Abstract
Background
Patients with Parkinson’s disease (PD) usually experience distress related not only to motor dysfunction, but also to nonmotor symptoms, including gastrointestinal dysfunction.
Objective
The purpose of this pilot study was to evaluate the efficacy and safety profile of a traditional Japanese medicine, rikkunshito (RKT), used for the treatment of gastrointestinal symptoms, associated with anorexia and dyspepsia, in patients with PD.
Methods
Patients were randomly assigned to either Group A (4-week treatment period with 7.5 g/d RKT followed by a 4-week off-treatment period) or Group B (4-week off-treatment period followed by a 4-week treatment period with 7.5 g/d RKT). Appetite, quality of life for gastrointestinal symptoms, and depression were assessed using a visual analog scale, the Gastrointestinal Symptom Rating Scale and the Self-Rating Depression Scale, respectively. The gastric emptying examination and assay of plasma acylated ghrelin level were performed using the 13C-acetate breath test and commercially available assay kits, respectively.
Results
RKT treatment produced a significant increase in the appetite score (1.84 [2.34]; P < 0.05), compared to a decrease in the score over the off-treatment period (−1.36 [2.94]). The mean score for abdominal pain, on the Gastrointestinal Symptom Rating Scale, and for self-reported depression, on the Self-Rating Depression Scale, also decreased significantly with RKT treatment (P < 0.05), compared with the off-treatment period scores. No effect of RKT on plasma acylated ghrelin level and rate of gastric emptying was identified.
Conclusions
RKT may improve anorexia in patients with PD. The positive effects of RKT on depression and anorexia may improve the overall quality of life of these patients. The benefits of RKT identified in our pilot study will need to be confirmed in a randomized, double-blind, controlled trial. UMIN Clinical Trial Registry identifier: UMIN000009626.
Key words: anorexia, depression, GSRS, Parkinson’s disease, rikkunshito
Introduction
Parkinson’s disease (PD) is a chronic neurodegenerative disorder characterized by motor dysfunction, such as resting tremor, rigidity, akinesia, and postural instability, resulting from a progressive degeneration of the nigrostriatal dopaminergic pathway.1 Patients with PD usually experience distress related not only to motor dysfunction, but also to nonmotor symptoms, such as hallucination, depression, anxiety, apathy, fatigue, and autonomic dysfunctions.2 Gastrointestinal symptoms, such as constipation, dyspepsia and anorexia are also very common in patients with PD, and may affect drug treatment.
Levodopa (L-dopa) is the most commonly used drug treatment for PD. However, L-dopa has been shown to inhibit gastric emptying in rats3 and in healthy human volunteers.4 For patients with PD, who generally develop delayed gastric emptying, treatment with L-dopa can further deteriorate the function of gastric emptying.5 Because impaired gastric emptying delays the arrival of L-dopa to the upper small intestine, irregular absorption of L-dopa is considered to be a major reason for the “delayed-on” and “no-on” phenomena of L-dopa.6, 7
Several studies have also reported alterations in body weight and food preference among patients with PD.8, 9, 10 Although the exact mechanism responsible for alterations in food intake/eating habit and body weight in these patients has not yet been identified, the loss of dopamine, which is known to have a role in the regulation of food intake, may be involved in the deterioration of feeding behavior in these patients.11, 12 Moreover, PD may affect the domains of taste and olfaction, cognition, mood, and motivation that influence food intake and, in turn, body weight.9 Beyer et al13 reported patients with PD to be at greater risk of poor nutritional status than an age- and sex-matched population. Ultimately, anorexia and delayed gastric emptying may affect the activities of daily living and quality of life (QOL) of patients with PD.
In Japan, the traditional medicine rikkunshito (RKT), in the form of extracted granules (product No. TJ-43; Tsumura & Co, Tokyo, Japan), has been approved for medicinal use by the Japanese Ministry of Health and Welfare and is widely prescribed for patients with upper gastrointestinal symptoms, such as gastroesophageal reflux,14 dyspeptic symptoms,15 chemotherapy-induced nausea,16 and anorexia.17 RKT has various pharmacologic actions, which include stimulation of gastric emptying,18, 19 regulation of ghrelin secretion20, 21 and a protective effect on the mucosa of the gastroesophageal region.22 A recent pilot clinical study indicated that RKT could ameliorate gastroparesis in patients with PD,23 as well as anorexia in elderly patients with dementia.24
The purpose of our study was to evaluate the efficacy and safety profile of RKT used in the treatment of gastrointestinal symptoms, including anorexia and dyspepsia, in patients with PD.
Patients and Methods
Participants
Seventeen patients with PD, treated on an outpatient basis at the Saitama Medical Center of Saitama Medical University, Saitama, Japan, between June 2013 and December 2015, were enrolled into our study. Prospective participants were screened according to the following set of inclusion criteria: age ≥20 years and ≤85 years, Hoehn-Yahr stages I–III, and symptoms of anorexia or dyspepsia. Exclusion criteria were intolerance to oral administration of medication; use of drugs known to be prohibited with the concomitant use of RKT, such as prokinetics and traditional Japanese medicine (Kampo medicines), during the observation period; presence of serious complications (heart, liver, kidney, blood disorders, or malignancy); history of allergy to Kampo medicine; and considered ineligible to participate by the principal investigator. All participants enrolled in the study provided their written informed consent.
A Consolidated Standards of Reporting Trial diagram of participant flow through the trial is shown in Figure 1A. Among the 17 patients enrolled into the study, 1 withdrew consent before group assignment. The remaining 16 patients were randomly allocated to Group A or B. Two patients showing Hoehn-Yahr stages IV and V were excluded from the full analysis set (FAS). The FAS, therefore, included 14 patients, 7 in Group A and 7 in Group B.
Figure 1.
Consort diagram and crossover study design. One Parkinson’s disease (PD) patient among the 17 patients initially enrolled withdrew consent before group assignment. The remaining 16 patients were randomly allocated to Group A or B. Two patients showing Hoehn-Yahr stages IV and V were excluded from the full analysis set (FAS) population (N = 14: Group A, n = 7 and Group B, n = 7). A 2-week washout period was completed before the start of the intervention phase. Patients in Group A received an initial 4-week treatment with rikkunshito (RKT) (7.5 g/d), followed by a 4-week nontreatment period. As a counterbalance, patients in Group B completed a 4-week nontreatment period, followed by a 4-week treatment with RKT at the same daily dose.
Drug
RKT extract granules for ethical use is a prescription drug that has been approved for medicinal use by the Japanese Ministry of Health and Welfare, at a usual dose of 7.5 g/d TID. Participants ingested 2.5 g RKT, 3 times per day, before each meal. The RKT was in the form of a powdered extract obtained by spray drying of a hot water extract mixture of the following 8 crude herbs: Atractylodes Lancea Rhizome (4.0g), Ginseng (4.0g), Pinellia Tuber (4.0g), Poria Sclerotium (4.0g), Jujube (2.0g), Citrus Unshiu Peel (2.0g), Glycyrrhiza(1.0g), Ginger (0.5g). The fingerprint pattern obtained using 3-dimensional high-performance liquid chromatography revealed that RKT contained several low molecular compounds, including hesperidin, liquilitin, liquilitigenin, isoliquilitin, isoliquilitigenin, formononetin glycycoumarin, glycyrrhizin, atractylodin, atractylodinol, 6-shogaol, and 6-gingerol.18 Pharmacokinetic profiles of active ingredients in RKT, as well as of the metabolites derived from RKT, were identified in healthy Japanese volunteers.25 Patients recorded their medication use, with patient interviews used to confirm adherence, as well as the use of any medication, other than the test drug RKT, at 4 and 8 weeks after the start of the trial. The reported treatment compliance was defined as the proportion of the prescribed test drug used (7.5 g/d × 21–28 days) during the treatment phase of the trial, with a reported treatment compliance of at least 75.0% considered acceptable for analysis.
Study design
This study (UMIN000009626) was conducted using a crossover design, with a 4-week “on-treatment” period (7.5 g/d RKT TID) and a 4-week “off-treatment” period (Figure 1B). A 2-week washout period was completed before the start of the intervention phase. The 16 participants in our study group were randomly allocated to Group A or B using a permuted block method, with a block size of 4, generated by the Web-based A2 Healthcare Corporation (Tokyo, Japan) response system. Because of the obvious differences between the RKT on- and off-treatment periods, we did not attempt to mask group allocation to patients and clinicians. Patients in Group A completed an initial 4-week RKT treatment (7.5 g/d), after which RKT was discontinued for 4 weeks (off-treatment period). In contrast, patients in Group B completed the 4-week off-treatment period first, followed by the 4-week RKT treatment at the same dosage.
This study was performed in accordance with the ethical guidelines for clinical studies and considered the patients’ human rights and privacy. The study protocol was approved by the Institutional Review Board of Saitama Medical Center, Saitama Medical University.
Study procedures and questionnaire
The following self-reported outcomes were obtained before the start of the trial (Week 0) and at 4 and 8 weeks after the trial initiation: appetite, QOL for gastrointestinal symptoms, and depression were assessed using a 100-mm visual analog scale (VAS), the Japanese version of Gastrointestinal Symptom Rating Scale (GSRS), and the Self-Rating Depression Scale (SDS), respectively.
The primary end point was the change in appetite score (VAS score) between the RKT treatment and nontreatment, after crossover (Period II). Secondary end points were the change in gastric emptying, plasma ghrelin concentration, depression (SDS score), and QOL for gastrointestinal symptoms (GSRS score) between RKT treatment and nontreatment, after crossover (Period II).
Appetite was assessed using questions of the following form: “How hungry are you?”, with VAS anchors at 0 (not at all) and 100 (extremely). Patients were instructed to rate their appetite by selecting the scale that was closest to their experience.
The GSRS questionnaire includes 15 items, rated on a 7-point Likert scale, from “no discomfort” to “very severe discomfort.” Based on a factor analysis, the 15 GSRS items break down into the following 5 scales: abdominal pain (abdominal pain, hunger pain, and nausea), reflux syndrome (heartburn and acid regurgitation), indigestion syndrome (borborygmus, abdominal distension, eructation, and increased flatus), diarrhea syndrome (diarrhea, loose stools, and urgent need for defecation), and constipation syndrome (constipation, hard stools, and a feeling of incomplete evacuation).
The SDS includes 20 items, with each item rated on a 4-point scale. The possible outcome score can range between 20 and 80, with a higher score indicative of stronger depression. Based on the following cutoff scores, patients can be classified into normal, neurotic, and depressive levels, with the following cutoffs (SD) used in the Japanese version of the SDS: 35 (12), 49 (12), and 60 (7) points for normal, neurotic, and depressive categories.
Measurement of gastric emptying
Gastric emptying was evaluated using the 13C-acetate breath test, according to the methods of Ghoos,26 with slight modifications. Once baseline measurements had been obtained after a 12-hour overnight fast, participants consumed a liquid test meal (protein 8.76 g, fat 4.46 g, carbohydrate 31.24 g, 200 kcal/200 mL [Racol; Otsuka Pharmaceuticals Co. Ltd, Tokyo, Japan]) containing 100 mg 13C-sodium acetate (Wako Pure Chemicals Industries, Osaka, Japan) within 5 minutes. All participants completed the examination in a sitting position. Breath samples were collected at 5, 10, 15, 20, 30, 40, 50, 60, 75, 90, 105, and 120 minutes after consuming the liquid test meal. Samples were analyzed using 13CO2 by an infrared spectrophotometer (UBiT-IR200; Otsuka Electronics Co Ltd, Osaka, Japan). Gastric emptying time was expressed as the 13CO2 excretion Tmax and the gastric emptying rate curve was determined from the 13CO2 excretion curve pattern. Tmax of gastric emptying was calculated using Excel software (Star Medical, Tokyo, Japan).
Measurement of plasma ghrelin levels
Blood samples were collected in a state of fasting. The blood samples were transferred into chilled tubes containing ethylenediaminetetraacetic acid and aprotinin and stored on ice during collection. The samples were immediately centrifuged at 10,000 g and 4°C for 3 minutes, and the supernatant fraction was collected. The supernatant was acidified with 1 mol/L hydrogen chloride (1/10 vol) and stored at −80°C until the ghrelin assay. The ghrelin level was determined using Active Ghrelin or Des-acyl Ghrelin Enzyme-Linked Immunoassay Kits (Mitsubishi Chemical Medicine, Tokyo, Japan) according to the manufacturer’s protocol. The minimal detection limits for acyl and des-acyl ghrelin were 2.5 fmol/mL and 12.5 fmol/mL, respectively. The intra- and interassay coefficients of variation for acyl-ghrelin were 6.5% and 9.8%, respectively, and 3.7% and 8.1%, respectively, for desacyl-ghrelin.
Adverse events, safety profile, and tolerability
Safety profile and tolerability were assessed by recording all adverse events, and changes in hematologic and clinical laboratory variables were measured at the screening visits. An adverse event was defined as any unfavorable or unintended sign, even if it was considered to be causally related to the drugs used in this study. The severity of PD at 0, 4, and 8 weeks of the study period was evaluated using the Unified Parkinson's Disease Rating Scale (UPDRS) to determine whether the use of RKT caused any deterioration of PD symptoms.
Statistical analysis
Baseline characteristics of participants in Groups A and B were based on the intention-to-treat sample. The efficacy analysis was based on the FAS population. Between-group comparisons were performed according to the standard 2 × 2 crossover method, using the change after crossover as the response.27
Baseline characteristics of participants in the 2 groups were compared using Fisher exact test for categorical variables and an unpaired Student t test or the Wilcoxon rank sum test for continuous variables, as appropriate for the data distribution. The assessment of RKT efficacy for the primary endpoint of appetite score was evaluated using the Wilcoxon rank-sum test, based on the normality of the data. The Wilcoxon rank-sum test was used to compare the change in GSRS, SDS, and UPDRS scores between Groups A and B. Similarly, the Wilcoxon rank-sum test was used to evaluate between-group differences in plasma ghrelin level, body weight, and gastric emptying rate.
In addition, for descriptive purposes, the intragroup changes over each period were analyzed using the Wilcoxon signed-rank test, with no adjustment for multiple comparisons performed. The data were expressed as mean (SD), with a 2-sided P value < 0.05 considered to be statistically significant.
All statistical analyses were performed using the Statistical Analysis System software version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
Background of patients enrolled in the study
Relevant characteristics of the 16 participants allocated to Group A or B are summarized in Table I, with no between-group differences identified for all measured outcome variables: appetite VAS score, overall GSRS score, SDS score, UPDRS score, Tmax of gastric emptying, and plasma ghrelin levels.
Table I.
Relevant background characteristics for patients in the study group.
| Characteristic | Total (n = 16) | n | Group A (n = 8) | n | Group B (n = 8) | n | P value |
|---|---|---|---|---|---|---|---|
| Age (year), mean (SD) | 70.47 (5.50) | 16 | 71.57 (5.50) | 8 | 69.50 (5.68) | 8 | 0.487* |
| Gender (Male/Female) | 8/8 | 16 | 5/3 | 8 | 3/5 | 8 | 0.619† |
| Body weight (kg) | 50.75 (9.68) | 16 | 54.38 (10.94) | 8 | 47.13 (7.16) | 8 | 0.139* |
| Hoehn–Yahr (I/II/III/IV/V) | 0/12/2/1/1 | 16 | 0/6/1/1/0 | 8 | 0/6/1/0/1 | 8 | 1.000† |
| UPDRS | 17.69 (4.17) | 13 | 17.71 (4.72) | 7 | 17.67 (3.88) | 6 | 0.886‡ |
| VAS of appetite | 4.55 (1.80) | 16 | 5.19 (1.39) | 8 | 3.91 (2.01) | 8 | 0.227‡ |
| GSRS (overall score) | 2.39 (0.84) | 14 | 2.19 (0.61) | 7 | 2.58 (1.03) | 7 | 0.701‡ |
| SDS | 48.80 (6.68) | 15 | 49.00 (6.37) | 8 | 48.57 (7.52) | 7 | 0.953‡ |
| Tmax of gastric emptying (min) | 50.90 (12.37) | 16 | 47.85 (8.86) | 8 | 53.95 (15.10) | 8 | 0.495‡ |
| Acylated ghrelin (fmol/mL) | 23.17 (13.35) | 16 | 24.46 (15.48) | 8 | 21.88 (11.78) | 8 | 0.958‡ |
| Desacylated ghrelin (fmol/mL) | 203.56 (178.23) | 16 | 182.84 (188.99) | 8 | 224.28 (177.13) | 8 | 0.372‡ |
GSRS = Gastrointestinal Symptom Rating Scale; SDS = Self-Rating Depression Scale; Tmax = the peak time to 13CO2 excretion UPDRS = Unified Parkinson’s Disease Rating Scale; VAS = visual analog scale.
t Test.
Fisher exact test.
Wilcoxon rank-sum test.
Efficacy of RKT in improving appetite and QOL for gastrointestinal symptoms
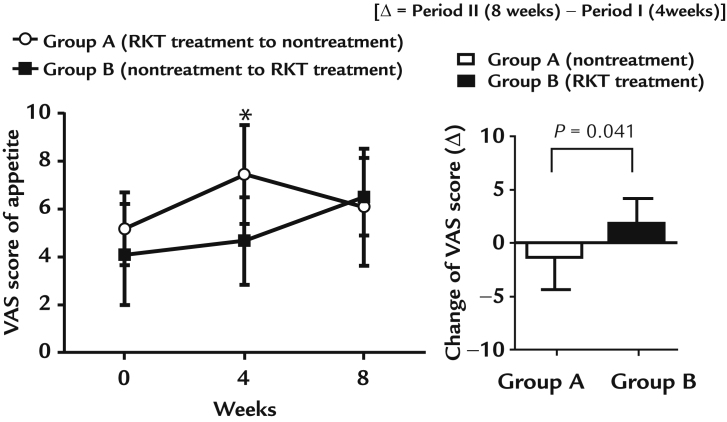
Efficacy of RKT treatment in improving appetite is shown in Figure 2. Seven patients in each of Group A and B completed the study protocol. In Group A, the VAS score significantly increased after the RKT treatment period (4 weeks after the start of the study), with the VAS score decreasing after the nontreatment period (8 weeks after the start of the study), as follows: Week 0, 5.19 (1.51); Week 4, 7.46 (2.04); and Week 8, 6.10 (2.44). In Group B, the VAS score did not change significantly after the nontreatment period (4 weeks after the start of the study) and the RKT treatment period (8 weeks after the start of the study: Week 0, 4.11 (2.08); Week 4, 4.67 (1.84); and Week 8, 6.51 (1.60). After crossover (Period II), there was significant change in the VAS score between the RKT treatment (1.84 [2.34]) and nontreatment (−1.36 [2.94]) period (P = 0.041).
Figure 2.
Effect of rikkunshito (RKT) on appetite scores. Values are expressed as mean (SD) *P < 0.05 versus Week 0 for Group A (Wilcoxon signed-rank test). After crossover (Period II), there was a significant change (Δ) in the visual analog scale (VAS) score (P = 0.041) between the RKT treatment (1.84 [2.34]) and nontreatment (−1.36 [2.94]) period.
No significant effect of RKT treatment was identified on the overall GSRS scale score or on the subscale scores for reflux, ingestion, diarrhea, and constipation. In Group A, the abdominal pain subscale score did not change significantly after RKT treatment period (4 weeks after the start of the study) and nontreatment period (8 weeks after the start-point of the study). In Group B, the abdominal pain subscale score did not change significantly after the nontreatment period (4 weeks after the start of the study) but significantly decreased after RKT treatment period (8 weeks after the start of the study). After crossover (Period II), there was a significant change in the abdominal pain subscale score between the RKT treatment and the off-treatment periods (Table II).
Table II.
Changes in Gastrointestinal Symptom Rating Scale (GSRS) scores.
| Group A RKT treatment to nontreatment |
|
Group B nontreatment to RKT treatment |
Group A vs Group B |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) (n) | Δ (n) | P value | Mean (SD) (n) | Δ (n) | P value | (ΔII-I) | |||
| Overall | 0.307 | ||||||||
| Baseline | 2.16 (0.66) (6) | 2.58 (1.03) (7) | |||||||
| Period I | 1.91 (0.63) (7) | (I–Baseline) −0.24 (0.70) (6) | 0.563 | 2.49 (0.63) (7) | (I-Baseline) −0.09 (0.69) (7) | 0.969 | |||
| Period II | 2.00 (0.62) (7) | (ΔII–I) 0.08 (0.64) (7) | 0.578 | 2.25 (0.84) (7) | (ΔII–I) −0.24 (0.43) (7) | 0.313 | |||
| Reflux syndrome | 0.178 | ||||||||
| Baseline | 1.64 (0.94) (7) | 1.86 (1.18) (7) | |||||||
| Period I | 1.57 (0.79) (7) | (I–Baseline) −0.07 (0.93) (7) | 1.000 | 2.07 (0.98) (7) | (I–Baseline) 0.29 (1.03) (7) | 0.594 | |||
| Period II | 1.43 (0.61) (7) | (ΔII–I) −0.14 (0.63) (7) | 1.000 | 1.64 (0.75) (7) | (ΔII-I) -0.43 (0.45) (7) | 0.125 | |||
| Abdominal pain | 0.012 | ||||||||
| Baseline | 1.57 (0.57) (7) | 1.95 (1.04) (7) | |||||||
| Period I | 1.29 (0.30) (7) | (I–Baseline) −0.29 (0.59) (7) | 0.375 | 2.24 (0.85) (7) | (I–Baseline) 0.29 (1.03) (7) | 0.656 | |||
| Period II | 1.33 (0.51) (7) | (ΔII-I) 0.05 (0.45) (7) | 0.875 | 1.48 (0.50)* (7) | (ΔII-I) −0.76 (0.53) (7) | 0.031 | |||
| Indigestion syndrome | 0.333 | ||||||||
| Baseline | 1.88 (0.85) (6) | 2.43 (0.89) (7) | |||||||
| Period I | 1.71 (0.76) (7) | (I–Baseline) −0.13 (0.41) (6) | 0.625 | 2.46 (0.96) (7) | (I–Baseline) 0.04 (0.37) (7) | 1.000 | |||
| Period II | 1.93 (0.97) (7) | (ΔII–I) 0.21 (0.55) (7) | 0.438 | 2.29 (0.67) (7) | (ΔII–I) −0.18 (0.79) (7) | 0.656 | |||
| Diarrhea syndrome | 0.948 | ||||||||
| Baseline | 1.94 (0.77) (6) | 2.38 (1.74) (7) | |||||||
| Period I | 1.71 (0.76) (7) | (I–Baseline) −0.28 (0.90) (6) | 0.750 | 1.95 (1.06) (7) | (I–Baseline) −0.43 (0.74) (7) | 0.250 | |||
| Period II | 1.76 (0.83) (7) | (ΔII–I) 0.05 (0.91) (7) | 0.875 | 2.05 (1.53) (7) | (ΔII–I) 0.10 (0.71) (7) | 0.813 | |||
| Constipation syndrome | 0.798 | ||||||||
| Baseline | 3.72 (1.45) (6) | 4.29 (1.77) (7) | |||||||
| Period I | 3.29 (1.30) (7) | (I–Baseline) −0.28 (1.45) (6) | 0.813 | 3.71 (1.52) (7) | (I–Baseline) −0.57 (1.13) (7) | 0.250 | |||
| Period II | 3.52 (1.30) (7) | (ΔII–I) 0.24 (1.10) (7) | 0.594 | 3.81 (1.81) (7) | (ΔII–I) 0.10 (0.94) (7) | 0.797 | |||
RKT = rikkunshito.
P < 0.05 significant differences (Wilcoxon signed-rank test) compared with period I (4 week). Group A (Period I, RKT treatment; Period II, nontreatment), Group B (Period I, nontreatment; Period II, RKT treatment). The statistically significant difference between RKT treatment (group B, period II–period I) and off-treatment (group A, period II–period I) was analyzed using the Wilcoxon rank sum test.
Changes in SDS scores after RKT treatments
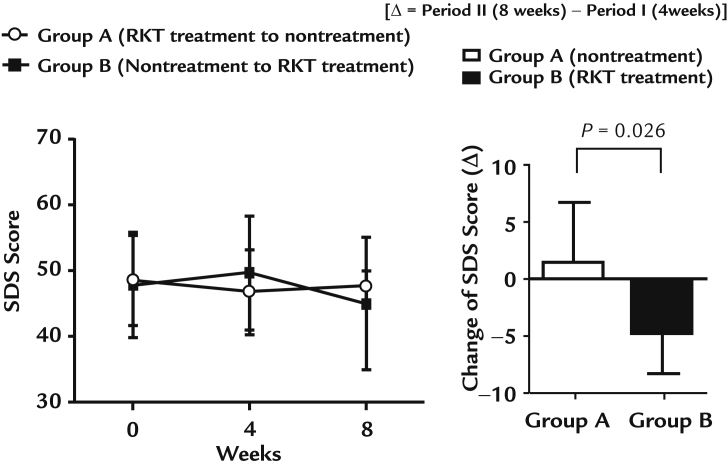
The efficacy of RKT treatment in improving depression symptoms is shown in Figure 3. In Group A, there was no significant change in the SDS score after RKT treatment period (4 weeks after the start of the study) and nontreatment period (8 weeks after the start of the study), as follows: Week 0, 48.57 (6.75); Week 4, 46.83 (6.40); and Week 8, 47.71 (2.29). In Group B, the SDS score did not change significantly after nontreatment period (4 weeks after the start of the study), with a tendency for the score to decrease after the RKT treatment period (8 weeks after the start of the study; P = 0.06): Week 0, 47.83 (7.96); Week 4, 49.71 (8.62); and Week 8, 45.00 (10.00). After crossover (Period II), there was a significant change in the SDS score between the treatment (−4.71 [3.55]) and nontreatment (1.50 [5.24]) period (P = 0.026).
Figure 3.
Effect of rikkunshito (RKT) on depression. Values are expressed as mean (SD). After crossover (Period II), there was a significant change (Δ) in the Self-Rating Depression Scale (SDS) score (P = 0.026, Wilcoxon rank sum test) between the treatment (−4.71 [3.55]) and nontreatment (1.50 [5.24]) period.
Effects of RKT on body weight, plasma ghrelin levels, and gastric emptying
Effects of RKT on body weight, plasma ghrelin levels, and gastric emptying in patients with PD are summarized in Table III. After crossover, no significant differences were observed in body weight, plasma ghrelin levels, and gastric emptying between the RKT treatment and off-treatment periods.
Table III.
Effects of rikkunshito (RKT) on body weight, plasma ghrelin levels and gastric emptying.*
| Group A RKT treatment to nontreatment |
|
Group B nontreatment to RKT treatment |
Group A vs Group B P value |
||||
|---|---|---|---|---|---|---|---|
| Mean (SD) (n) | Δ (n) | Mean (SD) (n) | Δ (n) | (ΔII–I) | |||
| (Body weight, kg) | |||||||
| Baseline | 55.29 (11.48) (7) | 48.14 (7.08) (7) | 0.938 | ||||
| Period I | 55.77 (11.40) (7) | (I–Baseline) 0.49 (0.50) (7) | 47.71 (7.57) (7) | (I–Baseline) −0.43 (1.64) (7) | |||
| Period II | 55.83 (11.48) (7) | (II–I) 0.06 (0.36) (7) | 48.58 (7.85) (6) | (II–I) −0.08 (0.80) (6) | |||
| (Acylated ghrelin, fmol/mL) | |||||||
| Baseline | 23.09 (16.18) (7) | 24.09 (10.78) (7) | 0.201 | ||||
| Period I | 17.89 (6.03) (7) | (I–Baseline) −5.21 (12.80) (7) | 23.73 (13.22) (7) | (I–Baseline) −0.36 (9.02) (7) | |||
| Period II | 18.70 (16.88) (7) | (II-I) 0.83 (13.76) (7) | 28.16 (15.06) (7) | (II–I) 4.43 (8.92) (7) | |||
| (Desacylated ghrelin, fmol/mL) | |||||||
| Baseline | 138.01 (151.43) (7) | 237.83 (186.79) (7) | 0.306 | ||||
| Period I | 180.07 (219.14) (7) | (I–Baseline) 42.06 (70.61) (7) | 175.31 (107.00) (7) | (I–Baseline) –62.51 (139.86) (7) | |||
| Period II | 143.07 (169.02) (7) | (II-I) −37.00 (72.84) (7) | 176.97 (103.33) (7) | (II-I) 1.66 (20.15) (7) | |||
| (Tmax of gastric emptying, min) | |||||||
| Baseline | 46.71 (8.91) (7) | 54.90 (16.05) (7) | 0.701 | ||||
| Period I | 53.54 (7.95) (7) | (I–Baseline) 6.83 (7.86) (7) | 60.29 (22.55) (7) | (I–Baseline) 5.39 (17.96) (7) | |||
| Period II | 51.16 (11.19) (7) | (II-I) −2.39 (7.81) (7) | 63.64 (40.52) (7) | (II-I) 3.36 (18.87) (7) | |||
Tmax = the peak time to 13CO2 excretion.
Group A (Period I, RKT treatment; Period II, nontreatment), Group B (Period I, nontreatment; Period II, RKT treatment). The change in plasma ghrelin level, body weight, and gastric emptying rate was analyzed using the Wilcoxon rank sum test.
Safety profile
UPDRS scores did not change during the RKT treatment period in both groups: Group A (Week 0, 16.50 [3.78] [n = 6]; Week 4, 13.43 [3.69] [n = 7]; and Week 8, 14.50 [2.59] [n = 6]) versus Group B (Week 0, 17.67 [3.88] [n = 6]; Week 4, 17.60 [2.70] [n = 5]; and Week 8, 17.25 [4.47] [n = 6]). After crossover, no significant difference was identified in the change in UPDRS score between RKT treatment and off-treatment periods, with a change in UPDRS score of 1.00 (2.74) (n = 5) during the treatment period compared with 1.00 (4.69) (n = 6) during the off-treatment period. Throughout the study period, no accumulation of adverse events or abnormal laboratory findings was identified with the RKT treatment.
Discussion
Gastrointestinal dysfunction is a common and clinically important symptom in patients with PD,28 with frequent occurrence of gastroparesis and constipation having been reported.29 In patients with PD who have already developed delayed gastric emptying, treatment with L-dopa can further deteriorate the function of gastric emptying.5 This decrease in gastric emptying can delay the arrival of L-dopa to the upper small intestine, with irregular absorption of L-dopa being considered as a major reason for the “delayed on” and “no-on” L-dopa phenomena.6, 7 RKT alleviates delayed gastric emptying induced by L-dopa/carbidopa in naïve and PD rat models, in part through ghrelin-related mechanisms.30 Doi et al22 reported that a 12-week treatment of RKT (15 g/d) significantly shortened the Tmax of the gastric emptying rate in patients with PD. However, a 4-week treatment of RKT (7.5 g/d) did not change the Tmax of the gastric emptying rate in our study. This difference may partly be due to differences in the dosage and duration of RKT treatment. At baseline, the average Tmax value for our study group (50.90 [12.37] minutes) (n = 16) was similar to the average Tmax for a healthy adult Japanese population (43.9 [10.3] minutes).31 Although RKT improves delayed gastric emptying in rats treated with L-dopa, RKT does not accelerate gastric emptying in naïve rats with a priori normal gastric emptying. Similarly, it might be difficult for RKT to have an addition beneficial effect in patients with normal gastric emptying a priori.
An improvement in abdominal pain with RKT treatment has been reported in patients with functional dyspepsia18 and in patients who have undergone endoscopic submucosal dissection.32 Abnormal gastric accommodation is a possible mechanism of visceral hypersensitivity. In a guinea pig model, the reduction in gastric accommodation induced by Nω-nitro-L-arginine was suppressed with the use of the 5-HT2B receptor antagonist SB215505, found in RKT and isoliquiritigenin.33 A clinical study using the gastric barostat method demonstrated that RKT also improves stress-induced gastric hypersensitivity, as well lowering the muscle tone of the gastric wall, in healthy volunteers.34 This effect of RKT on the muscle tone of the gastric wall likely explains the lowering of abdominal pain that we identified in our patients with PD.
Anorexia leads to weight loss and malnutrition, both of which are associated with a lowering of the health-related QOL.35 We identified an improvement in anorexia with a 4-week treatment of RKT (7.5 g/d), but with no significant effect on body weight. In general, changes in body weight appear later than a change in appetite or food intake. Therefore, it is likely that the period of observation was not sufficiently long to observe any increase in body weight. Regulation of the ghrelin system by RKT seems to be a possible mechanism underlying the improvement in appetite. Takeda et al19 reported an increase in the level of plasma ghrelin with the use of RKT in rats in whom ghrelin levels had been depleted by the use of cisplatin. Takeda et al19 suggested that this increase in ghrelin levels likely reflected the 5-HT2B receptor antagonist effects of RKT. In addition to the increase of plasma ghrelin level, RKT has pharmacologic effects on ghrelin, including enhancement of ghrelin secretion in the brain,36 upregulation of ghrelin receptors (growth hormone secretagogue receptors),37 and inhibition of circulating ghrelin degrading enzymes.38 In our study, RKT did not elevate the plasma acylated ghrelin level in patients with PD. However, there remains the possibility that RKT may act on the secretion of ghrelin, as well as inducing an upregulation of its receptors in brain, resulting in amplification of ghrelin signaling followed by an increase in appetite. In addition, 2 components of RKT (isoliquiritigenin and hesperetin) have been detected in the brain following oral administration of RKT to rats.39 These components are well-known CRF1 and 5-HT2C receptor antagonists, which enhance stress regulation and appetite, respectively. Accordingly, we assume that the effects of RKT on these receptor antagonists helped to improve mood and appetite in patients with PD, without affecting gastric emptying and ghrelin secretion.
The causes of appetite loss in patients with PD may include disturbed smell and taste, disturbed motility of the gastrointestinal tract, adverse effects of anti-Parkinsonian drugs, and depression.8, 9 Among our study group at baseline, SDS scores were classified as neurotic level. SDS scores improved during Period II, with significantly lower scores in Group A (RKT treatment) than Group B (off-treatment). Previous studies have reported that depression-like conditions are associated with a decreased density and hypofunction of astrocytes, combined with increased activation of microglia, in the frontolimbic regions, which is expected to contribute to the synaptic dysfunction present in depression.40, 41 A recent study using 3 different mouse models of accelerated or normal human aging showed that RKT inhibited microglia-mediated inflammation in the brain through an activation of the sirtuin 1 pathway.42 The pharmacologic mechanisms of RKT in improvement of depression have yet to be elucidated. However, inhibition of microglia-mediated inflammation by RKT can possibly be associated with an alleviation of depression symptoms, as shown by our findings. In addition, the influence of ghrelin on microglial behavior in the brain may influence the prognosis of some neurologic disorders, such as multiple sclerosis, supporting the above-mentioned possible mechanism.43, 44
The 4-week RKT treatment did not lead to deterioration in the motor function of our patients. In addition, no accumulation of adverse events for specific symptoms or abnormal laboratory findings was observed over the 4-week RKT treatment period. Although RKT may help improve the QOL of patients with PD, the effect of long-term RKT administration remains to be clarified.
The limitations of our study need to be acknowledged. First, the number of cases per group was limited, and the small sample size did not permit multiple comparisons. In addition, a placebo effect cannot be ruled in the absence of blinding of patients and clinicians. Further validation of our outcomes is required using a randomized, double-blind, controlled trial.
Conclusions
RKT may improve anorexia in patients with PD. The positive effects of RKT on depression and anorexia may improve the overall QOL of these patients. The benefits of RKT identified in our pilot study will need to be confirmed in a randomized, double-blind, controlled trial.
Conflicts of Interest
K. Yakabi and K. Nomura have received grant support from Tsumura & Co. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
Acknowledgments
Contributions to study concept and design, acquisition of data, data management, statistical analysis, interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content: K. Yakabi. Contributions to study concept and design, acquisition of data, statistical analysis interpretation of data and critical revision of the manuscript for important intellectual content: K. Nomura. Contributions to study concept and design acquisition of data and interpretation of data: N. Yakabi, S. Ono, E. Hosmi, K. Hayashi, and N. Yoshida. Contributions to data management and statistical analysis: M, Ochiai and K. Maezawa.
The authors thank Enago (www.enago.jp) and Elsevier for the English language review and editing. All authors approved the final version of the manuscript.
References
- 1.Kalia L.V., Lang A.E. Parkinson's disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.Chaudhuri K.R., Healy D.G., Schapira A.H., National Institute for Clinical Excellence Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang L., Murphy N.P., Stengel A. Ghrelin prevents levodopa-induced inhibition of gastric emptying and increases circulating levodopa in fasted rats. Neurogastroenterol Motil. 2012;24:e235–e245. doi: 10.1111/j.1365-2982.2012.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waller D.G., Roseveare C., Renwick A.G., Macklin B., George C.F. Gastric emptying in healthy volunteers after multiple doses of levodopa. Br J Clin Pharmacol. 1991;32:691–695. [PMC free article] [PubMed] [Google Scholar]
- 5.Hardoff R., Sula M., Tamir A. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord. 2001;16:1041–1047. doi: 10.1002/mds.1203. [DOI] [PubMed] [Google Scholar]
- 6.Melamed E., Ziv I., Djaldetti R. Management of motor complications in advanced Parkinson's disease. Mov Disord. 2007;22(Suppl 17):S379–S384. doi: 10.1002/mds.21680. [DOI] [PubMed] [Google Scholar]
- 7.Djaldetti R., Baron J., Ziv I., Melamed E. Gastric emptying in Parkinson's disease: patients with and without response fluctuations. Neurology. 1996;46:1051–1054. doi: 10.1212/wnl.46.4.1051. [DOI] [PubMed] [Google Scholar]
- 8.Kashihara K. Weight loss in Parkinson's disease. J Neurol. 2006;253(Suppl 7):VII38–VII 41. doi: 10.1007/s00415-006-7009-0. [DOI] [PubMed] [Google Scholar]
- 9.Aiello M., Eleopra R., Rumiati R.I. Body weight and food intake in Parkinson's disease. A review of the association to non-motor symptoms. Appetite. 2015;84:204–211. doi: 10.1016/j.appet.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Kistner A., Lhommée E., Krack P. Mechanisms of body weight fluctuations in Parkinson's disease. Front Neurol. 2014;5:84. doi: 10.3389/fneur.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro D.C., Cole S.L., Berridge K.C. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. 2015;9:90. doi: 10.3389/fnsys.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gugusheff J.R., Ong Z.Y., Muhlhausler B.S. The early origins of food preferences: targeting the critical windows of development. FASEB J. 2015;29:365–373. doi: 10.1096/fj.14-255976. [DOI] [PubMed] [Google Scholar]
- 13.Beyer P.L., Palarino M.Y., Michalek D., Busenbark K., Koller W.C. Weight change and body composition in patients with Parkinson's disease. J Am Diet Assoc. 1995;95:979–983. doi: 10.1016/S0002-8223(95)00269-3. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga K., Iwakiri R., Fujimoto K., GERD 4 Study Group Rikkunshito improves symptoms in PPI-refractory GERD patients: a prospective, randomized, multicenter trial in Japan. J Gastroenterol. 2012;47:284–292. doi: 10.1007/s00535-011-0488-5. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H., Matsuzaki J., Fukushima Y., Rikkunshito study group Randomized clinical trial: rikkunshito in the treatment of functional dyspepsia--a multicenter, double-blind, randomized, placebo-controlled study. Neurogastroenterol Motil. 2014;26:950–961. doi: 10.1111/nmo.12348. [DOI] [PubMed] [Google Scholar]
- 16.Seike J., Sawada T., Kawakita N. A new candidate supporting drug, rikkunshito, for the QOL in advanced esophageal cancer patients with chemotherapy using docetaxel/5-FU/CDDP. Int J Surg Oncol. 2011;2011:715623. doi: 10.1155/2011/715623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohno T., Yanai M., Ando H. Rikkunshito, a traditional Japanese medicine, suppresses cisplatin-induced anorexia in humans. Clin Exp Gastroenterol. 2011;4:291–296. doi: 10.2147/CEG.S26297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tominaga K., Kido T., Ochi M. The traditional Japanese medicine rikkunshito promotes gastric emptying via the antagonistic action of the 5-HT(3) receptor pathway in rats. Evid Based Complement Alternat Med. 2011;2011:248481. doi: 10.1093/ecam/nep173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusunoki H., Haruma K., Hata J. Efficacy of Rikkunshito, a traditional Japanese medicine (Kampo), in treating functional dyspepsia. Intern Med. 2010;49:2195–2202. doi: 10.2169/internalmedicine.49.3803. [DOI] [PubMed] [Google Scholar]
- 20.Takeda H., Sadakane C., Hattori T. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 2008;134:2004–2013. doi: 10.1053/j.gastro.2008.02.078. [DOI] [PubMed] [Google Scholar]
- 21.Arai M., Matsumura T., Tsuchiya N. Rikkunshito improves the symptoms in patients with functional dyspepsia, accompanied by an increase in the level of plasma ghrelin. Hepatogastroenterology. 2012;59:62–66. doi: 10.5754/hge11246. [DOI] [PubMed] [Google Scholar]
- 22.Miwa H., Koseki J., Oshima T. Rikkunshito, a traditional Japanese medicine, may relieve abdominal symptoms in rats with experimental esophagitis by improving the barrier function of epithelial cell in esophageal mucosa. J Gastroenterol. 2010;45:478–487. doi: 10.1007/s00535-009-0180-1. [DOI] [PubMed] [Google Scholar]
- 23.Doi H., Sakakibara R., Sato M. Dietary herb extract rikkunshi-to ameliorates gastroparesis in Parkinson's disease: a pilot study. Eur Neurol. 2014;71:193–195. doi: 10.1159/000355608. [DOI] [PubMed] [Google Scholar]
- 24.Utumi Y., Iseki E., Murayama N. Effect of Rikkunshi-to on appetite loss found in elderly dementia patients: a preliminary study. Psychogeriatrics. 2011;11:34–39. doi: 10.1111/j.1479-8301.2010.00347.x. [DOI] [PubMed] [Google Scholar]
- 25.Kitagawa H., Munekage M., Matsumoto T. Pharmacokinetic profiles of active ingredients and its metabolites derived from rikkunshito, a ghrelin enhancer, in healthy Japanese volunteers: a cross-over, randomized study. PLoS One. 2015;10:e0133159. doi: 10.1371/journal.pone.0133159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghoos Y.F., Maes B.D., Geypens B.J. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104:1640–1647. doi: 10.1016/0016-5085(93)90640-x. [DOI] [PubMed] [Google Scholar]
- 27.Mizukami K., Asada T., Kinoshita T. A randomized cross-over study of a traditional Japanese medicine (kampo), yokukansan, in the treatment of the behavioural and psychological symptoms of dementia. Int J Neuropsychopharmacol. 2009;12:191–199. doi: 10.1017/S146114570800970X. [DOI] [PubMed] [Google Scholar]
- 28.Fasano A., Visanji N.P., Liu L.W., Lang A.E., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. 2015;14:625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson's disease. Clin Neurosci. 1998;5:136–146. [PubMed] [Google Scholar]
- 30.Wang L., Mogami S., Karasawa H. Preventive effect of rikkunshito on gastric motor function inhibited by L-dopa in rats. Peptides. 2014;55:136–144. doi: 10.1016/j.peptides.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanaka M., Nakada K. Stable isotope breath tests for assessing gastric emptying: A comprehensive review. J Smooth Muscle Res. 2010;46:267–280. doi: 10.1540/jsmr.46.267. [DOI] [PubMed] [Google Scholar]
- 32.Uehara R., Isomoto H., Minami H. Characteristics of gastrointestinal symptoms and function following endoscopic submucosal dissection and treatment of the gastrointestinal symptoms using rikkunshito. Exp Ther Med. 2013;6:1083–1088. doi: 10.3892/etm.2013.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miwa H., Koseki J., Oshima T. Impairment of gastric accommodation induced by water-avoidance stress is mediated by 5-HT2B receptors. Neurogastroenterol Motil. 2016;28:765–778. doi: 10.1111/nmo.12775. [DOI] [PubMed] [Google Scholar]
- 34.Shiratori M., Shoji T., Kanazawa M., Hongo M., Fukudo S. Effect of rikkunshito on gastric sensorimotor function under distention. Neurogastroenterol Motil. 2011;23:323–329. doi: 10.1111/j.1365-2982.2010.01648.x. [DOI] [PubMed] [Google Scholar]
- 35.Akbar U., He Y., Dai Y. Weight loss and impact on quality of life in Parkinson's disease. PLoS One. 2015;10:e0124541. doi: 10.1371/journal.pone.0124541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yakabi K., Sadakane C., Noguchi M. Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin-induced anorexia. Endocrinology. 2010;151:3773–3782. doi: 10.1210/en.2010-0061. [DOI] [PubMed] [Google Scholar]
- 37.Fujitsuka N., Asakawa A., Uezono Y. Potentiation of ghrelin signaling attenuates cancer anorexia-cachexia and prolongs survival. Transl Psychiatry. 2011;1:e23. doi: 10.1038/tp.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadakane C., Muto S., Nakagawa K. 10-Gingerol, a component of rikkunshito, improves cisplatin-induced anorexia by inhibiting acylated ghrelin degradation. Biochem Biophys Res Commun. 2011;412:506–511. doi: 10.1016/j.bbrc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Mogami S., Sadakane C., Nahata M. CRF receptor 1 antagonism and brain distribution of active components contribute to the ameliorative effect of rikkunshito on stress-induced anorexia. Sci Rep. 2016;6:27516. doi: 10.1038/srep27516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rial D., Lemos C., Pinheiro H. Depression as a Glial-Based Synaptic Dysfunction. Front Cell Neurosci. 2016;9:521. doi: 10.3389/fncel.2015.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mechawar N., Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. 2016;6:e946. doi: 10.1038/tp.2016.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujitsuka N., Asakawa A., Morinaga A. Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Mol Psychiatry. 2016;21:1613–1623. doi: 10.1038/mp.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang G., Li J., Purkayastha S. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theil M.M., Miyake S., Mizuno M. Suppression of experimental autoimmune encephalomyelitis by ghrelin. J Immunol. 2009;183:2859–2866. doi: 10.4049/jimmunol.0803362. [DOI] [PubMed] [Google Scholar]