the interaction between drug history (CTA vs air) and cue pre-exposure (pre exposed, “PE” vs non pre exposed, “NPE”) on classical conditioning using a procedure based on Nonkes et al. (2012). CTA and air-treated rats were acclimatized to SCM one day before training began (see 2.3.1). On P60, rats were placed in operant chambers (Med Associates, St. Albans VT) for two sessions of food well location training. During these 60 min sessions, 45 µl of SCM were delivered on a variable interval (VI, 3 min average inter-trial interval) for 15 trials to ensure frequent visits to the well during future testing. For the next 6 days, half of the rats underwent cue pre exposure sessions. Rats were food deprived for 2 h before each session. These 60 min sessions consisted of a 60 s compound cue (tone + stimulus lights) delivered on a VI (3 min average inter-trial interval) for 15 trials with no reward delivery. On these training days, NPE rats were placed in operant boxes without any cues for the same amount of time as their PE peers. This training resulted in four groups (air-PE, air-NPE, CTA-PE, CTA-NPE).
2.5.2. Classical Conditioning Procedure
Rats next underwent eight days of classical conditioning sessions. Rats were food deprived for 2 h before each session. During these 40 min test sessions, a 60 s compound cue was delivered on a VI (3 min average inter-trial interval) for 10 trials. This cue was paired with the delivery of 45 µl SCM 30 s into cue presentation. The primary dependent variable used to measure cue-reward association strength was the elevation ratio, X/(X+Y). “X” equals the number of food well entries during the 30 s period following cue onset, when reward was available. “Y” equals the number of entries during the 30 s preceding cue onset, when reward was not available. Latency to approach the food well following cue onset and SCM delivery were also recorded. Cue-reward pairing was extinguished over the next three sessions using the same program in the absence of the SCM reward.
2.6. Dendritic Spine Analysis
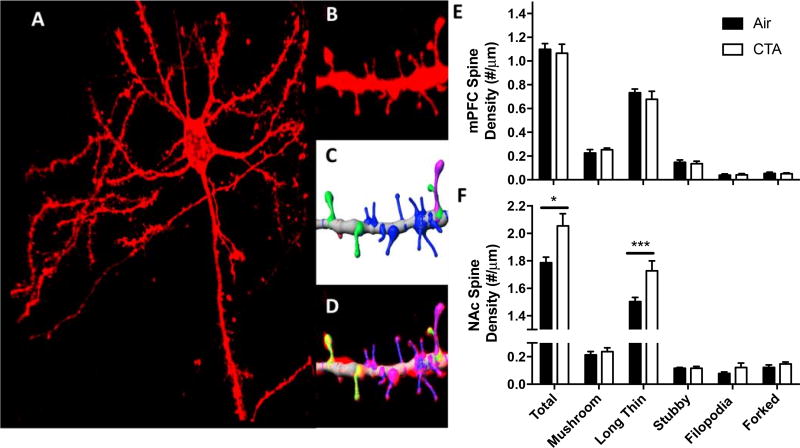
At PD 93–97 (7 days following the progressive ratio task, and approximately 7 weeks after the last toluene exposure) rats from the within-session behavioral flexibility test (group A) were processed for dendritic spine labeling and classification as previously reported (Uys et al., 2015). First, rats were anesthetized with urethane (3g/kg, i.p.) and perfused with 300ml saline-free 0.1M phosphate buffer (PB) followed by 300 ml 1.5% paraformaldehyde (PFA). Brains were blocked and post-fixed for 1 h in 1.5% PFA and then sliced into 150 µm coronal sections using a vibratome. Tungsten particles (1.3 µm diameter) were coated with DiI and then applied to coronal slices using a Helio Gene gun (Bio-Rad, Hercules, CA) fitted with a 3.0 µm polycarbonate filter (BD Biosciences, San Jose, CA). The lipophilic dye was allowed to spread through the samples overnight in PB at 4°C. The next day, slices were washed once in PB and then mounted onto slides with ProLong Gold (Life Technologies, Carlsbad, CA) and coverslipped. Slices were imaged using a Zeiss LSM 510 confocal microscope and 63× oil immersion objective (Plan-Apochromat; NA = 1.4). Voxel size (49 × 49 × 100 nm) was set according to the Nyquist theorem. On average, three to five dendritic segments 50 µm in size from basal dendrites were collected and imaged. Each segment was second-order, 25 µm from any branch point, and began 50–100 µm from the soma. Images were deconvolved using AutoQuant (Media Cybernetics, Rockville, MD) and subsequently modeled using Imaris (Bitplane, Zurich, Switzerland) software. Based on previously reported specifications (Trantham-Davidson et al., 2016; Lin, Lo, Lyu, & Lai, 2017), spines were classified into subtypes using the following parameters: spine length < 0.75 µm, stubby; spine length between 0.75 µm and 3.0 µm, long-thin; spine length < 3.5 µm, head width minimum > 0.3 µm, and head width maximum > minimum neck width *1.5, mushroom; spine length ≥ 3.0 µm, filopodia. Two-headed spines were counted manually.
2.7 Statistics
Lever press and visual discrimination data were analyzed with two-tailed unpaired t-tests using Prism 7 (Graphpad Software San Diego, CA). One rat (CTA, group B) failed to progress through phase 1 of training, assigned a value of 10 days for this training phase (max number of days to criteria observed), and then removed from the remainder of the study. Three rats (two air, group A; one CTA, group B) failed to meet criteria in phase 3 after 8 days, but subsequently passed visual cue training criteria and were included in the remainder of behavioral studies. Two outliers (one CTA, group A; one air, group A) as determined by Grubbs test were removed from the statistical analysis of figure 1A. Behavioral flexibility data were analyzed with 2-way ANOVA with task as the within subject factor and drug experience as the between subject factor (Prism 7). Error data from these experiments were analyzed with two-tailed unpaired t-tests. Classical conditioning and extinction data were analyzed using a three-way ANOVA using SPSS (SPSS, Armonk, NY) with cue and drug experience as between subject factors and test session (time) as the within subject, repeated factor. Since the purpose of these experiments was to explore the effects of CTA over time under a single set of cue experiences, we further analyzed any drug experience × time interactions revealed by the three-way ANOVA within each cue exposure condition using a two-way repeated measures ANOVA. Dendritic spine analyses were conducted using a mixed model (SAS Proc Mixed, SAS Institute Inc., Cary, NC) with a first order autoregressive covariance matrix across the sequential slices within rats.
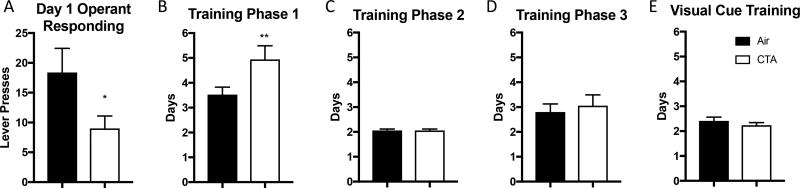
Figure 1.
A history of toluene exposure during adolescence impairs acquisition of operant behavior in adulthood. A) CTA exposed rats had fewer lever presses for a reward (20% sweetened condensed milk) on day one of operant training. B) CTA rats reached lever press training phase 1 criteria in significantly more days compared to control. C–E) CTA and air exposed rats progressed through the remainder of operant training in equivalent number of days. Data shown are mean ± SEM; *p<0.05, **p<0.01; air n=17, CTA n=17.
3. Results
3.1. Chronic toluene exposure attenuates weight gain
CTA rats weighed significantly less than air-treated controls by the fifth day of treatment. This difference persisted until the first day of lever press training (P60), but weights were not significantly different during behavioral flexibility testing (supplementary figure 1).
3.2 CTA causes operant conditioning deficits
In order to identify potential drug-induced deficits in operant conditioning, we noted the initial operant responding and number of days required to meet criteria during training. During initial lever press training, CTA rats pressed for reward significantly fewer times than air treated controls [t(31)=2.095, p<0.05] (Figure 1a). CTA rats took significantly longer to reach lever pressing criteria during phase 1 compared to air treated controls [t(33)=3.05, p<0.01] (Figure 1b). This deficit was not present during subsequent training: lever press training phase 2 [t(32)=0, p>0.999], lever press training phase 3 [t(32)=0.72, p=0.923], visual cue training [t(32)=0.36, p=0.994] (figure 1c–e). Both test groups (i.e. “A” or “B”, see 2.3.3.) progressed through training at comparable rates (supplementary table 1). Finally, the number of training days did not correlate with future strategy shifting (Pearson r=−0.033, p=0.86) or reversal learning (Pearson r=0.096, p=0.61) performance. Taken together, these data suggest that there is a toluene-induced deficit in operant conditioning, but not visual discrimination.
3.3 Within-Session Tests of Behavioral Flexibility
3.3.1 Within-Session Strategy Set-Shifting
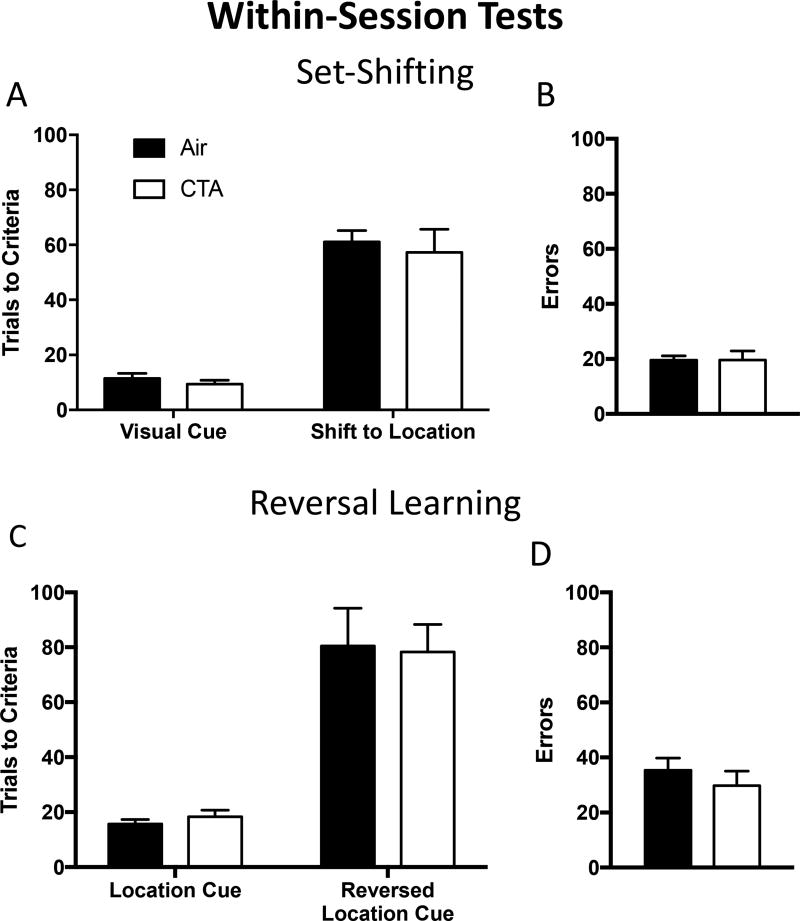
Eighteen rats (nine air, nine toluene; group A) were trained to lever press for a SCM reward in response to a visual cue. Once rats met the criteria of 8 correct lever presses in a row in under 30 trials, they were tested for their ability to shift to a new discrimination strategy (i.e. switch to a location based rule) using a within-session test design (i.e. testing occurred immediately after 20 training reminder trials at the start of the test session). Analysis of these data revealed no detectable differences in performance during the first twenty reminder trials on test day as measured by overall accuracy and response latency (all t’s< 1.09, p’s>0.29, supplementary figure 2a, b). A 2-way ANOVA revealed a main effect of task (F1,16=116.6, p<0.0001), but no task × drug interaction (F1,16=0.0373, p=0.8494) or main effect of drug (F1,16=0.3939, p=0.5397; figure 2a). Sidak’s post hoc revealed no differences in the number of trials to criteria between CTA rats and air treated controls during the reminder trials or the strategy set shift task itself (all t’s <0.59, p’s >0.81; figure 2a). There were no group differences in errors to criteria, response latency or errors committed in CTA rats compared to air treated controls (all p’s >0.05; figure 2b, supplementary figure 2c, 3).
Figure 2.
A history of adolescent toluene exposure did not affect within-session set-shifting or reversal learning in adulthood. (A & B) CTA and air exposed rats reached set-shift criteria in the same number of trials and committed the same number of errors during a within-session test design. (C & D) CTA and air exposed rats reached reversal learning criteria in the same number of trials and committed the same number of errors during a within-session test design. Data shown are mean ± SEM; air n=9, CTA n=9.
3.3.2 Within-Session Reversal Learning
Following completion of the set shift to the location-based rule, rats were tested on a reversal of this discrimination (i.e. learn that the previously inactive lever was now the only active lever) using a within-session test design (i.e. the reversal shift occurred immediately after 20 training reminder trials within the same session). There were no detectable differences between air and CTA rats in performance during the first twenty reminder trials on test day as measured by overall accuracy and response latency (all t’s<1.16, p’s>0.26; supplementary figure 2d, e). A 2-way ANOVA revealed a main effect of task (F1,16=56.6, p<0.0001), but no task × drug interaction (F1,16=0.0829, p=0.7772) or main effect of drug (F1,16=0.0004, p=0.9835; figure 2c). Sidak’s post hoc revealed no differences in the number of trials to criteria between CTA rats and air treated controls during the reminder trials or the reversal learning task itself (all t’s <0.21, p’s >0.97; figure 2c). There were no group differences in errors to criteria or error subtypes committed in CTA rats compared to air treated controls (p >0.05; figure 2d, supplementary figure 2f, 3).
3.4 Between-Session Tests of Behavioral Flexibility
Test of behavioral flexibility involve suppression of responding of an initial rule, exploring alternative rules, and the establishment/maintenance of an new rule (Block et al., 2007). Learning and extinction deficits have been observed both in acute and chronic toluene use in both humans (Yuncu et al., 2015) and rodents (Dick et al., 2014). Since a history of toluene exposure could be affecting the strength of the existing rule memory (Floresco & Jentsch, 2011) we expanded the amount of time between training and testing (“between-session” tests) and in a separate cohort of animals measured their behavioral flexibility. In this paradigm, testing occurred 24h after the last visual set training session.
3.4.1 Between-Session Strategy Set-Shifting
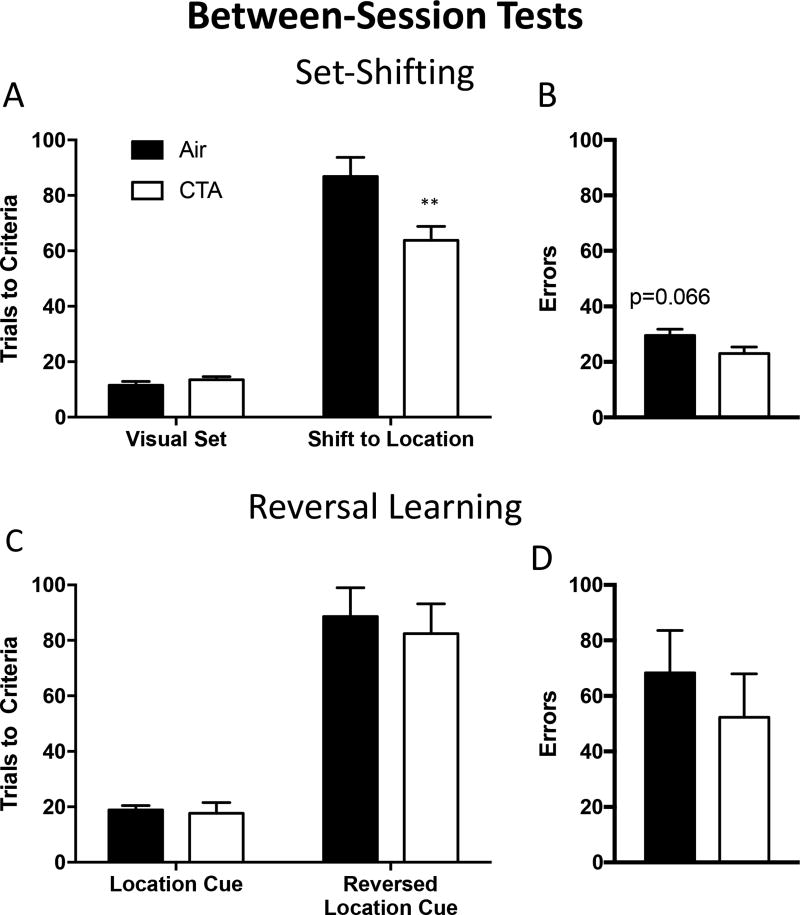
A separate group of sixteen rats (eight air, eight toluene; group B) were trained to lever press in response to a visual cue. Subjects were then tested on their ability to shift strategy between-session (i.e. with no reminder trials before the set shift and during the same session). A 2-way ANOVA revealed a task × drug interaction (F1,14=7.842, p<0.05) as well as main effects of task (F1,14=197.6, p<0.0001) and drug (F1,14=5.955, p<0.05). This effect was driven by CTA rats reaching criterion performance more rapidly than control when the rule switched to being dependent on lever-location (Sidak’s post-hoc p<0.01; figure 3a). There was no difference in performance during the last day of visual cue discrimination training (Sidak’s post-hoc p=0.9372; figure 3a). The enhanced performance was also reflected in the trend towards decreased errors committed by CTA rats compared to controls [t(14)=2.00, p=0.066; figure 3b]. There were no significant differences in the types of errors committed or number of trials omitted during testing (all p’s >0.08; supplementary figure 4).
Figure 3.
Toluene exposure during adolescence improved between-session set-shifting, but not reversal learning in adulthood. CTA rats reached set-shift criteria in fewer trials as compared to air-exposed controls (A) and showed a trend towards fewer errors (B). (C & D) CTA rats reached reversal learning criteria in the same number of trials and committed the same number of errors as air-exposed controls. Data shown are mean ± SEM; **p<0.01. Data shown are mean ± SEM; air n=8, CTA n=8.
3.4.2. Between-Session Reversal Learning
Once trained on the location-based rule, rats were tasked to reverse their responding. A 2-way ANOVA revealed a main effect of task (F1,14=84.55, p<0.001), but no task × drug interaction (F1,14=0.1186, p=0.7361) or main effect of drug F1,14=0.2026, p=0.6601; figure 3c). Sidak’s post hoc revealed no differences in the number of trials to criteria between CTA rats and air treated controls during last training task or during the reversal learning task itself (all t’s <0.57, p’s >0.82; figure 3c). There were no significant differences in total errors during testing [t(14)=0.73, p=0.4807; figure 3d]
3.5. Progressive Ratio
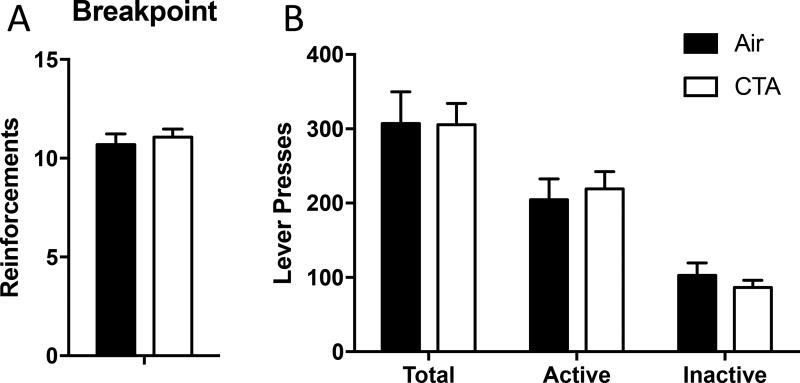
In order to determine if the operant conditioning deficits observed were due to a decreased motivation for obtaining SCM reward, we measured responding using a progressive ratio reinforcement regimen in these same rats. All rats from the behavioral flexibility experiments were included in this study. There were no differences between CTA and air-treated controls in response breakpoint, active lever presses, or inactive lever presses (all t’s<0.85, p’s>0.05; figure 4). These results suggest that the deficits in operant conditioning are not easily attributable to deficits in motivation to obtain a food reward.
Figure 4.
A history of toluene exposure during adolescence does not alter motivation for a food reward. A) CTA rats had a comparable breakpoint compared to controls using a progressive ratio schedule of reinforcement. B) The number of presses of active and inactive levers was not different between CTA and air-exposed rats. Data shown are mean ± SEM; air n=17, CA n=17.
3.6. Classical Conditioning
The associative learning phenomena of latent inhibition – where previous exposure to a non-reinforced cue blunts future conditioning to that cue – has also been proposed to play a role in behavioral flexibility (Chess et al., 2012; Nonkes et al., 2012). Blunted latent inhibition may increase exploration of previously irrelevant rule sets during a strategy shift that could lead to an apparent facilitation of shifting. Given that CTA resulted in somewhat more rapid shifting to a novel rule when rats were tested using a between-session protocol, we tested whether latent inhibition was altered in adult rats with CTA. In so doing, we characterized classically conditioned approach behavior, and extinction of this behavior, in both cue naïve and cue pre-exposed rats.
3.6.1. Acquisition and Latent Inhibition
Results from the between-session strategy shifting test suggest that toluene exposure may cause long term deficits in latent inhibition of a previously irrelevant cue to future cue-reward associations. By including both a cue pre-exposed (PE) and a non cue pre-exposed (NPE) group of air and CTA rats, this classical conditioning task design allowed us to test the effect of CTA on both classical conditioning and latent inhibition. We tested 8 rats in each of the drug × cue exposure combinations (air-PE, air-NPE, CTA-PE, CTA-NPE).
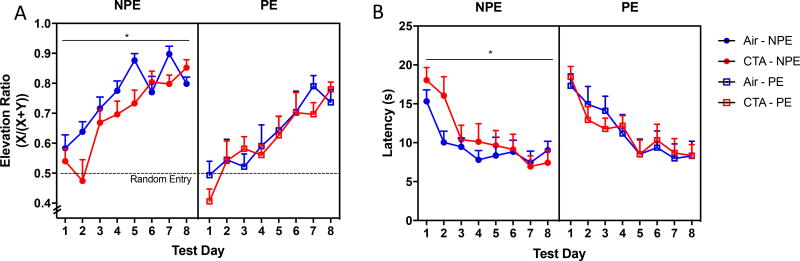
Conditioning was primarily measured using an elevation ratio, (X/(X+Y)), where X equals the number of food well approaches during the first 30 s of cue (when reward was available) and Y equals the number of approaches during the 30 s prior to cue onset (when reward was unavailable). As predicted by latent inhibition, cue pre-exposure blunted elevation ratios over the course of 8 test sessions (main effect of cue, F1,7=15.70, p<0.001). While there was no main effect of drug (F1,7=2.599, p=0.118), there was a drug × time interaction (F1,7=2.04, p<0.05). Subsequent partitioning of this interaction revealed a significant difference between treatment groups in the NPE condition (main effect of drug; F1,7=2.331, p<0.05) but not in PE condition (main effect of drug; F1,7=0.98, p=0.450] (Figure 5a). This interaction was reflected by the decreased latency in initiating food well approach behavior in response to cue over time in NPE (drug × time F1,7=2.626, p<0.05), but not PE (drug × time F1,7=0.689, p=0.681; Figure 5b). CTA decreased elevation ratio within the first day of training as well (main effect of drug; F1,2 = 4.597, p<0.05), but there was no evidence of a drug ×time interaction (F1,7=0.124, p=0.727; supplementary figure 5). These data indicate that CTA retards classical conditioning of a cue to a reward, but does not affect how a pre-exposure to a cue impedes subsequent associative learning about that cue.
Figure 5.
CTA mitigates the acquisition rate of classical conditioning without impairing latent inhibition. CTA and air treated animals were either naïve to the CS (NPE) or pre-exposed (PE) to the CS during the training phase. Conditioning was measured as the elevation ratio defined as X/(X+Y) where X is the number of food well entries during the first 30 s of cue and Y is the number of entries 30 s prior to cue onset. A) CTA blunted the conditioning acquisition curve over the course of eight test days in NPE but not PE rats. B) CTA also delayed food well approach in response to CS, indicative of blunted CS-US association. Data shown are mean + SEM; drug × test day interaction *p<0.05; all groups n=8.
3.6.2 Extinction of Classically Conditioned Approach Behavior
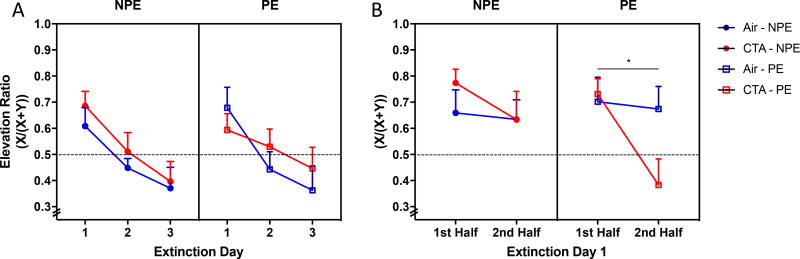
There were no main effects of drug (F1,7=0.599, p=0.455), cue (F1,7=0.011, p=0.918) or drug × time interactions (F1,7=0.507, p=0.605) on elevation ratios over the course of three extinction trials (Figure 6a). Interestingly, there was a drug × time interaction within the first extinction session (F1,2=4.711, p<0.05; Figure 6b) that reflected a significant difference between groups in the PE condition (F1,2=6.117, p<0.05), but not the NPE animals (F1,7=0.5637, p=0.47; Figure 6a). Neither a history of cue exposure nor CTA alone affected elevation ratios during this first extinction session (main effect of cue F1,2=0.591; main effect of drug, F1,2=0.448, p=0.593). Further, the drug × time interaction was not observed during acquisition of approach behavior (F1,7=0.124, p=0.727 supplementary figure 5). These data suggest that CTA enhances extinction of classically conditioned approach behavior only when the subject has previous experience of a non-reinforced cue within the first extinction session.
Figure 6.
CTA enhances extinction of classically conditioned approach behavior only within the first extinction session. CTA and air treated animals were either cue-naïve (NPE) or pre-exposed (PE) to cue during the training phase. Conditioning was measured as the elevation ratio defined as X/(X+Y) where X is the number of food well approaches during the first 30 s of cue and Y is the number 30 s prior to cue onset. A) CTA did not affect extinction progression over the course of three days. B) However, CTA-PE rats extinguished responding quicker than their air-PE counterparts within the first day of extinction testing. Data shown are mean + SEM; random well entry (dotted line); drug × time interaction *p<0.05; all groups n=8.
3.7. Dendritic Spines
To supplement findings from the behavioral studies, we measured dendritic spine density and morphology in neurons from two critical nodes in appetitive behavior, the mPFC and NAc (figure 7a–e). There were no detectable differences in dendritic spine density on basal dendrites of layer 5 prelimbic mPFC of CTA vs control rats (figure 7e). Conversely, toluene exposure during adolescence caused a lasting increase in dendritic spine density of NAc medium spiny neurons (main effect of drug F1,4=6.10, p<0.05; figure 7f) Post-hoc analysis revealed that this effect was driven by an increased prevalence of long-thin spines [t(60)=4.68; p<0.001]. There were no detectable differences in the length or head diameter of any spine subtype in either region tested (data not shown). Interestingly, there was a strong positive correlation between strategy-shifting (but not reversal learning) performance and medial PFC (but not NAc) spine density, further implicating the reliance of this behavior on the mPFC (Spearman r=0.667, n=17, p<0.01).
Figure 7.
Chronic exposure to toluene during adolescence causes persistent increases in dendritic spine density in a spine subtype and region-specific manner. (A) Representative image of a DiI-labeled pyramidal neuron from the prelimbic medial prefrontal cortex. Insets show representative spine segment image (B) after deconvolution, (C) modeling, and (D) overlay. (E) Chronic adolescent toluene exposure does not alter basal dendritic spine density in deep-layer mPFC neurons in adults. (F) Chronic adolescent toluene exposure increases spine density in medium spiny neurons of the NAc, an effect driven by the long-thin spine subtype. Data shown are mean +SEM; main effect F1,4=6.10 *p<0.05; post-hoc t(60)=4.68, *** p<0.001; air n=8, CTA n=9.
4. Discussion
4.1. The Effect of Toluene on Motivated Behavior
In this study, we investigated whether repeated exposures to abuse-level concentrations of toluene vapor (5700 ppm) during adolescence induced persistent cognitive effects (Dick et al., 2014; Gmaz, Yang, Ahrari, & McKay, 2012; Lubman et al., 2008). The results indicate that adolescent exposure to toluene retarded operant conditioning in adulthood consistent with observed instrumental learning deficits reported by Dick et al. (2014). The lever press training protocol used in this study had three phases. Phase 1 is a simple operant conditioning setup where levers remain extended throughout each session, and it was during this phase of training where deficits induced by toluene exposure were apparent. The next two phases contain an occasion setter, namely lever extension/retraction that signals reward availability. Here, no differences between groups were observed. The lack of effect during this phase may reflect the fact that the retraction of levers recruits cue-related attentional mechanisms that aid in task performance and could compensate for deficits in simple operant conditioning. The deficits shown in the present study are not likely attributable by a decreased motivation for SCM reward, as CTA rats reached a similar breakpoint under a progressive ratio level of responding compared to control, similar to that previously reported using a sucrose reward (Dick et al., 2014). Rather, these effects likely reflect impairments in the initial formation of action-outcome associations.
Exposure of adolescent rats to toluene vapor resulted in an initial reduction in weight gain. This finding mirrors reports in humans indicating that inhalant abusers often present as emaciated and is consistent with results from other rodent studies (Dick et al., 2014; Duncan et al., 2012; Ryu et al., 1998). The reduced body weight of CTA rats at the beginning of operant training could contribute to the observed deficits, as underweight rats might reach satiety quicker than heavier counterparts. However, both CTA and Air rats quickly consumed 5 ml of SCM in the days preceding operant training as part of the reward exposure protocol. This volume far exceeds the mean volumes consumed in lever press phase 1 (~1 ml air, ~0.5 ml CTA). Weight differences disappeared soon after operant training began and thus likely did not confound strategy shifting or reversal learning.
4.2. Behavioral Flexibility and Memory Retrieval
We next studied the effect of CTA on two types of behavioral flexibility – strategy set-shifting and reversal learning – in adulthood using either a within-session or between-session test design. There were no differences in behavioral flexibility in CTA rats that completed tasks within-session, suggesting that chronic toluene exposure does not produce long term deficits in set-shifting or reversal learning. Interestingly CTA rats performed a strategy shift quicker compared to controls when tested using a between-session design.
On the surface, the apparent improvement in set-shifting displayed by CTA rats may be interpreted as an enhancement in flexibility. While this is a possibility, it is important to note that CTA only accelerated shifting using a between-session shift, suggesting that these effects may not reflect a uniform enhancement in the mechanisms underlying shifting. Tasks which require behavioral flexibility typically present situations necessitating suppression of a previously relevant strategy alongside simultaneous acquisition and maintenance of a new strategy. With regards to the former, suppression of old strategies may be facilitated if there are underlying impairments in the consolidation, maintenance or retrieval of these strategies. In fact, pharmacologically destabilizing initial memories leads to enhanced reversal learning (Weiner et al., 1986) and extradimensional set-shifting (Crofts, Dalley, Collins, & Denderen, 2001). Furthermore, increasing the time between training and testing can also degrade memories and lead to poorer retrieval (Floresco & Phillips, 2001). With respect to the present study, retrieving the previously held response strategy is theoretically more important to completing between-session compared to within-session shifts because there are no reminder trials to jumpstart responding to the initial attentional set (visual cue). Interestingly, chronic exposure to 2000PPM toluene produces hippocampal cell loss that does not recover following 90 days of abstinence (Zhvania et al., 2012), and CTA alters hippocampus-dependent behaviors such as operant conditioning, delay discounting and contingency monitoring (Dick et al., 2014, Furlong et al., 2016). In attempting to explain the apparent improvement in set-shifting observed in one of our experiments, it is possible that CTA disrupted access to memories regarding the previously acquired discrimination rule via perturbations in hippocampal functioning. In turn, during between-session testing, poorer retrieval of this rule within the appropriate task context would lead to more rapid learning of the novel rule. On the other hand, the lack of improvement in shifting in CTA rats given reminder trials of the within-session shifts may be attributable to a reactivation of memories associated with the old rule that caused a comparable amount of interference of learning of the new rule in both groups. As such, when the within- and between- session data are considered together, it appears that CTA rats have persistent deficits in appetitive memory recall, but not necessarily enhanced behavioral flexibility.
One important caveat of these conclusions is the lack of differences when comparing within- vs between-session reversal learning. Two factors could explain this discrepancy. First, reversal learning was assessed after strategy set-shifting and side preference testing. This ensured that tasks proceeded in order of increasing difficulty, but also resulted in an incorrect lever choice that was 1) naturally preferred and 2) illuminated 50% of trials (a previously reinforced cue). Second, strategy set-shifting and reversal learning are dependent on two distinct prefrontal regions, the mPFC and orbitofrontal cortex, respectively (Floresco, Zhang, & Enomoto, 2009; Ghods-Sharifi, Haluk, & Floresco, 2008; Hamilton & Brigman, 2015). Nevertheless, the finding that CTA only affected set-shifting and not reversal learning suggests that impairments in memory retrieval induced by these manipulations may be more apparent when distinct discrimination strategies are required, rather than situations requiring the use of the same basic strategy (i.e. always press a lever in one location) and a mere shift between stimulus-reward associations.
4.3. The Effect of Latent Inhibition on Behavioral Flexibility
Upon changes in reinforcement contingencies that occur during strategy or reversal shifts, animals must acquire and then maintain a new strategy. Previous exposure to a non-reinforced cue has been repeatedly shown to block future conditioning to that cue, a phenomenon called latent inhibition. Although latent inhibition has traditionally been studied using Pavlovian conditioning paradigms, this idea can be conceptualized as “learned irrelevance” of cues in an operant conditioning scenario (although this definition is simplistic, for review: see (Meyer & Louilot, 2014)). Blunted latent inhibition has been observed in serotonin knockout (5HTT−/−) mice (Nonkes et al., 2012) and in spontaneously hypertensive rats, which are used as a in a rodent model of ADHD (Calzavara et al., 2009). Interestingly, both of these animal models also induce more rapid shifts in behavior upon changes in reinforcement contingencies that occurred between sessions (Chess et al., 2012; Nonkes et al., 2012). Furthermore, the enhanced shifting observed in spontaneously hypertensive rats was not observed following a within-session shift, in a manner similar to the present findings (Chess et al., 2012). The present studies reveal that CTA does not cause persistent latent inhibition deficits indicating that memory of previously non-reinforced cues likely do not play a role in the acquisition of new response strategies in the between-session tests of behavioral flexibility.
An additional novel finding from the latent inhibition study is that CTA enhances extinction progression within the first day of extinction compared to air-treated controls only when rats have previous experience with non-reinforced cues. This group was most similar to rats in the between-session behavioral flexibility tasks in that both had experience with a non-reinforced cue, and were tested 24h after the last training session. The enhanced extinction specific to CTA-NPE rats might be the cause of the decreased perseveration (and thus, enhanced performance) by CTA rats in between-session set-shifting.
4.4. Prolonged Abstinence from Addictive Substances Alters Postsynaptic Neuron Morphology in the NAc
Addictive substances affect frontal-striatal pathways and are thought to impair “top-down” regulation of compulsive drug-seeking behaviors. Chronic toluene exposure has been reported to decrease dendritic complexity in superficial cortical layers following 48 h of abstinence (Pascual et al., 2011; Pascual & Bustamante, 2010, 2011). The present findings add to these data by showing that protracted abstinence from toluene exposure (7 weeks) is associated with increases in long-thin spines in the NAc. The effect of drugs of abuse on spine morphology is variable, presumably due to different mechanisms of action, differences in drug treatment (chronic vs acute), and time of measurement (no abstinence vs hours or weeks; for review see: Mulholland, Chandler, & Kalivas, 2016; Spiga et al., 2014). Similar to toluene, chronic treatment with cocaine (Rasakham et al., 2014), alcohol (Peterson, McCool, & Hamilton, 2015), and nicotine (Gipson et al., 2013) causes selective and persistent enhancements in the NAc dendritic spine morphology.
The increased presence of long thin spines in the NAc might reflect an increase in silent synapses and thus deactivation of the region (Grueter et al., 2013). This could explain the impaired acquisition of operant and classical conditioning induced by CTA, both of which are blunted by NA lesions (Meredith et al., 2008). This explanation does not, however, address the specific enhancements in both extinction learning and delay dependent strategy set shifting caused by CTA since extinction is considered a novel memory rather than degradation of an initial memory (although unlearning can occur; for review see: Todd, Vurbic, & Bouton, 2014).
5. Conclusions
While strategy set-shifting was enhanced in CTA rats, this effect was only observed if there was long period of time before training and testing. This enhancement was not observed when a simple reversal of stimulus-reward associations was made. To assess the generality of these findings, future research should test whether CTA enhances behavioral flexibility exclusively when other dissimilar discrimination strategies are used (e.g. by using different textured levers or nose poke holes associated with different odors). Deficits in operant conditioning, classical conditioning and enhanced extinction following classical conditioning suggest that the behavioral flexibility “enhancements” in CTA rats may actually reflect specific impairments in appetitive memory recall. This would explain the lack of differences in behavioral flexibility when using a within-session test design that relies less on memory recall. Lasting changes in appetitive behavior may reflect specific anatomical alterations in neuroanatomy responsible for goal directed action. To this end, we observed an increase in immature dendritic spines in the NAc of CTA rats. This could prevent synaptic plasticity and thus behavioral flexibility in certain situations. Future studies should explore the cellular/molecular changes (e.g. enhanced GABAergic interneuron activity, upregulated D2 receptor expression, etc.) driving this aberrant NAc morphology.
Supplementary Material
Highlights.
Toluene exposure during adolescence (“CTA”) blunted operant conditioning
CTA improved strategy shifting when there was a delay between training and testing
CTA impaired classical conditioning without altering latent inhibition
CTA increased immature dendritic spine density in the nucleus accumbens core
Acknowledgments
SBF generously provided the core MedPC code and helped with data analysis in the behavioral flexibility experiments. JTG helped with the experimental design of the classical conditioning experiments, and PJM assisted with the dendritic spine analysis. KMB performed all experiments and data analyses. KMB and JJW were responsible for the study concept and drafted this manuscript. All authors provided critical revisions of this draft before its submission for publication. The authors would also like to thank postdoctoral scholar Wes N Wayman for his helpful comments in preparation of this manuscript.
Funding
This work was supported by the NIH National Institute on Drug Abuse (R01 DA013951) and the National Institute of Alcohol Abuse and Alcoholism (Collaborative Research on Addiction supplement to T32 AA007474) and a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada to SBF.
Abbreviations
- CTA
Chronic toluene exposure followed by protracted abstinence
- PE
cue pre exposed
- NPE
non cue pre exposed
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens core
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Batis JC, Hannigan JH, Bowen SE. Differential Effects of Inhaled Toluene on Locomotor Activity in Adolescent and Adult Rats. Pharmacology Biochemistry and Behavior. 2010;48(Suppl 2):1–6. doi: 10.1016/j.pbb.2010.07.003. http://doi.org/10.1097/MPG.0b013e3181a15ae8.Screening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydas G, Ozveren F, Akdemir I, Tuzcu M, Yasar A. Learning and memory deficits in rats induced by chronic thinner exposure are reversed by melatonin. Journal of Pineal Research. 2005;39(1):50–6. doi: 10.1111/j.1600-079X.2005.00212.x. http://doi.org/10.1111/j.1600-079X.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- Beckley JT, Evins CE, Fedarovich H, Gilstrap MJ, Woodward JJ. Medial prefrontal cortex inversely regulates toluene-induced changes in markers of synaptic plasticity of mesolimbic dopamine neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2013;33(2):804–13. doi: 10.1523/JNEUROSCI.3729-12.2013. http://doi.org/10.1523/JNEUROSCI.3729-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cerebral Cortex. 2007;17(7):1625–1636. doi: 10.1093/cercor/bhl073. http://doi.org/10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Hannigan JH, Cooper PB. Abuse pattern of gestational toluene exposure alters behavior in rats in a “waiting-for-reward” task. Neurotoxicology and Teratology. 2009;31(2):89–97. doi: 10.1016/j.ntt.2008.11.002. http://doi.org/10.1016/j.ntt.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Balster RL. The effects of abused inhalants on mouse behavior in an elevated plus-maze. European Journal of Pharmacology. 1996;312(2):131–136. doi: 10.1016/0014-2999(96)00459-1. http://doi.org/10.1016/0014-2999(96)00459-1. [DOI] [PubMed] [Google Scholar]
- Brady AM, Floresco SB. Operant Procedures for Assessing Behavioral Flexibility in Rats. Journal of Visualized Experiments. 2015;(96):1–13. doi: 10.3791/52387. http://doi.org/10.3791/52387. [DOI] [PMC free article] [PubMed]
- Brouette T, Anton R. Clinical review of inhalants. The American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2001;10(1):79–94. doi: 10.1080/105504901750160529. http://doi.org/10.1080/105504901750160529. [DOI] [PubMed] [Google Scholar]
- Bukowski Ja. Review of the epidemiological evidence relating toluene to reproductive outcomes. Regulatory Toxicology and Pharmacology : RTP. 2001;33(2):147–56. doi: 10.1006/rtph.2000.1448. http://doi.org/10.1006/rtph.2000.1448. [DOI] [PubMed] [Google Scholar]
- Calzavara MB, Medrano WA, Levin R, Kameda SR, Andersen ML, Tufik S, Abílio VC. Neuroleptic drugs revert the contextual fear conditioning deficit presented by spontaneously hypertensive rats: A potential animal model of emotional context processing in Schizophrenia? Schizophrenia Bulletin. 2009;35(4):748–759. doi: 10.1093/schbul/sbn006. http://doi.org/10.1093/schbul/sbn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess, Amy C, Raymond, Brittany E, Gardner-Morse, Ira G, Stefani, Mark R, Green JT. Set-Shifting in a Rodent Model of Attention-Deficit/Hyperactivity Disorder. Behavioral Neuroscience. 2012;29(6):997–1003. doi: 10.1037/a0023571. http://doi.org/10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Denderen Van JCM. Differential Effects of 6-OHDA Lesions of the Frontal Cortex and Caudate Nucleus on the Ability to Acquire an Attentional Set. 2001:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Dick A, L W, Axelsson M, Lawrence AJ, Duncan JR. Specific impairments in instrumental learning following chronic intermittent toluene inhalation in adolescent rats. Psychopharmacology. 2014;231:1531–1542. doi: 10.1007/s00213-013-3363-7. http://doi.org/10.1007/s00213-013-3363-7. [DOI] [PubMed] [Google Scholar]
- Duncan JR, Dick A, L W, Egan G, Kolbe S, Gavrilescu M, Wright D, Lawrence AJ. Adolescent Toluene Inhalation in Rats Affects White Matter Maturation with the Potential for Recovery Following Abstinence. PLoS ONE. 2012;7(9):1–12. doi: 10.1371/journal.pone.0044790. http://doi.org/10.1371/journal.pone.0044790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8(11):1481–9. doi: 10.1038/nn1579. http://doi.org/10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Block AE, Tse MTL. Inactivation of the medial prefrontal cortex of the rat impairs strategy set-shifting, but not reversal learning, using a novel, automated procedure. Behavioural Brain Research. 2008;190(1):85–96. doi: 10.1016/j.bbr.2008.02.008. http://doi.org/10.1016/j.bbr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Jentsch JD. Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2011;36(1):227–250. doi: 10.1038/npp.2010.158. http://doi.org/10.1038/npp.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Phillips Ga. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behavioral Neuroscience. 2001;115(4):934–939. http://doi.org/10.1037//0735-7044.115.4.934. [PubMed] [Google Scholar]
- Floresco SB, Zhang Y, Enomoto T. Neural circuits subserving behavioral flexibility and their relevance to schizophrenia. Behavioural Brain Research. 2009;204(2):396–409. doi: 10.1016/j.bbr.2008.12.001. http://doi.org/10.1016/j.bbr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Duncan JR, Corbit LH, Rae CD, Rowlands BD, Maher AD, Balleine BW. Toluene inhalation in adolescent rats reduces flexible behaviour in adulthood and alters glutamatergic and GABAergic signalling. J Neurochem. 2016 doi: 10.1111/jnc.13858. http://doi.org/10.1111/jnc.13858. [DOI] [PubMed]
- Gerasimov MR, Collier L, Ferrieri A, Alexoff D, Lee D, Gifford AN, Balster RL. Toluene inhalation produces a conditioned place preference in rats. European Journal of Pharmacology. 2003;477(1):45–52. doi: 10.1016/j.ejphar.2003.08.022. http://doi.org/10.1016/j.ejphar.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiology of Learning and Memory. 2008;89(4):567–573. doi: 10.1016/j.nlm.2007.10.007. http://doi.org/10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith ACW, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(22):9124–9. doi: 10.1073/pnas.1220591110. http://doi.org/10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmaz JM, Yang L, Ahrari A, McKay BE. Binge inhalation of toluene vapor produces dissociable motor and cognitive dysfunction in water maze tasks. Behavioural Pharmacology. 2012;23:669–677. doi: 10.1097/FBP.0b013e3283585923. http://doi.org/10.1097/FBP.0b013e3283585923. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Stayner DA, Chinman MJ, Wu P, Tebes JK, Davidson L. The relationship between anxiety and substance use disorders among individuals with severe affective disorders. Comprehensive Psychiatry. 2002;43(4):245–252. doi: 10.1053/comp.2002.33500. http://doi.org/10.1053/comp.2002.33500. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ΔFosB differentially modulates nucleus accumbens direct and indirect pathway function. Pnas. 2013;110(5):1923–8. doi: 10.1073/pnas.1221742110. http://doi.org/10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton Da, Brigman JL. Behavioral flexibility in rats and mice: contributions of distinct frontocortical regions. Genes, Brain and Behavior. 2015;14:4–21. doi: 10.1111/gbb.12191. http://doi.org/10.1111/gbb.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441(7096):979–983. doi: 10.1038/nature04783. http://doi.org/10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- Howard MO, Balster RL, Cottler LB, Wu L, Vaughn MG. Inhalant use among incarcerated adolescents in the United States : Prevalence, characteristics, and correlates of use. 2008;93:197–209. doi: 10.1016/j.drugalcdep.2007.08.023. http://doi.org/10.1016/j.drugalcdep.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Howard MO, Bowen SE, Garland EL, Perron BE, Vaughn MG. Inhalant use and inhalant use disorders in the United States. Addiction Science & Clinical Practice. 2011;6(1):18–31. [PMC free article] [PubMed] [Google Scholar]
- Huerta-Rivas A, López-Rubalcava C, Sánchez-Serrano SL, Valdez-Tapia M, Lamas M, Cruz SL. Toluene impairs learning and memory, has antinociceptive effects, and modifies histone acetylation in the dentate gyrus of adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2012;102(1):48–57. doi: 10.1016/j.pbb.2012.03.018. http://doi.org/10.1016/j.pbb.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Drug Use: 2014 Overview, Key Findings on Adolescent Drug Use 2015 [Google Scholar]
- Lin B-F, Ou M-C, Chung S-S, Pang C-Y, Chen H-H. Adolescent toluene exposure produces enduring social and cognitive deficits in mice: an animal model of solvent-induced psychosis. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry. 2010;11(6):792–802. doi: 10.3109/15622970903406234. http://doi.org/10.3109/15622970903406234. [DOI] [PubMed] [Google Scholar]
- Lin L, Lo LH-Y, Lyu Q, Lai K-O. Determination of dendritic spine morphology by the striatin scaffold protein STRN4 through interaction with the phosphatase PP2A. Journal of Biological Chemistry. 2017;(3) doi: 10.1074/jbc.M116.772442. jbc.M116.772442. http://doi.org/10.1074/jbc.M116.772442. [DOI] [PMC free article] [PubMed]
- Lopez-Rubalcava C, Hen R, Cruz SL. Anxiolytic-like actions of toluene in the burying behavior and plus-maze tests: Differences in sensitivity between 5-HT(1B) knockout and wild-type mice. Behavioural Brain Research. 2000;115(1):85–94. doi: 10.1016/s0166-4328(00)00241-2. http://doi.org/10.1016/S0166-4328(00)00241-2. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yücel M, Lawrence Ja. Inhalant abuse among adolescents: neurobiological considerations. British Journal of Pharmacology. 2008;154(2):316–326. doi: 10.1038/bjp.2008.76. http://doi.org/10.1038/bjp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154(2):358–368. doi: 10.1038/bjp.2008.132. http://doi.org/10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the striatum and its subdivisions. Brain Structure and Function. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. http://doi.org/10.1007/s00429-008-0175-3.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer F, Louilot A. Consequences at adulthood of transient inactivation of the parahippocampal and prefrontal regions during early development: new insights from a disconnection animal model for schizophrenia. Frontiers in Behavioral Neuroscience. 2014 Sep;7:118. doi: 10.3389/fnbeh.2014.00118. http://doi.org/10.3389/fnbeh.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser V, Balster R. The Effects of Acute and Repeated Toluene Exposure on Operant Behavior in Mice. Neurobehavioral Toxicology and Teratology. 1981;3(4):471–5. http://doi.org/7335141. [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ, Kalivas PW. Signals from the Fourth Dimension Regulate Drug Relapse. Trends in Neurosciences. 2016;39(7):472–485. doi: 10.1016/j.tins.2016.04.007. http://doi.org/10.1016/j.tins.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonkes LJP, van de Vondervoort IIGM, de Leeuw MJC, Wijlaars LP, Maes JHR, Homberg JR. Serotonin transporter knockout rats show improved strategy set-shifting and reduced latent inhibition. Learning & Memory. 2012;19(5):190–193. doi: 10.1101/lm.025908.112. http://doi.org/10.1101/lm.025908.112. [DOI] [PubMed] [Google Scholar]
- Pascual R, Bustamante C. Melatonin promotes distal dendritic ramifications in layer II/III cortical pyramidal cells of rats exposed to toluene vapors. Brain Research. 2010;1355:214–220. doi: 10.1016/j.brainres.2010.07.086. http://doi.org/10.1016/j.brainres.2010.07.086. [DOI] [PubMed] [Google Scholar]
- Pascual R, Bustamante C. Structural neuroplasticity induced by melatonin in entorhinal neurons of rats exposed to toluene inhalation. Acta Neurobiologiae Experimentalis. 2011;71(4):541–547. doi: 10.55782/ane-2011-1870. [DOI] [PubMed] [Google Scholar]
- Pascual R, Pilar Zamora-León S, Pérez N, Rojas T, Rojo A, JoséSalinas M, Bustamante C. Melatonin ameliorates neocortical neuronal dendritic impairment induced by toluene inhalation in the rat. Experimental and Toxicologic Pathology. 2011;63(5):467–471. doi: 10.1016/j.etp.2010.03.006. http://doi.org/10.1016/j.etp.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Peterson VL, McCool BA, Hamilton DA. Effects of ethanol exposure and withdrawal on dendritic morphology and spine density in the nucleus accumbens core and shell. Brain Research. 2015;1594:125–135. doi: 10.1016/j.brainres.2014.10.036. http://doi.org/10.1016/j.brainres.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasakham K, Schmidt HD, Kay K, Huizenga MN, Calcagno N, Pierce RC, Sadri-vakili G. Synapse Density and Dendritic Complexity Are Reduced in the Prefrontal Cortex following Seven Days of Forced Abstinence from Cocaine Self-Administration. 2014;9(7):1–6. doi: 10.1371/journal.pone.0102524. http://doi.org/10.1371/journal.pone.0102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. http://doi.org/10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Ryu YH, Lee JD, Yoon PH, Jeon P, Kim DI, Shin DW. Cerebral Perfusion Impairment in a Patient with Toluene Abuse. 1998;39(4) [PubMed] [Google Scholar]
- Spiga S, Mulas G, Piras F, Diana M. The Addicted Spine. Frontiers in Neuroanatomy. 2014 Oct;8:1–7. doi: 10.3389/fnana.2014.00110. http://doi.org/10.3389/fnana.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, LaLumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addiction Biology. 2013;18(1):50–3. doi: 10.1111/j.1369-1600.2012.00479.x. http://doi.org/10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Vurbic D, Bouton ME. Behavioral and neurobiological mechanisms of extinction in Pavlovian and instrumental learning. Neurobiology of Learning and Memory. 2014;108:52–64. doi: 10.1016/j.nlm.2013.08.012. http://doi.org/10.1016/j.nlm.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Centanni SW, Garr SC, New NN, Mulholland PJ, Gass JT, Chandler LJ. Binge-like alcohol exposure during adolescence disrupts dopaminergic neurotransmission in the adult prelimbic cortex. Neuropsychopharmacology Preview. 2016;(5):1–33. doi: 10.1038/npp.2016.190. http://doi.org/10.1038/npp.2016.190. [DOI] [PMC free article] [PubMed]
- Uys JD, McGuier NS, Gass JT, Griffin WC, Ball LE, Mulholland PJ. Chronic intermittent ethanol exposure and withdrawal leads to adaptations in nucleus accumbens core postsynaptic density proteome and dendritic spines. Addiction Biology. 2015 doi: 10.1111/adb.12238. n/a-n/a. http://doi.org/10.1111/adb.12238. [DOI] [PMC free article] [PubMed]
- Wise Ra, Koob GF. The Development and Maintenance of Drug Addiction. Neuropsychopharmacology. 2014;39(2):254–262. doi: 10.1038/npp.2013.261. http://doi.org/10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuncu Z, Zorlu N, Saatcioglu H, Basay B, Basay O, Zorlu PK, Gelal F. Abnormal white matter integrity and impairment of cognitive abilities in adolescent inhalant abusers. Neurotoxicology and Teratology. 2015;47:89–95. doi: 10.1016/j.ntt.2014.11.009. http://doi.org/10.1016/j.ntt.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Zhvania MG, Chilachava LR, Japaridze NJ, Gelazonia LK, Lordkipanidze TG. Immediate and persisting effect of toluene chronic exposure on hippocampal cell loss in adolescent and adult rats. Brain Research Bulletin. 2012;87(2–3):187–192. doi: 10.1016/j.brainresbull.2011.10.021. http://doi.org/10.1016/j.brainresbull.2011.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.