Abstract
This work includes the evaluation of 168 samples of raspberries ‘Glen Lyon’, representing whole maturation period, by colorimetric and near infrared imaging techniques, as well as the quantification of total phenols, total anthocyanins and antioxidant activity by chemical methods. Samples showed significant differences depending on the maturation stage using CIELAB colour parameters and total anthocyanins content. The application of partial least squares regression allowed predicting the chemical features from image analysis data, with coefficients of determination (R2) up to 0.75. The best prediction for total anthocyanins including colorimetric data was observed. The proposed methodology can be used as a reference method for assessing important quality attributes of raspberries. Moreover, it is useful, rapid and accurate automatic inspection method.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2716-3) contains supplementary material, which is available to authorized users.
Keywords: Raspberry ‘Glen Lyon’ (Rubus idaeus L), Image analysis, Hyperspectral image analysis, Phenolics, Partial least squares regression
Introduction
Raspberry (Rubus idaeus) is a fruit native to Europe and northern Asia. It is a good source of compounds, with recognized bioactive properties, such as phenolic compounds, vitamins and carotenoids (Kalt et al. 1999; Pantelidis et al. 2007). Raspberry ‘Glen Lyon’ produces very glossy orange-red fruit of medium size and excellent skin firmness and shelf life. This cultivar has good resistance to Spur Blight (Didymella applanata infection) and is a very heavy yielding variety when grown on fertile soils with good management (Jennings 2002).
The cultivation of berries in South-western Spain has experienced a huge development within the last 30 years, producing high-quality fruits with a great economic repercussion mainly due to exportations. This fact increases the need of developing rapid techniques for monitoring quality parameters along the maturation, when the biosynthesis of bioactive compounds such as anthocyanins occurs (Dobson et al. 2012). Anthocyanins are pigments accounting for the brilliant red, purple and blue colours of many fruits, such as berries and red grapes, as well as vegetables and derived food products (Gordillo et al. 2012).
Colour and appearance are features that define the quality of food products, being the first attributes perceived in tasting and, frequently, the only criterion for consumer’s acceptation. That is why producers take this issue so seriously (Fernández-Vázquez et al. 2011). In this respect, Image Analysis has revolutionised the way of evaluating appearance in food (Sun 2004). Computing advances every day, producing faster and capable systems that broaden the scope of application in food industry. Because of the unceasing rise of hygiene and safety standards, the need for objective quality determination in food products continues growing (Brosnan and Sun 2004). Foodstuff usually have peculiar sizes and shapes, and digital image analysis is the best way to satisfy the requirements of measuring heterogeneous samples in an objective manner. Moreover, it allows measuring not only colour but also other features related to appearance, such as texture or heterogeneity, which do not vary the colour but affect how the human eye perceive it (Valous et al. 2009). Colour can be measured by Tristimulus Colorimetry and expressed in terms of CIELAB colour space (1976 L* a* b* colour space). It is an international standard for colour measurements, recommended by the Commission Internationale de l’Eclairage (CIE) in 1976. Within CIELAB, L* is a qualitative attribute of relative luminosity, and ranges between black (L* = 0) and white (L* = 100). Coordinate a* takes positive values for reddish colours and negative values for greenish ones. In the same way, b* takes positive values for yellowish colours and negative for bluish ones. a* and b* become to C* ab (chroma) and h ab (hue) when are converted to polar coordinates (Fig. 1), which are more useful parameters with meanings easier to understand according to the human perception. Taking into account the CIE guidelines, the colour measurement includes the complete visible spectrum. However, there are several studies that uses simple RGB digital cameras for colorimetric purposes (Hutchings 2002; León et al. 2006; Fernández-Vázquez et al. 2011; Rodríguez-Pulido et al. 2013).
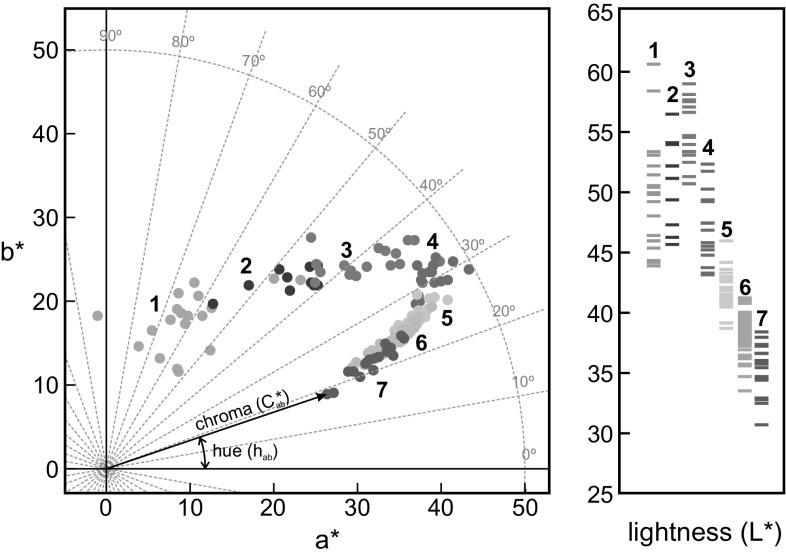
Fig. 1.
Scatterplot of colour data of raspberries samples regarding the stage of maturation (Lightness (L*), a* and b* are expressed in CIELAB units). a* b*-diagram also shows the correspondence between a*–b* and C*ab–hab)
Beyond the colour, near infrared (NIR) hyperspectral imaging is a technique that generates a spatial map of spectral information, making it a useful tool in many applications. These images, called hypercubes, are three-dimensional data matrices where two axes (x and y) represent the spatial coordinates, while the third (λ) axis portrays the spectral dimension. Hypercubes are easier to understand if we envision a battery of images of the same scene, each one representing the reflectance at one wavelength in greyscale. This way, hyperspectral imaging covers the potential of NIR spectroscopy and the versatility of computer vision.
Multivariate statistic techniques are essential for assessing the relationship between instrumental data and physicochemical properties of food products. Partial least squares regression (PLSR) is a common procedure used to relate a large number of independent variables, such spectra or colorimetric attributes, to one or few response variables (Kemsley et al. 1996; Esquerre et al. 2009). It is very effective in spectral analysis since it reduces a great number of redundant information (Lin et al. 2009; Pojić and Mastilović 2012).
The objective of this work was the overall study of the Glen Lyon raspberries maturation (from very early until overripe stages) by both colorimetric and NIR imaging techniques, assessing the capability of these techniques to predict the presence of some interesting compounds (phenols, anthocyanins) in these berries. The study considered one berry per sample to avoid possible errors of considering samples that include unequal berries in each maturation level group. To the best of our knowledge, there are not previous articles studying these berries individually, from very early stages of maturation, and merging these two kind of imaging techniques.
Materials and methods
Samples
Description and extraction procedure
One hundred and sixty-eight berries of Glen Lyon raspberry cultivar grown in South-western Spain (37.2º N, 6.5º W) and having very heterogeneous stages of maturation on February 2014 were considered in this study. Clearly unripe samples were small, hard, and pale green. On the contrary, overripe samples showed symptoms of degradation such as feeble flesh and sticky skin.
After collecting, the weight and size of every sample (it is highlighted that one sample means one berry) were measured, and the visible and NIR images were taken. Then, samples were stored at −20 °C until performing the chemical analyses.
The extraction of phenolic compounds was carried out following a modification of the methodology proposed by Seeram et al. (2006). Each berry was freeze-dried (lyophiliser Cryodos 80 Telstar, Spain) and grounded to a homogeneous powder (A 11 Analytical Mill, IKA, Staufen, Germany). Fifteen mg of powder were extracted with 500 μL of methanol containing 0.1% of HCl, sonicated during 15 min and centrifuged at 13,700 g (10 °C, 10 min) (Microfuge 22R, Beckman Coulter, Brea, CA), and the supernatant was collected. This procedure was performed four times more, until the solid residue had no coloration. The extract was concentrated at 30 °C under vacuum (Eppendorf Concentrator Plus, Hamburg, Germany) until elimination of methanol; finally, made up to 2 mL with HCl 0.1 M, and filtered through 0.5 μm pore-size filter. This amount of extract (2 mL) was enough to perform all the chemical analyses in triplicate.
Image analysis
Colorimetric analysis from digital images
The DigiEye® imaging system used (Luo et al. 2001) comprises an illumination cabinet (VeriVide Ltd., Leicester, UK) with fluorescent lamps that reliably simulate the CIE Standard Illuminant D65. A digital camera (Nikon D-80) attached at the top of the cabinet receives and stores the images in 48-bit TIFF format. By means of the certified colour chart DigiTizer (VeriVide Ltd., Leicester, UK), the software transforms from RGB to CIELAB colour spaces by applying advanced non-linear multivariate models. Besides colour of samples, heterogeneity was measured by using the mean colour difference from the mean (MCDM), proposed by Berns et al. (2000).
NIR hyperspectral image analysis
The acquisition of NIR hyperspectral images were made with an equipment that comprises the following parts: (a) Xenics® XEVA-USB InGaAs camera (Xenics Infrared Solutions, Inc., Leuven, Belgium); (b) spectrograph Specim ImSpector N17E Enhanced, that covers a range of 884–1717 nm (Spectral Imaging Ltd., Oulu, Finland); (c) two 70 W tungsten iodine halogen lamps as light source; (d) Mirror Scanner for scanning the sample surface (Spectral Imaging Ltd., Oulu, Finland); and (e) computer system to run the SpectralDAQ 3.62 acquisition software (Spectral Imaging Ltd., Oulu, Finland). A more detailed description as well as a scheme of this equipment are shown in Nogales-Bueno et al. (2014). Reflectance of images was normalised with respect to the reflectance of a white Spectralon® ceramic tile (Labsphere Inc., North Sutton, NH), and these reflectance values were transformed into absorbance (Log(1/R) units), better related to concentration and more adequate for the prediction models (Williams 2001; Williams et al. 2009; Rodríguez-Pulido et al. 2014).
Chemical analysis
Total phenolic content
The total phenolic content was determined using the Folin-Ciocalteu assay (Singleton and Rossi 1965). Briefly, 30 μL of extract, 900 μL of deionized water, 150 μL of Folin-Ciocalteu reagent, and 450 μL of a solution of sodium carbonate (20%) were mixed, and deionized water was added to make up a total volume of 3 mL. The solution was vortexed, reading the absorbance at 765 nm with an Agilent UV–vis 8453 spectrophotometer (Palo Alto, CA) after stand for 2 h to ensure the reaction takes place. The calibration standard used was gallic acid and results were expressed as gallic acid equivalents (mg gallic acid/g of fresh fruit).
Total anthocyanins
Total anthocyanins content was measured by the spectrophotometric method of Wrolstad et al. (2005). 200 μL of extract were added to 800 μL of buffer solution at pH 1.0. Another aliquot of 200 μL of extract was added to 800 μL of buffer solution at pH 4.5. Absorbance values at 520 and 700 nm were measured after 15 min to calculate the total amount of anthocyanins as follows:
being A = (A520–A700)pH1.0 − (A520–A700)pH4.5, MW = molecular weight (malvidin-3-glucoside = 493.2 g × mol−1), DF = dilution factor, ε = molar extinction coefficient, (malvidin-3-glucoside = 20,200 L × mol−1 × cm−1), and l = pathlength.
The results were transformed to mg malvidin-3-glucoside per gram of fresh fruit.
Antioxidant capacity
The FRAP method (Benzie and Strain 1996) was applied to determine the antioxidant capacity, with a modification adapted to the analysis of food samples (Pulido et al. 2000; Saura-Calixto and Goñi 2006), expressing the results as equivalents of Trolox, a water-soluble vitamin E analogue. The method consists on the reduction of the complex Fe3+-TPTZ to Fe2+-TPTZ in presence of antioxidants, and the reduced complex has a maximum of absorbance measurable at 593 nm by spectrophotometry.
Data treatment
The image processing and all the statistical treatments were performed by using Statistica 8.0 software and algorithms programmed under MATLAB R2012b.
Results and discussion
Sample grouping and physical analysis
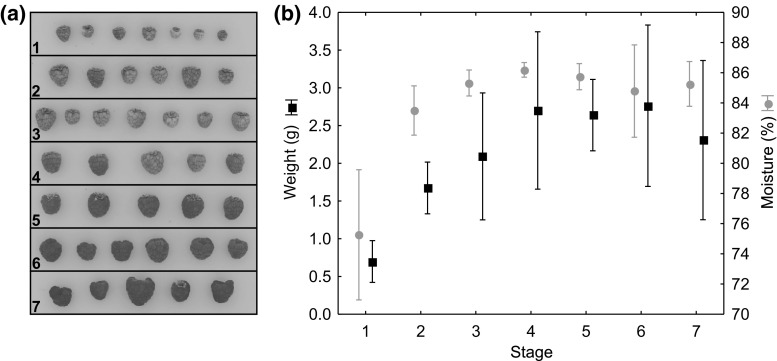
The wide range of maturation degree suggested us to make a pre-classification of the samples, firstly by simple visual inspection, dividing finally, the 168 samples into seven stages, from clearly unripe to overripe (Fig. 2a). The assessment of uncertain samples were made based on the reflectance spectra. The physical properties, moisture and weight showed the similar variations during maturation (Fig. 2b). Both weight and moisture increased quickly at stages 1 and 2 up to 2.5 grams and 84% of moisture per berry, approximately. The last stage showed a decrease in weight due to the phenomenon of over-ripening.
Fig. 2.
a Example of colour images of raspberries used grouped by stage of maturation. b Means with error bars (standard deviation) of weight and moisture of raspberries grouped by stage of maturation
Imaging techniques
Colorimetric analysis
The DigiEye imaging system stored the images having the colorimetric information in CIELAB colour space (16 bits per colorimetric variable, L* a* b*). The segmentation criterion recognized every fruit, removing automatically the pedicel when present, and so did not compute for colour measurements. The imaging analysis also determines size and heterogeneity. Figure 1 shows the colorimetric coordinates of each sample grouped by stage of maturation. The scatterplot at a* b*-diagram had a horseshoe shape. It can be seen a descent of hue from 70º until 20º at the same time that chroma increases at the beginning and decreases at final stages, showing the highest chroma at stage 4, which means that the fruits had the most saturated colour even when they had not reached the optimal features for being consumed. Regarding the lightness, L* values increased slightly at stage 3 and then, dropped up to 30–40 CIELAB units. These results are in agreement with other studies. Gordillo et al. (2012) reported that a greater amount of anthocyanins implies lower values in lightness because high concentration of pigments makes the sample absorbs more light, or rather reflects less light. Globular features of this fruits coupled with the unequal synthesis of pigments affected the overall appearance. These factors made the heterogeneity (measured as MCDM) increased quickly up to stage 4, and then, decrease when reached fully ripe or slightly before full ripeness. Table 1 shows the colorimetric results grouped according to maturation stage.
Table 1.
Colorimetric coordinates and chemical composition of raspberries at each stage of maturation (mean ± SD)
| Stage | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| L* | 49.73 ± 4.61a | 50.94 ± 3.62a | 54.67 ± 2.67b | 47.06 ± 2.91c | 41.58 ± 1.60d | 38.51 ± 1.62e | 35.54 ± 2.06f |
| C*ab | 20.98 ± 4.74a | 31.45 ± 3.41b | 39.95 ± 4.08c | 45.11 ± 2.04d | 40.99 ± 1.77c | 37.33 ± 2.59e | 34.07 ± 3.25b |
| hab | 61.66 ± 10.76a | 46.34 ± 5.14b | 39.06 ± 3.96c | 30.59 ± 1.66d | 26.24 ± 0.91e | 24.03 ± 1.07ef | 21.96 ± 1.74f |
| MCDM | 6.60 ± 0.80a | 6.71 ± 0.36ab | 7.29 ± 0.44bcd | 8.27 ± 0.54e | 7.87 ± 0.39e | 7.77 ± 0.68ce | 7.19 ± 0.69ad |
| Total phenols | 894 ± 333a | 407 ± 91b | 326 ± 74b | 251 ± 39b | 274 ± 43b | 281 ± 63b | 298 ± 60b |
| Total anthocyanins | 7.5 ± 6.8a | 14.4 ± 5.8ab | 14.4 ± 5.6ab | 22.4 ± 7.0b | 38.0 ± 8.2c | 48.9 ± 11.3d | 60.8 ± 16.5e |
| Antioxidant activity | 749 ± 395a | 324 ± 129b | 202 ± 117bc | 142 ± 62bc | 123 ± 64c | 141 ± 81c | 164 ± 90bc |
Lightness, chroma and MCDM are in CIELAB units. Hue values are expressed in degrees. Total phenols are in mg gallic acid/100 g of fresh fruit. Total anthocyanins are in mg malvidin-3-glucoside/100 g of fresh fruit. Antioxidant activity are in μequiv. Trolox/100 g fresh fruit
Different letters indicate significant differences (Tukey’s HSD test, α = 0.05)
Near infrared spectra (hyperspectral imaging)
After calibration, segmentation of hyperspectral images, and transformation into Log(1/R) units, the spectra from each berry were extracted, discarding the first and the last wavelengths to avoid noise. Hence, the useful interval of spectrum ranged between 950 and 1650 nm. The most advanced stages had higher absorbance values (data not shown), being more difference in absorbance (around 1400 nm) where the first O–H stretching overtone contributes, therefore, the moisture affects widely to this band. In this case, the influence can be attributed to the increase of water occurring along the maturation process (Osborne et al. 1993). Results also indicated that the first stages showed chemical changes more noticeable, while there were not significant changes (p < 0.05) between spectra belonging to stages 6 and 7.
Chemical analyses
Table 1 summarises the chemical analyses results. The initial stage showed the highest phenolic content which decreased dramatically at stages 2 and 3. This may be due to the presence of the lowest moisture at the first stages, which increased the phenolic content. However, the stage 1 had the highest standard deviation, so there were high variability among samples at this stage. Phenolic concentration slightly increased at stages 5, 6, and 7, probably due to the synthesis of anthocyanins, which are also phenolic compounds and these contributed to the total concentration of phenolics.
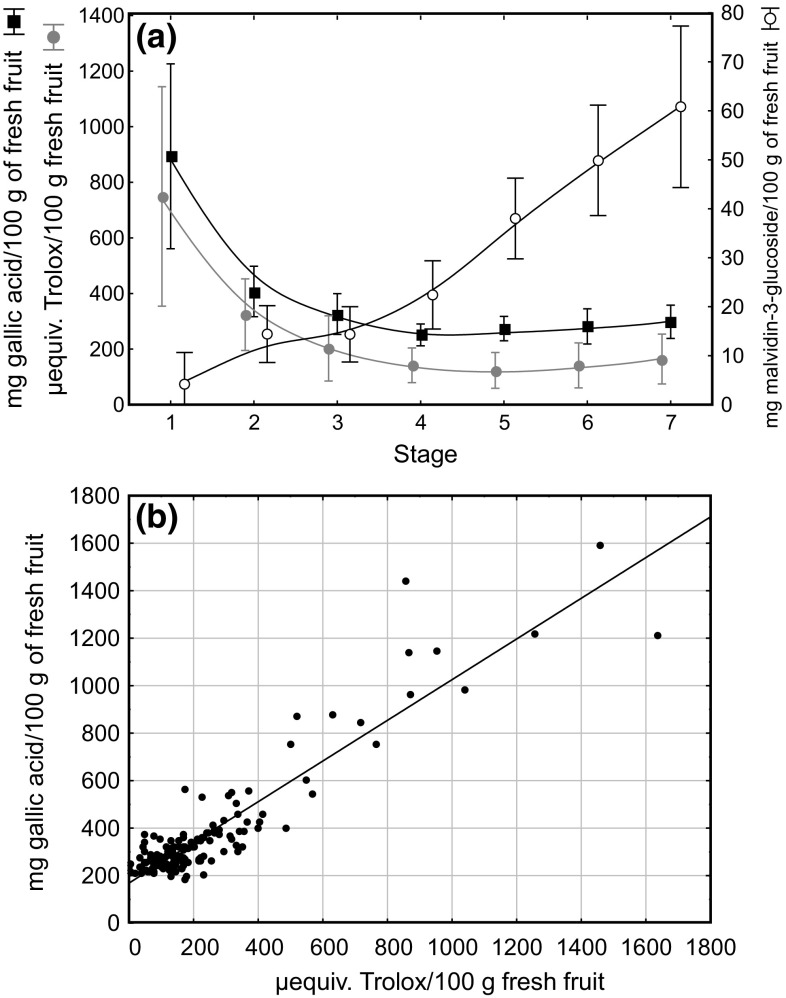
The antioxidant capacity showed the similar variation (Fig. 3a). On the other hand, it is well known that phenolics are one of the main compounds responsible for the antioxidant capacity in raspberries. Therefore, the direct relationship between these variables, studied by simple linear correlation (Fig. 3b), showed a value of 0.85 for the coefficient of determination (R2). There was not a suitable distribution of values along all the range of concentrations because only samples belonging to stages 1 and 2 had the highest concentration of phenolics. The remaining samples (stages 3–7) appeared located near to the origin of coordinates. On the other hand, the changes in anthocyanins occurred differently to that of total phenolics and antioxidant power. As expected, berries on stage 1 virtually had not anthocyanins, and small amounts appeared at stage 2, maintained at stage 3. From this point, the biosynthesis of anthocyanins follows an ascendant linear way until the full-ripe stage (Fig. 3a). In general, these results were in agreement with those found in literature (Sariburun et al. 2010; Piljac-Žegarac and Šamec 2011). However, some results reported that they may vary depending on variety and crop conditions (Manganaris et al. 2014).
Fig. 3.
a Means with error bars (standard deviation) of total phenols, antioxidant activity, and total anthocyanins along the maturation. b Scatterplot explaining the relationship between total phenols and antioxidant activity
Relationship between optical features and chemical composition
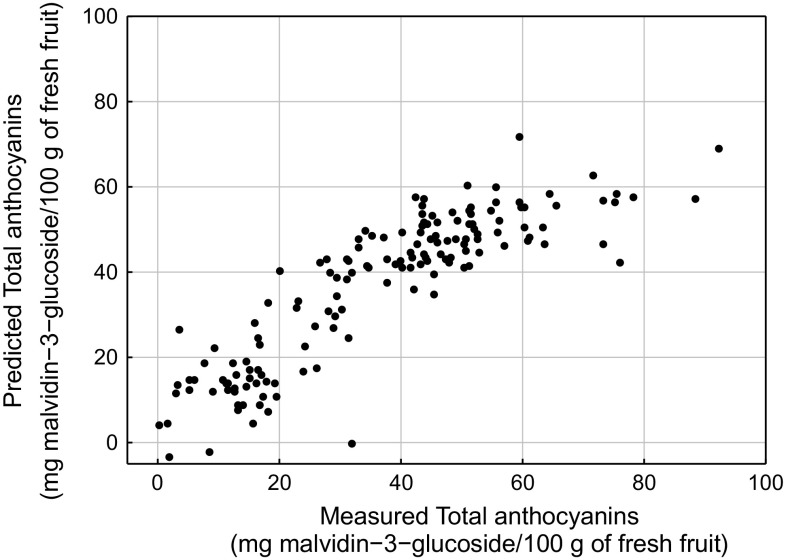
The ability of predicting the chemical composition from data obtained by both colorimetric and NIR imaging techniques was also assessed. To achieve this aim, the colorimetric and infrared datasets as independent variables, and the chemical analysis results as dependent variables were considered. The models calculated by merging both spectroscopic and colorimetric datasets did not yield better results than those obtained individually. PLSR built the models regardless the stage of maturation of berries. Table 2 shows the results. For total phenols, that ranged between 190 and 1592 mg gallic acid/100 g of fresh fruit, the prediction was almost the same regardless the data allocated as independent variables. The worst prediction were for antioxidant activity (all coefficient of determination <0.65), which ranged between 230 and 1638 μequiv. Trolox/100 g of fresh fruit, while the best prediction were for total anthocyanins, because their presence was clearly noticeable with naked eye. Figure 4 shows the scatterplot of predicted vs measured values of total anthocyanins considering colorimetric data as independent variables. Except for samples having a high anthocyanins concentration, all the samples were spread around the line of equality. The coefficient of determination above mentioned may appear insufficient to be a substitute of conventional chemical analysis, but they are quite acceptable comparing to those obtained by similar techniques (Clark et al. 2003; Downey and Kelly 2004; ElMasry et al. 2007; Nicolaï et al. 2007).
Table 2.
Calibration and cross validation results for the PLS models obtained from both visible and NIR datasets
| RMSEC | RMSECV | PLS factors | ||||
|---|---|---|---|---|---|---|
| Total phenols | VIS | 0.75 | 116.1 | 0.73 | 121.4 | 3 |
| NIR | 0.75 | 116.3 | 0.70 | 126.8 | 6 | |
| Total anthocyanins | VIS | 0.76 | 9.9 | 0.75 | 10.1 | 3 |
| NIR | 0.66 | 11.5 | 0.63 | 12.0 | 4 | |
| Antioxdant activity | VIS | 0.65 | 149.2 | 0.62 | 156.1 | 3 |
| NIR | 0.64 | 150.4 | 0.61 | 157.3 | 3 |
RMSEC and RMSECV were expressed as follows: for total phenols (mg gallic acid/100 g of fresh fruit), for total anthocyanins (mg malvidin-3-glucoside/100 g of fresh fruit), and for antioxidant activity (μequiv. Trolox/100 g fresh fruit)
Fig. 4.
Scatterplot of predicted versus measured values when total anthocyanins are predicted from colorimetric data obtained from images of raspberries by PLSR
Based on the definition of the chemical composition for the seven proposed stages, we developed a software under MATLAB to assess the maturation stage of each berry automatically from hyperspectral images (Supplementary video). The time needed to perform the loading, segmentation and assessing of images is less than one second, but video has to slow down for better results.
Conclusion
The methodology for inspecting single raspberries by colorimetric and NIR imaging techniques have been established. Thus, it is possible to predict important nutritional or bioactive properties by using a conventional digital camera, avoiding long and destructive chemical analyses. Moreover, the numerical methodology developed could become a reference method for the industry and satisfy the need of the on-line and in real time quality control of intact and individual berries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Consejería de Economía, Innovación, Ciencia y Empleo, Junta de Andalucía (PE11-AGR-7843). Moreover, the authors want to thank Surexport Cía. Agrícola S.L. for supplying the samples and collaborate with the Color y Calidad de Alimentos research group. Francisco J. Rodríguez-Pulido also thanks VPPI-Universidad de Sevilla for a postdoctoral grant. Finally, we are indebted to the staff of Biology Service (SGI, Universidad de Sevilla) for the technical assistance.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2716-3) contains supplementary material, which is available to authorized users.
Contributor Information
Francisco J. Rodríguez-Pulido, Email: rpulido@us.es
María Gil-Vicente, Email: margilvic@alum.us.es.
Belén Gordillo, Email: bgordillo@us.es.
Francisco J. Heredia, Email: heredia@us.es
M. Lourdes González-Miret, Phone: +34 954557017, Email: miret@us.es.
References
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Berns RS, Billmeyer FW, Saltzman M. Billmeyer and Saltzman’s principles of color technology. 3. New York: Wiley; 2000. [Google Scholar]
- Brosnan T, Sun D-W. Improving quality inspection of food products by computer vision—a review. J Food Eng. 2004;61:3–16. doi: 10.1016/S0260-8774(03)00183-3. [DOI] [Google Scholar]
- Clark CJ, McGlone VA, Requejo C, et al. Dry matter determination in “Hass” avocado by NIR spectroscopy. Postharvest Biol Technol. 2003;29:301–308. doi: 10.1016/S0925-5214(03)00046-2. [DOI] [Google Scholar]
- Dobson P, Graham J, Stewart D, et al. Over-seasons analysis of quantitative trait loci affecting phenolic content and antioxidant capacity in raspberry. J Agric Food Chem. 2012;60:5360–5366. doi: 10.1021/jf3005178. [DOI] [PubMed] [Google Scholar]
- Downey G, Kelly JD. Detection and quantification of apple adulteration in diluted and sulfited strawberry and raspberry purées using visible and near-infrared spectroscopy. J Agric Food Chem. 2004;52:204–209. doi: 10.1021/jf035019a. [DOI] [PubMed] [Google Scholar]
- ElMasry G, Wang N, ElSayed A, Ngadi M. Hyperspectral imaging for nondestructive determination of some quality attributes for strawberry. J Food Eng. 2007;81:98–107. doi: 10.1016/j.jfoodeng.2006.10.016. [DOI] [Google Scholar]
- Esquerre C, Gowen AA, O’Donnell CP, Downey G. Initial studies on the quantitation of bruise damage and freshness in mushrooms using visible-near-infrared spectroscopy. J Agric Food Chem. 2009;57:1903–1907. doi: 10.1021/jf803090c. [DOI] [PubMed] [Google Scholar]
- Fernández-Vázquez R, Stinco CM, Meléndez-Martínez AJ, et al. Visual and instrumental evaluation of orange juice color: a consumers’ preference study. J Sens Stud. 2011;26:436–444. doi: 10.1111/j.1745-459X.2011.00360.x. [DOI] [Google Scholar]
- Gordillo B, Rodríguez-Pulido FJ, Escudero-Gilete ML, et al. Comprehensive colorimetric study of anthocyanic copigmentation in model solutions. Effects of pH and molar ratio. J Agric Food Chem. 2012;60:2896–2905. doi: 10.1021/jf2046202. [DOI] [PubMed] [Google Scholar]
- Hutchings J. 14 Calibrated colour imaging analysis of food. In: MacDougall DB, editor. Colour in food: improving quality. Cambridge: CRC Press; 2002. [Google Scholar]
- Jennings DL. Breeding primocane-fruiting raspberries at medway fruits—progress and prospects. Acta Hort. 2002;85–89:2002. [Google Scholar]
- Kalt W, Forney CF, Martin A, Prior RL. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J Agric Food Chem. 1999;47:4638–4644. doi: 10.1021/jf990266t. [DOI] [PubMed] [Google Scholar]
- Kemsley EK, Holland JK, Defernez M, Wilson RH. Detection of adulteration of raspberry purees using infrared spectroscopy and chemometrics. J Agric Food Chem. 1996;44:3864–3870. doi: 10.1021/jf960089l. [DOI] [Google Scholar]
- León K, Mery D, Pedreschi F, León J. Color measurement in L∗ a∗ b∗ units from RGB digital images. Food Res Int. 2006;39:1084–1091. doi: 10.1016/j.foodres.2006.03.006. [DOI] [Google Scholar]
- Lin P, Chen Y, He Y. Identification of geographical origin of olive oil using visible and near-infrared spectroscopy technique combined with chemometrics. Food Bioprocess Technol. 2009;5:235–242. doi: 10.1007/s11947-009-0302-z. [DOI] [Google Scholar]
- Luo MR, Cui GH, Li C (2001) British Patent entitled apparatus and method for measuring colour (DigiEye System), Derby University Enterprises Limited
- Manganaris GA, Goulas V, Vicente AR, Terry LA. Berry antioxidants: small fruits providing large benefits. J Sci Food Agric. 2014;94:825–833. doi: 10.1002/jsfa.6432. [DOI] [PubMed] [Google Scholar]
- Nicolaï BM, Beullens K, Bobelyn E, et al. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: a review. Postharvest Biol Technol. 2007;46:99–118. doi: 10.1016/j.postharvbio.2007.06.024. [DOI] [Google Scholar]
- Nogales-Bueno J, Hernández-Hierro JM, Rodríguez-Pulido FJ, Heredia FJ. Determination of technological maturity of grapes and total phenolic compounds of grape skins in red and white cultivars during ripening by near infrared hyperspectral image: a preliminary approach. Food Chem. 2014;152:586–591. doi: 10.1016/j.foodchem.2013.12.030. [DOI] [PubMed] [Google Scholar]
- Osborne BG, Fearn T, Hindle PT, Osborne BG. Practical NIR spectroscopy with applications in food and beverage analysis. 2. Harlow, New York: Longman Scientific & Technical, Wiley; 1993. [Google Scholar]
- Pantelidis GE, Vasilakakis M, Manganaris GA, Diamantidis G. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007;102:777–783. doi: 10.1016/j.foodchem.2006.06.021. [DOI] [Google Scholar]
- Piljac-Žegarac J, Šamec D. Antioxidant stability of small fruits in postharvest storage at room and refrigerator temperatures. Food Res Int. 2011;44:345–350. doi: 10.1016/j.foodres.2010.09.039. [DOI] [Google Scholar]
- Pojić MM, Mastilović JS. Near infrared spectroscopy—advanced analytical tool in wheat breeding, trade, and processing. Food Bioprocess Technol. 2012;6:330–352. [Google Scholar]
- Pulido R, Bravo L, Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48:3396–3402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Pulido FJ, Gordillo B, Lourdes González-Miret M, Heredia FJ. Analysis of food appearance properties by computer vision applying ellipsoids to colour data. Comput Electron Agric. 2013;99:108–115. doi: 10.1016/j.compag.2013.08.027. [DOI] [Google Scholar]
- Rodríguez-Pulido FJ, Hernández-Hierro JM, Nogales-Bueno J, et al. A novel method for evaluating flavanols in grape seeds by near infrared hyperspectral imaging. Talanta. 2014;122:145–150. doi: 10.1016/j.talanta.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Sariburun E, Şahin S, Demir C, et al. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J Food Sci. 2010;75:C328–C335. doi: 10.1111/j.1750-3841.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- Saura-Calixto F, Goñi I. Antioxidant capacity of the Spanish Mediterranean diet. Food Chem. 2006;94:442–447. doi: 10.1016/j.foodchem.2004.11.033. [DOI] [Google Scholar]
- Seeram NP, Lee R, Scheuller HS, Heber D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem. 2006;97:1–11. doi: 10.1016/j.foodchem.2005.02.047. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Sun D-W. Computer vision—an objective, rapid and non-contact quality evaluation tool for the food industry. J Food Eng. 2004;61:1–2. doi: 10.1016/S0260-8774(03)00182-1. [DOI] [Google Scholar]
- Valous NA, Mendoza F, Sun D-W, Allen P. Colour calibration of a laboratory computer vision system for quality evaluation of pre-sliced hams. Meat Sci. 2009;81:132–141. doi: 10.1016/j.meatsci.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Williams P (2001) Variables affecting near-infrared reflectance spectroscopic analysis. In: Norris KH, American Association of Cereal Chemists (eds) Near-infrared technology in the agricultural and food industries, 2nd ed. American Association of Cereal Chemists, St. Paul, pp 143–167
- Williams P, Geladi P, Fox G, Manley M. Maize kernel hardness classification by near infrared (NIR) hyperspectral imaging and multivariate data analysis. Anal Chim Acta. 2009;653:121–130. doi: 10.1016/j.aca.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Wrolstad RE, Durst RW, Lee J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci Technol. 2005;16:423–428. doi: 10.1016/j.tifs.2005.03.019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.