Abstract
Antioxidant contents and activities of different extracts from four Tunisian pomegranate peels, locally called “Acide”, “Gabsi”, “Nebli” and “Tounsi”, were studied. Peels samples were extracted with three solvents (water, ethanol and acetone). For each extract, the total phenol contents and antioxidant activity were evaluated. The highest values of polyphenol, tannins, flavonoids and anthocyanins were recorded in the acetone extract of Acide ecotype with 304.6 mg gallic acid equivalent/g; 292.23 mg gallic acid equivalent/g; 15.46 mg Quercetin/g and 54.51 mg cy-3-glu/100 g, respectively. The acetone extract of Acide ecotype also showed the highest free radical-scavenging and reducing power activity compared to other extracts. Besides, the phytochemical analysis by LC–MS/MS revealed a high content of ellagitannins with punicalagin and punicalagin derivatives as the major compounds that might be responsible for promising antioxidant activity of pomegranate peel extracts. Two compounds (Castalagin derivative and Galloyl-bis-HHDP-hex derivative) were detected only in “Acide” ecotype in important contents.
Keywords: Antioxidant propreties, Punica granatum L., LC–MS/MS analysis, Punicalagin
Introduction
Over the last five decades, there has been a trend to find new sources of natural antioxidants, such as agronomic by-products that have traditionally been undervalued.
The toxicological effects of synthetic antioxidants together with the consumer preference for natural products have resulted in increased interest in the search for and use of natural antioxidants present in fruits and vegetables (Püssa et al. 2009). Some examples of fruit by-products that can show the profitability of bioactive compounds extraction are citrus fruits (oranges, lemons and mandarins). Rich in carotenoids, the citrus peel can be used as a natural colorant in the food industry (Wang et al. 2008). Other successful examples of exotic fruit by-products that may be considered as sources of bioactive compounds are coffee and mango (Miljkovic and Bignami 2002). Indeed coffee by-product showed an antioxidant capacity and could be considered as a new potential functional ingredient (Borrelli et al. 2004). Mango peels were also reported to be a good source of antioxidants such as polyphenols and carotenoids and can be utilized for the preparation of macaroni with improved antioxidant activity (Ajila et al. 2010).
Tropical exotic fruit by-products are known as sources of a great variety of antioxidants, and their particular properties may be useful in maintaining food quality and stability against oxydative phenomenon. Pomegrenate (Punica granatum) is one of these exotic fruits widely cultivated and consumed in the Mediterranean countries. In Tunisia, pomegranate has been cultivated since ancient times under diverse agroclimatic conditions. The production of pomegranate, consumed exclusively as fresh fruit and juice in Tunisia, is in a constant evolution. However, this progress of production and industrial transformation is accompanied with an increase of the low-value peel quantities. Pomegranate peel, which constitutes approximately 40% of the whole fruit, possess higher antioxidant activity than the edible portion (Venkataramanamma et al. 2016).
Pomegranates are reported to be a rich source of polyphenolic compounds that include flavonoids (anthocyanins, catechins and other complex flavonoids) and hydrolyzable tannins (punicalin, pedunculagin, punicalagin, gallagic acid and ellagic acid esters of glucose) which account for 92% of their antioxidant activities (Afaq et al. 2005). The pomegranate fruit was widely studied for its phenolic content (Gil et al. 2000; Nuncio-Jáuregui et al. 2015); however, there are only very few studies evaluating the phenolic content of pomegranate peel, particularly, Tunisian cultivars.
Thus, the purpose of this study was to investigate the effects of different extracting solvents on the total polyphenols and antioxidant activities of four Tunisian pomegranate peels ecotypes. An attempt was made to determine individual phenolic compounds, especially tannins, in pomegranate extracts using the high-performance liquid chromatography (HPLC)-linear ion trap mass spectrometry with negative electrospray ionization methodology.
Materials and methods
Plant material
Mature pomegranate fruits, ecotypes “Acide” (Ac), “Gabsi” (Ga), “Nebli” (Ne) and “Tounsi” (To), having no visible external cuts or spoilage, were collected from the same oasis at Gabes region (southeast of Tunisia). Fruits were manually peeled then the collected peels were cut into small pieces and maintained at −12 °C for further physicochemical characterization. For antioxidant extraction, samples were lyophilized (Thermo Electro Corporation, USA), ground and maintained at room temperature.
Physicochemical characterization of pomegranate peel
Fruits of each ecotype were individually analyzed for physical characteristics. Pomegranates were weighed, then the peels were manually separated from the fruits, and the percentage of pulps and peels per fruit were measured.
Proximate composition (dry matter, lipid, ash and protein content) was determined according to the method described by the Association of Official Analytical Chemists (AOAC 1997). Dry matter was determined by drying samples at 105 ± 3 °C to constant weights (AOAC 1997). The total ash was determined by calcination in muffle furnace at 550 °C until constant weight was obtained (AOAC 1997). The total nitrogen concentration was obtained using Kjeldahl method (AOAC 1997), and the protein concentration was estimated using a nitrogen conversion factor of 6.25. Fat content was determined by Soxhlet extraction with hexane at boiling point of the solvent (AOAC 1997). Total, soluble and insoluble fibre contents were determined according to the AOAC enzymatic–gravimetric method of Prosky et al. (1988). The total sugar content was determined by the phenol–sulfuric acid method of Dubois et al. (1956).
Colour measurements were carried out with a colorimeter (Minolta CR-300, Minolta, Osaka, Japan). Measurements were taken using the Commission International de l’Eclairage (CIE) L*a*b* system.
Extracts preparation
Five grams of finely-powdered peels were shaken for 1 h (25 °C- 180 rpm) with 25 ml of solvents (acetone or ethanol or distilled water). For each solvent, the mixture was centrifuged at 3000g for 15 min and the supernatant was recovered. The residue was re-extracted two times with the same procedure described above. Then the supernatants were combined and evaporated (concentrated to dryness) at the adequate temperature under vacuum (rotary evaporator, Heidolph, Schwabach, Germany), then stored at −20 °C.
Antioxidant compound quantification
Total phenolic content (TPC) of the concentrated extracts was determined spectrophotometrically (UV mini 1240, UV/VIS spectrophotometer, SHIMDZU, Kyoto, Japan) at 760 nm according to the Folin–Ciocalteu method (Slinkard and Singleton 1977). TPC was expressed as mg gallic acid equivalent (GAE) per gram of extract.
Total tannin content was determined by Folin–Ciocalteu procedure, after the removal of tannins by their adsorption on insoluble matrix (polyvinylpolypyrrolidone, PVPP) (Kollidon CL, BASF, Germany) and non-absorbed phenolics were determined as described. The calculated values were subtracted from the total polyphenol contents and total tannin contents expressed as mg gallic acid equivalent (GAE) per gram of extract.
Total flavonoid content of extracts was determined by the method described by Zhishen et al. (1999). The absorbance of the mixture was then measured spectrophotometrically at 510 nm. The total flavonoid content was expressed as mg Quercetin Equivalent (QE) per gram of extract.
The amount of carotenoids was determined according to the method described by kuti (2004) and was carried out on an aliquot of hexane extract by measuring absorbance spectrophotometrically at 450 nm. The total carotenoids were calculated using an extinction Coefficient of β-carotene, E1% = 2505.
The total anthocyanins of pomegranate peel extract were determined by a pH differential method using 2 buffer systems: potassium chloride buffer (pH 1.0, 0.025 M) and sodium acetate buffer (pH 4.5, 0.4 M). Peel extracts were mixed with the corresponding buffers and read against water as a blank at 510 and 700 nm (Cam et al. 2009).
Absorbance (A) was calculated using:
with a molar extinction coefficient ɛ of 29,600. The results were expressed as mg of cyanidin-3-glucoside equivalents (CGE) per 100 g of extract.
Antioxidant activity
DPPH free radical scavenging activity
The DPPH radical-scavenging activity of the extracts (50 µg/ml) was determined spectrophotometrically at 517 nm as described by Bersuder et al. (1998).
The control was conducted in the same manner, except that distilled water was used instead of extract. Butylatedhydroxyanisole (BHA) was used as a standard.
Reducing power assay
The ability of the extracts (20 µg/ml) to reduce iron (III) was determined spectrophotometrically at 700 nm according to the method of Kumaran and Joel Karunakaran (2007). The more the reducing power increased, the more the absorbance of the reaction mixture increased. Butylatedhydroxyanisole (BHA) was used as a standard.
Metal chelating activity
The chelating activity of the extracts (12 mg/ml) was measured spectrophotometrically at 562 nm according to the methods described by Dinis et al. (1994). The chelating anti-oxidant activity for Fe2+ was calculated according to the following formula:
where, Ac is the absorbance of the control reaction and As is the absorbance of the sample extract. Ethylenediaminetetraacetic acid (EDTA) was used as a standard.
LC–MS/MS analysis
LC–MS/MS (liquid chromatography–tandem mass spectrometry) analyses were performed as described by Ben Mansour et al. (2015) on a Thermo Scientific System consisting of an Accela U-HPLC unit with a photodiode array detector and an LTQ Orbitrap XL mass spectrometer fitted with an electrospray source. Chromatography was performed on 5 μL sample injections onto a 150 × 3 mm i.d., 3 um, Luna C18(2) column (Phenomenex) using a 400 μL/min linear mobile phase gradient of methanol/water/acetonitrile +1% formic acid changing from 0:90:10 to 90:0:10 over 20 min followed by an isocratic phase for 5 min and then a column wash phase and equilibrium of column for 3 min before the next injection. The electrospray ionization (ESI) source of the mass spectrometer was operated in negative mode since the phenolic compounds in question ionize better in this mode. The orbitrap mass analyzer was set to scan in range m/z 200–2000 at 30,000 resolutions in negative polarity, while the linear ion-trap analyzer performed MSn analyses on the most abundant ions in both polarities using an ion isolation window of ±2 m/z and relative collision energy of 35%. Phenolic compounds were identified on the basis of their Rt values, UV spectra and mass spectra, as well as by comparison of the spectra with those of the available authentic standards. The structure assignment of compounds for which no standards were available was based on a systematic search for molecular ions using extracted ion mass chromatograms and comparing those with data in the literature.
Statistical analysis
All experiments were conducted in triplicate and the differences between treatment means were determined by Duncan’s procedure at p < 0.05 using the SPSS statistics 19. The expressed values are mean ± standard deviation of triplicate measurements.
Results and discussion
Physicochemical characterization
The physical characteristics of analyzed pomegranate cultivars are described in Table 1. The average fruit weight of pomegranate cultivars ranged between 223.0 g (Ac) and 546.3 g (Ga). Similarly, the lowest (33.5%) and the highest (51.4%) peel’s percentages were observed in (Ac) and (Ga), respectively (Table 1). In general, the obtained results are close to those found by a previous study of pomegranate fruits grown in Iran, which are between 164.9 and 375.8 g (Sarkhosh et al. 2009).
Table 1.
Physicochemical characteristics of four selected pomegranate peels
| Parameters | Ac | Ga | Ne | To |
|---|---|---|---|---|
| Fruit weight (g) | 222.95 ± 65.12a | 546.28 ± 154.33b | 530.08 ± 87.29b | 456.43 ± 47.34b |
| Pulp percentage (%) | 64.33 ± 10.12b | 47.41 ± 1.85a | 60.97 ± 4.71b | 54.06 ± 6.09ab |
| Peel percentage (%) | 33.5 ± 8.04a | 51.37 ± 1.64b | 36.98 ± 5.22a | 42.99 ± 6.51ab |
| Moisture (%) | 67.26 ± 0.23a | 73.23 ± 0.15b | 72.58 ± 0.67b | 72.68 ± 0.79b |
| Proteins (%, DW) | 3.96 ± 0.49a | 7.13 ± 0.53c | 5.42 ± 0.01b | 5.84 ± 0.38b |
| Fat (%, DW) | nd | nd | nd | nd |
| Total sugar (%, DW) | 30.65 ± 0.70a | 33.58 ± 0.21ab | 33.00 ± 2.99ab | 34.83 ± 0.79b |
| Solubles | 27.33 ± 0.70a | 30.60 ± 0.79b | 30.14 ± 2.99ab | 32.33 ± 0.21b |
| Insolubles | 3.31 ± 0.07ab | 2.98 ± 0.37ab | 2.86 ± 0.04ab | 2.49 ± 0.44a |
| Total fibre (%, DW) | 28.27 ± 0.90a | 28.10 ± 1.20a | 33.81 ± 0.42b | 33.93 ± 0.66b |
| Insolubles | 27.11 ± 0.65a | 27.04 ± 0.77a | 32.51 ± 0.36b | 32.13 ± 0.46b |
| Solubles | 1.16 ± 0.11ab | 1.06 ± 0.04a | 1.35 ± 0.21b | 1.80 ± 0.09c |
| Ash (%, DW) | 4.97 ± 0.22b | 4.44 ± 0.31ab | 3.71 ± 0.37a | 4.52 ± 0.75ab |
| Carbohydrates (%, DW) | 32.14 ± 0.45d | 26.73 ± 0.48c | 24.04 ± 0.89b | 20.87 ± 0.50a |
| L* | 35.10±0.18a | 37.74 ± 0.26b | 47.83 ± 0.04d | 38.32 ± 0.22c |
| a* | 14.47 ± 0.09c | 15.22 ± 0.06d | 8.52 ± 0.02a | 12.57 ± 0.17b |
| b* | 10.43 ± 0.10a | 11.96 ± 0.19b | 22.61 ± 0.02d | 15.90 ± 0.28c |
Ac Acide, Ga Gabsi, Ne Nebli, To Tounsi, DW dry weight basis, L*a*b*, lightness and chromaticity coordinates in the L*a*b* color space (CIELAB)
Each value in the table is represented as mean ± SE (n = 3). Significant differences between values in the same row are indicated by different letters a–d (P < 0.05)
The results for chemical characteristics of pomegranate peel from different cultivars are displayed in Table 1. It can be noticed that moisture content in peel ranged from 67.26% in (Ac) ecotype to 73.23% in (Ga) ecotype. (Ga) ecotype has the highest protein content (7.13 g/100 g dry weight (dw)). These contents are higher than those found in other Turkish cultivars, ‘Lefon’, ‘Seedless’, ‘Kadi’, ‘Siyah’ and ‘Koycegiz’, whose contents are 3.19; 3.11; 3.06; 2.67 and 2.58% dw, respectively (Hepaksoy et al. 2000).
Total sugars account for 30.65–34.83%. This content, consisting mainly of soluble fraction (27.33–32.33% dw), was comparable to that reported for a Pakistan ecotype (Ullah et al. 2012) (31.38% dw). These carbohydrates would be held in the fibrous structure of the peel.
Pomegranate peel is considered a low-fat by-product, compared with that of other fruits such as bananas and the prickly pear (0.7 and 3.68, respectively) (Espiard 2002). Indeed, as shown in Table 1, the peels of all ecotypes are devoid of fat.
The peel of pomegranate could be considered as a rich source of dietary fibre, especially of insoluble fibres. It contains considerable contents of fibres ranging from 28.10 to 33.93% dw, respectively, in cultivars (Ga) and (To). These contents were higher than that of a Pakistan ecotype (Ullah et al. 2012) (21% dw). These quantities are relatively very important compared with those found in the peels of lemons, oranges and grapefruit; 14; 13.9 and 13.9%; respectively (Gorinstein et al. 2001). Singh et al. (2016) reported that total dietary fibre content of different fruits (pomegranate, kinnow, mango, banana, jambolan, grapes and sapodilla) was positively related with the insoluble dietary fibre content. It was also observed that fruit peels had higher total dietary fibre content than the respective pulps.
The four cultivars under study varied in colour from yellow to orange to red. The results show that all samples exhibited red coloration, with positive a* values (Table 1). (Ga) and (Ac) ecotypes showed the highest a* values (15.22 and 14.47, respectively) followed by (To) (12.57) and (Ne) (8.52). Compared with other ecotypes, (Ga), (Ac) and (To) had significantly higher red coloration (a* value) than ‘Jabal 2′ grown in the Sultanate of Oman (10.16 ± 1.41) (Al-Said et al. 2009). On the other hand, (Ac) ecotype had significantly less whiteness, while (Ne) had the most whiteness (L* value) and yellowness (b* value).
Skin colour is an important quality attribute in pomegranate marketing and the fruit with deep red colouration or blush tends to have greater consumer appeal in the local market.
As anthocyanins are a group of phenolics compounds which contributes to the red, blue, or purple colour of many fruits, especially of pomegranate juice, these characterizing values of colour in pomegranate peels may be due to the presence of anthocyanins.
Evaluation of antioxidant properties
Yield of extraction and antioxidant compounds content
Table 2 shows the percentage yield and antioxidant compounds content in different extracts. The pomegranate peel extracted with water gave the highest total extract yield (43.03–73.12%), followed by ethanol (16.88–32.16%) and acetone (1.68–9.72%). Thus, the majority of compounds are hydrosoluble. The highest yields were in (Ac) and (Ga) ecotypes, which indicates that these ecotypes are richer in hydrosoluble compounds than (Ne) and (To). Acetone was found to be the most efficient for extracting total phenolics from (Ne) ecotype, thus it can be considered as the richest in apolar compounds. This difference in extraction yield by different solvents can be explained by the fact that the yield of extraction and the extracts’ antioxidant activity extremely depend on the solvent polarity, which determines both quantitatively and qualitatively the extracted antioxidant compounds.
Table 2.
Yield and total phenolics content in different extracts
| Solvent | Ecotype | Yield (%) | Phenolic compounds (mg GAE/g) | Tannins (mg GAE/g) | Flavonoids (mg Quer/g) | Anthocyanins (mg cy-3-glu/100 g) |
|---|---|---|---|---|---|---|
| Water | Ac | 73.12 ± 5.31c | 209.83 ± 3.83b | 197.69 ± 0.12c | 11.67 ± 0.55b | 29.11 ± 2.10c |
| Ga | 63.08 ± 2.76b | 146.75 ± 1.75a | 134.50 ± 0.25a | 9.98 ± 0.08a | 21.68 ± 1.10b | |
| Ne | 43.96 ± 2.01a | 248.93 ± 14.50c | 236.69 ± 0.34d | 15.26 ± 0.43d | 16.8 ± 1.70a | |
| To | 43.03 ± 1.94a | 202.11 ± 4.95b | 185.03 ± 0.10b | 13.38 ± 0.17c | 20.37 ± 3.40ab | |
| Ethanol | Ac | 32.16 ± 2.31c | 140.93 ± 0.53c | 129.20 ± 0.27d | 7.49 ± 0.20d | 42.58 ± 2.00c |
| Ga | 30.48 ± 1.77c | 109.21 ± 0.34a | 99.18 ± 0.10a | 5.49 ± 0.17b | 29.55 ± 3.67b | |
| Ne | 16.88 ± 0.44a | 121.10 ± 6.20b | 110.55 ± 0.12c | 6.74 ± 0.43c | 14.27 ± 3.39a | |
| To | 25.08 ± 2.02b | 116.11 ± 5.15ab | 105.71 ± 0.12b | 5.00 ± 0.02a | 12.95 ± 0.05a | |
| Acetone | Ac | 4.68 ± 0.31b | 304.60 ± 14.20d | 292.23 ± 0.25d | 15.46 ± 0.11c | 54.51 ± 8.93d |
| Ga | 3.72 ± 0.12ab | 157.06 ± 0.00a | 144.96 ± 0.55a | 10.28 ± 0.26a | 30.05 ± 2.00c | |
| Ne | 9.72 ± 1.98b | 207.8 ± 0.00c | 196.26 ± 0.02c | 11.43 ± 0.43b | 6.84 ± 0.32a | |
| To | 1.68 ± 0.32a | 190.95 ± 6.91b | 179.35 ± 0.10b | 11.26 ± 0.60b | 13.86 ± 1.40b |
Ac Acide, Ga Gabsi, Ne Nebli, To Tounsi
Each value in the table is represented as mean ± SE (n = 3). Significant differences between the values in the same column for each solvent are indicated by different letters a–d (P < 0.05)
Quantitatively, our results are in agreement with the study of Wang et al. (2011) on pomegrante peel of ‘Wonderful’ variety from Madera, CA. They reported that the extract yield was 43.19% with water, 17.71% with ethanol and 3.81% with acetone. These results are nearly the same in (To), (Ne) and (Ga) ecotypes extracted with water, ethanol and acetone, respectively. It is clear that the yield of extraction in (Ac) ecotype is higher than that of «Wonderful» variety for all solvents.
As shown in Table 2, acetone was the best extracting solvent of phenolic compounds. As a matter of fact, the highest content was achieved with acetone in (Ac) ecotype (304 mg GAE/g = 541.89/100 g fresh weight). It should be noted that, because of polarity differences between solvents, the solubility of the solute into the solvent is expected to be different. Water and ethanol are polar protic solvents of dielectric constants of 80 and 24 respectively, while acetone is polar aprotic solvent of dielectric constant of 21 (Wang et al. 2011).
The comparison between the four ecotypes shows that the content of polyphenol in (Ac) ecotype is significantly higher than the other ecotypes using the ethanol and acetone as extracting solvent.
Since all four pomegranate cultivars used in this research were grown in the same location using similar agronomic practices, the differences in phenolic compounds have shown that the genetic variability leads to the variation in the biosynthesis of phenolic secondary metabolites in these cultivars.
The best contents in tannins were obtained in (Ac) ecotype by using the ethanol and the acetone with the contents of 129 and 290 mg GAE/g, respectively. Thus we come to the conclusion that the (Ac) ecotype is a potential source of tannins.
The amount of total tannins ranged from 90 to 95% of the total rate of polyphenols. We can deduce that polyphenols consist essentially of tannins.
The total flavonoids varied from 9.98 to 15.25; 5 to 7.49 and 10.27 to 15.46 mg Quercetin/g in water, ethanol and acetone extracts, respectively. Our results demonstrated that the amount of total flavonoids ranged from 4 to 6%. Therefore, the flavonoids can be said to constitute a small part of the total phenolics.
The results for the total anthocyanins of the pomegranate peel indicated that there were significant differences in the total anthocyanins content of the pomegranate cultivars. (Ac) ecotype had the highest amount of total anthocyanins compared to other cultivars (42.58 and 54.51 mg cy-3-glu/100 g) in ethanol and acetone extract, respectively. The results obtained from the anthocyanins content measurements were in accordance with L* measurements. As a matter of fact, (Ac) peel with the lowest L* values had the highest amounts of anthocyannins. Therefore, the variation in L* values can be attributed to anthocyanin content.
Hence, the Ac’s acetone extract showed the highest values of polyphenol, tannins, flavonoids and anthocyanins with 304.6 mg GAE/g; 292.23 mg GAE/g; 15.465 mg Quercetin/g and 54.51 mg cy-3-glu/100 g, respectively.
Antioxidant activity
DPPH free radical scavenging activity
As shown in Table 3, the radical-scavenging ranges from 71.11 to 84.16%, from 71.73 to 96.73% and from 77.32 to 97.82% in water, ethanol and acetone extracts, respectively. The highest activity was observed with acetone for all ecotypes. The results clearly indicate that the acetone extract of (Ac) ecotype exhibits the highest scavenging activity.
Table 3.
Antioxidant activity in different extracts
| Solvent | Ecotype | DPPH (%) | Reducing power | Metal chelating activity (%) |
|---|---|---|---|---|
| Water | Ac | 83.85 ± 0.62c | 0.25 ± 0.00b | 89.99 ± 0.54a |
| Ga | 71.11 ± 1.24b | 0.18 ± 0.01a | 89.20 ± 2.54a | |
| Ne | 84.16 ± 0.00c | 0.32 ± 0.02d | 97.37 ± 1.62b | |
| To | 84.16 ± 1.24c | 0.29 ± 0.02c | 94.90 ± 3.03b | |
| BHA | 64.03 ± 0.84a | 0.64 ± 0.00e | – | |
| Ethanol | Ac | 96.73 ± 0.15e | 0.24 ± 0.01c | 34.93 ± 0.01c |
| Ga | 71.73 ± 1.24b | 0.10 ± 0.02a | 26.26 ± 0.31b | |
| Ne | 84.47 ± 0.00c | 0.26 ± 0.02c | 21.00 ± 0.50a | |
| To | 90.83 ± 0.16d | 0.14 ± 0.00b | 19.72 ± 2.38a | |
| BHA | 64.03 ± 0.84a | 0.64 ± 0.00d | – | |
| Acetone | Ac | 97.82 ± 0.00e | 0.39 ± 0.01c | 75.25 ± 2.25c |
| Ga | 77.32 ± 0.62b | 0.24 ± 0.01a | 61.87 ± 1.25b | |
| Ne | 86.48 ± 0.46c | 0.28 ± 0.03b | 81.43 ± 0.06d | |
| To | 95.65 ± 0.62d | 0.26 ± 0.02ab | 54.56 ± 0.94a | |
| BHA | 64.03 ± 0.84a | 0.64 ± 0.00d | – |
Ac Acide, Ga Gabsi, Ne Nebli, To Tounsi, BHA butylatedhydroxyanisole
Each value in the table is represented as mean ± SE (n = 3). Significant differences between the values of the same column for each solvent are indicated by different letters a-e (P < 0.05)
According to the results displayed in Table 2, (Ac) and (Ga) acetone extracts have the highest and lowest levels of total phenolics and antioxidant activity, respectively. Thus it can be concluded that there is a close relationship between the total phenolics and antioxidant activity.
A study on eight species of fruits most commonly consumed and grown in Thailand (coconut (Cocos nucifera), mangosteen (Garcinia mangostana), dragon fruit (Hylocereus undatus), long-gong (Lansium domesticum), banana (Musa sapientum), rambutan (Nephelium lappaceum), passion fruit (Passiflora foetida) and pomegranate (Punica granatum) has shown that the extract of pomegranate peel has the highest scavenging activity followed by the peel extracts of rambutan and mangosteen (Okonogi et al. 2007).
Reducing power assay
Table 3 shows the reductive capability of water, ethanol and acetone extracts compared to BHA. The reducing activity is generally associated with the presence of reductones, which has been proven to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom (Gordon 1990). It was also reported that raductones react with certain precursors of peroxide, thus preventing the peroxide formation.
Acetone extracts have higher reducing power than that of ethanol extracts among all ecotypes. Like phenolic content, the greatest reducing power were detected in (Ac) extract acetone. Hence, this ecotype might contain the highest amount of reductones which could react with free radicals to stabilise and terminate the radical chain reactions.
The study of correlation between the phenolic content and antioxidant activity of extracts have shown that total phenols, tannins and flavonoids in peel are highly correlated with the reducing power assay with correlations coefficients 0.877, 0.874 and 0.844, respectively. This result suggests that the majority of the antioxidant capacity of pomegranate extracts results from the contribution of phenolic compounds, tannins and flavonoids.
Consequently, our peel extracts were electron donors and can react with free radicals to convert them into more stable products and terminate the radical chain reaction.
Therefore, the total phenolics yield might be one of the most important indicators of effective extraction processes for producing high quality products (Wang et al. 2011).
Secondary antioxidant activity: Metal chelating activity
The chelating activity of pomegranate peel may be considered low since such activity was recorded only at high concentration in spite of its important content in total phenolic compounds. Similar findings have been reported for bananas. In fact, these were reported to be a powerful secondary antioxidant, though had low phenolic content due to the presence of other active compounds that might bind to metal ions strongly (Lim et al. 2006).
As a result, all extracts under investigation may be regarded as unable to strongly obstruct the generation of ·OH radicals from Fenton reaction (Fe2+ + H2O2 ·Fe3+ + ·OH +OH−). Our results have shown that pomegranate’s peel has low chelating activity despite its important radical scavenging activity.
This result is confirmed by the study of Lim et al. (2006), who have found that although guava has a potent radical scavenging property, its function as a secondary antioxidant, as measured by chelating power, is rather low. This means that some fruits can possess high primary antioxidant activities, but low secondary antioxidant activities.
It can be concluded that the antioxidant activity of our extracts is not limited to phenolics. It may also be due to the presence of other antioxidant secondary metabolites, which is in agreement with a study on antioxidant activity of olive extracts (Hajimahmoodi et al. 2008).
Therefore, it was concluded from the three antioxidant activities that (Ac) ecotype exhibits the highest free radical-scavenging and reducing power activity. Hence, this ecotype might contain the highest amount of reductones which could react with free radicals to stabilise and terminate radical chain reactions. Pomegranate peel extract of (Ac) ecotype appeared to have strong antioxidant properties and merits further intensive study.
Characterization of phenolic compounds by LC–MS
To elucidate the structures of phenolic compounds in pomegranate peel, the acetone extracts were subjected to a combination of liquid chromatography–tandem mass spectrometry (LC–MS/MS) high resolution unit with a photodiode array detector and fitted with an electrospray ionization source (ESI) (Table 4). HPLC analysis of the fractions showed the presence of peaks with ellagitannins-type UV spectra (two bands, λmax of band 1 between 255 and 290 nm and λmax of band 2 between 326 and 378 nm).
Table 4.
Retention times, UV/Vis spectra and characteristic ions of phenolic compounds of pomegranate’s acetone extracts
| Comp | Assignment | Rt (min) | λmax(nm) | [M–H]− m/z | HPLC–ESI(_)-MSn experiment m/z | Ac | Ga | Ne | To |
|---|---|---|---|---|---|---|---|---|---|
| C1 | HHDP-hex | 1.93 | 326, 290 | 481 | 301;275 | + | + | + | |
| C2 | citric acid | 2.1 | 214 | 191 | 173;111 | + | + | ||
| C3 | HHDP-gallagyl-hex (punicalagin) | 2.33 | 378, 258 | 1083 | 601;781;575;721;549 | + | |||
| C4 | punicalagin derivative | 3.31 | 377, 256 | 541.31 | 532;481;301 | + | |||
| C5 | punicalagin derivative | 3.7 | 378, 258 | 541.33 | 532;481;301;275 | + | + | + | |
| C6 | punicalagin derivative | 4.33 | 377, 257 | 541.36 | 301;275;523;549;249 | + | + | + | |
| C7 | punicalagin derivative | 4.94 | 378, 258 | 541.37 | 301;781;601;532;275 | + | + | + | |
| C8 | HHDP-gallagyl-hex (punicalagin) | 4.93 | 378, 258 | 1083 | 1083;541 | + | |||
| C9 | Bis-HHDP-hex (pedunculagin II) | 5.36 | 359, 255 | 783 | 765;301;481;275 | + | |||
| C10 | Ellagic acid der | 5.62 | 265 | 799 | 781;479;301 | + | |||
| C11 | Digalloyl-HHDP-hex (pedunculagin II) | 6.06 | 274 | 785 | 483;301;633 | + | + | + | |
| C12 | Pgd-3-pentoside | 6.62 | 433, 271 | 401 | 271 | + | |||
| C13 | Galloyl-HHDP-hex | 6.8 | 365, 266, 330, 260 | 633 | 463;301;275 | + | + | + | |
| C14 | Galloyl-HHDP-DHHDP-hex (granatin B) | 7.83 | 365, 274 | 951 | 933;915;301 | + | + | + | |
| C15 | Digalloyl-HHDP-hex (pedunculagin II) | 8.55 | 272 | 785 | 615;301;275 | + | |||
| C16 | Castalagin der | 9.35 | 275 | 965 | 933;445;301 | + | |||
| C17 | Ellagic acid-pent | 10.01 | 267 | 433 | 301;300 | + | |||
| C18 | Galloyl-bis-HHDP-hex (casuarinin) derivative | 10.18 | 377, 258 | 979.14 | 932;445;301 | + | |||
| C19 | Ellagic acid-rhamnoside | 10.39 | 275 | 447 | 301;300 | + | + | ||
| C20 | Galloyl-bis-HHDP-hex (casuarinin) derivative | 10.4 | 378, 260 | 979.11 | 933;445;301 | + | |||
| C21 | Cyd-3-pentoside | 10.53 | 515, 278 | 417 | 287 | + | |||
| C22 | Ellagic acid | 10.74 | 367, 275 | 301 | 301;229;185 | + | + | + | |
| C23 | Cya-rut | 11.45 | 375, 268 | 593 | 285;257;229;547 | + | |||
| C24 | cyanidine 3 o-gluco | 11.66 | 518, 278 | 447 | 327;285;255 | + | + | + |
Ac Acide, Ga Gabsi, Ne Nebli, To Tounsi, Rt retention time, comp compound
Ellagitannins
Compound eluted at 1.93 min produced a molecular ion at m/z 481 [M_H]− and a fragment at m/z 301, indicating the presence of an ellagic acid moiety. This compound C1 was tentatively identified as HHDP-hex. Compound C9 eluted at 5.36 min showed a maxima in UV 359-255, which is characteristic of ellagitannins, and exhibited an [M_H]− ion at m/z 783. The loss of water and ellagic acid in the MS2 experiment produced fragments at 765 and 481m/z, respectively. Based on this fragmentation pathway and the occurrence of further typical fragments, compound C9 was identified as bis-HHDP-hexoside (pedunculagin I). Compound C10 produced an [M_H]− ion at m/z 799 and fragments at m/z 781 and at m/z 479, which came from the loss of water and ellagic acid, respectively. This compound (C10) may be attributed either to granatin A (HHDP-DHHDP-hexoside) or lagerstannin A (bis-HHDP-gluconic acid) (Sentandreu et al. 2013). Compound C11 and C15 exhibited an [M_H]− ion at m/z 785. The release of typical ellagitannin and gallotannin fragments at m/z 483 (digalloylhexoside), 301 (ellagic acid) and 633 (galloyl-HHDPhexoside), clearly suggests that C11 and C15 were characterised as digalloyl-HHDP-hexoside (pedunculagin II). Each of the two different retention times corresponds to an isomeric structure, also differing in their fragmentation patterns. Compound C13, showing an [M_H]− ion at m/z 633 and fragment at 301 in the MS2 experiment, was identified as galloyl-HHDP-hexoside. Compound C14 exhibited an [M_H]− ion at m/z 951 and compound C16 at m/z 965. Both compounds produced fragments at m/z 933 and at m/z 301 (ellagic acid) in the MS2 experiment. This fragment (m/z 933), generating fragments at m/z 915 from the loss of water, is typical for castalagin/vescalagin or galloyl-gallagyl-hexoside (galloylpunicalin, pedunculagin III). Nevertheless, the characteristic gallagyl-fragment at m/z 601 was not detected. Furthermore, castalagin/vescalagin exhibited molecular masses 18 Da lower than compound C14. Based on these results, compound C14 was identified as granatin B (galloyl-HHDPDHHDP- hexoside), which forms a part of type III-tannins (dehydroellagitannins) (Fischer et al. 2011). Granatin A and B were firstly identified as the major components of pomegranate leaves (Tanaka et al. 1986). Analysis of compound C16 shows the deprotonated molecule ion at m/z 965, which produced ions at m/z 933 and at m/z 301 in the MS2 experiment. Additionally, fragments were observed at m/z 915 and 897. The fragments at m/z 933, 915 and 897 are typical of the castalagin derivative (Fischer et al. 2011).
Two monoglycosylated ellagic acid derivatives were detected, such as an ellagic acid-pentoside (m/z 433; C17) and deoxyhexoside (m/z 447; C19). The [M-H]− ion of C17 was obtained at m/z 433. In the MS2 experiment, the ion at m/z 301 was generated by the loss of 132 Da, reasonably assigned as the elimination of pentose. The occurrence of the ion at m/z 300 was attributed to a homolytic rupture of the glycosidic bond (Ferreres et al. 2005). Ellagitannin C19 has been previously detected during a large scale purification of pomegranate husk polyphenols (Seeram et al. 2005).
C22 was identified as free ellagic acid. This compound was confirmed by its m/z 301 [M–H]− ion, yielding characteristic ions at m/z 185 and 229 upon dissociation. Ellagic acid has previously been reported for pomegranate husk and juices (Seeram et al. 2005).
Gallagic acid and gallagyl esters
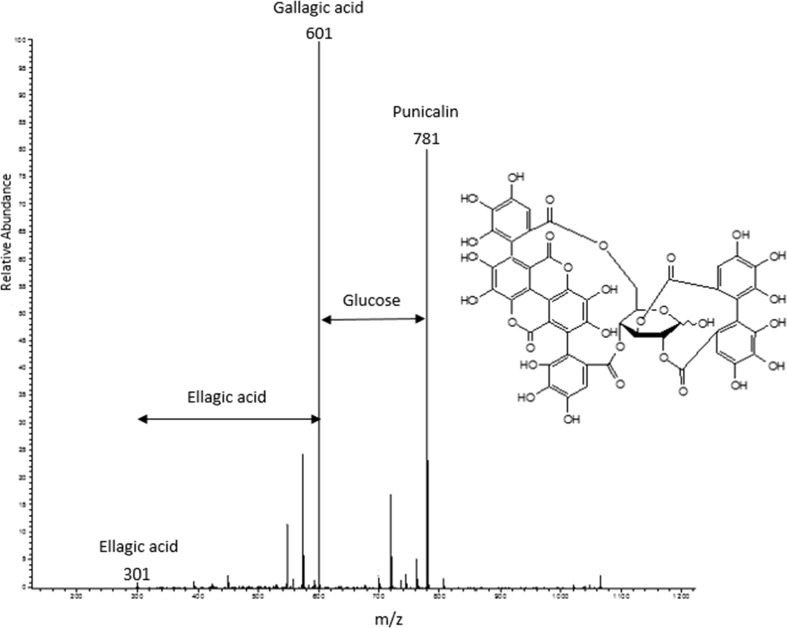
Punicalagin (2,3-HHDP-4,6 gallagylglucoside) is the main phenolic compound in pomegranate and has already been well characterised (Tanaka et al. 1986). This is a complex ellagitannin characteristic of pomegranate peel, which contains glucose, ellagic acid, and gallagic acid (Gil et al. 2000). Punicalagin was detected as doubly-charged ion species, displaying an [M_2H]2− ion at m/z 541, which is equivalent to a molecular weight of 1084 Da. The fragment at m/z 601 in the MS2 experiment indicated the loss of a gallagic acid moiety (Fischer et al. 2011). Several isomers have been previously described in pomegranate fruit peel and also in leaves and bark (Gil et al. 2000), which were confirmed in the present study as illustrated by different retention times of compound 1083. Fragments for the loss of ellagic acid (781 m/z) and for the gallagic (601 m/z) and ellagic acid (301 m/z) residues were the main fragments observed in the mass spectrum, supporting the nature of these compounds (Fig. 2).
Fig. 2.
MS–MS spectra of punicalagin (M–H m/z 1083) and the subsequent fragment ions of punicalin (M–H m/z 781), ellagic acid (M–H m/z 301) and then gallagic acid (M–H m/z 601)
According to the abovementioned informations, compounds (C3, C8) and (C4, C5, C6, C7) were identified as punicalagin and punicalagin derivatives, respectively.
The mass spectra in the negative mode of compounds C18 and C20 exhibited a base peak [M_H]− at m/z 979 with significant fragments at m/z 933, m/z 445 and m/z 301. This result suggest that they could be two isomers of Galloyl-bis-HHDP-hex (casuarinin) derivative.
Anthocyanins
The detected anthocyanin among the pomegranate peels were identified as mono-substituted anthocyanidin derivatives. A pelargonidin derivative was observed in pomegranate peel, such as Pgd-3-pentoside (m/z 401; C12). Cynanidin derivatives were also detected, such as cyanidin-3-rutinoside (m/z 593; C23), cyanidin-3-glucoside (m/z 447; C24) and Cyd-3-pentoside (m/z 417; C21).
Organic acid
Compound C2 exhibited an [M_H]− ion at m/z 191, which is typical for citric acid. The same compound was identified in pomegranate juice by Sentandreu et al. (2013).
Comparison between different ecotypes
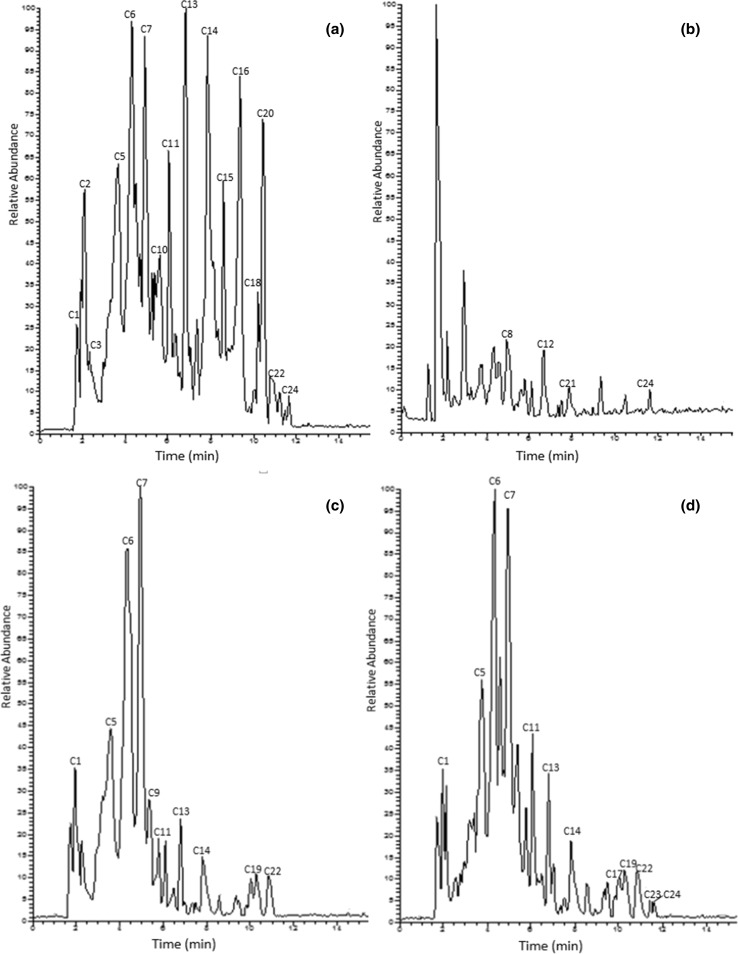
The analysis of the four extracts has shown the presence of punicalagins derivatives, galloyl-HHDP-hex, Galloyl-HHDP-DHHDP-hex (granatin B) and Digalloyl-HHDP-hex (pedunculagin II) in (Ac), (Ne) and (To) ecotypes (Fig. 1). However, the relative abundance of these compounds were more in (Ac) ecotype as compared to the others. These compounds were absent in (Ga) extract (Fig. 1b).
Fig. 1.
Identification of phenolic compounds in acetone extracts. a Acide ecotype, b Gabsi ecotype, c Nebli ecotype, d Tounsi ecotype
In addition to these phenolics, two other compounds were detected only in (Ac) ecotype in important content: Castalagin derivative (m/z 965) and Galloyl-bis-HHDP-hex (casuarinin) derivative (m/z 979.11) (Fig. 1a). Therefore, these two compounds might be responsible for the powerful antioxidative capacity of (Ac) ecotype.
Interestingly, among the various phenolic compounds (24 compounds) detected in pomegranate peel, the acetone fraction of (Ac) ecotype is rich in ellagitannins, a group of phenolics that could be responsible for demonstrated antioxidant properties (Fig. 2).
Acknowledgement
The authors would like to thank the “Ministère de l’Enseignement Supérieur et de la Recherche Scientifique, Tunisia” for the support of this research work. They also wish to express their gratitude to Mrs. Leila MAHFOUDHI, an English teacher at the Sfax Faculty of Science, for having proofread this paper.
References
- Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF- kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- Ajila CM, Aalami M, Leelavathi K, Prasada Rao UJS. Mango peel powder: a potential source of antioxidant and dietary fiber in macaroni preparations. Innov Food Sci Emerg Technol. 2010;11:219–224. doi: 10.1016/j.ifset.2009.10.004. [DOI] [Google Scholar]
- Al-Said FA, Opara LU, Al-Yahyai RA. Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman. J Food Eng. 2009;90:129–134. doi: 10.1016/j.jfoodeng.2008.06.012. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 16. Washington, DC: Association of Official Analytical Chemists; 1997. [Google Scholar]
- Ben Mansour A, Porter EA, Kite GC, Simmonds MS, Abdelhedi R, Bouaziz M. Phenolic profile characterization of Chemlali olive stones by liquid chromatography-ion trap mass spectrometry. J Agric Food Chem. 2015;63:1990–1995. doi: 10.1021/acs.jafc.5b00353. [DOI] [PubMed] [Google Scholar]
- Bersuder P, Hole M, Smith G. Antioxidants from a heated histidineglucose model system. I: investigation of the antioxidant role of histidine and isolation of antioxidants by high performance liquid chromatography. J Am Oil Chem. 1998;75:181–187. doi: 10.1007/s11746-998-0030-y. [DOI] [Google Scholar]
- Borrelli RC, Esposito F, Napolitano A, Ritieni A, Fogliano V. Characterization of a new potential functional ingredient: coffee silverskin. J Agric Food Chem. 2004;52:1338–1343. doi: 10.1021/jf034974x. [DOI] [PubMed] [Google Scholar]
- Cam M, Hisil Y, Durmaz G. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chem. 2009;112:721–726. doi: 10.1016/j.foodchem.2008.06.009. [DOI] [Google Scholar]
- Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Espiard E (2002) Introduction à la transformation industrielle des fruits. TEC&DOC-Lavoisier, p 106
- Ferreres F, Sousa C, Justin M, Valentão P, Andrade PB, Llorach R, Rodrigues A, Seabra RM, Leitão A. Characterisation of the phenolic profile of Boerhaavia diffusa L. by HPLC-PAD-MS/MS as a tool for quality control. Phytochem Anal. 2005;16:451–458. doi: 10.1002/pca.869. [DOI] [PubMed] [Google Scholar]
- Fischer UA, Carle R, Kammerer DR. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011;127:807–821. doi: 10.1016/j.foodchem.2010.12.156. [DOI] [PubMed] [Google Scholar]
- Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant Activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Gordon MH. The mechanism of antioxidant action in vitro. In: Hudson BJF, editor. Food antioxidants. London: Springer; 1990. pp. 1–18. [Google Scholar]
- Gorinstein S, Martín-Belloso O, Park YS, Haruenkit R, Lojek A, Ĉíž M, Caspi A, Libman I, Trakhtenberg S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001;74:309–315. doi: 10.1016/S0308-8146(01)00157-1. [DOI] [Google Scholar]
- Hajimahmoodi M, Sadeghi N, Jannat B, Oveisi MR, Madani S, Kiayi M, Akrami MR, Ranjbar AM. Antioxidant activity, reducing power and total phenolic content of Iranian Olive Cultivar. J Biol Sci. 2008;8:779–783. doi: 10.3923/jbs.2008.779.783. [DOI] [Google Scholar]
- Hepaksoy S, Aksoy U, Can HZ, Ui MA (2000) Determination of relationship between fruit cracking and some physiological responses, leaf characteristics and nutritional status of some pomegranate varieties. Cahiers Options méditerranéennes 87–92
- Kumaran A, Joel Karunakaran R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT. 2007;40:344–352. doi: 10.1016/j.lwt.2005.09.011. [DOI] [Google Scholar]
- Kuti JO. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004;85:527–533. doi: 10.1016/S0308-8146(03)00184-5. [DOI] [Google Scholar]
- Lim YY, Lim TT, Tee JJ. Antioxidant properties of guava fruit: comparison with some local fruits. Sunway Academic J. 2006;3:9–20. [Google Scholar]
- Miljkovic D, Bignami GS (2002) Nutraceuticals and methods of obtaining nutraceuticals from tropical crops. USA. Application number: 10/992.502. Published In. Google Patent
- Nuncio-Jáuregui N, Nowicka P, Munera-Picazo S, Hernández F, Carbonell-Barrachina AA, Wojdyło A. Identification and quantification of major derivatives of ellagicacid and antioxidant properties of thinning and ripe Spanish pomegranates. J Funct Foods. 2015;12:354–364. doi: 10.1016/j.jff.2014.11.007. [DOI] [Google Scholar]
- Okonogi S, Duangrat C, Anuchpreeda S, Tachakittirungrod S, Chowwanapoonpohn S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007;103:839–846. doi: 10.1016/j.foodchem.2006.09.034. [DOI] [Google Scholar]
- Prosky L, Asp NG, Schweizer TF, De Vries JW, Fruda I. Determination of insoluble, soluble and total dietary fibre in foods and food products. J Assoc of Anal Chem. 1988;71:1017–1023. [PubMed] [Google Scholar]
- Püssa T, Raudsepp P, Toomik P, Pällin R, Mäeorg U, Kuusik S. A study of oxidation products of free polyunsaturated fatty acids in mechanically deboned meat. J Food Comp Anal. 2009;22:307–314. doi: 10.1016/j.jfca.2009.01.014. [DOI] [Google Scholar]
- Sarkhosh A, Zamani Z, Fatahi R, Ranjbar H. Evaluation of genetic diversity among Iranian soft-seed pomegranate accessions by fruit characteristics and RAPD markers. Sci Hortic. 2009;121:313–319. doi: 10.1016/j.scienta.2009.02.024. [DOI] [Google Scholar]
- Seeram N, Lee R, Hardy M, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep Purif Technol. 2005;41:49–55. doi: 10.1016/j.seppur.2004.04.003. [DOI] [Google Scholar]
- Sentandreu E, Cerdan-Calero M, Sendra JM. Phenolic profile characterization of pomegranate (Punica granatum) juice by high-performance liquid chromatography with diode array detection coupled to an electrospray ion trap mass analyzer. J Food Compos Anal. 2013;30:32–40. doi: 10.1016/j.jfca.2013.01.003. [DOI] [Google Scholar]
- Singh JP, Kaur A, Shevkani K, Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol. 2016;53:4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analyses: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- Tanaka T, Nonaka G, Nishika I. Tannins and related compounds XL. Revision of the structures of punicalin and punicalagin, and isolation and characterization of 2-O-galloylpunicalin from the bark of Punica granatum L. Chem Pharm Bull. 1986;34:650–655. doi: 10.1248/cpb.34.650. [DOI] [Google Scholar]
- Ullah N, Ali J, Khan FA, Khurram M, Hussain A, Inayat-ur R, Zia-ur R, Shafqat U. Proximate composition, minerals content, antibacterial and antifungal activity evaluation of pomegranate (Punica granatum L.) Peels Powder. Middle East J Sci Res. 2012;11:396–401. [Google Scholar]
- Venkataramanamma D, Aruna P, Singh RP. Standardization of the conditions for extraction of polyphenols from pomegranate peel. J Food Sci Technol. 2016;53:2497–2503. doi: 10.1007/s13197-016-2222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Chuang YC, Hsu HW. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008;106:277–284. doi: 10.1016/j.foodchem.2007.05.086. [DOI] [Google Scholar]
- Wang Z, Pan Z, Ma H, Atungulu G. Extract of phenolics from pomegranate peels. Open Food Sci J. 2011;5:17–25. doi: 10.2174/1874256401105010017. [DOI] [Google Scholar]
- Zhishen J, Mengcheng T, Jianmin W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]