Abstract
Abstract
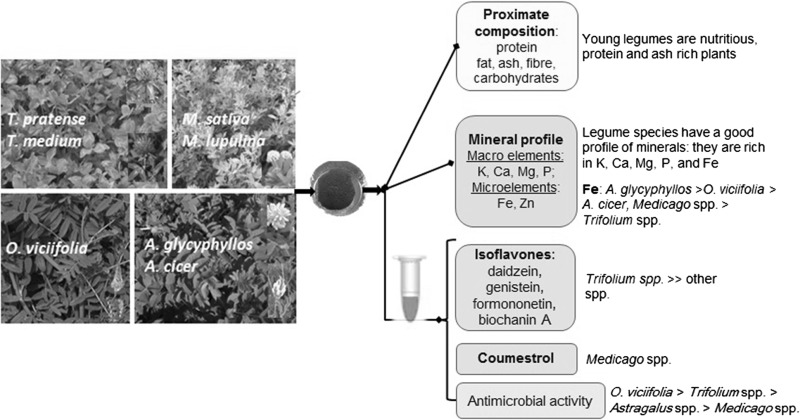
Perennial legumes have been used as edible or medicinal plants since ancient times. The focus of the current study are perennial legumes—Trifolium pratense L., T. medium L., Medicago sativa L., M. lupulina L., Onobrychis viciifolia Scop., Astragalus glycyphyllos L. and A. cicer L.—of branching stage as a potential source of value-added ingredients for healthy food. Freeze-dried samples were analysed for proximal composition, mineral, isoflavone and coumestrol contents as well as for antimicrobial activity. Legumes were protein-rich (23.0/100 g on average). Mineral contents in 100 g of plant dry matter averaged: K 2.64 g, Ca 1.81 g, Mg 0.475 g, P 0.324 g, Zn 2.76 mg and Fe 37.8 mg. According to the total amount of phytoestrogens, the species ranked as follows: T. medium (34.4 mg/g) ≫ T. pratense ≫ O. viciifolia ≥ M. sativa = A. cicer = M. lupulina ≥ A. glycyphyllos (0.207 mg/g). Extracts of legumes, especially that of O. viciifolia, exhibited noticeable potency to inhibit the growth of Gram-positive and Gram-negative bacteria. Perennial legumes of branching stage can be used as protein, mineral and phytoestrogen rich source for food ingredients and supplements.
Graphical Abstract
Keywords: Perennial legumes, Nutritious value, Minerals, Iron, Phytoestrogens, Antimicrobial activity
Introduction
Many legumes, generally pulses, are valuable components of staple and functional foods (Bouchenak and Lamri-Senhadji 2013; Prati et al. 2007; Singh 2017). They are characterized by high nutritional value, abundance of minerals and secondary metabolites. However, not only pulses but also some perennial legumes have been consumed as edible or medicinal plants since ancient times, although the composition of their nutritious substances or existence of bioactive compounds was unknown. Literature data confirm that leaves, young plants (shoots) and flowers of different genera and species of perennial legumes have been used in food or phytotherapy in different countries and ages (Chavan et al. 2015; Lim 2014; Small 2011). Nowadays, leaves and seeds of some species (e.g. alfalfa, red clover) are sold as bulk powdered herb, capsules, and tablets for nutritional supplement in health food stores (Silva et al. 2013). Potential health benefits of pulses and other legumes are associated with the presence of different phenolic compounds, including phytoestrogens (Prati et al. 2007; Singh et al. 2017). Literature sources confirmed that phytoestrogens are associated with a reduction in osteoporosis, cardiovascular disease, prevention of cancer, exhibit antidiabetic effect and relieve menopausal symptoms (Bouchenak and Lamri-Senhadji 2013; Pilšáková et al. 2010). For wellbeing, humans need to get about 25 minerals with food (White and Brown 2010). The deficiency of various minerals in different parts of the world led to cardiovascular disease and imbalance in majority of the biological pathways (Singh 2017). Therefore, mineral profile is an important attribute of nutraceuticals, alimentary products and food ingredients. Microorganisms are the cause of many infectious diseases in both immunosuppressed and immunocompetent individuals (Santos et al. 2012). Hence, antimicrobial activity is a very important trait for plant material examined as a source of nutraceuticals or functional ingredients for food.
Besides red clover (Trifolium pratense L.) and alfalfa (Medicago sativa L.), which have been more thoroughly examined for the aforementioned aspects, we involved in the investigation the species that had not been studied before or studied only scarcely: zigzag clover (T. medium L.), black medick (M. lupulina L.), liquorice milkvetch, cicer milkvetch (Astragalus glycyphyllos L., A. cicer L.) and sainfoin (Onobrychis viciifolia Scop.). Understanding that perennial fabaceous plants cannot become staples, we investigated their potential as medicinal plants and food ingredients (e.g. for salads, soups, stews, beverages, etc.) that could provide particular functional properties to staple diets. Therefore, the goal of the current study was to examine seven species of perennial legumes for proximate, mineral and isoflavone profiles as well as for coumestrol content and antimicrobial activity of plant extracts.
Materials and methods
Plant material
The species chosen for the study represent 7 cultivars (cv.) or wild ecotypes (WE) of legumes from the genera clover, medick, sainfoin and milkvetch (Table 1).
Table 1.
The list of germplasm collection of the perennial legumes spp. studied for nutritional, mineral composition and phytoestrogens
| Tribe | Trifolieae | Hedysareae | Galegeae | ||||
|---|---|---|---|---|---|---|---|
| Genus | Clover (Trifolium) | Medick (Medicago) | Sainfoin (Onobrychis) | Milkvetch (Astragalus) | |||
| Species | Trifolium pratense | Trifolium medium | Medicago sativa | Medicago lupulina | Onobrychis viciifolia | Astragalus glycyphyllos | Astragalus cicer |
| Accession | cv. Sadūnai | WE | cv. Malvina | cv. Arka | cv. Meduviai | WE | WE |
| Notation | Tpr | Tme | Msa | Mlu | Ovi | Agl | Aci |
WE Wild ecotype, cv cultivar
The germplasm collection was established in a field trial in 2014 in the Central Lowland of Lithuania (55°23′49″N; 23°51′40″E), at the Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry. The seeds of the species were sown in one row (2.5 m long and 0.5 m apart) in 4 replications. No herbicides were applied in the collection nursery.
For chemical analyses, plant samples were collected at branching stage. The samples were washed thoroughly with tap water, rinsed with distilled water and blotted on filter paper. Then they were freeze-dried and ground to pass a 1-mm screen. The samples were processed on the same day they were harvested.
Proximate analysis
Samples were analyzed for protein, fat, crude fiber, ash, and digestible (total) carbohydrate contents according to the methods described by Owusu-Apenten (2004). Crude protein was determined by the Kjeldahl method with a conversion factor of 6.25; crude fat—gravimetrically by the continuous Soxhlet extraction with hexane. Crude fiber was estimated by acid/alkaline hydrolysis of insoluble residues. Crude ash content was determined as the mass left after sample incineration at (550±10) °C. Total carbohydrate content was estimated by difference remaining after subtracting the contents of crude fiber, protein, fat and ash. Additionally, plant material was analyzed for soluble sugars and starch. Concentrations of soluble sugars in 40% ethanolic extracts after washing with 80% ethanol and water were measured spectrophotometrically (M107, Camspec, UK) using the anthrone reagent (Zhao et al. 2010). Starch was determined in plant material residue after SS washing. Remaining plant material was solubilized and hydrolyzed to glucose using enzymes α-amylase and amyloglucosidase and released glucose was assayed following the general procedures described by Zhao et al. (2010). Data of proximate analysis were expressed in g of nutritional component per 100 g on a dry matter basis (g/100 g).
Determination of minerals
Concentrations of potassium, sodium, calcium, magnesium, zinc and iron were quantified after the nitric acid plus hydrogen peroxide digestion (Leśniewicz et al. 2006) by flame atomic absorption spectroscopy (AAS) using a Perkin Elmer model AAnalyst 200 (US). Parameters of the instrument were chosen in accordance with the manufacturer’s instructions. Total phosphorous was determined after sulfuric acid digestion of the samples and reaction with molybdate‐vanadate. The absorbance was measured by UV–V spectrophotometer (Cary50, Varian, US) at 430 nm. Mineral content was expressed as g of macro-element and mg of micro-element per 100 g on a dry matter basis (g/100 g and mg/100 g, respectively).
Quantification of phytoestrogens
Biochanin A (≥98%), daidzein (≥98%), formononetin (≥99%), genistein (≥98%) and coumestrol (≥95%) were purchased from Sigma-Aldrich (St. Louis, US). LC–MS grade methanol and LC–MS grade acetic acid were obtained from Fluka (Sigma-Aldrich). All other reagents of ACS purity were purchased from Sigma-Aldrich. Water was obtained from a Milli-Q water purification unit (Millipore, Bedford, MA).
The stock standard solutions of isoflavones at 250 mg/L were prepared in aqueous methanol (1:1, v/v) and stored in the dark at 4 °C. The working standard solutions were prepared daily by dilution of the stock solutions with the aqueous methanol.
Free aglycones were released from their glycoside derivatives by acid hydrolysis. Both, hydrolysis and extraction were performed in a single step according to Saviranta et al. (2008), with minor modification (Lemežienė et al. 2015). Before chromatographic analysis, extracts of both clovers T. pratense and T. medium were diluted with aqueous methanol (1:1, v/v).
The four isoflavones (daidzein, genistein, and their 4′-methylated derivatives: formononetin and biochanin A) and coumestrol were quantified by ultra-performance liquid chromatography (UPLC) using a Waters Acquity UPLC system (Waters, Milford, US) equipped with diode array detector (DAD). Isoflavones and coumestrol were detected at 260 and 343 nm, respectively. Data were collected and managed using the HyStar 3.2 software (Bruker). Analytes in the extracts were identified according to our recently published procedure (Lemežienė et al. 2015; Taujenis et al. 2016). Quantification was performed by external calibration and the results were expressed in mg per 1 g of the dry matter (mg/g). Analyte standards were prepared covering a concentration range up to 100 mg/L (for coumestrol up to 50 mg/L) with seven levels and three replicates at each level. A relationship between peak area and concentration was linear with the correlation coefficient R ≥ 0.9989 for each of the four isoflavones and coumestrol. The limits of quantification (LOQ), defined as the concentration resulting in a signal of ten times the noise level, were 0.15 mg/L (0.006 mg/g) for biochanin A and formononetin, 0.20 mg/L (0.008 mg/g) for genistein and coumestrol, and 0.25 mg/L (0.010 mg/g) for daidzein.
Evaluation of antimicrobial properties
Freeze-dried plant material (15 g) was extracted sequentially with 150 mL of 70% ethanol using an ultrasonic bath for 30 min at 50 °C. The extracts were filtered through Whatman (No. 1) filter paper in sterile bottles. The filtrates were concentrated, then evaporated to dryness by placing them in a water bath at 50 °C. The concentrated crude extracts were then stored in the dark at 4–8 °C until use.
The ethanolic extracts of legume plant material were assayed for the in vitro antimicrobial activity against reference strains of 10 microbial species: Gram-positive no-spore forming bacteria Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228 and Enterococcus faecalis ATCC 29212; Gram-positive spore-forming bacteria Bacillus cereus ATCC 11778 and Bacillus subtilis ATCC 6633; Gram-negative bacteria Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 13883, Pseudomonas aeruginosa ATCC 27853, Proteus vulgaris ATCC 8427 and yeast Candida albicans ATCC 10231.
The antibacterial activity of plant extracts was evaluated as a minimum inhibitory concentration (MIC) by using an agar dilution technique (CLSI 2012) in the Mueller–Hinton agar (Becton, Dickinson and Company).
Test bacteria reference strains were cultivated at 37 °C for 18–20 h in a Trypticase Soy Agar (BBL, Cockeysville, USA) and C. albicans at 25 °C for 48 h—in a Sabouraud Dextrose Agar (BBL, Becton, Dickinson and Company). Cultures of test microorganisms were washed from the agar surface with 0.9% NaCl solution. The obtained suspensions were standardized using the 0.5 McFarland standards.
The concentrated plant extracts were dissolved in 3 mL of 30% ethanol. The dissolved extract was serially diluted with 30% ethanol in seven tubes using the dilution ratio 2:1. One millilitre of the solution from each tube was mixed with 9 mL of warmed-up to 45 °C Mueller–Hinton agar and poured into 9 cm diameter sterile petri dishes. The bottom of dishes was divided into 10 segments. Each segment was inoculated with the suspension of the reference strains of 10 microbial species.
The 30% (v/v) ethanol was used as positive control and 96% (v/v) ethanol—as negative control. The antimicrobial effect of the investigated solutions on bacterial culture was evaluated after 24 h of incubation at a temperature of 37 °C, and its effect on C. albicans—after 24–48 h after cultivation at a temperature of 25 °C. The lowest concentration of the extract that inhibits the growth of microorganism was determined as the MIC and expressed in mg/mL. MIC analyses were duplicated.
Statistical analysis
The experimental data were statistically processed using software Statistica 7.0 for Windows (StatSoft Inc., US). One-factor repeated-measures ANOVA was performed to evaluate the significance of differences among the species for the character tested. Analysis of variance (ANOVA) followed by Duncan’s test was carried out to test for simple main differences among species. All results are the means of three replicate determinations ± standard deviations (SD) and expressed on a dry matter (DM) basis.
Results and discussion
Proximate composition
The proximate composition of the legume species is presented in Table 2. All plants at branching stage were protein-rich (22.7/100 g on average). The highest protein content was determined in the plants of A. cicer (26.3/100 g) and M. sativa (24.6/100 g). Zigzag clover and sainfoin contained less protein (19.6 and 20.3/100 g, respectively). Ash content was relatively high with an average value of 10.4/100 g and ranged from 8.31/100 g for O. viciifolia to 11.1–11.4/100 g for Astragalus species. On average, the plants contained up to 15.3/100 g of readily digestible carbohydrates, composed of starch on a par with soluble sugars. A. cicer and Medicago species accumulated more starch than sugars. Both red and zigzag clovers as well as sainfoin had the lowest starch concentration (6.67, 7.19 and 6.20/100 g, respectively). Plants of zigzag clover and sainfoin accumulated more soluble sugars (>8.0/100 g DM) than the other legumes tested. The fat and crude fiber content was less variable among proximal characters. Fat ranged from 4.73/100 g in M. sativa to 6.15/100 g in T. pratense plants. Crude fiber varied from 12.3/100 g for M. lupulina to 15.7/100 g for T. pratense.
Table 2.
The proximate composition of perennial legumes (g/100 g)
| Species | Ash | Protein | Fat | Crude fiber | Total carbohydrates | Soluble sugars | Starch |
|---|---|---|---|---|---|---|---|
| Tpr | 11.0 ± 0.07c | 23.0 ± 0.04b | 6.15 ± 0.80b | 15.7 ± 1.44d | 44.1 ± 0.75a | 6.46 ± 0.45b | 6.67 ± 0.72a |
| Tme | 9.80 ± 0.11b | 19.6 ± 0.78a | 5.05 ± 1.12ab | 15.3 ± 0.93bcd | 50.3 ± 2.93bc | 8.79 ± 0.37e | 7.19 ± 0.20ab |
| Msa | 10.8 ± 0.18c | 24.6 ± 0.85c | 4.73 ± 0.14a | 15.3 ± 0.71bcd | 44.4 ± 1.89a | 5.58 ± 0.06a | 8.70 ± 1.00c |
| Mlu | 9.92 ± 0.04b | 22.9 ± 0.78b | 5.30 ± 0.07ab | 12.3 ± 0.55a | 49.6 ± 1.22bc | 7.41 ± 0.19c | 10.42 ± 0.70d |
| Ovi | 8.31 ± 0.01a | 20.3 ± 0.32a | 5.08 ± 0.57ab | 12.9 ± 0.19ab | 53.4 ± 0.69c | 8.20 ± 0.26de | 6.20 ± 0.45a |
| Agl | 11.1 ± 0.01c | 22.1 ± 0.35b | 5.09 ± 0.13ab | 15.5 ± 0.56 cd | 46.2 ± 0.80ab | 7.95 ± 0.06cd | 7.48 ± 0.56abc |
| Aci | 11.4 ± 0.04d | 26.3 ± 0.07d | 5.00 ± 0.55ab | 13.8 ± 1.57abcd | 43.5 ± 1.90a | 7.53 ± 0.43cd | 8.45 ± 0.13bc |
| Mean | 10.3 | 22.7 | 5.20 | 14.4 | 47.4 | 7.42 | 7.87 |
| LSD 05 | 0.140 | 0.909 | 0.808 | 1.61 | 2.58 | 0.421 | 0.873 |
| LSD 01 | 0.213 | 1.377 | 1.22 | 2.44 | 3.90 | 0.637 | 1.323 |
| P | 3 × 10−4 | 2 × 10−5 | 0.291 | 0.067 | 5 × 10−3 | 2 × 10−4 | 2.7 × 10−3 |
Means followed by the same letter in a column do not differ significantly by the Duncan’s multiple range test (< 0.05)
P Probability level of differences among species for component concentration
LSD 05 and LSD 01 show significant differences from mean at P < 0.05 and P < 0.01
Overall proximate composition for alfalfa, red clover and sainfoin agrees with the values presented in the datasheets of Feedipedia, which include systematized chemical composition and nutritional value of worldwide forage legumes (Feedipedia 2016). Reliable data on proximate composition for A. cicer and M. lupulina are limited and for T. medium and A. glycyphyllos are not available currently.
Since in this study we assessed young plants, assuming that they could be used in foods as components of salads, soups, stews or infusions, the proximate as well as further mineral and phytoestrogen composition of perennial legumes was compared with the respective composition of some plants traditionally used in food. The data from the different literature cited (Chongtham et al. 2011; Finglas et al. 2015; Hedges and Lister 2005) were recalculated into 100 g DM if the original source indicated otherwise. In summary, these literary sources suggest that in spinach, lettuce, parsley, Chinese cabbage protein content was comparable to that we found in our plant material. Amaranthus leaves, broccoli, watercress, rucola, rocket, wheat germ were noticeably protein-richer (30–50/100 g), cauliflowers, courgette, mint contained slightly more (27–30/100 g) protein than legumes in our study. However, the largest group of very important vegetables contained appreciably less protein. Only a few of the vegetables and potherbs surpassed legumes by contents of fat and ash. It is difficult to compare the values of fiber and carbohydrates because different methods have been applied in each specific case.
Mineral composition
Young plants of perennial legume species have a rich profile of minerals. The variation degree in mineral composition in legumes depends on the mineral and plant species (Table 3). Zinc concentrations were similar-sized and ranged from 2.33 mg/100 g in black medick to 3.04 mg/100 g in red clover and cicer milkvetch. Legumes differed at P < 10−4 in the concentration of potassium (2.11/100 g in sainfoin; 2.96/100 g in cicer milkvetch). Depending on the legume species, P concentration varied from 0.278 to 0.376/100 g, Ca ranged from 1.43 to 2.05/100 g and Mg—from 0.362 to 0.580/100 g. Sainfoin showed one of the most indigent mineral compositions among the legumes studied: plants contained the least concentration of ash (8.31/100 g, Table 1), K, Ca and Mg (2.11, 1.43 and 0.362/100 g, respectively, Table 2), nearly the lowest P (0.298/100 g) and Zn contents (2.64 mg/100 g). Summarized data of different scientific publications in datasheets of Feedipedia (Feedipedia 2016) support our observations concerning consistently lower content of ash and some macro elements in sainfoin than that in other legumes. Red clover proved to be one of the macro minerals-richest accessions among the tested ones, containing high K, Ca and Mg concentration; however, P concentration was low. Two Astragalus species were distinguished for abundance of different elements: A. cicer for high K, P and Zn concentrations, whereas A. glycyphyllos for richness in Mg and Fe. Our results on mineral contents of the selected legumes show only minor differences when compared with literature data (Feedipedia 2016). Some dissimilarity might be related to the pedoclimatic conditions, genotypic peculiarities, plant maturity, and eventually to analytical techniques. However, literary data on the mineral composition mostly concern red clover, alfalfa, less frequently sainfoin and cicer milkvetch; and studies for A. glycyphyllos, T. medium and M. lupulina are very scanty or inaccessible.
Table 3.
The concentration of minerals in whole aerial plant part of legumes at branching stage
| Species | K (g/100 g) | Ca (g/100 g) | Mg (g/100 g) | P (g/100 g) | Zn (mg/100 g) | Fe (mg/100 g) |
|---|---|---|---|---|---|---|
| Tpr | 2.84 ± 0.12d | 1.90 ± 0.01cd | 0.580 ± 0.024c | 0.302 ± 0.030a | 3.04 ± 0.42b | 19.0 ± 1.03a |
| Tme | 2.17 ± 0.05a | 2.05 ± 0.03d | 0.507 ± 0.012bc | 0.278 ± 0.023a | 2.98 ± 0.02ab | 23.2 ± 0.30a |
| Msa | 2.66 ± 0.13bc | 1.81 ± 0.08bc | 0.409 ± 0.015a | 0.355 ± 0.038bc | 2.33 ± 0.17a | 30.7 ± 0.24b |
| Mlu | 2.59 ± 0.01b | 1.77 ± 0.16bc | 0.431 ± 0.009ab | 0.347 ± 0.017bc | 2.72 ± 0.33ab | 38.9 ± 0.58c |
| Ovi | 2.11 ± 0.05a | 1.43 ± 0.06a | 0.362 ± 0.020a | 0.298 ± 0.021a | 2.64 ± 0.06ab | 46.3 ± 3.09d |
| Agl | 2.76 ± 0.03cd | 1.50 ± 0.08a | 0.546 ± 0.021c | 0.315 ± 0.007ab | 2.60 ± 0.22ab | 69.0 ± 3.47e |
| Aci | 2.96 ± 0.13e | 1.63 ± 0.04ab | 0.408 ± 0.035a | 0.376 ± 0.014c | 3.04 ± 0.35b | 37.6 ± 0.55c |
| Mean | 2.59 | 1.73 | 0.464 | 0.324 | 2.76 | 37.8 |
| LSD 05 | 0.071 | 0.131 | 0.051 | 0.027 | 0.455 | 3.13 |
| LSD 01 | 0.108 | 0.199 | 0.077 | 0.042 | 0.690 | 4.74 |
| P | 8 × 10−5 | 0.0024 | 0.0033 | 0.0098 | 0.2505 | 3 × 10−6 |
Means followed by the same letter in a column do not differ significantly by the Duncan’s multiple range test (< 0.05)
P Probability level of differences among species for mineral concentration
LSD 05 and LSD 01 show significant differences from mean at P < 0.05 and P < 0.01
In regard to Ca, Mg and, particularly Fe concentration, perennial legumes are ahead of many plants widely used as condiments, medicinal plants and, certainly, as staple food sources (Chongtham et al. 2011; Finglas et al. 2015; Hedges and Lister 2005). Currently, the most common micronutrients targeted are zinc and especially iron due to the high prevalence of deficiencies of these micronutrients among children under the age of 5 and women of childbearing age (La Frano et al. 2014). Therefore, we extended the discussion of our results on the Fe distribution in perennial legumes compared with traditional food sources and products. The herbage of legumes showed quite high Fe concentration (36.0 mg/100 g on average per trial); however, we revealed the significant differences (at P < 10−5) among species in Fe content: from 19.0 mg/100 g in T. pratense to 69.0 mg/100 g in A. glycyphyllos. Despite such large differences, perennial legumes, even the accessions containing less iron than an average per trial (Trifolium and Medicago species) are good sources of this micronutrient. Iron concentration in A. glycyphyllos, O. viciifolia and A. cicer plants at branching was higher than that in plants widely used as food components or in many medicinal plants (Özcan and Akbulut 2008) and incomparably much more than in traditional food sources (Chongtham et al. 2011; Finglas et al. 2015; Hedges and Lister 2005). According to the list of priorities of dietary supplement constituents (Dwyer et al. 2006), Ca, K, Mg, Zn and Fe are classified as components of the highest priority. As a result, young plants of perennial legumes can be considered as a potential source for fortification of staple foods with minerals.
Phytoestrogens
Numerous differences in both the qualitative and quantitative pattern of phytoestrogen composition among the species of legumes at branching stage were observed (Table 4). T. pratense and especially T. medium were distinguished for their abundance in isoflavones—19.9 and 34.4 mg/g, respectively. Despite the tremendous quantitative variation among the accessions of Fabaceae, formononetin was a dominant isoflavone (0.097–14.9 mg/g) and it was accompanied with comparable concentrations of biochanin A (0.0824–16.0 mg/g) in all entries investigated. There were only traces (below the LOQ value) of daidzein in extracts of Medicago, Onobrychis and Astragalus entries. As regards genistein, its presence was determined not only in Trifolium species (0.627 and 3.30 mg/g) but also in O. viciifolia and A. glycyphyllos, though in small concentrations (0.0134 and 0.0231 mg/g, respectively). Apart from formononetin and biochanin A, Medicago species contained another estrogenic compound belonging to coumestans—coumestrol (0.030 and 0.0326 mg/g). According to the total amount of phytoestrogens quantified (four isoflavones and coumestrol), the species ranked as follows: T. medium (34.4 mg/g) ≫ T. pratense (19.6 mg/g) ≫ M. lupulina (0.259 mg/g) = O. viciifolia (0.251 mg/g) = A. cicer (0.247 mg/g) ≥ M. sativa (0.239 mg/g) ≥ A. glycyphyllos (0.207 mg/g).
Table 4.
The concentration of isoflavones and coumestrol (mg/g) in perennial legumes
| Species | Isoflavone concentration | Coumestrol | Sum of phytoestrogens | |||
|---|---|---|---|---|---|---|
| Formononetin | Biochanin A | Daidzein | Genistein | |||
| Tpr | 9.27 ± 0.307 | 9.59 ± 0.257 | 0.123 ± 0.0108 | 0.627 ± 0.036 | <LOQ | 19.6 |
| Tme | 16.0 ± 0.356 | 14.9 ± 0.356 | 0.172 ± 0.0127 | 3.30 ± 0.138 | <LOQ | 34.4 |
| Msa | 0.097 ± 0.007 | 0.112 ± 0.009 | <LOQ | <LOQ | 0.0300 ± 0.002 | 0.239 |
| Mlu | 0.113 ± 0.009 | 0.113 ± 0.008 | <LOQ | <LOQ | 0.0326 ± 0.002 | 0.259 |
| Ovi | 0.110 ± 0.008 | 0.127 ± 0.008 | <LOQ | 0.0134 ± 0.002 | <LOQ | 0.251 |
| Agl | 0.102 ± 0.007 | 0.0824 ± 0.007 | <LOQ | 0.0231 ± 0.003 | <LOQ | 0.207 |
| Aci | 0.128 ± 0.010 | 0.119 ± 0.009 | <LOQ | <LOQ | <LOQ | 0.247 |
<LOQ The limit of quantification
Data < LOQ were not included for sum of phytoestrogens computation
Among the legume species discussed in the current paper only isoflavones present in red clover have been quantified fairly in detail (Lemežienė et al. 2015; Ramos et al. 2008; Saviranta et al. 2008). The concentration of total isoflavones we found in red clover (19.6 mg/g) agrees with the range reported by Ramos et al. (2008) and Saviranta et al. (2008) (12.3–24.1 and 15.5–24.1 mg/g, respectively). Lemežienė et al. (2015) found less isoflavones in red clover. The differences between the studies resulted from the dissimilarities in plants maturity, genotype and sample drying method. All studies confirmed our observation that formononetin and biochanin A are dominant isoflavones in red clover.
The results of the current study confirmed our previous observation (Butkutė et al. 2014) that T. medium plants proved to be a better source of isoflavones than red clover. The total mean content of isoflavones in different extracts from M. sativa (2.29–8.39 mg/kg) that was observed by Rodrigues et al. (2014) was noticeably lower than we found (0.209–0.226 mg/g, Table 4). Çölgeçen et al. (2014) did not detect isoflavones in herbal material of flowering and nonflowering plants of M. sativa cv. Elçi. The scientific literature points out that the species M. sativa exhibited phytoestrogenic activity not only due to small level of formononetin and biochanin A but mostly because of the presence of coumestrol (Saloniemi et al. 1995; Seguin and Zheng 2006). The contents we quantified in Medicago species were lesser than those reported by Seguin and Zheng (2006) and coincided with the range confirmed by Saloniemi et al. (1995). Reliable data on phytoestrogens quantification in other species of plants tested in our work are currently not available—only sporadic qualitative information can be found in the literature.
The concentrations of phytoestrogens in zigzag and red clover plants, obtained in the current study, are several times as high as the total concentration of isoflavones in soybeans (0.610–2.440 mg/g) (Bouchenak and Lamri-Senhadji 2013). In addition, the content of isoflavones in perennial legumes was compared with that reported by Kuhnle et al. (2009) in more than 240 foods. The concentrations of isoflavones in a variety of fruits and vegetables, including pulses, ranged from < 1 μg/100 g to 124.4 mg/100 g on wet mass basis. Only soya-based products (up to 124.4 mg/100 g) were isoflavone-richer than plant material of Medicago, Onobrychis and Astragalus accessions. At present, mostly soybeans are used as a source of isoflavones in the production of food supplements. Increasingly, genetically modified soy is being grown in the world, and many European consumers do not approve of such crops being used as a source of food or nutraceutical compound (Prati et al. 2007). The current study on perennial legume crops evidences that such GM-free sources of phytoestrogens and other bioactive compounds would become of particular interest. Furthermore, perennial legumes can also be seen as an intermediate source in the food chain that provides the functionality for milk products. Phytoestrogens from forages, fed to dairy cows, are partly transferred to their milk; consequently, such forages increase concentrations of isoflavones and especially nonsteroidal estrogen equol in bovine milk (Adler et al. 2014).
Antimicrobial activity
In this study, microorganisms had different biological characteristics; thereby different susceptibility to the studied extracts could be expected. According to that, the evaluation of the potential of plant extracts on a broad range of microorganism strains can be considered of high importance.
All investigated plant extracts were active against the tested microorganisms, except for E. coli (only extracts of A. glycyphyllos and A. cicer inhibited the growth of bacteria at MIC 39.1 and 38.8 mg/mL) and C. albicans (Table 5). Escherichia coli is known to be multi-resistant to drugs. The inefficacy of these extracts against C. albicans can be related to the complex yeast cell walls, preventing extracts from contact with cell structures (Rodrigues et al. 2013). Generally, Gram-positive bacteria were found to be more sensitive to the legume extracts tested than Gram-negative bacteria. The plant extracts from T. pratense, T. medium, and O. viciifolia were demonstrated to inhibit the growth of all the tested Gram-positive and Gram-negative (except E. coli) bacteria. Onobrychis viciifolia extract showed the highest antimicrobial activity among the investigated Fabaceae species (with MIC values against S. aureus, S. epidermidis, P. aeruginosa, K. pneumoniae and B. cereus of 3.18 mg/mL). The extracts from Medicago plants were the least active. The MIC values of these plant extracts against susceptible strains were also found largely higher and ranged from 9.93 to 45.8 mg/mL for M. sativa and from 9.93 to 34.7 mg/mL for M. lupulina.
Table 5.
The antimicrobial activity (MIC, mg/mL) of extracts of perennial legume plants
| Legume species | Gram-positive bacteria | Gram-negative bacteria | Yeast | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Bacillus cereus
ATCC 11778 |
Bacillus subtilis
ATCC 6633 |
Escherichia faecalis
ATCC 29212 |
Staphylococcus aureus
ATCC 25923 |
Staphylococcus epidermidis
ATCC 12228 |
Escherichia coli
ATCC 25922 |
Klebsiella pneumoniae
ATCC 13883 |
Proteus vulgaris
ATCC 8427 |
Proteus aeruginosa
ATCC 27853 |
Candida albicans
ATCC 10231 |
|
| Trifolium pratense | 4.21 | 11.7 | 32.3 | 7.00 | 11.7 | IS | 11.7 | 11.7 | 32.3 | IS |
| Trifolium medium | 5.20 | 8.66 | 24.0 | 8.66 | 14.4 | IS | 14.4 | 24.0 | 40.0 | IS |
| Medicago sativa | 27.5 | 45.8 | IS | 16.5 | 45.8 | IS | 9.93 | 11.8 | IS | IS |
| Medicago lupulina | 20.9 | 34.7 | IS | 20.9 | 20.9 | IS | 12.5 | IS | IS | IS |
| Onobrychis viciifolia | 3.18 | 24.5 | 24.5 | 3.18 | 3.18 | IS | 3.18 | 3.18 | 50.0 | IS |
| Astragalus glycyphyllos | 5.20 | 24.0 | IS | 8.67 | 8.67 | 39.1 | 40.0 | 40.0 | IS | IS |
| Astragalus cicer | 5.40 | 15.0 | IS | 8.99 | 15.0 | 48.8 | 5.40 | 41.5 | IS | IS |
IS The reference microbial strains were insusceptible to the extracts in their concentration ≤ 50 mg/mL
The results of this study are in agreement with those previously reported by Esmaeili et al. (2015), who revealed that extracts from in vivo and in vitro grown plants of red clover are potent antimicrobial agents against bacteria strains S. aureus, B. cereus. Rodrigues et al. (2013) found that M. sativa is active against Gram-positive bacteria—S. aureus, S. epidermidis and Gram-negative bacteria—E. coli, K. pneumonia, but inactive against P. aeruginosa and C. candida. We established that extracts of M. sativa and M. lupulina were active, though limitedly, against mentioned bacteria except for E. coli. Chavan et al. (2015) concluded that alfalfa plant may be a new source for novel antibacterial compound discovery for treating drug-resistant human pathogens as the methanol extracts of alfalfa leaves exhibited potent antibacterial activity. Lobanova and Yakimova (2012) observed that Astragalus glycyphyllos L. showed the antimicrobial activity against S. aureus and K. pneumoniae.
Conclusion
At branching stage the plants of perennial legumes were found to possess beneficial nutritional, mineral and bioactive value; properties studied are species-dependent. All young legume plants are rich in protein and minerals. A. glycyphyllos and O. viciifolia are extremely abundant in iron. Trifolium species are a promising source of isoflavones. Apart from formononetin and biochanin A, Medicago species contain coumestrol. According to the total amount of four isoflavones and coumestrol, the species ranked as follows: T. medium ≫ T. pratense ≫ O. viciifolia ≥ M. sativa = A. cicer = M. lupulina ≥ A. glycyphyllos. The extracts of legumes, especially those of O. viciifolia, exhibit noticeable potency to inhibit the growth of Gram-positive and Gram-negative bacteria. Young plants of perennial legumes can be added to food as healthy ingredients by choosing the species according to the individual need for a particular component or property.
Acknowledgements
This study was Funded by a Grant (No. SVE-06/2014) from the Research Council of Lithuania.
References
- Adler SA, Purup S, Hansen-Møller J, Thuen E, Gustavsson AM, Steinshamn H. Phyto-oestrogens and their metabolites in milk produced on two pastures with different botanical compositions. Livest Sci. 2014;163(1):62–68. doi: 10.1016/j.livsci.2014.02.006. [DOI] [Google Scholar]
- Bouchenak M, Lamri-Senhadji M. Nutritional quality of legumes and their role in cardiometabolic risk prevention: a review. J Med Food. 2013;16(3):185–198. doi: 10.1089/jmf.2011.0238. [DOI] [PubMed] [Google Scholar]
- Butkutė B, Lemežienė N, Dabkevičienė G, Jakštas V, Vilčinskas E, Janulis V. Source of variation of isoflavone concentrations in perennial clover species. Pharmacogn Mag. 2014;10:S181–S188. doi: 10.4103/0973-1296.127373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan SS, Jadhav RS, Khemnar KS, Tambe VB. Evaluation of antibacterial activity and phytochemical screening of Medicago sativa leaves. Int J Curr Res Acad Rev. 2015;3(5):308–313. [Google Scholar]
- Chongtham N, Bisht MS, Haorongbam S. Nutritional properties of bamboo shoots: potential and prospects for utilization as a health food. Compr Rev Food Sci Food Saf. 2011;10(3):153–168. doi: 10.1111/j.1541-4337.2011.00147.x. [DOI] [Google Scholar]
- CLSI, Clinical and Laboratory Standards Institute (2012) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard M07-A9. 9th edn. CLSI, Wayne. http://antimicrobianos.com.ar/ATB/wp-content/uploads/2012/11/03-CLSI-M07-A9-2012.pdf. Accessed 16 Oct 2015
- Çölgeçen H, Koca Çalişkan U, Kartal M, Büyükkartal HN. Comprehensive evaluation of phytoestrogen accumulation in plants and in vitro cultures of Medicago sativa L. ‘Elçi’ and natural tetraploid Trifolium pratense L. Turk. J Biol. 2014;38(5):619–627. [Google Scholar]
- Dwyer JT, Picciano MF, Betz JM, Fisher KD, Saldanha LG, Yetley EA, Coates PM, Radimer K, Bindewald B, Sharpless KE, Holden J, Andrews K, Zhao C, Harnly J, Wolf WR, Perry CR. Progress in development of an integrated dietary supplement ingredient database at the NIH Office of Dietary Supplements. J Food Compos Anal. 2006;19:S108–S114. doi: 10.1016/j.jfca.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili AK, Taha RM, Mohajer S, Banisalam B (2015) Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (red clover). Biomed Res Int 2015 (Article ID 643285):1–11. doi:10.1155/2015/643285 [DOI] [PMC free article] [PubMed]
- Feedipedia (2016) Feedipedia, a programme by INRA, CIRAD, AFZ and FAO. http://www.feedipedia.org/content/feeds?category=13594. Accessed 07 Apr 2016
- Finglas P, Roe M, Pinchen H, Berry R, Church S, Dodhia S, Powell N, Farron-Wilson M, McCardle J, Swan G (2015) McCance and Widdowson’s the composition of foods, 7th edn. Royal Society of Chemistry, Cambridge. https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid
- Hedges L, Lister C (2005) Nutritional attributes of salad vegetables. Nutritional attributes of salad vegetables. Crop & Food Research Confidential Report N. 1473. New Zealand Institute for Crop and Food Research Ltd, Christchurch. http://www.vegetables.co.nz/resources/1files/pdf/booklet_salad_foodreport.pdf. Accessed 07 Apr 2016
- Kuhnle GGC, Dell’Aquila C, Aspinall SM, Runswick SA, Joosen AMCP, Mulligan AA, Bingham SA. Phytoestrogen content of fruits and vegetables commonly consumed in the UK based on LC-MS and 13C-labelled standards. Food Chem. 2009;116(2):542–554. doi: 10.1016/j.foodchem.2009.03.002. [DOI] [Google Scholar]
- La Frano MR, de Moura FF, Boy E, Lönnerdal B, Burri BJ. Bioavailability of iron, zinc, and provitamin A carotenoids in biofortified staple crops. Nutr Rev. 2014;72(5):289–307. doi: 10.1111/nure.12108. [DOI] [PubMed] [Google Scholar]
- Lemežienė N, Padarauskas A, Butkutė B, Cesevičienė J, Taujenis L, Norkevičienė E, Mikaliūnienė J. The concentration of isoflavones in red clover (Trifolium pratense L.) at flowering stage. Zemdirbyste-Agriculture. 2015;102(4):443–448. doi: 10.13080/z-a.2015.102.057. [DOI] [Google Scholar]
- Leśniewicz A, Jaworska K, Żyrnicki W. Macro-and micro-nutrients and their bioavailability in polish herbal medicaments. Food Chem. 2006;99(4):670–679. doi: 10.1016/j.foodchem.2005.08.042. [DOI] [Google Scholar]
- Lim TK. Edible medicinal and non-medicinal plants—flowers. Dordrecht: Springer; 2014. Trifolium pratense; pp. 925–948. [Google Scholar]
- Lobanova IE, Yakimova YL (2012) The antimicrobic activity of oil and ethanol extracts of Astragalus glycyphyllos. Vestnik Novosibirskogo GU. Serija: Biologija, kliničeskaja medicina 10(2):79–83
- Owusu-Apenten R. Introduction to food chemistry. Boca Raton: CRC Press; 2004. [Google Scholar]
- Özcan MM, Akbulut M. Estimation of minerals, nitrate and nitrite contents of medicinal and aromatic plants used as spices, condiments and herbal tea. Food Chem. 2008;106(2):852–858. doi: 10.1016/j.foodchem.2007.06.045. [DOI] [Google Scholar]
- Pilšáková L, Riecanský I, Jagla F. The physiological actions of isoflavone phytoestrogens. Physiol Res. 2010;59(5):651–664. doi: 10.33549/physiolres.931902. [DOI] [PubMed] [Google Scholar]
- Prati S, Baravelli V, Fabbri D, Schwarzinger C, Brandolini V, Maietti A, Tedeschi P, Benvenuti S, Macchia M, Marotti I, Bonetti A, Catizone P, Dinelli G. Composition and content of seed flavonoids in forage and grain legume crops. J Sep Sci. 2007;30(4):491–501. doi: 10.1002/jssc.200600383. [DOI] [PubMed] [Google Scholar]
- Ramos GP, Dias PMB, Morais CB, Fröehlich PE, Dall’Agnol M, Zuanazzi JAS. LC determination of four isoflavone aglycones in red clover (Trifolium pretense L.) Chromatographia. 2008;67(1–2):125–129. doi: 10.1365/s10337-007-0450-0. [DOI] [Google Scholar]
- Rodrigues F, Palmeira-de-Oliveira A, das Neves J, Sarmento B, Amaral MH, Oliveira MB. Medicago spp. extracts as promising ingredients for skin care products. Ind Crops Prod. 2013;49:634–644. doi: 10.1016/j.indcrop.2013.06.015. [DOI] [Google Scholar]
- Rodrigues F, Almeida I, Sarmento B, Amaral MH, Oliveira MBPP. Study of the isoflavone content of different extracts of Medicago spp. as potential active ingredient. Ind Crop Prod. 2014;57:110–115. doi: 10.1016/j.indcrop.2014.03.014. [DOI] [Google Scholar]
- Saloniemi H, Wähälä K, Nykänen-Kurki P, Kallela K, Saastamoinen I. Phytoestrogen content and estrogenic effect of legume fodder. Exp Biol Med. 1995;208(1):13–17. doi: 10.3181/00379727-208-43825. [DOI] [PubMed] [Google Scholar]
- Santos ALS, Sodré CL, Valle RS, Silva BA, Abi-Chacra ÉA, Silva LV, Souza-Gonçalves AL, Sangenito LS, Gonçalves DS, Souza LOP, Palmeira VF, d’Avila-Levy CM, Kneipp LF, Kellett A, McCann M, Branquinha MH. Antimicrobial action of chelating agents: repercussions on the microorganism development, virulence and pathogenesis. Curr Med Chem. 2012;19(17):2715–2737. doi: 10.2174/092986712800609788. [DOI] [PubMed] [Google Scholar]
- Saviranta NMM, Anttonen MJ, von Wright A, Karjalainen RO. Red clover (Trifolium pretense L.) isoflavones: determination of concentrations by plant stage, flower colour, plant part and cultivar. J Sci Food Agric. 2008;88(1):125–132. doi: 10.1002/jsfa.3056. [DOI] [Google Scholar]
- Seguin P, Zheng W. Phytoestrogen content of alfalfa cultivars grown in eastern Canada. J Sci Food Agric. 2006;86(5):765–771. doi: 10.1002/jsfa.2412. [DOI] [Google Scholar]
- Silva LR, Pereira MJ, Azevedo J, Gonçalves RF, Valentão P, Guedes de Pinho P, Andrade PB. Glycine max (L.) Merr., Vigna radiata L. and Medicago sativa L. sprouts: a natural source of bioactive compounds. Food Res Int. 2013;50(1):167–175. doi: 10.1016/j.foodres.2012.10.025. [DOI] [Google Scholar]
- Singh N. Pulses: an overview. J Food Sci Technol. 2017;54(4):853–857. doi: 10.1007/s13197-017-2537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Singh JP, Shevkani K, Singh N, Kaur A. Bioactive constituents in pulses and their health benefits. J Food Sci Technol. 2017;54(4):858–870. doi: 10.1007/s13197-016-2391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small E. Alfalfa and relatives—evolution and classification of Medicago. Ottawa: NRC Research Press; 2011. p. 170. [Google Scholar]
- Taujenis L, Padarauskas A, Cesevičienė J, Lemežienė N, Butkutė B. Determination of coumestrol in lucerne by ultra-high pressure liquid chromatography-mass spectrometry. Chemija. 2017;27(1):60–64. [Google Scholar]
- White PJ, Brown PH. Plant nutrition for sustainable development and global health. Ann Bot. 2010;105(7):1073–1080. doi: 10.1093/aob/mcq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, MacKown CT, Starks PJ, Kindiger BK. Rapid analysis of nonstructural carbohydrate components in grass forage using microplate enzymatic assays. Crop Sci. 2010;50(4):1537–1545. doi: 10.2135/cropsci2009.09.0521. [DOI] [Google Scholar]