Abstract
The present study focuses on reduction in fermentation time of idli batter and its enrichment with calcium and iron using finger millet and pearl millet. Fermentation time decreased from 12 to 6 h with addition of finger millet and to 8 h with pearl millet. Rate of fermentation, bulk density, viscosity, microbial changes and particle morphology were assessed in the batter; nutritional quality, sensory, phytochemicals and internal characteristics of idli were investigated. The color and texture characteristics of both batter and idli were evaluated. The pH and titratable acidity of control batter at 6 h was 5.32 and 0.27%, addition of finger and pearl millet changed to 4.32, 0.45% and 4.53, 0.45% at respective fermentation times (6 and 8 h). The viable yeast, lactic acid bacteria and total bacterial count (log CFU g−1) in the batters increased with time, reaching 1.26, 3.85, 4.56 (control); 2.32, 9.84, 9.58 (finger millet) and 1.76, 7.34, 7.74 (pearl millet) respectively at the end of 6 h of fermentation. Addition of finger millet and pearl millet flour (10% w/w) to the batter enhanced the dietary fiber by 28 and 23%, calcium by 113 and 56%, iron by 51 and 258% in the respective idlies when compared with the control.
Keywords: Idli, Finger millet, Pearl millet, Accelerated fermentation
Introduction
Cereal and legume-based foods are a major source of dietary energy and nutrients. The use of desirable microorganisms, particularly those of lactic acid bacteria (LAB), yeasts and fungi in fermentation have been well documented (Steinkraus 1995). Among several of the Indian traditional foods, idli is popular and consumed throughout India.
The major ingredients are rice (Oryza sativa) and black gram dhal (Phaseolus mungo) in the ratio 3:1 (w/w). Reports have documented enhanced nutrition content of idli with mixed millet (Nazni and Shalini 2010). However, addition of finger millet and pearl millet has not been studied and hence selected for the following reasons.
Finger millet is considered equivalent to rice with respect to protein (6–8%) and fat (1–2%) but it is superior to rice and wheat in mineral and micronutrient contents. It also has important amino acids such as isoleucine (4.4 g), leucine (9.5 g), methionine (3.1 g) and phenyl alanine (5.2 g) that are deficient in other starchy meals. The high calcium content of finger millet makes it an exceptional dietary option to prevent osteoporosis and other calcium deficiencies.
The carbohydrate content of the millet included batter is less considerably; therefore, it is certainly useful for diabetic patients to include it in their diet (KK et al. 2013). Apparently, it would be a useful counter measure for obesity as well. Apart from imparting its nutritional qualities to idli batter, it can also substitute the black gram (Singh and Perez-Maldonado 2003) which is an important ingredient in idli preparation. This can be done by blending a suitable ratio of black gram and millet (Nazni and Shalini 2010).
Idli preparation in the conventional manner takes 12–18 h depending on environmental conditions. The available instant idli pre-mixes do not provide the desired textural characteristics and also lack the typical fermented aroma; further, idli prepared in households do not have consistent quality (Nisha et al. 2005). Idli in general has immense scope for commercialization as food with improved nutritional value as well as functional properties.
Idli batter fermentation has been the subject of many research investigations, covering aspects such as methods of preparation, microbiology and nutritive value (Rati Rao et al. 2006). Two significant changes occurring in idli batter fermentation are leavening and acidification (Jama and Varadaraj 1999). These two parameters have been used here as the criteria for evaluating the progress of fermentation.
The objectives of the present research work are to accelerate the fermentation of idli batter and extend its shelf life, along with enrichment of the idli batter with millet flour (finger millet and pearl millet).
Materials and methods
Procurement of raw materials
Rice (CR1009), urad dhal, finger and pearl millets were bought from the local market. Millets were ground to fine powder and stored at room temperature in air tight containers until used.
Idli batter preparation
Idli batter was prepared with the rice variety CR1009 and black gram dhal taken in the ratio 3:1 (w/w). They were washed with tap water individually and soaked in double the amount of drinking water at room temperature (27 ± 2 °C) for 4 h. Rice was coarsely ground and mixed with black gram dhal ground to a smooth batter with salt (2% w/w). The batter was mixed well with hand and allowed to ferment in an incubator at 30 °C for 12 h. Idli batter fortified with finger millet and pearl millet was prepared separately by adding respective millet powder (10% w/w) to non-fermented batter and mixed thoroughly. The fermentation was carried out for 24 h in both idli batters (with and without individual millet). The samples of fermented batter were drawn at every 4 h interval and subjected to physico-chemical analysis. Commercial idli batter obtained from the local market was used for the comparison studies. The proximate analysis of the commercial batter was done to confirm the proportion of rice and black gram dhal.
Preparation of idli
Idli was prepared by steaming the 12 h fermented batter in idli moulds for 15 min.
Batter characteristics
Moisture
Batter (2 g) was taken to determine the moisture content (moisture %) using a moisture analyzer (Sartorius, MA35).
pH and titratable acidity (TA)
Batter (10 g) was mixed with 100 mL of distilled water and centrifuged at 17,880 g for 20 min. The supernatant was separated to determine the pH using pH meter (Susima AP-1 plus, Chennai). An aliquot of the supernatant was titrated using 0.1 N NaOH and phenolphthalein as indicator to determine TA expressed as percent lactic acid (% Lactic acid = Titer value × 0.00908 × 100).
Bulk density
The bulk density of the batter sample was calculated as the ratio of mass by volume and expressed as kg m−3.
Viscosity measurement
The viscosity of the fermented batter (100 mL) at 20, 50 and 100 rpm was determined using Viscometer (Brookfield, DV-E) fitted with two types of disc spindles S-62 and S-18. The readings were taken after 1 min of revolution. The appropriate disc spindle was selected so that the torque readings were not below 10% of the total scale.
Texture
Texture of the idli batter was analyzed with a Texture Analyzer (TAXTplus, Stable Micro systems, UK) using a probe (back extrusion rig). The idli batter sample was placed on the platform attached to the instrument. When the probe touches the surface of the sample, the force is triggered. The measured force is plotted as a graph. Firmness is defined as the force (in grams or Newtons) required for penetrating the product.
Color
Using Ultra Scan VIS Spectrophotometer (Hunter Associates Laboratory, USA), the color of the idli batter was measured. The L*, a* and b* denotes whiteness to darkness, green to red and yellow to blue scale, respectively on which red and yellow are positive.
Microbiological changes
Total bacterial count (TBC)
Idli batter (10 g) was serially diluted in sterile saline (0.85%). The diluted samples were spread plated on plate count agar (PCA) and the plates were incubated at 37 °C for 48 h. After incubation the colonies were counted. Further, individual colonies were isolated and identified.
Total lactic acid bacteria (LAB) count
The serially diluted samples were spread plated on deMan, Rogosa and Sharpe (MRS) agar. The plates were incubated at 37 °C for 48 h. Further the colonies were counted, isolated and identified.
Total yeast and mold count (TYM)
Idli batter sample of 10 g was diluted in 20 mL of 0.85% sterile saline, serially diluted and spread plated on Sabouraud Dextrose Agar (SDA). Further the colonies were counted, isolated and identified after incubation of the plates for 3–5 days at 25 °C.
Idli characteristics
Color
The color measurements of idli samples were made as described for idli batter.
Texture
The texture of the idli was measured using a Texture Analyzer (TAXT2, Stable Micro systems, UK) attached with the cylindrical probe (36 mm). The peak corresponds to force at the maximum penetration depth of 75% of the sample’s original height.
Idli internal structure
The pores in the internal structure of idli were measured from the ink print of the cross-section (Nazni and Shalini 2010).
Particle morphology
The sample (idli and idli batter) was cut and spread as small pieces in hot air oven at 50 degrees till the sample gets dried. Then the dried sample was crushed using mortar and pestle. The course and fine particles were separated with 1 mm sieve. Fine particles were taken for SEM analysis. The samples were sprayed on a metal plate previously covered with double sided adhesive tape and sputter coated with gold palladium under vacuum in Ion Sputter equipment. The batter and idli samples were examined with a scanning electron microscope (Hitachi S-2400) operated at an accelerating voltage of 30 kV. The surface morphology at different fermentation time in the samples was observed.
Sensory analysis
The sensory characteristics (color, appearance, texture, mouth feel, taste, flavor and overall acceptability) of idli at two fermentation times were determined by a sensory panel consisting of 18 women and 12 men. The samples were scored using the 9-point hedonic scale.
Proximate composition
Moisture, ash, protein, fat and carbohydrate analysis was done according to Association of Official Analytical Chemists (AOAC 2000).
Dietary fiber analysis
Dietary fiber analysis was determined by method 991.43 of AOAC (2000).
Insoluble dietary fiber (IDF)
The precipitate was wet using 3 mL H2O. The beaker was rinsed, and then residue was washed two times with 10 mL H2O at 70 °C. The filtrate and water washings were combined, transferred to 500 mL beaker and reserved for determination of soluble dietary fiber. The residue was washed 2 times with 15 mL portions of 78% ethanol, 95% ethanol and acetone under vacuum.
Determination of soluble dietary fiber
The filtrate and water washings were combined in 500 mL beakers. The beaker was weighed with combined solution of filtrate and water washings, and the volumes were estimated. Four volumes of 95% ethanol pre-heated to 60 °C were added. A portion of ethanol at 60 °C was used to rinse the filtering flask from IDF determination. Weight of the combined solution of filtrate and water washings was adjusted to 80 g by addition of H2O and 320 mL of 95% ethanol at 60 °C was added. The precipitate was allowed to form at room temperature for 1 h. Soluble dietary fiber = (Weight of precipitate −Weight of ash)/Weight of sample taken for analysis.
Calcium and iron content analysis
Calcium and iron content was measured by the method (935.13) of AOAC (2000).
Bio-actives in finger millet and pearl millet
Preparation of extract
Finger millet and pearl millet were washed and dried at 30 °C and made into powder using a mechanical blender. Samples (5 g) were mixed with 20 mL ethanol and left for 24 h in (40 °C) after which it was allowed to evaporate. Chloroform (20 mL) was added to the dry extract in the same container and incubated at 40 °C for 24 h. Subsequently it was repeated with hexane. Final extract was filtered using a clean sterile filter paper and used for further experiments.
Detection of phytochemicals by GC–MS
The presence of active components in the methanol extracts of millet were performed on an Agilent 6890 N Series Gas Chromatography coupled with Mass Spectroscopy (GC–MS). The samples were injected into a DB-5MS capillary column (30 m length × 250 mm internal diameter × 25 μm film thickness). The flow rate was 1.0 mL min−1. The compounds were identified by comparison with the retention times of authentic compounds and the mass spectra in the GC–MS library.
Detection of functional groups by fourier transform infrared (FTIR) spectrophotometer
The functional groups were detected using FTIR (CARY 630, Agilent Technologies Pvt. Ltd., Germany) based on the Universal Diamond ATR and operated in spectral range between 4000 and 650 cm−1. The spectral resolution was 4 cm−1 coupled with Deuterated l-Alanine Tri-Glycine Sulfate (DLATGS) detector. The sample prepared was 3–5 mg of dry solid powder in 5–10 µL of liquid in the case of hexane extract.
All analytical tests were carried out in triplicates.
Results and discussion
Physicochemical changes in idli batter during fermentation
Acidity
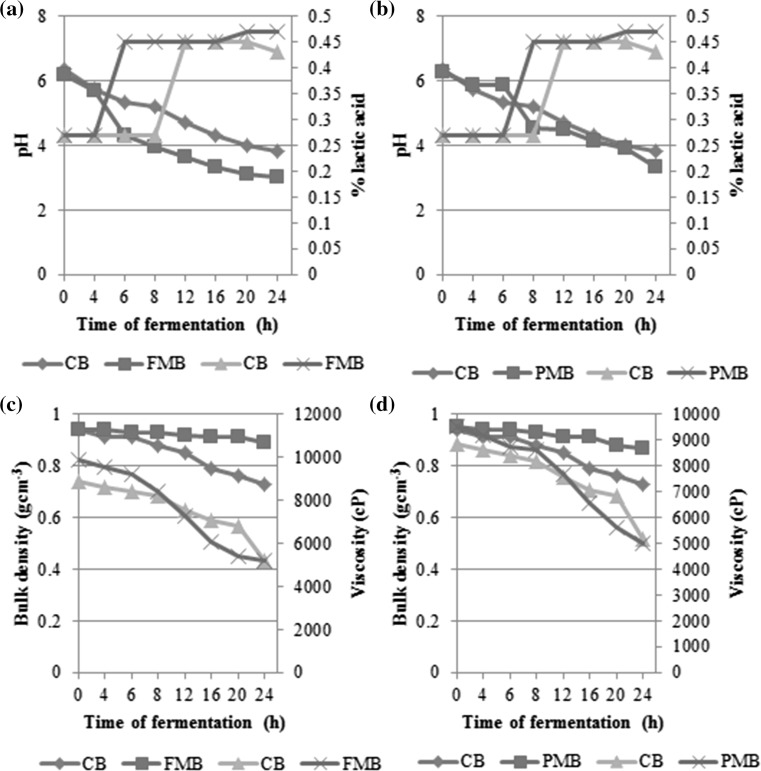
Figure 1a, b show the pH and titratable acidity of the millet fortified batters. The decrease in pH of the fortified batters (finger millet: 6.18–3.0; pearl millet: 6.29–3.33) when compared to the control (6.38–3.8) may be due to the rapid activity of the lactic acid bacteria in a nutrient enhanced medium resulting in increased level of lactic acid. All through the fermentation pearl millet fortified batter maintained a slightly higher pH than the finger millet fortified one. Rekha and Vijayalakshmi (2011) reported that in okara fortified batter acidity increased from 0.18 to 0.64 than in unfortified batter (0.15–0.43) even though there was not much change in the pH of the batters. The increase in acidity may be due to the high amount of soluble protein, amino acid and free fatty acid present in okra fortified batter.
Fig. 1.
pH, titratable acidity, bulk density and viscosity of control, finger millet and pearl millet idli batters
Bulk density and viscosity
Decrease in the bulk density of batter with increase in fermentation time was observed (Fig. 1c, d). This may be due to the release of carbon-dioxide by the lactic acid bacteria and yeast with hetero-fermentative metabolic pathway (Thyagaraja et al. 1992). The volume of control batter increased two-fold after 12 h of fermentation while with finger millet and pearl millet the increase in volume was achieved earlier (6 and 8 h respectively). Addition of finger millet and pearl millet did not alter the bulk density significantly up to 20 h of fermentation.
The viscosity of the finger millet batter (9851.2 cP) and pearl millet batter (9516 cP) were significantly higher prior to fermentation (0 h) compared to the control batter (8851.2 cP) (Fig. 1c, d). The viscosity of all batters decreased as the fermentation progressed and reached similar values at 12 h of fermentation. Subsequently, millet fortified batters showed a rapid decrease in the viscosity although reaching values similar to the control at 24 h.
Texture of fermented idli batter
From Fig. 2a, b it is evident that both firmness and cohesiveness which are measures of force of penetration and withdrawal respectively, increased till 12 h of fermentation in control batter contributed by the acid and gas production by the fermenting microbes. The decrease at later stages of fermentation till 24 h is probably from the increased acidity and loss of gas retention. However, in the presence of millets the values decreased till 8 h and then increased up to 24 h. The higher values of the above parameters in the unfermented fortified batters compared to the control batter suggest changes in texture from the addition of millets which persists through the fermentation. In contrast, the measure of consistency which takes into consideration the force per unit area decreased up to the 12 h in control batter and pearl millet fortified batter, after which an increase was seen. The finger millet batter showed similar changes in consistency although the minimum value was at 8 h. But all batters reached similar consistency both at 20 and 24 h of fermentation. The index of viscosity decreased with increase in fermentation time for all the batters and the changes were prominent after 16 h of fermentation (Fig. 2c, d). According to Soni and Sandhu (1989) spreadability of batter is inversely proportional to the air pockets and directly proportional to the moisture content as increase in air pockets decreased the density of batter.
Fig. 2.
Textural characteristics of control, finger millet and pearl millet idli batters and idlies
Color characteristics
The millet fortified batters showed variation in the L*, a*, b* values when compared to the control batter due to the addition of millets. The lightness decreased from 86.5 to 72.13 in the case of finger millet batter indicating a darker colored batter with slight pinkish tinge (−0.49, 7.20–3.30, 7.73) as shown from the color values given in Table 1a; whereas, pearl millet batter had slight yellowish tinge (−0.49, 7.20–0.12, 10.93). Similar color changes were also observed for the idli with a pinkish and yellowish product prepared from finger millet batter and pearl millet batter respectively rather than the white colored product prepared from conventional idli batter. A previous study by Manickavasagan et al. (2013) on fortified idli batter with date paste and date syrup reported that idli had light brown color.
Table 1.
Color characteristics of millet idli batters and idli and nutritional analysis and sensory scores of millet idlies
| L* | a* | b* | |
|---|---|---|---|
| 1a | |||
| CB | 86.50 | −0.49 | 7.20 |
| FMB | 72.13 | 3.30 | 7.73 |
| PMB | 80.79 | 0.12 | 10.93 |
| CI | 85.91 | −0.20 | 11.36 |
| FMI | 55.8 | 5.98 | 10.57 |
| PMI | 76.43 | 1.01 | 16.72 |
| COI | CI | FMI | PMI | |
|---|---|---|---|---|
| 1b | ||||
| Moisture (%) | 66 | 66 | 65.4 | 66 |
| Carbohydrate (%) | 17.14 | 17.13 | 17.19 | 17.12 |
| Crude protein (%) | 11.72 | 11.89 | 12.06 | 14.93 |
| Fat (%) | 0.17 | 0.19 | 0.32 | 3.1 |
| Ash (%) | 0.22 | 0.21 | 0.34 | 0.38 |
| Total dietary fiber (g/100 g) | 22.08 | 22.23 | 28.47 | 27.32 |
| Soluble fiber (g/100 g) | 7.86 | 7.53 | 20.25 | 19.43 |
| Insoluble fiber (g/100 g) | 14.63 | 14.7 | 12.22 | 7.89 |
| Calcium (mg/100 g) | 26.19 | 26.5 | 56.5 | 41.33 |
| Iron (mg/100 g) | 6.07 | 6.12 | 9.22 | 21.93 |
| CI | FM | PM | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time of fermentation (h) | ||||||||||||||||||||||||
| 0 | 4 | 6 | 8 | 12 | 16 | 20 | 24 | 0 | 4 | 6 | 8 | 12 | 16 | 20 | 24 | 0 | 4 | 6 | 8 | 12 | 16 | 20 | 24 | |
| 1c | ||||||||||||||||||||||||
| Appearance | 8 | 8 | 7 | 2 | 9 | 6 | 7 | 6 | 8 | 8 | 9 | 8 | 2 | 6 | 7 | 6 | 8 | 8 | 6 | 9 | 6 | 2 | 7 | 6 |
| Color | 6 | 6 | 6 | 4 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 4 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 4 | 6 | 6 |
| Texture | 6 | 7 | 6 | 4 | 8 | 7 | 6 | 6 | 6 | 7 | 8 | 7 | 4 | 7 | 6 | 6 | 6 | 7 | 7 | 8 | 7 | 4 | 6 | 6 |
| Flavor | 4 | 6 | 2 | 6 | 7 | 6 | 2 | 5 | 4 | 6 | 7 | 6 | 6 | 6 | 2 | 5 | 4 | 6 | 6 | 7 | 6 | 6 | 2 | 5 |
| Mouthfeel | 6 | 6 | 5 | 2 | 6 | 6 | 5 | 4 | 6 | 6 | 6 | 6 | 2 | 6 | 5 | 4 | 6 | 6 | 6 | 6 | 6 | 2 | 5 | 4 |
| Aftertaste | 4 | 5 | 2 | 6 | 8 | 7 | 2 | 2 | 4 | 5 | 8 | 5 | 6 | 7 | 2 | 2 | 4 | 5 | 7 | 8 | 7 | 6 | 2 | 2 |
Changes in microbial population dynamics in relation to fortification of batters
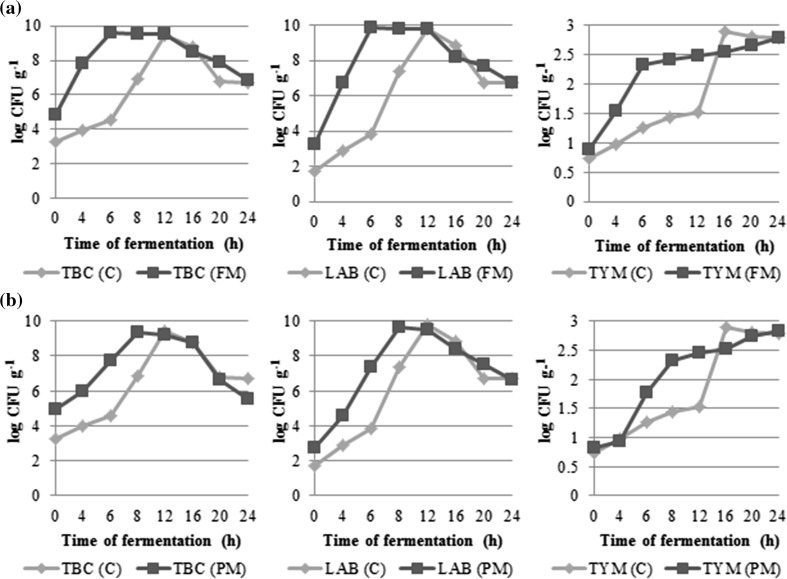
Majority of the microbial population was LAB in idli batter fermentation with a steady increase of LAB counts from 4 to 12 h for the control batter, which was reduced to 6 and 8 h for finger millet and pearl millet batters, respectively. At all points the fortified batters showed higher microbial counts compared to the control. TBC that was higher initially showed similar changes followed by a significant decrease (Fig. 3a, b). The production of hydrogen peroxide and bacteriocins (secondary metabolites) as well as the decrease in pH due to secretion of lactic acid by LAB resulted in unfavourable growth conditions for other groups of microorganisms (non-LAB). Yeasts contributed to the leavening of the batter with a steady increase throughout the 24 h fermentation in all batters and a rapid increase in the finger millet batter. The steady increase of yeast at later stages of fermentation was slower. This in turn affects the CO2 production in idli batter resulting in the higher bulk density in the millet batter than the control batter. Addition of finger millet and pearl millet powder to the unfermented batter resulted in rapid growth of bacteria and yeast and thereby reducing the fermentation time from 12 to 6 h and 12 to 8 h respectively, leading to accelerated fermentation. Reduction in fermentation time of idli batter is of great commercial significance for large-scale idli production and can be potentially achieved by addition of enzymes. Iyer and Ananthanarayan (2008) reported reduction (14–8 h) in fermentation time of idli batter by addition of an exogenous source of α-amylase enzyme. Rekha and Vijayalakshmi (2011) reported higher bacterial counts along with gradual increase in yeast and mould counts in okra fortified idli batter. Ghosh and Chattopadhyay (2011) suggested that black gram, as a source of protein in foods enhances the microbial population. Gobetti et al. (1994) observed an increase in lactic acid bacteria in sourdough due to the presence of amino acids.
Fig. 3.
Microbial changes during 24 h fermentation of control, finger millet and pearl millet idli batters
Nutritional analysis
The proximate composition of the prepared idlies from the batters (commercial, control, finger millet and pearl millet) is given in Table 1b. The carbohydrates content was similar and there was little change in moisture. Finger millet idli had 28.07% higher total dietary fiber content, 168.92% higher soluble dietary fiber, and 113.21% higher calcium content than control idli. While, pearl millet idli had 25.57% higher protein content, 15 fold increase in fat content, 158.03% higher soluble dietary fiber and 258.33% higher iron content than control idli.
Texture properties
Millet idlies were marginally more firm than the control idli but well within the softness range (Fig. 2e, f). The springiness (S) of the control idli increased till 12 h of fermentation and showed a slight decrease at 24 h, whereas springiness of the idli increased till 6 h of fermentation with addition of finger millet and 8 h with addition of pearl millet and maintained until 24 h of fermentation. Rekha and Vijayalakshmi (2011) reported that the hardness of idli decreased with okra substitution during 10 h of fermentation. The yeast growth was responsible for the springiness of the idli. The changes in the ions of the protein network induced by the alteration in the pH during fermentation may reduce the firmness and springiness of the idli.
Sensory analysis
Idli prepared with the addition of millet was preferred in the sensory analysis. However, idli from 6, 8 and 12 h fermentation was preferred most for finger millet, pearl millet and control batters respectively, in terms of appearance, color, texture, mouthfeel, flavour, after-taste (Table 1c) and overall acceptability (Fig. 4a). Although the acceptability was maintained till 16 h fermentation in all batters, decrease in scores was observed for idlies from 20 to 24 h fermentation for the control batter, as it turned sour. Low texture scores were due to loss of gas and decrease in porosity that correlated with high values of firmness and springiness (Fig. 2e, f). Springiness of the idli increased with the addition of millets as well as suppression of the sour taste.
Fig. 4.
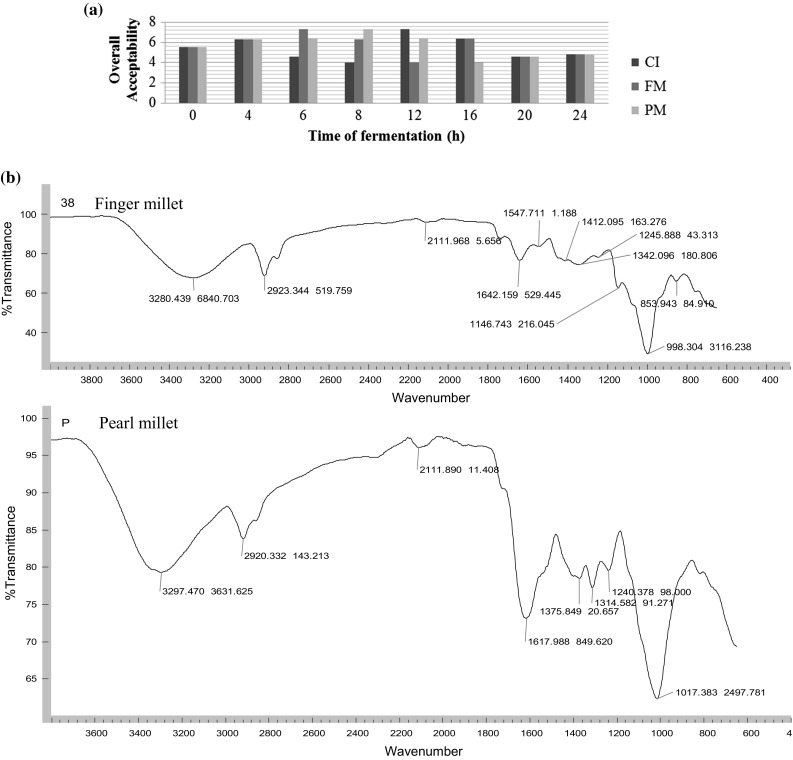
Sensory scores (overall acceptability) of idli prepared from control and millet idli batters and FTIR analysis of millet powders
GC–MS analysis
GC–MS analysis revealed the presence of several compounds in both finger millet (21) and pearl millet (23). Main compounds identified in finger millet were: Hexanoic acid, 2-ethyl-, anhydride (47.74%), Oleic acid (25.21%), 9,12-Octadecadienoic acid (Z,Z)- (5.71%) and Asparagine, N-dl-alanyl-, l- (5.36%). The main compounds in pearl millet were: 14-Pentadecenoic acid (32.43%), 9,12-Octadecadienoic acid (Z,Z)- (26.11%), n-Hexadecanoic acid (15.16%) and 2-Methyl-5-oxohexaethanoic acid, S-t-butyl ester (8.21%). All other compounds were present in significantly lower amounts from 0.03 to 4.87% in finger millet and 0.14 to 5.61% in pearl millet (Table 2). The bioactives in the end product (idli) were also estimated (Table 3). The phytochemicals prevent damage of pancreatic cells apart from oxidative stress relief (Arulselvan and Subramanian 2007).
Table 2.
GC-MS analysis of finger millet and pearl millet extracts
| Finger millet (Eleusine coracana) | Pearl millet (Pennisetum glaucum) | |||||
|---|---|---|---|---|---|---|
| S. No. | Phytochemicals | Area (%) | RT (min) | Phytochemicals | Area (%) | RT (min) |
| 1 | Butyl-tert-butyl-isopropoxyborane | 0.39 | 7.458 | Oxalic acid, cyclohexyl butyl este | 0.39 | 8.633 |
| 2 | 1-Pentanol, 2,2-dimethyl- | 0.63 | 7.805 | 2-Aminononadecane | 0.31 | 24.759 |
| 3 | Oxalic acid, cyclohexyl octyl este | 1.22 | 8.633 | n-Hexadecanoic acid | 15.16 | 25.484 |
| 4 | 1,2-Benzenedicarboxylic acid, butyl 8-methylnonyl ester | 0.5 | 23.553 | 1-Propanamine, N,2-dimethyl- | 0.14 | 25.729 |
| 5 | Propanamide | 4.27 | 25.443 | 2-Methyl-5-oxohexanethioic acid, S-t-butyl ester | 8.21 | 27.189 |
| 6 | Hexanoic acid, 2-ethyl-, anhydride | 47.74 | 27.189 | Nitro-l-arginine | 0.55 | 27.822 |
| 7 | 9,12-Octadecadienoic acid (Z,Z)- | 5.71 | 28.547 | 2-Dimethylaminomethyl-4-chloro-1-naphthol | 0.42 | 27.945 |
| 8 | Oleic acid | 25.21 | 28.65 | 9,12-Octadecadienoic acid (Z,Z)- | 26.11 | 28.599 |
| 9 | 2H-Azepin-2-one, hexahydro-1-methyl- | 0.6 | 28.986 | 14-Pentadecenoic acid | 32.43 | 28.721 |
| 10 | Asparagine, N-dl-alanyl-, l- | 5.36 | 29.078 | Cyclohexane, 1-(1,5-dimethylhexyl)-4-(4-methylpentyl)- | 5.61 | 29.099 |
| 11 | Acetic acid, hydroxy[(1-oxo-2-propenyl)amino]- | 0.03 | 30.253 | 3,3′-Iminobispropylamine | 1.04 | 30.314 |
| 12 | Hexanedioic acid, dioctyl ester | 1.33 | 32.867 | 1-Octadecanamine, N-methyl- | 0.39 | 31.131 |
| 13 | Methylpent-4-enylamine | 0.6 | 33.072 | 2H-Azepin-2-one, hexahydro-1-methyl- | 0.49 | 31.438 |
| 14 | Nitro-l-arginine | 4.18 | 33.347 | Hexanedioic acid, bis(2-ethylhexyl) ester | 0.6 | 32.867 |
| 15 | Benzenemethanol, alpha -(1-aminoethyl)-, (R*,R*)-( ± )- | 0.67 | 34.236 | Heptadecane | 0.85 | 33.072 |
| 16 | No matches found | 12.69 | 34.44 | 1-Propanamine, N1-methyl-2-methoxy | 1.06 | 33.358 |
| 17 | 2H-Azepin-2-one, hexahydro-1-methyl- | 4.87 | 34.583 | dl-Phenylephrine | 0.53 | 34.216 |
| 18 | Eicosane | 3.55 | 34.92 | 1,2-Benzenediol, 4-(2-amino-1-hydroxypropyl)- | 0.74 | 34.358 |
| 19 | 1,4-Benzenedicarboxamide, N,N′-bis(2-hydroxy-1-methyl-2-phenylethyl) | 0.43 | 35.032 | Benzenemethanol, 3-hydroxy- alpha-[(methylamino)methyl]-, (R)- | 0.74 | 34.481 |
| 20 | Eicosane | 1.15 | 35.216 | Heptadecane | 2.24 | 34.593 |
| 21 | Phenol, 4-(2-aminopropyl)-, ( ± )- | 0.49 | 35.41 | 2-(5-Aminohexyl)furan | 0.64 | 34.93 |
| 22 | 2-Oxo-3-methyl-cis-perhydro-1,3-benzoxazine | 0.27 | 34.961 | |||
| 23 | Eicosane | 1.07 | 35.227 | |||
Table 3.
GC-MS analysis of finger millet and pearl millet idlies
| Finger millet idli | Pearl millet idli | |||||
|---|---|---|---|---|---|---|
| S. No. | Phytochemicals | Area (%) | RT (min) | Phytochemicals | Area (%) | RT (min) |
| 1 | Propane, 1,1,3-diethoxy | 6.77 | 4.98 | Propane, 1,1,3-diethoxy | 5.31 | 4.98 |
| 2 | Ethylbenzene | 3.27 | 2.76 | Hexane, 2-nitro- | 0.57 | 7.458 |
| 3 | Pentane, 1,1-diethoxy | 2.31 | 3.55 | Oxalic acid, cyclohexyl octyl este | 1.22 | 8.633 |
| 4 | Propane, 1,1,3-diethoxy | 6.26 | 4.98 | 2-Pentanone | 0.27 | 10.2 |
| 5 | Oxalic acid, cyclohexyl octyl este | 1.22 | 8.633 | 2-Hexanone | 0.61 | 11.4 |
| 6 | 2-Pentanone | 0.02 | 10.2 | Ethanone | 0.82 | 13.8 |
| 7 | 3-Trifluoroacetoxypentadecane | 4.73 | 10.75 | 3-Trifluoroacetoxypentadecane | 4.73 | 10.75 |
| 8 | 2-Hexanone | 0.32 | 11.4 | Propanal | 0.1 | 21.2 |
| 9 | Ethanone | 0.6 | 13.8 | 3-Methylbutanal | 0.08 | 22.8 |
| 10 | 3-0-Methyl-d-glucose | 61.22 | 14.07 | 3-0-Methyl-d-glucose | 53.74 | 14.07 |
| 11 | Phthalic acid, butyl isohexyl ester | 4.41 | 15.83 | Phthalic acid, butyl isohexyl ester | 4.02 | 15.83 |
| 12 | Ethanol, 2-(9-octadecenyloxy) | 3.19 | 16.19 | Ethanol, 2-(9-octadecenyloxy) | 2.89 | 16.19 |
| 13 | Propanal | 0.1 | 21.2 | 9-Decenal | 0.4 | 23 |
| 14 | 3-Methylbutanal | 0.08 | 22.8 | n-Hexadecanoic acid | 5.51 | 25.443 |
| 15 | 9-Decenal | 0.4 | 23 | Decanoic acid | 0.11 | 26.89 |
| 16 | n-Hexadecanoic acid | 6.36 | 25.443 | 14-Pentadecenoic acid | 16.05 | 28.721 |
| 17 | Decanoic acid | 0.11 | 26.89 | 9,12-Octadecadienoic acid (Z,Z)- | 5.71 | 28.547 |
| 18 | Hexanoic acid, 2-ethyl-, anhydride | 16.22 | 27.189 | 2-Ethylacridine | 1.02 | 28.655 |
| 19 | 9,12-Octadecadienoic acid (Z,Z)- | 5.71 | 28.547 | 6-Octadecenoic acid, (Z)- | 2.67 | 28.67 |
| 20 | 2-Ethylacridine | 1.02 | 28.655 | Asparagine, N-dl-alanyl-, l- | 4.78 | 29.078 |
| 21 | Cis-9-Octadecenoic acid, (Z)- | 33 | 28.65 | 2-Ethylacridine | 1.02 | 28.655 |
| 22 | Asparagine, N-dl-alanyl-, l- | 5.36 | 29.078 | Octadecanoic acid | 0.45 | 29.07 |
| 23 | Octadecanoic acid | 0.45 | 29.07 | Hexadecanoic acid | 0.61 | 31.9 |
| 24 | Hexadecanoic acid | 0.61 | 31.9 | Undecanoic acid | 0.81 | 32.1 |
| 25 | Undecanoic acid | 0.81 | 32.1 | Nitro-l-arginine | 4.11 | 33.347 |
| 26 | Nitro-l-arginine | 4.18 | 33.347 | 2H-Azepin-2-one, hexahydro-1-methyl- | 4.74 | 34.583 |
| 27 | 2H-Azepin-2-one, hexahydro-1-methyl- | 4.87 | 34.583 | Eicosane | 3.05 | 34.92 |
| 28 | Eicosane | 3.55 | 34.92 | 3-Propoxyamphetamine | 0.52 | 34.93 |
| 29 | Tetratriacontane | 1.15 | 35.216 | Tetratriacontane | 2.04 | 35.216 |
| 30 | 1,4-Benzenedicarboxamide, N,N′-bis(2-hydroxy-1-methyl-2-phenylethyl) | 0.43 | 35.032 | 2H-Azepin-2-one, hexahydro-1-methyl- | 0.3 | 35.421 |
FTIR, SEM and ink-print analysis
Both the millet powders showed the presence of alcohol, alkanes, aldehydes, aromatic amines and halogen compounds in the FTIR analysis (Fig. 4b).
Variation in the particle size was observed during fermentation due to the microbial activity as seen from the SEM analysis of the batters and idli (Fig. 5a). In case of control batter, size reduction of 34% was observed which may be due to the enzymatic activity of the microbes during fermentation for 12 h. Size reduction was also observed earlier in the millet idlies at 6 and 8 h of fermentation for finger millet and pearl millet, respectively.
Fig. 5.
SEM analysis of control and millet idli batters and ink print test for idlies prepared from control and millet idli batters
The softness of the idli was indicated from the number of pores per square centimeter observed in ink-print test (Fig. 5b). The millet idlies had higher number of pores at their optimum fermentation times when compared to the control indicating a rapid increase in the microbial activity during fermentation. The distribution of air pockets is an indicator of the textural difference in the idli samples.
Conclusion
The best quality of idli in terms appearance, color, texture, flavor and taste was achieved with 12 h fermentation in the control idli batter with a pH of 4.5 ± 0.2. The batter prepared by using 10% of finger millet showed 6 h reduction in fermentation time and pearl millet at 10% showed 4 h reduction in fermentation time due to acceleration of fermentation of idli batter. The incorporation of both finger millet and pearl millet in the idli batter increased the nutrient content of the idli particularly soluble fiber, calcium and iron as well as it increased the flavor, aroma and appealing qualities of the idli. Reduction in the fermentation time of the idli batter is of great commercial significance for large-scale idli production and has been potentially achieved by addition of millet.
References
- AOAC . Official methods of analysis of AOAC International. Maryland: AOAC; 2000. [Google Scholar]
- Arulselvan P, Subramanian SP. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic β-cells in experimental diabetes in rats. Chem-Biol Interact. 2007;165:155–164. doi: 10.1016/j.cbi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Chattopadhyay P. Preparation of idli batter, its properties and nutritional improvement during fermentation. J Food Sci Technol. 2011;48:610–615. doi: 10.1007/s13197-010-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobetti M, Corsetti A, Rossi J. The sourdough microflora. Interactions between lactic acid bacteria and yeasts: metabolism of amino acids. World J Microbiol Biotechnol. 1994;10:275–279. doi: 10.1007/BF00414862. [DOI] [PubMed] [Google Scholar]
- Iyer BK, Ananthanarayan L. Effect of α-amylase addition on fermentation of idli: a popular south Indian cereal – Legume-based snack food. LWT-Food Sci Technol. 2008;41:1053–1059. doi: 10.1016/j.lwt.2007.07.004. [DOI] [Google Scholar]
- Jama YH, Varadaraj MC. Antibacterial effect of plantaricin LP84 on foodborne pathogenic bacteria occurring as contaminants during idli batter fermentation. World J Microbiol Biotechnol. 1999;15:27–32. doi: 10.1023/A:1008887201516. [DOI] [Google Scholar]
- Kk NM, Narmadha K, Gowri R, Baskar V. Review on incorporating pearl millet (Pennisetum glaucum) in the preparation of carbohydrate-based idly batter. J Biol Inf Sci. 2013;2:1–2. [Google Scholar]
- Manickavasagan A, Mathew TA, Al-Attabi ZH, Al-Zakwani IM. Dates as a substitute for added sugar in traditional foods: a case study with idli. Emir J Food Agric. 2013;25:899–906. doi: 10.9755/ejfa.v25i11.14920. [DOI] [Google Scholar]
- Nazni P, Shalini S. Standardization and quality evaluation of idli prepared from pearl millet (Pennisetum Glaucum) Int J Curr Res. 2010;5:84–87. [Google Scholar]
- Nisha P, Ananthanarayan L, Singhal RS. Effect of stabilizers on stabilization of idli (traditional south Indian food) batter during storage. Food Hydrocoll. 2005;19:179–186. doi: 10.1016/j.foodhyd.2004.03.007. [DOI] [Google Scholar]
- Rati Rao E, Vijayendra SVN, Varadaraj MC. Fermentation biotechnology of traditional foods of the Indian subcontinent. In: Shetty K, Paliyath G, Pometto A, Levin RE, editors. Food Biotechnology. 2. Florida: CRC Taylor and Francis; 2006. pp. 1759–1794. [Google Scholar]
- Rekha CR, Vijayalakshmi G. Accelerated fermentation of ‘idli’ batter using soy residue okara. J Food Sci Technol. 2011;48:329–334. doi: 10.1007/s13197-011-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DN, Perez-Maldonado R. Pearl millet as an alternative feed grain for pigs and poultry. Asia Pac J Clin Nutr. 2003;12:62–64. [Google Scholar]
- Soni SK, Sandhu DK. Nutritional improvement of Indian dosa batters by yeast enrichment and black gram replacement. J Ferment Bioeng. 1989;68:52–55. doi: 10.1016/0922-338X(89)90214-6. [DOI] [Google Scholar]
- Steinkraus K. Handbook of indigenous fermented foods. New York: Marcel Dekkar; 1995. [Google Scholar]
- Thyagaraja N, Otani H, Hosono A. Studies on microbiological changes during the fermentation of Idly. Lebensm Wiss Technol. 1992;25:77–79. [Google Scholar]