Abstract
Gelled emulsions with carrageenan are a novel type of emulsion that could be used as a carrier of unsaturated fatty acids in functional foods formulations. Lipid degradation through volatile compounds was studied in gelled emulsions which were high in polyunsaturated oils (sunflower or algae oil) after 49 days of storage. Aqueous Lavandula latifolia extract was tested as a natural antioxidant. Analysis of the complete volatile profile of the samples resulted in a total of 40 compounds, classified in alkanes, alkenes, aldehydes, ketones, acids, alcohols, furans, terpenes and aromatic hydrocarbons. During storage, the formation of the volatile compounds was mostly related to the oxidation of the main fatty acids of the sunflower oil (linolenic acid) and the algae oil (docosahexaenoic acid). Despite the antioxidant capacity shown by the L. latifolia extract, its influence in the oxidative stability in terms of total volatiles was only noticed in sunflower oil gels (p < 0.05), where a significant decrease in the aldehydes fraction was found.
Keywords: Lipid oxidation, Antioxidant, Lavandula latifolia, Functional ingredient, SPME
Introduction
Emulsions have widely been described as suitable delivery systems of functional ingredients in the food industry (McClements 2010). Additionally, modification of their characteristics and composition allows improving the nutritional properties of foodstuffs. In case these modifications imply to increase the polyunsaturated fatty acids/saturated fatty acids (PUFA/SFA) ratio, the resulting products might be more susceptible to oxidation, and the use of extra antioxidants could be necessary (Cofrades et al. 2014; Poyato et al. 2013).
The current growing demand for natural antioxidants justifies the search for new plant extracts as substitutes for synthetic antioxidants, which are lately been questioned due to health and safety issues (Moure et al. 2001). The Lamiaceae family gathers several widely known plants, some of them with bioactive species. Among them, extracts from some lavender species (Lavandula angustifolia and Lavandula viridis) have been described as a potential source of antioxidants and other functional compounds (Costa et al. 2013; Gallego et al. 2013). We have aimed our research at a member of this family which is naturally found in several regions in the East of Spain, Lavandula latifolia, commonly known as spike lavender (Herraiz-Peñalver et al. 2013). Most of the research conducted with the spike lavender has been focused on its essential oil, paying less attention to the antioxidant capacity of other type of the extracts from this plant (López et al. 2007). In this study, the evaluation of the antioxidant capacity of the L. latifolia extract was performed.
The study of the oxidative stability in lipid food matrices is a key factor since lipid oxidation products may lead to undesirable sensory and biological effects, and may contribute to reducing the storage stability of foods. In this sense, the determination of volatile compounds has been described as a more sensitive indicator of this process than classic assessment methods (Barriuso et al. 2013; Kerrihard et al. 2015). These compounds result from secondary lipid oxidation, and include alkanes, alkenes, aldehydes, ketones, alcohols, esters, acids and hydrocarbons. Among them, aldehydes are considered the best markers of lipid oxidation and the ultimate responsible party for the observed sensory defects (Ross and Smith 2006).
Our research group has previously developed a novel type of gelled emulsion enriched in PUFA that was successfully applied to different meat products, being a viable ingredient to design potentially functional reduced-fat products with a healthier lipid profile (Poyato et al. 2014a). The present study was focused on the study through volatile compounds of the lipid degradation formed during storage (25 °C, 49 days) from the optimized gelled emulsion formulated with two different polyunsaturated oils (sunflower and algae), with and without an aqueous extract from L. latifolia as a natural antioxidant.
Materials and methods
Material
The arial parts of L. latifolia were collected during October 2012 in the Southern part of Navarra (Spain) and afterwards were dried at room temperature and stored in the Laboratory of Pharmacognosy at the University of Navarra.
Gelled emulsion ingredients: sunflower oil (Urzante S.L., Navarra, Spain); algae oil (Martek Biosciences Corporation, Columbia, USA); kappa-carrageenan (kindly donated by Cargill, San Sebastián, Spain); Polysorbate 80 (Sigma-Aldrich, Steinheim, Germany).
Reagents: fatty acid methyl esters, NaCl, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid 97%), ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt), DPPH (2,2-diphenyl-1-picrylhydrazyl) and gallic acid were purchased from Sigma-Aldrich (Steinheim, Germany). Boron trifluoride/methanol and heptane were obtained from Merck (Whitehouse Station, NJ, USA). Folin–Ciocalteu reagent was supplied by Panreac (Barcelona, Spain).
Aqueous Lavandula latifolia extract
Preparation of the extract
The extraction of L. latifolia was carried out following a sequential extraction, using four different solvents of increasing polarity (dichloromethane, ethyl acetate, methanol and water) (López et al. 2007). The water extract was lyophilized (Cryodos-50, Telstar, Barcelona, Spain) and analyzed for antioxidant capacity.
Characterization of antioxidant capacity
Total phenolic compounds (TPC) TPC were spectrophotometrically determined following the Folin–Ciocalteu colorimetric method described by García-Herreros et al. (2010). The reaction mixture was placed in a 96 well microplate containing 3 μL of diluted sample (1.5–2 mg/mL), 237 μL of distilled water, 15 μL of Folin–Ciocalteu’s reagent, and 45 μL of 20% sodium carbonate anhydrous solution (adding 2 min after the Folin–Ciocalteu’s reagent). After 2 h in the dark at room temperature, the absorbance was read at 765 nm using a FLUOStar Omega spectrofluorometric analyzer (BMG Labtechnologies, Offenburg, Germany). The amount of total phenolic compounds was expressed in μg gallic acid/mg lyophilized extract. Determinations were performed in triplicate.
DPPH DPPH method was performed as described by García-Herreros et al. (2010). Briefly, a DPPH solution (0.04 mg/mL) was prepared in methanol and diluted to obtain an absorbance of 0.8 at 516 nm. Then, 750 µl of diluted extract (11–17.6 µg/mL) or control sample (methanol) were mixed with 750 µl of DPPH solution. After 30 min in the dark at room temperature, 200 µL of each solution were transferred into a 96 well micro-plate and the absorbance was measured at 516 nm (FLUOStar Omega spectrofluorometric analyser, BMG Labtechnologies, Offenburg, Germany). The inhibition percentage (%I) for each sample was calculated from the absorbance data obtained as follows:
where Abscontrol was the absorbance of the control and Abssample was the absorbance of the sample. The inhibition percentage was plotted versus the concentration of the extracts. Analyses were performed in triplicate and results were expressed as μg Trolox/mg lyophilized extract.
ABTS ABTS assay was carried out following the procedure described by García-Herreros et al. (2010). Briefly, ABTS.+ chromogenic radical was generated by a chemical reaction mixing an aqueous solution of ABTS with K2S2O4 (140 mM). The mixture was kept in the dark 12 h and its absorbance was adjusted to 0.7 at 741 nm using ethanol 50%. In a 96 well micro-plate, ABTS.+ working solution (182 µL) was allowed to react with 18 µL of each dilution of the extract (102–250 µg/mL) or control (ethanol 50%) for 6 min. The absorbance was then measured at 741 nm using a FLUOStar Omega spectrofluorometric analyzer (BMG Labtechnologies, Offenburg, Germany). The decrease in absorbance was recoded as inhibition percentage (%I) as follows:
where Abscontrol was the absorbance of the control and Abssample was the absorbance of the sample. The inhibition percentage was plotted versus the concentration of the extracts. Analyses were done in triplicate and results were expressed as μg Trolox/mg lyophilized extract.
Gelled emulsions
Sunflower and algae oil were used to prepare three types of gelled emulsions for each one of them: control without antioxidant (CTRL), antioxidant 1 (AOX1) and antioxidant 2 (AOX2). In order to study a potential dose–response effect, we prepared two different formulations for the antioxidant, AOX1 which had 0.655 g L. latifolia aqueous extract/kg of gel and AOX2 containing 1.31 g L. latifolia aqueous extract/kg of gel, which was added into the aqueous phase before the homogenization process. To establish the concentration of extract in AOX1 samples, we used an amount whose antioxidant capacity was that of Melissa officinalis aqueous extract (measured by the DPPH), which was previously successfully used in emulsions rich in unsaturated fatty acids (Poyato et al. 2013).
The gel formulation used in this study was optimized by Poyato et al. (2014a): oil 400 g/kg gel, carrageenan 15 g/kg gel and 0.003 surfactant-oil rate. The carrageenan and the lavender extract (when used) were dissolved in water and the surfactant (Polysorbate 80) was added to the oil phase. Both phases were heated separately up to 70 °C. Then, the oil phase was incorporated to the aqueous phase and the homogenization process was made with an Ultra-Turrax® T25 basic at 16,000 rpm.
Storage conditions
Gels prepared in sealed flasks were cooled down to room temperature allowing the carrageenan to polymerize. After that, gels were kept in a climate chamber at 25 °C and analyzed at day 1 and at day 49. The experiment was performed in triplicate.
Volatile compounds
Volatile compounds were analyzed by headspace solid phase microextraction (HS-SPME) combined with gas chromatography–mass spectrometry (GC–MS). The SPME fiber coating used was divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) (50/30 μm film thickness, Supelco). The gelled emulsion (2 g) was weighed into a 25 mL headspace vial and, before sealing, the air was replaced with nitrogen to eliminate air contaminants. The sample was equilibrated at 50 °C during 15 min and the adsorption time, with the fiber exposed to the headspace of the sample, was 60 min at the same temperature. The desorption time for the fiber in the injection port of the gas chromatograph was 30 min. The GC–MS instrumentation used was GC 6890 N coupled to a mass selective 5973 detector (Agilent Technologies, Santa Clara, US). Volatiles were separated using a capillary column HP-5MS, 5% phenyl methyl siloxane (30 m long × 0.25 mm inner diameter × 0.25 μm film thickness, Agilent Technologies, Santa Clara, US). Chromatographic conditions were as follows: the oven temperature was held for 5 min at 42 °C, then increased to 120 °C at 3 °C min−1 and to 250 °C at 10 °C min−1 (5 min hold); injector temperature, 250 °C; detector temperature 280 °C; ion source temperature, 230 °C; quadrupole mass analyzer temperature, 150 °C. Helium was used as carrier gas at 1 mL min−1. The mass spectrometer was operated by electronic impact at 70 eV and ions were scanned over the m/z range of 33–350 at a rate of 4.43 scan/s. Kovats Index (KI) was calculated for each detected peak using the following formula:
where z is the number of carbon atoms in alkane z; tR(i) is the retention time of compound i; tR(z) is the retention time of alkane z; tR(z+1) is the retention time of alkane z + 1.
The identification of each peak was made taking into account the KI reported in the literature (Kondjoyan and Berdagué 1996) and comparing their mass spectra with the one of a commercial library (Wiley 275.L, Mass Spectral Database). In the case of overlapping peaks, the quantification of the corresponding compound was done by a specific ion and taking into account the relative proportion in which this ion is present in each compound. Results are expressed in area ×103/g gelled emulsion. Samples were analyzed in triplicate.
Lipid profile
Fatty acids were determined in the oils by gas chromatography FID detection, previous preparation of the fatty acid methyl ester derivatives. Boron trifluoride/methanol was used for the preparation of fatty acid methyl esters (AOAC 2002). A Perkin-Elmer Clarus 500 gas chromatograph was used and chromatographic conditions were those applied by Poyato et al. (2014b). Results were expressed as g fatty acids/kg oil (Table 1).
Table 1.
Lipid profile of the oils used in the gelled emulsion (g fatty acids/kg oil) (mean ± standard deviation)
| Sunflower oil | Algae oil | |
|---|---|---|
| Caprilic C8:0 | nd | 3.76 ± 0.04 |
| Capric C10:0 | nd | 12.14 ± 0.09 |
| Lauric C12:0 | nd | 43.88 ± 0.21 |
| Myristic C14:0 | 0.84 ± 0.03 | 104.22 ± 0.48 |
| Palmitic C16:0 | 65.13 ± 0.41 | 73.67 ± 0.42 |
| t-Palmitoleic C16:1 | nd | 0.20 ± 0.00 |
| Palmitoleic C16:1 | 1.40 ± 0.06 | 21.61 ± 0.11 |
| Stearic C18:0 | 32.79 ± 0.16 | 6.19 ± 0.19 |
| Elaidic C18:1 | 0.81 ± 0.29 | 1.43 ± 0.14 |
| Oleic C18:1 (ω-9) | 314.68 ± 0.34 | 190.90 ± 1.07 |
| c-Vaccenic C18:1 (ω-7) | 8.14 ± 0.10 | 1.55 ± 0.04 |
| t-Linoleic C18:2 | 0.21 ± 0.06 | nd |
| c-t linoleic C18:1 | 1.29 ± 0.02 | 0.32 ± 0.02 |
| t-c linoleic C18:1 | 1.00 ± 0.07 | 0.46 ± 0.01 |
| Linoleic C18:2 (ω-6) | 565.57 ± 0.47 | 10.36 ± 0.07 |
| Arachidic C20:0 | 1.29 ± 0.24 | nd |
| γ-linolenic C18:3 (ω-6) | nd | nd |
| Eicosenoic C20:1 (ω-9) | 0.96 ± 0.06 | 0.59 ± 0.02 |
| α-linolenic C18:3 (ω-3) | 0.89 ± 0.17 | 0.57 ± 0.02 |
| Behenic C22:0 | 4.92 ± 0.13 | 1.70 ± 0.05 |
| Brassidic C20:1 | nd | nd |
| Erucic C22:1 | nd | nd |
| Eicosatrienoic C20:3 (ω-3) | nd | nd |
| Arachidonic C20:4 (ω-6) | nd | nd |
| Lignoceric C24:0 | nd | nd |
| Eicosapentaenoic C20:5 (ω-3) | nd | 1.19 ± 0.02 |
| Nervonic C24:1 (ω-9) | nd | nd |
| Docosapentaenoic C22:5 (ω-3) | nd | 5.13 ± 0.11 |
| Docosahexaenoic C22:6 (ω-3) | nd | 353.33 ± 10.00 |
| SFA | 104.98 ± 0.46 | 294.75 ± 4.09 |
| MUFA | 325.18 ± 0.21 | 257.64 ± 2.62 |
| PUFA | 566.46 ± 0.62 | 444.70 ± 6.61 |
| ω-3 | 0.89 ± 0.17 | 432.27 ± 6.73 |
| ω-6 | 565.57 ± 0.47 | 12.43 ± 0.12 |
| ω-6/ω-3 | 655.63 ± 147.24 | 0.03 ± 0.00 |
| Trans | 3.39 ± 0.29 | 2.91 ± 0.12 |
nd not detected, SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids
Data analysis
Significant differences in individual volatile compounds and volatile chemical classes between samples at day 1 and day 49 (CTRL, AOX1 and AOX2 samples) for each oil were determined using one-way analysis of variance (ANOVA) in combination with Tukey-b post hoc test (p < 0.05). A principal component analysis (PCA) was performed on the data applying orthogonal rotation. Principal component 1 (PC 1) and Principal component 2 (PC2) were shown. All the analyses were performed with the Stata 12 software (StataCorp LP, Texas, USA).
Results and discussion
Antioxidant activity of aqueous Lavandula latifolia extract
Lavender essential oils have traditionally been used both in cosmetics/perfumery and as a therapeutic agent, whereas other types of extracts from this plant have been poorly studied. Water extracts from lavenders have been pointed out as possible bleaching agents in foods (Hsu et al. 2007) and ethanolic extracts as potential food antioxidants (Gallego et al. 2013). This work showed that the antioxidant capacity of aqueous L. latifolia extract was 294.8 µg Trolox mg/lyophilized extract using the DPPH method and 839.1 µg Trolox/mg lyophilized extract using the ABTS method. Total phenolic compounds contributed to this antioxidant capacity and accounted for 194.1 µg gallic acid/mg lyophilized extract. These data reveal that the antioxidant capacity of aqueous L. latifolia extract fits within the range of other extracts which have previously been used as antioxidants in food and emulsions enriched in n-3 fatty acids. For instance, the antioxidant capacity of water extract of M. officinalis was 424.43 µg Trolox/mg lyophilized extract for DPPH, 436.42 µg Trolox/mg lyophilized extract for ABTS and 310 µg gallic acid/mg lyophilized extract (García-Iñiguez de Ciriano et al. 2010). On the other hand, water extracts of borage leaves showed values of 51.0 µg Trolox/mg lyophilized extract for DPPH, 50.2 µg Trolox/mg lyophilized extract for ABTS and 57.7 µg gallic acid/mg lyophilized extract (García-Iñiguez de Ciriano et al. 2009). However, the content of total phenolic compounds in other plant extracts proposed in the literature as antioxidants is very wide: aqueous extract of selected nutraceutical herbs (Psidium guajava, Camellia sinensis, Toona sinensis, Rosmarinus officinalis) showed 65–185 µg gallic acid/mg of total phenolics (Chen et al. 2007), whereas methanolic extracts from oregano, rosemary, sage, thyme, cinnamon and clove, among other plants, showed values between 45 and 144 µg gallic acid/mg dried weight (Shan et al. 2005). Additionally, ethanolic extracts from leaves, flowers and stems of rosemary, thyme and lavender showed values between 52 and 334 µg gallic acid/mg lyophilized extract (Gallego et al. 2013) and the quantity of total phenolics in potato peel extract used as antioxidant in fish was about 0.7–0.3 µg gallic acid/mg (Farvin et al. 2012). Taking into account all these data, in this study, an aqueous L. latifolia extract has been tested as a potential natural antioxidant in gelled emulsions prepared with oils rich in polyunsaturated fatty acids.
Volatile compounds profile after storage
Initial status
A total of 40 compounds were identified along the whole experiment, being classified in 9 chemical classes: alkanes (13 compounds), alkenes (3 compounds), aldehydes (8 compounds), ketones (5 compounds), acids (3 compounds), alcohols (4 compounds), furans (1 compound), terpenes (2 compounds) and aromatic hydrocarbons (1 compound) (Table 2).
Table 2.
Volatile compounds detected in gelled emulsions at the beginning and at the end of the storage (day 1 and 49)
| Ion1 | KI2 | RI3 | Sunflower oil gels | Algae oil gels | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 49 | Day 1 | Day 49 | ||||||||
| CRTL | AOX1 | AOX2 | CRTL | AOX1 | AOX2 | ||||||
| Alkanes | |||||||||||
| 2-Methylpentane | 57 | 564 | B | 306a | 624b | 588b | 316a | 385a | 289a | 330a | 402a |
| 3-Methylpentane | 579 | B | 117a | 174b | 82a | 231c | 204 | nd | nd | nd | |
| 4-Methylheptane | 43 | 754 | B | 597a | 3250b | 2587b | 2812b | nd | 1094b | 1596c | 776a |
| 2,4-Dimethylheptane | 43 | 819 | B | 1007a | 3584b | 3331b | 3014b | 567a | 1693b | 2154c | 1465b |
| 4-Methyloctane | 861 | B | 398a | 2406b | 1917ab | 1613a | 230a | 669b | 1149c | 697b | |
| Nonane | 900 | C | nd | 300c | 367bc | 252b | nd | 529a | 477a | 465a | |
| 4-Methylnonane | 961 | B | 252a | 997b | 1213b | 1149b | 142a | 1238b | 1809c | 1727c | |
| 2,2,4,6,6-Pentamethylheptane | 988 | C | 1787b | 1081a | 1339a | 1174a | nd | nd | nd | nd | |
| Decane | 57 | 1000 | B | 372a | 1316b | 1608c | 1410b | 394a | 949b | 1535c | 890b |
| 2,6-Dimethylnonane | 1008 | C | 1457a | 4596b | 3910b | 4410b | nd | nd | nd | nd | |
| 4-MethylDecane | 1061 | B | 459a | 1921b | 1785b | 1650b | 350a | 3481b | 4212b | 3454c | |
| Undecane | 1100 | C | 107a | 328b | 625c | 581c | 218a | 787b | 995c | 801b | |
| Dodecane | 1200 | A | 176a | 699b | 1009c | 931c | 331a | 1439b | 1689c | 1402b | |
| Alkenes | |||||||||||
| 2-Methyl-1-pentene | 593 | C | 151a | 339a | 691b | 999c | nd | nd | nd | nd | |
| 2,4-Dimethyl-1-heptene | 838 | C | 1671a | 5896b | 4180b | 4691b | 769a | 1824c | 2500d | 1537b | |
| 1-Decene | 991 | C | nd | nd | nd | nd | 2902a | 3775b | 4836c | 5407d | |
| Aldehydes | |||||||||||
| 2-Propenal | 56 | 507 | C | nd | nd | nd | nd | 663a | 3824c | 2981b | 2886b |
| (E)-2-Pentenal | 747 | B | nd | nd | nd | nd | nd | 8796a | 9692a | 8903a | |
| Hexanal | 56 | 800 | A | 1133a | 7726b | 2099a | 2387a | 348a | 428ab | 375a | 555b |
| Heptanal | 902 | A | 588b | 971c | 450a | 595b | 472a | 576a | 551a | 366a | |
| (E)-2-Heptenal | 83 | 956 | B | 492a | 1060b | 1237b | 1927c | nd | nd | nd | nd |
| (Z,E)-2,4-Heptadienal | 81 | 996 | B | nd | nd | nd | nd | 1170a | 37,458b | 32,793b | 33,249b |
| (E,E)-2,4-Heptadienal | 81 | 1009 | B | nd | nd | nd | nd | 378a | 13,549c | 11,068b | 11,323b |
| Nonanal | 1105 | B | 692a | 1796b | 903a | 1002a | 680a | 1431b | 1429b | 1395b | |
| Ketones | |||||||||||
| 2-Propanone | 43 | 511 | C | 769a | 2074b | 2162b | 1583ab | 595a | 641a | 1345c | 934b |
| 4-Methyl-2-heptanone | 939 | C | nd | 310a | 643b | 339a | nd | 286a | 387b | 475c | |
| 6-Methyl-5-hepten-2-one | 43 | 987 | B | nd | nd | nd | nd | 663a | 1860b | 1954b | 2429c |
| 3,5-Octadien-2-one (or isomer) | 95 | 1072 | C | nd | nd | nd | nd | nd | 5225c | 2586a | 4038b |
| 3,5-Octadien-2-one (or isomer) | 95 | 1092 | C | nd | nd | nd | nd | nd | 1061c | 401a | 681b |
| Acids | |||||||||||
| Acetic Acid | 60 | 584 | A | nd | nd | 731b | 531a | nd | nd | 413a | 876b |
| Hexanoic acid | 60 | 993 | B | nd | nd | 646a | 988b | nd | nd | 1410a | 1510a |
| Octanoic acid | 60 | 1179 | B | nd | nd | nd | nd | nd | nd | 95a | 186b |
| Alcohols | |||||||||||
| Hexanol | 868 | B | nd | 1275a | 1131a | 1223a | nd | nd | 708b | 519a | |
| 1-octen-3-ol | 980 | B | 95a | 1113c | 941bc | 830b | – | – | |||
| 2 -Ethylhexanol | 1029 | B | 281a | 2399b | 1732b | 2085b | 709b | 1673b | 2247c | 2582d | |
| Octanol | 56 | 1071 | B | nd | 655 | nd | nd | nd | nd | nd | nd |
| Furans | |||||||||||
| 2-Pentylfuran | 992 | B | 596a | 1522b | 1530b | 1372b | nd | nd | nd | nd | |
| Terpenes | |||||||||||
| Alpha-pinene | 931 | B | 335a | 309a | 314a | 250a | nd | nd | nd | nd | |
| Limonene | 68 | 1026 | A | 616a | 886a | 843a | 596a | nd | nd | nd | nd |
| Aromatic hydrocarbons | |||||||||||
| p-Xylene | 865 | B | 544a | 706a | 602a | 594a | 1096b | 657a | 944a | 793a | |
Results are expressed in total area counts × 103/g gelled emulsion
CTRL control without extra antioxidant, AOX1 0.655 g L. latifolia aqueous extract/kg gelled emulsion, AOX2 1.31 g L. latifolia aqueous extract/kg gelled emulsion, nd not detected
Different small letters in the same row denote significant differences (p < 0.05) among samples at day 1 and samples CTRL, AOX1 and AOX2 (day 49) for each oil
1 Ion used for quantification. Total area is estimated taking into account the proportion of this ion in the compound
2 Kovats Index calculated for a DB-5 column
3 Reliability of identification: A = retention time in agreement with injected standards, mass spectrum identified using Wiley library and KI in agreement with the literature; B = mass spectrum identified using Wiley library and KI in agreement with the literature; C = mass spectrum identified using Wiley library
A clear different initial distribution of volatiles was noticed depending on the type of oil used in the gelled emulsion. In sunflower oil containing samples, the main group was alkanes, reaching 47% of the total volatiles (Table 3), with 2,2,4,6,6-pentamethylheptane, 2,6-dimethylnonane and 2,4-dimethylheptane as the most abundant ones. Aldehydes was the second most abundant family (19%), with hexanal showing the highest amount, and alkenes was the third most abundant family (12%). Ketones, alcohols, aromatic hydrocarbons, furans and terpenes were minor compounds. In fact, these two last groups were detected only in gels with sunflower oil, being 2-pentylfuran clearly associated with linoleic acid oxidation and terpenes, as previously reported in sunflower oil samples, as a result of the metabolism of many plants (Guillén and Goicoechea 2008).
Table 3.
Volatile compounds by chemical classes
| Day | Sample | Alkanes | Alkenes | Aldehydes | Ketones | Acids | Alcohols | Furans | Aromatic hydrocarbons |
Terpenes | Total volatiles |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SF | 7036a
(47%) |
1821a
(12%) |
2905a
(19%) |
769a
(5%) |
nd | 376a
(3%) |
596a
(4%) |
544a
(6%) |
952a
(4%) |
14,999a |
| 49 | SF CTRL | 21,276b
(42%) |
6235b
(12%) |
11,554c
(23%) |
2385b
(5%) |
nd | 5442c
(11%) |
1522b
(3%) |
706a
(1%) |
1195a
(2%) |
50,317c |
| SFAOX1 | 20,360b
(49%) |
4871b
(12%) |
4689ab
(11%) |
2805b
(7%) |
1377a
(3%) |
3804b
(9%) |
1530b
(4%) |
602a
(1%) |
1157a
(3%) |
41,195b | |
| SF AOX2 | 19,542b
(47%) |
5690b
(14%) |
5911b
(14%) |
1922b
(5%) |
1519a
(4%) |
4138b
(10%) |
1372b
(3%) |
594a
(1%) |
846a
(2%) |
41,535b | |
| 1 | AO | 2820a
(21%) |
3671a
(28%) |
3712a
(28%) |
1258a
(10%) |
nd | 597a
(5%) |
nd | 1096b
(8%) |
nd | 13,153a |
| 49 | AO CTRL | 12,168b
(13%) |
5599b
(6%) |
66,063c
(69%) |
9071c* (10%) |
nd | 1673b
(2%) |
nd | 657a
(1%) |
nd | 95,232b |
| AO AOX1 | 15,945c
(17%) |
7336c
(8%) |
58,888b
(62%) |
6673b
(7%) |
1918a
(2%) |
2955c
(3%) |
nd | 944a
(1%) |
nd | 94,660b | |
| AO AOX2 | 12,078b
(13%) |
6944c
(7%) |
58,677b
(63%) |
8557c
(9%) |
2573b
(3%) |
3101c
(3%) |
nd | 793a
(1%) |
nd | 92,722b |
Results are expressed in total area counts × 103/g. Between brackets, the percentage in which family contributes to the total area is indicated
SF sunflower oil, AO algae oil, CTRL control without extra antioxidant, AOX1 0.655 g L. latifolia aqueous extract/kg gelled emulsion, AOX2 1.31 g L. latifolia aqueous extract/kg gelled emulsion, nd not detected
Different small letters in the same column denote significant differences between samples at day 1 and day 49 (CTRL, AOX1 and AOX2) for each oil (p < 0.05)
Regarding algae oil gels, at day 1, the type of volatile compounds comprised mostly alkanes, alkenes and aldehydes, accounting for approximately 80% of the total volatiles (21, 28 and 28%, respectively). The most abundant compounds were 1-decene, (Z,E)-2,4-heptadienal (both exclusively found in gels with algae oil) and p-xylene. 1-decene has been described as a compound generated by oxidation of oleic acid and with a role in the flavor of fish oil (Ritter and Budge 2012). Ketones, alcohols and aromatic hydrocarbons were the minor compounds.
Some of the identified compounds in sunflower and algae oil gels were previously described in crude sunflower and marine oils, respectively (Giogios et al. 2009; Uriarte et al. 2011). However, some other compounds, such as hexanal, heptanal, nonanal, (E)-2-heptenal, (Z,E)- 2,4-heptadienal and (E,E)-2,4-heptadienal are typical from heat treated oil samples where lipid oxidation occurs (Poyato et al. 2014b). In this sense, the presence of these aldehydes in gels at day 1 might be an indicative of the beginning of the lipid oxidation, which may be explained by the initial heating at 70 °C and the intense homogenization process during the gel preparation. Additionally, some other authors have described differences in the oxidative stability between oils and emulsions. On one hand, Paradiso et al. (2015) reported a moderate oxidative stress caused by the homogenization process in emulsion filled gels based on inulin and olive oil. In addition, Frankel et al. (2002) reported that oil-in-water emulsions of algae oil were less stable to oxidation than the corresponding oil. On the other hand, the emulsification process can modify the initial behavior of lipid oxidation during storage. O’Dwyer et al. (2013) pointed out that during storage, emulsification of bulk oils decreased the level of secondary oxidation products, compared to the initial concentration.
Effect of storage
A clear oil-effect was observed in the evolution of volatiles during the storage period. At day 49, alkanes continued being the most abundant family in sunflower oil gels (approximately 50% of total area counts), whereas in algae oil gels, aldehydes were clearly the major components, increasing their percentage up to 63–69% of total volatile compounds. Regarding absolute amounts, storage significantly increased total amount of volatiles in both oils regardless the presence of antioxidants (3-fold in sunflower oil gels and 7-fold in algae oil gels) (Table 3).
In particular, sunflower oil gels showed a significant increase in volatile compounds originated from the oxidation of linoleic acid (Cao et al. 2014; Goicoechea and Guillén 2014), which is the major fatty acid in this oil (423 g/kg oil, Table 1). These volatiles were hexanal, heptanal, (E)-2-heptenal and nonanal. The increment of hexanal after 49 days of storage was significantly higher in control versus antioxidant samples (7-fold in control samples, 2-fold in AOX1 and AOX2 samples). The same behaviour was observed for nonanal and heptanal, with a higher increment in the control samples in respect to those who contained antioxidant. It can be concluded that, in sunflower oil containing samples, the total amount of aldehydes at day 49 was significantly reduced in samples with added antioxidant, but without significant changes between AOX1 and AOX2 doses.
In algae oil gels, the decomposition of docosahexaenoic acid (DHA), the major fatty acid in this oil (353 g/kg oil, Table 1), resulted in the generation of aldehydes such as (Z,E)-2,4-heptadienal, (E,E)-2,4-heptadienal, 2-propenal and (E)-2-pentenal. These compounds have been reported by other authors as fish oil derivatives (Gómez-Cortés et al. 2015; Lee et al. 2003; Thomsen et al. 2013). Both (Z,E)-2,4-heptadienal and (E,E)-2,4-heptadienal were the aldehydes that showed the highest increment during storage, especially in the control samples (32–36-fold). A protective effect of the L. latifolia extract was noticed, contributing to a decrease in the amount of these aldehydes when compared to the control sample, being statistically different in the case of the (E,E) isomer.
The (E,E) isomers of alkadienals are usually more abundant than the (Z,E) ones, however it has been reported that, at early oxidation states, this pattern is however the opposite (Guillén and Goicoechea 2008; Guillén et al. 2009). This is in agreement with our present data, where the (Z,E)-2,4-heptadienal isomer was in a higher proportion than (E,E)-2,4-heptadienal isomer.
Moreover, storage also resulted in an increase in the number and concentration of ketones. Isomers of 3,5-octadien-2-one, which are derived from n-3 long chain fatty acids (Ryckebosch et al. 2013), were detected only in algae oil gels and at day 49, showing a significantly lower value when samples included the antioxidant (p < 0.05). In sunflower oil gels, only two ketones were identified, 2-propanone and 4-methyl-2-heptanone. In these samples, only the highest dose of antioxidant (AOX2) decreased the total amount of ketones with respect to the control, although the effect was not significant.
PCA analysis
A PCA model was performed to obtain an overview of the differences on the volatile compounds profile of gels for the two types of oils and the effect of the storage in the different conditions (with and without antioxidant). Volatiles with loading < 0.5 in both, component 1 and 2, were removed from the matrix in order to explain better the variability of the results. The resulting PCA analysis showed that the PC1 and PC2 explained 88% of the observed variance (45 and 43%, respectively). The loading plot (Fig. 1a) showed that PC1 was defined by those compounds mainly typical of algae oil gels ((Z,E)-2,4-heptadienal, (E,E)-2,4-heptadienal, 2-propenal, (E)-2- pentenal, isomers 3,5-octadien-2-one) with loading higher than 0.85. PC2 axis was characterized by high loadings (≥0.9) for compounds detected mainly in sunflower oil gels (2-pentylfuran, 2-propanone, hexanol, 2,6-dimethylnonane, 1-octen-3-ol, 2,4-dimethylheptane, 4-methylheptane) and were distributed in the upper side of the plot. In the score plot (Fig. 1b), segregation of volatiles was mainly due to the type of oil and period of storage. A clear distinction was noticed for samples analysed after the storage when compared to those analysed at day 1. This finding confirmed the different evolution of the lipid volatile profile of gels with the different oils. Nevertheless, despite the quantitative differences in the amount of detected compounds when the antioxidants were added, this was not reflected in the PCA analysis. Variability of results was mainly explained by the type of oils used, which had a greater influence than the presence of the natural antioxidant.
Fig. 1.
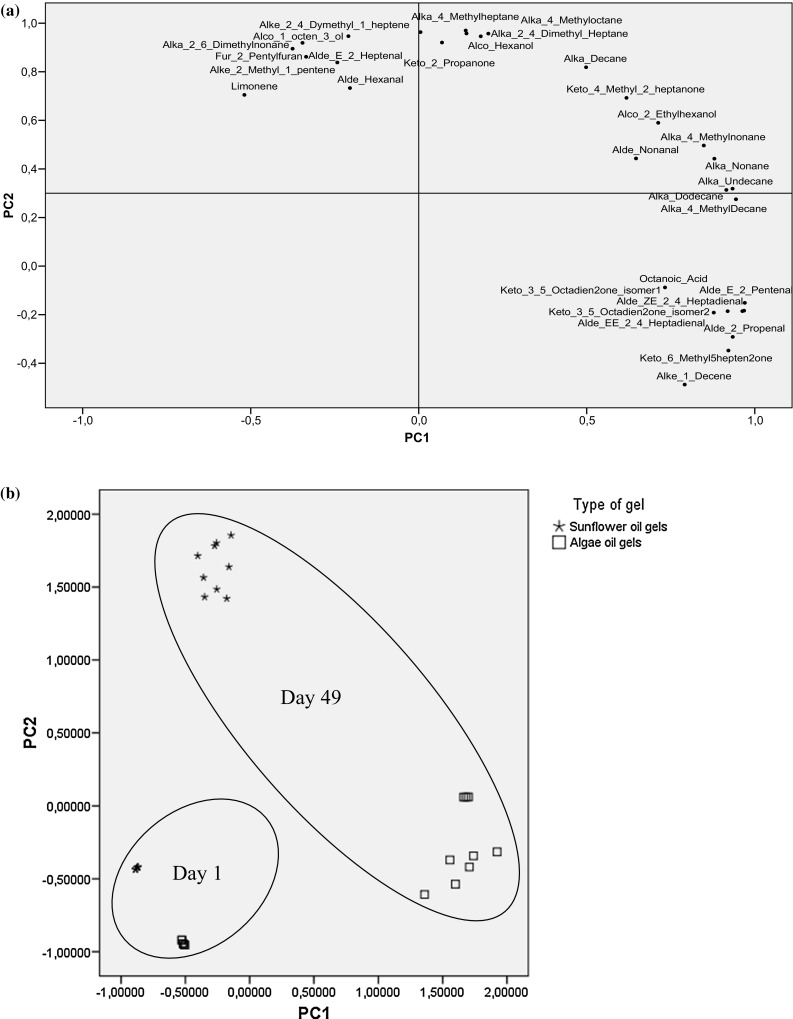
Loading plot (a) and score plot (b) of PC1 versus PC2 carried out on volatiles determined in sunflower and algae oil gels during storage
Conclusion
A novel type of gelled emulsion has been characterized through the lipid degradation compounds. The volatile pattern of sunflower and algae oil gels showed that the differences at day 1 and 49 could be attributed to the differences in the fatty acid profile of the two types of oils (with linoleic and DHA as most abundant fatty acids). The antioxidant effect of L. latifolia extract was significant only in sunflower oil containing samples and dose–response effect was not observed. Data from PCA analysis confirmed the different volatile pattern in both types of oils, and showed that the effect of storage had more influence than the effect of the aqueous extract on the volatile profile characterization. However, further studies are needed in order to test microbial stability during storage and to verify the efficacy of higher doses of L. latifolia extract.
Acknowledgements
We are grateful to the PIUNA (Plan de Investigación de la Universidad de Navarra) and to the Ministerio de Economía y Competitividad (AGL2014-52636-P) for the financial support and to “Red de Excelencia Consolider PROCARSE (AGL2014-51742-REDC)”. L. Gayoso and C. Poyato are grateful to “Asociación de Amigos de la Universidad de Navarra” for the grants received. Gobierno de Navarra is also acknowledged for the support to L. Gayoso.
Footnotes
Lucía Gayoso and Candelaria Poyato equally contributed to the paper.
References
- AOAC . Methyl esters of fatty acids in oils and fats. 969.33. Official methods of analysis. Gaithersburg: Association of Official Analytical Chemists; 2002. pp. 19–20. [Google Scholar]
- Barriuso B, Astiasarán I, Ansorena D. A review of analytical methods measuring lipid oxidation status in foods: a challenging task. Eur Food Res Technol. 2013;236:1–15. doi: 10.1007/s00217-012-1866-9. [DOI] [Google Scholar]
- Cao J, Deng L, Zhu X, Fan Y, Hu J, Li J, Deng Z. Novel approach to evaluate the oxidation state of vegetable oils using characteristic oxidation indicators. J Agric Food Chem. 2014;62:12545–12552. doi: 10.1021/jf5047656. [DOI] [PubMed] [Google Scholar]
- Chen H, Lin Y, Hsieh C. Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chem. 2007;104:1418–1424. doi: 10.1016/j.foodchem.2007.02.004. [DOI] [Google Scholar]
- Cofrades S, Santos-López JA, Freire M, Benedí J, Sánchez-Muniz FJ, Jiménez-Colmenero E. Oxidative stability of meat systems made with W-1/O/W-2 emulsions prepared with hydroxytyrosol and chia oil as lipid phase. Lwt- Food Sci Technol. 2014;59:941–947. doi: 10.1016/j.lwt.2014.06.051. [DOI] [Google Scholar]
- Costa P, Goncalves S, Valentao P, Andrade PB, Romano A. Accumulation of phenolic compounds in in vitro cultures and wild plants of Lavandula viridis L’Her and their antioxidant and anti-cholinesterase potential. Food Chem Toxicol. 2013;57:69–74. doi: 10.1016/j.fct.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Farvin KHS, Grejsen HD, Jacobsen C. Potato peel extract as a natural antioxidant in chilled storage of minced horse mackerel (Trachurus trachurus): effect on lipid and protein oxidation. Food Chem. 2012;131:843–851. doi: 10.1016/j.foodchem.2011.09.056. [DOI] [Google Scholar]
- Frankel EN, Satué-Gracia T, Meyer AS, German JB. Oxidative stability of fish and algae oils containing long-chain polyunsaturated fatty acids in bulk and in oil-in-water emulsions. J Agric Food Chem. 2002;50:2094–2099. doi: 10.1021/jf0111458. [DOI] [PubMed] [Google Scholar]
- Gallego GM, Gordon MH, Segovia FJ, Skowyra M, Almajano MP. Antioxidant properties of three aromatic herbs (Rosemary, Thyme and Lavender) in oil-in-water emulsions. J Am Oil Chem Soc. 2013;90:1559–1568. doi: 10.1007/s11746-013-2303-3. [DOI] [Google Scholar]
- García-Herreros C, García-Iñiguez M, Astiasarán I, Ansorena D. Antioxidant activity and phenolic content of water extracts of Borago Officinalis L.: influence of plant part and cooking procedure. Ital J Food Sci. 2010;22:156–164. [Google Scholar]
- García-Iñiguez de Ciriano M, García-Herreros C, Larequi E, Valencia I, Ansorena D, Astiasarán I. Use of natural antioxidants from lyophilized water extracts of Borago officinalis in dry fermented sausages enriched in omega-3 PUFA. Meat Sci. 2009;83:271–277. doi: 10.1016/j.meatsci.2009.05.009. [DOI] [PubMed] [Google Scholar]
- García-Iñiguez de Ciriano M, Rehecho S, Isabel Calvo M, Yolanda Cavero R, Navarro I, Astiasaran I, Ansorena D. Effect of lyophilized water extracts of Melissa officinalis on the stability of algae and linseed oil-in-water emulsion to be used as a functional ingredient in meat products. Meat Sci. 2010;85:373–377. doi: 10.1016/j.meatsci.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Giogios I, Grigorakis K, Nengas I, Papasolomontos S, Papaioannou N, Alexis MN. Fatty acid composition and volatile compounds of selected marine oils and meals. J Sci Food Agric. 2009;89:88–100. doi: 10.1002/jsfa.3414. [DOI] [Google Scholar]
- Goicoechea E, Guillén MD. Volatile compounds generated in corn oil stored at room temperature. Presence of toxic compounds. Eur J Lipid SciTechnol. 2014;116:395–406. doi: 10.1002/ejlt.201300244. [DOI] [Google Scholar]
- Gómez-Cortés P, Sacks GL, Brenna JT. Quantitative analysis of volatiles in edible oils following accelerated oxidation using broad spectrum isotope standards. Food Chem. 2015;174:310–318. doi: 10.1016/j.foodchem.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillén MD, Goicoechea E. Formation of oxygenated alpha, beta-unsaturated aldehydes and other toxic compounds in sunflower oil oxidation at room temperature in closed receptacles. Food Chem. 2008;111:157–164. doi: 10.1016/j.foodchem.2008.03.052. [DOI] [Google Scholar]
- Guillén MD, Carton I, Salmeron J, Casas C. Headspace composition of cod liver oil and its evolution in storage after opening. First evidence of the presence of toxic aldehydes. Food Chem. 2009;114:1291–1300. doi: 10.1016/j.foodchem.2008.11.007. [DOI] [Google Scholar]
- Herraiz-Peñalver D, Angeles Cases M, Varela F, Navarrete P, Sanchez-Vioque R, Usano-Alemany J. Chemical characterization of Lavandula latifolia Medik. essential oil from Spanish wild populations. Biochem Syst Ecol. 2013;46:59–68. doi: 10.1016/j.bse.2012.09.018. [DOI] [Google Scholar]
- Hsu C, Chang C, Lu H, Chung Y. Inhibitory effects of the water extracts of Lavendula sp on mushroom tyrosinase activity. Food Chem. 2007;105:1099–1105. doi: 10.1016/j.foodchem.2007.02.008. [DOI] [Google Scholar]
- Kerrihard AL, Pegg RB, Sarkar A, Craft BD. Update on the methods for monitoring UFA oxidation in food products. Eur J Lipid Sci Technol. 2015;117:1–14. doi: 10.1002/ejlt.201400119. [DOI] [Google Scholar]
- Kondjoyan N, Berdagué JL. A compilation of relative retention indices for the analysis of aromatic compounds. France: Laboratoire Flaveur; 1996. [Google Scholar]
- Lee H, Kizito SA, Weese SJ, Craig-Schmidt MC, Lee Y, Wei CI, An H. Analysis of headspace volatile and oxidized volatile compounds in DHA-enriched fish oil on accelerated oxidative storage. J Food Sci. 2003;68:2169–2177. doi: 10.1111/j.1365-2621.2003.tb05742.x. [DOI] [Google Scholar]
- López V, Akerreta S, Casanova E, García-Mina JM, Cavero RY, Calvo MI. In vitro antioxidant and anti-rhizopus activities of lamiaceae herbal extracts. Plant Foods Hum Nutr. 2007;62:151–155. doi: 10.1007/s11130-007-0056-6. [DOI] [PubMed] [Google Scholar]
- McClements DJ. Emulsion design to improve the delivery of functional lipophilic components. Annu Rev Food Sci Technol. 2010;1:241–269. doi: 10.1146/annurev.food.080708.100722. [DOI] [PubMed] [Google Scholar]
- Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, Nuñez MJ, Parajó JC. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- O’Dwyer SP, O’Beirne D, Eidhin DN, O’Kennedy BT. Effects of emulsification and microencapsulation on the oxidative stability of camelina and sunflower oils. J Microencapsul. 2013;30:451–459. doi: 10.3109/02652048.2012.752533. [DOI] [PubMed] [Google Scholar]
- Paradiso VM, Giarnetti M, Summo C, Pasqualone A, Minervini F, Caponio F. Production and characterization of emulsion filled gels based on inulin and extra virgin olive oil. Food Hydrocoll. 2015;45:30–40. doi: 10.1016/j.foodhyd.2014.10.027. [DOI] [Google Scholar]
- Poyato C, Navarro-Blasco I, Calvo MI, Cavero RY, Astiasarán I, Ansorena D. Oxidative stability of O/W and W/O/W emulsions: effect of lipid composition and antioxidant polarity. Food Res Int. 2013;51:132–140. doi: 10.1016/j.foodres.2012.11.032. [DOI] [Google Scholar]
- Poyato C, Ansorena D, Berasategi I, Navarro-Blasco Í, Astiasarán I. Optimization of a gelled emulsion intended to supply ω-3 fatty acids into meat products by means of response surface methodology. Meat Sci. 2014;98:615–621. doi: 10.1016/j.meatsci.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Poyato C, Ansorena D, Navarro-Blasco I, Astiasarán I. A novel approach to monitor the oxidation process of different types of heated oils by using chemometric tools. Food Res Int. 2014;57:152–161. doi: 10.1016/j.foodres.2014.01.033. [DOI] [Google Scholar]
- Ritter JCS, Budge SM. Key lipid oxidation products can be used to predict sensory quality of fish oils with different levels of EPA and DHA. Lipids. 2012;47:1169–1179. doi: 10.1007/s11745-012-3733-7. [DOI] [PubMed] [Google Scholar]
- Ross CF, Smith DM. Use of volatiles as indicators of lipid oxidation in muscle foods. CRev Food Sci Food Saf. 2006;5:18–25. doi: 10.1111/j.1541-4337.2006.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Ryckebosch E, Bruneel C, Termote-Verhalle R, Lemahieu C, Muylaert K, Van Durme J, Goiris K, Foubert I. Stability of Omega-3 LC-PUFA-rich photoautotrophic microalgal oils compared to commercially available Omega-3 LC-PUFA oils. J Agric Food Chem. 2013;61:10145–10155. doi: 10.1021/jf402296s. [DOI] [PubMed] [Google Scholar]
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- Thomsen BR, Haugsgjerd BO, Griinari M, Lu HFS, Bruheim I, Vogt G, Oterhals Å, Jacobsen C. Investigation of oxidative degradation and non-enzymatic browning reactions in krill and fish oils. Eur J Lipid Sci Technol. 2013;115:1357–1366. doi: 10.1002/ejlt.201300141. [DOI] [Google Scholar]
- Uriarte PS, Goicoechea E, Guillén MD. Volatile components of several virgin and refined oils differing in their botanical origin. J Sci Food Agric. 2011;91:1871–1884. doi: 10.1002/jsfa.4400. [DOI] [PubMed] [Google Scholar]


