Abstract
To effectively promote the industrial utilization of cocoyam (Xanthosoma sagittifolium) roots for enhanced food sustainability and security, there is a need to study their molecular, mechanical and physicochemical properties in detail. The physicochemical and textural characteristics of the red and white varieties of cocoyam roots were thus analysed by low field nuclear magnetic resonance relaxometry, multispectral imaging, uniaxial compression testing, and relevant physicochemical analysis in the current study. Both varieties had similar dry matter content, as well as physical and mechanical properties. However, up to four fast-interacting water populations were observed in the roots, dependent on the root variety and their degree of gelatinization during cooking. Changes in the relaxation parameters indicated weak gelatinization of starch at approximately 80 °C in both varieties. However, shorter relaxation times and a higher proportion of restricted water in the white variety indicated that this variety was slightly more sensitive towards gelatinization. A strong negative correlation existed between dry matter and all multispectral wavelengths >800 nm, suggesting the potential use of that spectral region for rapid analysis of dry matter and water content of the roots. The small, but significant differences in the structural and gelatinization characteristics of the two varieties indicated that they may not be equally suited for further processing, e.g. to flours or starches. Processors thus need to choose their raw materials wisely dependent on the aimed product characteristics. However, the spectroscopic methods applied in the study were shown to be effective in assessing important quality attributes during cooking of the roots.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2704-7) contains supplementary material, which is available to authorized users.
Keywords: Cocoyam (Xanthosoma sagittifolium), Physicochemical properties, Texture, Multispectral imaging, Low field nuclear magnetic resonance (LF-NMR), Cooking, Gelatinization
Introduction
The edible aroids, predominantly Xanthosoma sagittifolium (tannia) and Colocassia esculenta (taro), which commonly are called cocoyams, are consumed in many tropical and sub-tropical communities. Available statistics indicated an annual world production of 10 million tonnes in 2012 (FAO 2012), with Africa accounting for 74% of the global production (Onyeka 2014). Thus, their contribution to world food and nutrition security cannot be overstated. In West Africa, cocoyams are among the three most important root and tuber (R&T) staples for most communities (Ramanatha et al. 2010), with Xanthosoma sagittifolium, currently being the most cultivated and consumed edible aroid in Ghana. Research on the cocoyam species have been limited in Ghana, and the current knowledge is mainly in the hands of the farmers growing those. The red variety of Xanthosoma sagittifolium has been industrially processed into flour for production of fufu, a West African delicacy. Furthermore, the boiled cocoyam is used in the preparation of traditional cocoyam mash and porridge (εtɔ and mpotompoto), food for the elderly, and other local dishes for all age groups (such as ampesi and ɔgɔɔ). The fried or roasted roots are also a delicacy in the sub-region, eaten by all age groups (Opoku-Agyeman et al. 2004). It is also generally preferred over cassava (Manihot esculenta) by people who require foods of lower starch content. In spite of many similarities between the varieties of Xanthosoma sagittifolium, the cooking qualities of the roots are dependent on the chosen root variety (Opoku-Agyeman et al. 2004). Nonetheless, the available studies have largely focused on evaluating the macro and microscopic properties of flours and starches made from cocoyam (Eddy et al. 2012; Falade and Okafor 2013; Mweta et al. 2010; Perez et al. 1998; Sefa-Dedeh and Agyir-Sackey 2004; Sefa-Dedeh and Kofi-Agyir 2002), while less attention has been given to the molecular, mechanical and physicochemical properties of the roots. This leaves a gap in knowledge for the optimal use of the cocoyam roots on an industrial scale.
Most spectroscopic techniques can be used to study the molecular properties of foods (Feng et al. 2015). However, spectral data alone can only provide limited information on complex food matrices (Huang et al. 2014), due to its non-selective nature, necessitating the use for alternate analytical tools along with the spectroscopic techniques. New trends in food science therefore combine vision technology and spectroscopy to elucidate spatial and spectral information of foods at the molecular level using spectral imaging (Feng et al. 2015; Huang et al. 2014). Spectral imaging has been used e.g. in various food quality control and safety studies (Andresen et al. 2013; Dissing et al. 2013; Frosch et al. 2011), as well as for complex food analysis (Dissing et al. 2011; Frosch et al. 2011). The ability of spectral imaging to detect details in colour, size, surface chemistry and microstructure of food products (Dissing et al. 2013; Andresen et al. 2013), positions the technology as an efficient tool in studying the characteristics and quality of food crops.
Low field nuclear magnetic resonance (LF-NMR) relaxometry is another advanced spectroscopic technique that has gained increased relevance in the food industry. The method employs the spin properties of chosen nuclei (most commonly 1H) when exposed to an external magnetic field to elucidate information on their distribution and transitions, and thus also on the chemical and structural characteristics of the samples. The method has in recent years been employed for holistic assessments of the potential industrial use of some common root crops, such as potatoes (Solanum tuberosum). This includes the evaluation the mechanical, physical and chemical characteristics of potatoes, as well as the dynamic changes that occur in them during processing and cooking (Hansen et al. 2010; Martens and Thybo 2000; Thybo et al. 2000; Thygesen et al. 2001). However, limited studies exist on the characteristics and water dynamics of other tropical root crops, especially for Xanthosoma sagittifolium roots.
To effectively promote the industrial utilization of cocoyam for enhanced food sustainability and security, there is a need to study their molecular, mechanical and physicochemical properties in detail. This research therefore seeks to study the molecular, mechanical and physical characteristics of the raw and cooked roots of the two most common varieties of Xanthosoma sagittifolium on the market (the red and white varieties), using multispectral imaging, LF-NMR spectroscopy, along with relevant physicochemical and uniaxial compression analyses.
Materials and methods
Source of raw materials
Roots of the red and white varieties of Xanthosoma sagittifolium were obtained from Birim North District of the Eastern region of Ghana. Twenty roots (ten for each variety) were used in all determinations except the cooking experiment, which involved the use of six roots (three for each variety). Roots were harvested on March 30th 2015, and analysis was conducted in two batches; the first on May 29th 2015, and the second on June 2nd 2015. Unless otherwise stated, all determinations were made on the apical, middle and stem ends of both the raw and cooked roots.
Cooking experiment
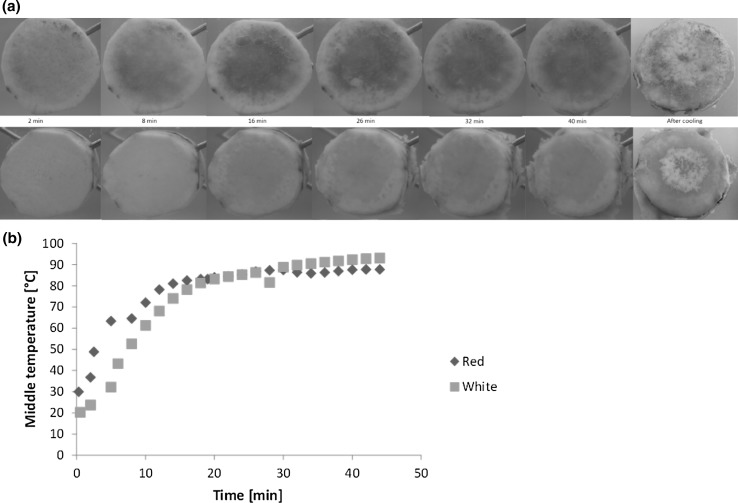
A cooking experiment was set-up to obtain information of the visual properties and changes which occur during cooking of cocoyam roots. About 40 mm long and 15 mm wide transverse sections cut from the middle portion of the roots were put in boiling water (simulating traditional cooking of the roots) in a cast iron half pot. The pot had been cut in half along the vertical axis and one side had been replaced by a glass plate to allow for visual evaluation of the cooking process, in a similar manner as described by Feyissa et al. (2015). Photographs were taken of the roots through the glass when the sample was placed in boiling water, and subsequently at every other minute during the cooking process, which lasted for 40 min. A digital camera (Nikon D60 with a Nikon DX AF-S NIKKOR 18–55 mm lens, with shutter speed 1/200, ISO 1600 taken both on raw (NEF) as well as JPEG format, using noise reduction and active D-lighting) was used for capturing the images. Temperatures for both the surrounding cooking water and from the core of the test samples (roots) were recorded with a digital thermometer at the same time as when the images were captured. The cooking experiment was mainly intended as a reference experiment, which focused on identifying visual properties and changes of the roots during cooking. These properties were used for verification of the analytical results obtained with the physicochemical methods described in the following section.
Analytical methods
Physical dimensions of roots
Xanthosoma sagittifolium roots vary in size and have contours requiring the use of a flexible/malleable device for measuring their physical dimensions. The length (inner and outer lengths), and width (measured as the circumference) of the apical, middle and distal portions of each root were measured with a thread, which was transferred onto a measuring ruler to assess the root dimensions. The mean and standard deviation of 10 roots for each variety was calculated during physicochemical analyses, unless stated otherwise.
Dry matter
The dry matter content was calculated on a fresh weight basis. Analysis was done by weighing 2 g of a minced portion of each root (n = 10 for each variety). The test sample was put in a pre-weighed aluminium foil pan and dried for 24 h at 110 °C in a hot air oven. The dry matter was then calculated as
| 1 |
CIE L* a* b* colour parameters
The L* a* b* colour parameters of the raw and cooked roots were determined using a calibrated Konica Minolta CM-600d chromametre, with the CM-S100w SpectraMagic™ NX colour data software (Konica Minolta Sensing Inc., Osaka, Japan). Lightness (L*) values ranged from 0 (black) to 100 (white), yellowness (b*-values) ranged from −b* (blue) to +b* (yellow), and redness (a*-values) ranged from −a (green) to +a* (red). The determinations were done on 5 mm thick circular transverse cuts from the apical, middle and distal portions, respectively, of each root analysed in the study. Measurements were performed on both sides of each slice. The mean of 8 replicates for each variety was reported for the colour analysis.
Texture analysis
The uniaxial compression was determined on 1 × 1 × 1 cm cubes of raw samples of the two cocoyam root varieties studied. The samples were taken from the middle portion of each root. Care was taken to discard the outermost cortical and vascular tissues. Each cube was compressed to 75% of its original height with a Stable Micro Systems Texture Analyzer (TA.XT2i texture analyser with the Exponent Lite software, Stable Micro Systems Ltd., Surrey, UK), using a 30 kg load cell. The deformation rate was set to 20 mm/min. The modulus of deformability (Ed), as well as the stress (σf) and strain (εf) at fracture were calculated from 10 replicates for each of the two root varieties. No detailed texture analysis was performed on the cooked roots, since pre-experiments indicated that they were too soft and did not show any significant difference between the two varieties with this method.
Multispectral imaging
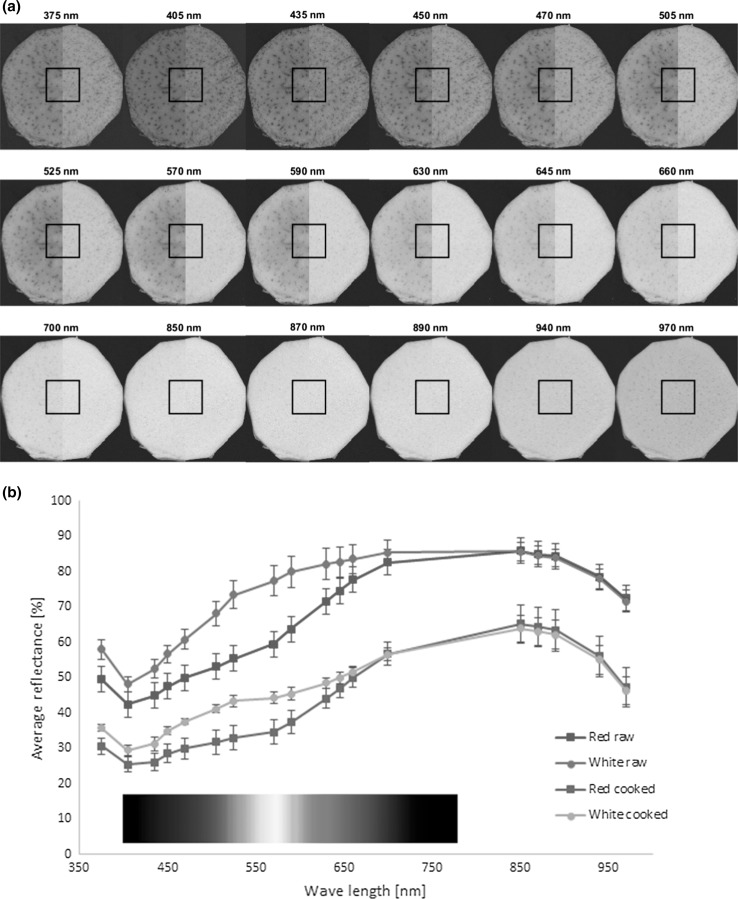
The surface reflection of 5 mm slices of both raw and cooked roots (same samples as used for the CIE Lab colour measurements) were used for multispectral measurements (n = 10 for each variety). A calibrated multispectral VideometerLab analyzer, (Videometer A/S, Hørsholm, Denmark), which consists of a CCD camera, LEDs, and an Ulbricht sphere with a matte white coating was used to obtain spectral images at 18 different wavelengths in the range from 375 to 970 nm, as described by Dissing et al. (2011). The mean reflective spectra from a square-shaped region of interest (ROI) from the middle of each sample slice were analysed and average reflection and standard deviations of the 10 replicates for each variety were compared. Data acquisition was first done on the raw slices, which were then cooked in boiling water for 10 min. The cooked samples were allowed to cool in covered petri dishes at ambient conditions for about 2 h before further data acquisition of the cooked slices.
Low field nuclear magnetic resonance (LF-NMR) studies
Three cylindrical samples (1.2–2.0 g) were taken from the apical, middle and stem ends, respectively, of each root. Care was taken not to include the outermost cortical and vascular tissues, as mentioned earlier in relation to the textural analysis. All samples were weighed, put in test tubes (45 × 15 × 0.6 mm), covered with a plastic cap, and put in longer outer test tubes (180 × 17.75 × 0.6 mm) for analysis. Measurements were performed at 25, 50, 70, 80 and 90 °C. The samples were allowed to equilibrate for at least 35 min at each corresponding temperature in a pre-set water bath prior to the NMR analysis. A 23.4 MHz MARAN LF H1 NMR benchtop analyser (Resonance Instruments, Witney, UK), equipped with an 18 mm temperature probe, was used to perform transverse relaxation time measurements of the roots. For this purpose a Carr-Purcell-Meiboom-Gill (CPMG) (Carr and Purcell 1954; Meiboom and Gill 1958) pulse sequence with a τ-value of 150 μs was used for the analysis, and every other, of the 2048 echoes obtained, were collected. Eight repetitive scans were used for data fitting and the repetition time between scans was set to 6 s. The Low-Field NMR Toolbox for Matlab (The Matworks Inc., Natric MA, USA) was then used for multi-exponential fitting of the data, as described by Pedersen et al. (2002).
Statistical analysis
An analysis of variance (ANOVA) was performed on all univariate data with the IBM SPSS (version 20.0) and Statgraphics centurion (version XV1) software packages. Discrete exponential fitting of the LF-NMR data, multispectral data analysis, and the multivariate analysis were all performed with MATLAB R2015a (The Mathworks, Natick, Mass., USA). The multivariate analysis consisted of a principal component analysis (PCA), which described the main differences and similarities between the raw and cooked samples of the two varieties with regards to the analysed parameters. The data was centred and variables were normalized with the inverse of their standard deviation to correct for the different scales and ranges of the variables. Furthermore, a correlation analysis was performed in Microsoft Excel to indicate the strength of the correlations between variables.
Results and discussion
Physicochemical characteristics of raw roots
The cormels of the two test varieties were similar in appearance. Both varieties were about 9–12 cm long, and of approximately the same width at the apical, middle and stem ends, respectively (Table 1). Thus, there were no significant differences (p > 0.05) between the physical dimensions of the two root varieties and both had a dark brown outer skin giving them a similar outer appearance. This similarity in the visual properties of the two varieties of Xanthosoma sagittifolium confirms earlier observations by Ramanatha et al. (2010) in an ethnobotanical study of the indigenous varieties of Xanthosoma sp in Ghana. Both studies corroborate the difficulty for consumers and processors in selecting the optimal root variety as a raw material for a certain production on the visual properties alone, since it is almost impossible to tell the difference between the two varieties until the root is peeled or even cooked.
Table 1.
The mean and standard deviations of the analysed colour, dry matter and textural properties of the tested cocoyam varieties
| Parameter | Red (raw) | Red (cooked) | White (raw) | White (cooked) |
|---|---|---|---|---|
| Width Apical (cm) | 8.9 (2.7) | 9.3 (3.2) | ||
| Middle (cm) | 14.9 (1.4) | 15.3 (1.5) | ||
| Distal (cm) | 15.3 (2.0) | 14.9 (2.0) | ||
| Dry matter content (%) | 44.4 (4.7)ab | 42.5 (1.7)a | 47.4 (3.3)ab | 44.0 (1.0)ab |
| L*-value Apical | 55.5 (4.3)a* | 48.4 (1.8)b | 60.7 (9.2)a | 50.6 (4.4)b* |
| Middle | 50.8 (3.5)a+ | 46.0 (4.3)b | 55.9 (5.7)a | 46.3 (2.7)b+ |
| Distal | 50.6 (5.6)a+ | 45.4 (3.6)b | 56.4 (6.6)a | 47.7 (2.9)b+ |
| Average | 52.1 (3.3)a | 46.8 (2.8)b | 57.8 (5.9)a | 48.0 (2.4)b |
| a*-value Apical | 0.8 (1.1)a | 1.2 (1.2)b | −3.6 (0.5)c* | −2.6 (0.6)d* |
| Middle | 0.4 (1.0)a | 0.5 (1.5)a | −2.9 (1.1)b* | −2.1 (0.3)b+ |
| Distal | 1.4 (0.9)a | 3.6 (1.0)b | −2.7 (0.6)c* | −2.0 (0.4)c+ |
| Average | 0.9 (0.6)a | 0.8 (0.9)a | −3.2 (0.6)b | −2.2 (0.3)c |
| b*-value Apical | 9.2 (1.8)a* | 6.4 (1.3)b* | 12.5 (1.6)c* | 7.3 (1.5)b* |
| Middle | 7.3 (1.0)a+ | 4.9 (1.1)b+ | 10.3 (1.5)c+ | 5.3 (0.6)b+ |
| Distal | 7.4 (1.6)a+ | 5.0 (1.3)b+ | 10.2 (1.8)a+ | 5.1 (0.8)b+ |
| Average | 7.9 (1.3)a | 5.5 (0.6)b | 11.1 (1.4)c | 5.9 (0.6)b |
| Strain (−) | 0.48 (0.18) | 0.42 (0.07) | ||
| Henckey strain (−) | 0.38 (0.10) | 0.35 (0.05) | ||
| Stress (MPa) | 2.15 (0.46) | 1.92 (0.53) | ||
| True.Stess (MPa) | 1.10 (0.42) | 1.09 (0.24) | ||
| Modulus_1% (GPa) | 0.17 (0.13) | 0.18 (0.13) | ||
| Modulus_10–25% (GPa) | 4.33 (1.59) | 4.74 (1.20) | ||
| Work to 0.75 (mJ) | 668 (280) | 684 (233) | ||
| Work to break (mJ) | 363 (102) | 321 (125) | ||
| Maximal work (mJ) | 913 (179) | 944 (232) |
Colour and dry matter measurements were performed on both raw and cooked roots, while texture analysis was only performed on the raw roots
Values represented as mean (st. dev) of 10 replicates
Values in the same row with different superscripts (a, b, c, d) indicate significant differences at p < 0.05 between the two varieties and/or treatment (raw/cooked). If no superscript is present, the values are not significantly different
Significant difference (p < 0.05) between root portions in a variable are indicated with different superscripted symbols (*, +)
Dry matter
The dry matter content for the two varieties ranged from 41.8 to 52.8% for the white and 37.9–51.8% for the red varieties, respectively (Table 1), but no significant difference (p > 0.05) was observed in the dry matter between the two varieties. The dry matter content of roots are influenced by genetic and environmental factors, as well as the maturity of crops at harvest (Falade and Okafor 2013; Mweta et al. 2010), and reflects the total starch content and textural properties of the roots to a large extent (Thygesen et al. 2001). However, Falade and Okafor (2013) reported different amylose contents of different varieties of Xanthosoma sagittifolium. This indicated that for the optimal industrial use of cocoyam roots, more detailed studies were needed to ascertain the differences and processability of the two varieties.
Cooking experiment
The temperature profiles of the roots during heating in 93 ± 4 °C hot water (Fig. 1b) indicated similar overall heat absorption by the two varieties. However, the white variety showed a slightly lower rate of heat absorption than the red variety during the first 15 min of cooking. During this time the core temperature Tc of the roots reached 80 ± 2 °C. However, the core temperature increased further during prolonged cooking, but no significant difference was observed in the core temperature between the two varieties at higher temperatures. The vascular and cortical tissues of the white variety visibly softened and started to peel off the root after approximately 26 min of cooking (Tc = 86 °C) until the end of cooking when the root looked very mushy (Fig. 1a). Similar behaviour was not observed in the red variety until after 32 min of cooking (also Tc = 86 °C). The softening and subsequent mushiness of roots is traditionally used to indicate well-cooked roots. Thus, this visual observation suggested a faster cooking rate of the white-fleshed variety, contrary to the report by Sefa-Dedeh and Kofi-Agyir (2002), who suggested bigger starch granule sizes of the red-fleshed variety with a corresponding lower pasting temperature compared to the white. Pigmentation of the roots seemed to be concentrated in the middle lamellae with almost no colouring in the cortex after cooking and upon cooling. The middle lamella of the cooked and cooled roots was purplish in colour, as compared to the initial reddish colour (for the red-fleshed variety) of the raw roots, and whiter for the white-fleshed variety although the cortical region of both varieties had darkened after cooking. This reflects the lower L*-values obtained for the different portions of both varieties after cooking, as compared to their initial instrumental lightness, as discussed in the following colour analysis section. There was also a marked change in the colour of the cooking water for both varieties from the 16th min on, but the red-fleshed variety had a visually higher intensity. This observation may be a result of the pigments leaching into the surrounding water during cooking (Srilakshmi 2003).
Fig. 1.
a Typical visual changes in red and white varieties of Xanthosoma sagittifolium roots during cooking. b Temperature profile of red and white varieties of Xanthosoma sagittifolium roots during cooking. The plot represents the mean temperature (°C) in the middle of the root samples (n = 3 for each variety), as affected by cooking time in 93 ± 4 °C hot water (colour figure online)
CIE L* a* b* colour
The major visual attribute to differentiate the two varieties were the surface colour of the peeled roots. In accordance with their names, the white-fleshed variety, whether raw or cooked, was lighter in colour with higher L*-values (Table 1) compared to the red-fleshed variety. The cooked roots had significantly lower (p < 0.05) L*-values than the raw roots for both varieties, signifying an apparent darkening of the roots upon cooking. According to the observed average redness values (a*-values) the red variety showed a slight red nuance to the colour, as expected, while the white variety showed a slightly stronger green nuance. However, this green colour faded significantly in the white variety during cooking, but no significant changes were seen in the a*-value in the red variety during cooking. Higher b*-values in the white variety indicated a more yellow colour compared to the red roots. However, both species lost yellowness during cooking. The apical portions were slightly different in colour compared to the middle and stem portions in both varieties, showing a slightly lighter (higher L*-value in the red raw and white cooked roots) and more yellow appearance (higher b*-value in all varieties and treatments). Also a slightly greener colour was observed in the apical portion of the cooked roots of the white variety, compared to the middle and distal parts. This large variability observed in the colour within the individual roots of the same variety indicated further the difficulties that breeders face towards producing crops that fulfil the industrial standards of colour uniformity of the roots.
Uniaxial compression test
Uniaxial compression tests were performed to assess the mechanical characteristics of raw roots of the two varieties. Both varieties had similar (p > 0.05) hardness with mean work to break/fracture of 363 ± 102 and 321 ± 125 mJ for the red and white variety, respectively (Table 1). No significant difference was observed in the rate of deformation (Henky strain) between the two varieties. These insignificant differences in the uniaxial compression parameters suggest a possible similar behaviour of the two varieties during further processing and treatment with respect to their mechanical properties. Traditionally, the two varieties are used for almost the same dishes because of their seeming similarities in cooking properties (Ramanatha et al. 2010). Thus, the findings confirm consumer assertion that except for indigenous dishes such as cocoyam fufu (pounded cocoyam), in which the red variety is of essence for colour appeal, the two varieties can be used interchangeably based on their mechanical attributes.
Multispectral imaging
Multispectral images were taken of both raw and cooked samples of the two varieties. Mean reflectance data from the squared region of interest in the middle of the roots (Fig. 2a) were used for further spectral analysis. For both raw and cooked samples an increase in mean reflectance was observed between 400 and 850 nm, followed by a decrease in reflection at higher wavelengths (Fig. 2b). Significantly (p < 0.05) higher reflection was observed in the raw samples compared to the cooked for both varieties, mostly reflecting the effect of the dry matter content of the roots before and after cooking (Table 1). Furthermore, a correlation analysis indicated an especially strong negative correlation (r = −0.72 to −0.77) between the dry matter content and the reflection at all wavelengths above 800 nm, indicating that this part of the spectra can be used for fast analysis of the dry matter, and thus also for water content assessment in the roots. This is in agreement with that water is an excellent absorber within the near infrared (NIR) spectral range. Due to the high water content of the roots, the reflectance of the NIR range decreases due to a bread water absorption around 970 nm, which relates to the second overtone of the O–H stretch (Thybo et al. 2000). A higher reflectance was seen in the white roots compared to the red in the visual part of the spectrum, especially in the blue, green and yellow spectral areas. These were in good agreement with the L*- and b*- colour measurements, but these colour parameters showed strong positive correlations (correlation coefficient r > 0.85) to the reflectance at all multispectral wavelengths, especially in the visual spectrum (r > 0.97). This analysis indicated furthermore that multispectral imaging can be effectively used to distinguish between the two varieties, both in the raw and cooked state.
Fig. 2.
a Typical multispectral images of red (left half) and white (right half) varieties of Xanthosoma sagittifolium roots at each of the investigated spectral wavelengths. Average reflectance spectra from the square region in the center of each cocoyam sample were used for spectral analysis. b Average multispectral reflectance of raw and cooked roots of the red and white varieties of Xanthosoma sagittifolium (n = 10 for each variety) (colour figure online)
The spectral region from 450 to 550 nm has also been ascribed for absorption of naturally occurring anthocyanins (Harborne 1958), suggesting the possible presence of these pigments, which are transmitted as red, violet and short blue to the human eye, as well as the presence of carotenoids (Thybo et al. 2000). The higher reflectance of the white variety in this region might thus indicate a lower content of these phytochemical compounds than in the red variety. Furthermore, the lower reflectance in the cooked samples compared to the raw may partly indicate that the content of these phytochemicals decrease during cooking. However, this hypothesis needs further analyses for confirmation.
Low-field nuclear magnetic resonance (LF-NMR) analysis
Assignation of water populations
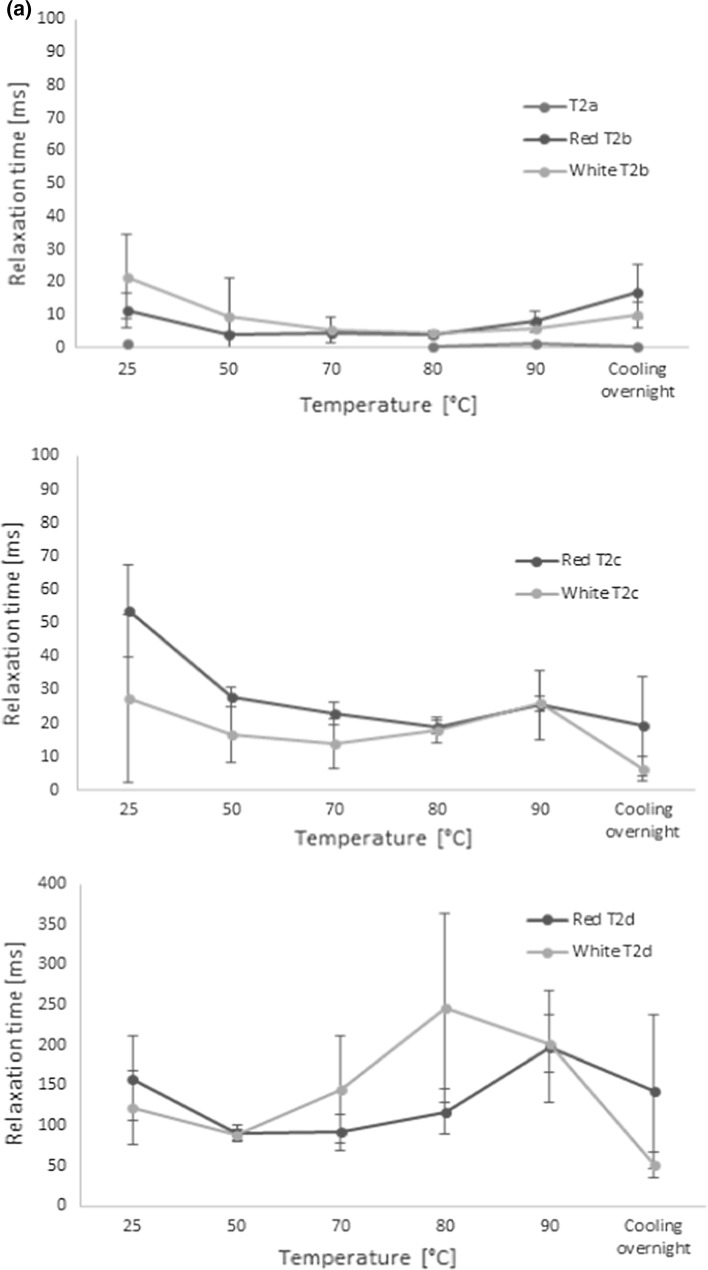
The effects of the cocoyam composition and characteristics on the water distribution and transitions were analysed by means of LF-NMR. Discrete exponential fitting of the NMR relaxation data indicated the presence of up to four water populations in the cocoyam roots during cooking (Fig. 3a, b). Hills and Le Floc’h (1994) observed four water populations in raw potato and assigned the components to water in starch granules (transverse relaxation time T2 of approximately 2–4 ms), water associated with cell walls (T2 around 10 ms), and two dominating components with transverse relaxation times around 100 and 500 ms to water in the cytoplasm and non-starch vacuoles, respectively. Although the values in cocoyam varied somewhat to those observed by Hills and Le Floc’h (1994) in potatoes, a similar interpretation of the populations could be applied in the present study. Thus, the fastest relaxing population in the cocoyam T2a (≈1.5 ms) was assigned to water in interaction with starch, T2b (5–10 ms) to cell wall associated water, T2c (13–54 ms) to water in the cytoplasm and the slowest relaxing component, T2d (51–246 ms), to water in vacuoles and other extracellular water, accordingly.
Fig. 3.
Mean transverse relaxation times (a) and their representative apparent water populations (b) of cocoyam roots as a function of cooking temperature (n = 10 for each variety). The T2 relaxation times were estimated by discrete exponential fitting of LF-NMR CPMG relaxation curves of the test samples at each temperature
Proton exchange during cooking and cooling
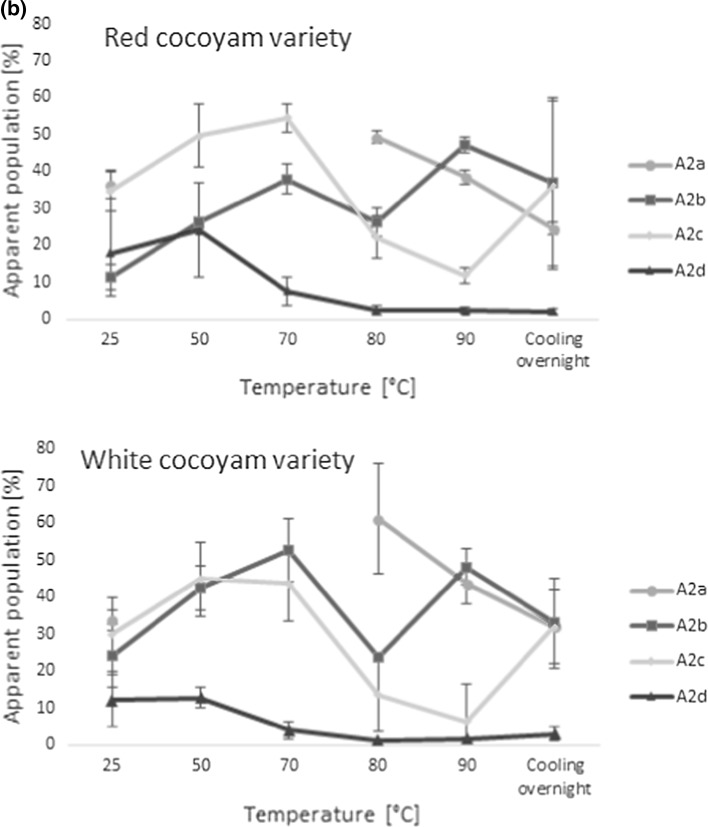
The relaxation parameters were highly affected by the cooking process and fast proton exchange between the different populations was indicated by merging and reappearing of populations during the cooking process (Fig. 4). Mortensen et al. (2005) also observed a small (up to 10%) fast-relaxing population (with relaxation time of 3–4 ms) in the potatoes for temperatures up to 60 °C when applying a tri-exponential fitting. When tri-exponential fitting was applied to the present data this population could also be seen in the raw cocoyam (T2a ~ 1.5 ms) at temperatures below 70 °C. According to Mortensen et al. (2005) this fast relaxing population, assigned to water in the starch granules, disappeared at temperatures above 60 °C in potato due to the gelatinization and disruption of cells.
Fig. 4.
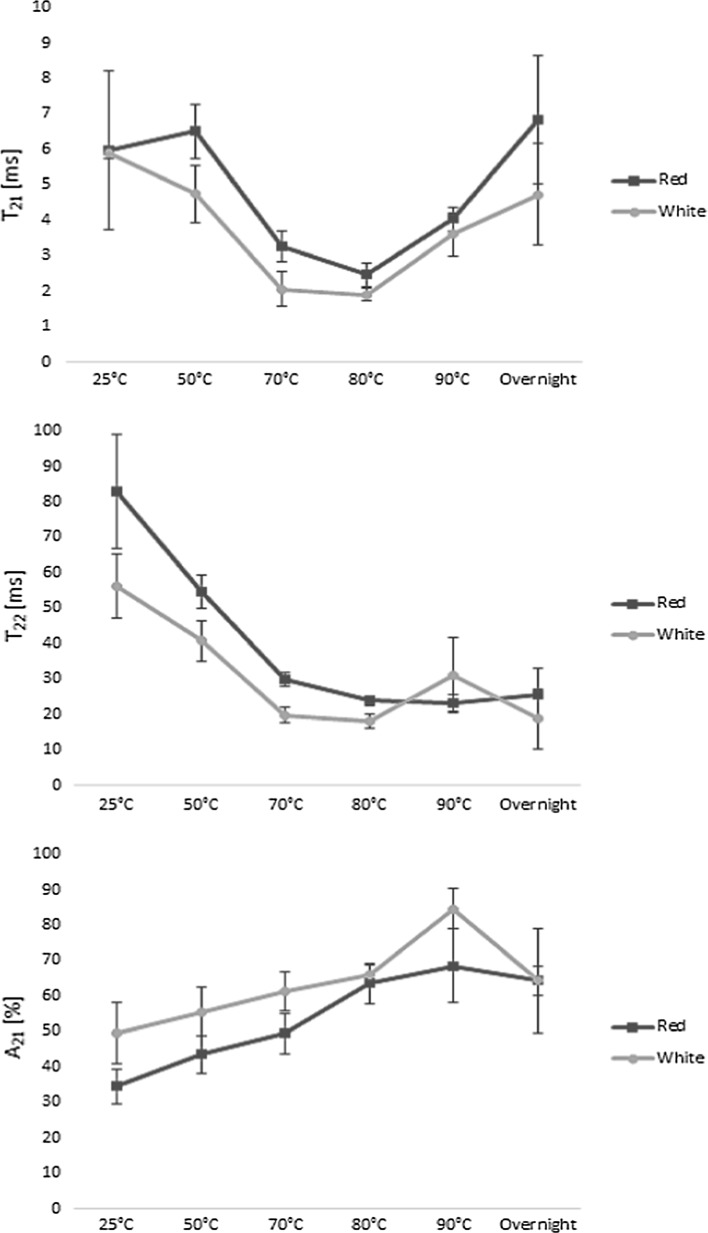
Bi-exponentially fitted transverse relaxation times of water in red and white cocoyam roots during cooking and overnight cooling (n = 10 for each variety). Changes in the faster relaxing component (T21) (top), the slower relaxing component (T22) (middle), and the apparent population of the faster relaxing component (A21) (bottom) are depicted as functions of cooking temperature (colour figure online)
However, to identify where substantial structural changes due to the cooking process were occurring a simplified bi-exponential model was built. This also allowed for comparison of the results to the studies of Micklander et al. (2008), Thybo et al. (2000), and Mortensen et al. (2005) on cooking of potatoes. The resulting faster relaxation component from the bi-exponential model, T21, ranged from 1.9 to 6.8 ms during the cooking process, and was ascribed to water within the starch granules and in cell walls, and the slower relaxing component, T22, believed to correspond to cytoplasmic and vacuole water, was observed in the range of 18–83 ms (Fig. 4)). Furthermore, the relative amount of water associated with the faster relaxing component (A21) was significantly lower in the red variety (34 ± 5%) compared to the white variety (50 ± 9%) at 25 °C. Although the relative amount of water in population A21 increased gradually during the whole cooking process, this proportion was always higher in the white variety, indicating more swelling of the starch granules in the white variety. Since no earlier studies have used LF-NMR for quality assessment of cocoyam, and since the values in the bi-exponential model of the cocoyams were quite different to what has been previously observed in potatoes (Thybo et al. 2000; Mortensen et al. 2005), a validation experiment using potatoes was conducted to validate the cocoyam results. This validation is described in the supplementary materials section of this manuscript. However, the shorter relaxation times observed in the cocoyam indicated a more compact cellular structure and a higher amount of highly restricted and unfreezable water compared to the analysed potatoes. This is further supported by the average starch granule size, which is typically larger in potatoes than in cocoyam (Owosu-Darko et al. 2014). Furthermore, generally lower T2 relaxation values were observed in the white variety, compared to the red, indicating more restriction of the water observed in the white variety.
When looking further at the changes in the bi-exponential relaxation parameters during cooking of the cocoyam roots (Fig. 4), a general decrease in the relaxation times was observed with increasing temperatures up to 80 °C, coupled with an increase in the more restricted apparent population A21 during cooking. This is believed to relate to the increased restriction of water, as water from the cytoplasm and vacuoles is lost from the matrix during cooking. This is in agreement with the study of Mortensen et al. (2005), who showed that increasing dry-matter content of potatoes lead to faster relaxation as well. Since the slower relaxation component T22 is thought to relate to more freely moving water in the extracellular cavities and not in direct interaction with the starch, the decrease in T22 up to 80 °C is furthermore believed to mostly relate to a decreased correlation time for chemical exchange.
The faster relaxation time T21 of both varieties and T22 in the white variety increased again between 80 and 90 °C, as starch gelatinization started, which allows for increased water exchange with the hydroxyls of the starch (Mortensen et al. 2005). This is in agreement with earlier stated gelling temperatures in cocoyam starch solutions, but Nwokocha et al. (2009) observed cocoyam starch gelatinization around 76 °C for average sized cocoyam starch granules. However, the presence of other chemical components, deviations in starch granule size, and impurities in the roots compared to the pure starch solutions may explain the somewhat higher gelatinization temperature of the roots in the present study. In addition to gelatinization, the increase in the relaxation times above 80 °C is furthermore believed to partially relate to softening of the cell walls, in agreement with the observations of Mortensen et al. (2005) and to an increase in thermal mobility (Micklander et al. 2008). The reappearance of the fast relaxing component (T2a around 1 ms, and corresponding dominating population A2a in Fig. 3) at 80 °C further supports the increased interaction of the water with the starch around this temperature due to gelatinization.
However, the gradual changes in the apparent bi-exponential populations A21 and A22 with increasing temperature indicated a continuous increase in swelling power of the cocoyam starch, in agreement with the observations of Lawal (2004). Furthermore, the gradual change in the water populations indicated that the gelatinization process is more subtle for the cocoyam varieties compared to gelatinization of potatoes. Lawal (2004) also showed that the solubility of the cocoyam starch increased with increasing temperatures during cooking, and thus changes in the cocoyam starch solubility might also contribute to the gradual increase in the two water populations during the cooking process. Furthermore, the gelatinization recurred at a much higher temperature (above 80 °C) than in potatoes, but according to Mortensen et al. (2005) gelatinization during cooking of potatoes happens at 53 °C, while Micklander et al. (2008) reported a gelatinization temperature of 58 °C. The gelatinization temperature in the current study is in good agreement with the study of Lawal (2004), who observed a gelatinization of cocoyam starch at a peak temperature of 79.80 °C as analysed with Differential Scanning Calorimetry (DSC). Moreover, the slightly steeper increase in A21 in the white variety in this temperature interval indicated that the white variety was slightly more sensitive towards gelatinizing than the red variety, and that it held a higher proportion of water after gelatinization during cooking. Several parameters such as differences in granule characteristics, weight, length and degree of branching, amylose/amylopectin ratios, as well as other physical conditions of the starch, may have influenced the gelling properties of the two varieties (Nwokocha et al. 2009). However, since no significant differences were observed in the relaxation times (T21 and T22) above 80 °C, the two varieties could be assumed to have similar gelatinization strength. Furthermore, the gelatinization temperature from the NMR analysis correlated well with the visual observations during the cooking experiment (Fig. 1, Tc = 86 °C).
After cooking, the roots were allowed to cool overnight. During the cooling stage an exchange between water associated with the starch granules and cell walls and water in the cytoplasm was observed, indicating the release of water from the gelatinized network. However, the amount of water of the slowest relaxing component A2d seemed unaffected by this water diffusion during cooling. A decrease in the slower relaxation times T2c and T2d (and T22 in the bi-exponential model) indicated a more compact structure of the cocoyams after cooling. This can possibly be explained by the expelling of water from the starch network to the cytoplasm, as well as the starch retrogradation process during cooling. These results correlate well with the study of Micklander et al. (2008) on gel retrogradation after cooking of potatoes, as well as the study of Thygesen et al. (2003) on the effects of amylose and starch phosphate on starch gel retrogradation.
Multivariate and correlation analysis
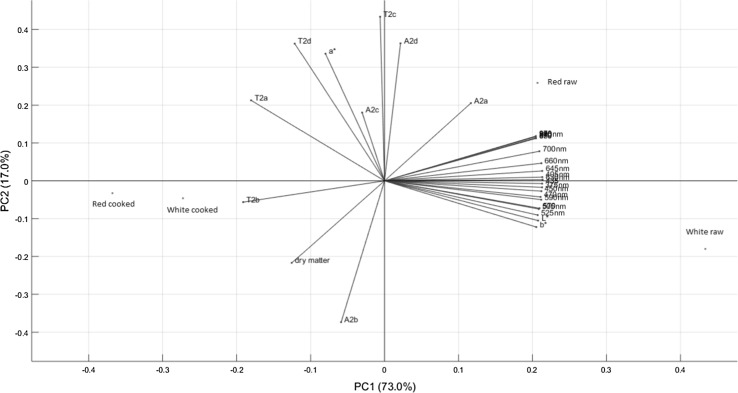
A multivariate analysis of the differences and similarities between the samples according to the applied analytical methods was performed. The resulting principal component analysis (PCA) bi-plot of scores and loadings can be viewed in Fig. 5. The first principal component (PC1) described 73.0% of the variation between the samples, indicating the clear differentiation between the raw and cooked samples, especially for the white variety. This difference is mostly portrayed in the changes in water and dry matter content during cooking, as reflected by traditional dry matter analysis and multispectral imaging, as well as the water characteristics of the more restrained water population (with corresponding relaxation time T2b) as assessed by LF-NMR. This was further supported by the correlation analysis, which showed a high correlation between the second fastest relaxing NMR component T2b and the dry matter content (r = 0.86), indicating that as the dry matter content increased the more restricted did the water within the roots become. The water present has an increased opportunity to interact and bind more strongly to macromolecules as their concentrations increase. Interestingly, a strong negative correlations was found between the two faster relaxing NMR components (T2a and T2b) and the multispectral reflectance data at all wavelengths (−0.94 < r<−0.70), as well as the L*- and b* colour values, while vague negative correlations (r ≈ −0.45) were observed between the dry matter content and the colour parameters. This indicated, firstly, that the water distribution had a larger effect on the colour appearance of the roots than the dry matter content itself, and secondly, that the multispectral imaging is quite superior to the colorimeter to evaluate the quality of the roots, since it can give more detailed indication about the physicochemical state of the sample.
Fig. 5.
Principal component analysis (PCA) bi-plot of scores and loadings on all tested variables, for raw and cooked roots of the red and white varieties of Xanthosoma saggitifolium. The uniaxial compression data was excluded from the PCA, since only data from the raw roots was available for this analytical technique (colour figure online)
The second component (PC2), describing 17.0% of the variation, portrayed the fast exchange between water populations, especially T2c and T2d. The slowest relaxing water component T2d also showed high positive correlation to the a*-value (r = 0.88), indicating that the red/green appearance of the roots was influenced by the extracellular and more freely moving water populations. Increases in T2a correlated well with changes in T2c and T2d indicating that the exchange rates between these populations was fast and highly affected by the type of variety and cooking progress. This was further supported by a strong negative correlation between the two fastest relaxing water populations A2a and A2b (r = −0.86), between A2b and A2d (r = −0.97) and a positive correlation between A2a and A2d (r = 0.83).
Conclusion
The phenotypic characteristics of the white and red varieties of Xanthosoma sagittifolium roots were shown to be similar, thus it is difficult to visually differentiate between the roots. Thus, more refined analytical methods have to be applied for root characterisation in order to assist processors in their selection of raw materials for optimal industrial use, as well as reduction of production losses and food wastage.
A significant difference (p < 0.05) existed between the colour of the raw and cooked roots, as well as in lightness (L*-values) and yellowness (b*-values) among the various portions of both varieties. This large colour variation within the same variety poses a hindrance to the effective industrial use of the roots. A strong positive correlation was furthermore obtained between the L* and b* measurements and reflectance intensity during multispectral imaging, especially in the visual region for both varieties. The raw roots showed higher reflectance than the cooked roots in both varieties at all investigated wavelengths, showing the influence of the dry matter content on the roots before and after cooking. Moreover, a strong negative correlation (r = −0.86) existed between dry matter and the reflection intensity at all wavelengths above 800 nm. Thus, there is the potential for this spectral part to be used in rapid testing of the dry matter and water content of Xanthosoma sagittifolium roots.
Up to four fast interacting water populations were identified in both varieties during cooking; T2a (≈1.5 ms), T2b (5–10 ms), T2c (13–54 ms) and T2d (51–246 ms). Furthermore, the LF-NMR measurements provided an in depth analysis of the gelatinization and retrogradation behaviour of the starch. Generally shorter relaxation times, and more swelling of the starch granules in the white variety during cooking, indicated that this variety was slightly more sensitive towards gelatinizing than the red variety, and that it held a higher proportion of water during cooking. These small, but still significant differences between the gelatinization characteristics of the two varieties indicated that they might be differently well suited for further processing (to flours or starches), depending on the wanted characteristics of the final product. Furthermore, detailed characterization of the roots opens the possibility for farmers to breed specific root varieties with certain phenotypic characteristics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the KNUST-DANIDA strengthening the root and tuber value chains project in Ghana for the financial support.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2704-7) contains supplementary material, which is available to authorized users.
References
- Andresen MS, Dissing BS, Løje H. Quality assessment of butter cookies applying multispectral imaging. Food Sci Nutr. 2013;1(4):315–323. doi: 10.1002/fsn3.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr HY, Purcell EM. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Am J Physiol. 1954;94:630–638. [Google Scholar]
- Dissing BS, Nielsen ME, Ersbøll BK, Frosch S. Multispectral imaging for determination of astaxanthin concentration in salmonids. PLoS ONE. 2011;6(5):E19032. doi: 10.1371/journal.pone.0019032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissing BS, Papadopoulou OS, Tassou C, Ersbøll BK, Carstensen JM, Panagou EZ, Nychas G-J. Using multispectral imaging for spoilage detection of pork meat. Food Bioprocess Technol. 2013;6(9):2268–2279. doi: 10.1007/s11947-012-0886-6. [DOI] [Google Scholar]
- Eddy NO, Essien E, Ebenso ENOE, Ukpe RA. Industrial potential of two varieties of cocoyam in bread making. E J Chem. 2012;9(1):451–464. doi: 10.1155/2012/635894. [DOI] [Google Scholar]
- Falade KO, Okafor CA. Physicochemical properties of five cocoyam (Colocasia esculenta and Xanthosoma sagittifolium) starches. Food Hydrocol. 2013;30(1):173–181. doi: 10.1016/j.foodhyd.2012.05.006. [DOI] [Google Scholar]
- FAO . FAO statistical yearbook 2012. Rome: World food and agriculture. Food and Agriculture Organization of the United Nations; 2012. [Google Scholar]
- Feng Y, Sun D, Feng Y, Sun D. Application of hyperspectral imaging in food safety inspection and control: a review. Crit Rev Food Sci Nutr. 2015;52(11):1039–1059. doi: 10.1080/10408398.2011.651542. [DOI] [PubMed] [Google Scholar]
- Feyissa AH, Christensen MG, Pedersen SJ, Hickman M, Adler-Nissen J. Studying fluid-to-particle heat transfer coefficients in vessel cooking processes using potatoes as measuring devices. J Food Eng. 2015;163:71–78. doi: 10.1016/j.jfoodeng.2015.04.022. [DOI] [Google Scholar]
- Frosch S, Dissing BS, Adler-Nissen J, Nielsen ME (2011) Spectral imaging as a tool in food research and quality monitoring of food production. In: Akyar I (ed) Wide spectra of quality control. Chapter 20. INTECH Open Access Publisher. pp 373–383
- Hansen CL, Thybo AK, Bertram HC, Viereck N, van den Berg F, Engelsen SB. Determination of dry matter content in potato tubers by low-field Nuclear Magnetic Resonance (LF-NMR) J Agric Food Chem. 2010;58(19):10300–10304. doi: 10.1021/jf101319q. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Spectral methods of characterizing anthocyanins. Biochem J. 1958;70(1):22–28. doi: 10.1042/bj0700022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills BP, Le Floc’h G. NMR studies of non-freezing water in cellular plant tissue. Food Chem. 1994;51:331–336. doi: 10.1016/0308-8146(94)90035-3. [DOI] [Google Scholar]
- Huang H, Liu L, Ngadi MO. Recent developments in hyperspectral imaging for assessment of food quality and safety. Sensors. 2014;14(4):7248–7276. doi: 10.3390/s140407248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal OS. Composition, physicochemical properties and retrogradation characteristics of native, oxidised, acetylated and acid-thinned new cocoyam (Xanthosoma sagittifolium) starch. Food Chem. 2004;87:205–218. doi: 10.1016/j.foodchem.2003.11.013. [DOI] [Google Scholar]
- Martens HJ, Thybo AK. An integrated microstructural, sensory and instrumental approach to describe potato texture. LWT Food Sci Technol. 2000;33(7):471–482. doi: 10.1006/fstl.2000.0688. [DOI] [Google Scholar]
- Meiboom S, Gill D. Modified spin-echo method for measuring nuclear times. Rev Sci Instrum. 1958;29:688–691. doi: 10.1063/1.1716296. [DOI] [Google Scholar]
- Micklander E, Thybo AK, van den Berg F. Changes occurring in potatoes during cooking and reheating as affected by salting and cool or frozen storage—a LF-NMR study. LWT Food Sci Technol. 2008;41:1710–1719. doi: 10.1016/j.lwt.2007.10.015. [DOI] [Google Scholar]
- Mortensen M, Thybo AK, Bertram HC, Andersen HJ, Engelsen SB. Cooking effect on water distribution in potatoes using nuclear magnetic resonance relaxation. J Agric Food Chem. 2005;53:5976–5981. doi: 10.1021/jf0479214. [DOI] [PubMed] [Google Scholar]
- Mweta DE, Labuschagne MT, Bonnet S, Swarts J, Saka JDK. Isolation and physicochemical characterisation of starch from cocoyam (Colocasia esculenta) grown in Malawi. J Sci Food Agric. 2010;90(11):1886–1896. doi: 10.1002/jsfa.4029. [DOI] [PubMed] [Google Scholar]
- Nwokocha LM, Aviara NA, Senan C, Williams PA. A compartive study of some properties of cassava (Manihot esculenta, Crantz) and cocoyam (Colocasia esculenta, Linn) starches. Carbohydrate Poly. 2009;76:362–367. doi: 10.1016/j.carbpol.2008.10.034. [DOI] [Google Scholar]
- Onyeka J (2014) Status of cocoyam (Colocasia esculenta and Xanthosoma spp) in West and Central Africa: production, household importance and the threat from leaf Blight. CGIAR Research Program on Roots, Tubers and Bananas (RTB). Report available online at www.rtb.cgiar.org. Accessed 24 Feb 2016
- Opoku-Agyeman MO, Bennett-Lartey SO, Markwei C (2004) Agro-morphological and sensory characterization of cocoyam (Xanthosoma sagittifolium (L) (Schott) germplasm in Ghana. Ghana J Agric Sci vol 37:pp 23–31 (http://www.csir.org.gh). Accessed 14 May 2013)
- Owosu-Darko P, Paterson A, Omenyo EL. Cocoyam (corms and cormels)—an underexploited food and feed resource. J Agric Chem Environ. 2014;3(1):22–29. [Google Scholar]
- Pedersen HT, Bro R, Engelsen SB. Towards rapid and unique curve resolution of low-field NMR relaxation data: trilinear SLICING versus two-dimensional curve fitting. J Magn Res. 2002;157(1):141–155. doi: 10.1006/jmre.2002.2570. [DOI] [PubMed] [Google Scholar]
- Perez EE, Breene WM, Bahnassey YA. Gelatinization profiles of Peruvian carrot, cocoyam and potato starches as measured with the brabender viscoamylograph, rapid visco-analyzer, and differential scanning calorimeter. Starch. 1998;50(1):14–16. doi: 10.1002/(SICI)1521-379X(199801)50:1<14::AID-STAR14>3.0.CO;2-P. [DOI] [Google Scholar]
- Ramanatha RV, Matthews PJ, Eyzaguirre PB, Hunter D, editors. The global diversity of taro: ethnobotany and conservation. Rome: Bioversity International; 2010. [Google Scholar]
- Sefa-Dedeh S, Agyir-Sackey EK. Chemical composition and the effect of processing on oxalate content of cocoyam Xanthosoma sagittifolium and Colocasia esculenta cormels. Food Chem. 2004;85(4):479–487. doi: 10.1016/S0308-8146(02)00244-3. [DOI] [Google Scholar]
- Sefa-Dedeh S, Kofi-Agyir SE. Starch structure and some properties of cocoyam (Xanthosoma sagittifolium and Colocasia esculenta) starch and raphides. Food Chem. 2002;79(4):435–444. doi: 10.1016/S0308-8146(02)00194-2. [DOI] [Google Scholar]
- Srilakshmi B. Food Science. 3. New Delhi: New Age International; 2003. [Google Scholar]
- Thybo AK, Bechmann IE, Martens M, Engelsen SB. Prediction of sensory texture of cooked potatoes using uniaxial compression, near infrared spectroscopy and low field 1H NMR spectroscopy. LWT Food Sci Technol. 2000;33(2):103–111. doi: 10.1006/fstl.1999.0623. [DOI] [Google Scholar]
- Thygesen LG, Thybo AK, Engelsen SB. Prediction of sensory texture quality of boiled potatoes from low-field 1H NMR of raw potatoes. The role of chemical constituents. LWT Food Sci Technol. 2001;34:469–477. doi: 10.1006/fstl.2001.0788. [DOI] [Google Scholar]
- Thygesen LG, Blennow A, Engelsen SB. The effects of amylose and starch phosphate on starch gel retrogradation studied by low-field 1H NMR relaxometry. Starch. 2003;55:241–249. doi: 10.1002/star.200390062. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.