Abstract
Active edible films have been proposed as an alternative to extend shelf life of fresh foods. Most essential oils have antimicrobial properties; however, storage conditions could reduce their activity. To avoid this effect the essential oil (EO) can be microencapsulated prior to film casting. The aim of this study was to determine the effects of the type of encapsulating agent (EA), type of EO and storage time on physical properties and antimicrobial activity of alginate-based films against Escherichia coli ATCC 25922. Trehalose (TH), Capsul® (CAP) and Tween 20 (Tw20) were used as EA. Lemongrass essential oil (LMO) and citral were used as active agents. The results showed that the type of EA affected the stability of the film forming-emulsions as well as the changes in opacity and colour of the films during storage but not the antimicrobial activity of them. Both microencapsulated EOs showed a prolonged release from the alginate films during the 28 days of storage. Trehalose was selected to encapsulate both active compounds because the films made with this microencapsulated EA showed the greatest physical stability and the lowest color variation among all the films studied.
Keywords: Alginate, Edible films, Lemongrass oil, Citral, Microencapsulation
Introduction
Food industry faces the permanent challenge of extending the shelf life of fresh products. Edible films and coatings are used for preservation of fresh and minimally processed foods. This technology allows to place a physical barrier on the food surface, reducing moisture and solutes migration as well as gas exchange, preventing oxidative reactions and food deterioration during storage (Fakhreddin et al. 2013). However, storage time could affect the physical and organoleptic properties of the films and the food products that is expected to protect (Riquelme et al. 2015; Bustos et al. 2016). Alginate is one of the preferred hydrocolloids for edible film manufacturing given its generally recognized as safe (GRAS) status, availability, low relative cost, well-known physical behavior in food systems and it is ease to cast films in the presence of calcium (Córdova et al. 2015). Besides, it has also been demonstrated that alginate films have the capacity of carrying different active ingredients such as antimicrobials and antioxidants (Bustos et al. 2016; Navarro et al. 2016). Alginate is a natural product extracted from brown seaweeds and it is made of β-D-manopiranosiluronic acid (M) and α-L-gulopiranosiluronic acid (G). Several emerging technologies utilize alginate as a key component, for example, edible coatings for fresh products (Matiacevich et al. 2015), microencapsulation of labile compounds such as phytochemicals, pharmaceutical, enzymes and others (Soukoulis et al. 2014), and transport of antimicrobial agents (Matiacevich et al. 2015).
The addition of antimicrobial agents such as EOs to edible films would increase considerably the protection of fresh foods, reducing microbial spoilage and, consequently, extending the shelf life (Raybaudi-Massilia et al. 2008; Burt 2004). It is worth mentioning that EOs and their components have a GRAS status under US legislation and equivalent status in the legislation of other countries.
Lemongrass (Cymbopogon citratus) is a perennial herb, with a strong lemon-like aroma, widely grown in the tropics and subtropics, farmed in the western regions of India. Lemongrass essential oil (LMO) and its main component, citral, are hydrophobic compounds and show antimicrobial activity against enterohemorrhagic E. coli O157:H7 which is comparable to the effect observed for oregano oil and only surpassed by thyme oil (Burt 2004). Antimicrobial activity of these oils can be lost for volatilisation of their active compounds or by degradation of them when exposed to high temperatures, oxygen and UV light (Beyki et al. 2014; Navarro et al. 2016). In order to achieve a stable and homogenous distribution of these natural antimicrobial compounds inside an alginate film, proper formulation and protection mechanisms have to be considered. Several technologies have been developed lately in order to achieve that goal: emulsification, micro and nano-encapsulation, liposome entrapment, coacervation and others. Among these, emulsification is considered a technique that allows the microencapsulation of active compounds with high efficiencies, homogeneous distribution, and controlled release kinetics (McClements 2005). However, selection of the suitable encapsulating agent (EA) is crucial in order to improve stability of the system. Among the different agents normally utilized are: Capsul®, Caseinate, Maltodextrin, Trehalose and Tween 20 (Elizalde et al. 2002; Finotelli and Rocha 2005; Álvarez-Cerimedo et al. 2008; Raybaudi-Massilia et al. 2008; Santagapita et al. 2012; Navarro et al. 2016).
Tween 20 is a common emulsifier for EOs in edible films (Matiacevich et al. 2015; Navarro et al. 2016). Trehalose is considered a promising EA because of its high vitreous transition temperature, providing a great stabilizing capacity (Álvarez-Cerimedo et al. 2008; Riquelme et al. 2015). On the other hand, Capsul® is an amphipathic starch derivative that shows excellent emulsifying properties, allowing to obtain very stable oil in water emulsions (Finotelli and Rocha 2005). Additionally, the low viscosity of aqueous solutions of Capsul® allows the preparation of emulsions with high solids content, and the polymeric interphase generated leads to microparticles with high retention of the active compounds presents in the EOs (Finotelli and Rocha 2005).
The main objective of this study was to evaluate the effects of the type of EO (LMO or its active component citral), type of EA and storage time on the physical and antimicrobial properties of alginate films against Escherichia coli ATCC 25922.
Materials and methods
Materials
Lemongrass oil (LMO) (from Cymbopogon citratus, East Indian origin, Kosher), citral 99.9% (Kosher), sodium carbonate and Tween 20 (Tw20) were obtained from Sigma-Aldrich (St. Louis, USA). Sodium alginate, trehalose (TH) and sorbitol were supplied by Blumos (Santiago, Chile). Capsul® (CAP) was purchased from Quimatic S.A. (Santiago, Chile). Mueller–Hinton broth and agar for microbiological analysis were obtained from Biokar Diagnostics (Beauavais Cedex, France). Sodium hydroxide and hydrochloric acid were purchased from Merck (Darmstadt, Germany). All reagents were analytical grade.
Minimum inhibitory concentration (MIC) of film forming emulsions
Antimicrobial activity of film forming-emulsions with the active compounds were tested against Escherichia coli ATCC 25922. The bacteria obtained from the Public Health Institute of Chile (ISP Chile) and was stored at −18 °C in Mueller–Hinton broth containing glycerol (15% v/v). Prior to use the bacteria was grown in Mueller–Hinton broth at 37 °C in 10 mL test tubes for 24 h. After that time, absorbance at 625 nm was measured in a spectrophotometer (Shimadzu UVmini-1240, Japan) and the concentration of E. coli in CFU/mL (Colony Forming Units/mL) was calculated applying the factor 0.05 of McFarland turbidity scale (approximately 108 CFU/mL) (CDCP and WHO 2003).
For the MIC determination, dilutions of 1 mL were prepared, which contained E. coli at 1 × 106 CFU/mL, 10 × concentrated Mueller–Hinton broth, antimicrobial agent (LMO or citral) at concentrations between 0.1 and 2% v/v and either water or 1.0% v/v alginate solution as solvent. These samples were placed in 96 well microplates and absorbance changes at 625 nm were recorded using a Multiskan GO equipment (Thermo Scientific, Finland) operating with agitation at 37 °C for a period of 30 h. The MIC value corresponded to the minimum concentration necessary to avoid any bacterial growth under the incubation conditions.
Film-forming emulsions
Suspensions were prepared with 1%w/w alginate and 1%w/w sorbitol. The encapsulating agents (Tw20, TH and CAP) were added to the suspensions in different stages of the process depending on the water solubility of each one. Capsul® was previously dissolved in water at 70 °C, trehalose was added at the same time than sorbitol, and Tween20 was added mixed with the antimicrobial oil (lemongrass or citral). All the encapsulating agents were added at the same concentration (1.5%w/w) and mixtures were prepared using a thermoregulated magnetic stirrer (Velp Scientific, Italy) set up at 40 °C until complete dispersion of all components was achieved. After that, the antimicrobial agents were added at concentrations (%w/v) equivalent to their previously determined MICs. Finally, suspensions were emulsified using an ultra turrax homogenizer (Tristor Regler TR50, Germany) operating at 10,000 rpm, for 2 min intervals and 15 s pauses to avoid overheating and the pH of the emulsion was adjusted to 5.5.
Film casting procedure
Films were prepared by a hot casting method. 0.02%w/w Calcium carbonate solution was added to the film forming-emulsions as crosslinking agent (Benavides and Villalobos 2012). 40 mL aliquots of this mixture were poured into petri dishes (8.5 cm diameter), and were placed in a drying oven with forced air circulation (Wiseven Daihan Scientific WOF-105, Korea) kept at 40 °C for 18 h.
Emulsion characterization
Microcapsule droplet size (d32)
To determine the droplet size of emulsion three samples were taken from each film-forming emulsion and were observed under a light microscope (Carl Zeiss, Germany), using a 100× magnification. Images were captured with a digital camera (Canon EOS Rebel T3, Canon Inc., Tokyo, Japan) and the droplet size was determined from those images using the calibrated software Motic Images Plus 2.0 (Causeway Bay, Hong Kong). At least 200 microcapsules were measured from each replicate. The droplet size values were reported using the Sauter diameter (d32) (Eq. 1; Di Scipio et al. 2008):
| 1 |
where is the sumatory of the d32 corresponding to the droplet volume. is the sumatory of the d32 corresponding to the particle area.
Stability of the film-forming emulsion
Stability was measured using Turbiscan technology (Thermo Scientific, Germany). Backscattering profiles were obtained at constant temperature of 22.5 ± 0.5 °C under quiescent conditions. Measurements were performed immediately after emulsion preparation and at regular time intervals during 72 h of storage at three different temperatures (20, 30 and 40 °C).
Films characterization at initial time
Moisture content and thickness
The film thickness was determined with a digital micrometer (0.1 mm resolution). Six measurements taken at random places from each film duplicate were considered, and values were expressed as the mean with its respective standard deviation. The moisture content was determined from gravimetric measurements obtained by oven drying at 105 °C for 24 h until constant weight. Results were expressed as % dry basis (%db, g water/100 g of dry film) and the average of three replicates of each film was reported.
Molecular mobility by nuclear magnetic resonance (NMR) spectroscopy analysis
The molecular mobility of different populations of protons present in each film were obtained from Free Induction decay (FID) signals of the NMR spectra. FID analysis was used to evaluate proton mobility associated to the solid matrix in the range 15–70 °C. A pulsed nuclear magnetic resonance (1H-NMR) Bruker Minispec spectrometer model mq20 (Rheinstetten, Germany) operating with a magnetic field of 0.47 T and a frequency of 20 MHz at 40 °C, was used for the measurements. The working parameters were: pulse frequency 2.74 s, pulse angle of 90°, 4 scans, and different temperatures (15, 25, 30, 40, 50, 60 and 70 °C). Films were placed in NMR glass tubes and let equilibrating at the selected measurement temperatures in a water thermostated bath (Thermo Haake C35P, Germany). Since measurements were taken within a very short time (around 10 s), no detectable modifications of the samples were expected to take place. The decay curves were fitted to bi-exponential behaviour applying the following equation:
| 2 |
where I is the intensity of protons signals, A1 and A2 are constants, and T2FID1 and T2FID2 correspond to relaxation times of protons that are part of the polymeric chains and protons of water tightly bound to the polymer, respectively.
Interaction between components by Fourier transform infrared spectrometry (FT-IR) analysis
The infrared spectra of the samples were registered utilizing a FT-IR spectrometer (Spectrum Two System, Perkin-Elmer, USA). Analyses were performed utilizing a universal attenuated total reflectance unit (UATR) within a spectral range of 4000–400 cm−1, with a resolution of 4 cm−1 and a constant pressure of 20 N. Three measurements from random places of three replicates of each film sample were analysed.
Microstructure of surface by scanning electron microscopy (SEM) analysis
To observe the microstructure of films, these were cut into pieces of 1 × 1 mm approximately, which were previously covered with gold, and then surface and cross sectional cuts were analysed in a Scanning Electron Microscope (Supra 40, Carl Zeiss NTS, Oberkohen, Germany).
Characterization of films during storage
Films were stored at 4 °C and 75% of relative humidity (%RH) on desiccators. Relative humidity was obtained using a saturated solution of NaCl (Greenspan 1977). These storage conditions were selected taken into account the environmental standard conditions at which the films can be exposed during food storage.
Optical properties
Digital images from three replicates of each film were captured on white and black background through a calibrated computer vision system (LabVisionQ, Chile), according to Matiacevich et al. (2015). All images were acquired at the same conditions, with a remote camera controlled by the EOS Utility software (Cannon Inc., Japan). Images were analyzed using Adobe Photoshop v7.0 software to obtain RGB space parameters, which were then converted into CIEL*a*b* standard colour space. Opacity was calculated from the values of lightness (L*), obtained from films placed over white (L*white) and black (L*black) background surfaces (Eq. 2; Matiacevich et al. 2013).
| 3 |
Color variations between each film sample and the control film were calculated using the CIE∆E2000 equation (Luo et al. 2001).
Release kinetics of microencapsulated LMO and citral from alginate films
Release kinetics of the active compounds (LMO and citral) from films were performed following the methodology of Bustos et al. (2016), with some modifications. Simulant D1 (UE 10/2011, 50:50 ethanol:water) was utilized as solvent for migration studies. Briefly, film pieces of 2 × 2 cm were placed inside dialysis bags (cut off of 12,000 Dalton, Thomas Scientific, NJ, USA) and submerged in 30 mL of simulant D1 within 100 mL glass bottles. Prior to release studies, dialysis bags were hydrated for 6 h with distilled water at room temperature. Calibration curves were performed for each oil under study using dilutions of oil with the D1 simulant and measuring the absorbance at a wavelength of 274 nm. Then, absorbance vs concentration curves were plotted and a linear adjustment was performed to determine the concentration of oil released in the simulant. Release data from films were fitted to two different models, Weibull (Eq. 4; Papadopoulou and Kosmidis 2006) and Peppas (Eq. 5; Bustos et al. 2016) according to the following equations:
| 4 |
| 5 |
Q = Fraction of bioactive released, a and Kp are constants, n and b are constants indicative of the release mechanism and t is time. Release kinetics of each sample was performed for each storage time, by triplicate. Mean values of kinetics parameters were used to compare different samples over time.
Antimicrobial activity against E. coli
The antimicrobial activity of films was determined according to Matiacevich et al. (2013), following the agar diffusion methodology. Film pieces (1 cm2) were placed on Muller Hinton agar inoculated with E. coli (103 CFU/mL) by the pour plate technique and were stored at 37 °C for 24 h. The growth inhibition percentage was determined as the relation between the number of colonies that grew underneath the film piece and the number of colonies that grew within a 1 cm2 area of the control zone (without film) multiplied by 100 (Bustos et al. 2016). Inhibition percentage was obtained for each sample at each storage time by triplicate. Data reported is the mean value with their corresponding standard deviation.
Statistical analysis
One factor ANOVA test was used for statistical analysis of physical parameters. Significant differences were taken using a post-test Tukey with a confidence interval of 95%. All statistical analysis was determined using software XLSTAT2015 v17.1 (Adinsoft, France). All measurements were performed by triplicate.
Results and discussion
Minimum inhibitory concentration (MIC)
To determine the MIC value for LMO and citral two aqueous systems were considered: one in which EOs were dispersed in water and another where EOs were dispersed in a 1%w/w alginate solution, in order to estimate the potential antimicrobial effects that alginate by itself could have in addition to EO effect. For the oil–water system, bacterial growth took place only at concentrations below 0.6%v/v. At any LMO concentration above that value no growth of E. coli was observed, being such concentration the MIC value for the corresponding organism (data not shown). However, in the alginate-LMO suspension, growth inhibition took place at a concentration of 0.3%v/v, half the concentration required in the oil–water system, which suggested that the film-forming suspension mixture doubled the inhibitory effect on E. coli showed by the oil–water system.
The MIC value for citral was also determined. In the citral-water system growth inhibition took place at a citral concentration of 0.6%v/v, the same MIC value obtained for LMO. In the alginate-citral system, growth inhibition was observed at all the concentrations equal or greater than 0.2%v/v, 2/3 lower than for the citral-water system. For both oils, the MIC value were lower in presence of alginate because of this polymer is able to immobilize some bacteria as demonstrated by Yabur et al. (2007) with alginates extracted from a macroalgae. With these results, the MIC values obtained in presence of alginate were chosen to prepare film forming emulsions.
Emulsion droplet size and stability
The droplet size has a strong impact on stability over time (gravitational separation, flocculation and/or coalescence) and optical properties (color and transparency) of an emulsion (McClements 2005). Droplet sizes obtained for the emulsions prepared with LMO were: 1.6 ± 0.4; 2.0 ± 0.3 and 1.7 ± 0.4 μm and for the emulsions with citral values were: 2.3 ± 0.4; 2.4 ± 0.5 and 1.9 ± 0.4 μm using Tw20, TH and CAP, respectively. Thus, no significant differences in microcapsules size (p > 0.05) were observed, independently of the type of EA and EO utilised.
Emulsion stability was analysed during storage at three different temperatures: 20, 30 and 40 °C. As expected, stability decreased faster at higher temperatures. Figure 1 corresponds to the stability of the LMO emulsions with different EAs where significant differences in stability behaviours can be observed. At initial time, all emulsions were homogenous but, after a while, the principal destabilization mechanism observed was flocculation/coalescence (data not shown). The effect of each EA on the stability of emulsions was evaluated subtracting the initial backscattering percentage in the central zone of the sample tube (15–55 mm sample height) from the percentage of backscattering obtained at a selected time. Trehalose was the EA that gave place to the most stable emulsion at the three temperatures considered since it showed a little backscattering percentage variation during storage time compared to the values of the emulsions containing Tw20 or Capsul®. Besides, the behavior of the Tw20 containing emulsion was markedly different from the behaviour of the emulsion with Capsul®. In the first case, there was a fast destabilization within the first hours and then no more changes in back scattering profiles were observed for the rest of the time. On the contrary, emulsions containing Capsul® remained stable for the first hours and destabilize afterwards.
Fig. 1.

Normalized backscattering variation during storage time at different temperatures of emulsions containing alginate and lemongrass oil using different encapsulating agents a Tween 20, b Trehalose, c Capsul®
Results also demonstrated that droplet size does not always influence emulsion stability. Droplet sizes were similar (p > 0.05) between the three emulsions but their stabilities were greatly different. The higher stabilizing effect observed for trehalose, in addition to its encapsulating activity, could also be a consequence of its effect on the viscosity of the continuous phase of emulsion. Specifically, trehalose increases viscosity of the medium, which make it more difficult for oil droplets to collide and coalesce to destabilize the emulsion (Álvarez-Cerimedo et al. 2008).
Physical characterization of films at initial time
Moisture content and thickness
No significant differences (p > 0.05) in moisture content were observed among all films compared to the control sample (11 ± 2%db). Although addition of an oily ingredient to a film decrease water content, this principle does not apply in this case, since oil concentration in the films was too low (0.3–0.2%v/w for LMO and citral, respectively) to have a measurable effect on the moisture content of the films.
Films thicknesses varied between 0.15 to 0.22 mm following the trend Tw20 < CAP < TH. However, neither the EA nor the antimicrobial oil had significant effect (p > 0.05) on the thickness of the alginate films. The thicknesses of all the films were below 0.25 mm, which is within the value normally accepted for films for food applications (Skurtys et al. 2011). A structure with a thickness value above 0.25 mm should rather be called a sheet; and a structure with a thickness value below 0.10 mm would be too weak and with very limited physical properties as to be useful for practical applications. Therefore, moisture content and thickness of films were not affected neither by the type of EA nor by the type of EO.
Analysis of molecular mobility of films components by NMR
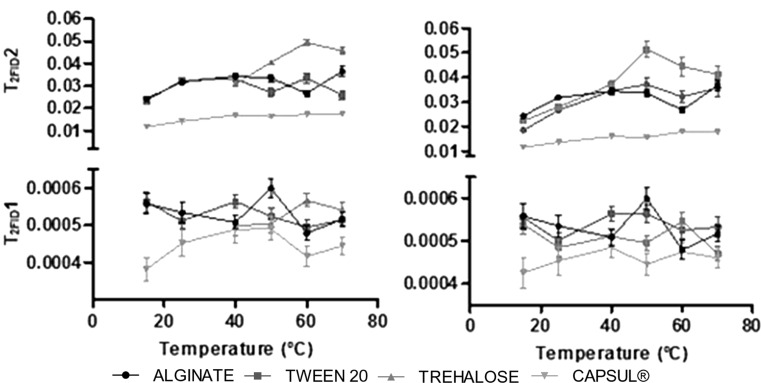
The study of the relaxation time by H-NMR (T2FID) as a function of the EA and temperature gives information about the mobility of the protons bond to water and/or to the solids present in the film matrix (Farroni et al. 2008). Curves obtained were fitted using a biexponential equation (Eq. 3), obtaining two relaxation time (T2) values, one short and other long. T2FID1 (short) represents multilayer water interacting with the alginate matrix; and T2FID2 (long) corresponds to water molecules that have a weak interaction with the solid matrix (Farroni et al. 2008). Figure 2 shows that as temperature increases, T2FID2 also increases because protons get greater mobility as it is the particular case of the trehalose containing films. Chung et al. (2000) found that the changes in T2FID with temperature for several food powders are highly associated with physical changes in the product such as agglomeration and water evaporation. Films that showed the highest values for T2FID2 had a global increase in the mobility of their matrixes, which was observed in the films with trehalose. The films that contained Capsul® showed the lowest values for T2FID1 and T2FID2. The lower mobility of water molecules in this film can be a consequence of matrix (oil droplets and alginate) agglomeration resulting from the hot casting (40 °C) method applied for the films manufacture.
Fig. 2.
Relaxation time values (T2FID) obtained by RMN using FID measurements of alginate films containing a lemongrass oil and b citral oil using different encapsulating agents. T2FID1 is a short relaxation time associated to mobility of matrix and T2FID2 is a long relaxation time associated to mobility of water associated to matrix
From an ANOVA analysis (data not shown) it was possible to find that the relaxation time (T2FID) was dependent on the EA utilized in the films (p < 0.05), whereas the type of EO did not have any significant effect (p > 0.05). For the films containing Capsul®, significant differences (p < 0.05) were observed for both T2FID1 (short) and T2FID2 (long), with respect to what happened in films with the other EAs and in alginate control film.
Analysis of molecular interactions between film components by FT-IR
The FT-IR spectra of the films containing LMO and the different EAs are shown in Fig. 3. Same patterns were observed for the films with citral. In general, the spectra were affected by the type of EA but not by the type of oil neither by the storage time of the films. All films presented wave numbers in three different spectral zones: 3500–3200 cm−1, 2900–2930 cm−1 and 1000–1030 cm−1, which can be assigned to bond stretching of the groups OH, CH and COC, respectively (Navarro et al. 2016) present in the alginate structure. Also, peaks at the wave numbers of 1600–1605 cm−1 and 1409–1412 cm−1 can be assigned to asymmetrical and symmetrical stretching of the COO bond, respectively (Li et al. 2015).
Fig. 3.
FT-IR spectra of films containing lemongrass oil using different encapsulating agents a control film of alginate, b Tween 20, c Trehalose and d Capsul®
When the EA was added to alginate film new peaks appeared in the FT-IR spectra. For the films with Tw20, a peak for the stretching of the C=O group at a wavenumber of 1736 cm−1 (Ren et al. 2012) and another peak at 2870–2873 cm−1 corresponding to the CH3 group appeared. When trehalose was added, new peaks appeared between 991 and 1148 cm−1, that belong to the glucosidic bond of the sugar as it has been also observed by Santagapita et al. (2012) in similar films to the ones utilized in this work. The films containing Capsul® presented two new peaks not present in the alginate control film. The first peak was at a wave number of 1765 cm−1 and the second at 1148 cm−1, which corresponded to the stretching of the groups C=O and C–OH, respectively. In all the films that contained EAs, the peaks that belong to alginate were all also present but with lower intensity than in films without EAs.
Analysis of the surface microstructure of the alginate films by SEM
Figure 4 shows photomicrographs of the cross sectional area of the films containing LMO. The pictures clearly show that the type of EA utilized for film manufacture had an effect on the microstructure of films. Specifically, the films with Capsul® showed a granular structure with several slightly spherical elements. On the contrary, the films with trehalose and Tw20 showed a very smooth and homogenous microstructure with no granular elements present, but some oval dark elements of regular size can be observed that might correspond to holes left by oil droplets that could have evaporated during the application of the gold covering. In the samples with trehalose, there are also some oval cavities that could also be interpreted as if the LMO would have become uniformly incorporated within the films in a similar way to what has been described for cinnamon oil within chitosan films (Ojagh et al. 2010).
Fig. 4.
Microstructure morphology by SEM at ×20,000 of transversal section of alginate films containing lemongrass oil and different encapsulating agents a Tween 20, b Trehalose, c Capsul®, d control film of alginate
Solubility of films
Solubility is an important property to select the industrial application of an edible film. High solubility (>70%) is desirable if a coating application is required, where the film and the food are consumed together. In contrast, films with low solubility are normally used as packaging (Campos et al. 2011). Mean solubility value of all films studied was 88 ± 3%, where no significant differences were observed regardless of EA or EO used.
Characterization of films during storage
Optical property changes
In food products, optical properties (opacity and color variation) are the most important factors to be considered when a new technology is being developed. For the intended purpose of edible films, colour is usually considered one of the main parameters that have to be tightly controlled in order to achieve a successful industrial application.
Colour and opacity variations of films prepared with the different oils and EAs with respect to the alginate control film were recorded during 35 days of storage. Figure 5 shows that films containing LMO and trehalose significantly increased (p < 0.05) their opacity during storage. The most likely explanation for this change would be the crystallization of trehalose that can take place at the low temperature and the high moisture content of the film (Elizalde et al. 2002). The same behaviour was observed in the films containing citral.
Fig. 5.
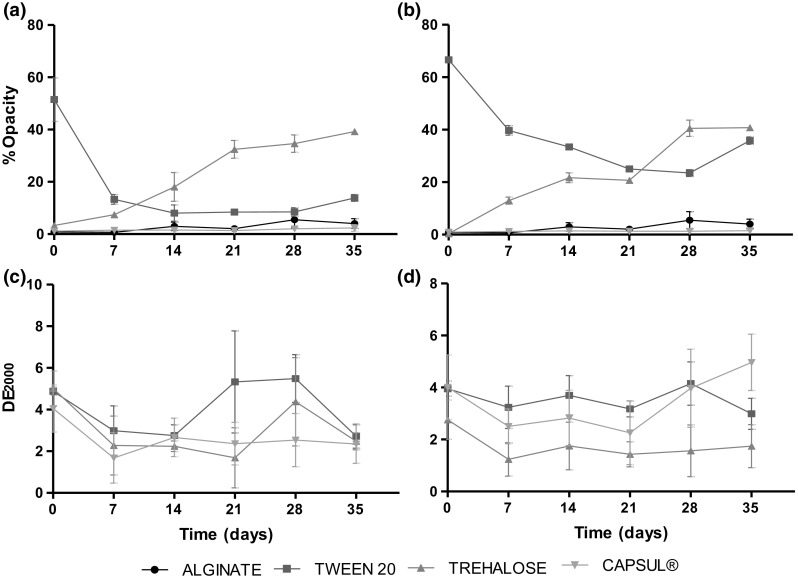
Opacity and color variation (CIEDE2000) of films containing different encapsulating agents for different essential oils: a and c lemongrass oil, b and d citral oil
The films containing Tw20 decreased their opacity during storage to values close to the opacity of the control film. There could be different explanations for this but one the most likely is the increased solubility of Tw20 and of some EO at lower temperatures, which determines that LMO and Tw20 droplets of lower sizes completely dissolve in water and, therefore, the amount of droplets that remain dispersed in the film decreases which, in turn, determines that a large proportion of the incident light can cross the film making it less opaque. This “clarifying” effect is further enhanced by the water that films absorbed from the surrounding environment with 75%RH (Ziani et al. 2008). The films containing Capsul® did not suffer any significant change in opacity (p > 0.05) with respect to control film throughout the storage period studied.
With respect to colour variation, films containing Capsul® as EA were the ones that showed the lowest colour variation compared to films with the other EAs (CIE∆E2000 < 5). When films containing LMO were compared with the films containing citral, it was observed that the latter suffered the lowest colour changes (CIE∆E2000 < 3) during storage time, even reaching values close to 1. These values correspond to what the perception by the human eye would recognize as between slight and noticeable different (Yang et al. 2012). However, for the films that contained LMO, colour differences had CIE∆E2000 values in the range of 2–4 which indicates that the human eye perception of the difference would be between noticeable and appreciable (Yang et al. 2012).
Release kinetics of EO from alginate film
Samples of the different films stored at 4 °C and 75%RH were taken at different time intervals and the release kinetics of the active principles was measured. The data sets obtained were fitted to different kinetics models (Eqs. 4, 5). It was observed that most of the release data fitted well to the Weibull and Peppas models (R2 close to 1) (data not shown). For all EAs films containing LMO showed release kinetics according to Weibull model and remained the same throughout the storage period, as expected. Only the films containing Capsul® with LMO showed a minor change in the release pattern after the day 14, when the experimental data fitted better to Peppas model (data not shown). On the other hand, for the films containing citral, the release kinetics of the active principle followed the Weibull model when Tw20 or trehalose (Fig. 6a shows an example of the fitting of Weibull model to the release data for both oils) were used and this behaviour did not change during all the storage time, which is a desirable result. However, for films containing Capsul® as the EA of citral, the release kinetics data fitted to Peppas model (Fig. 6b) throughout the storage time.
Fig. 6.

Example of kinetics release of citral oil with a Trehalose using Weibull model and b Capsul® using Peppas model a white cross symbol zero and white diamond symbol 9 days; release rate constant (k) of the active compounds using different encapsulating agents and inhibition growth of E. coli of films stored at 4 °C–75% RH containing c, e lemongrass oil, d, f citral oil and control film of alginate without encapsulating agents and essential oils
For all the films that followed the Weibull model (Eq. 4) the b constant of the equation was analysed in order to find out the type of release mechanism that is involved in the liberation of the EO’s components. In general, the b constant took values lower than 0.75 (data not shown) which indicates that the release of the active principles followed a Fickian type of diffusion (Papadopoulou and Kosmidis 2006). On the other hand, the n constant of the Peppas model (Eq. 5), like the b constant of the Weibull model, indicates the type of release mechanism involved. Values of n corresponding to the films containing Capsul® were below 0.5, which indicates that the release mechanism is a combination of partial diffusion through a swollen matrix and pores filled with water (Aragón et al. 2010). Based on these results, it can be concluded that LMO and citral are released by diffusional mechanism that follow the first Fick law when Tw20 and trehalose are used as EA. However, the films with Capsul® showed partial diffusion through a swollen matrix. This mechanism was corroborated with swelling experiment for both oils, where films containing Capsul® showed lower moisture uptake (0.005 g water/min) during storage time than the other EAs (0.01 g water/min), indicating that the swelling is slower in these films, so permitting the partial diffusion of components.
For each storage time, the release rate of each film was calculated and compared. Figure 6c, d report the release rate of the active components LMO or citral from EAs. No significant differences (p > 0.05) in release rate were observed for LMO regardless of the EA present in the film or storage time. However, the films with citral showed an increase of the release rate at the 9th day for all the EA used. This probably happen because at this time alginate and microencapsulating molecules reach the maximum hydration and swollen volume (data not shown), which could facilitate the release of the active principles contained inside the films.
Antimicrobial activity of films
In order to establish which EA or EO had the greatest effect on the growth inhibition of E. coli, their effect on the growth curves were compared. Figure 6e, f show the growth inhibition of the films containing LMO and citral, respectively. These figures show that the EA type affected bacterial growth (p < 0.05), regardless of the active ingredient (p > 0.05). This is not surprising since the main active principle of LMO is citral (Seow et al. 2014). It is important to note that all films containing EO showed prolonged growth inhibition during storage time. However, the EAs that promoted the greater and most perdurable inhibitory activity of the films were trehalose with citral, and Capsul® with LMO, indicating the importance of the selection of EA to control the antimicrobial effect of edible films. Additionally, it can be observed that the maximum inhibitory effect is achieved after nine days of storage regardless of EA type, which was observed principally in citral samples, according to the highest release rate of active compounds (Fig. 6c, d).
Conclusion
The type of EA selected generated more differences on physical and antimicrobial properties than the type of active compound in both systems studied: suspensions and films. Film forming emulsions had similar droplet sizes and stabilities during the first hours of storage at 20 °C, for the three EAs studied; however, the emulsions with trehalose showed the highest stability at higher temperatures, being this EA the most suitable for industrial application. The films with Tw20 showed the highest opacity and colour variation during storage time. Therefore, these films could affect the organoleptic properties of coated foods. The films with Capsul® showed the lowest opacity and, the films with trehalose, the lowest color variation, being both suitable to use as edible coatings for fresh foods. The release of active compounds and the growth inhibition of E. coli were similar for both EOs studied, showing a dominantly Fickian release mechanism during the whole storage time. The maximum release rate and microbial growth inhibition was displayed on the 9th day of storage regardless of the EA used in the microcapsules.
Taking into account the different physical and antimicrobial properties of the edible films based on alginate, the active compound selected was LMO because of their large availability and low cost, comparing to their pure active compound, citral. Finally, the best EA for LMO was trehalose because this one generated higher emulsion stability and lower film colour variation, comparing to the other EAs.
Acknowledgements
Authors acknowledge financial support of Fondecyt Regular Project No. 1160463, Dicyt Projects and Basal Program USA1555 from University of Santiago de Chile (VRIDEI-USACH), UBACYT Project No. 20020130100136BA from University of Buenos Aires and Programa Escala Docente AUGM–University of Buenos Aires-University of Santiago de Chile.
References
- Álvarez-Cerimedo M, Cerdeira M, Candal RJ, Herrera ML. Microencapsulation of a low-trans fat in trehalose as affected by emulsifier type. J Am Oil Chem Soc. 2008;85(9):797–807. doi: 10.1007/s11746-008-1267-1. [DOI] [Google Scholar]
- Aragón J, González R, Fuentes G (2010) Estudio in vitro de liberación de fármacos desde un biomaterial compuesto. Rev CENIC Cienc Quím 41:1–8. http://www.redalyc.org/articulo.oa?id=181620500008. Accessed 16 Feb 2016
- Benavides S, Villalobos R. Physical, mechanical and antibacterial properties of alginate film: effect of the crosslinking degree and oregano essential oil concentration. J Food Eng. 2012;110(2):232–239. doi: 10.1016/j.jfoodeng.2011.05.023. [DOI] [Google Scholar]
- Beyki M, Zhaveh S, Tahere S, et al. Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind Crops Prod. 2014;54:310–319. doi: 10.1016/j.indcrop.2014.01.033. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Bustos R, Alberti F, Matiacevich S. Edible antimicrobial films based on microencapsulated lemongrass oil. J Food Sci Technol. 2016;53(1):832–839. doi: 10.1007/s13197-015-2027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CA, Gerschenson LN, Flores SK. Development of edible films and coatings with antimicrobial activity. Food Bioprocess Tech. 2011;4(6):849–875. doi: 10.1007/s11947-010-0434-1. [DOI] [Google Scholar]
- CDCP (Center for Disease Control and Prevention) and WHO (World Health Organization) Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world. Georgia: World Health Organization; 2003. pp. 204–209. [Google Scholar]
- Chung MS, Ruan R, Chen P, et al. Study of caking in powdered foods using nuclear magnetic resonance spectroscopy. J Food Sci. 2000;65:134–138. doi: 10.1111/j.1365-2621.2000.tb15968.x. [DOI] [Google Scholar]
- Córdova K, Tello F, Bierhalz A, et al. Protein adsorption on to alginate-pectin microparticles and films produced by ionic gelation. J Food Eng. 2015;154:17–24. doi: 10.1016/j.jfoodeng.2014.12.020. [DOI] [Google Scholar]
- Di Scipio S, Escalona Y, Quijada K, Millán F (2008). Estudio del mezclado de emulsiones aplicando la metodología de superficie respuesta. Rev Fac Ing Univ Cent Venez 23:3. http://www.scielo.org.ve/scielo.php?script=sci_arttext&pid=S0798-40652008000300006. Accessed 2 Mar 2016
- Elizalde BE, Herrera ML, Buera MP. Retention of β-carotene encapsulated in trehalose-based matrix as affected by water content and sugar crystallization. J Food Sci. 2002;67(8):3039–3045. doi: 10.1111/j.1365-2621.2002.tb08856.x. [DOI] [Google Scholar]
- Fakhreddin S, Zandi S, Rezaei M, Farahmandghavi F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohyd Polym. 2013;95(1):50–55. doi: 10.1016/j.carbpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Farroni A, Matiacevich S, Guerrero S, et al. Multilevel approach for the analysis of water effects in corn flakes. J Agric Food Chem. 2008;56:6447–6453. doi: 10.1021/jf800541f. [DOI] [PubMed] [Google Scholar]
- Finotelli P, Rocha M (2005) Microencapsulation of ascorbic acid in maltodextrin and capsul using spray-drying. In: Second mercosur congress on chemical engineering
- Greenspan L. Humidity fixed points of binary saturated aqueous solutions. J Res Natl Stand. 1977;81(1):89–96. doi: 10.6028/jres.081A.011. [DOI] [Google Scholar]
- Li J, He J, Huang Y, et al. Improving surface and mechanical properties of alginate films by using ethanol as co-solvent during external gelation. Carbohyd Polym. 2015;123:208–216. doi: 10.1016/j.carbpol.2015.01.040. [DOI] [PubMed] [Google Scholar]
- Luo MR, Cui G, Rigg B. The development of the CIE2000 colour-difference formula: CIEDE2000. Color Res Appl. 2001;26(5):340–350. doi: 10.1002/col.1049. [DOI] [Google Scholar]
- Matiacevich S, Celis-Cofré D, Schebor C, Enrione J. Physicochemical and antimicrobial properties of bovine and salmon gelatin-chitosan films. CyTA-J Food. 2013;11:366–378. doi: 10.1080/19476337.2013.773564. [DOI] [Google Scholar]
- Matiacevich S, Acevedo N, López D. Characterization of edible active coating based on alginate-thyme oil-propionic acid for the preservation of fresh chicken breast fillets. J Food Proc Preserv. 2015;39(6):2792–2801. doi: 10.1111/jfpp.12530. [DOI] [Google Scholar]
- McClements DJ. Food emulsions: principles, practice and techniques. Boca Raton: CRC Press; 2005. pp. 269–339. [Google Scholar]
- Navarro R, Arancibia C, Herrera ML, Matiacevich S. Effect of type of encapsulating agent on physical properties of edible films based on alginate and thyme oil. Food Bioprod Process. 2016;97:63–75. doi: 10.1016/j.fbp.2015.11.001. [DOI] [Google Scholar]
- Ojagh S, Rezaei M, Razavi S, Hosseini S. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010;120(1):193–198. doi: 10.1016/j.foodchem.2009.10.006. [DOI] [Google Scholar]
- Papadopoulou V, Kosmidis V. On the use of the Weibull function for the discernment of drug release mechanisms. Int Pharm. 2006;309(1–2):44–50. doi: 10.1016/j.ijpharm.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Raybaudi-Massilia R, Mosqueda-Melgar J, Martin-Belloso O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int J of Food Microbiol. 2008;121(3):313–327. doi: 10.1016/j.ijfoodmicro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Ren W, Tian G, Jian S, et al. Tween coated NaYF4:Yb, Er/NaYF4 core/shell upconversion nanoparticles for bioimaging and drug delivery. RSC Adv. 2012;2:7037–7041. doi: 10.1039/c2ra20855e. [DOI] [Google Scholar]
- Riquelme N, Miranda M, Matiacevich S. Effect of processing conditions on the optical properties of films based on alginate, caseinate and lemongrass oil. CyTA–J Food. 2015;14(2):219–226. doi: 10.1080/19476337.2015.1086440. [DOI] [Google Scholar]
- Santagapita PR, Mazzobre MF, Buera MP. Invertase stability in alginate beads: effect of trehalose and chitosan inclusion of drying methods. Food Res Int. 2012;47:321–330. doi: 10.1016/j.foodres.2011.07.042. [DOI] [Google Scholar]
- Seow Y, Yeo Ch, Chung H, Yunk H. Plant essential oils as active antimicrobial agents—review. Food Sci Nutr. 2014;54(5):625–644. doi: 10.1080/10408398.2011.599504. [DOI] [PubMed] [Google Scholar]
- Skurtys O, Acevedo C, Pedreschi F, et al. Food hydrocolloid edible films and coatings. Santiago: Nova; 2011. [Google Scholar]
- Soukoulis C, Yonekura L, Gan HH, et al. Probiotic edible films as a new strategy for developing functional bakery products: the case of pan bread. Food Hydrocol. 2014;39:231–242. doi: 10.1016/j.foodhyd.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabur R, Bashan Y, Hernández-Carmona G. Alginate from the macroalgae Sargassum sinicola as a novel source for microbial immobilization material in wastewater treatment and plant growth promotion. J Appl Phycol. 2007;19:43–53. doi: 10.1007/s10811-006-9109-8. [DOI] [Google Scholar]
- Yang Y, Ming J, Yu N. Color image quality assessment based on CIEDE2000. Adv Multimed. 2012;2012:1–6. [Google Scholar]
- Ziani K, Oses J, Coma V, Maté JI. Effect of the presence of glicerol and Tween 20 on the chemical and physical properties of films based on chitosan with different degree of deacetylation. LWT-Food Sci Tech. 2008;41(10):2159–2165. doi: 10.1016/j.lwt.2007.11.023. [DOI] [Google Scholar]