Abstract
Hepatocellular carcinoma (HCC) is more prevalent in men than in women. Previously we have found that some stromal cells, including hepatic stellate cells (HSCs), neutrophils and macrophages, play crucial roles in promoting sex disparity in kras V12-induced zebrafish HCC. The activation of HSCs is mediated by serotonin while activation of neutrophils and macrophages is mediated by cortisol. To ensure that these findings are also applicable to other oncogene induced tumors, stromal cell activation was compared between male and female fish during liver tumorigenesis initiated by xmrk or Myc oncogene. Consistently, we observed male-biased liver tumorigenesis in the xmrk and Myc models. In both models, there was a higher rate of HSC activation accompanied with a higher level of serotonin in male liver tumors. For tumor-infiltrated neutrophils and macrophages, significantly higher densities in male liver tumors were observed in both xmrk and Myc models. However, the male-biased increase of cortisol was observed only in xmrk- but not apparently in Myc expressing liver tumors. Overall, these observations are consistent with the observations in the kras liver tumor model, indicating that the serotonin- and cortisol-mediated pathways also play roles in sex disparity of liver tumors caused by other molecular pathways.
Subject terms: Cancer models, Cancer microenvironment
Introduction
Hepatocellular carcinoma occurs more frequently and aggressively in men than in women1. Based on the animal model studies, the gender disparity might be owing to a sex hormone related mechanism, with a stimulating role of androgen and an inhibitory role of estrogen2. Administration of estrogens inhibits HCC development in diethylnitrosamine (DEN)-treated male mice. On the contrary, ovariectomy or testosterone supplement increase occurrence of HCC in female mice3. However, clinical trials targeting sex hormone pathways, e.g. by using the estrogen receptor modulator tamoxifen, synthetic progestin (megestrol) and androgen antagonist flutamide, produced inconclusive results as these treatments in the clinical trials did not show significant improvement4–7.
Most of HCC arise on a background of hepatic fibrosis and/or inflammation and it has been increasingly recognized that some stromal cells in the tumor microenvironment (TME) are abnormally activated in both HCC animal models and HCC patients. Hepatic stellate cells (HSCs) are the main matrix-producing cells in the TME and play key roles in the progression of liver fibrosis. Co-culture of HSCs with HCC cells showed upregulated expression of proinflammatory cytokines and proangiogenic genes8. Depletion of HSCs from pre-established fibrosis attenuated fibrogenesis in a mouse liver disease model9. Co-transplantation of human intratumoral HSCs with HCC into nude mice showed enhanced HCC progression by HSCs and HSC density is correlated with the overall and recurrence-free survival, thus providing promising prognostic biomarkers10. Consistent with this, we also reported recently that HSC has a higher density and activation ratio in kras V12-expresing tumor in the zebrafish11.
Tumor associated macrophages (TAMs) and tumor associated neutrophils (TANs) play key roles in hepatic inflammation and cytokines produced by TAMs promote tumor growth, angiogenesis and suppression of adaptive immunity12. DEN administration promotes IL-6 production in Kupffer cells in male mice and ablation of IL-6 attenuates liver carcinogenesis in male and abolishes the gender difference13. In HCC patients after resection, the number of intratumoral neutrophils is significantly correlated with the early recurrence which could be a poor prognostic factor14. In our kras V12-expresing zebrafish liver tumor model, Infiltrations of TAMs and TANs are significantly higher in male tumors than in female tumors. Pro-tumor genes are also more strongly expressed in the TAMs and TANs of male tumors, correlating to a faster tumor progression15.
As we previously reported, the activation of stromal cells has a close correlation with tumor progression, especially HSCs, TAMs and TANs11, 15, 16. Interestingly, these stromal cells appear to also contribute to the sex disparity of HCC in the kras V12 model. In male kras V12 transgenic zebrafish, a higher level of serotonin activates HSCs and causes accelerated liver tumor progression11. Male kras V12 transgenic zebrafish also produced higher of cortisol to cause enhanced TAN and TAM infiltration to accelerate liver tumor progression15. However, whether the importance of these stromal cells is universal to other oncogene-induced tumors or only specific to kras V12–induced tumors remains unclear. Previously, our laboratory has generated two other oncogene induced HCC models in transgenic zebrafish; one is by inducible expression of xmrk oncogene17 and the other is inducible expression of the mouse Myc oncogene18. Both transgenic lines have been generated with the same Tet-on transgenic system where the oncogenes are expressed under the hepatocyte-specific fabp10a promoter and the expression was induced only by addition of the chemical inducer, doxycycline. In the present study, we first confirmed the sex disparity in HCC development in these two oncogene transgenic models. Then we found that positive correlations of these stroma cells to the progression of liver tumors in both the xmrk and Myc transgenic models, including conserved molecular pathways such as serotonin activated HSCs and cortisol enhanced TANs and TAMs. These mechanisms also similarly contribute to sex disparity of liver tumors in the xmrk and Myc models.
Results
Enhanced cell proliferation during hepatocarcinogenesis in male zebrafish with transgenic expression of xmrk or Myc oncogene
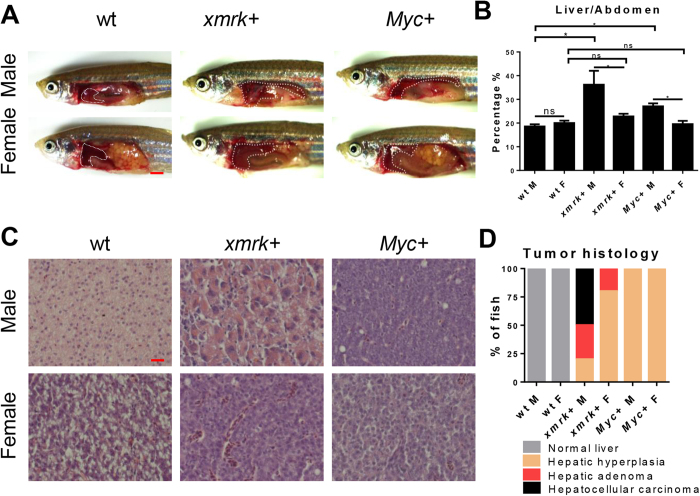
As we reported previously, krasV12-expressing male tumors shows an accelerated HCC progression11, 15, 19. To investigate if xmrk- or Myc–induced HCC has similar sex disparity, male and female xmrk+ or Myc+ fish were exposed to doxycycline for 7 days. 2D liver size was measured after the treatment (Fig. 1A). In the xmrk+ and Myc+ expressing fish of both sexes, male tumor livers were bigger than the female tumor livers in both xmrk and Myc-expressing fish (Fig. 1B). Histologically, male developed more aggressive tumors than female in xmrk-expressing liver. In wildtype livers, the 2-cell hepatic plate was well organized while in xmrk-expressing male tumor, oncogenic hepatocytes had prominent and multiple nucleoli and lost the 2-cell plate organization (Fig. 1C). In xmrk-expressing female tumors, most of the tumors were at early HCC stage with loss of 2-cell plate and prominent nucleoli. However, in Myc-expressing fish, all of the liver tumors were at the hyperplastic stage without apparent sex disparity. As summarized in Fig. 1D, 50% of male xmrk-expressing tumor showed carcinoma, 30% showed adenoma and the remaining 20% had hepatic hyperplasia. In contrast, 80% of female xmrk-expressing tumor showed hepatic hyperplasia and the remaining 20% had adenoma histology. However, the Myc-expressing livers in both female and male had similar hepatic hyperplasia histology.
Figure 1.
Characterization of sex disparity in xmrk and Myc-induced HCC progression. Three-month-old, xmrk+, Myc+ and wildtype zebrafish were treated with 60 μg/ml dox for 7 days. 10 fish were analysed in each group and the experiment was repeated multiple times. (A) Gross morphology of male and female fish (left lateral view). The livers are outlined. (B) Quantification of percentage of liver area to abdomen area. (C) Representative images of liver sections of male and female fish after H&E staining. (D) Quantification of tumor histology in male and female fish. *P < 0.05. Scale bars: 2 mm in (A) and 20 μm in (C).
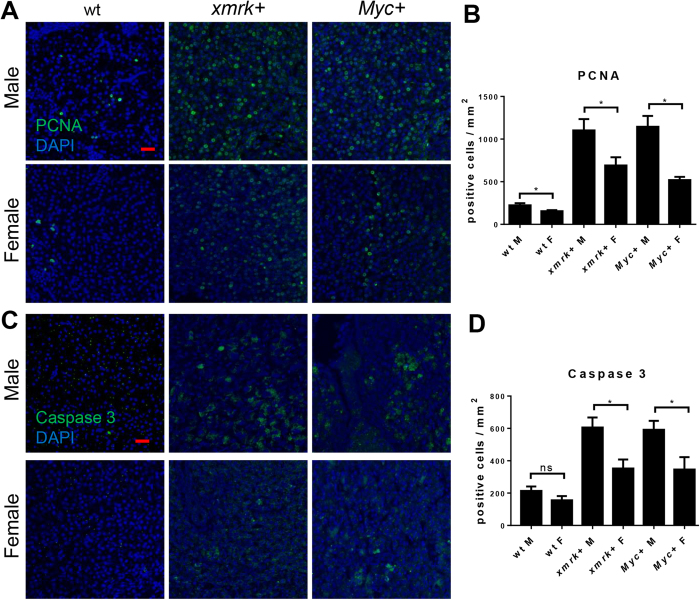
To further investigate the molecular mechanism in both transgenic lines, the cell proliferation and apoptosis were examined by immunofluorescence (IF) staining of PCNA and caspase-3, respectively. The proliferating cells were comparable and had a low percentage in both sexes of normal livers of wildtype fish, while xmrk and Myc-expression promoted hepatocyte proliferation significantly (Fig. 2A,B). Levels of hepatocyte apoptosis had also been accelerated by xmrk and Myc-expression in both sexes (Fig. 2C,D). In wildtype fish, there were no significant difference in both proliferating cells and apoptotic cells between female and male.
Figure 2.
Proliferation and apoptosis in the livers of male and female xmrk+ and Myc+ fish following oncogene activation. 10 fish were analysed in each group and the experiment was repeated multiple times. Proliferation and apoptosis were examined by PCNA and Caspase 3 staining respectively. (A) IF staining of PCNA in liver sections. (B) Quantification of densities of proliferating liver cells (PCNA+). (C) IF staining of Caspase-3 in liver sections. (D) Quantification of densities of apoptotic liver cells (Caspase 3+). *P < 0.05. Scale bars: 20 μm.
Correlation of serotonin level, activated HSCs and higher induction of male carcinogenesis
Previously we have found that both the density of total HSCs and the percentages of activated HSCs are significantly higher in male krasV12–induced liver tumors than female krasV12–induced liver tumors11. As a higher HSC density also indicates a poor prognosis in HCC patients20, HSC density and activation ratio were determined in xmrk and Myc-expressing model. Glial fibrillary acidic protein (Gfap) has been used as a marker of HSCs as it marks both quiescent and activated HSC21. A-SMA (alpha smooth muscle Actin), in contrast, only labels the activated HSC22. By immunofluorescence (IF) co-staining of Gfap and a-SMA, both quiescent and activated HSC could be detected. As shown in Fig. 3A and B, Gfap marked total HSCs were increased in xmrk- and Myc-expressing livers with a higher density in males than in females. A-SMA+/Gfap+ cells indicated activated HSCs and the percentage of activated HSCs was also significantly increased after overexpression of xmrk or Myc, but the sex disparity only existed in the xmrk-expressing model (Fig. 3A,C).
Figure 3.
Determination of HSCs and activated HSCs in the livers of male and female xmrk+ and Myc+ fish following oncogene activation. 10 fish were analyzed in each group and the experiment was repeated once for reproducibility. (A) IF co-staining of GFAP (red) and a-SMA (green) in liver sections. White boxes indicate the enlarged area as shown on the right. (B) Quantification of total HSC density in liver sections. (C) Quantification of activated ratio of HSCs in liver sections. *P < 0.05. Scale bars: 20 μm.
Serotonin has been shown to specifically activate HSCs through 5-hydroxytryptamine receptor 2B (Htr2b)9 and there is a higher level of serotonin in the krasV12-expressing livers in male zebrafish than female zebrafish11. Tryptophan hydroxylase 1b (Tph1b) is the rate limiting enzyme of serotonin synthesis23. To investigate if xmrk and Myc-expressing liver also have the sex difference in the serotonin level, IF staining of serotonin with HNF4α (hepatocyte nuclear factor 4 alpha, for marking the hepatocytes) as well as IF staining of phoso-Tph1 with HNF4α were carried out. In both xmrk- and Myc-expressing livers, males had a higher level of serotonin than females (Fig. 4A,B). Compare between xmrk- and Myc-expressing livers in the same sex, xmrk-expressing liver tumors had a higher serotonin level than Myc-expressing liver tumor. IF staining of phoso-Tph1 led to similar and consistent results. Phoso-Tph1 was higher in males than in females in xmrk and Myc-expressing liver (Fig. 4C,D). Male fish had higher levels of phoso-Tph1 than female fish across all three comparing groups and male xmrk-expressing tumor had a higher phoso-Tph1 level than Myc-expressing tumor.
Figure 4.
Immunofluorescent staining for serotonin and P-Tph1 in the livers of male and female xmrk+ and Myc+ fish following oncogene activation. 10 fish were analyzed in each group and the experiment was repeated once for reproducibility. (A) IF co-staining of serotonin (red) and HNF4a (green) in liver sections. (B) Quantification of ratio of serotonin-productive hepatocytes in liver sections. (C) IF co-staining of P-Tph1 (red) and HNF4a (green) in liver sections. (D) Quantification of ratio of P-Tph1-expressed hepatocytes in liver sections. *P < 0.05. Scale bars: 20 μm. ns, non-significance.
Correlation of cortisol level, TAMs/TANs and higher induction of male carcinogenesis
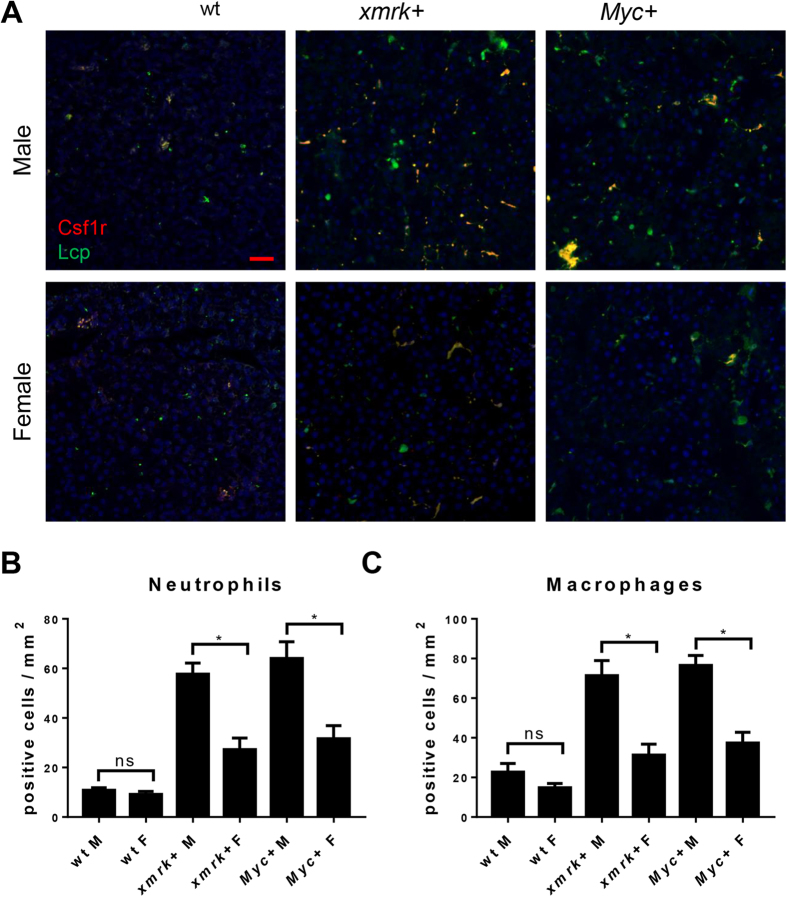
In HCC patients, immune cell density has a close correlation with tumor progression and could be a poor prognostic factor of HCC14. In our previously reported krasV12 expressing zebrafish model, TAN and TAM infiltrations were much severer in male fish than in female fish15. To investigate the immune cell infiltration in xmrk- and Myc-expressing livers, IF co-staining of Csf1r (colony stimulating factor 1 receptor) and Lcp (L. pneumophila-containing phagosome) were conducted. Csf1r is a macrophage-specific receptor which controls the differentiation and survival of macrophages24. Lcp marks both neutrophils and macrophages25. By co-staining of Csf1r and Lcp, neutrophils and macrophages could be identified simultaneously. In both xmrk- and Myc-expressing liver tumors, neutrophils and macrophages were significantly higher in males than in females (Fig. 5A,C). Compare between the same sex, xmrk- or Myc-expressing liver tumors had the similar density of neutrophils and macrophages.
Figure 5.
Determination of neutrophils and macrophages in the livers of male and female xmrk+ and Myc+ fish following oncogene activation. 10 fish were analyzed in each group and the experiment was repeated once for reproducibility. (A) IF co-staining of Csf1r (red) and Lcp (green) in liver sections. (B) Quantification of neutrophil densities in liver sections. (C) Quantification of macrophage densities in liver sections. *P < 0.05. Scale bars: 20 μm.
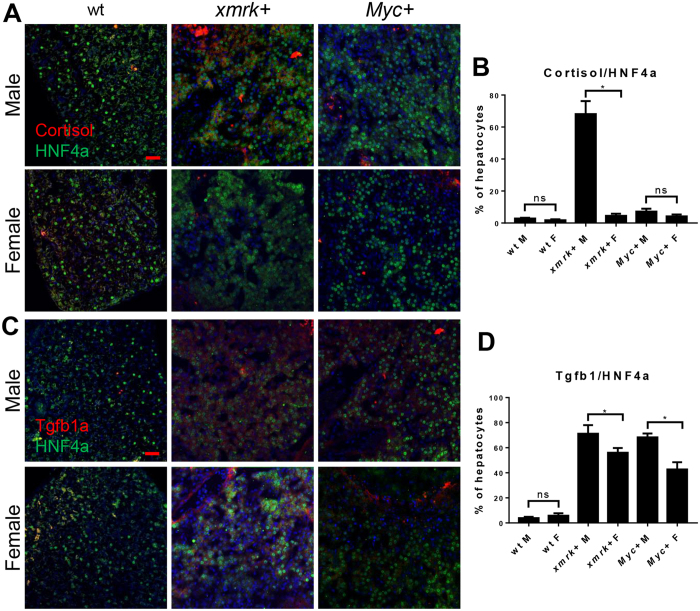
Cortisol has been shown to affect neutrophil and macrophage gene expression profile26, 27. In krasV12-expressing livers, cortisol is upregulated after krasV12 expression with a higher level of production in male liver tumors, which in turn induces Tgfb1a expression15. To investigate if similar phenomena existed in xmrk- and myc-expressing livers, levels of cortisol and Tgfb1a were examined by IF staining together with Hnf4α. As shown in Fig. 6A,B, cortisol was only greatly upregulated in male xmrk-expressing livers. In both sexes of Myc-expressing livers, cortisol remained at the same level to that in wildtype fish. Tgfb1a expression was higher in both male xmrk- and myc-expressing livers than those in female counterparts (Fig. 6C,D). It has been well documented that Tgfb1a promotes tumor progression through polarization of TANs and TAMs28. Here, the Tgfb1a expression showed a consistent sex disparity with TAN/TAM density and tumor progression in xmrk- and Myc-expressing tumors.
Figure 6.
Immunofluorescent staining for cortisol and Tgfb1a in the livers of male and female xmrk+ and Myc+ fish following oncogene activation. 10 fish were analyzed in each group and the experiment was repeated once for reproducibility. (A) IF co-staining of cortisol (red) and HNF4a (green) in liver sections. (B) Quantification of ratio of cortisol-expressing hepatocytes in liver sections. (C) IF co-staining of Tgfb1a (red) and HNF4a (green) in liver sections. (D) Quantification of ratio of Tgfb1a-expressing hepatocytes in liver sections.
Discussion
In recent years, increasing evidence indicates that the crosstalk between the cancer cells and stromal cells plays a significant role in tumor progression29. In human patients, MYC triggers hepatocyte proliferation and is associated with liver fibrosis. Myc overexpression in an HCC mice model activates HSCs and facilitates liver fibrosis30. Xmrk is a fish oncogene and is basically a mutated form of EGFR with hyperactivity31. In human HCC patients, EGFR mutations also cause increased tumor infiltration of immune cells and necrosis32. Thus, both oncogenes studied in the present report are actively involved in stromal cell activities in human tumors.
In our previous studies on kras V12-expressing liver tumors in the zebrafish, stromal cells including HSCs, neutrophils and macrophages are activated after kras V12 oncogene induction. We have observed that following induction of kras V12 expression, both total HSCs and activated HSCs are increased and so is infiltration of neutrophils and macrophages in the kras V12-expressing liver tumors15. In the present study, consistent observations were also made in the xmrk- and Myc-expressing liver tumors. In the previous studies, we have also found that serotonin level is crucial for HSC activation11; this is consistent in the xmrk- and Myc-expressing liver tumors. Recently, the involvement of serotonin in human HCC and identification of plasma serotonin as a marker for HCC diagnosis have been reported33. Our studies in the zebrafish liver tumor models should help elucidate the potential mechanism of how serotonin promote HCC progression. As the importance of serotonin in HCC has been observed consistently in three different oncogene-induced liver tumors in the zebrafish models, the serotonin-mediated mechanism in HCC could be quite universal and thus our zebrafish studies support the use of serotonin as a promising diagnostic marker of HCC.
In the kras V12-expressing liver tumor model in zebrafish, there is an apparent sex disparity with significantly faster liver tumor progression in males than in females11, 15, 19. In the present study, we found the same sex disparity on HCC progression in the xmrk model and, to a less extent, in the Myc model. One week of oncogene activation causes apparent HCC in some of the male fish of both kras- and xmrk-expressing tumors, while the female kras- and xmrk-expressing fish could only reach the adenoma stage. In contrast, both male and female Myc-expressing fish were only at the hyperplastic stage without much discrimination histologically; however, molecularly, male Myc fish had more proliferating and apoptotic cells than female Myc fish (Fig. 2). These observations are consistent with our initial reports of these oncogene transgenic models over a longer term (a few months) of oncogene activation, in which kras+ and xmrk+ fish could develop advanced HCC while Myc+ fish generally develop only adenoma17, 18, 34.
Previous studies in the kras V12-induced liver tumor models in zebrafish have also shown that the higher level of cortisol induced stronger Tgfb1a expression in male kras V12-expressing tumors to attract more neutrophils and macrophages, thus stimulating tumor progression15. In xmrk and Myc-expressing tumor, we found that intratumoral TAN and TAM density were higher in male liver tumors than in female liver tumors. However, the increased cortisol in the liver was observed only in male xmrk+ fish but not significantly in male Myc+ fish. Nevertheless, TAN and TAM infiltrations were similarly higher in males than in females in both xmrk+ and Myc+ fish, which may indicate that other signals maybe responsible for sex-biased activation of TANs and TAMs in Myc-induced liver tumors.
Compared to our previous findings in the kras model11, 15, we found that the xmrk overexpression model showed more similar characteristics to the kras model in activation of stromal cells including HSCs, neutrophils and macrophages during liver tumor initiation. This is also consistent with our previous observation by transcriptomic analysis that the xmrk and kras liver tumors models have more similar deregulated pathways compared to that in the Myc liver tumor model35. In the present study, we also found that the Myc model has a mild sex disparity in liver tumorigenesis and this may be due to a slower progression of the liver tumor in the Myc model; for example, histologically diagnosed HCC could be induced in the kras and xmrk models within one week of oncogene activation while it may takes 4 months to develop HCC under a high dose of induction in the Myc model17, 18, 34. Similarly in mouse, Myc overexpression could only lead to less severe liver tumors instead of development of HCC in vivo 36.
HCC develops from chronic inflammatory and/or fibrosis tissue, which promote tumor progression and resist medical therapy. A better understanding of the interaction between tumor cells and various stromal cells as well as relevant signaling pathways should help gain more knowledge on potential mechanisms in tumor progression. A better understanding of sex disparity of liver cancer should be important for identification of sex-based therapeutic targets and thus for improving therapeutic efficiency.
Methods
Zebrafish Husbandry
All zebrafish experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the National University of Singapore (Protocol Number: 096/12), Tg(fabp10:TA; TRE:xmrk; krt4:GFP)17 and Tg(fabp10:TA; TRE:Myc; krt4:GFP)18 zebrafish in a Tet-on system for inducible hepatocyte-specific expression of oncogenic xmrk and Myc were used in this study and referred to as xmrk and Myc, respectively.
Induction of transgene expression and gross examination
Induction of transgene expression were conducted in 3-month-old adult fish for 7 days with 60 μg/ml doxycycline (D9891; Sigma). In preliminary experiments, we found no histological change of liver histology after one day of doxycycline induction but histological transformation of liver cells (hyperplasia) was observed from 3 days post-induction (data not shown). At the end of doxycycline treatment, >10 fish in each group were used for imaging analyses. All the zebrafish were anesthetized in 0.08% tricaine (E10521; Sigma) and immobilized in 3% methylcellulose (M0521; Sigma) before imaging. Each fish was photographed individually from the left lateral side with an Olympus microscope.
Histological and immunocytological Analyses
All of the adult livers were fixed in 4% paraformaldehyde in phosphate-buffered saline (P6748; Sigma) overnight, embedded in paraffin, and sectioned at 5-mm thickness using a microtome, followed by hematoxylin and eosin (H&E), immunohistochemistry (IHC), or immunofluorescence (IF) stainings. H&E (H-3404; Vector) staining were conducted according to the manufacturers’ protocols. For IHC and IF-stainings, the primary antibodies derived from rabbit or mouse were purchased commercially, including anti-PCNA (FL-261; Santa Cruz Biotechnology, Dallas, TX), anti-caspase 3 (C92–065; BD Biosciences, Singapore), anti-Gfap (154474; Abcam, Singapore), anti–a-smooth muscle actin (a-Sma) (ab15734; Abcam, Singapore), anti-serotonin (C5545; Sigma, USA), anti-PTph (SC135716; Santa Cruz, CA, USA), anti-Hnf4a (MA5-14891; Thermo, Singapore), anti-Lcp (40898; GeneTex, USA), anti-Csf1r (128677; GeneTex, USA), anti-cortisol (C8409; Sigma, USA) and anti-Tgfb1a (55450; Anaspec, CA, USA). Anti-rabbit or anti-mouse secondary antibodies were purchased from Thermo Fisher Scientific (Singapore). At least eight fish from each treatment group were examined and one high-power field was selected randomly from each fish liver as a representative image. IF signals were counted manually for quantitative analyses.
Statistical Analysis
For statistical significance between two groups, 2-tailed unpaired Student t test was performed using GraphPad Prism version 7.00 for Windows. Statistical data are presented as means ± SEM. Same statistical results were obtained by one way ANOVA analysis.
Acknowledgements
This work was supported by grants from Ministry of Education of Singapore (R154000667112 and R154000A23112).
Author Contributions
Q.Y., C.Y. and Z.G. conceived the experiments and wrote the paper. Q.Y. and C.Y. performed the experiments and analyzed data.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiqi Yang and Chuan Yan contributed equally to this work.
References
- 1.Lee CM, et al. Age, gender, and local geographic variations of viral etiology of hepatocellular carcinoma in a hyperendemic area for hepatitis B virus infection. Cancer. 1999;86:1143–1150. doi: 10.1002/(SICI)1097-0142(19991001)86:7<1143::AID-CNCR7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Yeh SH, Chen P. J. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology. 2010;78(Suppl 1):172–179. doi: 10.1159/000315247. [DOI] [PubMed] [Google Scholar]
- 3.Nakatani T, Roy G, Fujimoto N, Asahara T, Ito A. Sex hormone dependency of diethylnitrosamine-induced liver tumors in mice and chemoprevention by leuprorelin. Jpn J Cancer Res. 2001;92:249–256. doi: 10.1111/j.1349-7006.2001.tb01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Maio M, et al. Hormonal treatment of human hepatocellular carcinoma. Ann N Y Acad Sci. 2006;1089:252–261. doi: 10.1196/annals.1386.007. [DOI] [PubMed] [Google Scholar]
- 5.Yeh YT, Chang CW, Wei RJ, Wang SN. Progesterone and related compounds in hepatocellular carcinoma: basic and clinical aspects. BioMed research international. 2013;2013:290575. doi: 10.1155/2013/290575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow PK, et al. Randomised double-blind trial of megestrol acetate vs placebo in treatment-naive advanced hepatocellular carcinoma. British journal of cancer. 2011;105:945–952. doi: 10.1038/bjc.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow PK, et al. High-dose tamoxifen in the treatment of inoperable hepatocellular carcinoma: A multicenter randomized controlled trial. Hepatology. 2002;36:1221–1226. doi: 10.1053/jhep.2002.36824. [DOI] [PubMed] [Google Scholar]
- 8.Coulouarn C, et al. Hepatocyte-stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res. 2012;72:2533–2542. doi: 10.1158/0008-5472.CAN-11-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebrahimkhani MR, et al. Stimulating healthy tissue regeneration by targeting the 5-HT(2)B receptor in chronic liver disease. Nat Med. 2011;17:1668–1673. doi: 10.1038/nm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun B, et al. Intratumoral hepatic stellate cells as a poor prognostic marker and a new treatment target for hepatocellular carcinoma. PLoS One. 2013;8:e80212. doi: 10.1371/journal.pone.0080212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Q, Yan C, Yin C, Gong Z. Serotonin Activated Hepatic Stellate Cells Contribute to Sex Disparity in Hepatocellular Carcinoma. Cell Mol Gastroenterol Hepatol. 2017;3:484–499. doi: 10.1016/j.jcmgh.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Naugler WE, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 14.Li YW, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 15.Yan C, Yang Q, Gong Z. Tumor-Associated Neutrophils and Macrophages Promote Gender Disparity in Hepatocellular Carcinoma in Zebrafish. Cancer Res. 2017;77:1395–1407. doi: 10.1158/0008-5472.CAN-16-2200. [DOI] [PubMed] [Google Scholar]
- 16.Yan C, Huo X, Wang S, Feng Y, Gong Z. Stimulation of hepatocarcinogenesis by neutrophils upon induction of oncogenic kras expression in transgenic zebrafish. J Hepatol. 2015;63:420–428. doi: 10.1016/j.jhep.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, et al. Inducible and repressable oncogene-addicted hepatocellular carcinoma in Tet-on xmrk transgenic zebrafish. J Hepatol. 2012;56:419–425. doi: 10.1016/j.jhep.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, et al. A transgenic zebrafish liver tumor model with inducible Myc expression reveals conserved Myc signatures with mammalian liver tumors. Disease models & mechanisms. 2013;6:414–423. doi: 10.1242/dmm.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Li H, Spitsbergen JM, Gong Z. Males develop faster and more severe hepatocellular carcinoma than females in krasV12 transgenic zebrafish. Sci Rep. 2017;7:41280. doi: 10.1038/srep41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji J, et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology. 2015;62:481–495. doi: 10.1002/hep.27822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morini S, et al. GFAP expression in the liver as an early marker of stellate cells activation. Ital J Anat Embryol. 2005;110:193–207. [PubMed] [Google Scholar]
- 22.Carpino G, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37:349–356. doi: 10.1016/j.dld.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem. 1999;68:355–381. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- 24.Pyonteck SM, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijer AH, et al. Identification and real-time imaging of a myc-expressing neutrophil population involved in inflammation and mycobacterial granuloma formation in zebrafish. Dev Comp Immunol. 2008;32:36–49. doi: 10.1016/j.dci.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Castro R, Zou J, Secombes CJ, Martin SA. Cortisol modulates the induction of inflammatory gene expression in a rainbow trout macrophage cell line. Fish Shellfish Immunol. 2011;30:215–223. doi: 10.1016/j.fsi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Davis, J. M. et al. Increased neutrophil mobilization and decreased chemotaxis during cortisol and epinephrine infusions. J Trauma31, 725–731; discussion 731–722 (1991). [PubMed]
- 28.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bremnes RM, et al. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol. 2011;6:209–217. doi: 10.1097/JTO.0b013e3181f8a1bd. [DOI] [PubMed] [Google Scholar]
- 30.Nevzorova YA, et al. Overexpression of c-myc in hepatocytes promotes activation of hepatic stellate cells and facilitates the onset of liver fibrosis. Biochim Biophys Acta. 2013;1832:1765–1775. doi: 10.1016/j.bbadis.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Gomez A, Wellbrock C, Gutbrod H, Dimitrijevic N, Schartl M. Ligand-independent dimerization and activation of the oncogenic Xmrk receptor by two mutations in the extracellular domain. J Biol Chem. 2001;276:3333–3340. doi: 10.1074/jbc.M006574200. [DOI] [PubMed] [Google Scholar]
- 32.Brzezniak C, et al. RRx-001-Induced Tumor Necrosis and Immune Cell Infiltration in an EGFR Mutation-Positive NSCLC with Resistance to EGFR Tyrosine Kinase Inhibitors: A Case Report. Case Rep Oncol. 2016;9:45–50. doi: 10.1159/000443605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdel-Razik A, et al. Could serotonin be a potential marker for hepatocellular carcinoma? A prospective single-center observational study. Eur J Gastroenterol Hepatol. 2016;28:599–605. doi: 10.1097/MEG.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 34.Chew TW, et al. Crosstalk of Ras and Rho: activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafish models. Oncogene. 2014;33:2717–2727. doi: 10.1038/onc.2013.240. [DOI] [PubMed] [Google Scholar]
- 35.Zheng W, et al. Xmrk, kras and myc transgenic zebrafish liver cancer models share molecular signatures with subsets of human hepatocellular carcinoma. PLoS One. 2014;9:e91179. doi: 10.1371/journal.pone.0091179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zender L, et al. Generation and analysis of genetically defined liver carcinomas derived from bipotential liver progenitors. Cold Spring Harb Symp Quant Biol. 2005;70:251–261. doi: 10.1101/sqb.2005.70.059. [DOI] [PMC free article] [PubMed] [Google Scholar]