Abstract
Objective
Personality traits related to negative emotionality and low constraint are strong correlates of alcohol use disorder (AUD), but few studies have evaluated the prospective interplay between these traits and AUD symptoms from adolescence to young adulthood.
Method
The Minnesota Twin Family Study (N = 2,769) was used to examine the developmental interplay between AUD symptoms and three personality measures of constraint, negative emotionality, and aggressive undercontrol from ages 17 to 29.
Results
Results from random-intercept cross-lagged panel models showed that low constraint and aggressive undercontrol predicted subsequent rank-order increases in AUD symptoms from ages 17 to 24. AUD symptoms did not predict rank-order change in these traits from ages 17 to 24. There was support for both cross-effects from ages 24 to 29. Biometric analysis of the twin data showed genetic influences accounted for most of the phenotypic correlations over time.
Conclusion
Results are consistent with the notion that personality traits related to low constraint and aggressive undercontrol are important vulnerability/predisposition factors for the development of early adult AUD. In later young adulthood, there is more evidence for the simultaneous co-development of personality and AUD. Implications are addressed with attention to personality-based risk assessments and targeted AUD prevention approaches.
Keywords: Alcohol Use Disorder, Aggressive Undercontrol, Behavioral Disinhibition, Constraint, Negative Emotionality
Alcohol Use Disorder (AUD) is characterized by frequent and heavy alcohol use that then leads to problems in psychosocial functioning and physiological dependence (withdrawal and tolerance) (American Psychological Association, 2013). AUD is associated with a myriad of poor psychosocial and health outcomes, such as school failure and loss of work place productivity, divorce, legal problems, and poor physical health, including early death (CDC, 2014; Hicks et al., 2010; Foster et al., 2014; Huang et al., 2013; Greig et al., 2006, Meier et al., 2010; Rehm et al., 2009). Nearly 20% of those age 18–25 qualify for a AUD, whereas only about 6% of adults age 26 or older meet an AUD diagnosis (Substance Use and Mental Health Administration [SAMSHA], 2012). Thus, identifying etiological processes that operate in the developmental transition from adolescence through young adulthood is critical for understanding and addressing the adverse outcomes attributed to AUD.
One of the most consistent predictors of AUD and problematic substance use in general are personality traits related to negative emotionality and low constraint (Boschloo et al., 2012; James & Taylor, 2007; Kotov, Gamez, Schmidt, & Watson, 2010; McGue, Slutske, & Iacono, 1999; McGue, Slutske, Taylor, & Iacono, 1997; Sher & Trull, 1994; Slutske et al., 2002; Vrieze, Vaidyanathan, Hicks, Iacono, & McGue, 2014; Zucker, Heitzeg, & Nigg, 2011). Negative emotionality is characterized by the propensity to experience emotions and mood states related to anger, contempt, disgust, guilt, fear, and nervousness (Watson, Clark, & Tellegen, 1988), particularly in response to stressful situations. Negative emotionality captures dimensions identified in the Big Five personality traits of high neuroticism and low agreeableness (Church, 1994). Persons high in constraint (reverse of disinhibition) have strong self-control, avoid physically dangerous or thrilling activities, and endorse conventional values and norms for behavior (Tellegen & Waller, 2008). Constraint captures aspects of the Big Five personality traits of conscientiousness as well as openness to experiences (Church, 1994). Aggressive undercontrol is an intermediate trait between high negative emotionality and low constraint that has been shown to be a core feature of a general liability to substance use disorders and antisocial behavior in adolescence and young adulthood and accounts for most of the predictive power between personality traits and externalizing disorders (Hicks, Schalet, Malone, Iacono, & McGue, 2011).
Although several longitudinal studies have demonstrated that traits related to negative emotionality and low constraint in adolescence are predictors of alcohol and substance use problems in adulthood (Caspi et al., 1997; Chassin, Fora, & King, 2004; Elkins, King, McGue, & Iacono, 2006), less research has examined the co-development (i.e., reciprocal or bi-directional associations) between these personality traits and AUD over time. We aimed to fill this gap by testing multiple theoretical models of personality-AUD development (vulnerability/predisposition, scar/complication, and common-cause) in the developmental transition from late adolescence (age 17) through early adulthood (age 24) and into later young adulthood (age 29). We first review developmental trends in the prevalence of AUD symptoms and mean-level change in personality traits consistent with a maturation process. Next we describe the theoretical models relevant to personality-AUD co-development. Finally we review how our analytic plan to test these models and confirm or disconfirm the model’s predictions.
Co-Development between Personality and AUD
Epidemiological surveys have consistently shown that problematic alcohol use peaks in the age 18–25 time period (SAMSHA, 2014; Blanco et al., 2008; Chen & Kandel, 1995) and that there is a normative decline in problematic drinking by age 30 (SAMSHA, 2012). Normative maturation of personality in the form of decreases in negative emotionality and increases in constraint become evident across this same developmental window (Blonigen, Carlson, Hicks, Krueger, & Iacono, 2008; Durbin, Hicks, Blonigen, Johnson, Iacono, & McGue, 2016; Hicks, Durbin, Blonigen, Iacono, & McGue, 2012). Changes across personality and AUD constructs appear to be intertwined in this developmental period. For example, Littlefield, Sher, and Wood (2009) showed that declines in problematic alcohol use between ages 18 and 35 were correlated with declines in neuroticism and impulsivity, suggesting there may be a “maturing out” process of problematic alcohol use partially attributable to normative maturational changes in broader aspects of personality. Consistent with this, Hicks et al. (2012) showed that those with persistent AUD failed to show normative declines in negative emotionality from ages 17 to 24. Similar results have been found for serious juvenile offenders in that decreasing substance use from ages 15 to 22 was correlated with increases in psychosocial maturity during this same period (Chassin et al., 2010).
Rather than merely spurious co-occurring phenomena, mean-level changes in personality traits of negative emotionality and constraint and problematic drinking may be functionally related as antecedents and/or consequences of one another. Consistent with this, Quinn, Stappenbeck, and Fromme (2011) showed that over the period from prior to freshman year to after college graduation, increases in novelty-seeking and impulsivity predicted increases in subsequent heavy drinking and that increases in heavy drinking predicted subsequent increases in novelty-seeking and impulsivity. Littlefield, Verges, Wood, and Sher (2012) similarly showed that higher levels of novelty-seeking/impulsivity at age 21 significantly predicted increases in heavy drinking from ages 21 to 25, with some evidence that heavy drinking at age 21 also predicted increases in novelty-seeking/impulsivity in this same time frame.
Describing the direction and relative magnitude of these longitudinal associations across domains lays out the basic observations to be explained by theoretical models of how personality traits and heavy drinking influence one another. Replicating and extending these explorations to multiple developmental periods is also critical for testing whether functional associations between personality traits and heavy drinking vary across development (Durbin & Hicks, 2014). Much of the research to date focuses on the ages of 18 to 25, when alcohol use is most prevalent (SAMSHA, 2012). However, personality traits and alcohol use may covary to a greater degree when evaluated earlier in time when problematic alcohol use is less normative (e.g., adolescence), or later in life (e.g., past age 25) when reductions in heavy drinking desistence and psychosocial and personality maturation are more normative (Blonigen et al., 2008; Hicks et al., 2012). If such differences were found, they might indicate more dynamic patterns of associations reflecting different causal influences between traits and AUD across development.
Theoretical Models of Personality-AUD Development
Several theoretical models of personality-AUD associations make predictions relevant to the developmental unfolding of personality-AUD associations (see Durbin & Hicks, 2014; Klein, Kotov, & Bufferd, 2011; Tackett, 2006), although it is important to point out these models are not mutually exclusive. Two of these models privilege one direction of effect. First, the vulnerability/predisposition model posits that individual differences in key personality traits such as negative emotionality or constraint capture processes that put individuals at increased risk for subsequent AUD. Second, the scar/complication model posits that problems such as AUD set into motion processes that change personality functioning as captured by higher negative emotionality and/or lower constraint. Evidence for both vulnerability and scar processes playing a role in personality trait-AUD associations is consistent with a transactional model of AUD-personality development - that is, the origins of their covariance are not isolated to one causal direction. Rather, bidirectional processes exist that can be detected by modeling pathways across the two domains in longitudinal designs. At present, evidence from longitudinal studies best support transactional models (Blonigen, Durbin, Hicks, Johnson, McGue, 2015; Chassin et al., 2010; Hicks et al., 2010; Littlefield et al., 2009; 2012; Quinn et al., 2011) although the direction of effects using a wider developmental perspective than from ages 18 to 25 remains unclear.
Finally, AUD and personality trait associations may emerge from common cause processes, such that their overlap is not driven by functional associations across domains, but by shared etiological factors that contribute to the development of each construct (Klein et al., 2011). In the strongest version of the common cause model, AUD and personality traits would not have any direct causal relationship with one another after accounting for their common causes (Durbin & Hicks, 2014). One powerful strategy for demonstrating common cause processes is to identify the potential cause(s) and model its contributions to traits and AUD in the same model. Twin and family studies have shown that both personality and AUD are substantially influenced by additive genetic influences (Matteson, McGue, & Iacono, 2013; Verhulst, Neale, & Kendler, 2015; Vukasovic & Bratko, 2015) and that the cross-sectional associations between key personality traits and AUD and related externalizing disorders are predominately attributable to shared additive genetic influences (Krueger et al., 2002; Khremiri, Kuja-Halkola, Larsson, & Jayaram-Lindstrom, 2016; Littlefield et al., 2011; Slutske et al., 2002). Few studies, however, have incorporated both the longitudinal design and twin methodology to evaluate relationships between personality and AUD over time. Thus, a central goal of this investigation was to synthesize these methods to better understand which predictions of different models of personality development are supported (vulnerability/predisposition, scar, transactional, and/or common cause).
Study Overview
We aimed to extend prior research and evaluate antecedent vs. consequence in the associations between key personality traits (constraint, negative emotionality, aggressive undercontrol) and AUD symptoms across critical time points in adolescence (age 17) through early adulthood (age 24) and into later young adulthood (age 29) via prospective analysis. Results were expected to garner support for vulnerability/predisposition, scar, or transactional models of AUD-personality co-development. As described earlier, each of these models makes predictions regarding the direction of prospective associations across constructs and testing these together in the same model allows for determination of which longitudinal pathways (i.e., personality traits to AUD or vice versa) contribute most to the covariance between the constructs.
Based on prior research (Littlefield et al., 2012; Quinn et al., 2011), we might expect equally strong AUD to personality as personality to AUD effects - thus supporting a transactional model of personality-AUD development. However, it is unclear whether such effects are specific to this time period in which both traits and alcohol use are exhibiting normative mean-level changes, or whether the strength of effects across the two domains may be different in earlier or later developmental periods characterized by lower overall rates of AUD. For example, early onset AUD may have more potential to result in deleterious effects on normative personality development than AUD with onset later in life, leading to greater support for scar processes in earlier developmental intervals. Alternatively, personality processes may crystalize ahead of the onset of AUD and then become an important predictor of subsequent AUD at a time with heavy alcohol use is more normative, leading to greater support for a vulnerability/predisposition model.
To further evaluate whether the longitudinal associations we observed between personality and AUD were due to common causes, we took advantage of our twin design and conducted a second set of analyses that evaluated the extent to which the prospective associations between these three personality traits in relation to AUD symptoms are explained by common genetic versus environmental influences. Based on prior cross-sectional research on adolescents (Krueger et al., 2002) and adults (Littlefield et al., 2011; Slutske et al., 2002), we hypothesized that longitudinal associations between personality and AUD would be predominately due to common additive genetic influence.
Method
Participants
The Minnesota Twin Family Study (MTFS) is a longitudinal study of twins born in Minnesota, designed for the purpose of investigating the etiology of SUDs and related psychopathology (Iacono, Carlson, Taylor, Elkins, & McGue, 1999). The MTFS is an accelerated cohort investigation that includes a younger cohort originally assessed when twins were 11 years-old, and an older cohort that was first assessed when twins were 17 years-old. Follow-up assessments were conducted for both cohorts every 3–5 years through age 29 (with overlapping assessments at ages 17, 20, 24, and 29). Twins and their parents were identified using publicly available birth certificates through the use of several public databases (target birth years: 1972 – 1984). Eligibility criteria included living within a day’s drive to the University laboratory. Families were excluded if either twin had a mental or physical handicap that would impair study participation. Nearly all eligible twins (90%) were located successfully and 83% of those eligible and located families agreed to participate. The University of Minnesota Institutional Review Board approved all study protocols. All twins provided informed consent or assent depending on their age of assessment.
The sample for the current analyses included 2,769 individuals (52% female) from 1,382 same-sex twin pairs (65% monozygotic, including 5 triplets). Zygosity was determined through parent-reports via a standard zygosity questionnaire, staff evaluations of physical similarity of eyes, hair, face, ears, and fingerprint ridge counts. DNA markers were used to resolve any discrepancies in these reports. The majority of participants were of European ancestry (95%), which is consistent with the demographics of Minnesota for the relevant birth years (Holdcraft & Iacono, 2004; U.S. Census, 2000). There was considerable diversity in socio-economic status. For example, the highest education completed for the majority of parents was a high school diploma or equivalent (63.5% for fathers, and 62.6% for mothers); 28.5% of fathers and 25.1% of mothers earned at least a BA/BS degree. The median household income was $45,001 to $50,000 at the initial assessment, but 25% of families earned ≤ $40,000 and 25% of families earned ≥ $60,001.
Data used in the present study included assessments for the target ages of 17 (M = 17.8 years, SD = 0.69), 24 (M = 25.0 years, SD = 0.90), and 29 (M = 29.4 years, SD = 0.67) years (personality data were not collected at age 20, thus the age 20 assessment was not used here). Participation rates ranged from 88% to 93% across assessments. Potential attrition effects were evaluated by comparing mean differences in symptoms of alcohol use disorder at age 17 for those who did or did not complete the follow-up assessments (at ages 20, 24, and 29). Those who participated in adult assessments had slightly fewer alcohol symptoms at age 17 than those who did not participate; however, the effect sizes were small (Cohen’s d = .20, .22, and .14 at ages 20, 24, and 29, respectively). Thus, there was little evidence of meaningful attrition effects.1
Measures
Personality
The Multidimensional Personality Questionnaire (MPQ) (Tellegen & Waller, 2008) was used to assess personality at ages 17, 24, and 29. The MPQ is a 198-item self-report survey that assesses 10 primary scales that correspond to the higher order traits of Positive Emotionality, Negative Emotionality, and Constraint (an 11th primary scale of Absorption is also measured by the MPQ that does not load onto the higher order factors and is not used in the present analyses). Specifically, the scales that load on to the higher-order factor of Positive Emotionality are the Achievement, Well-Being, Social Potency, and Social Closeness scales. Higher scores in Positive Emotionality relate to higher scores in working hard, reaching goals, and valuing close relationships. The scales that load onto the higher-order factor of Negative Emotionality are the Alienation, Aggression, and Stress Reaction scales. Thus, higher scores in Negative Emotionality relate to feeling more negative emotions, antagonism, particularly in the context of stress. The scales that load onto the higher-order factor of Constraint are the Traditionalism, Harm Avoidance, and Control scales. Thus, higher scores in Constraint are associated as endorsing conservative values, being risk adverse, planful, and cautious. Internal consistency reliability estimates (α) for all primary MPQ scales ranged from .77 to .92 across the age 17, 24, and 29 assessments.
Negative emotionality and constraint are two of the primary scores evaluated in this study, given prior research showing strong linkages between these scores and alcohol and substance use variables (Boschloo et al., 2012; James & Taylor, 2007; Kotov, Gamez, Schmidt, & Watson, 2010; McGue, Slutske, & Iacono, 1999; McGue, Slutske, Taylor, & Iacono, 1997; Sher & Trull, 1994; Slutske et al., 2002; Vrieze, Vaidyanathan, Hicks, Iacono, & McGue, 2014; Zucker, Heitzeg, & Nigg, 2011). Additionally, we explored whether results were consistent for aggressive undercontrol, a facet scale developed by Hicks et al. (2011) that has been shown to be particularly relevant to and representative of an overall liability towards substance use and externalizing problems in adolescence and young adulthood. This scale contains items from the scales that load onto constraint (including 5 items from the traditionalism scale = assessing dislike versus like of rebellion, cursing, and traditional values, 5 items from the control scale - assessing degree of impulsivity versus planning, and 3 items from the harm avoidance scale - assessing preference for boring but safe activities versus thrilling but potentially dangerous activities), as well as 7 items from the aggression scale (which loads onto negative emotionality) - assessing violent behaviors; α’s ranged from .80 to .81 across all assessments.
Alcohol use disorder (AUD) symptom counts
AUD symptom counts at ages 17, 24, and 29 were assessed using the Substance Abuse Module (SAM) (Robins, Babor, & Cottler, 1987) which was developed as a supplement to the World Health Organization’s Composite International Diagnostic Interview (CIDI) (Robins et al., 1988). Clinical interviews were conducted by trained interviewers. Symptoms were assigned based on a subsequent review of the interview by pairs of clinically-trained staff members, who were blind to diagnoses of other family members (kappa exceeded .95). Symptoms were evaluated using the Diagnostic and Statistical Manual of Mental Disorders (3rd edition, revised) (DSM-IIIR) (APA, 1987), the diagnostic system in place at the time of assessment (DSM-IV was added at later assessments, III-R and IV symptom counts r = .95, p < .001). Symptoms present since the last assessment were used to measure AUD at ages 24 and 29. A lifetime report of AUD symptoms was used at age 17.
Analysis plan
All phenotypic analyses were conducted in Mplus, version 7.2 (Muthén & Muthén, 1998–2012). The CLUSTER specification was used to control for non-independence of cases (i.e., shared family variance of including twins as cases). To better approximate normality assumptions, AUD symptom counts were log-transformed prior to analysis. Missing data were handled using full information maximum likelihood (FIML) in both phenotypic and multivariate genetic analyses, which has been shown to be superior to other handling of missing data (Enders & Bandalos, 2001).
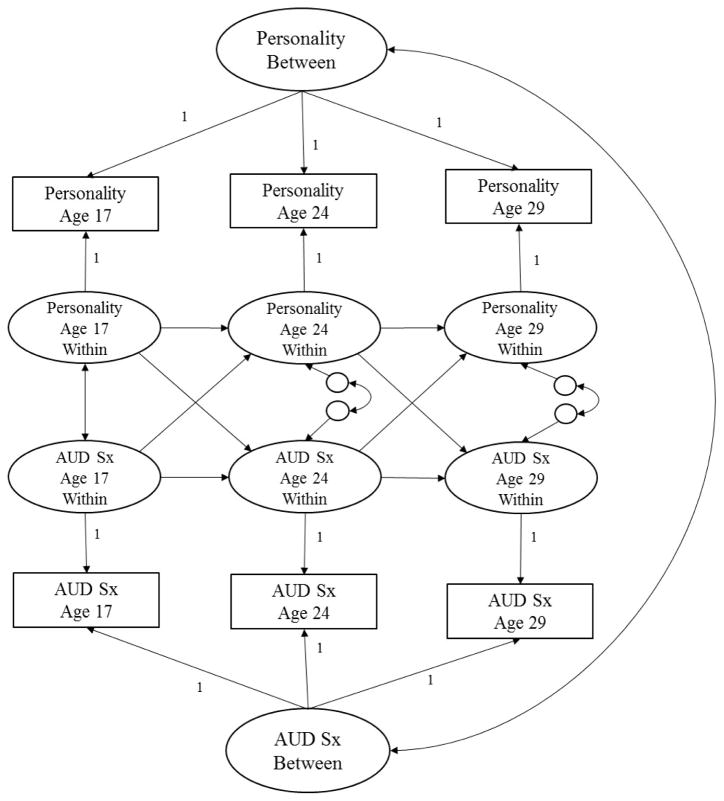
Phenotypic data were prospectively analyzed using Random-Intercept Cross-Lagged Panel Models (RI-CLPM), developed by Hamaker, Kuiper, and Grasman (2015) (Mplus script available in supplemental materials). Three RI-CLPMs were evaluated to estimate the phenotypic associations between each of the three personality scales (negative emotionality, constraint, and aggressive undercontrol) and AUD symptoms overtime. The traditional cross-lagged model allows us to detect ordering of effects (i.e., whether personality predicts subsequent AUD or AUD subsequent personality) after accounting for the stability of personality traits and AUD over time and residual correlations at each time point. RI-CLPM takes this one step further by modeling between-person parameters in addition to within-person parameters. As shown in Figure 1, within-person parameters refer to the autoregressive paths (e.g., constraint at age 17 predicting constraint at age 24). With RI-CLPM, these paths are estimated after accounting for time-invariant individual differences that may contribute to stability of constructs across time via the incorporation of correlated latent factors indicated by wave-specific variables (e.g., constraint at ages 17, 24, and 29 loads onto a “constraint” latent factor). This approach corrects for potentially incorrect conclusions derived by the traditional cross-lagged panel model in terms of presence, predominance, and sign of causal influences (Hamaker et al., 2015; also see Keijsers, 2015 and Poel, 2016 for additional examples of the RI-CLPM and how it compares to the CLPM).
Figure 1. Random-Intercept Cross-Lagged Panel Model (RI-CLPM; Hamaker et al., 2015) Showing the Prospective Associations between Personality and Alcohol Use Disorder Symptoms (AUD Sx) from Adolescence (Age 17) to Young Adulthood (29).
This figure shows two random intercepts (Personality between and AUD Sx between) that reflect time-invariant between-person differences. Within-person stability is modeled over time (as shown by the autoregressive paths of Personality and AUD Sx) as well as reciprocal cross-effects (e.g., the effect of Personality on subsequent AUD Sx and AUD Sx on subsequent Personality), the correlation between Personality and AUD at Time 1, and the residual correlations at Times 2 and 3.
We judged support for the predisposition/vulnerability, scar, or transactional models based on the significance and magnitude of effect for the evaluated cross-paths across the three personality trait-AUD models. If cross-paths from the personality trait to subsequent AUD symptoms were significant and cross-paths from AUD symptoms to the subsequent personality trait were not, results would provide support vulnerability/predisposition causal processes. On the other hand, if cross-paths from AUD symptoms to subsequent personality trait were significant and cross-paths from personality trait to subsequent AUD symptoms were not, results would be more consistent with scar model of AUD-Personality development. If both cross-paths were significant, this would be consistent with a reciprocal/transactional model of AUD-personality development.
Finally, we evaluated the contribution of genetic and environmental influences on the personality scale(s) most relevant to AUD by conducting bivariate and multivariate Cholesky decomposition using the Mx software (Neale, Boker, Xie, & Maes, 2006). Consistent with prior research, all phenotypes were age, sex, age*age, and age*sex adjusted prior to twin modeling. Specifically, age at the current assessment was regressed out of each log-transformed score (as well as sex and interactions between age and sex) and the residualized score was used in subsequent analyses. As illustrated by McGue and Bouchard (1984), it is recommended that age and sex effects are regressed out of phenotypes prior to analysis in order to reduce the overestimation of the twin correlation.
Additive genetic (A), shared environmental (C), and nonshared environmental (E) parameters were estimated using matrix algebra based on the degree of genetic relatedness among twin pairs. Additive genetic variance (A) refers to the additive effects of individual genetic variants summed over all genetic loci. Shared environment variance (C), also known as the common environment, refers to anything that makes siblings similar other than genes. Finally, nonshared environmental variance (E) refers to anything that makes siblings different—other than genes—including measurement error. Thus, the additive genetic path was set to 1.0 for monozygotic (MZ) twins (as they share all additive genetic variance) and .5 for dizygotic (DZ) twins (as they share half of the additive genetic variance). The shared environmental path between siblings is set to 1.0 for both MZ and DZ twins. The nonshared environmental path between siblings is set to 0.0 for both MZ and DZ twins (see Rijsdijk & Sham, 2002 for an overview of ACE modeling).
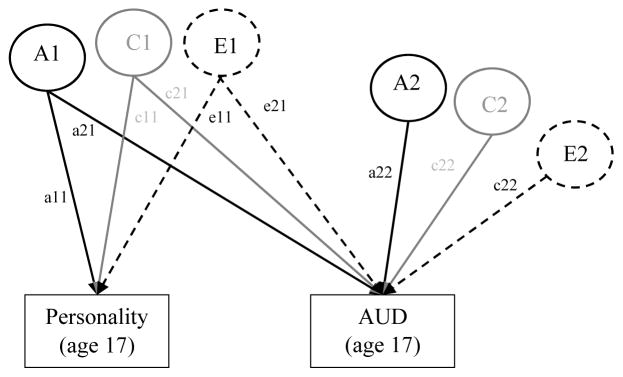
In conjunction with our twin modeling, we first evaluated the genetic and environmental influences on the bivariate associations between each personality trait and AUD at each time point (17, 24, and 29). As diagrammed in Figure 2, ACE influence unique to both personality (a11, c11, e11) and AUD (a22, c22, e22) were evaluated, as well as the ACE influences common to the association between personality and AUD (a21, c21, e21). Genetic and environmental correlations were calculated by standardizing the genetic and environmental covariance. Squaring the genetic and environmental correlations shows the proportion of ACE influences shared by personality and AUD.
Figure 2. Bivariate Cholesky Decomposition Conceptual Model: Genetic and Environmental Influences on the Prospective Associations between Personality and AUD.
AUD = Alcohol Use Disorder symptom count. The genetic and environmental influences on both personality and AUD are evaluated, as well as the association between personality and AUD (separately evaluated at age 17, 24, and 29). Variance of each phenotype is decomposed into additive genetic effects (A1, A2), shared environmental effects (C1, C2), and nonshared environmental effects (E1, E2). A labels and paths are shown in black, C in gray, and E are dashed. Paths labels are represented by lowercase letters followed by two numbers (e.g., a11). Paths a11, c11, and e11 refer to the ACE influences on personality. Paths a21, c21, and e21 refer to the ACE influences on the covariance between personality and AUD. Paths a22, c22, and e22 refer to the unique ACE influences on AUD. Paths can be squared and summed to determine the total proportion of ACE variance explained. For example, to determine the proportion of A variance explained in AUD by personality, path a21 would be squared then divided by all the squared and summed paths leading to Age 29 AUD (a212/a212+a222).
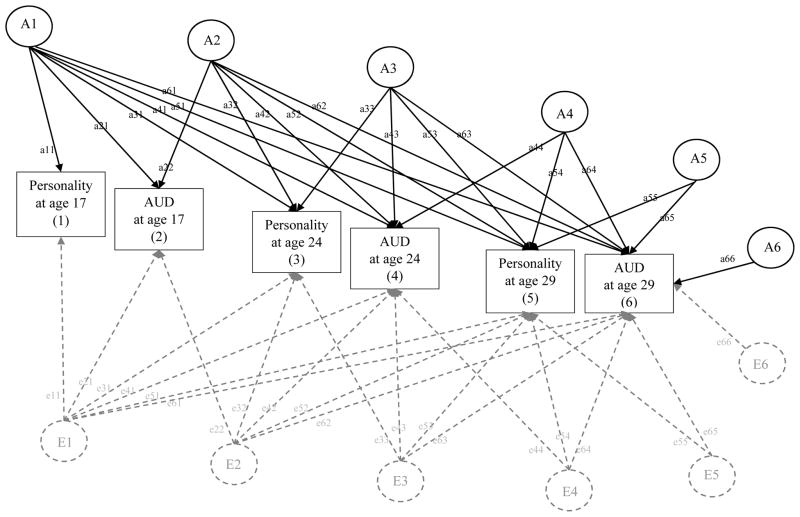
After estimating the common and unique ACE influences on personality and AUD at each time point, we then estimated the common and unique ACE influences on each personality trait and AUD across time using a multivariate Cholesky decomposition (see Figure 3). This model was specifically chosen, as it is a way to evaluate common genetic and environmental influences on the covariance of phenotypes over time, as well as account for unique ACE influences above and beyond common ACE influences. Following the same conceptual model of the bivariate decomposition (discussed above), the variance and covariance involving six phenotypes (i.e., the corresponding personality trait and AUD at ages 17, 24, and 29) was decomposed in genetic and environmental influences. The most parsimonious model was identified by dropping all non-significant parameters; that is, those paths whose 95% confidence intervals included zero. Comparison of model fit was evaluated by using the −2 × log-likelihood (−2lnL) and using the chi-square difference test to evaluate significant decrements in model fit. Standardized coefficients can be squared and summed to determine the total proportion of predicted ACE variance explained. For example, to determine the proportion of A variance explained in AUD at age 29 by personality at age 17, path a61 (see Figure 3) would be squared then divided by all the squared and summed paths leading to Age 29 AUD (a612/a612+a622+a633+a642+a652+a662).
Figure 3. Multivariate Cholesky Decomposition Conceptual Model: Genetic and Environmental Influences on the Prospective Associations between Personality and AUD From Age 17 to Age 29.
AUD = Alcohol Use Disorder symptom count. Following the same conceptual model as the bivariate decomposition, in the multivariate decomposition, variance and covariance of each phenotype is decomposed into additive genetic effects (A1, A2, A3, A4, A5, A6, shown in black), nonshared environmental effects (E1, E2, E3, E4, E5, E6, shown in dashed gray), and shared environmental effects (not shown for clarity of presentation but follow the same pattern for A and E). Paths labels are represented by lowercase letters followed by two numbers (e.g., a11, e11). Paths represent AE influence unique and common to phenotypes across time. For example, path a11 refers to additive genetic influence unique to personality at age 17. Path a21 refers to additive genetic influence common to AUD at age 17 and personality at age 17. Path a31 refers to additive genetic influence common personality at age 24 and personality at age 17, etc. Paths can be squared and summed to determine the total proportion of ACE variance explained. For example, to determine the proportion of A variance explained in AUD at age 29 by personality at age 17, path a61 would be squared then divided by all the squared and summed paths leading to Age 29 AUD (a612/a612+a622+a632+a642+a652+a662).
Results
Preliminary Analyses
Table 1 shows descriptive statistics of AUD symptoms at each assessment by sex. Following national statistics (SAMSHA, 2014), males had significantly greater average AUD symptom counts; Cohen’s d for sex differences in AUD symptoms ranged from .30 to .67 across assessments, indicating moderate effect sizes. The highest rates of those meeting AUD diagnosis were found at ages 20 and 24 (see Table 1 for details).
Table 1.
Descriptive Statistics for Alcohol Use Disorder for Males (n = 1,333) and Females (n = 1,436)
| Variable | M Symptom Count (SD) | Cohen’s d | % Meeting AUD Diagnosis | ||
|---|---|---|---|---|---|
|
|
|||||
| Males | Females | Males | Females | ||
|
|
|||||
| AUD at age 17 | .86 (1.78) | .40 (1.24) | .30 | 18.5 | 8.7 |
| AUD at age 24 | 1.56 (2.02) | .60 (1.42) | .55 | 37.3 | 13.1 |
| AUD at age 29 | 1.03 (1.80) | .34 (1.09) | .46 | 22.6 | 7.2 |
Notes. M = Mean, SD = Standard Deviation. Males had significantly higher average AUD symptoms than females across assessments (all p’s < .05). AUD diagnosis criteria was defined as meeting two of any of the abuse or dependence criteria (consistent with DSM-5, APA, 2013)
Descriptive statistics and zero-order correlations between AUD symptoms, constraint, negative emotionality, and aggressive undercontrol at ages 17, 24, and 29 are shown in Table 2. AUD exhibited moderate rank-order stability across time (r’s ranged from .28 to .45, all p’s < .001). Conversely, personality traits exhibited substantial rank-order stability (r’s ranged from .60 to .79 for constraint, from .53 to .74 for negative emotionality, and from .61 to .78 for aggressive undercontrol; all p’s < .001; see Table 2). AUD symptoms were significantly correlated with each personality trait both within and across time (correlations for AUD with constraint ranged from −.17 to −.29; correlations for AUD with negative emotionality ranged from .13 to .22; correlations for AUD with aggressive undercontrol ranged from .25 to .41; all p’s < .001; see Table 2).
Table 2.
Correlations and Raw Descriptive Statistics for AUD Symptoms, Constraint, Negative Emotionality, and Aggressive Undercontrol from Ages 17 to 29 (N = 2,769)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| 1. AUD Symptoms at age 17 | -- | |||||||||||
| 2. AUD Symptoms at age 24 | .36* | -- | ||||||||||
| 3. AUD Symptoms at age 29 | .28* | .49* | -- | |||||||||
| 4. Constraint at age 17 | −.29* | −.25* | −.24* | -- | ||||||||
| 5. Constraint at age 24 | −.20* | −.31* | −.28* | .64* | -- | |||||||
| 6. Constraint at age 29 | −.17* | −.27* | −.28* | .60* | .79* | -- | ||||||
| 7. Negative Emotionality at age 17 | .16* | .16* | .14* | −.10* | −.03 | −.02 | -- | |||||
| 8. Negative Emotionality at age 24 | .13* | .22* | .18* | −.13* | −.08* | −.08* | .55* | -- | ||||
| 9. Negative Emotionality at age 29 | .14* | .19* | .19* | −.12* | −.10* | −.08* | .53* | .74* | -- | |||
| 10. Aggressive Undercontrol at age 17 | .36* | .32* | .29* | −.81* | −.51* | −.49* | .40* | .29* | .27* | -- | ||
| 11. Aggressive Undercontrol at age 24 | .27* | .41* | .36* | −.57* | −.82* | −.66* | .20* | .39* | .31* | .61* | -- | |
| 12. Aggressive Undercontrol at age 29 | .25* | .36* | .36* | −.56* | −.69* | −.81* | .20* | .31* | .38* | .61* | .78* | -- |
| M (SD) | .62 (1.54) | 1.06 (1.79) | .67 (1.51) | 134.48 (15.89) | 140.68 (15.73) | 143.48 (15.55) | 89.29 (14.14) | 81.46 (13.59) | 79.46 (13.59) | 47.08 (7.97) | 43.12 (7.54) | 41.61 (7.28) |
| % Valid | 94.4 | 89.9 | 90.1 | 88.1 | 81.2 | 86.8 | 88.1 | 81.2 | 86.8 | 90.0 | 83.2 | 88.1 |
Notes. AUD = Alcohol Use Disorder, M = mean, SD = Standard Deviation. This table shows the zero-order correlations between AUD, constraint, negative emotionality, and aggressive undercontrol across time. Raw means, standard deviations, and percent of valid (non-missing) data for each variable are also shown.
denotes correlations that were significant at p < .05.
Means and standard deviations are also shown in Table 2. The mean number of AUD symptoms increased from age 17 to age 24 (Cohen’s d = .26) and then decreased from age 24 to age 29 (Cohen’s d = −.24). Conversely, mean scores of constraint increased from ages 17 to 24 (Cohen’s d = .39) and then increased again from ages 24 to 29 (Cohen’s d = .18). Negative emotionality and aggressive undercontrol both decreased from ages 17 to 24 (Cohen’s d for negative emotionality = −.57; Cohen’s d for aggressive undercontrol = −.51) and then decreased again from ages 24 to 29 (Cohen’s d for negative emotionality = −.15; Cohen’s d for aggressive undercontrol = −.20). This pattern of mean changes is consistent with the developmental literature on these constructs (Hicks, Durbin et al., 2012; SAMSHA, 2012) and suggests a “maturing out” of alcohol use by age 29–30 that is concurrent with maturation of personality with time.
Prospective Phenotypic Analyses
Results for the phenotypic cross-lagged panel models for each of the three personality traits (constraint, negative emotionality, aggressive undercontrol) and AUD symptoms are shown in Figures 4–6.
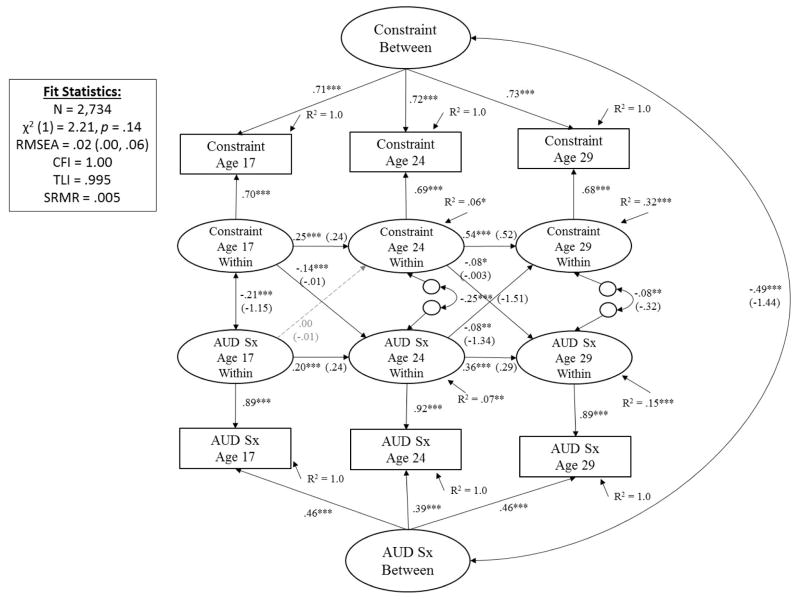
Figure 4. Prospective Associations between Constraint and Alcohol Use Disorder Symptoms (AUD Sx) from Adolescence (Age 17) to Young Adulthood (29).
Showing standardized coefficients (unstandardized coefficients); with exception of latent factor loadings, where only standardized coefficients are shown (all unstandardized coefficients for factor loadings = 1.0). Significance is noted by *** p < .001, ** p < .01, * p < .05 (paths that are not significantly different from zero are also shown in dashed gray for clarity of presentation).
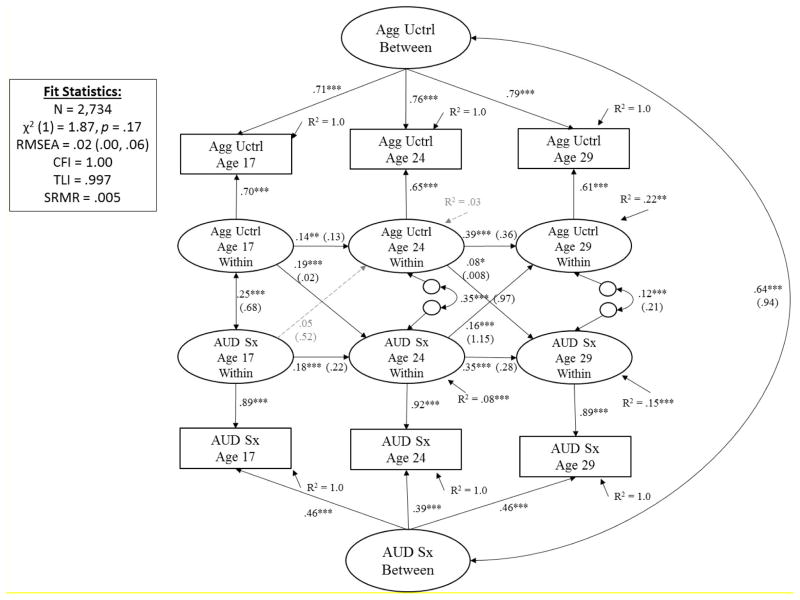
Figure 6. Prospective Associations between Aggressive Undercontrol (Agg Uctrl) and Alcohol Use Disorder Symptoms (AUD Sx) from Adolescence (Age 17) to Young Adulthood (29).
Showing standardized coefficients (unstandardized coefficients); with exception of latent factor loadings, where only standardized coefficients are shown (all unstandardized coefficients for factor loadings = 1.0). Significance is noted by *** p < .001, ** p < .01, * p < .05 (paths that are not significantly different from zero are also shown in dashed gray for clarity of presentation).
Constraint and AUD
As shown in Figure 4, after accounting for time-invariant individual differences that contribute to stability of constraint and AUD symptoms across time (indicated by the covarying latent factors of constraint and AUD symptoms; r = −.49, p < .001), and stability of constructs across time (β’s ranged from .20 to .36 for AUD and from .25 to .54 for constraint, all p’s < .001), results showed that constraint at age 17 significantly predicted subsequent AUD symptoms at age 24 (β = −.14, p < .001). Conversely, AUD symptoms at age 17 did not significantly predict subsequent constraint at age 24 (β = .00, p = .99). For the age 24 to 29 transition, both cross-effects were significant (both β’s = −.08; see Figure 4 for details). Follow-up analyses confirmed results were consistent across males and females, although cross-effects did not reach statistical significance for either sub-group due to smaller sample size for each gender relative to the larger sample (see eFigures 1 and 2 in the supplementary materials for details).
Altogether, results from phenotypic analyses on constraint and AUD support a predisposition/vulnerability model of AUD-personality development from ages 17 to 24 and a reciprocal/transactional model of AUD-personality development from ages 24 to 29. It is important to note that effect sizes were small, as indicated by the standardized coefficients. Squaring the standardized coefficient (β) and dividing by the total variance explained (R2) gives the proportion of predicted variance explained by that predictor. For example, constraint at age 17 explained (−.142/.07) ~28% of the predicted variance of AUD at age 24. Constraint at age 24 explained (−.082/.15) ~4% of the predicted variance of AUD at age 29. AUD at age 17 and 24 explained less than 3% of the predicted variance in constraint at ages 24 (.0012/.06 = <1%) and 29 (−.082/.32 = 2%).
Negative Emotionality and AUD
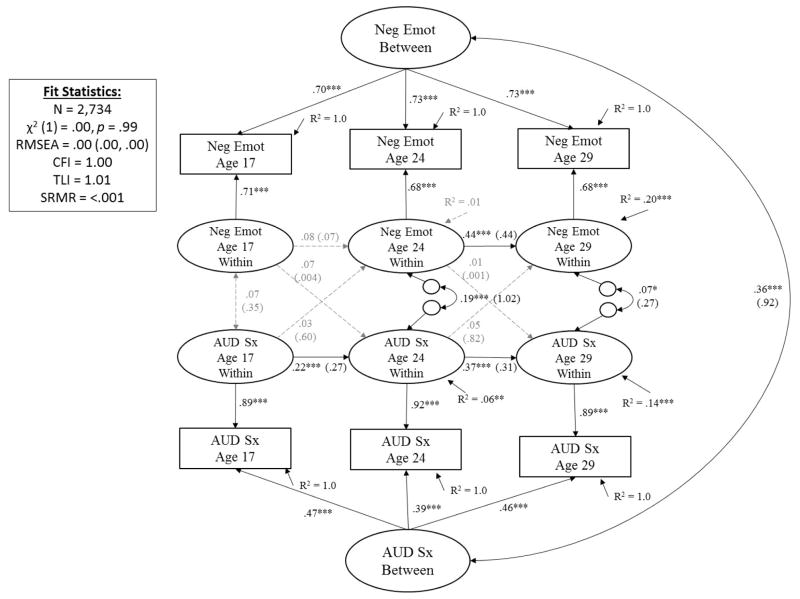
As shown in Figure 5, after accounting for time-invariant individual differences that contribute to stability of constraint and AUD symptoms across time (r = .36, p < .001) and stability of constructs across time (β’s ranged from .22 to .37 for AUD and from .08 to .44 for negative emotionality, see Figure 5 for associated p-values), results showed no significant cross-effects for either negative emotionality on subsequent AUD or AUD on subsequent negative emotionality for both developmental transitions (ages 17 to 24 and age 24 to 29). Thus, there was little support for any negative emotionality-AUD co-development in the transition from adolescence through young adulthood.
Figure 5. Prospective Associations between Negative Emotionality (Neg Emot) and Alcohol Use Disorder Symptoms (AUD Sx) from Adolescence (Age 17) to Young Adulthood (29).
Showing standardized coefficients (unstandardized coefficients); with exception of latent factor loadings, where only standardized coefficients are shown (all unstandardized coefficients for factor loadings = 1.0). Significance is noted by *** p < .001, ** p < .01, * p < .05 (paths that are not significantly different from zero are also shown in dashed gray for clarity of presentation).
It is worth noting that the stability coefficient for negative emotionality from ages 17 to 24 was not significantly different than zero. This result was unlike results for the stability of constraint from ages 17 to 24 (Figure 4) or results for the stability for negative emotionality from ages 24 to 29 or results for the stability of AUD symptoms from ages 17 to 24 and 24 to 29. Follow-up analyses (eFigures 3–4 in supplemental materials) showed these results were consistent across males and females (e.g., lack of significant stability of negative emotionality from ages 17 to 24, lack of significant cross-paths), with one exception. For males, AUD symptoms at age 24 significantly predicted subsequent negative emotionality at age 29 (β = .11, b = 1.59, S.E. = .62, p = .01). This cross-path was not significantly different than zero for females (β = −.03, b = −.57, S.E. = .77, p = .46). A comparison of unstandardized coefficients and standard errors across gender showed this difference was significant (z = 2.20, p = .03).
In total, results for the phenotypic analyses on negative emotionality and AUD showed support for the scar model of personality-AUD development, but only for males, and only for the age 24 to 29 developmental transition. Effect sizes were quite small. For males, AUD symptoms at age 24 explained (.112/.22) less than 1% of the predicted variance of negative emotionality at age 29.
Aggressive Undercontrol and AUD
As shown in Figure 6, after accounting for time-invariant individual differences that contribute to stability of aggressive undercontrol and AUD symptoms across time (r = .64, p < .001) and stability of constructs across time (β’s ranged from .18 to .35 for AUD and from .14 to .39 for aggressive undercontrol, all p’s < .01), results showed aggressive undercontrol at age 17 significantly predicted subsequent AUD symptoms at age 24 (β = .19, p < .001). AUD symptoms at age 17 did not significantly predict subsequent aggressive undercontrol at age 24 (β = .05, p = .19). For the age 24 to 29 transition, both cross effects were significant, and the effect of AUD symptoms at age 24 on subsequent aggressive undercontrol at age 29 (β = .16, p < .001) was about two times greater than the effect of aggressive undercontrol at age 24 on subsequent AUD symptoms at age 29 (β = .08, p = .03). Follow-up analyses showed results were consistent across males and females (see eFigures 5–6 in supplementary materials).
Altogether, results for aggressive undercontrol were similar to those of constraint in that they support a predisposition/vulnerability model of AUD-personality development from ages 17 to 24 and a reciprocal/transactional model of AUD-personality development from ages 24 to 29. Effect sizes are somewhat larger than those of constraint; aggressive undercontrol at age 17 explained (.192/.08) ~45% of the predicted variance of AUD symptoms at age 24. Aggressive undercontrol at age 24, however, only explained (.082/.15) ~4 % of the predicted variance of AUD symptoms at age 29. Finally AUD symptoms at age 24 explained (.162/.22) ~12% of the predicted variance of aggressive undercontrol at age 29.
Bivariate Genetic and Environmental Influences
Table 3 shows results for the bivariate decomposition analyses, which decompose the variance and cross-sectional covariance between each personality trait and AUD symptoms into genetic and environmental influences. Both full ACE and AE models are shown as all C parameters could be dropped without a significant decrement in model fits (Δχ2 ranged from .00 to 3.27 on 3 df change). Results from the full ACE models show about half the variance on each phenotype was due to additive genetic influences and the other half due to nonshared environmental influences, with the exception of AUD at age 29, in which there was less additive genetic influence (12%–14%) and greater nonshared environmental influence (73% see Table 3 for details). Results from the AE models were consistent with this pattern of results, with one exception: there was greater additive genetic influence on AUD symptoms at age 29 (28–29%) relative to the full ACE models (12–14%) with essentially no change to nonshared environmental influence by ACE vs. AE model (73% vs. 71–72%; see Table 3 for details). As AUD symptoms were assessed as lifetime estimates at age 17 and covered the time period of symptoms since the last assessment at ages 24 and 29, results from both full ACE and AE models suggests there is less genetic influence and more nonshared environmental influence on AUD symptomology that presents after age 24.
Table 3.
Genetic and Environmental Influences on Constraint, Negative Emotionality, Aggressive Undercontrol and Alcohol Use Disorder (AUD) at Each Time Point: Results from Full ACE and AE models
| Bivariate Decomposition | Variance Components from Full ACE Bivariate Decomposition | Genetic and Environmental ACE Correlations (between each personality trait and AUD at each time point) | Variance Components from AE Bivariate Decomposition | Genetic and Environmental AE Correlations (between each personality trait and AUD at each time point) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| A | C | E | rA | rC | rE | A | E | rA | rE | |
|
|
||||||||||
| Age 17 | ||||||||||
| 1. Constraint | .52* (.41, .57) | .00 (.00, .10) | .48* (.43, .53) | −.34* (−.47, −.24) | .42 (−1.0, 1.0) | −.17* (−.24, −.10) | .52* (.47, .57) | .48* (.43, .53) | −.34* (−.42, −.25) | −.17* (−.24, −.10) |
| AUD Symptoms | .59* (.50, .63) | .00 (.00, .07) | .41* (.37, .46) | .59* (.54, .63) | .41* (.37, .46) | |||||
| 2. Negative Emotionality | .47* (.30, .52) | .00 (.00, .15) | .53* (.48, .59) | .25* (.07, .36) | .99 (−1.0, 1.0) | .07 (−.00, .14) | .47* (.41, .52) | .53* (.48, .59) | .25* (.16, .35) | .07 (−.00, .14) |
| AUD Symptoms | .59* (.48, .63) | .00 (.00, .09) | .41* (.37, .46) | .59* (.54, .63) | .41* (.37, .46) | |||||
| 3. Aggressive undercontrol | .50* (.34, .55) | .00 (.00, .14) | .50* (.45, .55) | .46* (.36, .62) | −.65 (−1.0, 1.0) | .19* (.12, .26) | .50* (.45, .55) | .50* (.45, .55) | .45* (.37, .53) | .19* (.13, .26) |
| AUD Symptoms | .59* (.50, .63) | .00 (.00, .07) | .41* (.37, .46) | .59* (.54, .63) | .41* (.37, .46) | |||||
| Age 24 | ||||||||||
| 4. Constraint | .53* (.44, .58) | .00 (.00, .07) | .47* (.42, .53) | −.38* (−.67, −.22) | 1.0 (−1.0, 1.0) | −.16* (−.23, −.08) | .53* (.47, .58) | .47* (.42, .53) | −.35* (−.45, −.25) | −.16* (−.23, −.09) |
| AUD Symptoms | .37* (.18, .46) | .04 (.00, .20) | .59* (.54, .65) | .41* (.35, .46) | .59* (.54, .65) | |||||
| 5. Negative Emotionality | .44* (.26, .51) | .02 (.00, .18) | .54* (.48, .60) | .38* (.08, .76) | −1.0 (−1.0, 1.0) | .12* (.05, .49) | .46* (.41, .52) | .54* (.48, .59) | .28* (.17, .39) | .12* (.05, .19) |
| AUD Symptoms | .36* (.18, .46) | .04 (.00, .19) | .60 (.54, .66) | .41* (.35, .46) | .59* (.54, .65) | |||||
| 6. Aggressive undercontrol | .51* (.38, .56) | .00 (.00, .11) | .49* (.44, .55) | .53* (.33, .85) | −1.0 (−1.0, 1.0) | .21* (.14, .28) | .51* (.45, .56) | .49* (.44, .55) | .46* (.36, .56) | .22* (.15, .28) |
| AUD Symptoms | .36* (.18, .46) | .05 (.00, .20) | .59* (.54, .66) | .41* (.36, .46) | .59* (.54, .65) | |||||
| Age 29 | ||||||||||
| 7. Constraint | .49* (.40, .54) | .00 (.00, .07) | .51* (.46, .57) | −.46* (−1.0, −.04) | 1.0 (−1.0. 1.0) | −.18* (−.24, −.11) | .49* (.43, .54) | .51* (.46, .57) | −.30* (−.42, −.17) | −.18* (−.24, −.11) |
| AUD Symptoms | .13 (.00, .32) | .14 (.00, .28) | .73* (.67, .80) | .29* (.22, .34) | .71* (.66, .78) | |||||
| 8. Negative Emotionality | .39* (.23, .48) | .05 (.00, .18) | .56* (.51, .62) | .05 (−1.0, 1.0) | 1.0 (−.65, 1.0) | .12* (.05, .18) | .44* (.38, .50) | .56* (.51, .62) | .29* (.15,. 42) | .11* (.04, .17) |
| AUD Symptoms | .12 (.00, .31) | .15 (.00, .29) | .73* (.67, .80) | .28* (.22, .34) | .72* (.66, .78) | |||||
| 9. Aggressive undercontrol | .47* (.32, .52) | .00 (.00, .13) | .53* (.48, .59) | .57* (.08, 1.0) | 1.0 (−1.0, 1.0) | .20* (.13, .27) | .47* (.41, .52) | .53* (.48, .59) | .45* (.33, .56) | .20* (.14, .26) |
| AUD Symptoms | .14* (.001, .33) | .13 (.00, .27) | .73* (.67, .80) | .28* (.22, .34) | .72* (.66, .78) | |||||
Notes. AUD = Alcohol Use Disorder. This table shows results from bivariate decompositions between the three examined personality traits (constraint, negative emotionality, aggressive undercontrol) and AUD symptoms at each time point (each numbered row represents one cross-sectional correlation). Total additive genetic (A), shared (or common) environment (C), and nonshared environmental (E) variance are shown (variance components add to 1.0). rA, rC, and rE refer to additive genetic and nonshared environmental correlations between the three examined personality traits and AUD symptoms. Results in gray text (left) show results from the full ACE models. Results in black text (right) show results from the more parsimonious AE models (all C parameters could be dropped without a significant decrement in model fit; Δχ2 ranged from .00 to 3.27 on 3 df change). Parameters are significantly different from zero if 95% Confidence Intervals (shown in parentheses) do not cross zero (also indicated by * p < .05).
In addition to providing rough estimates of genetic vs. environmental influence on each phenotype, results from Table 3 also show genetic and environmental correlations between each personality trait and AUD symptoms at each time point. Results show evidence for substantial genetic correlations (rAs) between each personality trait and AUD at each time point. Across ACE and AE models, rAs ranged from −.46 to −.30 for associations between constraint and AUD symptoms. A similar magnitude of effect was found for the association between negative emotionality and AUD symptoms (rAs ranged from .25 to .38) and between aggressive undercontrol and AUD symptoms (rAs ranged from .46 to .57; see Table 3 for details). There was significant but smaller nonshared environmental correlations (rEs) for all pairs of phenotypes. Across ACE and AE models, rEs ranged from −.18 to −.16 for associations between constraint and AUD symptoms. Similar magnitudes of effect were found for associations between negative emotionality and AUD symptoms (rEs ranged from .07 to .12) and between aggressive undercontrol and AUD symptoms (rEs ranged from .19 to .22; see Table 3 for details).
Multivariate Genetic and Environmental Influences
Our final goal was to decompose the prospective associations between each of the three personality traits and AUD symptoms from ages 17 to 29 into genetic and environmental influences. Model fit statistics from the full ACE models were compared to models that removed paths that were not significantly different than zero. Results from the more parsimonious are shown in Figures 7, 8, and 9 for constraint and AUD, negative emotionality and AUD, and aggressive undercontrol and AUD results, respectively. Results from the full ACE models are provided in the supplementary materials, eFigure 7–9.
Figure 7. Genetic and Environmental Influences on Constraint and Alcohol Use Disorder (AUD) Symptoms from Age 17 to Age 29.
Showing standardized coefficients. This figure illustrates the most parsimonious model (Δχ2 = 10.96 on 21 df change, p = 1.0). Results from the full model are provided in the supplementary materials (eFigure 7). Variance of each phenotype is decomposed into additive genetic effects (A1, A2, A3, A4, A5, A6) and nonshared environmental effects (E1, E2, E3, E4, E5, E6); all shared environmental effects (and corresponding C paths) were not significantly different than zero and thus dropped in this more parsimonious model. For clarity of presentation, A labels and paths are shown in black and E paths and labels are shown in dashed gray. Significance is denoted by * p < .05 and bolded coefficients. Paths can be squared and summed to determine the total proportion of A and E variance explained. For example, to determine the proportion of A variance explained in AUD at age 29 by personality at age 17, path a61 (−.16) would be squared then divided by all the squared and summed paths leading to Age 29 AUD (−.162/−.162 + .312 + −.102 + .292 + .292); thus confirming that ~9% of the predicted additive genetic influence on AUD at age 29 can be explained by constraint at 17.
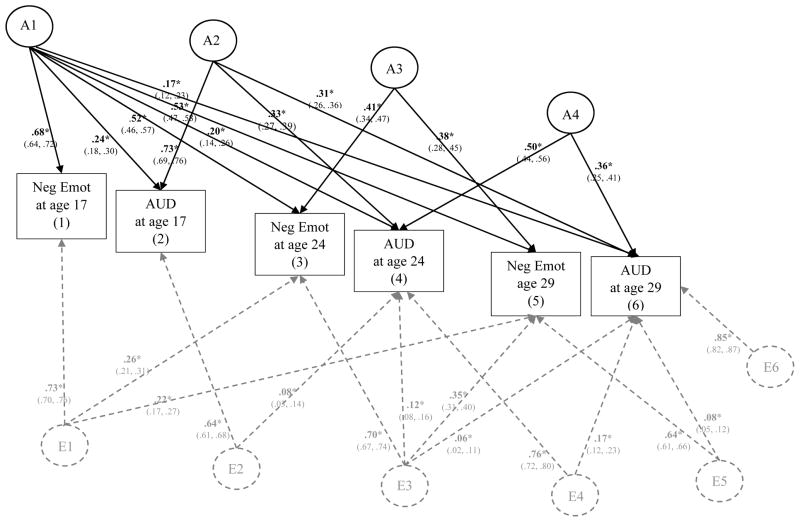
Figure 8. Genetic and Environmental Influences on Negative Emotionality (Neg Emot) and Alcohol Use Disorder (AUD) Symptoms from Age 17 to Age 29.
This figure illustrates the most parsimonious model (Δχ2 = 32.54 on 36 df change, p = .32). Results from the full model are provided in the supplementary materials (eFigure 8). Variance of each phenotype is decomposed into additive genetic effects (A1, A2, A3, A4, A5, A6) and nonshared environmental effects (E1, E2, E3, E4, E5, E6); all shared environmental effects (and corresponding C paths) were not significantly different than zero and thus dropped in this more parsimonious model. A labels and paths are shown in black and E paths and labels are shown in dashed gray. Significance is denoted by * p < .05 and bolded coefficients. Paths can be squared and summed to determine the total proportion of A and E variance explained. For example, to determine the proportion of A variance explained in AUD at age 29 by negative emotionality at age 17, path a61 (.17) would be squared then divided by all the squared and summed paths leading to Age 29 AUD (.172/.172+.312+.362); thus confirming that ~11% of the predicted additive genetic influence on AUD at age 29 can be explained by negative emotionality at age 17.
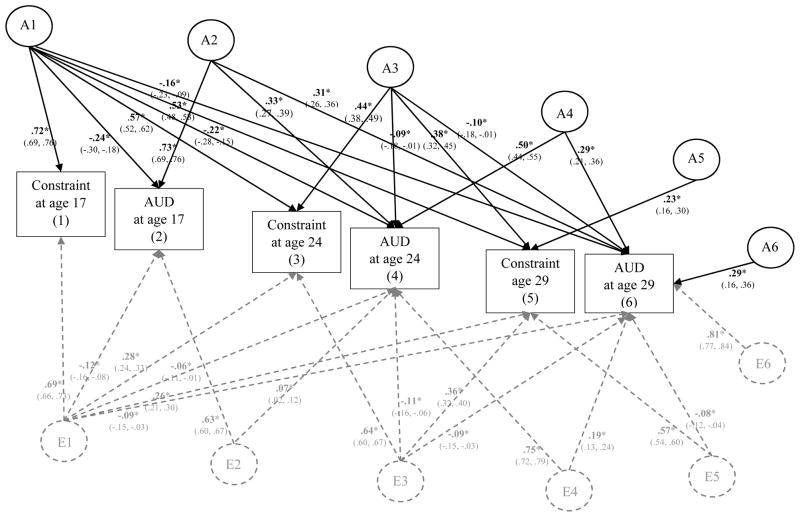
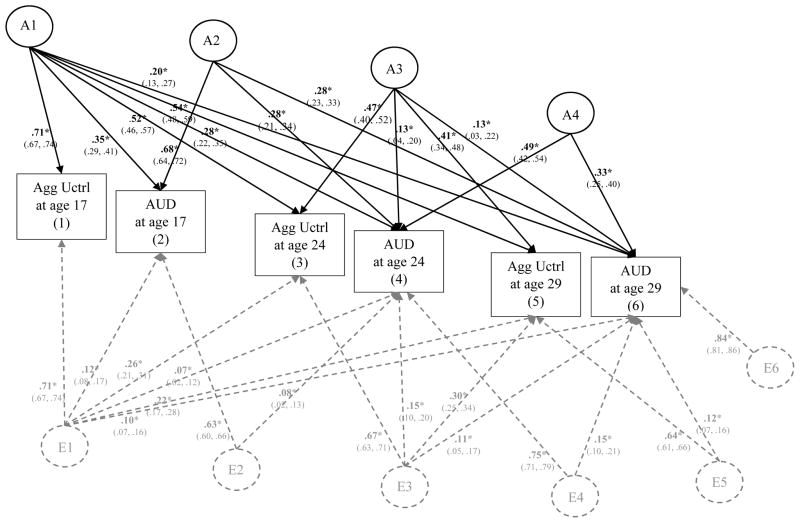
Figure 9. Genetic and Environmental Influences on Aggressive Undercontrol (Agg Uctrl) and Alcohol Use Disorder (AUD) Symptoms from Age 17 to Age 29.
This figure illustrates the most parsimonious model (Δχ2 = 34.08 on 31 df change, p = .32). Results from the full model are provided in the supplementary materials (eFigure 9). Variance of each phenotype is decomposed into additive genetic effects (A1, A2, A3, A4, A5, A6) and nonshared environmental effects (E1, E2, E3, E4, E5, E6); all shared environmental effects (and corresponding C paths) were not significantly different than zero and thus dropped in this more parsimonious model. For clarity of presentation, A labels and paths are shown in black and E paths and labels are shown in dashed gray. Significance is denoted by * p < .05 and bolded coefficients. Paths can be squared and summed to determine the total proportion of A and E variance explained. For example, to determine the proportion of A variance explained in AUD at age 29 by aggressive undercontrol at age 17, path a61 (.20) would be squared then divided by all the squared and summed paths leading to Age 29 AUD (.202/.202+.282+.132+.332); thus confirming that ~16% of the total predicted additive genetic influence on AUD at age 29 can be explained by aggressive undercontrol at age 17.
Constraint and AUD
In the most parsimonious model, all shared environmental (or C) paths were dropped, as well as a several non-significant A and E paths (specifically, a32, a52, a54, a65, e32, e52, e54, and e62) without a significant decrement to model fit; Δχ2 = 10.96 on 21 df change, p = 1.0. As shown in Figure 7, there was significant additive genetic influence on constraint at age 17 (β = .72, p < .05) that was shared with additive genetic influence on constraint at each of the following ages (age 24 β = .57, p < .05; age 29 β = .53, p < .05) as well as AUD at all ages (age 17 β = −.24, age 24 β = −.22, age 29 β = −.16, all ps < .05). This indicates the prospective associations between constraint and AUD are at least to some degree accounted for by one shared additive genetic factor (A1), present as early as age 17. These results generally support a common cause model of personality-AUD associations, consistent with our expectations. Effect sizes are also important to note. Squaring the standardized coefficient and dividing it by the total proportion of A or E variance explained gives the proportion of predicted additive genetic variance explained by that unique predictor. Thus, constraint at age 17 explained ~10% of predicted additive genetic variance on AUD at age 17 [(−.242)/(−.242 + .732); 95% CI: 5.6% to 14.1%], ~12% of the predicted additive genetic variance on AUD at age 24 [(−.222)/(−.222 + .332 + −.092 + .502); 95% CI: 5.8% to 19.3%], and ~9% of the additive genetic variance on AUD at age 29 [(.162)/(−.162 + .312 + −.102 + .292 + .292; 95% CI: 5.2% to 16.6%].
There was evidence for additional additive genetic influence above and beyond the A1 latent factor. These mostly concerned the stability of measures over time (see top half of Figure 7). Specifically, a second latent genetic factor (A2) showed evidence of residual additive genetic influence (over and above what contributed to the prospective associations between constraint and AUD symptoms over time) contributing to the stability of AUD from ages 17 to 24 (β = .33, p < .05) and age 24 to 29 (β = .31, p < .05). A third genetic latent factor (A3) showed evidence for significant residual additive genetic influence contributing mostly to the stability of constraint from age 24 to age 29 (β = .38, p < .05) but also showed small but significant shared additive genetic influence between constraint at age 24 and 29 with AUD at ages 24 and 29 (βs ranged from −.10 to −.09, ps < .05). Effect sizes were very small here: constraint at age 24 explained only 2%–3% of the predicted additive genetic influences on AUD at ages 24 and 29, respectively. A fourth latent genetic factor (A4) showed evidence for significant residual additive genetic influence contributing to the stability of AUD symptoms from ages 24 to 29 (β = .29, p < .05). Finally, there was also small but significant residual additive genetic influence on both constraint at age 29 (A5; β = .23, p < .05) and AUD at age 29 (A6; β = .29, p < .05). Altogether, constraint and AUD (at ages 17 and 24) explained a total of ~79% of the predicted additive genetic influence on constraint at age 29 [(−.222 + .382)/(−.222 + .382 + .232)] and ~72% of the predicted additive genetic influence on AUD at age 29 [(−.162 + .312 + −.102 + .292)/(−.162 + .312 + −.102 + .292 + .292)].
In addition to additive genetic influences, there was significant nonshared environmental influence on constraint at age 17 (β = .69, p < .05) that was shared with constraint and AUD at the following ages (βs ranged from −.12 to .26, all ps < .05), represented by the E1 latent factor (see bottom half of Figure 7). This supports another common-cause model of personality-AUD development; however, it is important to note that E1 contributes mostly to the nonshared environmental influence common to constraint across time (βs range from .26 to .28), with quite small but significant cross-effects with AUD across time (βs range from −.12 to −.06, ps < .05). Effect sizes are small (especially in comparison to A1 effects), with constraint at age 17 explaining < 1% to ~4% of the predicted nonshared environmental influences on AUD at ages 17, 24, and 29. This suggests that for all practical purposes, results best support additive genetic influence as a source of common cause.
There was evidence of significant but small residual nonshared environmental influences that contributed to the stability of AUD from ages 17 to 24 (see E2, β = .07, p < .05) and the stability of AUD from ages 24 to 29 (see E4, β = .19, p < .05). There was also unique nonshared environmental influence (E3) contributing mostly to the stability of constraint from ages 24 to 29 (β = .36, p < .05), with small but significant cross-effects to AUD (βs range from −.11 to −.09, ps < .05). These effects were also quite small, with constraint at age 24 explaining less than 2% of the total nonshared environmental influence on AUD at ages 24 to 29. Similarly, there was also small but significant nonshared environmental influence shared between constraint at age 29 and AUD at age 29 (E5), that was small in effect size (β = −.08, p < .05); constraint at age 29 explained less than 1% of the predicted nonshared environmental variance on AUD symptom at age 29. Finally, there was substantial residual nonshared environmental influence on AUD at age 29 (β = .81, p < .05), suggesting much of the nonshared environmental influence on AUD at age 29 was not explained by earlier measures of AUD or constraint. Altogether, constraint and AUD symptoms at ages 17 to 24 explained ~39% of the predicted nonshared environmental influence on constraint at age 29 [(.262 + .362)/(.262 + .362 + .572)] and just ~8% of the predicted nonshared environmental influence on AUD symptoms at age 29 [(−.092 + −.092 + .192 + −.082)/(−.092 + −.092 + .192 + −.082 + .812)]. Following earlier phenotypic results, follow-up analyses confirmed an essentially identical pattern of results males and females (see eFigures 10–11 in supplemental materials).
In sum, results from the multivariate analyses that evaluated the proportion of the genetic and environmental influence on the prospective associations between constraint and AUD symptoms over time showed evidence for a common cause model of personality-AUD development, as at least some of the prospective associations were explained by one source of common additive genetic influence – thus supporting our expectations. There was some evidence these associations were significantly influenced by common nonshared environmental influence as well, however nonshared environmental influences were much smaller in effect and explained very little (i.e., <5%) of the total nonshared environmental influence on either constraint or AUD across time.
Negative Emotionality and AUD
In the most parsimonious model, all shared environmental (or C) paths were dropped, as well as several non-significant A and E paths (a32, a43, a52, a54, a55, a63, a65, a66, e21, e32, e41, e52, e54, e61, e62) without a significant decrement to model fit; Δχ2 = 32.54 on 36 df change, p = .32. As shown in Figure 8, there was significant additive genetic influence on negative emotionality at age 17 (β = .68, p < .05) that was shared with negative emotionality at the following ages (βs range from .53 to .73, ps < .05), as well as AUD from ages 17 to 29 (βs ranged from .17 to .24, ps < .05). This follows the multivariate genetic results for constraint and supports the notion that that the prospective associations between negative emotionality and AUD symptoms from ages 17 to 29 are largely explained by common additive genetic influences (A1), thus supporting a common-cause model of personality-AUD development. Effect sizes were moderate, with negative emotionality at age 17 explaining a total of ~10% of the predicted additive genetic variance on AUD at age 17 [(.242)/(.242 + .732); 95% CI: 5.7% to 15.2%], ~10% of the predicted additive genetic variance on AUD at age 24 [(.202)/(.202 + .332 + .502); 95% CI: 4.9% to 16.3%], and ~11% of the predicted additive genetic variance on AUD at age 29 [(.172)/(.172 + .312 + .362); 95% CI: 5.8% to 22.4%].
As was what was found for constraint, there was evidence for residual additive genetic influence that contributed to the stability of AUD symptoms (represented by A2 and A4 in Figure 8) and the stability of negative emotionality from age 24 to 29 (A3). As the residual additive genetic influence beyond A4 was not significant than zero and therefore dropped from the parsimonious model presented in Figure 8, we can conclude that negative emotionality and AUD at ages 17 and 24 explained 100% of the predicted additive genetic variance on both traits at age 29.
Unlike what was found for constraint, there was no evidence that nonshared environmental influence accounted for the prospective associations between negative emotionality and AUD over time. Instead, there was evidence for common nonshared environmental influence that contributed to the stability of constraint from ages 17 to 29 (represented by E1 in Figure 8 – note the lack of cross-over effects to AUD symptoms) and residual nonshared environmental influence that contributed to the stability of AUD from ages 17 to 24 (E2) and the stability of AUD from ages 24 to 29 (E4). Residual nonshared environmental influence was also common to mostly the stability of negative emotionality from ages 24 to 29 (E3), with evidence of small but significant effects with AUD at those ages (βs ranged from .06 to .12, ps < .05). Effect sizes were quite small, with negative emotionality explaining less than 3% of the predicted nonshared environmental influence on AUD at ages 24 and 29. Finally, there was evidence for small but significant effects of negative emotionality at age 29 explaining residual nonshared environmental influence on AUD at age 29 (β = .08, p < .05); less than 1% of the predicted nonshared environmental influence on AUD at age 29 was explained by negative emotionality at age 29. Consistent with these small effect sizes, there was evidence for substantial residual nonshared environmental influence on negative emotionality at age 29 (β = .64, p < .05) and AUD at age 29 (β = .85, p < .05). Altogether, negative emotionality and AUD symptoms (from ages 17 to 24) explained a total of ~29% of the predicted nonshared environmental influence on negative emotionality at age 29 [(.222 + .352)/(.222 + .352 + .642)] and just ~5% of the predicted nonshared environmental influence on AUD symptoms at age 29 [(.062 + .172 + .082)/(.062 + .172 + .082 + .852)]. Follow-up analyses confirmed an essentially identical pattern of results males and females (see eFigures 12–13 in supplemental materials).
In sum, results from the multivariate analyses that evaluated the proportion of the genetic and environmental influence on the prospective associations between negative emotionality and AUD symptoms over time showed evidence for a common cause model of personality-AUD development, as much of the prospective associations were explained by one source of common additive genetic influence – thus supporting our expectations. There was some evidence these associations were significantly influenced by common nonshared environmental influence as well, however nonshared environmental influences were much smaller in effect, limited to age 24 to 29 prospective associations, and explained very little (i.e., <5%) of the total nonshared environmental influence on either negative emotionality or AUD across time.
Aggressive Undercontrol and AUD
In the most parsimonious model, all shared environmental (or C) paths were dropped, as well as a several non-significant A and E paths (a32, a52, a54, a55, a65, a66, e32, e52, e54, and e62) without a significant decrement to model fit; Δχ2 = 34.08 on 31 df change, p = .32. As shown in Figure 9, there was significant additive genetic influence on aggressive undercontrol at age 17 (β = .71, p < .05) that was shared with aggressive undercontrol at each of the following ages (βs ranged from .52 to .54, ps < .05) as well as AUD from ages 17 to 29 (βs ranged from .20 to .35, ps < .05). This follows results for both constraint and negative emotionality in relation to AUD and supports the notion that the prospective associations between aggressive undercontrol and AUD symptoms from age 17 to 29 is at least in part explained by common additive genetic influences (A1). Effect sizes were substantial, with aggressive undercontrol at age 17 explaining ~21% of the predicted additive genetic variance on AUD at age 17 [(.352)/(.352 + .682); 95% CI: 14.6% to 28.3%], ~20% of the predicted additive genetic variance on AUD at age 24 [(.282)/(.282 + .282 + .132 + .492); 95% CI: 11.7% to 29.7%], and ~16% of the predicted additive genetic variance on AUD at age 29 [(.202)/(.202 + .282 + .132 + .332); 95% CI: 7.3% to 29.4%].
As was found for constraint and negative emotionality, there was additional evidence for significant residual additional additive genetic influence on the stability of AUD from ages 17 to 29 (as represented by the A2 latent factor in Figure 9) and the stability of AUD from age 24 to 29 (as represented by A4). There was also significant residual additive genetic influence concerning mostly the stability of aggressive undercontrol from ages 24 to 29 (see A3; βs ranged from .41 to .47, ps < .05) with some small but significant cross-effects on AUD at ages 24 and 29 (βs = .13, ps < .05). Effect sizes were small, with aggressive undercontrol at age 24 explaining ~4% to 7% of the predicted additive genetic influence on AUD at ages 24 and 29 (above and beyond age 17 aggressive undercontrol and AUD). As the residual additive genetic influence beyond A4 was not significant than zero and therefore dropped from the parsimonious model (depicted in Figure 9), we can conclude that aggressive undercontrol and AUD at ages 17 and 24 explained 100% of the predicted additive genetic variance on both traits at age 29.
As was found for constraint, there was some evidence for nonshared environmental influences contributing to aggressive undercontrol at age 17 (β = .71, p < .05) that was shared with nonshared environmental influence on aggressive undercontrol at ages 24 and 29 (βs ranged from .22 to .26, ps < .05) as well as AUD at ages 17 to 29 (βs ranged from .07 to .12). These effects were quite small in magnitude, with aggressive undercontrol explaining less than 1% to ~4% of the predicted nonshared environmental influence on AUD at ages 17, 24, and 29. There were also small but significant nonshared environmental influences common to the residual stability of AUD from ages 17 to 24 (E2) and 24 to 29 (E4). There was evidence for residual nonshared environmental influence contributing mostly to the stability of aggressive undercontrol from ages 24 to 29 (E3), with small but significant cross effects to AUD at ages 24 and 29 (βs ranged from .11 to .15; explaining less than 4% of nonshared environmental influence on AUD). There was also evidence for residual nonshared environmental influence on aggressive undercontrol at age 29 that was shared with nonshared environmental influence on AUD at age 29, but effect sizes were again quite small (β = .12, p < .05; explaining less than 2% of nonshared environmental influence on AUD). Following this, there was a large residual nonshared environmental influence on AUD at age 29 (β = .84, p < .05). Altogether, aggressive undercontrol and AUD symptoms (from ages 17 to 24) explained a total of ~25% of the predicted nonshared environmental influence of aggressive undercontrol at age 29 [(.222 + .302)/(.222 + .302 + .642)] and just ~8% of the predicted nonshared environmental influence on AUD symptoms at age 29 [(.102 + .112 + .152 + .122)/(.102 + .112 + .152 + .122 + .842)]. Follow-up analyses confirmed an essentially identical pattern of results males and females (see eFigures 14–15 in supplemental materials).
In sum, results from the multivariate analyses that evaluated the proportion of the genetic and environmental influence on the prospective associations between aggressive undercontrol and AUD symptoms over time showed evidence for a common cause model of personality-AUD development, as much of the prospective associations were explained by one source of common additive genetic influence – thus supporting our expectations. There was some evidence these associations were significantly influenced by common nonshared environmental influence as well, however nonshared environmental influences were much smaller in effect and explained very little (i.e., <5%) of the total nonshared environmental influence on either negative emotionality or AUD within and across time. Altogether, our set of three multivariate genetic analyses evidenced consistent and clear support for additive genetic influence as a common cause to personality-AUD development.
Discussion
Although a large research literature has demonstrated robust associations between personality traits related to constraint and negative emotionality with alcohol and substance use problems (Boschloo et al., 2012; Hicks et al., 2011; James & Taylor, 2007; Kotov et al., 2010; McGue et al., 1999; McGue et al., 1997; Sher & Trull, 1994; Slutske et al., 2002; Vrieze et al., 2014; Zucker et al., 2011), few studies have evaluated the direction of effects of these associations to better inform theoretical models of personality-AUD development (i.e, vulnerability vs. scar models; Klein et al., 2011; Tackett, 2006). Notable exceptions to this include recent research by Littlefield et al. (2012) and Quinn et al. (2011), which showed support for transactional and reciprocal influences among personality traits (novelty-seeking/impulsivity) and heavy drinking during and after the college years. We extended this work by evaluating prospective relationships between personality and AUD over a longer time span (from ages 17 to 29) with larger time intervals between assessments, as well as evaluating the impact of shared additive genetic and environmental influences on these prospective associations.
Results from our phenotypic analyses showed that low constraint and greater aggressive undercontrol at age 17 prospectively predicted rank-ordered increases in AUD symptoms at age 24. AUD symptoms at age 17, however, did not predict subsequent rank-order change in low constraint or aggressive undercontrol at age 24. Together, these results provide evidence that vulnerability/predisposition processes are a larger contributor to AUD development than are scar processes – at least concerning the developmental transition of late adolescence (age 17) to early adulthood (age 24) and involving the personality traits of constraint and aggressive undercontrol. From early adulthood (ages 24) to later young adulthood (age 29), results showed significant effects of both lower constraint and higher aggressive undercontrol on subsequent rank-ordered increases in AUD symptoms as well as AUD symptoms on subsequent rank-ordered increases in constraint and aggressive undercontrol – thus aligning with a reciprocal/transactional model of personality-AUD development.
These findings somewhat contradict prior studies (Littlefield et al., 2012; Quinn et al., 2011) which found support for a transactional/reciprocal model of personality-AUD development involving impulsive and sensation-seeking personality traits in relation to heavy/problematic drinking around ages 18 to 25. One important reason why results may differ from prior research in this area may be due to the age span evaluated and/or the interval of time between assessments. Quinn et al. evaluated how impulsivity, sensation-seeking, and heavy drinking in high school predicted subsequent heavy drinking, impulsivity, and sensation-seeking in the sophomore and senior year at college. Littlefield et al. evaluated how novelty seeking and heavy drinking in the freshman year of college predicted novelty seeking and heavy drinking in the sophomore and senior years. Thus, both studies evaluated smaller time intervals and focused on samples that evaluated change from 18 to 25. However, Littlefield and colleagues found some support that the transacational/reciprocal relationships between novelty seeking and heavy drinking seems more relevant to ages 21 to 25 than from 18 to 21 or 25 to 35. Our results are consistent with this as we evidenced bidirectionality between personality and AUD symptoms from ages 24 to 29 but not ages 17 to 24. Although further research is needed, results support the notion that personality in late adolescence is a fairly strong and robust predictor of early adult alcohol problems.
Contrary to expectations, we did not find any evidence from our phenotypic models that negative emotionality significantly predicted subsequent AUD symptoms or vice versa (after accounting for within-person stability and between-person correlation of traits over time). One reason why we may have failed to detect any significant cross-effects with negative emotionality may be due to the less stable nature of this construct relative to constraint and aggressive undercontrol. Results showed that the stability of negative emotionality from age 17 to 24 was not significantly different than zero – and this was consistent across gender. This is consistent with prior research, which has demonstrated a greater degree of change in negative emotionality from ages 17 to 24 relative to positive emotionality or constraint (Blonigen, Carlson, Hicks, Krueger, & Iacono, 2008). This lower stability of negative emotionality might also be due to it’s emphasize on mood states where as constraint and aggressive undercontrol are more based on behavioral patterns.
It is noteworthy that prior research has demonstrated links between negative emotionality and alcohol problems using mostly middle-aged samples (ages 35–45) or (McGue et al., 1997; 1999) with some studies demonstrating smaller effects for negative emotionality/neuroticism compared to behavioral undercontrol/disinhibition (Kotov et al., 2010; Slutske et al., 2002). It may be that negative emotionality becomes more relevant to later middle-age AUD – perhaps because AUD across multiple decades over the lifespan results in relatively larger increases in negative emotionality relative to AUD over shorter spans. More research is needed to address this important hypothesis.
Our multivariate genetic results were also consistent with a common cause model of personality-AUD development. The prospective associations between all three traits (low constraint, negative emotionality, aggressive undercontrol) and AUD were largely due to one source of shared additive genetic influence. This follows prior research demonstrating substantial genetic influences common to the cross-sectional associations between key personality traits and SUDs using both adolescent (Krueger et al., 2002) and adult samples (Littlefield et al., 2011; Slutske et al., 2002) and this is the first study that we are aware of that evaluates and shows support for shared genetic influences as a source of common cause for prospective personality-AUD development. Altogether then, our results support the notion that particularly the traits of lower constraint and greater aggressive undercontrol indexes a relatively stable, biologically-influenced vulnerability factor for AUD.
Results from both our phenotypic and multivariate genetic models suggest it may be useful to target personality-based risk for AUD and implement targeted AUD prevention programs for those at high-risk s (e.g., see Conrod et al., 2013; Krank et al., 2011). Conrod and colleagues (2013) evidenced success for such a personality-based risk assessment and tailored alcohol use prevention program in the high school population. Specifically, students over 100 secondary schools in London completed a personality questionnaire in 9th grade and those scoring one standard deviation above the average on one of the four personality risk scores (including anxiety sensitivity, hopelessness, impulsivity, and sensation seeking) and were then randomly selected into a treatment or control group (by school). Treatment consisted of those right-risk students attending two 90-minute sessions that incorporated components of motivation enhancement and cognitive behavior therapy and included exercises related to goal setting and identification of risky versus adaptive coping responses. Importantly, the program did not aim to change personality – only change the relationship between personality risk and alcohol use. This brief intervention was associated with relatively large and robust decrements on drinking and binge drinking rates up to two years post-intervention. Moreover, “herd effects” were demonstrated – drinking and binge drinking among low-risk students from intervention schools also showed slower growth in and rates of drinking and binge drinking than low-risk students from control schools. It is unclear how such a program might operate in college or older-aged samples but does provide evidence that personality-based risk assessment may have an important role in AUD prevention – perhaps more so if it is implemented prior to the development of more severe and persistent drinking problems characterized by AUD.
These findings should be interpreted in light of limitations and possible alternative explanations. First and foremost, and as discussed above, a relatively large lag was used to evaluate personality-AUD models at the front end (ages 17 to 24) then the back end (ages 24 to 29). It is important for future research to evaluate these associations in smaller time-lapses, such as annual assessments, and there is some evidence that transactional effects may be more relevant to smaller time lags (Littlefield et al., 2011; Quinn et al., 2012). There are also important limitations of generalizability; participants of this study were almost entirely white and it is imperative for future research to address how results may or may not replicate in ethnic minority populations. It is also unclear of how results might differ with evaluating other SUDs or substance use measures. Finally, more research is needed that evaluates more complex processes of person-environment interplay – involving an assessment of how personality-based risk may influence exposure or selection into key environments (such as enrollment in “party” schools or fraternity or sorority organizations) as well as how those environments may amplify or offset personality-based risk for AUD (see Grekin & Sher, 2006). Strengths of this study include the large sample size with limited attrition, the equivalent number of males and females and evaluation of gender differences, the use of a standardized (self-reported) personality assessment across data collection points, the incorporation of structured clinical interviews to assess AUDs, and the use of prospective and biometric twin analyses to foster support for multiple apriori defined theoretical models,
In sum, results from this study indicate that personality traits related to low constraint and aggressive undercontrol are strong and salient predictors of subsequent AUD during the developmental transition from late adolescence to young adulthood. These traits are relatively stable and substantially influenced by additive genetic factors. Results suggest targeting those low in constraint and high in aggressive undercontrol (via personality-based risk assessments) may be a useful tactic for future AUD and potentially other substance use disorder prevention and intervention programs (Conrod et al., 2013; Krank et al., 2011). More research is needed to evaluate the prospective relationships between key personality traits and other substance use and substance use disorder outcomes, as well as research taking a more nuanced approach to evaluate how personality trait risk for AUD varies as a function of environmental context (e.g., Grekin & Sher, 2006).
Supplementary Material
Acknowledgments
Funding
This research was supported by Grant DA05147, DA034606, and DA024417 from the National Institute on Drug Abuse and Grant AA09367 and AA024282 from the National Institute of Alcohol Abuse and Alcoholism. The first author was also supported by Grant AA024282 from the National Institute of Alcohol Abuse and Alcoholism and the USDA National Institute of Food and Agriculture, Hatch project 1006129. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
These results support the notion that our data was not missing completely at random (MCAR) but missing at random (MAR), as later alcohol use (at ages 20, 24, and 29) missing data were conditional on earlier (age 17) alcohol use. Our modeling approach accounts for this by including age 17 alcohol use symptoms in all analyses and incorporating full information maximum likelihood (FIML), which is warranted under MAR and recommended (see Enders & Bandalos, 2001); see the analysis plan for more details of modeling strategy.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Substance Abuse and Mental Health Administration (SAMSHA) 2008–2010 National Survey on Drug Use and Health Substate Age Group Tables. 2012 Retrieved August 18, 2015, from http://archive.samhsa.gov/data/NSDUH/substate2k10/AgeGroupTables/NSDUHsubstateAgeGroupTabs2010.pdf.
- SAMSHA. Results from the 2013 national survey on drug use and health: Summary of national findings. NSDUH Series. 2014 Retrieved Publication No. (SMA) 14-4863, H-48, from http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf.
- American Psychological Association (APA) Diagnostic and Statistical Manual of Mental Disorders (3rd ed., revised; DSM–III–R) Washington, DC: Author; 1987. [Google Scholar]
- American Psychological Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: Author; 2013. [Google Scholar]
- Blanco C, Okuda M, Wright C, Hasin DS, Grant BF, Liu SM, Olfson M. Mental health of college students and their non-college-attending peers: results from the National Epidemiologic Study on Alcohol and Related Conditions. Arch Gen Psychiatr. 2008:1429–1437. doi: 10.1001/archpsyc.65.12.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonigen DM, Carlson MD, Hicks BM, Krueger RF, Iacono WG. Stability and change in personality traits from late adolescence to early adulthood: a longitudinal twin study. J Pers. 2008:229–266. doi: 10.1111/j.1467-6494.2007.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonigen DM, Durbin CE, Hicks BM, Johnson W, McGue M. Alcohol use initiation is associated with changes in personality trait trajectories from early adolescence to young adulthood. Alcohol Clin Exp Res. 2015:2163–2170. doi: 10.1111/acer.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschloo L, Vogelzangs N, van den Brink W, Smit JH, Beekman AT, Penninx BW. Predictors of the 2-year recurrence and persistence of alcohol dependence. Addiction. 2012:1639–1640. doi: 10.1111/j.1360-0443.2012.03860.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Begg D, Dickson N, Harrington H, Langley J, Moffitt TE, Silva PA. Personality differences predict health-risk behaviors in young adulthood: evidence from a longitudinal study. J Pers Soc Psychol. 1997:1052–1063. doi: 10.1037//0022-3514.73.5.1052. [DOI] [PubMed] [Google Scholar]
- Census. Minnesota Population by Race, 1980–2000. 2000 from http://www.censusscope.org/us/s27/chart_race.html.
- Chassin L, Dmitrieva J, Modecki K, Steinberg L, Cauffman E, Piquero AR, … Losoya SH. Does Adolescent Alcohol and Marijuana Use Predict Suppressed Growth in Psychosocial Maturity Among Male Juvenile Offenders? Psychology of Addictive Behaviors. 2010:48–60. doi: 10.1037/a0017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Fora DB, King KM. Trajectories of alcohol and drug use and dependence from adolescence to adulthood: the effects of familial alcoholism and personality. J Abnorm Psychol. 2004:483–498. doi: 10.1037/0021-843X.113.4.483. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel DB. The natural history of drug use from adolescence to the mid-thirties in a general population sample. Am J Public Health. 1995:41–47. doi: 10.2105/ajph.85.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church AT. Relating the Tellegen and five-factor models of personality structure. J Pers Soc Psychol. 1994:898–909. doi: 10.1037//0022-3514.67.5.898. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, O’Leary-Barrett M, Newton N, Topper L, Castellanos-Ryan N, Mackie C, Girard A. Effectiveness of a selective, personality-targeted prevention program for adolescent alcohol use and misuse: a cluster randomized controlled trial. JAMA Psychiatry. 2013:334–342. doi: 10.1001/jamapsychiatry.2013.651. [DOI] [PubMed] [Google Scholar]
- Durbin CE, Hicks BM. Personality and psychopathology: A stagnant field in need of development. European Journal of Personality. 2014:362–386. doi: 10.1002/per.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin CE, Hicks BM, Blonigen DM, Johnson W, Iacono WG, McGue M. Personality trait change across late childhood to young adulthood: Evidence for nonlinearity and sex differences in change. European Journal of Personality. 2015:31–44. doi: 10.1002/per.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, King SM, McGue M, Iacono WG. Personality traits and the development of nicotine, alcohol, and illicit drug disorders: prospective links from adolescence to young adulthood. J Abnorm Psychol. 2006:26–39. doi: 10.1037/0021-843X.115.1.26. [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The Relative Performance of Full Information Maximum Likelihood Estimation for Missing Data in Structural Equation Models. Structural Equation Modeling-a Multidisciplinary Journal. 2001:430–457. doi: 10.1207/S15328007sem0803_5. [DOI] [Google Scholar]
- Grekin ER, Sher KJ. Alcohol dependence symptoms among college freshmen: Prevalence, stability, and person-environment interactions. Experimental and Clinical Psychopharmacology. 2006:329–338. doi: 10.1037/1064-1297.14.3.329. [DOI] [PubMed] [Google Scholar]
- Hamaker EL, Kuiper RM, Grasman RPPP. A critique of the cross-lagged panel model. Psychological Methods. 2015:102–116. doi: 10.1037/a0038889. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Durbin CE, Blonigen DM, Iacono WG, McGue M. Relationship between personality change and the onset and course of alcohol dependence in young adulthood. Addiction. 2012:540–548. doi: 10.1111/j.1360-0443.2011.03617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behav Genet. 2011:459–475. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cross-generational effects on gender differences in pscyhoactive drug abuse and dependence. Drug and Alcohol Dependence. 2004:147–158. doi: 10.1016/j.drugalcdep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- James LM, Taylor J. Impulsivity and negative emotionality associated with substance use problems and Cluster B personality in college students. Addictive Behaviors. 2007:714–727. doi: 10.1016/j.addbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Keijsers L. Parental monitoring and adolescent problem behaviors: How much do we really know? International Journal of Behavioral Development. 2015:271–281. doi: 10.1177/0165025415592515. [DOI] [Google Scholar]
- Kenny DA, Harackiewicz JM. Cross-lagged panel correlation: Practice and promise. Journal of Applied Psychology. 1979:372–379. [Google Scholar]
- Keyes MA, Malone SM, Elkins IJ, Legrand LN, McGue M, Iacono WG. The enrichment study of the Minnesota twin family study: Increasing the yield of twin families at high risk for externalizing psychopathology. Twin Research and Human Genetics. 2009:489–501. doi: 10.1375/twin.12.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khremiri L, Kuja-Halkola R, Larsson H, Jayaram-Lindstrom N. Genetic overlap between impulsivity and alcohol dependence: A large-scale national twin study. Psychological Medicine. 2016:1091–1102. doi: 10.1017/S0033291715002652. [DOI] [PubMed] [Google Scholar]
- Klein DN, Kotov R, Bufferd SJ. Personality and Depression: Explanatory Models and Review of the Evidence. Annual Review of Clinical Psychology. 2011:269–295. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Gamez W, Schmidt F, Watson D. Linking "Big" Personality Traits to Anxiety, Depressive, and Substance Use Disorders: A Meta-Analysis. Psychological Bulletin. 2010:768–821. doi: 10.1037/a0020327. [DOI] [PubMed] [Google Scholar]
- Krank M, Stewart SH, O’Connor R, Woicik PB, Wall AM, Conrod PJ. Structural, concurrent, and predictive validity of the Substance Use Risk Profile Scale in early adolescence. Addict Behav. 2011:37–46. doi: 10.1016/j.addbeh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002:411–424. doi: 10.1037//0021-843x.111.3.411. [DOI] [PubMed] [Google Scholar]
- Littlefield AK, Agrawal A, Ellingson JM, Kristjansson S, Madden PAF, Bucholz KK, … Sher KJ. Does Variance in Drinking Motives Explain the Genetic Overlap Between Personality and Alcohol Use Disorder Symptoms? A Twin Study of Young Women. Alcoholism-Clinical and Experimental Research. 2011:2242–2250. doi: 10.1111/j.1530-0277.2011.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Sher KJ, Wood PK. Is "maturing out" of problematic alcohol involvement related to personality change? J Abnorm Psychol. 2009;118:360–374. doi: 10.1037/a0015125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Verges A, Wood PK, Sher KJ. Transactional Models Between Personality and Alcohol Involvement: A Further Examination. Journal of Abnormal Psychology. 2012:778–783. doi: 10.1037/a0026912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson LK, McGue M, Iacono WG. Shared Environmental Influences on Personality: A Combined Twin and Adoption Approach. Behavior Genetics. 2013:491–504. doi: 10.1007/s10519-013-9616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Keyes M, Sharma A, Elkins I, Legrand L, Johnson W, Iacono WG. The environments of adopted and non-adopted youth: Evidence on range restriction from the Sibling Interaction and Behavior Study (SIBS) Behavior Genetics. 2007:449–462. doi: 10.1007/s10519-007-9142-7. [DOI] [PubMed] [Google Scholar]
- McGue M, Slutske W, Iacono WG. Personality and substance use disorders: II. Alcoholism versus drug use disorders. J Consult Clin Psychol. 1999:394–404. doi: 10.1037//0022-006x.67.3.394. [DOI] [PubMed] [Google Scholar]
- McGue M, Slutske W, Taylor J, Iacono WG. Personality and substance use disorders: I. Effects of gender and alcoholism subtype. Alcohol Clin Exp Res. 1997:513–520. [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx Statistical Modeling. 7. Richmond, VA: Authors; 2006. [Google Scholar]
- Poel FT, Baumgartner SE, Hartmann T, Tanis M. The curious case of cyberchondria: A longitudinal study on the reciprocal relationship between health anxiety and online health information seeking. Journal of Anxiety Disorders. 2016:32–40. doi: 10.1016/j.janxdis.2016.07.009. doi:10.1016/j.janxdis.2016.07.009 0887-6185. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Stappenbeck CA, Fromme K. Collegiate Heavy Drinking Prospectively Predicts Change in Sensation Seeking and Impulsivity. Journal of Abnormal Psychology. 2011:543–556. doi: 10.1037/a0023159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Robins LM, Babor T, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis: Authors; 1987. [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, … Burke J, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Madden PA, Bucholz KK, Statham DJ, Martin NG. Personality and the genetic risk for alcohol dependence. J Abnorm Psychol. 2002:124–133. [PubMed] [Google Scholar]
- Tackett JL. Evaluating models of the personality-psychopathology relationship in children and adolescents. Clinical Psychology Review. 2006:584–599. doi: 10.1016/j.cpr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Waller NG. Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire. In: Boyle GL, Matthews G, Saklofske DH, editors. Handbook of personality theory and testing: Vol.II. Personality measurement and assessment. Thousand Oaks, CA: Sage; 2008. pp. 261–292. [Google Scholar]
- Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychological Medicine. 2015:1061–1072. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Vaidyanathan U, Hicks BM, Iacono WG, McGue M. The role of constraint in the development of nicotine, marijuana, and alcohol dependence in young adulthood. Behav Genet. 2014:14–24. doi: 10.1007/s10519-013-9629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukasovic T, Bratko D. Heritability of Personality: A Meta-Analysis of Behavior Genetic Studies. Psychol Bull. 2015:769–785. doi: 10.1037/bul0000017. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the Undercontrol/Disinhibition Pathway to Substance Use Disorders: A Multilevel Developmental Problem. Child Dev Perspect. 2011:248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.