Abstract
Objective
Consuming low‐value health care not only highlights inefficient resource use but also brings an important concern regarding the economics of disparities. We identify the relation of socioeconomic characteristics to the use of low‐value cancer screenings in Medicare fee‐for‐service (FFS) settings, and quantify the amount subsidized from nonusers and taxpayers to users of these screenings.
Data Sources
2007–2013 Medicare Current Beneficiary Survey, Medicare FFS claims, and the Area Health Resource Files.
Study Design
Our sample included enrollees in FFS Part B for the entire calendar year. We excluded beneficiaries with a claims‐documented or self‐reported history of targeted cancers, or those enrolled in Medicaid or Medicare Advantage plans. We identified use of low‐value Pap smears, mammograms, and prostate‐specific antigen tests based on established algorithms, and estimated a logistic model with year dummies separately for each test.
Data Collection/Extraction Methods
Secondary data analyses.
Principal Findings
We found a statistically significant positive association between privileged socioeconomic characteristics and use of low‐value screenings. Having higher income and supplemental private insurance strongly predicted more net subsidies from Medicare.
Conclusions
FFS enrollees who are better off in terms of sociodemographic characteristics receive greater subsidies from taxpayers for using low‐value cancer screenings.
Keywords: Distributional effects, Medicare, low‐value cancer screenings
Growing evidence highlights the overuse of various low‐value medical services in the United States (Goodwin et al. 2011; Kale et al. 2013; Kepka et al. 2014; Schwartz et al. 2014; Colla et al. 2015). Among these services are low‐value cancer screenings: unnecessarily frequent tests or those given to broader populations that provide limited benefits, which may not justify their harms and costs (U.S. Preventive Services Task Force [USPSTF] 2009, 2012a,b; Wilt, Harris, and Qaseem 2015). For example, multiple medical guidelines indicate that preventive Pap smears used by women without a cervix are unlikely to generate value. Similarly, they consider prostate‐specific antigen (PSA) tests for men older than 74 as low‐value services. Because the receipt of clinical preventive care occurs to a large extent at the individual's discretion, differences exist in using preventive services, and they can be both overused and underused—a challenging two‐sided conundrum.
An extensive body of literature underscored racial and socioeconomic (SES) variations in preventive care, which is traditionally perceived as underused (Paskett et al. 1997; Sambamoorthi and McAlpine 2003; O'Malley et al. 2005; Agency for Healthcare Research and Quality 2014a). However, few studies have analyzed use of low‐value preventive care (potential overuse) by individual socioeconomic characteristics, such as income or education, although policy discussions have increasingly focused on reducing waste in the health care system and promoting value‐based health care.
A recent literature review reported an inconsistent relationship between race/ethnicity and overuse of a variety of medical procedures, for example, inappropriate antipsychotic drugs or radiographic imaging (Kressin and Groeneveld 2015). While a few studies presented differences in overusing preventive services by patient group, they are limited to identifying groups only by geographic boundaries like Hospital Referral Regions (Goodwin et al. 2011; Sheffield et al. 2013; Schwartz et al. 2014; Colla et al. 2015), or to presenting self‐reported service with only a 1‐year study period (Kepka et al., 2014). Few studies looked at how low‐value care utilization varies by individual SES—an important issue that could inform policy makers of what groups to target, and help explore group‐specific strategies to reduce waste in health care effectively.
Socioeconomic disparities in service use are usually approached as a problem of access to care or as potential inequities in health outcomes. However, these aspects are not the only concern: the economics of disparities in discretionary service use has long been ignored. Insurance coverage of any service shifts (part of) individuals’ out‐of‐pocket costs to the premiums paid by all enrollees in the pool, while benefits are dispensed only to service users. Thus, users are subsidized by nonusers through insurance (cross‐subsidies). When enrollees face exogenous risk of using a service (e.g., getting sick), this redistribution by risk pooling reflects the underlying purpose of insurance. In contrast, when the use of covered services is voluntary or discretional (with no stochastic risk of illness associated with consumption), some cross‐subsidies are unintended. Levels of utilization of those discretionary services reflect disadvantages in certain individuals’ SES or other characteristics related to access to care. Because the cost of the service is shifted to the premium that is borne by every enrollee, nonusers of discretionary care subsidize users. Utilization of low‐value cancer screenings is one such example.
For Medicare, the program created equal health insurance entitlements for the elderly, yet enrollees who consume discretionary services (e.g., low‐value cancer screenings) at higher rates are subsidized more than equally by both nonusers and taxpayers. In Medicare Part B, 75 percent of the program costs are financed through general federal tax revenue; the remaining 25 percent comes from beneficiary premiums. Medicare benefits are the same for all beneficiaries. If use of low‐value cancer screenings is related to beneficiary SES, “unintended” cross‐subsidies would occur from taxpayers and low‐SES beneficiaries to high‐SES beneficiaries.
The 2010 Affordable Care Act (ACA) required Medicare to cover Grade A and B screenings recommended by the U.S. Preventive Services Task Force (USPSTF) without patient cost sharing. Even before the ACA, Medicare had gradually expanded coverage of preventive care, including various cancer screenings. The consideration of value, however, has rarely been factored into binding requirements of insurance benefits in those coverage expansion efforts. For example, Medicare covers screenings at a broader level than USPSTF guidelines. It reimburses certain cancer screenings (e.g., breast cancer screening) without an upper age cap (CMA, 2015), whereas USPSTF recommendations typically vary by age (Table 1). These arrangements may have led beneficiaries to overuse low‐value services—potentially at different rates by SES.
Table 1.
Outcome Measures, Medicare Coverage, and USPSTF Guidelines
| Outcome Measures | Medicare Coverage Policiesa , b | Current USPSTF Guidelines c Relevant to Our Measures | Major USPSTF Guideline Changes during 2007–2013 |
|---|---|---|---|
| Mammograms performed on women ≥75 |
All female Medicare beneficiaries ≥35 are eligible (annually for those ≥40) |
Grade B: biennial screening mammography for women 50–74 years; Grade I: women ≥75 |
The current guideline was released in 2009, updating the 2002 guideline by providing specific recommendations for mammography screening by age. Previously, screening mammography was recommended every 1–2 years for all women ≥40 |
| Pap smears used by women >65 or those without a cervix |
All female Medicare beneficiaries are eligible (biennially for women at normal risk) |
Grade A: screening for cervical cancer in women age 21–65 years with cytology (Pap smear) every 3 years; Grade D: women >65, who have had adequate prior screening; Grade D: women who have had a hysterectomy |
The current guideline was released in 2012, updating the 2003 recommendations. It differs from the previous recommendation in that Pap smear every 3 years. But the D grade assigned to screenings on women older than 65 or those without a cervix remained unchanged |
| Prostate‐specific antigen (PSA) tests by men >74 | All male Medicare beneficiaries ≥50 are eligible (annually) | Grade D: screening for prostate cancer | The current guideline was released in 2012. Since 2008, the USPSTF had recommended against PSA screening for prostate cancer in men aged 75 years and older. It now recommends against PSA screening for prostate cancer in all age groups |
Medicare coverage policies: Medicare Learning Network®, Centers for Medicare and Medicaid Services, October 2015. “Preventive Services Chart.” ICN 006559.
The Affordable Care Act requires Medicare to provide coverage to services recommended by USPSTF at grades A or B without out‐of‐pocket payments from beneficiaries.
Grade A and Grade B: USPSTF recommends the service; Grade D: USPSTF recommends against the service; Grade I: Evidence is insufficient to assess the balance of benefits and harms of the service (Grade Definitions. U.S. Preventive Services Task Force. October 2014. http://www.uspreventiveservicestaskforce.org/Page/Name/grade-definitions).
Low‐value screenings may only take up a small share of the total Medicare spending (Colla et al. 2015), but their unintended redistribution is of concern. If insurance benefits accrue disproportionately toward beneficiaries who consume discretionary low‐value services, it is not only an issue of inefficient resource use but also undesirable distributional effects in health insurance settings. Despite their importance, such financial implications have not been explored or discussed in the prior literature on health care overuse.
To fill these gaps, we first examined the relation of SES characteristics to use of low‐value cancer screenings in Medicare, focusing on the importance of socioeconomic status (income and education) and access barriers (e.g., having supplementary coverage or a usual source of care) in explaining differences in utilization. Next, we quantified the amount subsidized from nonusers and taxpayers to users of low‐value cancer screenings in Medicare based on individual characteristics and access barriers. We found that beneficiaries with higher income or private supplementary coverage were more likely to use low‐value screenings and, in turn, received larger net subsidies from Medicare.
Methods
Study Data
Our primary data source comes from the 2007–2013 Medicare Current Beneficiary Survey (MCBS) Access to Care files. This nationally representative survey provides comprehensive information on demographics, socio‐economic status, access to care, and insurance sources of the U.S. elderly population. For fee‐for‐service (FFS) beneficiaries, the survey is linked to the Medicare claims data, which were used to identify overused low‐value health services, including low‐value cancer screenings, in prior literature (Chan et al. 2013; Schwartz et al. 2014; Colla et al. 2015; Segal et al. 2015). The Carrier and Outpatient claims files were searched to obtain the diagnoses (International Classification of Diseases, Ninth Revision [ICD‐9]) and procedures (the Health Care Financial Agency's common procedures coding system [HCPCS] codes & Current Procedural Terminology [CPT] codes) for billed tests, and the Medicare‐approved reimbursement amounts.
Study Sample
We analyzed a sample of community‐dwelling Medicare FFS beneficiaries who were continuously enrolled in Part B programs for the entire calendar year and who met the gender specification for each cancer screening. Because our objective is to examine the unintended cross‐subsidies arising from equal premiums (a share of Part B costs) paid but different financial benefits taken, we excluded those who enrolled in Medicaid and helped with Medicare Part B premiums because they do not contribute to the premium and thus this cross‐subsidy issue is not relevant to them. We further excluded end‐stage renal disease entitled beneficiaries and beneficiaries who were likely to have screenings to monitor existing chronic conditions: those with a claims‐documented or self‐reported history of prostate cancer (ICD‐9: 185, V10.46) or prostatectomy (ICD‐9: 60.21, 60.29, 60.3‐60.6, 60.61, 60.62, 60.69; CPT: 55810, 55812, 55815, 55801, 55821, 55831, 55842, 55845), mastectomy (HCPCS: 19180, 19200, 19220, 19303,‐19307; ICD‐9‐CM: 85.24, 85.44, 85.46, 85.48, 85.41, 85.43, 85.45, 85.47), lumpectomy (ICD‐9‐CM: 85.20‐85.21; CPT: 19120, 19125, 19126), or high cervical cancer risk (V15.89). Enrollees in Medicare managed care plans were also excluded because claims data are not available for them.
Low‐Value Screening Measures
For each low‐value screening measure, we first identified the eligible beneficiaries (denominator) for the screening as those who met the age specification in the USPSTF guideline of low‐value screenings and who did not have a diagnosis of disease symptoms that would otherwise make their cancer tests diagnostic rather than preventive, based on established algorithms (Freeman et al. 2002; Randolph et al. 2002; Freeman et al. 2003; Walter et al. 2006; National Committee for Quality Assurance [NCQA] 2007, 2010, 2013; Ma et al. 2014; Xu and Dowd 2015). For example, the denominator for low‐value mammograms includes women ≥75 with no symptom diagnosis. The USPSTF recommendations have been commonly used to define low‐value services in prior work (Kale et al. 2013), although they are not a basis of Medicare coverage policies (Table 1 compares Medicare policies and USPSTF guidelines). Second, within this denominator group for each low‐value test, we constructed an outcome variable of use of the low‐value test—an indicator capturing the receipt of the test in a year. Thus, a value of 1 is assigned to preventive Pap smear use by women past the age of 65 years or those without a cervix, PSA test use by men older than 74, and mammograms performed on women ≥75.
Key SES Measures
Our key SES measures are income and education. We expect both variables to be positively associated with low‐value service use because people with high SES generally use preventive care more than those with low SES. In our data, income was available in ranges. We grouped the income ranges into four categories1 (Agency for Healthcare Research and Quality 2014b): Poor ($0–$10,000), Near Poor ($10,001–$20,000), Middle Income ($20,001–$40,000), and Top Income level (more than $40,000). MCBS income data capture total income, collected from 13 sources of income and assets (e.g., pensions, Social Security benefits, investment earnings) of the respondent and spouse (if married). In cases of missing data, hot deck imputation was used by the survey to generate responses based on previously reported income sources, amounts, and demographic characteristics (Alecxih et al. 2001). Despite their comprehensiveness in survey methods, the MCBS income data may be subject to underreporting, particularly among married beneficiaries (Goldman and Smith 2001; Shoemaker et al. 2012). To gauge the potential impacts of this issue, we conducted two robustness tests: (1) we limited our analysis to those who were not married and (2) we combined the two lowest income groups (Poor and Near Poor) to address the possibility of underreporting in low‐income groups.
For education, we constructed three categories—College, High School, and Less than High School—based on self‐reported education information in MCBS.
Another SES indicator was whether beneficiaries had supplemental private insurance (e.g., Medigap or an employer‐sponsored health plan). Because supplemental private insurance helps to pay for Part B coinsurance and deductibles, we expect beneficiaries with supplemental coverage to use more screening tests than those without it. In sensitivity analyses, we used separate indicators for different sources of supplemental coverage.
Analytic Models
We first examined SES‐related differences in overutilization. We used logistic regression to predict the probability of receiving a low‐value screening (Test it). We estimated the model separately for mammogram, Pap smear, and PSA. We accounted for the complex survey design of MCBS data by applying cross‐sectional sample weights and the overlapping sample by clustering standard errors within individuals. The unit of analysis is person i in year t. Our model is as follows:
| (1) |
Coefficients β 1, β 2, and β 3 capture the income effects (the reference group is Poor), and β 4 and β 5 represent the education effects (the reference group is “Less than high school”). Private Insurance it is the supplemental insurance indicator, and Year t represents year dummies that control for time‐specific effects common to all of the sample. We expect the use of low‐value screenings to decrease over time due to rising attention to the issue and updates in clinical guidelines, whereas an increasing trend may be observed due to the removal of cost‐sharing requirements.
The control variables (X it) include individual and market characteristics that are associated with preventive care use. Individual‐level factors are beneficiary's age, race, marital status, and access to a usual source of care: individuals who are older, white, married, and have a usual source of care are more likely to obtain preventive care (Lerman et al. 1990; Paskett et al. 1997; Fiscella et al. 2002; Sambamoorthi and McAlpine 2003). Skeptical views toward health care providers (McAlearney et al. 2012) and language barriers (Woloshin et al. 1997; Fiscella et al. 2000) are related to fewer preventive screenings. We captured them with indicators of English proficiency and of trust and communication between patients and doctors. Certain health characteristics, such as being a current smoker or having poorer health conditions, also correlate with screening behaviors (Deshpande, McQueen, and Coups 2012; Xu, Dowd & Abraham, 2016).
Market characteristics were obtained from the Area Health Resource Files. We used physician capacity, which is measured by primary care physician availability per 100,000 people, as greater supply of physicians is associated with higher rates of cancer screenings (Ferrante et al. 2000; Roetzheim et al. 2001). We controlled for the percentage of the Medicare population in managed care to capture spill‐over effects of managed care on Medicare FFS: beneficiaries in areas with higher managed care penetration are likely to use more preventive care (Lawrence, Mattingly, and Ludden 1997; Greene, Blustein, and Laflamme 2001; Chernew and Baicker 2010).
Our second analysis measured the unintended financial subsidies that arise from different utilization probabilities estimated in Equation (1). Our model assumed all enrollees are in a large insurance pool where they share the same premiums.2 The cost of a screening ($test) was represented by the average Medicare‐approved payment associated with a screening test,3 which was adjusted for the Geographic Practice Cost Index and computed in 2013 dollars. Until 2010, Medicare Part B had paid 80 percent of screening costs and had required 20 percent coinsurance from FFS beneficiaries (20 percent of $test). Taxpayers share 75 percent of the Part B costs and FFS enrollees pay the remaining 25 percent through premiums. Thus, an enrollee's expected premium related to a screening can be computed as 25 percent of the Medicare costs for the test, times the average expected probability of using it (), whereas Medicare's financial subsidy to the enrollee was a function of individual probability () of consumption. We calculated the net financial subsidies as a function of expected Medicare subsidy minus the individual's expected premium.4 Since 2011, coinsurance for preventive cancer screenings has been 0 by ACA decree. We thus estimated the net financial subsidies separately for before and after 2010 (Equations 2a and 2b).
| (2a) |
| (2b) |
We restricted the analysis to contemporaneous subsidies to our sample enrollees who are eligible for the Medicare‐financed test in question. For example, the net subsidy of using low‐value mammograms was calculated based on women who are age 75 or older. Our analysis did not attempt to evaluate intergenerational income transfers of the entire Medicare program, nor did we attempt to estimate the effect of overriding Medicare policy, for example, women subsidizing prostate exams for men.
Results
Table 2 presents the descriptive statistics of screening samples. In the mammogram or Pap smear samples, around 40 percent of enrollees fell into poor or near‐poor income levels.5 These income groups represented only 26 percent of PSA sample enrollees. A similar pattern is observed in the distribution of education: 43 percent college education for the PSA sample versus 31 percent and 37 percent for the mammogram and Pap smear samples, respectively. Over half of the sample had private supplemental coverage. About 23 percent of the female sample ≥75 used low‐value mammograms. Utilization rates of low‐value Pap smears were 9 percent. About 24 percent of men older than 74 underwent low‐value PSA tests. Overall, the utilization rates decreased from 2007 to 2013 (Figure 1). The descriptive statistics indicated a clear, disparate pattern of low‐value screenings by income and education levels that persisted over time.
Table 2.
Characteristics of Sample
| Mammogram (N = 16,396) | Pap Smear (N = 30,830) | PSA Test (N = 10,559) | |
|---|---|---|---|
| Low‐value screening rate | 0.23 | 0.09 | 0.24 |
| Having a private supplemental insurance | 0.60 | 0.63 | 0.57 |
| Living in metro area | 0.76 | 0.76 | 0.76 |
| Income level: poor | 0.09 | 0.08 | 0.05 |
| Income level: near poor | 0.37 | 0.30 | 0.21 |
| Income level: middle | 0.37 | 0.38 | 0.43 |
| Income level: top | 0.17 | 0.24 | 0.32 |
| Education level: less than high school | 0.10 | 0.07 | 0.12 |
| Education level: high school | 0.59 | 0.56 | 0.45 |
| Education level: college | 0.31 | 0.37 | 0.43 |
| Current smoker | 0.04 | 0.06 | 0.07 |
| Being married | 0.31 | 0.42 | 0.67 |
| Age | 82.84 | 77.79 | 81.57 |
| Race: black | 0.07 | 0.07 | 0.05 |
| Race: other race | 0.03 | 0.03 | 0.04 |
| Race: white | 0.90 | 0.90 | 0.91 |
| Self‐reported health: excellent | 0.16 | 0.18 | 0.17 |
| Self‐reported health: very good | 0.33 | 0.34 | 0.32 |
| Self‐reported health: good | 0.32 | 0.31 | 0.32 |
| Self‐reported health: fair | 0.15 | 0.14 | 0.15 |
| Self‐reported health: poor | 0.04 | 0.04 | 0.04 |
| Having a usual source of care | 0.97 | 0.97 | 0.96 |
| Not proficient in English | 0.03 | 0.03 | 0.03 |
| Distrust doctor | 0.07 | 0.07 | 0.06 |
| Bad communication with doctor | 0.22 | 0.20 | 0.20 |
| MA plan penetration | 16.31 | 16.41 | 16.47 |
| Physician number/100,000 population | 48.22 (35.48) | 48.40 (36.04) | 48.09 (36.22) |
Figure 1.
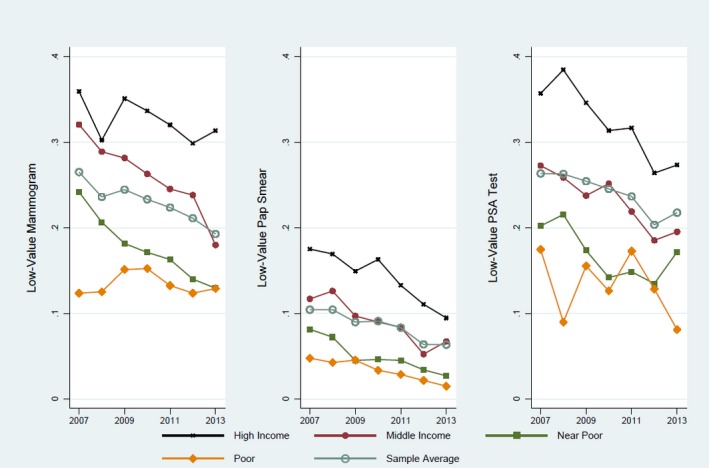
Descriptive Utilization Level of Low‐Value Cancer Screenings
Associations between SES Characteristics and Low‐Value Tests
Table 3 displays the marginal effects on the probability of using each low‐value screening. We found a significant, positive association between income and consumption. Women whose income belonged to “Top” and “Middle” levels were 6.43 percentage points and 4.24 percentage points more likely to use low‐value mammograms, respectively, compared with the “Poor” category. Women in the top income group also had a higher probability of receiving low‐value Pap smears (3.17 percentage points). Similarly, men in the top income group used more low‐value PSA tests than their poor peers (5.79 percentage points). We found that the income‐related disparate patterns in consumption persisted during the entire observation period.
Table 3.
Predicted Probability of Using Low‐Value Screenings (Marginal Effects, Robust Standard Errors)
| Low‐Value Mammogram | Low‐Value Pap Smear | Low‐Value PSA Test | |
|---|---|---|---|
| Year 2013 | −8.77*** (1.28) | −5.67*** (0.84) | −3.31** (1.63) |
| Year 2012 | −6.39*** (1.23) | −5.23*** (0.80) | −4.42*** (1.67) |
| Year 2011 | −4.68*** (1.20) | −2.66*** (0.75) | −1.06 (1.62) |
| Year 2010 | −4.12*** (1.13) | −1.43** (0.72) | −0.75 (1.58) |
| Year 2009 | −2.94*** (1.06) | −1.35* (0.70) | −0.83 (1.52) |
| Year 2008 | −2.50** (0.98) | 0.16 (0.68) | 1.33 (1.48) |
| Having a private supplemental insurance | 25.76*** (0.78) | 9.21*** (0.46) | 25.85*** (0.97) |
| Income level: near poor (Ref: poor) | 1.90 (1.63) | 1.18 (1.25) | 0.44 (2.78) |
| Income level: middle | 4.24** (1.71) | 2.04* (1.23) | 2.47 (2.70) |
| Income level: top | 6.43*** (2.01) | 3.17** (1.35) | 5.79** (2.93) |
| Education level: high school (Ref: less than high school) | 1.13 (1.73) | 1.97 (1.35) | −0.05 (1.80) |
| Education level: college | 2.71 (1.92) | 4.34*** (1.43) | 0.94 (1.92) |
| Current smoker | −12.03*** (1.57) | −3.49*** (0.79) | −3.38* (1.77) |
| Living in metro area | −2.46** (1.00) | 1.22** (0.53) | −2.03* (1.21) |
| Being married | −0.10 (0.94) | 1.66*** (0.55) | 0.10 (1.16) |
| Age | −1.54*** (0.08) | −0.62*** (0.04) | −0.95*** (0.11) |
| Race: black (Ref: white) | −2.22 (1.80) | 0.19 (1.10) | −1.75 (2.63) |
| Race: other race | −4.84* (2.82) | −2.89** (1.40) | −3.77 (3.15) |
| Having usual source of care | 14.74*** (1.65) | 5.16*** (1.06) | 14.70*** (1.83) |
| Self‐reported health: very good | −0.26 (1.04) | −0.18 (0.62) | 1.07 (1.32) |
| Self‐reported health: good | −1.71 (1.10) | −0.69 (0.66) | 0.34 (1.38) |
| Self‐reported health: fair | −3.16** (1.31) | −1.55* (0.79) | −2.79* (1.59) |
| Self‐reported health: poor | −10.81*** (1.71) | −3.73*** (1.07) | −9.01*** (2.03) |
| Not proficient English | −4.63 (3.50) | 0.50 (2.00) | −13.14*** (3.12) |
| Distrust doctor | −0.07 (1.59) | 1.73 (1.10) | −3.17 (2.03) |
| Bad communication with doctor | −2.80*** (1.04) | −1.17* (0.62) | 1.41 (1.38) |
| MA plan penetration | −0.12*** (0.04) | 0.02 (0.03) | −0.06 (0.05) |
| Physician number/100,000 population | 0.06*** (0.02) | 0.01 (0.01) | −0.02 (0.03) |
| N | 16,396 | 30,830 | 10,559 |
***Significance at .01; **significance at .05; *significance at .10.
Women with a college education were 4.34 percentage points more likely to use a low‐value Pap smear than those with less than a high school education. Part of the education effects could have been explained away by income effects. After controlling for income, the associations between education and low‐value mammograms or PSA tests were not statistically significant.
Having private supplemental coverage and having a usual source of care were both strongly related to increased use of all low‐value screenings. Whites were more likely to receive low‐value Pap smears than nonblack racial minorities. Being a smoker and having lower levels of perceived health in general decreased use of low‐value screenings. Clinicians may have played an important role in use of low‐value mammograms. Specifically, a 1 SD increase (35.48) in the number of primary care doctors per 100,000 people was related to a 2.13 percentage point utilization increase (35.48*0.06). Enrollees who indicated a lack of communication with their providers were less likely to use low‐value mammograms.
Our regression results indicated that use of low‐value mammogram and Pap smear tests continuously dropped over time, at increasing rates. These results are encouraging yet surprising, given the removal of cost sharing for these tests since November 2010. A significant decreasing trend in low‐value PSA tests started only in 2012, possibly due to the recent controversial debates on PSA screenings (Hoffman and Nguyen 2011).
Robustness Tests
Analyses with different specifications showed consistent results with the primary analysis. Results from the analysis excluding married beneficiaries were very similar to the primary finding, suggesting that potential underreporting of income among married beneficiaries did not influence our finding. Another test that combined the two low‐income groups (Poor and Near Poor) also confirmed the main result of the positive relationship between income and use of low‐value mammograms and Pap smears.
We also estimated the model only among people with private supplemental coverage—that is, those who are likely to have higher income and/or be better educated (Atherly, 2001)—and found similar results: differences in low‐value mammograms or PSA tests by income remained significant. We then used separate indicators for different sources of supplementary plans (self‐purchased, employer‐sponsored supplemental insurance [ESI] for Medicare retirees, both self‐purchased and ESI, other source, and no coverage) because ESI plans are often more generous than self‐purchased plans (McArdle, Neuman, and Huang 2014). The results on income and education effects were similar to the primary analysis. Having any source of private coverage increased utilization of low‐value screening, and beneficiaries with both ESI and self‐purchased extra coverage had the highest likelihood of using low‐value screenings.
We conducted separate analyses by race and found income effects on low‐value mammogram and Pap smear use among white enrollees. However, income was not consistently associated with low‐value screenings among other racial groups. Finally, we examined the role of contacts with the health care system by using the number of ambulatory (physician office, outpatient services, or preventive medicine) visits. We found that additional visits significantly increased the probabilities of receiving low‐value screenings.
Economics of Disparities
Based on Equations (2a) and (2b), the coverage would provide a financial benefit (net subsidy) to an enrollee if the expected subsidy from Medicare is greater than beneficiary's expected share of the premium. Table 4 shows the net subsidy amounts from Medicare coverage. On average, each woman received an average net subsidy of $13.02 for a low‐value mammogram before 2011, and $16.56 afterward. With a 26 percent consumption rate for low‐value mammograms prior to 2011, each of them shared $4.34 of Medicare costs ((1 – 20 percent) × 25 percent × $test × 0.26), and taxpayers would pay about $13.02 for each service. With more than 47 percent of female Medicare beneficiaries aged 75 and older (U.S. Department of Health and Human Services (HHS) 2013), the total cost to taxpayers would be about $150 million per year. The contributions from Medicare were $17.36 and $22.07 before and after 2011, respectively. Although the individual subsidy amounts appear modest, in the long run, the cumulative subsidy amounts for all users can be large.
Table 4.
Net Subsidies from Using Low‐Value Screening Tests
| Characteristics | Low‐Value Mammogram | Low‐Value Pap Smear | Low‐Value PSA Test | |||
|---|---|---|---|---|---|---|
| Pre‐ACA | Post‐ACA | Pre ‐ACA | Post‐ACA | Pre‐ACA | Post‐ACA | |
| Sample average | 13.02 | 16.56 | 2.07 | 0.98 | 8.50 | 9.52 |
| Income level: top | 19.99a | 25.80a | 3.87a | 1.74a | 12.15a | 13.61a |
| Income level: middle | 15.36a | 18.04a | 2.27 | 0.89 | 8.60 | 8.55 |
| Income level: near poor | 10.19 | 10.12 | 1.12 | 0.34 | 5.30 | 4.21 |
| Income level: poor (Ref) | 5.98 | 6.96 | 0.60 | 0.16 | 3.26 | 3.10 |
| Having a usual source of care | 13.37a | 16.97a | 2.12a | 1.00a | 8.82a | 9.88a |
| Not having a usual source of care (Ref) | 1.69 | 2.56 | 0.56 | 0.21 | 0.92 | 0.26 |
| White (Ref) | 13.70 | 17.42 | 2.14 | 1.01 | 8.91 | 9.95 |
| Black | 7.66 | 10.33a | 1.63 | 0.83 | 4.30 | 5.76 |
| Other race | 5.14 | 4.43 | 0.65a | 0.37a | 3.32 | 3.86 |
| Has supplemental insurance | 19.26a | 26.45a | 2.96a | 1.50a | 13.44a | 16.13a |
| No supplemental insurance (Ref) | 0.53 | 0.76 | 0.24 | 0.10 | 0.73 | 0.79 |
| Obtained less than high school education (Ref) | 6.73 | 6.90 | 0.36 | 0.10 | 5.48 | 5.11 |
| Obtained high school education | 12.43 | 14.75 | 1.64 | 0.65 | 7.75 | 8.36 |
| Obtained college education | 16.70 | 21.69 | 3.22a | 1.51a | 10.35 | 11.74 |
Statistically significant effect at .05, estimated in Equation (1).
Medicare's coverage of a more expensive service is associated with more net subsidies to users. Thus, the amount of expected individual premium share and net subsidies are smaller for low‐value Pap tests and PSA tests. Eligible female enrollees received an average of $2.07 net subsidy from using a low‐value Pap smear test before 2011. The net subsidy to a male enrollee was $8.50 for a PSA test. The subsidy amounts remained relatively similar in post‐ACA years.
Having higher income and supplemental private insurance strongly predicted more net subsidies, suggesting a regressive redistribution through premiums.6 In recent years, Medicare financially contributed more to these screenings due to the zero cost‐sharing requirement. Enrollees with socioeconomic advantages maintained higher probabilities to consume low‐value screening tests; as a result, the gap in net subsidies widened. For instance, relative to poor women, $14.01 extra net subsidies were distributed to the highest‐income women from using low‐value mammograms before 2011, but this gap increased to $18.84 afterward. Similarly, receiving a low‐value PSA test brought increasingly larger net subsidies to men who had supplemental insurance coverage ($12.71 and $15.34 more than those without, prior to and after 2011, respectively).
Discussion and Conclusion
Lately, health care organizations and insurance companies have turned attention to designing strategies to reduce low‐value services. As a result, there is an urgent need to identify targeted populations to curb use of low‐value care. Our study generates timely information by empirically assessing the effects of individual socioeconomic characteristics on the use of low‐value cancer screenings in Medicare. Despite decreasing trends, use of these screenings occurs in all population groups. We found substantial SES variations in consumption patterns, largely stemming from income and private supplemental insurance status, but also driven by educational and racial differences. Physician availability in a market and a beneficiary's contacts with the health care system also played noticeable roles in increasing the use of low‐value screenings.
Our study also revealed the unintentional redistributions associated with using discretionary services covered by insurance. On average, beneficiaries received positive net financial subsidies from using screenings not recommended by the guidelines. About 10–15 percent of our sample (mostly the socioeconomically disadvantaged) received negative net subsidies because of the premium built into the coverage for these low‐value screenings. The Part B premium needed to cover the costs of these services is borne by all enrollees, regardless of their likelihood of utilization. These results suggest that more resources are transferred to individuals with better SES status and have less access to care barriers. These unintended cross‐subsidies would be much larger when accounting for costs of follow‐up services, such as supplementary imaging, biopsies, and treatments as a result of cancer screenings.
With rising health care costs, coverage or benefit expansions are often contentious issues. It is important to note that our results do not advocate for equal access to excessive screenings, which would not be a desirable equality. In fact, preventive services may bring negative consequences, such as risks of excessive radiation from mammograms, or unnecessary and costly treatments from positive test results. In addition, we do not argue for rationing health care (e.g., denying necessary mammograms to women at 78 years of age). Rather, high‐value preventive care should be made affordable and accessible, whereas use of low‐value services should be discouraged. This is a challenging task, as major clinical guidelines can change over time and vary in their recommendations. For example, the American Cancer Society (ACS) significantly modified their mammogram recommendations at least six times since the 1980s (ACS 2013), and the ACS and USPSTF recommendations on age and frequency of mammogram screenings still do not align. Reactions by providers to limiting low‐value care have also been mixed. For instance, there are still controversies over USPSTF's recommendations against PSA screening in asymptomatic men (Hoffman and Nguyen 2011) and over the age and frequency when a mammogram could be beneficial to women (Anderson et al. 2014).
Our findings raise a question of whether Medicare's eliminating cost sharing for all screening tests (including low‐value services) and for both low‐income and high‐income beneficiaries is a reasonable or fair policy from the perspective of redistribution. Taxpayers and socioeconomically disadvantaged Medicare enrollees subsidize better‐off enrollees for low‐value services. Selectively increasing consumer cost sharing for high‐income groups and/or for low‐value screenings may partially counter the unfair distribution of insurance benefits resulted from overuse of screenings.
The financial and behavioral implications from our study are meaningful for other discretionary health services. There is a continued interest in reducing or eliminating consumer out‐of‐pocket costs for preventive care among Medicare beneficiaries. For example, policy makers have proposed to expand Medicare coverage to a preoperative physician visit prior to a preventive colonoscopy (PRNewswire, 2013). In the meantime, recent literature has pointed out various overused services in the current health care system, ranging from screening, diagnostic tests, to expensive therapeutic care and medication treatments (Chan et al. 2013; Kale et al. 2013). However, socioeconomically disadvantaged beneficiaries could also fail to use high‐value, recommended preventive services, and as a result contemporary unintended cross‐subsidies also happen. As utilization of many covered services is likely to vary based on individual characteristics, lower‐income beneficiaries (who are not eligible for Medicaid) facing additional access barriers would fare the worst in terms of net benefits in Medicare FFS.
With the continuous discussions of expanding insurance benefits, the issue of unintentional cross‐subsidies is important to both beneficiaries and taxpayers. Coverage of higher‐cost discretionary care would be associated with larger unintended cross‐subsidies. Medicare may consider decreasing payments to socially undesirable services that lead to minimal health benefits but generate regressivity. In addition, an insurance benefit design that applies means‐tested coinsurance rates for services that are at the discretion of consumers could help offset the regressive effects of coverage.
Our study has a few limitations. A common challenge faced by researchers comes from using the administrative claims data to definitively identify overuse/low‐value health care (Chan et al. 2013; Colla et al. 2015; Segal et al. 2015). Claims may not provide enough details of important disease (e.g., cancer) history or symptoms that clinically justified the screening, which would appear as low value in claims data. Claims data may also miss screenings not reimbursed by Medicare (e.g., paid entirely out‐of‐pocket) and include potentially inconsistent or inaccurate coding of services by different providers (Freeman et al. 2002; Tan, Kuo, and Goodwin 2012; Xu and Dowd 2015). While Medicare claims data have limited information on clinical risks or symptoms, they were commonly relied upon in past research that measured overuse of low‐value care (Chan et al. 2013; Colla et al. 2015; Segal et al. 2015). Taking these limitations into consideration, we used algorithms that are likely to provide conservative estimates of the use of services.
As discussed earlier, the income data of MCBS are also subject to underreporting, which is a common problem in survey data. Our estimates of net subsidies may be imprecise due to the lack of objective income data. In particular, the unintended cross‐subsidies may be larger than calculated if higher‐income families tended to underreport their income. We may have overestimated the redistribution issue if the underreporting is concentrated among low‐income beneficiaries. However, the MCBS survey is still the most comprehensive socioeconomic dataset available for a representative sample of Medicare beneficiaries, with income data collected from multiple sources of income and assets. Furthermore, our robustness tests—one limiting the sample to single beneficiaries and one combining low‐income groups—both showed a clear decreasing pattern in utilization by income group, and produced similar estimates to the primary analysis. Thus, any bias in our cross‐subsidy results is likely to be very small.
Despite these limitations, our study is the first to report variation in the use of low‐value screenings by SES in Medicare. We also showed that such variation led to an unequal distribution of insurance benefits in a direction favoring socioeconomically advantaged beneficiaries. This suggests that the undesirable distributional effect associated with discretionary care utilization needs to be considered in efforts to discourage the use of low‐value services and/or to reduce the gap in use of preventive care.
Supporting information
Appendix SA1: Author Matrix.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This study was funded by the AcademyHealth New Investigator Small Grant Program. We would like to thank Dr. Thomas Wickizer from the Ohio State University for being the project mentor.
Disclosures: None.
Disclaimers: None.
Notes
The near poor category included some records with the value “less than $25,000” and the middle income category included some records with the value “$25,000 or more.”
Medicare Part B premium has been means tested since 2007, impacting beneficiaries whose income ranked the top 5 percent.
Costs of the follow‐up tests or treatments from a positive screening result were not accounted in this analysis.
Medicare annual deductibles were waived for these cancer screenings during our study period (Center for Medicare Advocacy [CMA] 2015).
The income distributions in our samples are in line with statistics in prior research using MCBS data (Jacobson, Huang, and Neuman 2014).
By controlling for covariates that may also influence the screening consumption, we captured partial effects. We ran robustness tests that estimate the full effects of insurance and income, respectively. The estimates of net subsidies from those tests were very similar to the main results.
References
- Agency for Healthcare Research and Quality . 2014a. “2010 National Healthcare Quality and Disparities Reports” [accessed on September 7, 2015]. Available at http://archive.ahrq.gov/research/findings/nhqrdr/nhqrdr10/qrdr10.html
- Agency for Healthcare Research and Quality . 2014b. “Medicare Current Beneficiary Survey (MCBS): 2012 National Healthcare Quality and Disparities Reports: Detailed Methods Appendix” [accessed on February 10, 2015] Available at http://archive.ahrq.gov/research/findings/nhqrdr/nhqrdr12/methods/mcbs.html
- Alecxih, L. , Corea J., Gross D., Caplan C., Brangan N., and Gibson M. J.. 2001. “Reply.” Health Services Research 35 (6): 1365–70. [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society (ACS) . 2014. “Chronological History of ACS Recommendations for the Early Detection of Cancer in People without Cancer Symptoms” [accessed on October 29, 2014]. Available at http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/chronological-history-of-acs-recommendations
- Anderson, B. L. , Urban R. R., Pearlman M., and Schulkin J.. 2014. “Obstetrician‐Gynecologists’ Knowledge And Opinions about the United States Preventive Services Task Force (USPSTF) Committee, the Women's Health Amendment, and the Affordable Care Act: National Study After the Release of the USPSTF 2009 Breast Cancer Screening Recommendation Statement.” Preventive Medicine 59: 79–82. [DOI] [PubMed] [Google Scholar]
- Atherly, A. 2001. “Supplemental insurance: Medicare's accidental stepchild.” Medical Care Research and Review 58 (2): 131‐61. [DOI] [PubMed] [Google Scholar]
- Center for Medicare Advocacy (CMA) . 2015. “Part B” [accessed on July 28, 2015]. Available at http://www.medicareadvocacy.org/medicare-info/medicare-part-b/
- Chan, K. S. , Chang E., Nassery N., Chang H. Y., and Segal J.. 2013. “The State of Overuse Measurement: A Critical Review.” Medical Care Research and Review 70 (5): 473–96. [DOI] [PubMed] [Google Scholar]
- Chernew, M. , and Baicker K.. 2010. “Spillovers in Health Care Markets: Implications for Current Law Projections” [accessed on April 6, 2015]. Available at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/ReportsTrustFunds/downloads/spillovereffects.pdf
- Colla, C. H. , Morden N. E., Sequist T. D., Schpero W. L., and Rosenthal M. B.. 2015. “Choosing Wisely: Prevalence and Correlates of Low‐Value Health Care Services in the United States.” Journal of General Internal Medicine 30 (2): 221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande, A. D. , McQueen A., and Coups E.. 2012. “Different Effects of Multiple Health Status Indicators on Breast and Colorectal Cancer Screening in a Nationally‐Representative US Sample.” Cancer Epidemiology 36 (3): 270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante, J. M. , Gonzalez E. C., Pal N., and Roetzheim R. G.. 2000. “Effects of Physician Supply on Early Detection of Breast Cancer.” Journal of the American Board of Family Medicine 13 (6): 408–14. [DOI] [PubMed] [Google Scholar]
- Fiscella, K. , Franks P., Gold M. R., and Clancy C. M.. 2000. “Inequality in Quality: Addressing Socioeconomic, Racial, and Ethnic Disparities in Health Care.” Journal of the American Medical Association 283 (19): 2579–84. [DOI] [PubMed] [Google Scholar]
- Fiscella, K. , Franks P., Doescher M. P., and Saver B. G.. 2002. “Disparities in Health Care by Race, Ethnicity, and Language among the Insured: Findings from a National Sample.” Medical Care 40 (1): 52–9. [DOI] [PubMed] [Google Scholar]
- Freeman, J. L. , Klabunde C. N., Schussler N., Warren J. L., Virnig B. A., and Cooper G. S.. 2002. “Measuring Breast, Colorectal, and Prostate Cancer Screening with Medicare Claims Data.” Medical Care 40 (8): IV36–42. [DOI] [PubMed] [Google Scholar]
- Freeman, J. L. , Goodwin J. S., Zhang D., Nattinger A. B., and Freeman D. H.. 2003. “Measuring the Performance of Screening Mammography in Community Practice with Medicare Claims Data.” Women and Health 37 (2): 1–15. [DOI] [PubMed] [Google Scholar]
- Goldman, D. P. , and Smith J. P.. 2001. “Methodological Biases in Estimating the Burden of Out‐of‐Pocket Expenses.” Health Services Research 35 (6): 1357–70. [PMC free article] [PubMed] [Google Scholar]
- Goodwin, J. S. , Singh A., Reddy N., Riall T. S., and Kuo Y.. 2011. “Overuse of Screening Colonoscopy in the Medicare Population.” Archives of Internal Medicine 171 (15): 1335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene, J. , Blustein J., and Laflamme K. A.. 2001. “Use of Preventive Care Services, Beneficiary Characteristics, and Medicare HMO Performance.” Health Care Financing Review 22 (4): 141–53. [PMC free article] [PubMed] [Google Scholar]
- Hoffman, K. F. , and Nguyen P. L.. 2011. “The Debate over Prostate Cancer Screening Guidelines.” American Medical Association Journal of Ethics 13 (1): 10–5. [DOI] [PubMed] [Google Scholar]
- Jacobson, G. , Huang J., and Neuman T.. 2014. “Medigap Reform: Setting the Context for Understanding Recent Proposals.” The Henry J. Kaiser Family Foundation, January 2014 Issue Brief [accessed on March 3, 2016]. Available at https://kaiserfamilyfoundation.files.wordpress.com/2014/01/8235-02-medigap-reform-setting-the-context-for-understanding-recent-proposals1.pdf
- Kale, M. S. , Bishop T. F., Federman A. D., and Keyhani S.. 2013. “Trends in the Overuse of Ambulatory Health Care Services in the United States.” Journal of the American Medical Association Internal Medicine 173 (2): 142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepka, D. , Breen N., King J. B., Benard V. B., and Saraiya M.. 2014. “Overuse of Papanicolaou Testing among Older Women and among Women without a Cervix.” Journal of the American Medical Association Internal Medicine 174 (2): 293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kressin, N. , and Groeneveld P.. 2015. “Race/Ethnicity and Overuse of Care: A Systematic Review.” Milbank Quarterly 93 (1): 112–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, K. M. , Mattingly P. H., and Ludden J. M.. 1997. “Trusting in the Future: The Distinct Advantage of Nonprofit HMOs.” Milbank Quarterly 75 (1): 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman, C. , Rimer B., Trock B., Balshem A., and Engstrom P. F.. 1990. “Factors Associated with Repeat Adherence to Breast Cancer Screening.” Preventive Medicine 19 (3): 279–90. [DOI] [PubMed] [Google Scholar]
- Ma, X. , Wang R., Long J. B., Ross J. S., Soulos P. R., Yu J. B., Makarov D. V., Gold H. T., and Gross C. P.. 2014. “The Cost Implications of Prostate Cancer Screening in the Medicare Population.” Cancer 120: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlearney, A. S. , Oliveri J. M., Post D. M., Song P. H., Jacobs E., Waibel J., Harrop J. P., Steinman K., and Paskett E. D.. 2012. “Trust and Distrust among Appalachian Women Regarding Cervical Cancer Screening: A Qualitative Study.” Patient Prevention and Counseling 86 (1): 120–6. doi: 10.1016/j.pec.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle, F. , Neuman T., and Huang J.. 2014. “Retiree Health Benefits at the Crossroads.” The Henry J. Kaiser Family Foundation, April 14, 2014 [accessed on March 3, 2016]. Available at http://kff.org/report-section/retiree-health-benefits-at-the-crossroads-overview-of-health-benefits-for-pre-65-and-medicare-eligible-retirees/
- National Committee for Quality Assurance (NCQA) . 2007. HEDIS 2008, Volume 2: Technical Specifications. Washington, DC: NCQA. [Google Scholar]
- National Committee for Quality Assurance (NCQA) . 2010. “HEDIS 2010 Quick Reference Guide” (As cited in Health America website) [accessed August, 2014]. Available at http://healthamerica.coventryhealthcare.com/web/groups/public/documents/webcontent/c051476.pdf
- National Committee for Quality Assurance (NCQA) . 2013. “HEDIS Technical Specifications.” HEDIS Code Quick Reference Guide—Women's Health Services. (As cited in United Healthcare Insurance Company website) [accessed on August 8, 2014]. Available at https://www.uhccommunityplan.com/content/dam/communityplan/healthcareprofessionals/providerinformation/MI-Provider-Information/MI_Womens_Health_OBGYN_HEDIS_Quick_Reference_Guide.pdf
- O'Malley, A. S. , Forrest C. B., Feng S., and Mandelblatt J.. 2005. “Disparities Despite Coverage Gaps in Colorectal Cancer Screening among Medicare Beneficiaries.” Archives of Internal Medicine 165 (18): 2129–35. [DOI] [PubMed] [Google Scholar]
- Paskett, E. D. , Rushing J., D'Agostino R. Jr, Tatum C., and Velez R.. 1997. “Cancer Screening Behaviors of Low‐income Women: The Impact of Race.” Women's Health 3 (3–4): 203–26. [PubMed] [Google Scholar]
- PRNewswire . 2013. SCREEN Act Introduced in House and Senate for Colorectal Cancer Awareness Month. Bethesda, MD: PRNewswire‐USNewswire; Available at http://www.prnewswire.com/news-releases/screen-act-introduced-in-house-and-senate-for-colorectal-cancer-awareness-month-199517591.html [Google Scholar]
- Randolph, W. M. , Mahnken J. D., Goodwin J. S., and Freeman J. L.. 2002. “Using Medicare Data to Estimate the Prevalence of Breast Cancer Screening in Older Women: Comparison of Different Methods to Identify Screening Mammograms.” Health Services Research 37: 1643–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roetzheim, R. G. , Gonzalez E. C., Ramirez A., Campbell R., and Van Durme D. J.. 2001. “Primary Care Physician Supply and Colorectal Cancer.” Journal of Family Practice 50 (12): 1027–31. [PubMed] [Google Scholar]
- Sambamoorthi, U. , and McAlpine D. D.. 2003. “Racial, Ethnic, Socioeconomic, and Access Disparities in the Use of Preventive Services among Women.” Preventive Medicine 37: 475–84. [DOI] [PubMed] [Google Scholar]
- Schwartz, A. L. , Landon B. E., Elshaug A. G., Chernew M. E., and McWilliams J. M.. 2014. “Measuring Low‐Value Care in Medicare.” Journal of the American Medical Association Internal Medicine 174 (7): 1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal, J. B. , Nassery N., Chang H. Y., Chang E., Chan K., and Bridges J. F.. 2015. “An Index for Measuring Overuse of Health Care Resources with Medicare Claims.” Medical Care 53 (3): 230–6. [DOI] [PubMed] [Google Scholar]
- Sheffield, K. M. , Han Y., Kuo Y. F., Riall T. S., and Goodwin J. S.. 2013. “Potentially Inappropriate Screening Colonoscopy in Medicare Patients: Variation by Physician and Geographic Region.” Journal of the American Medical Association Internal Medicine 173 (7): 542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker, J. S. , Davidoff A. J., Stuart B., Zuckerman I. H., Onukwugha E., and Powers C.. 2012. “Eligibility and Take‐Up of the Medicare Part D Low‐Income Subsidy.” Inquiry 49 (3): 214–30. [DOI] [PubMed] [Google Scholar]
- Tan, A. , Kuo Y. F., and Goodwin J. S.. 2012. “Integrating Age and Comorbidity to Assess Screening Mammography Utilization.” American Journal of Preventive Medicine 42 (3): 229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (HHS) . 2013. “CMS Statistics 80146‐23597” [accessed on July 28, 2015]. Available at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/CMS-Statistics-Reference-Booklet/Downloads/CMS_Stats_2013_final.pdf
- U.S. Preventive Services Task Force (USPSTF) . 2009. “Final Update Summary: Breast Cancer: Screening” [accessed on November 18, 2014]. Available at http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening
- U.S. Preventive Services Task Force (USPSTF) . 2012a. “Final Update Summary: Prostate Cancer: Screening” [accessed on November 18, 2014]. Available at http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening
- U.S. Preventive Services Task Force (USPSTF) . 2012b. “Final Update Summary: Cervical Cancer: Screening” [accessed on November 18, 2014]. Available at http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening
- Walter, L. C. , Bertenthal D., Lindquist K., and Konety B. R.. 2006. “PSA Screening among Elderly Men with Limited Life Expectancies.” Journal of the American Medical Association 296 (19): 2336–42. [DOI] [PubMed] [Google Scholar]
- Wilt, T. J. , Harris R. P., and Qaseem A.. 2015. “Screening for Cancer: Advice for High‐Value Care from the American College of Physicians.” Annals of Internal Medicine 162 (10): 718–25. [DOI] [PubMed] [Google Scholar]
- Woloshin, S. , Schwartz L. M., Katz S. J., and Welch H. G.. 1997. “Is Language a Barrier to the Use of Preventive Services?” Journal of General Internal Medicine 12 (8): 472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W. Y. , and Dowd B.. 2015. “Lessons from Medicare Coverage of Colonoscopy and Prostate‐Specific Antigen Test.” Medical Care Research Review 72 (1): 3–24. [DOI] [PubMed] [Google Scholar]
- Xu, W. Y. , Dowd B., and Abraham J.. 2016. “The Impact of State Mandated Benefits of Preventive Cancer Screenings on Consumers.” European Journal of Health Economics 17 (2): 203‐15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


