Abstract
Sertraline (Zoloft) and fluoxetine (Prozac) are selective serotonin reuptake inhibitors whose antidepressant mechanism of action is classically attributed to an elevation of the extracellular levels of serotonin in the synaptic cleft. However, the biological effects of these drugs seem to be more complex than their traditionally described mechanism of action. Among their actions is the inhibition of different types of Na+ and K+ channels, as well as of glutamate uptake activity. The clearance of extracellular glutamate is essential to maintain the central nervous system within physiological conditions, and this excitatory neurotransmitter is removed from the synaptic cleft by astrocyte transporters. This transport depends upon a hyperpolarized membrane potential in astrocytes that is mainly maintained by Kir4.1 K+ channels. The impairment of the Kir4.1 channel activity reduces driving force for the glutamate transporter, resulting in an accumulation of extracellular glutamate. It has been shown that sertraline and fluoxetine inhibit Kir4.1 K+ channels. Recently, we demonstrated that sertraline reduces glutamate uptake in human platelets, which contain a high-affinity Na+-dependent glutamate uptake system, with kinetic and pharmacological properties similar to astrocytes in the central nervous system. Considering these similarities between human platelets and astrocytes, one might ask if sertraline could potentially reduce glutamate clearance in the synaptic cleft and consequently modulate glutamatergic transmission. This possibility merits investigation, since it may provide additional information regarding the mechanism of action and perhaps the side effects of these antidepressants.
Key words: Glutamate modulator, Zoloft, Prozac, Kir4.1 potassium channels, sertraline, fluoxetine
Introduction
Sertraline (Zoloft) is a selective serotonin reuptake inhibitor (SSRI) that is widely prescribed as an antidepressant1 and proposed as a potential first-line drug to treat people with major depression.2 In addition to its classic mechanism of action, several lines of evidence suggest that sertraline has other biological effects that appear to be unrelated to its inhibition of serotonin reuptake.3 Although it is widely recognized that the action of an antidepressant that inhibits reuptake of serotonin (such as fluoxetine, Prozac) can elevate the extracellular levels of this neurotransmitter in the synaptic cleft,4, 5 additional mechanisms might also be involved with its biological and clinical effects.6, 7, 8, 9, 10
The biological activity of sertraline in fact appears to be more complex than its traditionally described mechanism of action. For instance, sertraline was recently reported to inhibit several types of Na+ and K+ channels3, 7, 11, 12, 13, 14, 15 as well as to reduce glutamate uptake in human platelets.8 Moreover, an effect on Ca2+ channels and on levels of cytosolic free Ca2+ in cancer cells was also reported.16, 17, 18 Recently, several investigators have attributed a wide range of biological effects to sertraline that are unrelated to its action as an inhibitor of serotonin reuptake,3, 7, 8, 12, 15, 16, 17 and that have been suggested to be implicated in the therapeutic and/or adverse effects of this SSRI.7
Especially interesting is the inhibitory effect of sertraline (and also fluoxetine) on K+ channels, such as the astroglial inwardly rectifying channel Kir4.1, which is responsible for astroglial K+ buffering7 and the G protein-activated inwardly rectifying K+ channels, Kir3.12 Particularly because the blockade of the astroglial Kir4.1 channels was related to reduction of both K+ buffering and glutamate uptake, which could potentially lead to hyperexcitability of neurons and seizure activity.7, 19, 20, 21 An eventual effect of sertraline (and fluoxetine) on glutamate neurotransmission may have significant consequences for the neuroactivity of the central nervous system (CNS), and also potentially contribute to its clinical activity.
Glutamate uptake
Glutamate is the major excitatory neurotransmitter in the mammalian CNS22 and is removed from the synaptic cleft by astrocyte uptake activity, a role that is essential for the physiological functionality of neurons.23 The removal of extracellular glutamate mediated by excitatory amino-acid transporters (EAATs) limits the temporal and spatial extent of glutamatergic transmission, a rapid dynamic process that allows local modulation of signaling in the synaptic cleft.24 Although glutamate uptake activity is widely studied, it remains an object of great interest since it involves different levels of complexity and is modulated at all known levels.23, 24, 25 To date, five subtypes of EAATs (EAAT 1-5) have been identified in mammalian tissues; EAAT1 (GLAST) and EAAT2 (GLT1) are preferentially expressed in astrocytes, and EAAT2 is the major isoform responsible for cerebral clearance of glutamate.23 Uptake mediated by astrocyte transporters is a high-affinity Na+-dependent process that removes glutamate from the synaptic cleft, using the electrochemical Na+ and K+ gradients across the plasma membranes as the driving force.23
The subtypes of glutamate transporters EAAT1-3 are expressed not only in the CNS, but also in a variety of peripheral tissues,23 including platelets,26 where EAAT2 proved to be functional as well as the predominant carrier.27 In addition to the presence of the main EAATs, several other similarities are shared between platelets and astrocytes regarding glutamate uptake. Human platelets contain a high-affinity Na+-dependent glutamate uptake system, with similar kinetic and pharmacological properties to astrocytes.28 Additionally, as observed in the CNS, glutamate may rapidly upregulate the activities of different EAAT subtypes of human platelets, and in this substrate-induced modulation, EAAT2 activity plays an important role.29 Another similarity shared between platelets and astrocytes is related to EAAT functionality, which uses the Na+/K+-electrochemical gradient as the driving force for glutamate uptake, and is reduced by membrane depolarization.19, 30, 31, 32, 33
Kir4.1 K+ channels
In the nervous system, the restoration of the resting potential of the excitable cells depends on opening the K+ channels, which is fundamentally mediated by outwardly rectifying K+ channels.34 However, there is another K+ current referred to as inwardly rectifying K+ currents (Kir), where the current flow is reduced in the depolarized membrane and increased when it shows a more negative potential. Among the several types of Kir channels, Kir4.1 is considered the principal pore-forming subunit in astrocytes, and is responsible for the strongly negative resting potential and high resting permeability for K+ ions in these cells. Besides their action in K+ buffering, the activity of Kir4.1 channels is also related to glutamate uptake.32
Proper functioning of glutamate transport depends on a hyperpolarized membrane potential in astrocytes, which is maintained mainly by Kir4.1 K+ channels.19, 31, 32, 33 The reduction of Kir4.1 channel activity evokes astrocyte membrane depolarization and impairs the driving force for the glutamate transporter, resulting in accumulation of extracellular glutamate.21 This impairment of glutamate uptake resulting from the depolarization inhibits the glutamate translocation mediated by the transporter.35, 36 An imbalance in the glutamate transport efficiency related to Kir4.1 was previously shown both in vitro21 and in vivo.37
Astrocytes have been considered a potential target of antidepressants,6, 38, 39 and inhibition of Kir4.1-mediated astroglial K+ buffering by sertraline and fluoxetine was suggested to be involved with their potential pharmacological action.7
Discussion
Glutamatergic transmission shows wide potential for modulation and a high complexity of the targets involved with its functioning, which includes different types of receptors and transporters. As well as the possibility of pharmacological modulation of their receptors, the performance of their transporters can also be affected by drugs. It was previously reported that some medications, such as riluzole,40 ceftriaxone41 and ketamine,42 may potentially affect glutamate transporter activity. This opened new possibilities for their therapeutic application as glutamatergic modulators.42, 43, 44, 45
The functioning of the glutamatergic system is highly dependent on glutamate uptake, which must be closely controlled and whose transporters may be regulated at all known levels.23, 25 Glutamate transporters show an affinity similar to that of receptors, and compete with these for glutamate in the synaptic cleft, reducing their activity and consequently affecting the duration of excitatory transmissions. Moreover, they maintain the specificity of glutamatergic signaling by avoiding spillover of glutamate between synapses (for review, see Murphy-Royal et al.43). Hence, the functionality of glutamatergic transmission requires fine adjustment to be maintained under physiological conditions. However, this system is complex and it seems to be highly subject to modulation. The absence of extracellular metabolism for glutamate and the high dependence on transporter activity to clear this neurotransmitter are important elements of this complexity.
The uptake activity is essential to remove glutamate released by neurons, and is mediated by astrocytic transporters that are electrogenic and hence are much more effective at negative resting potentials.21, 23 The maintenance of this important function depends on the hyperpolarized membrane potential of astrocytes, which is mediated mainly by Kir4.1 channels.19, 31, 32, 33 Therefore, inhibition of astroglial channel Kir4.1 may depolarize these cells, disrupting the driving force necessary to maintain glutamate clearance. As a consequence of this, both the concentration and the permanence of glutamate in the synaptic cleft would be increased, potentiating the action of this neurotransmitter on the glutamatergic system (Fig. 1). It is crucial to consider the inhibitory effect of sertraline and fluoxetine on astroglial Kir4.1 channels, since blockade of these channels by these SSRIs reduces glutamate uptake in astrocytes.7 Notably, a sertraline-mediated decrease in glutamate uptake was recently described in human platelets,8 and considering the several similarities of these cells with astrocytes, this may suggest an analogous effect in the CNS. The comparison of a peripheral and cerebral glutamatergic system is supported by several resemblances between platelets and astrocytes, such as the presence of the main glutamate transporters,27 an equivalent functionality regarding uptake28 and the possibility that uptake is upregulated by glutamate.29 An additional similarity is the need for a Na+/K+-electrochemical gradient as a driving force for glutamate uptake, which is disrupted by membrane depolarization.19, 30, 31, 32, 33
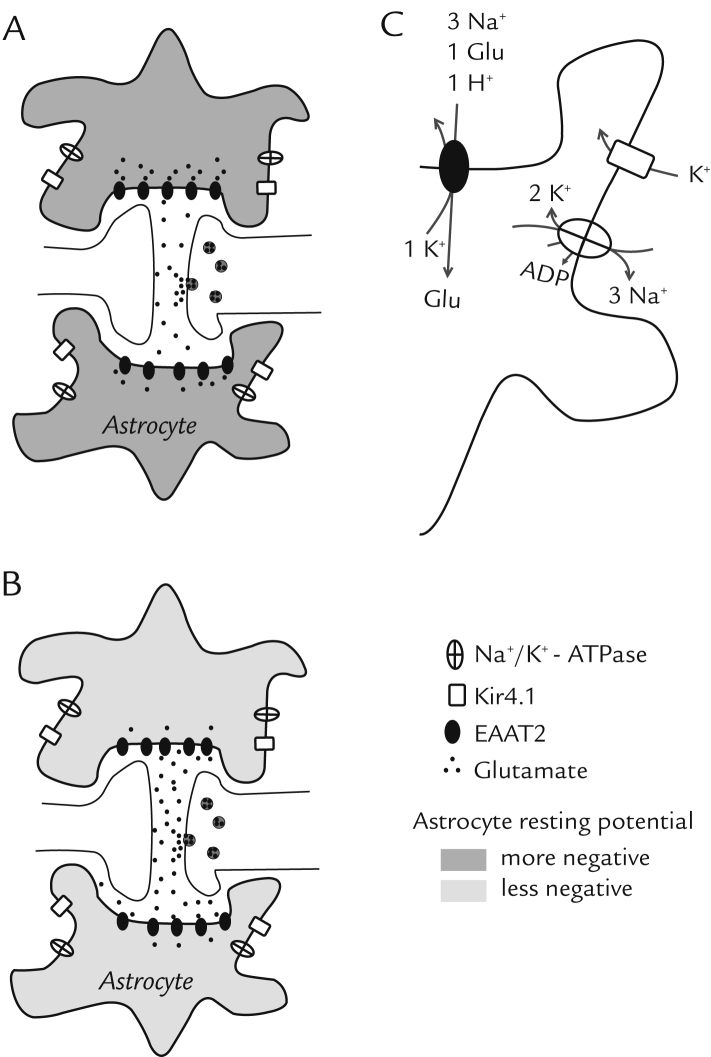
Figure 1.
Representation of the tripartite glutamatergic synapse. Astrocytes are responsible for removing glutamate released from the presynaptic terminal, mainly through excitatory amino-acid transporter 2 (EAAT2). This uptake depends on the electrochemical gradient of Na+ established by Na+/K+-ATPase activity, and also on a hyperpolarized membrane potential in astrocytes that is maintained mainly by the inwardly rectifying potassium channel 4.1 (Kir4.1). The inhibition of Kir4.1 evokes astrocyte membrane depolarization and impairs the driving force for the glutamate transporter, resulting in accumulation of extracellular glutamate. The schematic diagrams illustrate the relationship between astrocytes with more-negative (A) and less-negative (B) resting potentials, and physiological and reduced glutamate uptake, respectively. Diagram B shows an astrocyte with less-negative resting potential due to inhibition of Kir4.1 channels and higher levels of glutamate in the synaptic cleft as a consequence of this effect. According to this proposal, a potential inhibition of Kir4.1 channels by a selective serotonin reuptake inhibitor might cause astrocyte depolarization and disrupt the driving force necessary to maintain glutamate uptake. As a consequence, the temporal and spatial extent of glutamatergic signaling would be increased, resulting in an indirect modulation of glutamatergic transmission. Diagram C shows the glutamate transport mechanism with stoichiometric details of EAAT2 and Na+/K+-ATPase activities.
The comment presented here notes that an effect of SSRIs (such as sertraline and fluoxetine) on rectifying-K+ channels might affect the astrocyte resting potential, making it less negative and consequently reducing the function of glutamate transporters. Although an action of sertraline on the glutamatergic system might not seem obvious at first, the extensive data showing an inhibitory action on K+ channels, particularly Kir4.1, provide robust evidence that this SSRI might affect glutamate uptake activity. It is important to determine if glutamate transporters might undergo a modulation of their functionality in response to SSRI action, considering their physiological importance and also because they have been considered good potential therapeutic targets.43 The possibility that SSRIs disrupt the driving force necessary for glutamate uptake suggests that these drugs might extent the time that this neurotransmitter remains in the synaptic cleft, and consequently might have an unexpected modulating effect on the glutamatergic system.
In spite of the above evidence, an appropriate experimental evaluation is necessary to clarify if the glutamate uptake system may actually be modulated by SSRIs. Bearing in mind the several similarities with astrocytes, particularly with regard to the glutamatergic system, the ex vivo use of human platelets seems to be a reliable model to investigate this potential effect on glutamate uptake. Human platelets were proposed as a model for studying the cerebral glutamatergic system28 and were used to investigate the effects of different neuroactive drugs under experimental conditions in vitro.8, 46 Moreover, platelets are considered by many researchers as peripheral markers of cerebral glutamatergic dysfunction,29, 47 and the suitability of their use to evaluate glutamatergic alterations in the CNS is supported by the impairment of glutamate transport in platelets in a variety of neurodegenerative diseases.26, 48, 49, 50, 51, 52 Taking into account that several investigations with these cells have been carried out based on their similarities to astrocytes,8, 48, 49, 50, 51, 52 we consider that additional in vitro studies are essential to test our hypothesis. One possible way to evaluate this proposal would be to measure the glutamate uptake in human platelets obtained from patients under SSRI treatment, comparing the kinetic profile of glutamate uptake and the clinical information obtained during therapeutic use of the drugs.
Conclusion
An eventual confirmation that SSRIs may act as glutamatergic modulators would have significant importance, since this neurotransmitter system has a pivotal role in several physiological activities of the CNS and also in different acute or chronic neurological disorders. The potential confirmation of this hypothesis could contribute to complement the information about the mechanism of action and safety of SSRIs, as well as suggest the possibility of reprofiling these drugs in the future.
Conflict of Interest
Author reports that there is no conflict of interest. Funding for previous research that allowed the present study was provided by grants from the Brazilian National Research Council (CNPq). The CNPq had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
References
- 1.Sheehan D.V., Kamijima K. An evidence-based review of the clinical use of sertraline in mood and anxiety disorders. Int Clin Psychopharmacol. 2009;24:43–60. doi: 10.1097/yic.0b013e3282f4b616. [DOI] [PubMed] [Google Scholar]
- 2.Cipriani A., La Ferla T., Furukawa T.A. Sertraline versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2009:2. doi: 10.1002/14651858.CD006117.pub2. CD006117. [DOI] [PubMed] [Google Scholar]
- 3.Lee H.M., Hahn S.J., Choi B.H. Blockade of Kv1.5 channels by antidepressant drug sertraline. Korean J Physiol Pharmacol. 2016;20(2):193–200. doi: 10.4196/kjpp.2016.20.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez C., Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol. 1999;19:467–489. doi: 10.1023/A:1006986824213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frazer A. Serotonergic and noradrenergic reuptake inhibitors: prediction of clinical effects from in vitro potencies. J Clin Psychiatry. 2001;62:16–23. [PubMed] [Google Scholar]
- 6.Fuchs E., Czeh B., Kole M.H.P., Michaelis T., Lucassenm P.J. Alterations of neuroplasiticity in depression: the hippocampus and beyond. Eur Neuropsychopharmacol. 2004;14:S481–S490. doi: 10.1016/j.euroneuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Ohno Y., Hibino H., Lossin C., Inanobe A., Kurachi Y. Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res. 2007;1178:44–51. doi: 10.1016/j.brainres.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues D.O., Bristot I.J., Klamt F., Frizzo M.E. Sertraline reduces glutamate uptake in human platelets. Neurotoxicology. 2015;51:192–197. doi: 10.1016/j.neuro.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Sitges M., Chiu L.M., Reed R.C. Effects of Levetiracetam, Carbamazepine, Phenytoin, Valproate, Lamotrigine, Oxcarbazepine, Topiramate, Vinpocetine and Sertraline on Presynaptic Hippocampal Na+ and Ca2+ Channels Permeability. Neurochem Res. 2016;41:758–769. doi: 10.1007/s11064-015-1749-0. [DOI] [PubMed] [Google Scholar]
- 10.Gill J.S., Jamwal S., Kumar P., Deshmukh R. Sertraline and venlafaxine improves motor performance and neurobehavioral deficit in quinolinic acid induced Huntington's like symptoms in rats: Possible neurotransmitters modulation. Pharmacol Rep. 2017;69(2):306–313. doi: 10.1016/j.pharep.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Aldana B.I., Sitges M. Sertraline inhibits pre-synaptic Na+ channel-mediated responses in hippocampus-isolated nerve endings. J Neurochem. 2012;121(2):197–205. doi: 10.1111/j.1471-4159.2012.07674.x. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T., Washiyama K., Ikeda K. Inhibition of G Protein-Activated Inwardly Rectifying K+ Channels by Different Classes of Antidepressants. PLoS ONE. 2011;6(12):e28208. doi: 10.1371/journal.pone.0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H.A., Kim K.S., Hyun S.A., Park S.G., Kim S.J. Wide spectrum of inhibitory effects of sertraline on cardiac ion channels. Korean J Physiol Pharmacol. 2012;16:327–332. doi: 10.4196/kjpp.2012.16.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G.K., Mitchell J., Wang S.Y. Block of persistent late Na+ currents by antidepressant sertraline and paroxetine. J Membr Biol. 2008;222:79–90. doi: 10.1007/s00232-008-9103-y. [DOI] [PubMed] [Google Scholar]
- 15.Yeh J.H., Sun T.K., Chou C.T. Effect of sertraline on Ca2+ fluxes in rabbit corneal epithelial cells. Chinese Journal of Physiology. 2015;58(2):85–94. doi: 10.4077/CJP.2015.BAC255. [DOI] [PubMed] [Google Scholar]
- 16.Chien J.M., Chou C.T., Pan C.C. The mechanism of sertraline-induced [Ca2+]i rise in human OC2 oral cancer cells. Hum Exp Toxicol. 2011;30(10):1635–1643. doi: 10.1177/0960327110396523. [DOI] [PubMed] [Google Scholar]
- 17.Huang J.K., Chang H.T., Chou C.T. The mechanism of sertraline-induced [Ca2+]i rise in human PC3 prostate cancer cells. Basic Clin Pharmacol Toxicol. 2011;109:103–110. doi: 10.1111/j.1742-7843.2011.00690.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen S., Xuan J., Wan L. Sertraline, an antidepressant, induces apoptosis in hepatic cells through the mitogen-activated protein kinase pathway. Toxicol Sci. 2014;137:404–415. doi: 10.1093/toxsci/kft254. [DOI] [PubMed] [Google Scholar]
- 19.Bay V., Butt A.M. Relationship between glial potassium regulation and axon excitability: a role for glial Kir4.1 channels. Glia. 2012;60:651–660. doi: 10.1002/glia.22299. [DOI] [PubMed] [Google Scholar]
- 20.Jansen L.A., Uhlmann E.J., Crino P.B., Gutmann D.H., Wong M. Epileptogenesis and reduced inward rectifier potassium current in tuberous sclerosis complex-1-deficient astrocytes. Epilepsia. 2005;46:1871–1880. doi: 10.1111/j.1528-1167.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 21.Kucheryavykh Y.V., Kucheryavykh L.Y., Nichols C.G. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia. 2007;55:274–281. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- 22.Fonnum F. Glutamate: A neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- 23.Danbolt N.C. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 24.Armbruster M., Hanson E., Dulla C.G. Glutamate clearance is locally modulated by presynaptic neuronal activity in the cerebral cortex. J Neurosci. 2016;36(40):10404–10415. doi: 10.1523/JNEUROSCI.2066-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morel L., Regan M., Higashimori H. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem. 2013;288(10):7105–7116. doi: 10.1074/jbc.M112.410944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zoia C., Cogliati T., Tagliabue E. Glutamate transporters in platelets: EAAT1 decrease in aging and in Alzheimer’s disease. Neurobiol Aging. 2004;25:149–157. doi: 10.1016/s0197-4580(03)00085-x. [DOI] [PubMed] [Google Scholar]
- 27.Hoogland G., Bos I.W., Kupper F., van Willigen G., Spierenburg H.A., van Nieuwenhuizen O., de Graan P.N. Thrombin-stimulated glutamate uptake in human platelets is predominantly mediated by the glial glutamate transporter EAAT2. Neurochem Int. 2005;47:499–506. doi: 10.1016/j.neuint.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Mangano R.M., Schwarcz R. The human platelet as a model for the glutamatergic neuron: platelet uptake of L-glutamate. J Neurochem. 1981;36:1067–1076. doi: 10.1111/j.1471-4159.1981.tb01701.x. [DOI] [PubMed] [Google Scholar]
- 29.Begni B., Tremolizzo L., D'Orlando C., Bono M.S., Garofolo R., Longoni M., Ferrarese C. Substrate-induced modulation of glutamate uptake in human platelets. Br J Pharmacol. 2005;145:792–799. doi: 10.1038/sj.bjp.0706242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasatnika L., Borisova T. Impaired Na+-dependent glutamate uptake in platelets during depolarization of their plasma membrane. Neurochem Int. 2010;56:711–719. doi: 10.1016/j.neuint.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Olsen M.L., Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 2008;107(3):589–601. doi: 10.1111/j.1471-4159.2008.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbour B., Brew H., Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. J Physiol. 1991;436:169–193. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brew H., Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature. 1987;327:707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- 34.Yuan L.-L., Chen X. Diversity of potassium channels in neuronal dendrites. Prog Neurobiol. 2006;78:374–389. doi: 10.1016/j.pneurobio.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Mennerick S., Shen W., Xu W. Substrate turnover by transporters curtails synaptic glutamate transients. J Neurosci. 1999;19:9242–9251. doi: 10.1523/JNEUROSCI.19-21-09242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otis T.S., Kavanaugh M.P. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J Neurosci. 2000;20:2749–2757. doi: 10.1523/JNEUROSCI.20-08-02749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djukic B., Casper K.B., Philpot B.D., Chin L.S., McCarthy K.D. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercier G., Lennon A.M., Renouf B. MAP kinase activation by fluoxetine and its relationship to gene expression in cultured rat astrocytes. J Mol Neurosci. 2004;24:207–216. doi: 10.1385/JMN:24:2:207. [DOI] [PubMed] [Google Scholar]
- 39.Czeh B., Simon M., Schmelting B., Hiemks C., Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 40.Frizzo M.E., Dall'Onder L.P., Dalcin K.B., Souza D.O. Riluzole enhances glutamate uptake in rat astrocyte cultures. Cell Mol Neurobiol. 2004;24(1):123–128. doi: 10.1023/B:CEMN.0000012717.37839.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rothstein J.D., Patel S., Regan M.R., Haenggeli C., Huang Y.H., Bergles D.E., Jin L., Dykes Hoberg M., Vidensky S., Chung D.S., Toan S.V., Bruijn L.I., Su Z.Z., Gupta P., Fisher P.B. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433(7021):73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X., Ye G., Wang Z., Luo J., Hao X. Sub-anesthetic doses of ketamine exert antidepressant-like effects and upregulate the expression of glutamate transporters in the hippocampus of rats. Neurosci Lett. 2017;639:132–137. doi: 10.1016/j.neulet.2016.12.070. [DOI] [PubMed] [Google Scholar]
- 43.Murphy-Royal C., Dupuis J., Groc L., Oliet S.H.R. Astroglial Glutamate Transporters in the Brain: Regulating Neurotransmitter Homeostasis and Synaptic Transmission. J Neurosci Res. 2017;00 doi: 10.1002/jnr.24029. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Arellano J.J., Parpura V., Zorec R., Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2016;323:170–182. doi: 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Pereira A.C., Gray J.D., Kogan J.F., Davidson R.L., Rubin T.G., Okamoto M., Morrison J.H., McEwen B.S. Age and Alzheimer's disease gene expression profiles reversed by the glutamate modulator riluzole. Mol Psychiatry. 2017;22(2):296–305. doi: 10.1038/mp.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Türck P., Frizzo M.E. Riluzole Stimulates BDNF Release from Human Platelets. BioMed Research International. 2015:6. doi: 10.1155/2015/189307. Article ID 189307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Luca M., Colciaghi F., Pastorino L., Borroni B., Padovani A., Cattabeni F. Platelets as a peripheral district where to study pathogenetic mechanism of Alzheimer disease: the case of amyloid precursor protein. Eur J Pharmacol. 2000;405:277–283. doi: 10.1016/s0014-2999(00)00559-8. [DOI] [PubMed] [Google Scholar]
- 48.Ferrarese C., Zoia C., Pecora N. Reduced platelet glutamate uptake in Parkinson's disease. J Neural Transm. 1999;106:685–692. doi: 10.1007/s007020050189. [DOI] [PubMed] [Google Scholar]
- 49.Ferrarese C., Begni B., Canevari C. Glutamate uptake is decreased in platelets from Alzheimer’s disease patients. Ann Neurol. 2000;47:641–643. [PubMed] [Google Scholar]
- 50.Ferrarese C., Sala G., Riva R. Decreased platelet glutamate uptake in patients with amyotrophic lateral sclerosis. Neurology. 2001;56:270–272. doi: 10.1212/wnl.56.2.270. [DOI] [PubMed] [Google Scholar]
- 51.Rainesalo S., Keränen T., Peltola J., Saransaari P. Glutamate uptake in blood platelets from epileptic patients. Neurochem Int. 2003;43:389–392. doi: 10.1016/s0197-0186(03)00026-3. [DOI] [PubMed] [Google Scholar]
- 52.Mangano R.M., Schwarcz R. Platelet glutamate and aspartate uptake in Huntington's disease. J Neurochem. 1981;37:1072–1074. doi: 10.1111/j.1471-4159.1981.tb04502.x. [DOI] [PubMed] [Google Scholar]