Abstract
The DESolve (Elixir Medical Corporation, Sunnyvale, California, USA) is a poly-L lactide-based polymer scaffold coated with the antiproliferative and anti-inflammatory drug novolimus. The scaffold biodegrades within one year with a complete resorption in two years and in vitro bench test have shown the ability to supply the necessary radial strength to support the vessel for the critical 3- to 4-month period after implant. The DESolve showed the unique self-correction property, which may reduce the incidence of minor malapposition after deployment. Overexpansion with DESolve is safe since a high capability resistance to fracture has been demonstrated with this scaffold. The aim of this review is to provide a comprehensive overview of the available preclinical and clinical data regarding the DESolve.
Keywords: DESolve, Scaffold, bioresorbable scaffolds (BRS)
Introduction
The current second generation drug eluting stent (DES) have dramatically improved the outcome of percutaneous coronary interventions (PCI) thanks to the very low incidence of stent thrombosis and restenosis (1). In the last few years biodegradable stents, commonly addressed as scaffolds, have entered the interventional arena has raising stars due to their potentiality.
Indeed, bioresorbable scaffolds (BRS) made of biodegradable polymers or biocorrodible metals hold the promise to work as DES when needed (i.e., until vessel scaffolding is required) and then disappear leaving an healthy and regenerated vessel (2).
Several companies have developed poly-L-lactic-acid (PLLA)-based polymer scaffolds with the Abbott (Abbott Vascular, North Chicago, Illinois, US) producing the firstly commercially available scaffold: the Absorb.
Few years later Elixir (Elixir Medical Corporation, Sunnyvale, California, USA) obtained the CE mark for the DESolve which is currently available with the first generation (150 µm struts) and the second generation (120 µm struts). The DESolve theoretically differs from the Absorb for its self-expansion properties and for a greater tolerance to overexpansion. So far there are limited data exploring the clinical results of PCI with the DESolve (3). On the contrary some insights on the peculiar characteristics of this scaffold have been obtained performing experimental bench tests (4).
This review is aimed to provide a comprehensive overview on the available clinical data and peculiar characteristics of the DESolve.
DESolve: a novolimus-eluting bioresorbable coronary scaffold
Technical features: the scaffold structure
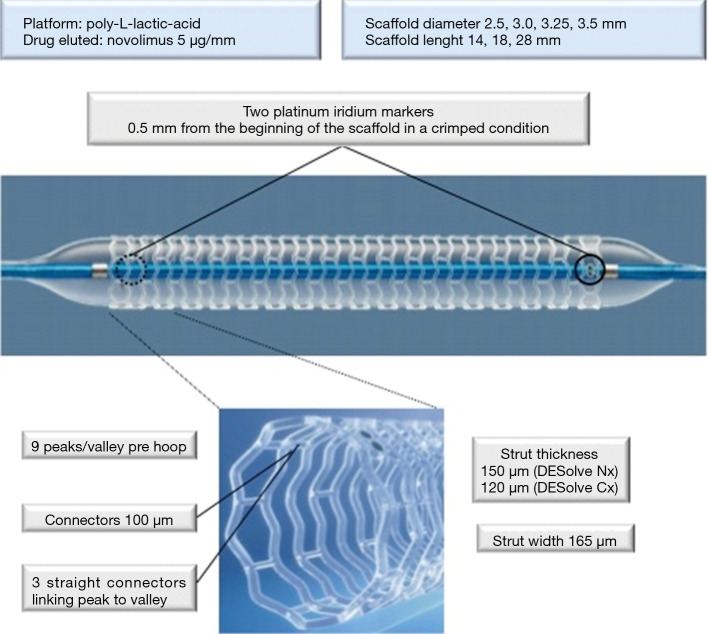
The DESolve Novolimus-Eluting Bioresorbable Coronary Scaffold System (Elixir Medical Corporation, Sunnyvale, CA) is poly-L-lactic-acid based polymer scaffold. The antiproliferative and anti-inflammatory drug novolimus is coming this scaffold (Figure 1) which is mounted on a rapid exchange balloon catheter delivery system. The strut thickness of 150 µm (in its first generation) incorporating 2 platinum-iridium based radiopaque markers at either end between the last 2 rings to aid in placement (0.5 mm to its beginning in a crimped condition), the polymer prepared using proprietary techniques, made the DESolve a unique device (4). A matrix of polylactide-based polymer is coating the scaffold and realising novolimus which is incorporated approximately 5 µg per mm of scaffold length; approximately 85% of the drug is released over 4 weeks in vivo (5). The crossing profile of the scaffold is of 1.44 mm (±0.02 mm) and is 6-F catheter compatible. The available diameters are ranging from 2.5 to 4.0 mm with lengths of 14 to 28 mm; significant oversizing is not recommended (6) although DESolve has a greater margin of safety with a reduced risk of single strut fracture during post-dilation as compared with Absorb. Currently Elixir has developed and is testing a new novolimus-eluting BRS with thinner struts (120 µm), the DESolve CX, with the same drug coating dose. This new scaffold is expected to maintain radial strength and vessel support for the necessary period of vessel healing and to degrade at the same rate of the first generation DESolve. The second generation, the DESolve CX, has recently shown promising safety and efficacy results at 6 months (7).
Figure 1.
DESolve Nx scaffold. Sinusoidal rings are connected to consecutive ring by three connectors. Two platinum iridium markers are localized at each end. DESolve Nx has CE mark for sizes overwritten, DESolve XL for large vessel 4.0 mm is recently evaluated in a First-In-Man registry.
The resorption process
Potentially a BRS has three major aims: revascularization of the target vessel with restoration of a normal blood flow, temporary support to the vessel wall in order to avoid recoil and finally complete resorption with degradation of the polymers up to no longer been identified at the histological analysis.
The main chemical process responsible for in vivo molecular weight degradation of polylactide-based polymers, including their copolymers that are used for resorbable implants, is hydrolysis. The degradation and subsequent absorption develops through five steps: hydration, depolymerisation by hydrolysis with reduction of molecular weight, loss of mass with scission of amorphous tie chains and subsequent loss of radial strength, dissolution of the monomer by macrophages via phagocytosis and final formation of carbon dioxide and water into the Krebs cycle (8). In a comparable manner occurs the PLLA-based DESolve degradation process resulting in consistent loss in molecular weight from one year (more than 90%) with a complete bioresorption of the scaffold in 2 years. After this period the polymer is replaced by a provisional matrix made of proteoglycan and then by de novo connective tissue similar a thin neointimal lining. Bench testing and clinical data have shown the ability of DESolve to supply the necessary radial strength to support the vessel for the critical 3- to 4-month period of vessel healing (3,4).
Novolimus
Novolimus is a metabolite of rapamycin (sirolimus) with immunosuppressive and anti-proliferative properties. Rapamycin, a macrocyclic lactones, is produced by a strain of Streptomyces hygroscopicus and it has been proven safe and effective to prevent organ transplant rejection by systemic administration. Analogs of Rapamycin are a polymer already in widespread use (with application such as resorbable sutures, soft-tissue implants, orthopedic implants, and dialysis media) with all the key mechanical properties to be the candidate material for coronary indications. Other analogs of rapamycin such as everolimus and zotarolimus have been clinically tested and shown safe and efficacious on DES (5). Gallo et al. demonstrated that systemic rapamycin administration significantly reduced the arterial proliferative response after balloon angioplasty in the pig by increasing the level of the CDKI p27kip1 and inhibition of the protein retinoblastoma protein phosphorylation within the vessel wall (9). Pharmacodynamics of novolimus is similar to other macrocyclic lactones: they bind to immunophilins, a specific cytosolic proteins (for example, FK506 binding protein 12), to gain their immunosuppressive activity.
Rapamycin obstructs the progression from G1 to S cell cycle by interacting with mTOR, (mammalian target of rapamycin) and inhibits its activation (10). mTOR is an important regulatory kinase and its inhibition results in several important effects which ultimately determinants an antiproliferative effect on T and B cells, peripheral blood mononuclear cells, mast cell and also inhibit the proliferation and migration of smooth muscle cells and fibroblast (11).
Experience from bench tests
Several experience from bench test have demonstrated that the DESolve has minimal propensity to acute recoil, as well as Absorb scaffold, with subsequent increase in diameter; Schmidt and colleagues reported an acute recoil of 7.85% (12), while Ormiston and colleagues demonstrated that DESolve 3.0 mm recoiled 0.1 mm after deployment (4). Furthermore, differently from others BRS and drug-eluting stents, it shows the unique ability to enlarge (“self-correction”) and to return to the post-deployment diameter, between 10 minutes and one hour. The DESolve permits a wide range of overexpansion before fracturing; preclinical data demonstrated that the sinusoidal rings of the DESolve straightened and stretched before fracturing with increasing pressure (4).
Serial OCT analysis after the deployment of BRS in porcine model demonstrated an initial reduction in minimal and mean luminal area between the immediate postprocedure and 6-month follow-up but observed a late enlargement of the luminal area between 6 months and 2 years (13). This fact, differently from metallic stents, could be the consequence of vessel “un-caging” through scaffold bioresorption and thin neointimal lining formation.
A major concern about BRS is the return to normal vascular function after the scaffold resorption to restore a physiological response to vasoactive stimulus. Direct evidence for vasomotion restoration was obtained after infusion of acetylcholine at the 2-year time point: five of nine patients tested with acetylcholine exhibited vasodilatation during the highest infused doses (14). In Table 1 are listed the main mechanical and physical properties evaluated in bench tests
Table 1. Mechanical and physical properties evaluated in bench tests.
| Mechanical and physical properties | Bench comparison ABSORB, DESolve and ML8/Xpedition (scaffolds size 3.0) (4) | Bench comparison ABSORB, DESolve, MAGMARIS (12) |
|---|---|---|
| Crossing profile (trackability; pushability) | Method: The diameter of the scaffold mounted on its delivery system was photographed in air, rotated 90° then photographed again for measurements; results: 1.44±0.02 mm (major than Xpedition) | Trackability: the system is able to measure proximal and distal forces during the passage of the stent system through the guiding catheter and the vessel model with a simulated vessel curvature; results: mean track force 0.64±0.02 N (more trackability than ABSORB and MAGMARIS); pushability: It was measured using the trackability setup, simulating vessel curvature and total occlusion model at the end; results: 36.27%±1.30% (minor pushability than MAGMARIS but major than ABSORB) |
| Acute recoil | Method: 3.5 mm scaffolds were expanded unconstrained in a water bath at 37 °C at nominal pressure and photographed at one, 10 and 60 minutes; results: 1 min → DESolve recoiled 0.1 mm; 10 min → no change; 60 min → DESolve increased in diameter up to 3.52±0.14 mm (self-correction) (different behaviour than ABSORB and Xpedition) | Method A: recoil without outer loading acute and after 1 h (scaffold expansion up to the nominal pressure 9 atm in 2 s intervals per atm); results: acute elastic recoil (7.85%), after 1 h the diameter increased by about 3%. Method B: Recoil with outer loading through mock vessel (light 40 mm, inner diameter 2.7±0.2 mm, radial compliance 5–7% per 100 mmHg. 72 bpm); results: after 1 h diameter decreased by more 11% (different behaviour than ABSORB and MAGMARIS) |
| Radial strength | Method: the pressures needed to reduce the device cross-sectional area by 25% were assessed; results: 1.1±0.1 atm (minor than ABSORB and Xpedition) | No data available |
| Strut fracture (main branch post-dilatation) | Method: post-dilatation with increasing NC balloon size up to 20 atm pressure or until a strut fracture; results: stent fracture caused by balloon 5.0 mm at 21 atm (better than ABSORB and Xpedition) | No data available |
| Scaffold fracture (side branch dilatation) | Method: side branches were dilated with NC balloons of increasing diameters; results: no fracture (better than ABSORB) | No data available |
| Bending stiffness | No data available | Method: experimental setup where the test sample is fixed at the free bending length l and deflected by the distance F; results: 21.25 N·mm−2 (twice more flexible than ABSORB and MAGMARIS) |
Self-correction characteristic
Self-correction characteristic in unique to the DESolve. Self-correction is obtained by proprietary processing technique of the PLLA that allows the scaffold to reach a predefined diameter. Differently by self-expanding metallic stents which exert continuous long term outward force on the vessel wall, the DESolve is able to expand (self-correct) itself up to nominal diameter (5). This property was inquired into vitro bench testing; under physiological conditions were performed serial OCT imaging after deployment of DESolve: in the first minute after deployment DESolve recoiled similarly to others BRS, however, unlike these it subsequently self-correction, returning to one hour after deployment to a similar diameter to the original. This characteristic was confirmed in another bench test by Schmidt and colleagues which pointed out that self-correction comes with very low radial forces when introducing an outer load from a stretched mock vessel and that these forces cannot contribute to a relevant vessel support but can improve contact of struts and vessel wall (12). DESolve unique self-correction property could be an advantageous feature in cases in which stents may be undersized, for instance in acute myocardial infarction or after chronic total occlusion treatment. For the setting of acute coronary syndromes Elixir has designed a specific fully BRS, Amity®; it has been presented last year and results in clinical practice are ongoing.
Clinical experience
The first generation of this BRS, the DESolve NX, had its efficacy evaluated in the single-arm, multicentric DESolve NX trial, which enrolled 126 patients with non-complex coronary lesions. Device success was achieved in 97% of the cases and acute recoil was low (6.6%). The 6-month invasive assessment was obtained in 93% of the cases and showed QCA late loss of 0.20±0.32 and 0.21±0.31 mm, respectively, with 3.5% binary restenosis. Of note, in the IVUS (n=40) and OCT (n=38) substudies, this BRS showed an early and significant increase in its area from after the procedure to the 6-month follow-up, attributed to its early resorption properties, resulting in early vessel restoration. Additionally, at 6 months, the strut coverage was nearly 98% by OCT, with a very thin layer of tissue (30.6 mm) on top of them. The clinical safety profile was confirmed both at 6 and 24 months follow-up with major adverse cardiac events (MACE) rate being 3.3% (n=4 of 122) at 6 months and 7.4% (n=9 of 122) at 24 months, including 1 probable scaffold thrombosis within the first month (3).
A post-market observational study was designed to evaluate long-term safety and performance of the DESolve NX. The clinical endpoint included MACE [MACE, which includes cardiac death, target vessel myocardial infarction, and clinically indicated target lesion revascularization (TLR)] at 1, 6 months. In total, 102 patients with stable coronary artery disease were enrolled in this prospective registry. Excessive tortuosity, angulation, and moderate or heavy calcification were excluded. After 6 months overall MACE rate was 2.0%. There was no cardiac death and only one TLR resulting in one target vessel MI. Rate of definite or probable scaffold thrombosis was 1.0% (15).
Currently, the second generation DESolve CX (120 µm strut) is being evaluated in a first-in-man registry enrolling about 150 patients.
Table 2 listed the main clinical experience with DESolve.
Table 2. Clinical studies completed or ongoing.
| Study | Patients | Endpoints | Results |
|---|---|---|---|
| DESolve First-in-Man trial (16) | 16 | Clinical | |
| • MACE (cardiac death, MI, and clinically TLR) at 1, 6, 12 months | 1 patients (1 months), 2 patients (6 months), 3 patients (12 months) | ||
| • Stent thrombosis | 0% | ||
| QCA at 6 months: | |||
| • Acute recoil | 6.42% | ||
| • In segment LLL | 0.31±0.54 | ||
| IVUS e OCT at 6 months: | |||
| • Neointimal volume | 7.19%±3.56% | ||
| • Covered struts | 98.70% | ||
| Device and procedural success | 87.5%, 100% | ||
| DESolve Nx clinical study (3,17) (to assess performance of DESolve Nx through clinical and imaging endpoints) | 126 | Clinical: | |
| • MACE (cardiac death, target vessel MI, clinically TRL) at 1, 6, 12 months and 2, 3, 4, 5 years | 0.8% (1 month), 3.3% (6 months), 5.7% (12 months), 7.4% (2 years), 8.2% (3 years), 9.0% (4 years) | ||
| • Scaffold thrombosis | 0.8% (1, 6, 12, 18 months) | ||
| QCA at 6, 18 months: | |||
| • Acute recoil | 6.60% | ||
| • In-segment LLL | 0.20±0.32 mm (6 months), 0.29±0.34 mm (18 months) | ||
| • Binary restenosis | 3.5% (6 months) | ||
| • % diameter stenosis | 18% (6 months), 20.8% (18 months) | ||
| IVUS (40 patients) at 6 months: | |||
| • In-scaffold % volume obstruction | 5.13%±4.19% | ||
| • Malapposition | 1 patients | ||
| OCT (38 patients) at 6.18 months: | |||
| • In-scaffold % obstruction | 11.27±4.19 (6 months), 22.05±5.94 (18 months) | ||
| • Strut coverage | 98.79% (6 months), 99.98% (18 months) | ||
| Device and procedural success | 97%, 100% | ||
| DESolve Post Market Registry (18) | 102 | MACE (6 months) | 2.00% |
| Scaffold thrombosis (6 months) | 1.00% | ||
| ABSORB vs. DESolve retrospective OCT analysis (19) | 72 | OCT: | |
| • MLA and mean luminal area | No significant differences | ||
| • Eccentricity Index | 0.85±0.05 (Absorb) vs. 0.80±0.05 (DESolve), P<0.01 | ||
| Overlapping implantation of DESolve: observational OCT study (20) | 23 patients single DESolve vs. 15 patients with DESolve in overlapping | OCT: | |
| • Mean lumen area and residual stenosis | No significant differences | ||
| • Eccentricity index | 0.77±0.07 (single DESolve) vs. 0.81±0.03 (overlapping group), P=0.07 | ||
| DESolve XL for large coronary arteries: OCT analysis (21) | 10 patients | • Adverse clinical events (6 months) | 0% |
| • MLA and mean luminal area | NO significant differences | ||
| • Strut fracture | 0% | ||
| DESolve Cx clinical study (to assess performance of DESolve Cx through clinical and imaging endpoints) (7) (NCT02086045) | 50 patients | Clinical | |
| • MACE (cardiac death, target vessel MI, clinically TLR) at 1, 6, 12, 24 months | 0% (1 month), 0% (6 months) | ||
| • Scaffold thrombosis | 0% | ||
| QCA (25 patients) at 6 months: | |||
| • In-segment LLL | 0.18±0.29 mm | ||
| • % diameter stenosis | 14.70% | ||
| IVUS (22 patients) at 6 months: | |||
| • In-scaffold % volume obstruction | 5% | ||
| OCT (25 patients) at 6 months: | |||
| • Strut coverage | 98.9% (6 months) | ||
| DESolve NxT clinical study (to assess performance of DESolve NxT through clinical and imaging endpoints) (7) | Ongoing | Same parameters analysed in DESolve Nx and DESolve Cx Clinical Study | No data available |
| BIFSORB study (randomized 1:1 Absorb vs. DESolve in Bifurcation) (20) (NCT02973529) | Ongoing | Clinical, angiographic and OCT parameters | No data available |
MLA, minimal lumen area.
Large vessels
Large vessel are a major concern with current BRS since 3.5 mm is the largest available Absorb with a well-known overexpansion limit of 3.75 mm. Overexpansion beyond this limit is not recommended with the 3.5 mm Absorb because it can lead to strut fracture, resulting in focal loss of mechanical support. Elixir has produced a dedicated scaffold for large vessels, the DESolve XL.
Boeder et al. firstly demonstrated good performance of DESolve in large vessels (reference diameters >4 mm). They performed a prospective analysis of ten consecutive patients treated with DESolve® XL BRS using OCT for guidance and final post deployment analysis: the QCA analysis revealed a reference vessel diameter of 3.17±0.6 mm with a minimum lumen diameter of 1.31±0.77 mm. In the assessment of geometrical OCT analysis revealed a mean eccentricity index of 0.81±0.05, a percentage of malapposed struts of 0.09±0.26 and prolapse area of 0.25±0.7 mm; OCT did not revealed any strut fractures or edge dissections at distal or proximal ends (21).
In vivo acute performance in comparison to Absorb
Our group recently performed a study aimed to assess the acute mechanical performance of the Absorb compared to the DESolve NX. This OCT study compared 35 patients treated with 63 Absorb scaffolds to a well-matched group of 37 patients treated with 50 DESolve. The quantitative parameters were assessed with OCT. The minimal lumen area (MLA) (Absorb vs. DESolve: 5.8±1.9 vs. 6.1±2.6 mm2, P=0.43) and mean luminal area (Absorb vs. DESolve: 7.1±2.2 vs. 7.2±1.9 mm2, P=0.77) were similar with the two device. Also the incomplete scaffold apposition was similar. A smaller prolapse area was found with Absorb (Absorb vs. DESolve 1.0±1.1 vs. 3.6±6.2 mm2, P<0.01). The main difference was the eccentricity (0.85±0.05 with Absorb and 0.80±0.05 with DESolve, P<0.01) suggesting that the DESolve might have a more elastic behavior with a greater tendency to adapt to the vessel shape (19).
Future perspective
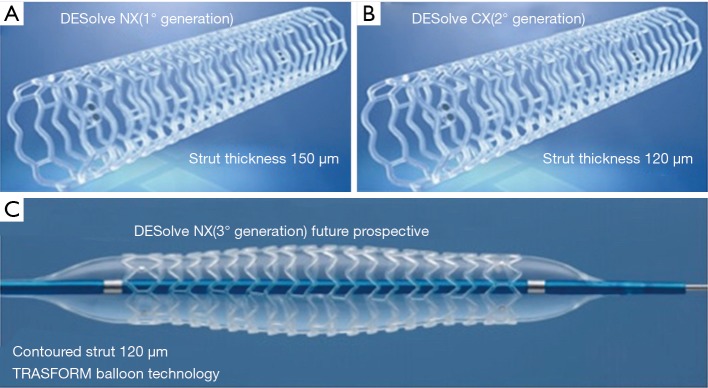
A criticism of currently available scaffolds is the strut thickness, which is close to two times that of the market leading metallic DES (≥150 vs. ∼80 µm). These thick struts possibly cause reduced endothelial shear stress leading to platelet activation and consecutively an increased risk of thrombosis and a prolonged dual antiplatelet therapy to avoid the adverse effects (22). Thin, circular struts retain physiologic endothelial shear stress, which favors platelet quiescence on top of struts and enhances re-endothelialization and production of antithrombotic factors downstream of struts. Elixir is developing a third generation of scaffolds, the DESolve NTx (clinical trials on-going): this scaffold is constituted of 120 µm contoured struts to improve acute performance (optimization of lesion expansion, increase of scaffold embedding into the vessel) and enhanced visibility angiography. It is pre-mounted in a dedicated balloon, the TRASFORM balloon which is characterized by a central segment expansion 0.25 mm larger than the end segments (3 mm) at nominal pressure. This reduction in the strut width, together with better visibility on X-ray and dedicated delivery balloon has the potential to significantly improve the scaffold performance (Figure 2).
Figure 2.
The DESolve family. (A) DESolve Nx (first generation): strut thickness 150 µm; (B) DESolve Cx (second generation): 20% reduction of strut thickness (120 μm) to minimize areas of flow disturbance, without compromising radial strength or recoil; (C) DESolve NTx (third generation, clinical trials ongoing): 120 μm contoured struts to improve acute performance (optimization of lesion expansion, increase of scaffold embedding into the vessel) and improvement of scaffold visibility. TRASFORM balloon technology is characterized by a central segment expansion 0.25 mm larger than the end segments (3 mm) at nominal pressure.
Conclusions
There are several well-known theoretical long-term benefits with BRS for the treatment of coronary artery disease. Nevertheless the first generation of these devices, with bulky struts and high crossing prolife, prolonged resorption time (>24 months), lack of X-ray visibility and limited tolerance to post dilation, have restricted their clinical application. The trend with Absorb to higher thrombotic events as compared to current generation of metallic drug-eluting stents as limited the clinical use of BRS. The clinical experience with DESolve is limited and definitive answer on safety profile of this scaffold cannot be derived. Indeed, Elixir has developed and is currently evaluating a thinner strut (120 µm) scaffold design, which has the same drug dose, radial strength and degradation rate as the DESolve Nx (150 µm) design. This reduction in the strut width has the potential to significantly improve scaffold performance and safety profile. Large clinical trial and registries are warranted in order to understand if the DESolve will be the new raising star between the currently BRS.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Palmerini T, Kirtane AJ, Serruys PW, et al. W. Stent thrombosis with everolimus-eluting stents: meta-analysis of comparative randomized controlled trials. Circ Cardiovasc Interv 2012;5:357-64. 10.1161/CIRCINTERVENTIONS.111.967083 [DOI] [PubMed] [Google Scholar]
- 2.Caiazzo G, Kilic ID, Fabris E, et al. Absorb bioresorbable vascular scaffold: What have we learned after 5 years of clinical experience? Int J Cardiol 2015;201:129-36. 10.1016/j.ijcard.2015.07.101 [DOI] [PubMed] [Google Scholar]
- 3.Abizaid A, Costa RA, Schofer J, et al. Serial Multimodality Imaging and 2-Year Clinical Outcomes of the Novel DESolve Novolimus-Eluting Bioresorbable Coronary Scaffold System for the Treatment of Single De Novo Coronary Lesions. JACC Cardiovasc Interv 2016;9:565-74. 10.1016/j.jcin.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 4.Ormiston JA, Webber B, Ubod B, et al. An independent bench comparison of two bioresorbable drug-eluting coronary scaffolds (Absorb and DESolve) with a durable metallic drug-eluting stent (ML8/Xpedition). EuroIntervention 2015;11:60-7. 10.4244/EIJY15M02_03 [DOI] [PubMed] [Google Scholar]
- 5.Nef HM, Wiebe J, Foin N, et al. A new novolimus-eluting bioresorbable coronary scaffold: Present status and future clinical perspectives. Int J Cardiol 2017;227:127-33. 10.1016/j.ijcard.2016.11.033 [DOI] [PubMed] [Google Scholar]
- 6.Foin N, Lee R, Mattesini A, et al. Bioabsorbable vascular scaffold overexpansion: insights from in vitro post-expansion experiments. EuroIntervention 2016;11:1389-99. 10.4244/EIJY15M07_02 [DOI] [PubMed] [Google Scholar]
- 7.TCT 2016 first report investigations examine potential for novel bioresorbable stent technologies. Available online: http://www.crf.org/tct/press/press-releases/64-tct-2016/press-release/1847-tct-2016-first-report-investigations-examine-potential-for-novel-bioresorbable-stent-technologies
- 8.Ang HY, Bulluck H, Wong P, et al. Bioresorbable stents: Current and upcoming bioresorbable technologies. Int J Cardiol 2017;228:931-9. 10.1016/j.ijcard.2016.11.258 [DOI] [PubMed] [Google Scholar]
- 9.Gallo R, Padurean A, Jayaraman T, et al. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation 1999;99:2164-70. 10.1161/01.CIR.99.16.2164 [DOI] [PubMed] [Google Scholar]
- 10.Serruys PW, Regar E, Carter AJ. Rapamycin eluting stent: the onset of a new era in interventional cardiology. Heart 2002;87:305-7. 10.1136/heart.87.4.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc 2003;35:7S-14S. 10.1016/S0041-1345(03)00211-2 [DOI] [PubMed] [Google Scholar]
- 12.Schmidt W, Behrens P, Brandt-Wunderlich C, et al. In vitro performance investigation of bioresorbable scaffolds - Standard tests for vascular stents and beyond. Cardiovasc Revasc Med 2016;17:375-83. 10.1016/j.carrev.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Onuma Y, Serruys PW. Bioresorbable scaffold: the advent of a new era in percutaneous coronary and peripheral revascularization? Circulation 2011;123:779-97. 10.1161/CIRCULATIONAHA.110.971606 [DOI] [PubMed] [Google Scholar]
- 14.Sarno G, Bruining N, Onuma Y, et al. Morphological and functional evaluation of the bioresorption of the bioresorbable everolimus-eluting vascular scaffold using IVUS, echogenicity and vasomotion testing at two year follow-up: a patient level insight into the ABSORB A clinical trial. Int J Cardiovasc Imaging 2012;28:51-8. 10.1007/s10554-010-9769-y [DOI] [PubMed] [Google Scholar]
- 15.Nef H, Hauptmann KE, Latib A, et al. TCT-515 multi-center, post-marketing evaluation of the elixir DESolve novolimus eluting bioresorbable coronary stent system: 6-month results from the DESolve PMCF study. J Am Coll Cardiol 2015;66:B210 10.1016/j.jacc.2015.08.532 [DOI] [PubMed] [Google Scholar]
- 16.Verheye S, Ormiston JA, Stewart J, et al. A next-generation bioresorbable coronary scaffold system: from bench to first clinical evaluation: 6- and 12-month clinical and multimodality imaging results. JACC Cardiovasc Interv 2014;7:89-9. 10.1016/j.jcin.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 17.Verheye S, Morrison L, Schofer J, et al. TCT-32 Prospective, Multi-Center Evaluation of the DESolve Novolimus-Eluting Bioresorbable Coronary Scaffold: Imaging Outcomes and 4-Year Clinical and Imaging Results. J Am Coll Cardiol 2015;66:B13 10.1016/j.jacc.2015.08.061 [DOI] [Google Scholar]
- 18.Blachutzik F, Boeder N, Wiebe J, et al. Overlapping implantation of bioresorbable novolimus-eluting scaffolds: an observational optical coherence tomography study. Heart Vessels 2017;32:781-9. 10.1007/s00380-016-0932-9 [DOI] [PubMed] [Google Scholar]
- 19.Mattesini A, Boeder N, Valente S, et al. Absorb vs. DESolve: an optical coherence tomography comparison of acute mechanical performances. EuroIntervention 2016;12:e566-73. 10.4244/EIJV12I5A96 [DOI] [PubMed] [Google Scholar]
- 20.The Bioresorbable Implants for Scaffolding Obstructions in Randomized Bifurcations (BIFSORB) Study (BIFSORB). Available online: https://clinicaltrials.gov/ct2/show/NCT02973529
- 21.Boeder NF, Koepp T, Dorr O, et al. A new novolimus-eluting bioresorbable scaffold for large coronary arteries: an OCT study of acute mechanical performance. Int J Cardiol 2016;220:706-10. 10.1016/j.ijcard.2016.06.160 [DOI] [PubMed] [Google Scholar]
- 22.Foin N, Mattesini A, Wong P, et al. Bioresorbable Scaffold Thrombosis: Why BRS Size Matters. J Am Coll Cardiol 2016;68:571-2. 10.1016/j.jacc.2016.03.603 [DOI] [PubMed] [Google Scholar]