Abstract
From ancient times, plants and plant derived products are exploited as a prominent source of folkloric medicines with tremendous therapeutic potential for an array of health disorders. In the present study, ethanolic leaf extract of Hibiscus sabdariffa and Croton caudatus were evaluated for free radical scavenging activity in Saccharomyces cerevisiae model system. H. sabdariffa and C. caudatus showed tremendous DPPH free radical scavenging potential with an IC50 value of 184.88 and 305.39 µg/mL respectively at a concentration of 500 µg/mL. The ethanolic leaf extract of H. sabdariffa and C. caudatus also showed significant hydoxyl radical scavenging and total antioxidant activity. Ascorbic acid was used as positive control. The in vitro antioxidant activity was further supported by in vivo studies using radical scavenging mechanism in S. cerevisiae wild type and its isogenic deletion strains sod1∆ and tsa1∆. The mutant yeast cells substantially scavenged the stress generated by H2O2 when supplemented with ethanolic leaf extract of H. sabdariffa and C. caudatus as evident from spot assays followed by fluorescence assay (DCF-DA) using fluorescence microscopic and intensity studies. H. sabdariffa and C.caudatus significantly neutralize the ROS level in yeast mutants with concomitant decrease in fluorescence intensity as compared to the untreated yeast cells. The results suggested the efficacy of H. sabdariffa and C. caudatus as potent antioxidants in yeast system and thus their futuristic applications in therapeutics.
Keywords: Antioxidant activity, DPPH, Dichlorofluorescin, Saccharomyces cerevisiae, Reactive oxygen species
Introduction
In living organisms free radicals are invariably generated as a result of normal metabolic processes. However, when the production of these free radicals such as hydroxyl, peroxyl and superoxide radicals exceeds the threshold limit due to some physiological factors it leads to generation of oxidative stress (Benharlal and Arumughan 2007). This oxidative stress can cause severe oxidative damage to the biological macromolecules such as proteins, lipids, carbohydrates, nucleic acids directly or indirectly. Besides, oxidative stress can trigger several cellular processes like vasodilation, signal transduction, cell differentiation, development and a number of degenerative diseases such as diabetes, atherosclerosis, inflammatory diseases, cancer etc. (Islam et al. 2013).
Though the generation of excessive oxidative stress induces a remarkable impact on cellular and physiological processes of living systems; living cells constitute a unique and powerful radical scavenging machinery to counteract cellular injury. However, under the influence of ageing and other physiological factors these endogenous systems become inefficient and require exogenous antioxidant supplementation. In this context, there is an increasing attention towards the use of natural antioxidants such as ascorbic acid, phenolic compounds from plant origin in attenuating oxidative stress by maintaining the redox system of the living system (Mohamed et al. 2013).
Hibiscus sabdariffa L., (Malvaceae), commonly known as “roselle”, is an important medicinal plant native to India and Malaysia. H. sabdariffa is rich in protocatechuic acid and anthocyanin which enables the plant extracts in effective treatment of cancer, hypertension, inflammation, mutagenecity, leukaemia and gastrointestinal disorders (Mohd-Esa et al. 2010; Yin et al. 2011). H. sabdariffa extracts from calyces have been reported for significant antioxidant properties due to presence of bioactive compounds such as delphinidin-3-O-glucoside, delphinidin-3-O-sambubioside, cyanidin-3-O-sambubioside, polyphenolic compounds and organic acids (Formagio et al. 2015; Tahir et al. 2016).
Croton caudatus Geisel. is an extensive plant of family Euphorbiaceae with potent therapeutic uses in convulsions, malaria, gastrointestinal disorders, rheumatic arthritis, liver disorders, cancer etc. (Nath et al. 2013; Rosangkima and Jagetia 2015). C. caudatus stem extract contains a majority of flavonoids such as sinensetin, kaemferol, nobiletin, crotoncaudatin, tangeretin etc. which aid in free radical scavenging activity (Zou et al. 2010).
In the present study, the antioxidant potential of H. sabdariffa and C. caudatus leaf extract was evaluated in Saccharomyces cerevisiae model system using the antioxidant mutants, sod1∆ and tsa1∆. Hence, the present study aims to exploit the mechanism of radical scavenging in the sub-cellular system of yeast mutants, deficient in endogenous antioxidant mechanism. As Superoxide dismutase (SOD) and thioredoxin peroxidase (TSA) are mainly responsible for determining the oxidative stress response in S. cerevisiae, sod1∆ and tsa1∆ are frequently exploited for evaluating the scavenging efficacy of antioxidants in deficient endogenous antioxidant system (Kerdsomboon et al. 2015). Therefore, in the present study, antioxidant potential of ethanolic leaf extract of H. sabdariffa and C. caudatus were evaluated in S. cerevisiae model system.
Materials and methods
Chemicals and reagents
The chemicals used in the present study are ascorbic acid, 2, 2-diphenyl-1-picrylhydrazyl (DPPH), potassium ferricyanide [K3Fe(CN)6], trichloroacetic acid (TCA), ferric chloride (FeCl3), sulphuric acid (H2SO4), ammonium molybdate ([NH4]6Mo7O2·4H2O), 2-deoxyribose, ethylene diamine tetraacetic acid (EDTA), thiobarbituric acid (TBA), ferrous sulphate (FeSO4), hydrogen peroxide (H2O2), sodium hydroxide (NaOH) and dichloro-fluorescin diacetate (DCF-DA). All the chemicals were purchased from Himedia Labratories, India.
Collection of plant samples
The leaf samples of plants H. sabdariffa and C. caudatus were collected from Manipur, India. The leaf samples were shade dried and grounded to coarse powder form before extraction.
Extract preparation
For sample preparation, 50 g of samples were extracted twice in 200 mL of ethanol and kept for 2–3 days in continuous shaking condition (150 rpm). The obtained extracts were filtered and the filtrates were concentrated using rotary evaporator at 40 °C (Saeed et al. 2012).
Determination of in vitro antioxidant activity
DPPH radical scavenging assay
The free radical scavenging activity of ethanolic extract of H. sabdariffa and C. caudatus was determined in vitro by DPPH radical scavenging assay according to the method described by Eshwarappa et al. 2014 with slight modification. Briefly, 0.2 mM DPPH (in methanol) was mixed with different concentration of the sample (100–500 μg/mL) and incubated in the dark for 30 min at room temperature. The absorbance was taken at 517 nm. The control was prepared as above without any sample. The scavenging activity was estimated based on the percentage of DPPH radical scavenged as the following equation:
Reducing power
The reducing power assay was based on the reduction of ferric to ferrous form indicated by the formation of Prussian blue complex at 700 nm. Briefly, different concentrations of the sample were mixed with phosphate buffer (0.2 M, pH 6.6) and 1% K3Fe(CN)6. The reaction mixture was incubated at 50 °C for 20 min followed by addition of 10% TCA. The mixture was centrifuged at 3000 rpm for 10 min. The supernatant was mixed with deionized water and 0.1% (w/v) of freshly prepared FeCl3. After 10 min of reaction, the absorbance was measured at 700 nm (Aqil et al. 2012).
Hydroxyl radical scavenging assay
The effect of ethanolic leaf extract of H. sabdariffa and C. caudatus on hydroxyl radicals was determined by using the deoxyribose method described by Tounkara et al. 2014 with slight modifications (Tounkara et al. 2014). The reaction mixture contained 0.2 M sodium phosphate buffer (pH 7.0), 10 mM 2-deoxyribose, 10 mM FeSO4-EDTA, 10 mM H2O2 and different concentration of sample (100–500 µg/mL). The reaction was started by the addition of H2O2 and incubated at 37 °C for 4 h followed by the addition of 2.8% TCA and 1% TBA in 50 mM NaOH. The resulting solution was boiled in a water bath for 10 min and then cooled to room temperature. The absorbance of the solution was measured at 532 nm. The ability to scavenge the hydroxyl radical was calculated using the following equation:
Total antioxidant assay
The total antioxidant activity of ethanolic extract of H. sabdariffa and C. caudatus was determined by phosphomolybdate method. Briefly, different concentration of sample (100–500 µg/ml) solution was mixed with reagent solution (comprising of 0.6 M H2SO4, 28 mM sodium phosphate and 4 mM ([NH4]6Mo7O2·4H2O)). The reaction mixture was incubated in a water bath at 95 °C for 90 min followed by cooling at room temperature. The absorbance of the mixture was measured at 695 nm. The total antioxidant activity of the ethanolic fractions of the plants was expressed as ascorbic acid equivalents (Kalaivani et al. 2011).
GC–MS analysis
The presence of important secondary metabolites in ethanolic leaf extract of H. sabdariffa and C. caudatus was determined by GC–MS analysis. The spectrums of the components were compared with the GC–MS NIST (2008) library (de Almeida et al. 2013).
Determination of in vivo antioxidant activity
Yeast strain, media and growth condition
In the present study, wild type strain BY4741 and their isogenic deletion strains sod1∆ and tsa1∆ of S. cerevisiae were used to validate the antioxidant efficacy of ethanolic leaf extract of H. sabdariffa and C. caudatus. Yeast cells were obtained after the middle of first exponential phase (OD600 = 0.5) in liquid YPD medium (1% yeast extract, 2% peptone and 2% glucose) using an orbital shaker at 160 rpm and 30 °C (Frassinetti et al. 2012).
H2O2 sensitivity assay
To evaluate the sensitivity against oxidative agents (H2O2), overnight cultures of yeast strains were used. Briefly, 20 µL of exponential phase of the S. cerevisiae cultures were taken and serially diluted in a 96-well plate and spotted onto different concentration of H2O2 (1, 1.5, 2, 2.5, 3 and 4 mM) treated YPD agar (YPDA) plates and incubated at 30 °C for 24–48 h to assess the sensitivity of the yeast mutants to a varied degree of stressing agent (Wu et al. 2011).
Spot assay
Wild type S. cerevisiae and their mutant strains were used to assess the antioxidant efficacy of ethanolic leaf extracts of H. sabdariffa and C. caudatus. Briefly, overnight S. cerevisiae cells (OD600 = 0.5) with and without exposure to ethanolic plant extracts (500 µg/mL) and oxidative agent (2.5 mM) were incubated for 1 h each in YPD medium at 160 rpm and 30 °C. After incubation, subsequent dilutions (10−1, 10−2, 10−3 and 10−4) were made for all the strains in a 96 well-plate. The cells were then spotted onto YPDA plates and incubated at 30 °C for 24 h (Golla and Raj Bhimathati 2014).
ROS detection assay
DCF-DA method was performed to detect the level of cellular ROS and their subsequent removal by ethanolic leaf extract of H. sabdariffa and C. caudatus as described by Azad et al. 2014 with slight modifications (Azad et al. 2014). Briefly, cells were grown in YPD medium until A600 reached 0.5. The cultures were treated with different concentration of ethanolic leaf extracts (100–500 µg/mL) and incubated at 30 °C for 1 h H2O2 (1 mM) was added to the reaction mixture and incubated for 30 min followed by centrifugation (6000 rpm, 10 min) to collect the cells. The cells were then washed thrice with 1X phosphate-buffered saline (PBS). Cells were resuspended in PBS with 0.8 µL of 20 µM 2′, 7′-dichlorofluorescein diacetate (DCF-DA) and incubated at 30 °C for 1 h in dark. The fluorescent dye reacts specifically with H2O2 to give a highly fluorescent DCF. Cells were collected, washed three times with PBS, of which 10 µL was mounted onto slides and examined under fluorescent microscope with an excitation and emission wavelength of 485 and 529 nm respectively. The remaining cells were transferred into 96-well plate and fluorescent intensity of the samples was measured using plate reader. A blank was prepared consisting of PBS without any sample.
Statistical analysis
All the experiments were conducted in triplicate. All the values were represented as mean ± standard deviation. All the in vitro antioxidant results and datas obtained from the fluorescent intensity studies were subjected for Tukey–Kramer multiple comparison test (Q-test) followed by one-way ANOVA to analyze whether there exist significant difference in the antioxidant activities shown by the test samples as compared to the control at varied level of significance. For statistical analyses, p values <0.05 were considered significant (Huang et al. 2015).
Results and discussion
Determination of in vitro antioxidant activity
DPPH radical scavenging assay
The ability of ethanolic leaf extracts to scavenge free radicals was determined by DPPH assay. DPPH free radical scavenging activity exhibited scavenging potential of ethanolic leaf extract of H. sabdariffa and C. caudatus in a concentration dependent manner. From the results, it was observed that H. sabdariffa showed a scavenging potential of 65.19 ± 1.63% at 500 µg/mL with an IC50 value of 184.88 µg/mL which was comparatively higher than the previous report (Tahir et al. 2016). Meanwhile, C. caudatus showed 57.86 ± 1.69% of free radical scavenging (Table 1). The IC50 value for C. caudatus was observed to be 305.39 µg/mL is significantly higher than the earlier report with an IC50 of 396.20 µg/mL (Qiasar et al. 2013).
Table 1.
DPPH free radical scavenging and hydroxyl radical scavenging potential of ethanolic leaf extract of Croton caudatus and Hibiscus sabdariffa at 500 µg/mL
| Ethanolic leaf extract and positive control | DPPH radical scavenging activity | OH radical scavenging activity | ||
|---|---|---|---|---|
| Percentage of DPPH activity (%) | IC50 (µg/mL) | Percentage of OH· radical scavenging (%) | IC50 (µg/mL) | |
| C. caudatus | 57.86 ± 1.69* | 305.39 | 59.99 ± 1.52*** | 335.07 |
| H. sabdariffa | 65.19 ± 1.63* | 184.88 | 65.55 ± 2.45 | 281.42 |
| Ascorbic acid | 74.68 ± 3.19 | 2.83 | 67.37 ± 2.88 | 3.83 |
Radical scavenging percentage values were represented as Mean ± standard deviation (SD) of 3 replicates (n = 3). Ascorbic acid was used as positive control
All the values are expressed as Mean ± SD (n = 3)
* Values are significantly different from positive control (ascorbic acid) at p < 0.05
** Values are significantly different from positive control (ascorbic acid) at p < 0.01
*** Values are significantly different from positive control (ascorbic acid) at p < 0.001
Reducing power
In the present study, an increase in the absorbance was observed with a concomitant increase in the concentration of ethanolic leaf extracts with a characteristic change in colour from yellow to green in colour which eventually enhances the reducing power. In the present study, out of the 2 plants studied, H. sabdariffa showed higher reducing power as compared to C. caudatus as evident from their absorbance of 0.43 ± 0.01 and 0.375 ± 0.01 respectively at a concentration of 500 µg/mL (Table 2). The result of present study was in accordance with the previous report (Tounkara et al. 2014).
Table 2.
Reducing Power and total antioxidant activity of ethanolic extract of Croton caudatus and Hibiscus sabdariffa at 500 µg/mL
| Ethanolic leaf extract and positive control | Reducing power (absorbance at 700 nm) | Total antioxidant activity (Ascorbic acid equivalents in µg/mL) |
|---|---|---|
| C. caudatus | 0.387 ± 0.02*** | 77.07 ± 4.16* |
| H. sabdariffa | 0.439 ± 0.016* | 96.72 ± 4.88 |
| Ascorbic acid | 0.479 ± 0.026 |
All the values were represented as Mean ± standard deviation (SD) of 3 replicates (n = 3). Ascorbic acid was used as positive control
All the values are expressed as Mean ± SD (n = 3)
* Values are significantly different from positive control (ascorbic acid) at p < 0.05
** Values are significantly different from positive control (ascorbic acid) at p < 0.01
*** Values are significantly different from positive control (ascorbic acid) at p < 0.001
Hydroxyl radical scavenging
From the OH· radical scavenging results, it was observed that the ethanolic leaf extract scavenged highly reactive OH· radical in a concentration dependent manner. In the present study, H. sabdariffa showed relatively higher OH· radical scavenging (65.55 ± 2.45%) with an IC50 of 281.42 µg/mL at a concentration of 500 µg/mL This result was comparatively higher than the previous report with a scavenging percentage of 62.30% at a concentration of 10 mg/mL (Tounkara et al. 2014). Meanwhile, C. caudatus extract exhibited 59.99 ± 1.52% of OH· radical scavenging activity with an IC50 of 335.07 µg/mL (Table 1). The potential of scavenging highly reactive OH· radicals by the ethanolic extract of H. sabdariffa and C. caudatus suggested its efficacy in minimizing the effects of lipid peroxidations in biological systems.
Total antioxidant activity
The total antioxidant activity of ethanolic leaf extract of H. sabdariffa and C. caudatus revealed that with an increase in concentration there is an increase in absorbance which corresponds to the enhanced total antioxidant activity. The ethanolic extract of H. sabdariffa and C. caudatus showed an ascorbic acid equivalent of 96.72 ± 4.88 and 77.07 ± 4.16 µg/mL respectively (Table 2). This result coincide with the previous report where the ethanolic extract showed the most prominent antioxidant activity as compared to other extraction medium (Kalavani et al. 2011).
GC–MS analysis
GC–MS analysis of ethanolic leaf extract of H. sabdariffa showed the presence of phytoconstituents like phytol, alpha-tocopherol, methyl linolenate, ethyl palmitate and ethyl linolenate in substantial quantity. Meanwhile, ethanolic leaf extract of C. caudatus showed the presence of phytol and hexamethyl cyclotrisiloxane in significant quantity in the GC–MS analysis (Table 3).
Table 3.
List of phytochemicals present in the ethanolic leaf extract of H. sabdariffa and C. caudatus from the GC–MS analysis
| Sl no. | Compound name | Common name | Retention time (RT) (min) | Biological activities | References |
|---|---|---|---|---|---|
| Ethanolic leaf extract of Hibiscus sabdariffa | |||||
| 1. | Hexadecanoic acid ethyl ester | Ethyl palmitate | 18.13 | Antioxidant | Sudha et al. 2013 |
| 2. | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | Phytol | 16.759 | Antiradical, antimicrobial, antioxidant, antityrosinase, antinociceptive activity, anti-inflammatory activity | Santos et al. 2013; Silva et al. 2013; Pejin et al. 2014; Zhang et al. 2016 |
| 3. | Alpha tocopherol | Vitamin E | 27.173 | Radical scavenging activity, antioxidant activity | Vargas et al. 2014; Shah et al. 2016 |
| 4. | 9,15-octadecadienoic acid, methyl ester | Methyl linolenate | 19.66 | Antioxidant activity | Sharma et al. 2015 |
| 5. | 9,12,15-octadecatrienoic acid, ethyl ester | Ethyl linolenate | 19.75 | Antioxidant activity | Sharma et al. 2015 |
| Ethanolic leaf extract of Croton caudatus | |||||
| 1. | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | Phytol | 17.02 | Antioxidant | Pejin et al. 2014; Zhang et al. 2016; Shibula and Velavan 2015 |
| 2. | Cyclotrisiloxane, hexamethyl | Hexamethyl Cyclotrisiloxane |
29.32 | Antioxidant | Falowo et al. 2016 |
The presence of natural antioxidants such as phytol and alpha tocopherol as phytoconstituents in the ethanolic extracts of H. sabdariffa leaves suggested the enhancement in the antioxidant activity in a multivariate manner both in vitro as well as in cellular stress response system in S. cerevisiae (Santos et al. 2013; Silva et al. 2013; Pejin et al. 2014; Vargas et al. 2014; Zhang et al. 2016; Shah et al. 2016). In addition to these phytoconstituents, the prevalence of fatty acids and fatty acid esters also aid to a significant increase in the antioxidant potential of H. sabdariffa in S. cerevisiae system which is reported for the first time (Sudha et al. 2013; Sharma et al. 2015). In the present study, C. caudatus leaf extract also exhibited significant antioxidant activity due to presence of substantial amount of phytol and hexamethyl cyclotrisiloxane as evident from the GC–MS analysis (Shibula and Velavan 2015; Falowo et al. 2016).
In vivo antioxidant activity
H2O2 sensitivity
H2O2 sensitivity test revealed that S. cerevisiae isogenic deletion mutants (sod1∆ and tsa1∆) donot showed any sign of sensitivity until the concentration of H2O2 reached 2.5 mM. However, with an increase in concentration, both mutant strains invariably showed varied degree of sensitivity. Hence, 2.5 mM of H2O2 was used for subsequent in vivo studies.
Spot assay
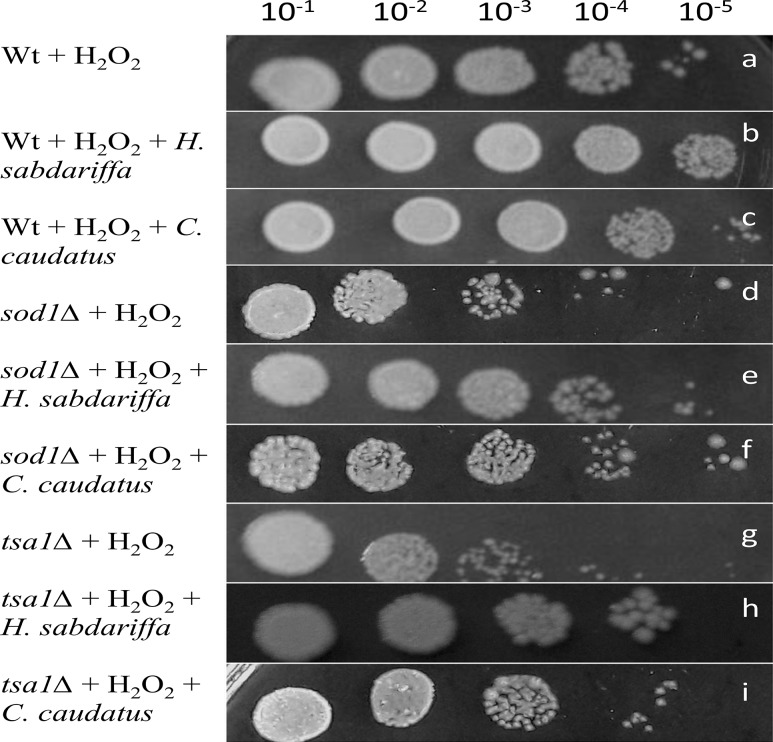
A significant difference in the growth pattern was observed in the stressed yeast cells in presence or absence of ethanolic leaf extract of H. sabdariffa and C. caudatus. This suggested the efficacy of the plant extract in ameliorating the stress generated by H2O2 in antioxidant deficient sod1∆ and tsa1∆ mutants. In the present study, out of the 2 test plants H. sabdariffa showed comparatively higher potential to scavenge the stress generated by H2O2 (Fig. 1). The spot assay suggested the efficacy of H. sabdariffa and C. caudatus leaf extract by decreasing the cytotoxicity generated by H2O2 (Slatnar et al. 2012).
Fig. 1.
Effect of ethanolic leaf extracts of Hibiscus sabdariffa and Croton caudatus on the growth of yeast cells (wild type and mutants) by spot assay. a Wt yeast cells treated with H2O2, negative control; b Wt yeast cells treated with H2O2 and ethanolic leaf extract of H. sabdariffa; c Wt yeast cells treated with H2O2 and ethanolic leaf extract of C. caudatus; d sod1∆ yeast cells treated with H2O2, negative control; e sod1∆ yeast cells treated with H2O2 and ethanolic leaf extract of H. sabdariffa; f sod1∆ yeast cells treated with H2O2 and ethanolic leaf extract of C. caudatus; g tsa1∆ yeast cells treated with H2O2, negative control; h tsa1∆ yeast cells treated with H2O2and ethanolic leaf extract of H. sabdariffa; i tsa1∆ yeast cells treated with H2O2 and ethanolic leaf extract of C. caudatus
ROS detection
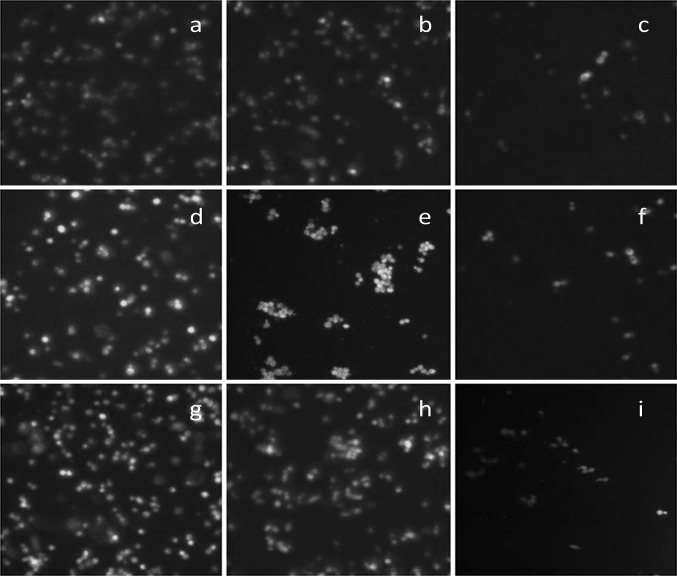
The ability of ethanolic extract of H. sabdariffa and C. caudatus to protect the yeast cells from the damaging effect of oxidative stress was examined by DCF-DA assay by measuring the level of intracellular H2O2. The evaluation of intracellular ROS level acts as a prime marker to determine the level of oxidative damage in yeast cells in absence/presence of plant extracts (Frassinetti et al. 2012). The results showed that the level of fluorescence was comparatively higher in stressed S. cerevisiae mutant strains in absence of any plant extracts (negative control), as the fluorescent stain specifically fluoresce more with concomitant higher level of subcellular H2O2. However, on treatment with H. sabdariffa and C. caudatus leaf extract, the fluorescence significantly decreased as evident from fluorescent intensity studies that can be correlated with concomitant decrease in the intracellular ROS level in S. cerevisiae mutants (Figs. 2, 3).
Fig. 2.
Determination of intracellular reactive oxygen species (ROS) in the S. cerevisiae cells (both Wt and mutants) by fluorescent microscopic studies using DCF-DA assay under the influence of ethanolic extract of Hibiscus sabdariffa and Croton caudatus. a Wt yeast cells treated only with H2O2, negative control; b Wt yeast cells treated with H2O2 and C. caudatus; c Wt yeast cells treated with H2O2 and H. sabdariffa; d sod1∆ yeast cells treated with H2O2, negative control; e sod1∆ yeast cells treated with H2O2 and C. caudatus; f sod1∆ yeast cells treated with H2O2 and H. sabdariffa; g tsa1∆ yeast cells treated only with H2O2, negative control; h tsa1∆ yeast cells treated with H2O2 and C. caudatus; i tsa1∆ yeast cells treated with H2O2 and H. sabdariffa
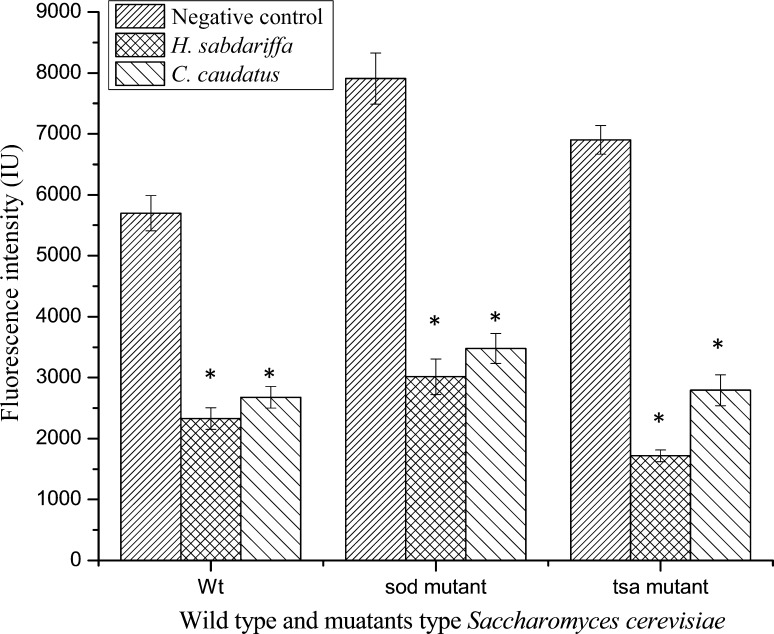
Fig. 3.
Effect of ethanolic leaf extract of Hibiscus sabdariffa and Croton caudatus on the intracellular ROS levels estimated by the intensity of fluorescence. All the values were represented as mean ± SD (n = 3). *** Values are significantly different from positive control (ascorbic acid) at p < 0.001, ** values are significantly different from positive control (ascorbic acid) at p < 0.01, * values are significantly different from positive control (ascorbic acid) at p < 0.05
H. sabdariffa showed the highest scavenging potential with a percentage decrease of 59.13, 61.88 and 75.10% in the fluorescence intensity in wild type, sod1∆ and tsa1∆ mutant strains respectively as compared to the negative control. Meanwhile, ethanolic leaf extract of C. caudatus exhibited 53.03, 56.01 and 59.65% decrease in fluorescence intensity in wild type, sod1∆ and tsa1∆ mutant strains respectively as compared to the negative control. From the results, it was observed that both H. sabdariffa and C. caudatus are highly reactive towards tsa1∆ mutant as evident from higher percentage decrease in fluorescence intensityas compared to the negative control. The reason behind the decrease in fluorescence intensity in the plant extract treated yeast cells might be the low level of intracellular ROS which is insufficient to oxidize DCF-DA to highly fluorescent DCF (Wu et al. 2009).
Due to excessive production of ROS, the endogenous antioxidant system may fail to scavenge the free radicals in an effective manner. In this context, there is a growing demand for supplementing natural antioxidants exogenously to maintain a proper balance in the system as the application of synthetic antioxidants possess severe carcinogenic properties and other health ailments (Wang et al. 2014). Plants and plant derived products are the richest source of phytoconstituents and can be utilized for attenuating the oxidative stress and their side effects (Aderogba et al. 2011). In that context, in the present study, an attempt was made to evaluate the scavenging potential of ethanolic leaf extract of H. sabdariffa and C. caudatus in attenuating the level of ROS generated in the stress response system in S. cerevisiae wild type and their mutants as model system for the 1st time.
From the in vitro and in vivo antioxidant studies of H. sabdariffa and C. caudatus leaf extract unwrapped the efficacy of these plant extracts as an efficient antioxidant in S. cerevisiae model system which was reported for the first time. This result suggested the use of these plants as remarkable alternative to synthetic antioxidants and thus established their use in ethnopharmacological applications.
Conclusion
In the present study, both H. sabdariffa and C. caudatus leaf extract showed significant antioxidative potential in vitro as well as in the S. cerevisiae model system specifically targeting the antioxidant deficient mutant yeast cells, suggesting their multitudinal application in radical scavenging activity and widespread pharmacological potential.
Compliance with ethical standards
Conflict of interest
The authors are declaring no conflict of interests in this work.
References
- Aderogba MA, McGaw LJ, Bezabih M, Agebaz BM. Isolation and characterization of novel antioxidant constituents of Croton zambesicus leaf extract. Nat Prod Res. 2011;25(13):1224–1233. doi: 10.1080/14786419.2010.532499. [DOI] [PubMed] [Google Scholar]
- Aqil F, Gupta A, Munagala R, Jeyabalan J, Kausar H, Sharma R, Singh IP, Gupta RC. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry) Nutr Cancer. 2012;64(3):428–438. doi: 10.1080/01635581.2012.657766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad GK, Singh V, Tomar RS. Assessment of the biological pathways targeted by isocyanate using N-Succinimidyl N-methylcarbamate in budding yeast Saccharomyces cerevisiae. PLoS ONE. 2014;9(3):e92993. doi: 10.1371/journal.pone.0092993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benharlal PS, Arumughan C. Chemical composition and in vitro antioxidant studies on Syzigium cumini fruit. J Sci Food Agr. 2007;87:2560–2569. doi: 10.1002/jsfa.2957. [DOI] [PubMed] [Google Scholar]
- De Almeida TS, Rocha JBT, Rodrigues FFG, Campos AR, Da Costa JGM. Chemical composition, antibacterial and antibiotic modulatory effect of Croton campestris essential oils. Indust Crops Prod. 2013;44:630–633. doi: 10.1016/j.indcrop.2012.09.010. [DOI] [Google Scholar]
- Eshwarappa RSB, Iyer RS, Subbaramaiah SR, Richard SA, Dhanajaya BL. Antioxidant activity of Syzigium cumini leaf gall extracts. Bioimpacts. 2014;4(2):101–107. doi: 10.5681/bi.2014.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falowo AB, Muchenje V, Hugo A, Aiyegoro OA, Fayemi PO. Antioxidant activities of Moringa oleifera L. and Bidens pilosa L. leaf extracts and their effects on oxidative stability of ground raw beef during refrigeration storage. CYTA J Food. 2016;15(2):1–8. doi: 10.1080/19476337.2016.1162847. [DOI] [Google Scholar]
- Formagio ASN, Ramos DD, Vieira MC, Ramalho SR, Silva MM, Zarate NAH, Foglio MA, Carvalho JE. Phenolic compounds of Hibiscus sabdariffa and influence of organic residues on its antioxidant and antitumoral properties. Braz J Biol. 2015;75(1):69–76. doi: 10.1590/1519-6984.07413. [DOI] [PubMed] [Google Scholar]
- Frassinetti S, Croce CMD, Caltavuturo L, Longo V. Antimutagenic and antioxidant activity of Lisosan G in Saccharomyces cerevisiae. Food Chem. 2012;135:2029–2034. doi: 10.1016/j.foodchem.2012.06.090. [DOI] [PubMed] [Google Scholar]
- Golla U, Raj Bhimathati SS (2014) Evaluation of antioxidant and DNA damage protection activity of the hydroalcoholic extract of Desmostachya bipinnata L. Stapf. Sci World J 2014: Article ID: 215084 [DOI] [PMC free article] [PubMed]
- Huang C, Song P, Fan P, Hou C, Thacker P, Ma X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J Nutr. 2015;145(12):2774–2780. doi: 10.3945/jn.115.217406. [DOI] [PubMed] [Google Scholar]
- Islam S, Nasrin S, Khan MA, Hossain ASMS, Islam F, Khandokhar P, Mollah MNH, Rashid M, Sadik G, Rahman MAA, Alam AHMK. Evaluation of antioxidant and anticancer properties of the seed extracts of Syzigium fruticosum Roxb. Growing in Rajshahi, Bangladesh. BMC Compl Altern Med. 2013;13:142–151. doi: 10.1186/1472-6882-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaivani T, Rajasekaran C, Suthindhiran K, Mathew L (2011) Free radical scavenging, cytotoxic and hemolytic activities from leaves of Acacia nilotica (L.) wild ex. Delile subsp. indica (Benth.) Brenan. Evid Compl Altern Med 2011:Article ID:274741 [DOI] [PMC free article] [PubMed]
- Kerdsomboon K, Tatip S, Kosasih S, Auesukaree C. Soluble Moringa oleifera leaf extract reduces intracellular cadmium accumulation and oxidative stress in Saccharomyces cerevisiae. J Biosci Bioeng. 2015;21(5):543–549. doi: 10.1016/j.jbiosc.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Mohamed AA, Ali SI, El-Baz FK. Antioxidant and antibacterial activities of crude extracts and essential oils of Syzigium cumini leaves. PLoS ONE. 2013;8(4):e60269. doi: 10.1371/journal.pone.0060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd-Esa N, Hern FS, Ismail A, Yee CL. Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem. 2010;122:1055–1060. doi: 10.1016/j.foodchem.2010.03.074. [DOI] [Google Scholar]
- Nath R, Roy S, De B, Choudhury MD. Anticancer and antioxidant activity of Croton: a review. Int J Pharm Pharmaceut Sci. 2013;5(2):63–70. [Google Scholar]
- Pejin B, Savic A, Sokovic M, Glamoclija J, Ciric A, Nikolic M, Radotic K, Mojovic M. Further in vitro evaluation of antiradical and antimicrobial activities of phytol. Nat Prod Res. 2014;28(6):372–376. doi: 10.1080/14786419.2013.869692. [DOI] [PubMed] [Google Scholar]
- Qaisar MN, Chaudary BA, Uzair M, Hussain SN. Evaluation of antioxidant and cytotoxic capacity of Croton bonplandianum. Baill. Am J Plant Sci. 2013;4:1709–1712. doi: 10.4236/ajps.2013.49208. [DOI] [Google Scholar]
- RosangkimaG Jagetia GC. Anticancer, antioxidant and analgesic properties of Croton caudatus Geisel leaf extracts. Int J Curr Res. 2015;7(9):20640–20646. [Google Scholar]
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Compl Altern Med. 2012;12:221–232. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CCMP, Salvadori MS, Mota VG, Costa LM, de Almeida AAC, de Oliveira GAL, Costa JP, de Sousa DP, de Freitas RM, de Almeida RN (2013) Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci J 2013:Article ID: 949452 [DOI] [PMC free article] [PubMed]
- Shah AA, Khan MS, Khan S, Ahmad N, Alhidary IA, Khan RU, Shao T. Effect of different levels of alpha tocopherol on performance traits, serum antioxidant enzymes, and trace elements in Japanese quail (Coturnix coturnix japonica) under low ambient temperature. Revista Brasileira de Zootecnia. 2016;45(10):622–626. doi: 10.1590/S1806-92902016001000007. [DOI] [Google Scholar]
- Sharma K, Pasricha V, Satpathy G, Gupta RK. Evaluation of phytochemical and antioxidant activity of raw Pyrus communis (l), an under exploited fruit. J Pharmacognosy Phytochem. 2015;3(5):46–50. [Google Scholar]
- Shibula K, Velavan S. Determination of phytocomponents in methanolic extract of Annona muricata leaf using GC-MS technique. Int J Pharmacognosy Phytochem Res. 2015;7(6):1251–1255. [Google Scholar]
- Silva RO, Sousa FBM, Damasceno SRB, Carvalho NS, Silva VG, Oliveira FRMA, Sousa DP, Aragao KS, Barbosa ALR, Freitas RM, Medeiros JVR. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fund Clin Pharmacol. 2013;28(4):455–464. doi: 10.1111/fcp.12049. [DOI] [PubMed] [Google Scholar]
- Slatnar A, Jakopic J, Stampar F, Veberic R, Jamnik P. The effect of bioactive compounds on in vitro and in vivo antioxidant activity of different berry juices. PLoS ONE. 2012;7(10):e47880. doi: 10.1371/journal.pone.0047880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudha TC, Mohan VR. GC-MS Analysis of Bioactive Components of Aerial parts of Fluggea leucopyrus Willd. (Euphorbiaceae) J Appl Pharmceut Sci. 2013;3(5):126–130. [Google Scholar]
- Tahir HE, Xiaobo Z, Jiyong S, Mariod AA, Wiliam T. Rapid determination of antioxidant compounds and antioxidant activity of Sudanese Karkade (Hibiscus sabdariffa L.) using near infrared spectroscopy. Food Anal Method. 2016;9:1228–1236. doi: 10.1007/s12161-015-0299-z. [DOI] [Google Scholar]
- Tounkara F, Bashari M, Le GW, Shi YH. Antioxidant activities of Roselle (Hibiscus sabdariffa L.) seed protein hydrolysate and its derived peptide fractions. Int J Food Prop. 2014;17(9):1998–2011. doi: 10.1080/10942912.2013.779700. [DOI] [Google Scholar]
- Vargas FS, Soares DG, Ribeiro APD, Hebling J, Costa CA (2014) Protective effect of alpha-tocopherol isomer from vitamin E against the H2O2 induced toxicity on dental pulp cells. Biomed Res Int 2014:Article ID:895049 [DOI] [PMC free article] [PubMed]
- Wang J, Cao X, Jiang H, Qi Y, Chin KL, Yue Y. Antioxidant activity of leaf extracts from different Hibiscus sabdariffa accessions and simultaneous determination five major antioxidant compounds by LC-Q-TOF-MS. Molecules. 2014;19:21226–21238. doi: 10.3390/molecules191221226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Steffen J, Eide DJ. Cytosolic Superoxide Dismutase (SOD1) is critical for tolerating the oxidative stress of zinc deficiency in yeast. PLoS ONE. 2009;4(9):e7061. doi: 10.1371/journal.pone.0007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MJ, O’Doherty PJ, Fernandez HR, Lyons V, Rogers PJ, Dawes IW, Higgins VJ. An antioxidant screening assay based on oxidant-induced growth arrest in Saccharomyces cerevisiae. FEMS Yeast Res. 2011;11:379–387. doi: 10.1111/j.1567-1364.2011.00726.x. [DOI] [PubMed] [Google Scholar]
- Yin G, Cao L, Xu P, Jeney G, Nakao M. Hepatoprotective and antioxidant effects of Hibiscus sabdariffa extract against carbon tetrachloride-induced hepatocyte damage in Cyprinus carpio. Vitro Anim Cell Dev Biol. 2011;47:10–15. doi: 10.1007/s11626-010-9359-2. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen J, Wang L, Cao J, Xu L. Chemical composition and biological activities of the essential oil from Rubus pungens var. oldhamii. Nat Prod Res. 2016;6:1–5. doi: 10.1080/14786419.2016.1253076. [DOI] [PubMed] [Google Scholar]
- Zou G, Su ZH, Zhang HW, Wang Y, Yang J, Zou ZM. Flavonoids from the stems of Croton caudatus Geisel. var. tomentosus Hook. Molecules. 2010;15:1097–1102. doi: 10.3390/molecules15031097. [DOI] [PMC free article] [PubMed] [Google Scholar]