Abstract
Objective
To examine the effects of facility‐level acute–postacute continuity on probability of community discharge and 30‐day rehospitalization following inpatient rehabilitation.
Data Sources
We used national Medicare enrollment, claims, and assessment data to study 541,097 patients discharged from 1,156 inpatient rehabilitation facilities (IRFs) in 2010–2011.
Study Design
We calculated facility‐level continuity as the percentages of an IRF's patients admitted from each contributing acute care hospital. Patients were categorized into three groups: low continuity (<26 percent from same hospital that discharged the patient), medium continuity (26–75 percent from same hospital), or high continuity (>75 percent from same hospital). The multivariable models included an interaction term to examine the potential moderating effects of facility type (freestanding facility vs. hospital‐based rehabilitation unit) on the relationships between facility‐level continuity and our two outcomes: community discharge and 30‐day rehospitalization.
Principal Findings
Medicare beneficiaries in hospital‐based rehabilitation units were more likely to be referred from a high‐contributing hospital compared to those in freestanding facilities. However, the association between higher acute–postacute continuity and desirable outcomes is significantly better in freestanding rehabilitation facilities than in hospital‐based units.
Conclusions
Improving continuity is a key premise of health care reform. We found that both observed referral patterns and continuity‐related benefits differed markedly by facility type. These findings provide a starting point for health systems establishing or strengthening acute–postacute relationships to improve patient outcomes in this new era of shared accountability and public quality reporting programs.
Keywords: Referrals and referral networks, rehabilitation services, Medicare, hospitals, quality of care/patient safety (measurement)
Several initiatives within the Affordable Care Act (ACA) (Public Law 111‐148, 2010) extend inpatient providers’ accountability for patient well‐being beyond the services provided and outcomes achieved during the isolated stay in their facility. The assumption underlying many programs such as accountable care organizations, bundled payments, and care transitions is that shared accountability will lead to better patient experiences and improved outcomes resulting from improvements in the continuity and coordination of care across settings and over time. The emphasis on acute–postacute continuity is reflected in the Centers for Medicare and Medicaid Services’ (CMS) list of quality measures under consideration for December 2016, which includes a process measure on the transfer of health information and care preferences during these transitions (Centers for Medicare & Medicaid Services, 2016).
The concepts of care continuity and care coordination are often used interchangeably. Neither term has a consensus definition or definitive measure. McDonald et al. (2007) defined care coordination as “the deliberate organization of patient care activities between two or more participants (including the patient) involved in a patient's care to facilitate the appropriate delivery of health care services.” While this definition clearly captures the spirit of care coordination, it also conveys the difficulty in creating a practical coordination measure. Some consider coordination to be a distinct subdomain within care continuity, whereas others argue that coordination is the direct result of continuity (McDonald et al. 2007). Regardless of the overlap, distinction, and/or serial nature of the two concepts, continuity is more easily operationalized and measured in health claims data (Chen and Ayanian 2014). Traditionally, continuity has been viewed as the ongoing relationship a patient has with a single provider over time, and several continuity indices are available to measure different aspects of these relationships at the individual patient level (Jee and Cabana 2006). However, the routine use of multiple specialists for the standard delivery of care in the U.S. health care system and current initiatives within the ACA have led to a new view of continuity. In this modern context, continuity reflects the degree of communication, coordination, and integration between providers (Haggerty et al. 2003; Gulliford, Naithani, and Morgan 2006). There are currently no validated measures to quantify interfacility continuity.
Older adults who require intensive postacute services are most in need of well‐coordinated care to manage their prolonged recovery. They are also most vulnerable to discontinuity as they transition from setting to setting and ultimately back to the community (Greenwald and Jack 2009; Naylor et al. 2011). Inpatient rehabilitation facilities (IRFs) provide the most intensive postacute rehabilitative care. Nearly 400,000 Medicare beneficiaries received IRF services in 2014, more than 85 percent of whom were discharged from an acute care hospital within 30 days prior to IRF admission (Medicare Payment Advisory Commission, 2016). Thus, most patients experience a minimum of three difficult transitions (home‐to‐hospital, hospital‐to‐IRF, and IRF‐to‐community or other inpatient setting) over a relatively short timeframe. These transitions in care teams and locations of services occur while recovering from their recent illness, injury, or functional impairments. Ineffective and fragmented care transitions lead to higher hospital readmissions, costs, and other adverse events (Forster et al. 2003; Jencks, Williams, and Coleman 2009).
Preferred referral networks and shared‐savings plans are not new concepts. Health maintenance organizations and other forms of “managed” care have been around for decades. However, the current health care reform environment has renewed interest among providers and health care systems to establish and/or expand their collaborative relationships with others across the continuum of care. These organizational relationships are well‐suited to target processes of care and, to some extent, overall costs of care. Less is known regarding the potential impacts of these provider relationships on patient experiences and outcomes. Rahman and colleagues (2013) recently reported lower readmission rates for hospitals that concentrate their skilled nursing facility (SNF) discharges to a preferred SNF. Patient flow to IRFs has not been studied and the influence on outcomes is unknown.
The objectives of this study were twofold: (1) to operationalize a facility‐level continuity variable reflecting the relative contributions from various admitting hospitals to an IRF's total patient population and (2) to test whether this facility‐level continuity measure was associated with important patient outcomes. We used the 100 percent Medicare hospital claims and IRF assessment files to calculate the percentages of an IRF's patients that were admitted from each referring hospital. We used this continuity measure as a proxy for the degree of communication, coordination, and integration between providers, and we presumed it would be positively associated with important patient‐level outcomes. Outcomes of interest included community discharge and all‐cause 30‐day rehospitalization.
Methods
Data Source and Population
We used the 100 percent Medicare inpatient (Part A) files, Beneficiary Summary, Medicare Provider and Analysis Record (MedPAR), and Inpatient Rehabilitation Facility‐Patient Assessment Instrument (IRF‐PAI) files for 2009–2011. The University's institutional review board approved this study, and we had a data use agreement from the CMS.
Medicare beneficiaries discharged from an inpatient rehabilitation facility between January 1, 2010 and November 30, 2011 were identified in the IRF‐PAI file. Functional assessment variables from the IRF‐PAI were linked to claims data from 2009 to 2011 MedPAR file. The 2009 claims were included to capture prior acute admissions in the year preceding the index stays. The initial sample included 744,801 cases. We excluded patients with a prior IRF stay within 30 days of an IRF admission (n = 32,160), without an acute hospital stay within 30 days of IRF admission (n = 68,577), admitted for any reason other than initial rehabilitation (n = 33,348), who enrolled in the Medicare Advantage program anytime from 12 months prior to their index rehabilitation stays through the end of the observation period at 30 days after discharge (n = 101,209), and patients who died prior to IRF discharge or within 30 days of discharge without a preceding rehospitalization (n = 9,062). To ensure stable estimates from the multilevel (mixed) models, we excluded IRFs with fewer than 20 total patients over the study period (n = 119). In all, we excluded 203,704 (27 percent) cases—summing the individual numbers yields a slightly larger value as some patients met more than one exclusion criterion. The final sample included 541,097 patients from 1,156 IRFs, including 238 freestanding facilities and 918 hospital‐based rehabilitation units.
Variables
Outcomes
We studied two patient outcomes: discharge setting after the IRF stay and all‐cause 30‐day rehospitalization. The IRF‐PAI lists 14 categories within the discharge to living setting variable. We collapsed categories to create a dichotomous discharge setting variable (community vs. institution). The community category included home, board and care, transitional living, or assisted living. A rehospitalization (yes vs. no) was counted if the patient had at least one claim in the MedPAR file from a short‐term or critical‐access hospital within 30 days of discharge from the index rehabilitation stay.
Independent Variable
Facility‐level continuity was calculated as the percentage of an IRF's patients that were admitted from each contributing hospital. Patients were categorized into three groups: low continuity (<26 percent of IRF admissions from the same hospital that discharged the patient), medium continuity (26–75 percent of IRF admissions from the same hospital that discharged the patient), or high continuity (>75 percent of IRF admissions from the same hospital that discharged the patient).
Covariates
Patient demographic variables included age, sex, race/ethnicity (white, black, Hispanic, other), social support, and disability status (yes/no). Social support was a three‐level variable (family/friends, paid/other, or none) based on integrating responses from two variables in the IRF‐PAI file: marital status and living with others prior to admission. All married patients were classified in the family/friends category, whereas currently unmarried patients were classified based on their response to living with others prior to admission. Disability status reflects whether a patient's original eligibility for Medicare benefits was due to disability.
Clinical measures included dialysis (yes vs. no) during the prior hospital stay, primary medical condition, comorbid medical conditions, number of hospital admissions in prior year, and admission functional status. Each patient is assigned to 1 of 21 rehabilitation impairment categories (RICs) (US Department of Health and Human Services, 2016) based on his or her primary condition at admission to rehabilitation. We used the methodology described by Stineman et al. (1997) to combine impairment categories with similar functional prognoses to create a reduced list of six categories: central nervous system, spinal cord injury, neurological, musculoskeletal, endurance‐related, and other. Comorbidity burden was calculated using the Elixhauser comorbidity index (Elixhauser et al. 1998). Number of prior hospital admissions was the count of acute hospitalization claims over the 12 months preceding the index rehabilitation stay. Admission functional status is assessed within 72 hours of admission to inpatient rehabilitation. The IRF‐PAI contains 18 items from the Functional Independence Measure (FIM) (UB Foundation Activities, 2004). Each item is rated on a scale from one (totally dependent) to seven (totally independent). Thus, scores range from 18 to 126 points.
Facility‐level variables included total patient volume and IRF type. The provider number and special unit code variables in the MedPAR were used to classify IRFs into two distinct categories: freestanding facilities and hospital‐based units.
Statistical Analysis
Descriptive summaries (means and standard deviations or counts and percentages) were computed for all covariates and the independent variable (facility‐level continuity), and stratified by the two dichotomous outcome variables: discharge setting (community vs. institution) and rehospitalization (no vs. yes). Group differences were tested using chi‐square and independent t‐tests as appropriate. Histograms were produced to show patient distribution across the facility‐level continuity variable with the bins representing consecutive 10 percent intervals in the percentage of IRF patients admitted from a given hospital. Generalized linear mixed models were used to calculate the adjusted odds ratios (ORs) for community discharge and 30‐day rehospitalization. Mixed modeling accounts for the clustering of patients within IRFs (Bingenheimer and Raudenbush 2004). All covariates listed above were included in the models. We also tested an interaction term for facility type‐by‐continuity in both models. Parameter estimates from those models were converted to probabilities and plotted by the three‐level continuity variable for both freestanding facilities and hospital‐based units. IBM SPSS v23 software (Armonk, NY) was used for all analyses.
Results
Descriptive summaries of patient characteristics stratified by the two dichotomous outcome measures are shown in Table 1. Overall, 75 percent of patients were discharged back to the community immediately following inpatient rehabilitation, and 22 percent of patients were rehospitalized at some point during the first 30 days following rehabilitation discharge. All characteristics listed in Table 1 were significantly (p<.001) associated with both outcomes. Compared to patients in hospital‐based rehabilitation units, those in freestanding facilities demonstrated slightly lower community discharge rates (75 percent vs. 74 percent) and slightly higher 30‐day rehospitalization rates (21 percent vs. 22 percent). Regarding the continuity measure, categorical increases in continuity (<26, 26–75, and >75 percent of patients from same referring hospital) were associated with slight increases in community discharge rates (73, 75, and 75 percent, respectively) and slight decreases in rehospitalization rates (22, 22, and 21 percent, respectively).
Table 1.
Sample Characteristics by the Two Outcome Measures: Values Are Reported as Row Percent or Mean (SD)
| Total | Discharge Setting | Total | Rehospitalization | |||
|---|---|---|---|---|---|---|
| Community | Institution | No | Yes | |||
| Total | 540,908 | 74.6% | 25.4% | 541,097 | 78.4% | 21.6% |
| Age | 75.9 (10.9) | 75.3 (10.9) | 77.6 (11.0) | 75.9 (10.9) | 76.0 (10.8) | 75.6 (11.3) |
| Sex | ||||||
| Female | 317,310 | 75.2% | 24.8% | 317,418 | 80.1% | 19.9% |
| Male | 223,598 | 73.7% | 26.3% | 223,679 | 75.8% | 24.2% |
| Race/ethnicity | ||||||
| White | 445,033 | 74.4% | 25.6% | 445,198 | 78.8% | 21.2% |
| Black | 54,518 | 74.8% | 25.2% | 54,527 | 75.1% | 24.9% |
| Hispanic | 27,936 | 75.8% | 24.2% | 27,947 | 76.6% | 23.4% |
| Other | 12,744 | 76.1% | 23.9% | 12,748 | 79.7% | 20.3% |
| Social support | ||||||
| Family/friends | 358,236 | 77.0% | 23.0% | 358,349 | 77.7% | 22.3% |
| Paid/other | 5,044 | 71.0% | 29.0% | 5,045 | 76.1% | 23.9% |
| None | 177,102 | 69.8% | 30.2% | 177,177 | 79.8% | 20.2% |
| Disability | ||||||
| No disability | 421,450 | 74.1% | 25.9% | 421,605 | 79.1% | 20.9% |
| Disability | 119,458 | 76.5% | 23.5% | 119,492 | 75.7% | 24.3% |
| Prior acutes | 1.5 (0.9) | 1.5 (0.9) | 1.6 (1.0) | 1.5 (0.9) | 1.4 (0.8) | 1.8 (1.2) |
| Dialysis | ||||||
| No | 522,509 | 74.8% | 25.2% | 522,695 | 79.1% | 20.9% |
| Yes | 18,399 | 68.1% | 31.9% | 18,402 | 58.7% | 41.3% |
| Rehab impairment | ||||||
| CNS | 146,966 | 67.4% | 32.6% | 147,025 | 77.1% | 22.9% |
| SCI | 23,138 | 72.8% | 27.2% | 23,142 | 77.5% | 22.5% |
| Neuro | 48,116 | 75.6% | 24.4% | 48,126 | 72.8% | 27.2% |
| Musculoskeletal | 203,752 | 78.5% | 21.5% | 203,807 | 84.9% | 15.1% |
| Endurance | 41,699 | 79.5% | 20.5% | 41,709 | 69.8% | 30.2% |
| Other | 77,237 | 75.3% | 24.7% | 77,288 | 71.9% | 28.1% |
| Elixhauser sum | 4.5 (2.1) | 4.4 (2.1) | 4.8 (2.1) | 4.5 (2.1) | 4.4 (2.0) | 5.1 (2.2) |
| Admit FIM | 60.7 (16.6) | 64.1 (15.4) | 50.8 (16.2) | 60.7 (16.6) | 61.9 (16.3) | 56.5 (16.9) |
| Facility volume | 1,227 (945) | 1,214 (932) | 1,264 (982) | 1,226 (945) | 1,218 (946) | 1,259 (941) |
| IRF type | ||||||
| Hospital unit | 319,764 | 75.0% | 25.0% | 319,949 | 78.9% | 21.1% |
| Freestanding | 221,144 | 73.9% | 26.1% | 221,148 | 77.6% | 22.4% |
| Continuity | ||||||
| <26% | 176,127 | 73.2% | 26.8% | 176,171 | 77.8% | 22.2% |
| 26–75% | 192,608 | 75.2% | 24.8% | 192,641 | 78.5% | 21.5% |
| >75% | 172,173 | 75.3% | 24.7% | 172,285 | 78.7% | 21.3% |
p<.001 for all bivariate comparisons.
Three variables had missing values: discharge setting (n = 189), race/ethnicity (n = 677), and social support (n = 526).
CNS, central nervous system; FIM, Functional Independence Measure; SCI, spinal cord injury.
Figure 1 shows the distribution of total IRF patients by the proportion of patients from the same referring hospital. More patients in freestanding facilities were admitted from relatively low‐contributing hospitals, whereas more patients in hospital‐based units were admitted from relatively high‐contributing hospitals.
Figure 1.
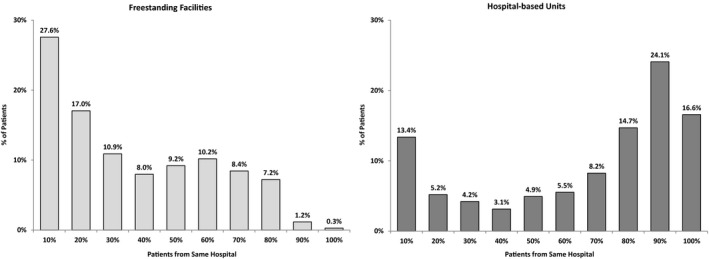
Distribution of Total IRF Patients by the Proportion of Patients from the Same Referring Hospital
Table 2 lists ORs and 95 percent confidence intervals (95 percent CIs) from the multivariable models estimating community discharge and 30‐day rehospitalization. The facility type X continuity interaction terms were significant in both models. In the community discharge model, IRF type also demonstrated a significant main effect with freestanding facilities exhibiting 20 percent higher odds of community discharge compared to hospital‐based units. The interaction term further added to this difference with the moderate and high‐continuity groups in freestanding facilities yielding 6 and 18 percent higher odds, respectively. In the rehospitalization model, the continuity variable demonstrated a significant main effect with the high‐continuity group exhibiting 4 percent higher odds of readmission compared to the low‐continuity group. The interaction term countered the main effect in freestanding facilities with moderate and high continuity yielding 4 and 11 percent lower odds, respectively.
Table 2.
Odds Ratios (95 Percent Confidence Intervals) from the Two Generalized Linear Mixed Models
| Community Discharge | Rehospitalization | |||
|---|---|---|---|---|
| OR (95% CIs) | p‐value | OR (95% CIs) | p‐value | |
| Age, 5 years | 0.93 (0.93, 0.94) | <.001 | 1.01 (1.01, 1.02) | <.001 |
| Male | 0.92 (0.91, 0.93) | <.001 | 1.15 (1.14, 1.17) | <.001 |
| Race/ethnicity (white) | 1.00 | 1.00 | ||
| Black | 1.19 (1.16, 1.22) | <.001 | 0.99 (0.97, 1.01) | .396 |
| Hispanic | 1.30 (1.26, 1.35) | <.001 | 0.95 (0.92, 0.99) | .005 |
| Other | 1.15 (1.10, 1.21) | <.001 | 0.89 (0.85, 0.93) | <.001 |
| Missing | 1.11 (0.91, 1.35) | .313 | 0.99 (0.82, 1.20) | .933 |
| Social support (family/friends) | 1.00 | 1.00 | ||
| Paid/other | 1.03 (0.96, 1.10) | .434 | 0.95 (0.89, 1.02) | .156 |
| None | 0.56 (0.55, 0.57) | <.001 | 1.00 (0.98, 1.01) | .609 |
| Missing | 0.47 (0.39, 0.58) | <.001 | 0.98 (0.80, 1.21) | .865 |
| Disability | 0.90 (0.88, 0.91) | <.001 | 1.10 (1.07, 1.12) | <.001 |
| Prior acutes | 0.90 (0.89, 0.90) | <.001 | 1.34 (1.33, 1.35) | <.001 |
| Dialysis | 0.59 (0.57, 0.61) | <.001 | 1.76 (1.70, 1.82) | <.001 |
| Impairment (CNS) | 1.00 | 1.00 | ||
| SCI | 0.77 (0.75, 0.80) | <.001 | 1.20 (1.16, 1.24) | <.001 |
| Neuro | 1.04 (1.01, 1.07) | .003 | 1.26 (1.23, 1.30) | <.001 |
| Musculoskeletal | 1.00 (0.98, 1.02) | .941 | 0.81 (0.79, 0.82) | <.001 |
| Endurance | 1.02 (0.99, 1.05) | .198 | 1.59 (1.55, 1.63) | <.001 |
| Other | 0.98 (0.96, 1.00) | .058 | 1.40 (1.37, 1.43) | <.001 |
| Elixhauser sum | 0.95 (0.94, 0.95) | <.001 | 1.11 (1.10, 1.11) | <.001 |
| Admit FIM, 5 points | 1.34 (1.34, 1.35) | <.001 | 0.91 (0.91, 0.91) | <.001 |
| Facility volume, 100 cases | 1.01 (1.00, 1.01) | .042 | 1.00 (1.00, 1.00) | .136 |
| Freestanding IRF | 1.20 (1.11, 1.29) | <.001 | 0.99 (0.95, 1.02) | .473 |
| Continuity (<26%) | 1.00 | 1.00 | ||
| 26–75% | 1.00 (0.97, 1.03) | .951 | 1.03 (1.00, 1.05) | .066 |
| >75% | 0.99 (0.96, 1.03) | .613 | 1.04 (1.01, 1.07) | .011 |
| Facility type × continuity interaction | ||||
| Hospital unit × < 26% | 1.00 | 1.00 | ||
| Hospital unit × 26–75% | 1.00 | 1.00 | ||
| Hospital unit × >75% | 1.00 | 1.00 | ||
| Freestanding × <26% | 1.00 | 1.00 | ||
| Freestanding × 26–75% | 1.06 (1.02, 1.11) | .002 | 0.96 (0.93, 1.00) | .047 |
| Freestanding × >75% | 1.18 (1.04, 1.33) | .008 | 0.89 (0.81, 0.97) | .011 |
CNS, central nervous system; FIM, Functional Independence Measure; SCI, spinal cord injury.
Adjusted probabilities derived from the multivariable models for community discharge and 30‐day rehospitalization are displayed in Figures 2 and 3, respectively. The figures include values for the three‐category facility‐level continuity measure plotted for both freestanding facilities and hospital‐based rehabilitation units.
Figure 2.
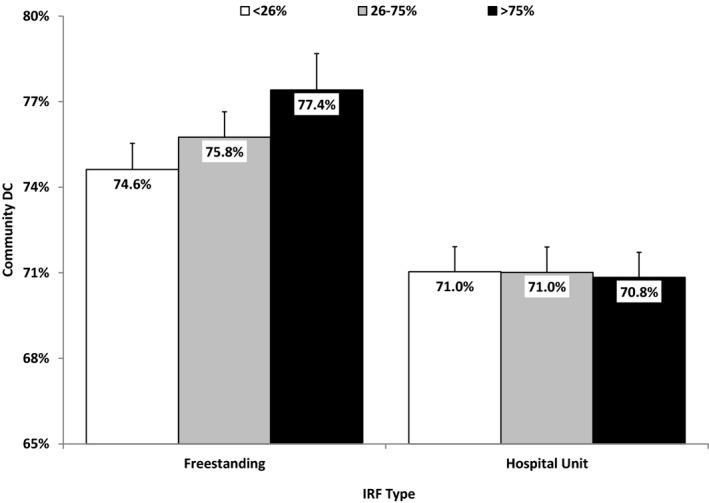
Adjusted Probabilities for Community Discharge by IRF Type and the Three‐Category Facility‐Level Continuity Variable
Note. Values are Based on the average patient and parameter estimates from Table 2.
Figure 3.
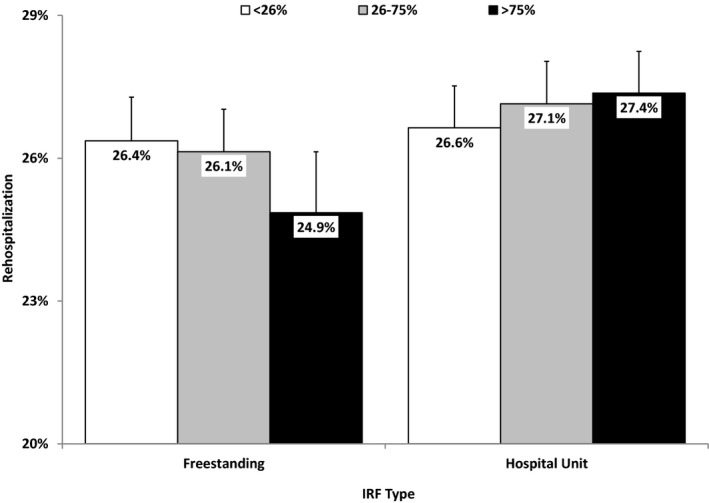
Adjusted Probabilities for 30‐Day Rehospitalization by IRF Type and the Three‐Category Facility‐Level Continuity Variable
Note. Values are based on the average patient and parameter estimates from Table 2.
Discussion
This is the first study examining relationships between a hospital‐IRF continuity measure and patient outcomes following inpatient rehabilitation. Thirty‐day unplanned rehospitalization is a current quality metric, and community discharge is a proposed quality metric for the IRF quality reporting program (US Department of Health and Human Services 2016). We quantified continuity as the proportions of an IRF's volume they receive from each referring hospital and then examined the associations between continuity and the two quality indicators. Our measure divided referral volume into low (<26 percent), medium (26–75 percent), and high (>75 percent) continuity categories. Medicare beneficiaries in hospital‐based rehabilitation units were more likely to experience high‐continuity transitions compared to those in freestanding facilities. However, the association between higher continuity and desirable outcomes seems limited to patients in freestanding rehabilitation facilities.
Many studies convey the importance of continuity in care transitions (Helleso and Lorensen 2005); however, transition quality is difficult to quantify. Continuity concepts are generally classified into three broad types: informational, relational, or management continuity (Holland and Harris 2007; van Walraven et al. 2010). Donaldson (2001) applied an “agency theory” perspective to continuity and contends that information transfer and goal alignment are the fundamental elements of uninterrupted care. Thus, an effective handoff from one inpatient setting to another may be facilitated through improved communication across providers (e.g., shared access to electronic medical records) and/or shared care plans that extend patient preferences and goals across settings. Regardless of the specific elements, the intent of continuity in care transitions is to increase the efficiency and quality of longitudinal care, which should lead to better patient experiences and outcomes (van Walraven et al. 2010). We calculated the percentages of an IRF's patients that were admitted from each referring hospital and used the resultant measure as a proxy for facility‐level continuity. We presumed that stronger (more exclusive) referral patterns, whether simply due to geographic proximity or to a purposeful affiliation, would reflect better communication and greater familiarity with care processes between acute and postacute facilities.
Our unadjusted comparisons showed small benefits on both outcomes for patients in hospital‐based rehabilitation units compared to those in freestanding facilities. Greater continuity was also associated with improvements in overall rates on both outcomes. However, the adjusted models with the facility type × continuity interaction terms seemed to switch the more favorable setting from hospital units to freestanding facilities, which was further evident at higher levels of continuity. Using hospital units and low continuity as the reference groups for the interaction term, patients in freestanding facilities and in the medium‐ and high‐continuity groups were significantly more likely to be discharged to the community and less likely to be rehospitalized over the 30 days following discharge. Additional research is needed to determine whether these setting‐specific patterns can be linked to clear differences in patient management strategies during care transitions or simply whether additional case mix adjustments may be able to explain these findings.
Rahman et al. (2013) examined the effect of hospital to SNF “referral linkages” on 30‐day rehospitalization following discharge to an SNF. The facility‐level continuity variable was operationalized from the acute rather than the postacute perspective, that is, proportion of patients from the originating hospital who were discharged to the treating SNF. Overall, hospitals with a higher proportion of referrals going to a single SNF had lower rehospitalization rates; the effects were stronger in hospitals that did not own an SNF and within the first week following discharge (Rahman et al. 2013). Methodological differences aside, together our findings suggest that the relative strength of acute–postacute referral patterns may independently impact near‐term patient outcomes. Moreover, the associations between facility‐level continuity and outcomes seem to vary based on the perceived financial relationships between the acute and postacute settings, for example, IRFs or SNFs affiliated with acute hospitals versus independent facilities.
The ACA established the mandate for the IRF Quality Reporting Program (QRP) (Public Law 111‐148, 2010). Subsequent legislation and demonstration projects have provided the fundamental structure for the program. Thirty‐day unplanned rehospitalization is a current quality metric and community discharge is a proposed quality metric for the IRF quality reporting program (US Department of Health and Human Services, 2016). CMS plans to launch the “IRF Compare” website in the Winter of 2016 (Centers for Medicare & Medicaid Services, 2016). Similar to the existing Hospital Compare and SNF Compare websites, IRF Compare will publicly report summaries of quality indicators for each of the more than 1,100 IRFs in the United States. The goals of these QRPs are twofold: (1) allow patients, family members, and other providers to compare IRFs based on their quality rankings and make more informed care decisions; and (2) encourage IRFs to improve the quality of the care they provide. As reporting of postacute provider quality rankings progresses, it will be interesting to examine whether the rankings influence the affiliations with and/or referral patterns from acute hospitals. Further research should also examine the extent to which the quality rankings of the upstream and/or downstream providers mediate the relationships between facility‐level referral patterns and patient outcomes.
Our study has some limitations. Our sample was restricted to Medicare fee‐for‐service beneficiaries, so our findings may not generalize to younger patients with other insurance coverage or Medicare beneficiaries in managed care programs. We excluded patients who died within 30 days of IRF discharge if they were not rehospitalized first. There are likely other unmeasured factors such as patient preferences, health behaviors, and environmental issues associated with our selected outcomes. We modeled all‐cause 30‐day rehospitalizations and did not differentiate planned or preventable readmissions. In addition, our data were from discharges that occurred in 2010–2011, but the penalty for acute readmissions began in 2012. Lastly, our facility‐level continuity measure is based solely on the percentage of an IRF's patients that were admitted from a given hospital and does not capture the extent of true coordination and integration across settings. The National Quality Forum has established a list of 25 consensus standards for care coordination (National Quality Forum, 2010), many of which are directly related to effective care transitions. Prospective research is needed to assess whether greater transfer volume leads to better care coordination based on those standards.
Conclusions
Referral patterns differed markedly by facility type. Hospital‐based rehabilitation units generally received a higher percentage of patients from a particular acute hospital. Interestingly, freestanding facilities demonstrated a distinct advantage over hospital‐based units in terms of the observed benefits of higher continuity on odds of community discharge and 30‐day rehospitalization. Quality research is needed to examine the mechanisms underlying successful interfacility transitions, common barriers to community discharge, common reasons for rehospitalization, and whether they differ for hospital‐based units versus freestanding IRFs. These findings and related research have implications in this new era of shared accountability and public quality reporting programs as health systems continue establishing and/or strengthening acute–postacute relationships to improve patient outcomes.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This study was funded in part by grants from the National Institutes of Health (NIH: P2C HD065702, R01 HD069443) and the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR: 90IF0071).
Disclaimer: None.
Disclaimer: None.
References
- Bingenheimer, J. B. , and Raudenbush S. W.. 2004. “Statistical and Substantive Inferences in Public Health: Issues in the Application of Multilevel Models.” Annual Review of Public Health 25: 53–77. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services . (2016). “List of Measures Under Consideration” [accessed on December 1, 2016]. Available at http://www.qualityforum.org/Project_Pages/MAP_Coordinating_Committee.aspx
- Chen, L. M. , and Ayanian J. Z.. 2014. “Care Continuity and Care Coordination: What Counts?” JAMA Internal Medicine 174 (5): 749–50. [DOI] [PubMed] [Google Scholar]
- Donaldson, M. S. 2001. “Continuity of Care: A Reconceptualization.” Medical Care Research and Review 58 (3): 255–90. [DOI] [PubMed] [Google Scholar]
- Elixhauser, A. , Steiner C., Harris D. R., and Coffey R. M.. 1998. “Comorbidity Measures for Use with Administrative Data.” Medical Care 36 (1): 8–27. [DOI] [PubMed] [Google Scholar]
- Forster, A. J. , Murff H. J., Peterson J. F., Gandhi T. K., and Bates D. W.. 2003. “The Incidence and Severity of Adverse Events Affecting Patients after Discharge from the Hospital.” Annals of Internal Medicine 138 (3): 161–7. [DOI] [PubMed] [Google Scholar]
- Greenwald, J. L. , and Jack B. W.. 2009. “Preventing the Preventable: Reducing Rehospitalizations through Coordinated, Patient‐Centered Discharge Processes.” Professional Case Management 14 (3): 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliford, M. , Naithani S., and Morgan M.. 2006. “What Is ‘Continuity of Care’?” Journal of Health Services Research & Policy 11 (4): 248–50. [DOI] [PubMed] [Google Scholar]
- Haggerty, J. L. , Reid R. J., Freeman G. K., Starfield B. H., Adair C. E., and McKendry R.. 2003. “Continuity of Care: A Multidisciplinary Review.” British Medical Journal 327 (7425): 1219–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleso, R. , and Lorensen M.. 2005. “Inter‐Organizational Continuity of Care and the Electronic Patient Record: A Concept Development.” International Journal of Nursing Studies 42 (7): 807–22. [DOI] [PubMed] [Google Scholar]
- Holland, D. E. , and Harris M. R.. 2007. “Discharge Planning, Transitional Care, Coordination of Care, and Continuity of Care: Clarifying Concepts and Terms from the Hospital Perspective.” Home Health Care Services Quarterly 26 (4): 3–19. [DOI] [PubMed] [Google Scholar]
- Jee, S. H. , and Cabana M. D.. 2006. “Indices for Continuity of Care: A Systematic Review of the Literature.” Medical Care Research and Review 63 (2): 158–88. [DOI] [PubMed] [Google Scholar]
- Jencks, S. F. , Williams M. V., and Coleman E. A.. 2009. “Rehospitalizations Among Patients in the Medicare Fee‐for‐Service Program.” New England Journal of Medicine 360 (14): 1418–28. [DOI] [PubMed] [Google Scholar]
- McDonald, K. M. , Sundaram V., Bravata D. M., Lewis R., Lin N., Kraft S. A., McKinnon M., Paguntalan H., and Owens D. K.. 2007. “Care Coordination – Volume 7 (AHRQ Publication No. 04(07)‐0051‐7)” In Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies, edited by Shojania K. G., McDonald K. M., Wachter R. M., and Owens D. K. Rockville, MD: Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Medicare Payment Advisory Commission . March 2016. Report to Congress: Medicare Payment Policy, pp. 237–257. Washington, DC: Author. [Google Scholar]
- National Quality Forum . 2010. Preferred Practices and Performance Measures for Measuring and Reporting Care Coordination: A Consensus Report. Washington, DC: NQF. [Google Scholar]
- Naylor, M. D. , Aiken L. H., Kurtzman E. T., Olds D. M., and Hirschman K. B.. 2011. “The Care Span: The Importance of Transitional Care in Achieving Health Reform.” Health Affairs 30 (4): 746–54. [DOI] [PubMed] [Google Scholar]
- Patient Protection and Affordable Care Act (Public Law 111‐148) . 2010. “111th Congress.”
- Rahman, M. , Foster A. D., Grabowski D. C., Zinn J. S., and Mor V.. 2013. Effect of Hospital‐SNF Referral Linkages on Rehospitalization. Health Services Research 48(6 Pt 1): 1898–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stineman, M. G. , Goin J. E., Tassoni C. J., Granger C. V., and Williams S. V.. 1997. “Classifying Rehabilitation Inpatients by Expected Functional Gain.” Medical Care 35 (9): 963–73. [DOI] [PubMed] [Google Scholar]
- UB Foundation Activities . (2004). “The IRF‐PAI Training Manual” [accessed on February 14, 2017]. Available at http://www.cms.hhs.gov/InpatientRehabFacPPS/downloads/irfpaimanual040104.pdf
- US Department of Health and Human Services . 2016. Federal Register, Vol 81(79). [Google Scholar]
- van Walraven, C. , Oake N., Jennings A., and Forster A. J.. 2010. “The Association Between Continuity of Care and Outcomes: A Systematic and Critical Review.” Journal of Evaluation in Clinical Practice 16 (5): 947–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


